Abstract
Reactive oxygen species (ROS) form a class of molecules with both positive and negative impacts on cellular health. Negatively, ROS may react with cellular constituents including proteins, lipids, and DNA to generate an array of oxidative lesions. These lesions may compromise genome stability which is critical for long-term cellular homeostasis and healthy progeny. Paradoxically, ROS also function as strong signalling molecules that mediate various growth-related responses, so their presence is also essential for cellular metabolism. While ROS are generated in an unregulated manner by physical stresses such as exposure to ionizing radiation and biochemical malfunctions such as mitochondrial leakage, cells also contain the NADPH oxidases NOXs and DUOXs, which specifically generate ROS in a wide variety of tissues. While the NOXs/DUOXs may be involved in maintaining optimal cellular redox levels, there is also accumulating evidence that NADPH oxidases-derived ROS may elevate the risk for genomic instability and cancer. Cancer cells may produce high levels of ROS, and in some cases, the source of these ROS has been linked to NOX/DUOX deregulation as reported for prostate cancer (NOX1 and NOX5), melanoma and glioblastoma (NOX4) among others. In addition, recent studies reveal that targeting NADPH oxidases with NOXs inhibitors may impair tumor growth in vivo; indicating that these proteins may be useful targets in future clinical strategies to fight cancer. This review provides an overview of the current knowledge concerning these enzymes, their roles in cancer, and their potential as targets in future cancer therapies.
Keywords: NADPH oxidases, ROS, Cancer, Cancer therapy, Tumors, NOXs inhibitors
INTRODUCTION
Cellular imbalance in reactive oxygen species (ROS) leads to oxidative stress and has been linked to the pathogenesis of many diseases, including cancer [1]. ROS refer to a group of chemically reactive molecules in which the superoxide anion (O(2)(−)) appears as the main precursor of other species including hydrogen peroxide (H(2)O(2) and the hydroxyl radical (OHo). Generated by the addition of one electron to an oxygen molecule, the negatively charged superoxide anion is short-lived and unable to readily cross membranes, restricting it to local reactions. However, H(2)O(2) derived from superoxide dismutation can readily cross the membrane barrier, resulting in more persistent and widespread effects [2]. The hydroxyl radical (OHo), which can be generated from Fenton and Haber-Weiss reactions in the presence of H(2)O(2) and ions such as Fe(II) and Cu(I), is able to react with many cellular biomolecules [1, 3] (Fig. 1).
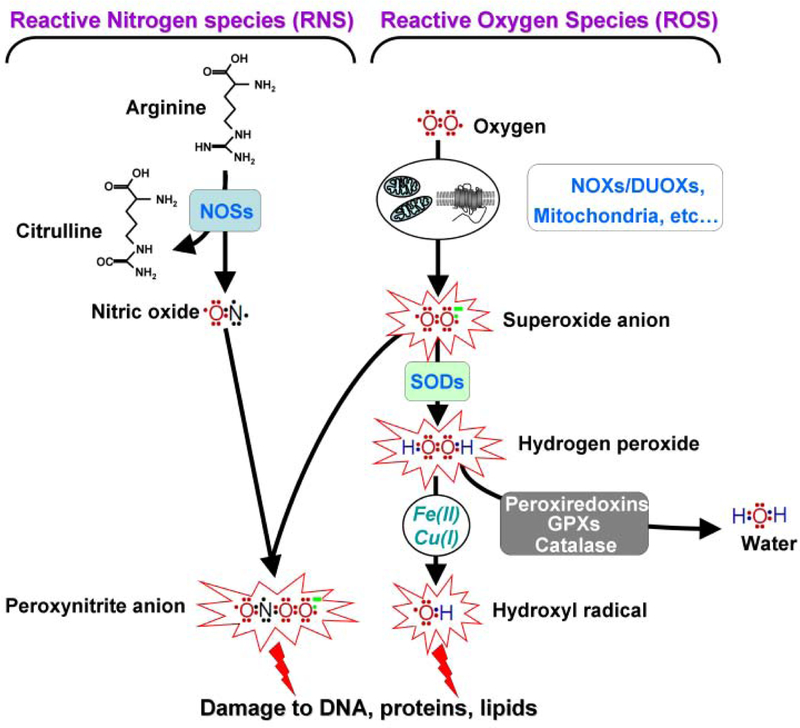
Fig. (1). Roles of the NADPH oxidase family members, the NOXs and DUOXs, in the production of reactive nitrogen (RNS) and oxygen species (ROS).
The NOXs, DUOXs, and the mitochondria are involved in the formation of the superoxide anion O(2)(−) While O(2)(−) is rapidly converted into H(2)O(2) by the superoxide dismutases (SODs), it can react with nitrite oxide (NO) to produce peroxynitrite (ONOOº). NO formation occurs following the oxidation of L-arginine by members of the nitric oxide synthase (NOSs) family. The Fenton and Haber-Weiss reaction generate and ONOOº are highly the hydroxyl radical (OHº) from the superoxide anion O(2)(−) and H(2)O(2) in the presence of metal ions such as Fe(II) and Cu(I). H(2)O(2) can also be converted into H(2)O by the actions of multiple enzymes such as peroxiredoxins, glutathione peroxidase (GPXs) and catalase. Both, OHº reactive molecules that are able to react with many cellular biomolecules including proteins, lipids, and DNA to generate an array of oxidative lesions.
While ROS can be generated by the interaction of ionizing radiation with water molecules, the great majority of ROS has a metabolic origin as natural by-products of cellular respiration [4]. Some of the important biological functions that are attributed to ROS include apoptosis, homeostasis, wound healing, immunity and cell signaling [5]. Cellular ROS signaling has been shown to occur in caveolae and lipid rafts, at cell–matrix adhesions, at cell–cell contacts, in lamellipodial leading edge focal complexes, in endosomes and in the nucleus. These signals regulate many cellular processes including cell proliferation, differentiation and gene expression [5, 6].
Elevated ROS levels may cause significant cellular damage manifested as cytotoxicity and mutagenicity [1, 5, 7] and have been implicated in numerous chronic diseases including atherosclerosis, hypertension, inflammation, carcinogenesis and tumor growth among others (reviewed in [5, 8]). Additionally, elevated ROS levels are found in many tumors where they are believed to play a major role in cancer progression (see [9] for review).
While the mitochondria are major contributor of ROS primarily as a byproduct, the recently described NOX/DUOX family of NADPH oxidases appears to be unique in that its physiological function is specifically the generation of ROS [10]. This family encompasses 7 homologs: NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1 and DUOX2 and is known as the NOX/DUOX family (Fig. 2). NADPH oxidases members transfer an electron from NADPH to molecular oxygen, thus producing superoxide anion which is the basis for both reactive oxygen and nitrogen species (Fig. 1). The NADPH oxidases share a common core structure consisting of 6 transmembrane domains with two heme binding sites and a relatively long cytoplasmic C-terminus containing FAD and NADPH-binding sites.
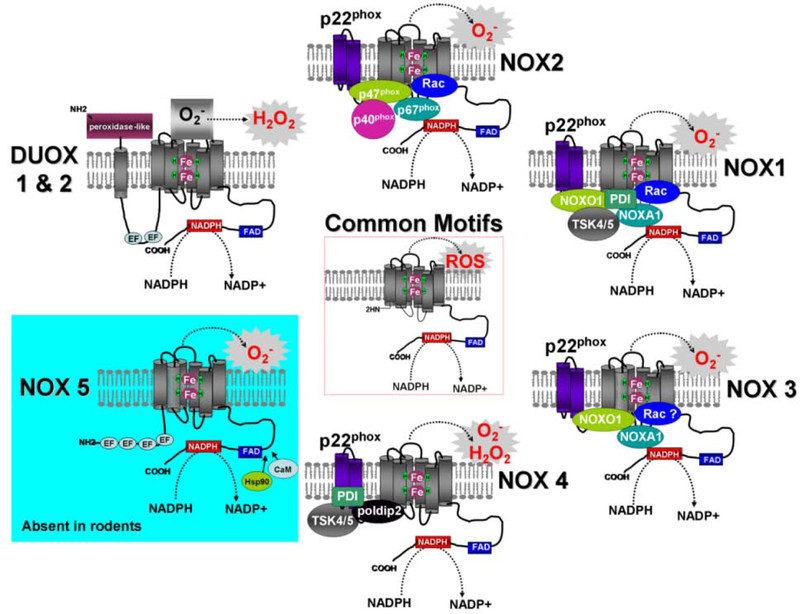
Fig. (2). Stucture and activation of the NOXs and DUOXs.
While members of the NADPH family of oxidases share similar structures and enzymatic functions, these proteins rely on different mechanisms for activation. NOX2 (top) requires p47(phox) phosphorylation along with the presence of p22(phox), p40(phox), p67(phox) and small GTPases Rac proteins. NOX1 (upper right) activity is mediated by p22(phox), NOXO1 or its homologue p47phox, NOXA1, the small GTPases Rac as well as a protein disulfide isomerase (PDI) and the p47(phox) analogue tyrosine kinase substrate (Tsk4/5). Similarly, NOX3 (lower right) activity requires p22(phox), NOXO1, NOXA1 and possibly Rac. In contrast to NOX1/2/3, NOX4 (bottom) is constitutively active. Its activity requires p22(phox), and two regulatory proteins, named poldip2 and NOXR1 (not shown), which have been recently described to enhance NOX4 activity. PDI and Tsk4/5 have been reported to bind and activate NOX4. Additionally, Rac1 could be implicated in the function of NOX4 in endothelial and mesangial cells (see text for details). Both NOX5 (lower left) and DUOX1 and DUOX2 (upper right) are activated by Ca(2+) in regard to the presence of calmodulin-like domain (EF) but do not require other protein partners for activity. NOX5 activity can be regulated by the binding of Hsp90 or Calmodulin (CaM) to the C-terminus of the protein. However, the full processing of DUOXs to the plasma membrane requires the maturation factors DUOX Activator 1 (DUOXA1) and DUOX Activator 2 (DUOXA2). Contrary to the NOXs, DUOXs display an extracellular peroxidase-like domain in addition to their C-terminal NOX-like region. It should be noted that NOX5 is not present in rodent genomes (mouse, rat).
Cellular transformation has been associated with increased ROS production and there are now accumulating evidences showing roles for the participation of members of the NOX/DUOX family in cancer biogenesis and/or progression [9, 11]. For example, inhibition of some members leads to cancer cell death and to retarded tumor growth [12, 13]. In addition, antioxidants alone or in combination with other cancer therapies are used in oncology for cancer prevention or to mitigate unwanted side effects [14]. For all these reasons, targeting NOXs and/or DUOXs is seen as a potential powerful tool against cancer. The aim of this review is to summarize the recent findings regarding the structure and function of NOXs, particularly in reference to their roles in cancer and their utility as putative targets for cancer treatment.
1. STRUCTURE OF THE NADPH OXIDASES NOXs AND DUOXs
1.1. NOX2
This review focuses first on NOX2 because it was the first NOX identified; thus much of what is known about the structure of the other NOXs is derived from comparisons to NOX2. NOX2 has been proposed as a primary component of the microbicidal oxidase system of phagocytes. NOX2 is present in the phagosomal membrane of phagocytic cells including macrophages, monocytes and neutrophils [10]. The integral membrane proteins NOX2 and p22(phox) form heterodimers called flavocytochrome b(558). After exposure of phagocytic cells to microorganisms or inflammatory mediators, cytosolic factors such as p67(phox), p47(phox), p40(phox) and Rac1 or Rac2 move to the membranes where they come together with NOX2 and p22(phox) to form an activated NADPH oxidase complex [15, 16] (Fig. 2).
NOX2 converts cytosolic NADPH into NADP+ and gives rise to the production of two electrons and one proton. While the proton remains in the cytosol, the two electrons are transported through the phagosomal membrane to reduce two oxygen molecules leading to the formation of two superoxide anions in the intraphagosomal space [17]. Researchers initially believed that the NOX protein was restricted to phagocytic cells, but human genome analysis revealed that NOX2 was representative of a new protein family that included six other homologs that were found in a large array of human tissues: NOX1, NOX3, NOX4, NOX5, DUOX1 and DUOX2 (Fig. 2 and Table 1). All NADPH oxidases are transmembrane proteins with conserved structural domains involved in electron transport across biological membranes to reduce oxygen to superoxide [18]. Their common structural features include (1) a NADPH-binding site located at the COOH terminus, (2) six conserved transmembrane domains, (3) a FAD-binding site located between the NADPH-binding site and the transmembrane domains, and (4) four highly conserved heme-binding sites located in the third and fifth, and in the fourth and sixth transmembrane domains of the NOXs and DUOXs respectively (Fig. 2, Common Motifs). In addition, an amino-terminal calmodulin-like domain containing binding sites for Ca(2+) (EF-hands) is present in NOX5 and DUOX1/2 and an additional NH2-terminal transmembrane domain plus a peroxidase homology domain are present in DUOX1/2 (Fig. 2). Structure and functions of all NOXs and DUOXs have been extensively reviewed previously [16–19].
Table 1.
Tissue Distribution and Pathophysiological Roles of the NADPH Oxidases NOXs/DUOXs
| Nadph Oxidase | Tissue Distribution | Physiological & Pathophygiologiacal Roles | Ref. |
|---|---|---|---|
| NOX1 | Colon, stomach, uterus, prostate, kidney, vascular smooth muscles, arteries, etc. | Host defense (bacterial killing), cell division, arthrosclerosis, cancers, cardiovascular diseases, etc. | [10, 18, 21] |
| NOX2 | White blood cells of myeloid lineage (neutrophiles, monocytes/macrophages, eosinophiles), ovary, placenta, adipocytes, cardiomyocytes, microglia, etc. | Host defense (microbicidal action), development of cardiovascular disease, HIV pathogenesis, neurodegeneration, angiogenesis, cancers (role in immune suppressive network and angiogenesis). | [10, 18, 53, 54] |
| NOX3 | Inner ear (sensory epithelia and ganglia), foetal tissues. | Senescence, ocotonia formation. | [10, 18, 58] |
| NOX4 | Kidney, thyroid, endothelial cells, smooth and skeletal muscle cells, heart, pancreas, ovary, testis, osteaclasts, fibroblasts, astrocytes, adipocytes, neurons, etc. | Oxygen sensing, senescence, oncogene-induced senescence, cell transformation (melanoma), apoptosis, insulin-dependent signaling, cardiovascular disease, diabetes, angiogenesis, obesity, cancers, etc. | [10, 25, 27, 63, 68] |
| NOX5 | Lymphoid tissue (lymphocytes, macrophages), testis, ovary, placenta, arteries. | Ca(2+)-dependent ROS generation, male fertility, spermatogenesis (early steps, capacitation reaction, sperm oocyte fusion), putative role in lymphocytes differentiation, prostate adenocarcinoma, etc. | [18, 32, 34] |
| DUOX1 | Thyroid, respiratory epithelia, lung, intestine, aortic smooth muscle, etc. | Thyroid hormone synthesis, host defense, hypothyroidism, crosslinking of extracellular matrix proteins. | [18, 37, 38] |
| DUOX2 | Thyroid, intestine, salivary and rectal gland epithelia, lung, etc. |
This table is meant to be illustrative rather than comprehensive, as these enzymes are found to be expressed in many other tissues.
Medically, NOX2 deficiency is associated with chronic granulomatous disease (CGD) [20]. In this human disorder, the phagocytic cells are able to engulf pathogens including bacteria but they are unable to kill them in the phagocytic vacuoles because of an inability to produce free radicals or other small molecules.
1.2. NOX1
Discovered in 1999, NOX1 shares 56% sequence identity with NOX2 [21]. NOX1 requires p22(phox), p47(phox) (or its homologue NOXO1 (NOX Organizer1)), the p67(phox) (or its homologue NOXA1 (NOX Activator 1)) and small G protein Rac1 for its activity [18]. The requirement for Rac1 may depend on the cell type, but the mechanism of action remains to be clarified (Fig. 2). Two recently identified proteins, Tks4 and Tks5 (tyrosine kinase substrates 4 and 5) belonging to the p47(phox)/ NOXO1 protein family are able to interact with NOXA1 and have been recently described as NOX1 regulators by localizing ROS production to particular cellular compartments [22] (Fig. 2). Furthermore, another partner known as protein disulfide isomerase (PDI) has been reported to bind and activate NOX1 in vascular smooth muscle cells [23] (Fig. 2).
1.3. NOX3
The widely less studied NOX3 shares 58% of sequence homology with NOX2 [24]. Like NOX1, NOX3 function requires p22(phox), NOXO1 and NOXA1. So far, it is unknown if the presence of small GTPases Rac proteins is required for NOX3 activity [18] (Fig. 2).
1.4. NOX4
NOX4 shares 39% sequence homology with NOX2, and like the NOX1/2/3 proteins, NOX4 requires p22(phox) for stabilization. NOX4 displays a high constitutive ROS-generating activity [25, 26]. Initially and in contrast to NOX1/2/3 proteins, NOX4 was first thought to be unaffected by interacting factors, except in kidney mesangial cells where its activity was reported to be mediated by the small GTPase Rac1 under the control of angiotensin-II stimulation [27]. Two novel partners referred as poldip2 [28] and NOXR1 [29] were recently described to positively regulate NOX4 activity (Fig. 2). As previously reported for NOX1, PDI and Tsk4/5 have been described to activate NOX4 in vascular smooth muscle cells [22, 23, 30]. Four other splice variants of NOX4 known as NOX4B, NOX4C, NOX4D and NOX4E have been identified in the human lung cancer cell line A549 as well as in lung tissues [31]. Cells over-expressing NOX4B and NOX4C have been reported to exhibit decreased ROS levels, suggesting that these isoforms may act as dominant negative downregulators for ROS production. Moreover, both NOX4D and NOX4E variants were shown to lack the transmembrane domains and are not considered to be directly associated with cell membranes. However, NOX4D, which displays both FAD and NADPH binding motifs, has a ROS-generating activity similar to the one of NOX4 prototype [31].
1.5. NOX5
NOX5, the most recent NADPH oxidase to be identified [32], shares only 27% homology with NOX2. NOX5 activity has been shown to be regulated by calcium and contains an amino-terminal calmodulin-like domain containing four Ca2+ binding sites (Fig. 2, EF-hands). Also, NOX5 activity can be regulated by the binding of Hsp90 or Calmodulin (CaM) to its C-terminus [33] (Fig. 2). Five other splice variants of NOX5 (NOX5α, β, δ, γ and a truncated variant NOX5-S or ε) have been identified in endothelial cells [34, 35]. Finally, it is important to note that NOX5 is absent from the rodent (rat, mouse) [18] but is present in lagomorphs, making the rabbit an interesting model for NOX5 functional studies [36].
1.6. DUOX1 and DUOX2
DUOX1 and DUOX2, also known as dual oxidases, were first discovered in the thyroid gland [37], acting as the main source of H(2)O(2) production required for the organification of iodine and the biosynthesis of thyroid hormones [38]. DUOX1 and DUOX2 share 83% sequence homology and are the largest members of the NADPH oxidase family at 180–190 kDa compared to 55–90 kDa for other NOXs [18]. The name DUOX refers to the presence in the proteins of an extracellular peroxidase-like domain in addition to their C-terminal NOX-like region (Fig. 2). Two maturation factors, DUOX Activator 1 (DUOXA1) and DUOX Activator 2 (DUOXA2), were recently found to be required for DUOX1 and DUOX2 localization to the plasma membrane [39]. In addition, DUOX1 and DUOX2 contain two EF-hands in the N-terminal region, which are involved in the calcium-dependence of DUOXs for ROS-generation (Fig. 2).
2. NADPH OXIDASES AND CANCER - PUTATIVE TARGETS IN CANCER THERAPY
The last decades have seen the identification of the NOXs/DUOXs proteins and their isoforms. At the same time, both NOXs and DUOXs were shown to have critical roles for both carcinogenesis and tumor growth (see below). These explain the reason why there has been an increasing interest for these proteins in the last few years, with the number of scientific publication focusing on NADPH oxidases going from one a day on average in 2000 to 4–5 a day in the last two years (Fig. 3). ROS have been implicated in the etiology of a wide array of human diseases, including cancer [1], and a large body of evidence emphasizes a role for the NADPH oxidases in carcinogenesis and tumor growth (summarized in Fig. 4). It is generally accepted that increased levels of NADPH oxidases or enzymatic dysregulation, would result in increased ROS levels, leading to oxidative stress, genomic instability and ultimately to cancer. In addition, ROS over-production may have a crucial role for cancer immune suppression and tumor growth. As summarized in Table 1, the NADPH oxidases are found in a large array of tissues and have several pathophysiological roles in addition to their normal ones.
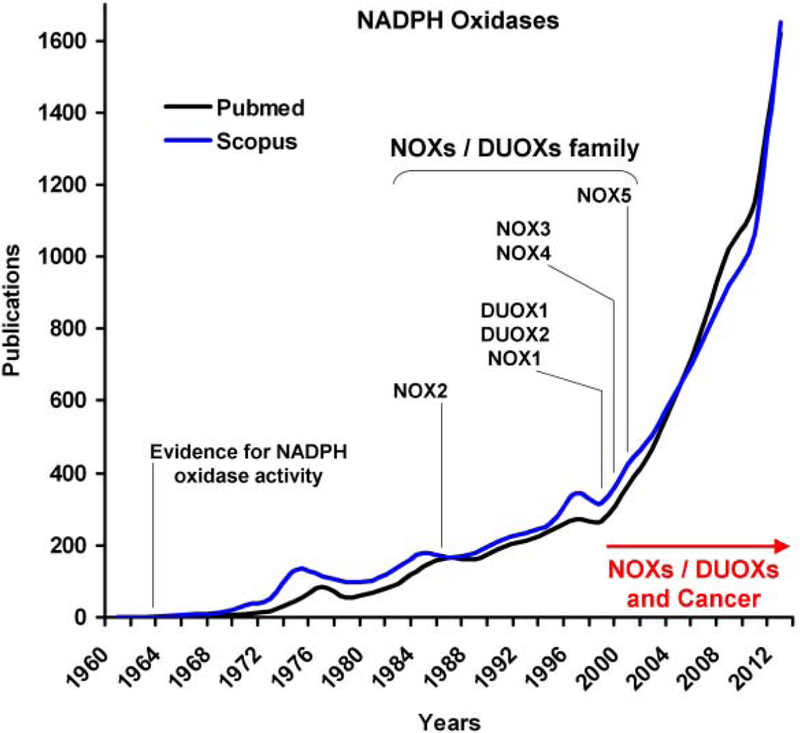
Fig. (3). The emerging roles for the NADPH oxidases NOXs and DUOXs in cancer.
While the first NADPH oxidase activity was described in rat liver microsomes in 1964, it took more than 20 years after that to identify the first NADPH oxidase, NOX2, while analyzing patients with chronic granulomatous disease (CGD) (see text for details). Within the following decade, six other NOX2 homologs were identified in mammals and were referred to as the NOXs/DUOXs family. Since the first study linking NOX1 and cell transformation was published in 1999, there has been an increasing body of evidence establishing a role for the NADPH oxidases NOXs and DUOXs in cancer (see text for details). This growing interest raises the published studies on NADPH oxidases over the last decade, ranging from 200 publications per year in 1999 to almost 1400 publications per year in 2011 (Pubmed and Scopus). The number of publications for the year 2012 is an estimation made from the number of articles that were published in the first quarter of the same year.
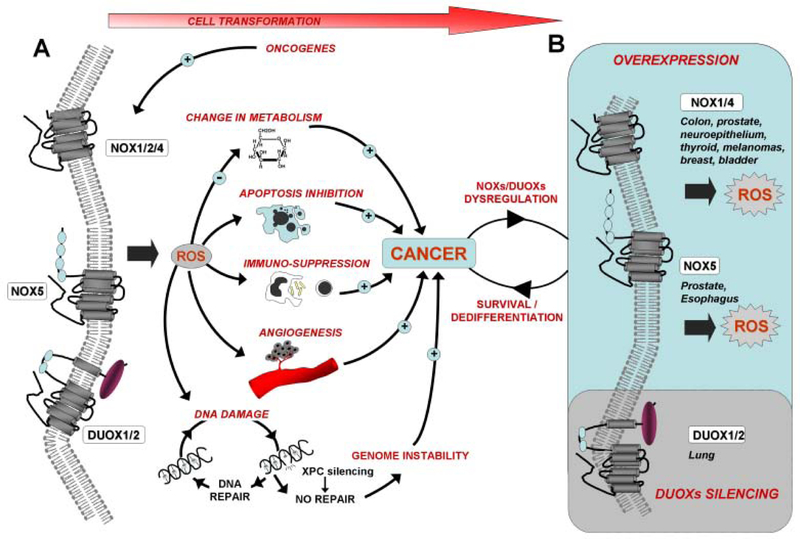
Fig. (4). Model for the role of the NADPH oxidases NOXs and DUOXs in cancer development.
(A) Oncogenes such as Ras and Epstein-Barr virus (EBV) nuclear antigen (EBNA)-1 constitutively activate the NADPH oxidases (i.e., NOX1 and NOX4 by Ras and NOX2 by (EBNA)-1). Oncogenic NOX activation results in accrued ROS production and the formation of both DNA damage and chromosomal aberrations, ultimately leading to genomic instability and cell transformation. Therefore proficient DNA repair is crucial to minimize the risk of tumorigenesis (i.e., mutated xeroderma pigmentosum C (XPC) leads to increased genomic instability; see text for details). As major sources for ROS generated by VEGF and Angiotensin 1 (Ang1), NOX2 and to some extent, NOX1, play critical roles in angiogenesis during carcinogenesis. NOXs, via ROS production, may also play a critical role in the cancer immune suppressive network, especially in the myeloid-derived suppressor cells (MDSC). Additionally, the tumor-associated macrophages (TAMs) robustly express NOX2. The immunosuppressive properties of NOX2 and H(2)O(2) have been demonstrated while ROS generated by NOXs are involved in invasive behavior and cell proliferation. NOX1 and NOX2 favor survival in numerous cell types through the inhibition of apoptosis. Other mechanisms involved in tumorigenesis, such as metabolism alteration (glucose transporter Glut1 activation by NOX2 and NOX4) and cell dedifferentiation (through DUOXs downregulation in airway epithelium), are also attributed to the NOXs. (B) Cell transformation results in NOXs overexpression in many cancers while DUOX silencing was observed in lung cancer cells. NOX1 is prominently expressed in colon and prostate cancers while increased expression of NOX4 is found in a large range of cancers including melanoma, prostate, thyroid, breast, bladder, kidney and colon. NOX5 overexpression was observed in esophageal cancer. NOX overexpression leads to accrued ROS production, which in turn, may increase the survival and proliferation of cancer cells. In contrast, DUOX silencing may promote cell dedifferentiation and promote cancer progression.
2.1. NOX1
Evidence implicating NOXs in cancer came from a study of the relationship between NOX1 and tumor growth [21]. Cells overexpressing NOX1 displayed a transformed phenotype, with an anchorage-independent growth and the ability to produce tumors in vivo in athymic mice. However, further characterization of the original NIH 3T3 cell lines used in this study (YA26 and YA28) revealed the presence of human RasV12 oncogene in their genomes (see [16] for review) raising the issue, currently unresolved that the transformation observed in the NIH 3T3 cells utilized in that study may be attributable solely to NOX1 alone. In fact, a cooperative relationship between NOX1 overexpression and the Ras oncogene has been confirmed [40]. The transduction of normal rat kidney cells (NRK cells) by Ras oncogene was found to be accompanied by increased NOX1 expression [40]. Moreover, when NOX1 was silenced in these cells, their transformed phenotype including anchorage-independent growth was reversed [40]. The link between NOX1 and cancer was later confirmed in a study implicating NOX1 in the autocrine-mediated growth of liver tumor cells through the upregulation of a pathway involving the epidermal growth factor [41]. The authors of this study hypothesized that the role of NOX1 in tumor growth might, at least in part, be due to decreased apoptosis induced by NOX1-generated ROS. The same authors also showed that rat hepatoma cells display a NOX1-dependent resistance to TGF-beta-induced apoptosis, thus suggesting that NOX1 is a prosurvival player in tumor cells [41]. Similar observations were obtained with human bladder cancer cells in which the leukotriene B4 receptor (BLT2) plays a pivotal role in their survival [42]. BLT2 was found to mediate cancer cell survival via up-regulation of both NOX1 and NOX4, resulting in elevated ROS levels. Conversely, inhibition of ROS production by silencing NOX1 or NOX4 or the treatment with a ROS-scavenging drug resulted in increased cell death. The authors hypothesized the existence of a ‘BLT2-NOX1/4-ROS’ cascade to be crucial for cancer survival and highlighted BLT2 and NOXs as potential targets for anti-bladder cancer therapy. It is interesting to note that ROS scavenging by antioxidants or vitamins is already used in the clinic to reduce bladder cancer recurrence [14].
The role of NOX1 in cancer development is further supported by the fact that this enzyme is involved in angiogenesis, a crucial process involved in tumor growth. NOX1-derived H(2)O(2) was shown to be responsible for increased tumor vascularization and for the presence of elevated angiogenesis markers such as vascular endothelial growth factor (VEGF), VEGF receptors and elevated matrix metalloproteinase activity [43]. Again, it is important to point out that oncogenic Ras is present in these NOX1-overexpressing cells (NIH-3T3/YA28 cells). Therefore these findings must be interpreted with caution. Nevertheless, the role of NOX1 in angiogenesis was later supported by a study showing ROS-dependent leukocyte adhesion to endothelial cells [44]. This mechanism is known to induce the disruption of the cell-to-cell junction, leading ultimately to the endothelial cell migration, proliferation and angiogenesis (see [9] for review). A recent study showed that, in the presence of the NOX1 and NOX4 inhibitor GKT136901, endothelial cells did not exhibit ROS production after VEGF and bFGF stimulation, supporting the notion that NOX1 is a key factor in the angiogenesis process [45]. In another study, NOX1 was depicted as a key player in primary melanoma cells invasion through a mechanism involving the loss of epithelial characteristics and the gain of mesenchymal ones, known as epithelial-to-mesenchymal transition (EMT) [46]. EMT, in which the protein matrix metalloproteinase-2 (MMP-2) is involved, is characterized by loss of cell adhesion and increased cell mobility, and results in the loss of epithelial characteristics accompanied by the gain of mesenchymal cell morphology [46].
Because persistent DNA damage is known to be a precursor of carcinogenesis, the role of NOX1 in genomic instability has been explored. It has been recently shown that the genomic instability resulting from xeroderma pigmentosum C (XPC) deficiency is mediated by the simultaneous activation of AKT1 and NOX1, thus suggesting that NOX1 may play a role in the transformation of human keratinocytes [47]. Moreover, in a model of chronic oxidative stress involving the coexpression of NOX1 and its cofactors NOXO1 and NOXA1, it has been shown that NOX1 increased the levels of DNA-8-oxo-7,8-dihydroguanine and the HPRT mutation rate in HeLa cells [48]. This finding raises the question of whether chronic oxidative induced by NOX1 may lead to significant genomic instability by saturating the cell’s DNA repair capacity and lead to significant genomic instability.
NOX1 overexpression has been demonstrated in human prostate cancer. In fact, 80% of human prostate tumors samples display markedly increased NOX1 protein and transcript levels [49]. Similar observations were made in several cancer cells lines developed from 12 cancer patients [49]. In contrast, utilizing an in situ hybridization approach on a large range of tumor samples from ovary, breast, prostate, colon, and lung as well as from melanomas and lymphomas, another study found that NOX1 was exclusively expressed in the colon cancer samples [50].
2.2. NOX2
As with NOX1, the role of NOX2 in cancer development is related to angiogenesis. Indeed, NOX2 is a major source for ROS generated by VEGF and Angiotensin 1, two factors involved in angiogenesis (see [9] for review). Neovascularization following ischemia or treatment with VEGF was inhibited in NOX2-deficient mice. Similar observations were obtained when wild-type mice were treated with the antioxidant ebselen or the NOX inhibitor apocynin [9, 51]. Another role for NOX2 in cancer development may be linked to the cancer immune suppressive network. While ROS production is important for the immune defense to pathogens, recent studies have also shown that ROS may be involved in the silencing of the immune response to cancer [52]. For example, the myeloid-derived suppressor cells (MDSC), known to induce the immune suppression in tumors in a ROS dependent manner, exhibited elevated ROS levels in a variety of tumors [53]. Importantly, in the absence of NOX2 activity, MDSC lost the ability to suppress the response of a subset of T cells, suggesting that ROS-induced immune suppression by MDSC may be due to NOX2 [53]. Additionally, the relationship between increased density of tumors-associated macrophages (TAMs) and decreased survival has been recently discussed in terms of NOX2 expression. TAMs display high NOX2 expression and NOX2-derived ROS were proposed to contribute to cancer cell growth in anaplastic thyroid carcinomas, one of the most aggressive malignancies in humans [54].
Yet, NOX2 function in cancers is not solely restricted to angiogenesis and immune regulation. For example, the malignant transformation and tumor progression driven by the Epstein-Barr virus (EBV) nuclear antigen (EBNA)-1 were attributed to NOX2-derived ROS [55]. While EBNA-1 is known to induce DNA double-strand breaks, chromosomal aberrations and the DNA damage response, these hallmarks of genomic instability were abrogated by NOX2 silencing [55].
2.3. NOX3
NOX3 has been shown to be expressed in rat inner ear and primarily in human fetal tissues [24, 56] and may have a role in organogenesis [57]. While there is no clear link between cancer and NOX3, a recent study pointed out the high NOX3 expression in the human HepG2 hepatoma cells [58]. In these cells, NOX3 was shown to be a new effector for insulin signaling with NOX3-derived H(2)O(2) activating the transcription factor Sp1 via the p42/44 MAPK signaling pathway, and ultimately leading to VEGF-A expression [59]. Because both insulin and VGFF-A play a role in tumor cell proliferation, studying the effect of NOX3 silencing/ inhibition in these cancer cells may reveal if this NOX isoform plays a role in some cancers.
2.4. NOX4
NOX4 has been reported to play a role in angiogenesis and in cardiovascular diseases (see [2] for review) as well as genomic instability, cell death, inflammation-related diseases and cancer [60]. NOX4 plays a major role in cytokines secretion (IL-6 and IL-8) due to hypoxia-induced chronic inflammation, and the subsequent renal cell carcinoma cell invasion [61]. As it is the case for NOX1 and EMT [46], a recent evidence suggests that NOX4 may be a key player in TGF-beta-induced epithelial-to-mesenchymal transition (EMT) and in the migration of normal (MCF10A) and metastatic (MDA-MB-231) human breast epithelial cells. The mechanism underlying these processes which are critical for cancer development, involves the transcriptional regulation of NOX4 by TGF-beta through the SMAD3 transcription factor [62]. However, it is important to note that NOX4 overexpression alone may not be sufficient to induce EMT and other molecular factors may be involved.
NOX4 has been depicted as a crucial mediator in the Ras oncogene-induced DNA damage response and in the subsequent establishment of senescence in human thyroid cells [63]. In normal breast epithelial cells, NOX4 overexpression resulted in multiple phenotypes such as cellular senescence, transformation and apoptosis resistance, some of these characteristics classifying this enzyme, as an oncoprotein [64]. Other observations also point to a critical role for NOX4 in apoptosis resistance and sustained tumor cell proliferation. For example, the specific silencing of NOX4 by RNA interference resulted in cell-growth inhibition while enhancing cell death in glioma cells induced by chemotherapeutic agents like cisplatin [65]. The role of NOX4 in apoptosis resistance was also described in human adenocarcinoma MIAPaCa-2 and PANC-1 cells [66]. The mechanism underlying this resistance to cell death was found to involve the inhibition of tyrosine phosphatases (PTPs) such as LMW-PTP (low molecular weight-protein tyrosine phosphatase) and the enhancement and sustained phosphorylation of JAK2, a major antiapoptotic kinase. Finally, a NOX4-mediated prosurvival pathway was also observed in pancreatic cancer cells [67].
There are other clues connecting NOX4 with cancer incidence. NOX4 was shown to be the most frequently expressed NOX isoform in several tumor cell lines [24]. A large body of evidence within the last decade showed that NOX4 was prominently expressed in various neuroepithelial tumors [65], in more than 60% of human melanoma cells, and in 31% of melanoma tumor biopsies [13]. This pattern of NOX4 overexpression was also reported in both human papillary and follicular thyroid carcinomas [68], in human colon cancers [69], in urothelial carcinogenesis of urinary bladder [70] and in a large range of breast cancer cell lines and primary breast tumors [64].
Beyond its selective overexpression in human tumors, NOX4 appears as a critical mediator in cell transformation and tumor growth, a role highlighted by the finding that NOX4 silencing resulted in decreased ROS production and inhibition of melanoma tumorigenesis in nude mice [13]. In this study, the role of NOX4 in melanoma growth was linked to its regulation of cell cycle progression.
Another study linking NOX4 and cancer revealed that Akt upregulation led to increased levels of NOX4-derived ROS that correlated with enhanced tumor growth in vivo. NOX4-generated ROS scavenging, along with Akt silencing, were proposed by the authors of this study as a potential therapeutic strategy in the treatment of advanced melanoma [71]. The use of NOX4 as a therapeutic target was also suggested for the treatment of hemangiomas [12], the most common type of tumor in infants, whose growth is regulated by the autocrine-acting Tie2 ligand angiopoietin (Ang2). NOX4 silencing in the brain endothelial cell line (bEnd) impaired hemangioma growth in vivo in the same manner as fulvene-5, a novel class of NOX inhibitors [12]. Supporting these observations, another study showed later that NOX4 inactivation markedly attenuated the formation of hemangioendothelioma formation, one of the most common soft tissue tumors in infants [72].
Another anti-cancer strategy targeting NOX4 relies on the finding that because NOX2 and NOX4 may be the sources for a ROS-induced activation of Glut1, a glucose transporter upregulated in cancer cells [73], inhibition of NOX4 may disrupt the cancer cell metabolism, slowing tumor growth.
2.5. NOX5
Just a few years after its discovery, NOX5 was described as essential for the proliferation of the DU 145 prostate cancer cells [74]. Later, the role of NOX5 was depicted in Barrett’s esophageal adenocarcinoma where the expression of NOX5-S, a NOX5 isoform lacking the EF-hand calcium binding domain, was upregulated compared to normal oesophageal squamous epithelial cells. This NOX5 dysregulation was shown to correlate with high grade dysplasia, contributing to increased cell proliferation and decreased apoptosis [75]. It was proposed that acid reflux could activate NOX5-S, resulting in an increase in ROS. ROS overproduction, would then, induce p16 promoter methylation and its subsequent downregulation in a process that may contribute to the progression from Barrett’s esophagus to esophageal adenocarcinoma [76]. Finally, elevated NOX5 was found in some breast tumors and melanomas when compared to the adjacent nontumor tissues [77].
2.6. DUOXs
Because of the widely reported overexpression of other NOXs in many cancer cells, the discovery that DUOX1 and DUOX2 were silenced through hypermethylation of CpG-rich regions in their promoters in lung cancer cells was unexpected [78]. While these differences observed in protein expression between the NOXs and DUOXs were not explained, it was suggested that the DUOXs may be involved in maintaining the differentiated phenotypes of the airway epithelium [78]. Therefore, it may be a possibility that DUOX downregulation participates in cell dedifferentiation towards cell transformation. Additionally, DUOXs may be involved in the regulation of proteins critical for cell cycle or for the DNA damage response and their silencing might allow the cancer cells to escape programmed cell death.
On the other hand, DUOX expression was reported as a predictive marker for the prognosis of thyroid cancers. High DUOX expression was associated with a reduced risk of death in poorly differentiated follicular thyroid carcinoma (PDFC), a cancer known for its aggressive behavior compared to other well-differentiated carcinomas [79].
3. INHIBITORS OF NADPH OXIDASES AND CANCER
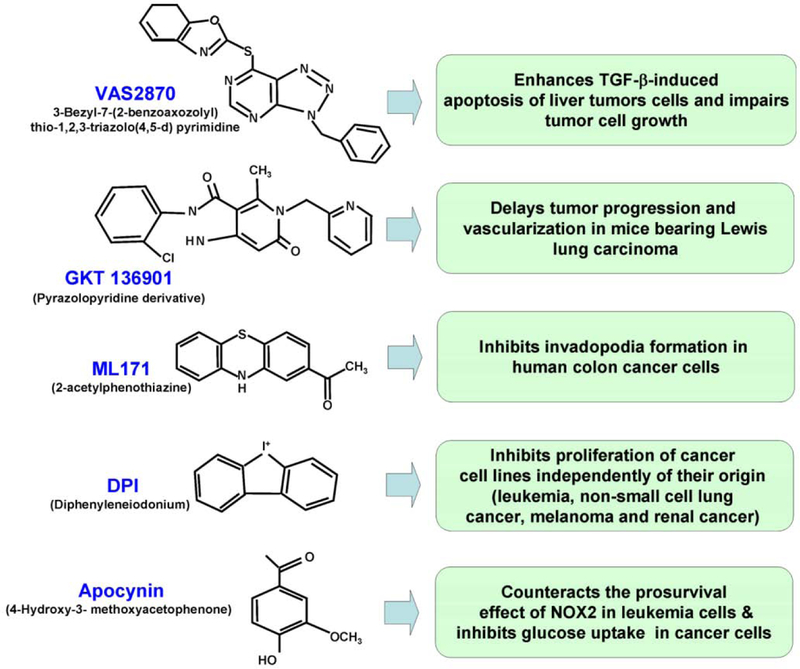
There are no highly specific inhibitors known for any of the NADPH oxidase isoforms. Consequently, compound screening has been increasingly focused on finding isoform-specific inhibitors (see text below). This also explains why some of the studies focusing on the role of these proteins in cancer utilize genetic inhibition by RNA interference. Non-peptides inhibitors of the NADPH oxidases have been developed by several research groups over the last decade, and employed to explore the role of NADPH oxidases in diseases such as angiogenesis, idiopathic fibrosis and cancer both in vitro and in vivo [12, 80]. Some of these compounds, reviewed elsewhere [81], are discussed here in terms of structure and mechanisms of action in cancer therapy.
In addition to non-peptide inhibitors, several studies have demonstrated the potential advantages of using peptide inhibitors. These peptides which mimic specific protein sequences of the NADPH oxidase subunits alter the formation of these different subunits complexes, and may show more specificity when compared to other small molecules [82]. To inhibit ROS production in living cells, these peptides may be coupled to cell-penetrating peptides such as TAT or other protein transduction domains. TAT-coupled peptide inhibitors have been shown to inhibit ROS production in vitro (decreased NADPH oxidase activity in neutrophils) and in vivo (decreased systolic blood pressure in mouse) (reviewed in [82]). Although these studies are encouraging, the effects of NADPH peptide inhibitors in cancer remain to be evaluated. Some of the non-peptide compounds, reviewed elsewhere [81], are discussed here in terms of structure and mechanisms of action in cancer therapy.
3.1. VAS2870
Discovered by high-throughput screening for a selective NADPH oxidase inhibitor, VAS2870 [3-Bezyl-7-(2-benzoaxozolyl) thio-1,2,3-triazolo(4,5-d) pyrimidine] is a triazolopyrimidine derivative, and its analogue VAS3947 are NADPH oxidase-specific and do not inhibit other flavoproteins [83] (Fig. 5). The detailed mechanism of action of the VAS compounds has been described elsewhere [83]. NADPH oxidase inhibition by VAS compounds was detected in vivo against different NOX isoforms and gave IC50 values of 1–2 μM to 13 μM in cell-free assays depending on the targeted NOX isoform [84]. Specificity of NADPH oxidase inhibition by the VAS compounds was confirmed by the fact that VAS2870 shows no intrinsic antioxidant activity. However, VAS inhibitors are not NOX isoform specific. In cells, VAS2870 concentrations of 10 to 20 µM were shown to abolish PDGF-mediated NADPH oxidase activation and ROS production in vascular smooth muscle cells [83]. VAS2870 has been used in a recent study on hepatocellular carcinoma (HCC) [85]. HCC is a major cause of cancer-related death, due in part, to high malignancy and lack of effective therapy. Interestingly, ROS production has been described to play a major role in the growth of hepatic tumor cells [86]. NOX1, NOX2 and NOX4 isoforms are expressed in hepatocytes even though they might have opposing effects with NOX4 favoring apoptosis and NOX1 sustaining survival (see [85] for details). Interestingly, liver tumor cells showed an altered pattern of NOX expression: the pro-apoptotic NOX4 is repressed while NOX1 and other NOX isoforms were expressed (see [85, 87] for details). In addition, NOX1 targeted knockdown by siRNA experiments increased caspases-2 activity and cell death [87]. Because NOX1 may protect liver cancer cells from apoptosis, the potential antitumor activity of VAS2870 was testing in vitro. Indeed, VAS2870 was shown to block ROS production, decreases cell proliferation and enhances the apoptotic response induced by TGF-beta in HCC. Finally, VAS2870 pharmacological inhibition was comparable to those achieved by siRNA experiments suggesting that NADPH oxidase inhibition by VAS compounds might be a useful therapeutic approach to treat certain cancers.
Fig. (5). Non exhaustive list of inhibitors for NADPH oxidases NOXs and DUOXs showing anticancer activities.
While NOXs inhibitors show anticancer activities, only high specificity and low toxicity will allow NADPH oxidase inhibitor to be used in future cancer therapies. The recently discovered NADPH oxidases inhibitors VAS2870, GKT136901, and ML171 show high specificity for NADPH oxidases, displaying marginal effects on other flavoproteins. However the inhibitory effect is not NADPH oxidase isoform specific. A derivative of GKT136901 (GKT137831) is the only clinically available specific NADPH oxidase inhibitor as of this writing. This drug, which shows no signs of toxicity in healthy patients, is being evaluated for the treatment of diabetic nephropathy. In contrast, DPI shows very poor specificity towards NADPH oxidases, targeting other flavoproteins. Therefore DPI may have other nonspecific effects in humans. Apocynin is described to inhibit the translocation of the cytosolic NADPH oxidase components p47(phox) and p67(phox) from the cytoplasm to the membrane. There are also reports that apocynin may act as an antioxidant rather than a NOX inhibitor.
3.2. GKT136901
Also discovered by high-throughput screening, several pyrazolopyridine derivatives have recently been reported as specific NADPH oxidase inhibitors (reviewed by [88]). Compounds such as GKT136901 and its derivatives (Fig. 5) developed by GenKyoTex exhibit strong specificities towards NOX1 and NOX4 with IC50 values lower than 100 nM together with high safety profiles [80]. GKT136901 is more specific for NOX1 and NOX4 with a ten-fold lower potency on NOX2 [45]. The potential of GKT136901 against cancer was tested in vivo with mice bearing Lewis lung carcinoma cells [45]. Compared to vehicle-treated controls, mice receiving one week of oral daily administration of GKT136901 exhibited tumor shrinkage of 34% together with a 59% reduction of tumor vasculature. When animals with established tumors were treated with GKT136901 or the anti-VEGFR2 antibody DC101, both treatments exhibited similar delayed tumor progression and vascularization. Additionally, GKT136901 exhibited no toxicity issues. Because they showed that GKT136901 did not interfere with cancer cell proliferation and apoptosis in vitro, the authors of this study concluded that the delayed tumor growth was due solely to an anti angiogenic activity through decreased NOX1-induced ROS production in endothelial cells.
A derivative compound of GKT136901, named GKT137831, is undergoing clinical evaluation for the treatment of diabetic nephropathy by targeting NOX4. NOX4 is the most abundant NOX isoform expressed in the kidney [24, 25] and has been described to be upregulated in diabetic nephropathy, where it plays a key role in glomerular damage [89]. In fact, a successful completion of a phase 1a clinical study (screening for drug safety) for GKT137831 was recently completed and showed that oral administration of this drug to 36 healthy human subjects displayed no toxicity (see “http://www.genkyotex.com/genkyotex/” for more details). Therefore, to date, this drug is the only clinically approved NOX inhibitor and this may open new opportunities for its use in future clinical studies involving cancer therapies.
3.3. ML171
The compound 2-acetylphenothiazine, also referred to as ML171 (chemical probe #171 from the Molecular Libraries Initiative of the National Institutes of Health), belongs to the class of phenothiazines, drugs known to display various antipsychotic effects [90]. ML171 was first identified by high-throughput screening of 16, 000 compounds [91] by using a luminol-based chemiluminescence assay on human HT29 colon cancer cells which are known to endogenously express only NOX1 isoform (Fig. 5). In HT29 cells, ML171 and its derivative 2-(trifluoromethyl)-phenothiazine inhibited ROS production with a IC50 (HT29) of 0.129 µM (its derivative 2-(trifluoromethyl)-phenothiazine showing a IC50 (HT29) of 0.32 µM) while harboring no cytotoxicity even at 10 µM. Selectivity for NOX1-dependent ROS generation was confirmed with IC50 (Nox1) of 0.25 µM for ML171 and 1 µM for the trifluo derivative coupled with only a marginal activity against the other flavoenzymes.
In human colon cancer cells, NOX1 is necessary for the formation of invadopodia. Invadopodia are specialized cell structures involved in cancer invasion [22] and are characterized by dynamic phospho-tyrosine-rich structures with actin cores, and abundant actin regulatory proteins involved in extracellular matrix (ECM) degradation. When the the effects of ML171were analyzed in DLD1 colon cancer cells, NOX1-dependent invadopodia formation as well as ECM degradation were strongly decreased in such cells [91]. Because ECM remodeling is often linked to cancer metastasis and invasion [46], studies of ML171 and cancer growth/spread in vivo appears as the next critical step towards the use of this drug in future cancer therapy.
3.4. Diphenyleneiodonium (DPI)
DPI is a fulvene analog which has long been used to inhibit NADPH oxidases at low micromolar to submicromolar concentrations (Fig. 5). This compound is known to inhibit NOX2 in neutrophils by abstracting an electron from FAD to create a radical, which in turn forms covalent adducts with the FAD motif of the protein. This process blocks the NADPH oxidase rapidly and irreversibly [92]. At a dose of 500nM, DPI was shown to decrease the angiogenic ability of ovarian cancer cells through the inhibition of NOX4-generated ROS [93]. A recent study looking into the growth inhibitory effect of DPI in the NCI-60 tumor cell panel showed that a DPI concentration of 10 nM altered the proliferation of seven different tumor cell lines (four leukemia cell lines, one non-small cell lung cancer line, one melanoma cell line and one renal cell line) [94]. It is important to note that DPI may have effects independent of NADPH oxidases in cell culture through the targeting of other flavoenzymes including the mitochondrial complex 1 and NOS [95]. Many other small molecules, derivatives of DPI, have been synthesized including triphenylmethane dyes and fulvene derivatives [12]. Unfortunately, despite the efforts developed to make these compounds more potent and specific than DPI, they still lack specificity by targeting both NOX4 and NOX2.
3.5. Apocynin
Apocynin (4-Hydroxy-3-methoxyacetophenone) is a methoxy-substituted catechol, a plant-derived polyphenol extracted from the leaves of Picrorhiza kurroa (Fig. 5). [96] (Fig. 5). It should be emphasized that while many studies utilized apocynin, this inhibitor failed to inhibit ROS produced by cells overexpressing NOX1, NOX2 and NOX3. In fact, apocynin is a prodrug that is activated (apocynin dimerization) by leukocyte myeloperoxidase. Thus, NADPH oxidase inhibition by apocynin may be specific in cells expressing myeloperoxidases (i.e., NOX2 in leukocytes) whereas in other cells (i.e. vascular cells), the compound could act as an antioxidant (see [97] for detailed mode of action).
However, at a concentration of 300 µM, apocynin is able to markedly reduce the translocation of the cytosolic NADPH oxidase components p47(phox) and p67(phox) from the cytoplasm to the membrane, suggesting that apocynin may be an intracellular inhibitor of the assembly of the NADPH oxidase complex in neutrophils and eosinophils (NOX2) [98]. The anticancer activity of apocynin was demonstrated in several studies. Apocynin was shown to counteract the prosurvival effect of NOX2 in human leukaemic megakaryocytic M07 cells. Short exposure to 1mM apocynin inhibited glucose uptake while decreasing ROS production in M07 cells [73, 99]. Furthermore, ROS decrease by apocynin was shown to abrogate the anti-apoptotic effect of the growth factor IL-3 in these particular cancer cells. Thus, ROS production mediated at least in part by NOX2, has a pro-survival role in leukaemia cells. Another study revealed that apocynin and its structurally related compound vanillin inhibited human lung cancer cell migration as well as angiogenesis. Both compounds were shown to inhibit selectively the hepatocyte growth factor (HGF) induced-Akt phosphorylation as well as the enzymatic activity of phosphoinositide 3-kinase (PI3K) [100]. This study suggested a role for apocynin in the inhibition of PI3K activity as a mechanism underlying its inhibitory effect on cancer cell migration. Another clue supporting the anticancer effect of apocynin was the finding that it suppressed angiopoetin-1-stimulated ROS production while stalling cell migration [101], suggesting that this compound may inhibit both angiogenesis and metastasis. Finally, apocynin has been proven to be effective in the experimental treatment of several inflammatory diseases [102], diseases that are often associated with an increased cancer risk [1].
3.6. Prodigiosins
Prodigiosins are natural bacterial red pigments that show anti-malarial, anti-microbial, immunosuppressive and anti-cancer activities [103]. Prodigiosins are potential anti-cancer agents as they were shown to induce apoptosis in numerous types of cancer cells including heamatopoietic, gastric, colon and lung cancer cells among others [103]. In addition, prodigiosins demonstrated anti-cancer activity was shown both in vitro and in vivo [104] and were proven to be efficient against cancer cells resistant to other drugs [103]. However, there may be several mechanisms of action for these compounds as prodigiosins were also shown to act on mitochondria and topoisomerases I and II [105]. However, a prodigiosin analogue (PG-L-1), was shown to inhibit superoxide anion production in activated macrophages by a mechanism involving the inhibition of p47(phox) and Rac binding to the membrane components of NOX2 [106]. Further studies are needed to demonstrate whether the antitumor activity of prodigiosins is linked to NADPH oxidase inactivation.
3.7. RNA Silencing
Besides the potential use of small NOXs inhibitors for cancer treatment, the use of RNA interference may be a promising tool for future therapies. Cancer is one of the major targets of RNAi-based therapy, although proof-of-concept studies in animal models are just beginning to emerge (reviewed in [107]). A recent study looking into the molecular mechanisms of hypoxia involved in the development of glioblastoma showed that NOX4 knockdown by siRNA technology in GBM8401 glioblastoma cells prior to their injection in mice suppressed both tumor ROS production and tumor growth in vivo [11]. Glioblastoma multiforme (GM) is the most common and lethal malignant brain tumor in humans. Present therapies are based on the use of surgical resection of the metastatic tumors followed by chemotherapy and radiation therapy [11], and offer GM patients a median survival of less than 1 year from the time of diagnosis [11]. One reason for this poor prognosis is emphasized by the radioresistant nature of glioblastoma. Interestingly, NOX4 inactivation by RNA interference was shown to enhance radiosensitivity of glioblastoma xenografts in mice [108]. Using bioluminescent imaging to assess the growth of glioblastoma cells in mice in which NOX4 inactivation was inducible by doxycycline treatment, the authors showed that NOX4 silencing prior to initiation of radiotherapy significantly delays GM tumors growth.
Similarly, using an orthotopic implantation model of human bladder cancer KU-7 cells in mice, NOX4 was reported to have a critical role in bladder tumor growth in vivo. [70]. KU-7 cells carrying a green fluorescent protein were inoculated into the bladders of mice by catheterization. Tumor growth was monitored by in situ imaging. Intravesical injection of siRNA against NOX4 resulted in a significant reduction in tumor growth, suggesting that NOX4-derived ROS may be a critical mediator in the growth of urothelial carcinoma cells and therefore a useful target in bladder cancer treatment [70].
CONCLUSION AND PERSPECTIVES
ROS are increasingly recognized as important players in many cellular physiological processes, as well as in many pathophysiologies like cancer and cancer-prone conditions such as chronic inflammation. The NADPH oxidase family of proteins is central to ROS production for signaling purposes and for the elevated ROS levels often found associated with cancer. As further progress is being made towards understanding the mechanisms of action of these proteins, they may become potential targets for treatment of cancer and other diseases. However, this potential will be realized only after the development of highly specific inhibitors for the different NADPH oxidase family members and further insights into the mechanisms controlling their expression.
ACKNOWLEDGEMENTS
We thank Dr. Thomas L. Leto (National Institutes of Health, NIH) for the critical reading of the manuscript. This research was supported by the NIAID Radiation/Nuclear Countermeasures Program and the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, NIH.
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- [1].Sedelnikova OA; Redon CE; Dickey JS; Nakamura AJ; Georgakilas AG; Bonner WM Role of oxidatively induced DNA lesions in human pathogenesis. Mutat. Res, 2010, 704 (1–3), 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lassegue B; Griendling KK NADPH oxidases: functions and pathologies in the vasculature. Arterioscler. Thromb. Vasc. Biol, 2010, 30 (4), 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mladenka P; Simunek T; Hubl M; Hrdina R The role of reactive oxygen and nitrogen species in cellular iron metabolism. Free Radical Res, 2006, 40 (3), 263–272. [DOI] [PubMed] [Google Scholar]
- [4].Finkel T Signal transduction by reactive oxygen species. J. Cell Biol, 2011, 194 (1), 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lambeth JD Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radical Biol. Med, 2007, 43 (3), 332–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brown DI; Griendling KK Nox proteins in signal transduction. Free Radical Biol. Med, 2009, 47 (9), 1239–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Naka K; Muraguchi T; Hoshii T; Hirao A Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid. Redox Signal, 2008, 10 (11), 1883–1894. [DOI] [PubMed] [Google Scholar]
- [8].Kamata T Roles of Nox1 and other Nox isoforms in cancer development. Cancer Sci, 2009, 100 (8), 1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ushio-Fukai M; Nakamura Y Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett, 2008, 266 (1), 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Krause KH Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn. J. Infect. Dis, 2004, 57 (5), S28–S29. [PubMed] [Google Scholar]
- [11].Hsieh CH; Shyu WC; Chiang CY; Kuo JW; Shen WC; Liu RS NADPH oxidase subunit 4-mediated reactive oxygen species contribute to cycling hypoxia-promoted tumor progression in glioblastoma multiforme. PloS one, 2011, 6 (9), e23945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bhandarkar SS; Jaconi M; Fried LE; Bonner MY; Lefkove B; Govindarajan B; Perry BN; Parhar R; Mackelfresh J; Sohn A; Stouffs M; Knaus U; Yancopoulos G; Reiss Y; Benest AV; Augustin HG; Arbiser JL Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J. Clin. Investigation, 2009, 119 (8), 2359–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yamaura M; Mitsushita J; Furuta S; Kiniwa Y; Ashida A; Goto Y; Shang WH; Kubodera M; Kato M; Takata M; Saida T; Kamata T NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res, 2009, 69 (6), 2647–2654. [DOI] [PubMed] [Google Scholar]
- [14].Mazdak H; Zia H Vitamin e reduces superficial bladder cancer recurrence: a randomized controlled trial. Int. J. Prev. Med, 2012, 3 (2), 110–115. [PMC free article] [PubMed] [Google Scholar]
- [15].Aliverti A; Piubelli L; Zanetti G; Lubberstedt T; Herrmann RG; Curti B The role of cysteine residues of spinach ferredoxin-NADP+ reductase As assessed by site-directed mutagenesis. Biochemistry, 1993, 32 (25), 6374–6380. [DOI] [PubMed] [Google Scholar]
- [16].Lambeth JD NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol, 2004, 4 (3), 181–189. [DOI] [PubMed] [Google Scholar]
- [17].Rada B; Leto TL Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib. Microbiol, 2008, 15, 164–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bedard K; Krause KH The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev, 2007, 87 (1), 245–313. [DOI] [PubMed] [Google Scholar]
- [19].Montezano AC; Touyz RM Reactive Oxygen Species and Endothelial Function - Role of Nitric Oxide Synthase Uncoupling and Nox Family Nicotinamide Adenine Dinucleotide Phosphate Oxidases. Basic Clin. Pharmacol. Toxicol, 2012,110(1),87–94 [DOI] [PubMed] [Google Scholar]
- [20].Royer-Pokora B; Kunkel LM; Monaco AP; Goff SC; Newburger PE; Baehner RL; Cole FS; Curnutte JT; Orkin SH Cloning the gene for an inherited human disorder--chronic granulomatous disease--on the basis of its chromosomal location. Nature, 1986, 322 (6074), 32–8. [DOI] [PubMed] [Google Scholar]
- [21].Suh YA; Arnold RS; Lassegue B; Shi J; Xu X; Sorescu D; Chung AB; Griendling KK; Lambeth JD Cell transformation by the superoxide-generating oxidase Mox1. Nature, 1999, 401 (6748), 79–82. [DOI] [PubMed] [Google Scholar]
- [22].Gianni D; Diaz B; Taulet N; Fowler B; Courtneidge SA; Bokoch GM Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Science Signaling, 2009, 2 (88), ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Janiszewski M; Lopes LR; Carmo AO; Pedro MA; Brandes RP; Santos CX; Laurindo FR Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J. Biol. Chem, 2005, 280 (49), 40813–9. [DOI] [PubMed] [Google Scholar]
- [24].Cheng G; Cao Z; Xu X; van Meir EG; Lambeth JD Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene, 2001, 269 (1–2), 131–140. [DOI] [PubMed] [Google Scholar]
- [25].Geiszt M; Kopp JB; Varnai P; Leto TL Identification of renox, an NAD(P)H oxidase in kidney. Proc. Nat. Acad. Sci.U.S.A, 2000, 97 (14), 8010–8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ambasta RK; Kumar P; Griendling KK; Schmidt HH; Busse R; Brandes RP Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J. Biol. Chem, 2004, 279 (44), 45935–45941. [DOI] [PubMed] [Google Scholar]
- [27].Gorin Y; Ricono JM; Kim NH; Bhandari B; Choudhury GG; Abboud HE Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. American Journal of Physiology. Renal Physiology, 2003, 285 (2), F219–29. [DOI] [PubMed] [Google Scholar]
- [28].Lyle AN; Deshpande NN; Taniyama Y; Seidel-Rogol B; Pounkova L; Du P; Papaharalambus C; Lassegue B; Griendling KK Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circulation Res, 2009, 105 (3), 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sedeek M; Hebert RL; Kennedy CR; Burns KD; Touyz RM Molecular mechanisms of hypertension: role of Nox family NADPH oxidases. Curr. Opin. Nephrol. Hypertension, 2009, 18 (2), 122–127. [DOI] [PubMed] [Google Scholar]
- [30].Diaz B; Shani G; Pass I; Anderson D; Quintavalle M; Courtneidge SA Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Science Signaling, 2009, 2 (88), ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Goyal P; Weissmann N; Rose F; Grimminger F; Schafers HJ; Seeger W; Hanze J Identification of novel Nox4 splice variants with impact on ROS levels in A549 cells. Biochem. Biophys. Res. Commun, 2005, 329 (1), 32–39. [DOI] [PubMed] [Google Scholar]
- [32].Banfi B; Molnar G; Maturana A; Steger K; Hegedus B; Demaurex N; Krause KH A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem, 2001, 276 (40), 37594–37601. [DOI] [PubMed] [Google Scholar]
- [33].Chen F; Pandey D; Chadli A; Catravas JD; Chen T; Fulton DJ Hsp90 regulates NADPH oxidase activity and is necessary for superoxide but not hydrogen peroxide production. Antioxidants & Redox Signaling, 2011, 14 (11), 2107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jay DB; Papaharalambus CA; Seidel-Rogol B; Dikalova AE; Lassegue B; Griendling KK Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Rad. Biol. Med, 2008, 45 (3), 329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Montezano AC; Burger D; Paravicini TM; Chignalia AZ; Yusuf H; Almasri M; He Y; Callera GE; He G; Krause KH; Lambeth D; Quinn MT; Touyz RM Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circulation Res, 2010, 106 (8), 1363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rizvi F; Heimann T; O’Brien WJ Expression of NADPH Oxidase (NOX) 5 in Rabbit Corneal Stromal Cells. PloS one, 2012, 7 (4), e34440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dupuy C; Ohayon R; Valent A; Noel-Hudson MS; Deme D; Virion A Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J. Biol. Chem, 1999, 274 (52), 37265–9. [DOI] [PubMed] [Google Scholar]
- [38].De Deken X; Wang D; Many MC; Costagliola S; Libert F; Vassart G; Dumont JE; Miot F Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J. Biol. Chem,2000, 275 (30), 23227–23233. [DOI] [PubMed] [Google Scholar]
- [39].Grasberger H; Refetoff S Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J. Biol. Chem, 2006, 281 (27), 18269–18272. [DOI] [PubMed] [Google Scholar]
- [40].Mitsushita J; Lambeth JD; Kamata T The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res, 2004, 64 (10), 3580–3585. [DOI] [PubMed] [Google Scholar]
- [41].Sancho P; Fabregat I NADPH oxidase NOX1 controls autocrine growth of liver tumor cells through up-regulation of the epidermal growth factor receptor pathway. J. Biol. Chem, 2010, 285 (32), 24815–24824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Seo JM; Cho KJ; Kim EY; Choi MH; Chung BC; Kim JH Up-regulation of BLT2 is critical for the survival of bladder cancer cells. Experimental Mol. Med, 2011, 43 (3), 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Arbiser JL; Petros J; Klafter R; Govindajaran B; McLaughlin ER; Brown LF; Cohen C; Moses M; Kilroy S; Arnold RS; Lambeth JD Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc. Nat. Acad. Sci. U.S.A, 2002, 99 (2), 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sorescu GP; Song H; Tressel SL; Hwang J; Dikalov S; Smith DA; Boyd NL; Platt MO; Lassegue B; Griendling KK; Jo H Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circulation Res, 2004, 95 (8), 773–779. [DOI] [PubMed] [Google Scholar]
- [45].Garrido-Urbani S; Jemelin S; Deffert C; Carnesecchi S; Basset O; Szyndralewiez C; Heitz F; Page P; Montet X; Michalik L; Arbiser J; Ruegg C; Krause KH; Imhof BA Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARalpha mediated mechanism. PloS One, 2011, 6 (2), e14665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Liu F; Gomez Garcia AM; Meyskens FL Jr. NADPH Oxidase 1 Overexpression Enhances Invasion via Matrix Metalloproteinase-2 and Epithelial-Mesenchymal Transition in Melanoma Cells. J. Invest. Dermatol, 2012, 132(8), 2033–2041 [DOI] [PubMed] [Google Scholar]
- [47].Rezvani HR; Kim AL; Rossignol R; Ali N; Daly M; Mahfouf W; Bellance N; Taieb A; de Verneuil H; Mazurier F; Bickers DR XPC silencing in normal human keratinocytes triggers metabolic alterations that drive the formation of squamous cell carcinomas. J. Clin. Investigation, 2011, 121 (1), 195–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chiera F; Meccia E; Degan P; Aquilina G; Pietraforte D; Minetti M; Lambeth D; Bignami M Overexpression of human NOX1 complex induces genome instability in mammalian cells. Free Rad. Biol. Med, 2008, 44 (3), 332–342. [DOI] [PubMed] [Google Scholar]
- [49].Lim SD; Sun C; Lambeth JD; Marshall F; Amin M; Chung L; Petros JA; Arnold RS Increased Nox1 and hydrogen peroxide in prostate cancer. The Prostate, 2005, 62 (2), 200–207. [DOI] [PubMed] [Google Scholar]
- [50].Geiszt M; Lekstrom K; Brenner S; Hewitt SM; Dana R; Malech HL; Leto TL NAD(P)H oxidase 1, a product of differentiated colon epithelial cells, can partially replace glycoprotein 91phox in the regulated production of superoxide by phagocytes. J. Immunol, 2003, 171 (1), 299–306. [DOI] [PubMed] [Google Scholar]
- [51].Tojo T; Ushio-Fukai M; Yamaoka-Tojo M; Ikeda S; Patrushev N; Alexander RW Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation, 2005, 111 (18), 2347–55. [DOI] [PubMed] [Google Scholar]
- [52].Kusmartsev S; Nefedova Y; Yoder D; Gabrilovich DI Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol, 2004, 172 (2), 989–999. [DOI] [PubMed] [Google Scholar]
- [53].Corzo CA; Cotter MJ; Cheng P; Cheng F; Kusmartsev S; Sotomayor E; Padhya T; McCaffrey TV; McCaffrey JC; Gabrilovich DI Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol, 2009, 182 (9), 5693–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Caillou B; Talbot M; Weyemi U; Pioche-Durieu C; Al Ghuzlan A; Bidart JM; Chouaib S; Schlumberger M; Dupuy C Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PloS One, 2011, 6 (7), e22567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gruhne B; Sompallae R; Marescotti D; Kamranvar SA; Gastaldello S; Masucci MG The Epstein-Barr virus nuclear antigen-1 promotes genomic instability via induction of reactive oxygen species. Proc. Nat. Acad. Sci. U.S.A, 2009, 106 (7), 2313–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Banfi B; Malgrange B; Knisz J; Steger K; Dubois-Dauphin M; Krause KH NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem, 2004, 279 (44), 46065–46072. [DOI] [PubMed] [Google Scholar]
- [57].Paffenholz R; Bergstrom RA; Pasutto F; Wabnitz P; Munroe RJ; Jagla W; Heinzmann U; Marquardt A; Bareiss A; Laufs J; Russ A; Stumm G; Schimenti JC; Bergstrom DE Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Gene. Dev, 2004, 18 (5), 486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kikuchi H; Hikage M; Miyashita H; Fukumoto M NADPH oxidase subunit, gp91(phox) homologue, preferentially expressed in human colon epithelial cells. Gene, 2000, 254 (1–2), 237–43. [DOI] [PubMed] [Google Scholar]
- [59].Carnesecchi S; Carpentier JL; Foti M; Szanto I Insulin-induced vascular endothelial growth factor expression is mediated by the NADPH oxidase NOX3. Experimental Cell Res, 2006, 312 (17), 3413–24. [DOI] [PubMed] [Google Scholar]
- [60].Weyemi U; Dupuy C The emerging role of ROS-generating NADPH oxidase NOX4 in DNA-damage responses. Mutation Res, 2012Available online on 2012 May 8. [DOI] [PubMed]
- [61].Fitzgerald JP; Nayak B; Shanmugasundaram K; Friedrichs W; Sudarshan S; Eid AA; DeNapoli T; Parekh DJ; Gorin Y; Block K Nox4 mediates renal cell carcinoma cell invasion through hypoxia-induced interleukin 6- and 8- production. PloS One, 2012, 7 (1), e30712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Boudreau HE; Casterline BW; Rada B; Korzeniowska A; Leto TL Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radical Biol. Med, 2012. Available online on 2012 Jun 19 [DOI] [PMC free article] [PubMed]
- [63].Weyemi U; Lagente-Chevallier O; Boufraqech M; Prenois F; Courtin F; Caillou B; Talbot M; Dardalhon M; Al Ghuzlan A; Bidart JM; Schlumberger M; Dupuy C ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene ,2012, 31 (9), 1117–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Graham KA; Kulawiec M; Owens KM; Li X; Desouki MM; Chandra D; Singh KK NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol. Ther, 2010, 10 (3), 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Shono T; Yokoyama N; Uesaka T; Kuroda J; Takeya R; Yamasaki T; Amano T; Mizoguchi M; Suzuki SO; Niiro H; Miyamoto K; Akashi K; Iwaki T; Sumimoto H; Sasaki T Enhanced expression of NADPH oxidase Nox4 in human gliomas and its roles in cell proliferation and survival. Int. J. Cancer, 2008, 123 (4), 787–92. [DOI] [PubMed] [Google Scholar]
- [66].Vaquero EC; Edderkaoui M; Pandol SJ; Gukovsky I; Gukovskaya AS Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J. Biol. Chem, 2004, 279 (33), 34643–34654. [DOI] [PubMed] [Google Scholar]
- [67].Lee JK; Edderkaoui M; Truong P; Ohno I; Jang KT; Berti A; Pandol SJ; Gukovskaya AS NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology, 2007, 133 (5), 1637–1648. [DOI] [PubMed] [Google Scholar]
- [68].Weyemi U; Caillou B; Talbot M; Ameziane-El-Hassani R; Lacroix L; Lagent-Chevallier O; Al Ghuzlan A; Roos D; Bidart JM; Virion A; Schlumberger M; Dupuy C Intracellular expression of reactive oxygen species-generating NADPH oxidase NOX4 in normal and cancer thyroid tissues. Endocrine-related Cancer, 2010, 17 (1), 27–37. [DOI] [PubMed] [Google Scholar]
- [69].Wang R; Dashwood WM; Nian H; Lohr CV; Fischer KA; Tsuchiya N; Nakagama H; Ashktorab H; Dashwood RH NADPH oxidase overexpression in human colon cancers and rat colon tumors induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Int. J.Cancer, 2011, 128 (11), 2581–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shimada K; Fujii T; Anai S; Fujimoto K; Konishi N ROS generation via NOX4 and its utility in the cytological diagnosis of urothelial carcinoma of the urinary bladder. BMC Urology, 2011, 11, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Govindarajan B; Sligh JE; Vincent BJ; Li M; Canter JA; Nickoloff BJ; Rodenburg RJ; Smeitink JA; Oberley L; Zhang Y; Slingerland J; Arnold RS; Lambeth JD; Cohen C; Hilenski L; Griendling K; Martinez-Diez M; Cuezva JM; Arbiser JL Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J. Clin. Invest, 2007, 117 (3), 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gordillo G; Fang H; Park H; Roy S Nox-4-dependent nuclear H2O2 drives DNA oxidation resulting in 8-OHdG as urinary biomarker and hemangioendothelioma formation. Antioxidants & Redox Signaling, 2010, 12 (8), 933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Prata C; Maraldi T; Fiorentini D; Zambonin L; Hakim G; Landi L Nox-generated ROS modulate glucose uptake in a leukaemic cell line. Free Rad. Res, 2008, 42 (5), 405–414. [DOI] [PubMed] [Google Scholar]
- [74].Brar SS; Corbin Z; Kennedy TP; Hemendinger R; Thornton L; Bommarius B; Arnold RS; Whorton AR; Sturrock AB; Huecksteadt TP; Quinn MT; Krenitsky K; Ardie KG; Lambeth JD; Hoidal JR NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am. J. Physiol. Cell Physiol, 2003, 285 (2), C353–C369. [DOI] [PubMed] [Google Scholar]
- [75].Fu X; Beer DG; Behar J; Wands J; Lambeth D; Cao W cAMP-response element-binding protein mediates acid-induced NADPH oxidase NOX5-S expression in Barrett esophageal adenocarcinoma cells. J. Biol. Chem, 2006, 281 (29), 20368–20382. [DOI] [PubMed] [Google Scholar]
- [76].Hong J; Resnick M; Behar J; Wang LJ; Wands J; DeLellis RA; Souza RF; Spechler SJ; Cao W Acid-induced p16 hypermethylation contributes to development of esophageal adenocarcinoma via activation of NADPH oxidase NOX5-S. Am.J. Physiol. Gastrointestinal Liver Physiol, 2010, 299 (3), G697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Juhasz A; Ge Y; Markel S; Chiu A; Matsumoto L; van Balgooy J; Roy K; Doroshow JH Expression of NADPH oxidase homologs and accessory genes in human cancer cell lines, tumours and adjacent normal tissues. Free Rad. Res, 2009, 43 (6), 523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Luxen S; Belinsky SA; Knaus UG Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res, 2008, 68 (4), 1037–45. [DOI] [PubMed] [Google Scholar]
- [79].Pulcrano M; Boukheris H; Talbot M; Caillou B; Dupuy C; Virion A; De Vathaire F; Schlumberger M Poorly differentiated follicular thyroid carcinoma: prognostic factors and relevance of histological classification. Thyroid, 2007, 17 (7), 639–46. [DOI] [PubMed] [Google Scholar]
- [80].Gaggini F; Laleu B; Orchard M; Fioraso-Cartier L; Cagnon L; Houngninou-Molango S; Gradia A; Duboux G; Merlot C; Heitz F; Szyndralewiez C; Page P Design, synthesis and biological activity of original pyrazolo-pyrido-diazepine, -pyrazine and -oxazine dione derivatives as novel dual Nox4/Nox1 inhibitors. Bioorg. Med. Chem, 2011, 19 (23), 6989–6999. [DOI] [PubMed] [Google Scholar]
- [81].Jaquet V; Scapozza L; Clark RA; Krause KH; Lambeth JD Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid. Redox Sign, 2009, 11 (10), 2535–52. [DOI] [PubMed] [Google Scholar]
- [82].El-Benna J; Dang PM; Perianin A Towards specific NADPH oxidase inhibition by small synthetic peptides. Cellular and molecular life sciences: CMLS, 2012, 69 (14), 2307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].ten Freyhaus H; Huntgeburth M; Wingler K; Schnitker J; Baumer AT; Vantler M; Bekhite MM; Wartenberg M; Sauer H; Rosenkranz S Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovascular Res,2006, 71 (2), 331–341. [DOI] [PubMed] [Google Scholar]
- [84].Altenhofer S; Kleikers PW; Radermacher KA; Scheurer P; Rob Hermans JJ; Schiffers P; Ho H; Wingler K; Schmidt HH The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell. Mol. life Sci, 2012, 69 (14), 2327–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Sancho P; Fabregat I The NADPH oxidase inhibitor VAS2870 impairs cell growth and enhances TGF-beta-induced apoptosis of liver tumor cells. Biochem. Pharmacol, 2011, 81 (7), 917–24. [DOI] [PubMed] [Google Scholar]
- [86].Dong-Yun S; Yu-Ru D; Shan-Lin L; Ya-Dong Z; Lian W Redox stress regulates cell proliferation and apoptosis of human hepatoma through Akt protein phosphorylation. FEBS Lett, 2003, 542 (1–3), 60–64. [DOI] [PubMed] [Google Scholar]
- [87].Sancho P; Bertran E; Caja L; Carmona-Cuenca I; Murillo MM; Fabregat I The inhibition of the epidermal growth factor (EGF) pathway enhances TGF-beta-induced apoptosis in rat hepatoma cells through inducing oxidative stress coincident with a change in the expression pattern of the NADPH oxidases (NOX) isoforms. Biochim. Biophys. Acta, 2009, 1793 (2), 253–63. [DOI] [PubMed] [Google Scholar]
- [88].Kim JA; Neupane GP; Lee ES; Jeong BS; Park BC; Thapa P NADPH oxidase inhibitors: a patent review. Expert Opin. Therapeutic Patents, 2011, 21 (8), 1147–1158. [DOI] [PubMed] [Google Scholar]
- [89].Etoh T; Inoguchi T; Kakimoto M; Sonoda N; Kobayashi K; Kuroda J; Sumimoto H; Nawata H Increased expression of NAD(P)H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibity by interventive insulin treatment. Diabetologia, 2003, 46 (10), 1428–37. [DOI] [PubMed] [Google Scholar]
- [90].Mitchell SC Phenothiazine: the parent molecule. Curr. Drug Targets, 2006, 7 (9), 1181–1189. [DOI] [PubMed] [Google Scholar]
- [91].Gianni D; Taulet N; Zhang H; DerMardirossian C; Kister J; Martinez L; Roush WR; Brown SJ; Bokoch GM; Rosen H A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem. Biol, 2010, 5 (10), 981–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].O’Donnell BV; Tew DG; Jones OT; England PJ Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. The Biochem. J, 1993, 290 ( Pt 1), 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Xia C; Meng Q; Liu LZ; Rojanasakul Y; Wang XR; Jiang BH Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res, 2007, 67 (22), 10823–10830. [DOI] [PubMed] [Google Scholar]
- [94].Doroshow JH; Juhasz A; Ge Y; Holbeck S; Lu J; Antony S; Wu Y; Jiang G; Roy K Antiproliferative mechanisms of action of the flavin dehydrogenase inhibitors diphenylene iodonium and di-2-thienyliodonium based on molecular profiling of the NCI-60 human tumor cell panel. Biochem. Pharmacol, 2012, 83 (9), 1195–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Hutchinson DS; Csikasz RI; Yamamoto DL; Shabalina IG; Wikstrom P; Wilcke M; Bengtsson T Diphenylene iodonium stimulates glucose uptake in skeletal muscle cells through mitochondrial complex I inhibition and activation of AMP-activated protein kinase. Cellular Signalling, 2007, 19 (7), 1610–20. [DOI] [PubMed] [Google Scholar]
- [96].Beukelman CJ; Van den Berg AJ; Kroes BH; Labadie RP; Mattsson EE; Van Dijk H Plant-derived metabolites with synergistic antioxidant activity. Immunol.Today ,1995, 16 (2), 108. [DOI] [PubMed] [Google Scholar]
- [97].Heumuller S; Wind S; Barbosa-Sicard E; Schmidt HH; Busse R; Schroder K; Brandes RP Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension, 2008, 51 (2), 211–217. [DOI] [PubMed] [Google Scholar]
- [98].Stolk J; Rossie W; Dijkman JH Apocynin improves the efficacy of secretory leukocyte protease inhibitor in experimental emphysema. Am.J. Respir. Crit. Care Med, 1994, 150 (6 Pt 1), 1628–1631. [DOI] [PubMed] [Google Scholar]
- [99].Maraldi T; Prata C; Vieceli Dalla Sega F; Caliceti C; Zambonin L; Fiorentini D; Hakim G NAD(P)H oxidase isoform Nox2 plays a prosurvival role in human leukaemia cells. Free Rad. Res, 2009, 43 (11), 1111–21. [DOI] [PubMed] [Google Scholar]
- [100].Lirdprapamongkol K; Kramb JP; Suthiphongchai T; Surarit R; Srisomsap C; Dannhardt G; Svasti J Vanillin suppresses metastatic potential of human cancer cells through PI3K inhibition and decreases angiogenesis in vivo. J. Agri. Food Chem, 2009, 57 (8), 3055–63. [DOI] [PubMed] [Google Scholar]
- [101].Chen JX; Zeng H; Lawrence ML; Blackwell TS; Meyrick B Angiopoietin-1-induced angiogenesis is modulated by endothelial NADPH oxidase. Am. J. Physiol. Heart Circulatory Physiology 2006, 291 (4), H1563–72. [DOI] [PubMed] [Google Scholar]
- [102].Van den Worm E; Beukelman CJ; Van den Berg AJ; Kroes BH; Labadie RP; Van Dijk H Effects of methoxylation of apocynin and analogs on the inhibition of reactive oxygen species production by stimulated human neutrophils. Eur. J. pharmacol, 2001, 433 (2–3), 225–30. [DOI] [PubMed] [Google Scholar]
- [103].Pandey R; Chander R; Sainis KB Prodigiosins as anti cancer agents: living upto their name. Curr. Pharmaceutical Design, 2009, 15 (7), 732–41. [DOI] [PubMed] [Google Scholar]
- [104].Perez-Tomas R; Montaner B; Llagostera E; Soto-Cerrato V The prodigiosins, proapoptotic drugs with anticancer properties. Biochem. Pharmacol, 2003, 66 (8), 1447–52. [DOI] [PubMed] [Google Scholar]
- [105].Montaner B; Castillo-Avila W; Martinell M; Ollinger R; Aymami J; Giralt E; Perez-Tomas R DNA interaction and dual topoisomerase I and II inhibition properties of the anti-tumor drug prodigiosin. Toxicol. Sci, 2005, 85 (2), 870–879. [DOI] [PubMed] [Google Scholar]
- [106].Nakashima T; Iwashita T; Fujita T; Sato E; Niwano Y; Kohno M; Kuwahara S; Harada N; Takeshita S; Oda T A prodigiosin analogue inactivates NADPH oxidase in macrophage cells by inhibiting assembly of p47phox and Rac. J. Biochem, 2008, 143 (1), 107–115. [DOI] [PubMed] [Google Scholar]
- [107].Bora RS; Gupta D; Mukkur TK; Saini KS RNA interference therapeutics for cancer: challenges and opportunities (review). Mol. Med. Reports, 2012, 6 (1), 9–15. [DOI] [PubMed] [Google Scholar]
- [108].Hsieh CH; Wu CP; Lee HT; Liang JA; Yu CY; Lin YJ NADPH oxidase subunit 4 mediates cycling hypoxia-promoted radiation resistance in glioblastoma multiforme. Free Radical. Bio. Med, 2012. Available online on 2012 Jun 16. [DOI] [PubMed]