Abstract
Copper plays an essential role in normal human physiology. Copper misbalance affects heart development, CNS and liver function, influences lipid metabolism, inflammation, and resistance to chemotherapeutic drugs. Recent studies yielded new information on the structure, function, and regulation of human copper transporters, uncovered unanticipated functions for copper chaperones, and established connections between copper homeostasis and other metabolic pathways. It has become apparent that the copper trafficking machinery is regulated at several levels and that the cross-talk between cell compartments contributes to the intracellular copper balance. The human copper regulon is emerging.
Introduction
Copper is required for respiration, connective tissue formation, iron metabolism, and many others processes. In human cells, copper is utilized in several cells compartments, and the intracellular distribution of copper is regulated in response to metabolic demands and changes in cell environment. Significant progress has been made in characterizing the copper trafficking pathways in mammalian cells. This review will discuss recent advances and emerging concepts in the following areas:
Copper entry: machinery and regulation
Copper chaperones: an expanding repertoire of functions and mechanisms
Cross-talk between copper trafficking pathways
Regulation of copper delivery to the secretory pathway and copper efflux
Copper homeostasis and cells’ resistance to cisplatin await integration
Copper entry: machinery and regulation
The transporter CTR1 plays the key role in high affinity copper uptake. In cells, CTR1 is found in two locations: at the plasma membrane and in intracellular vesicles (Figure 1). The distribution of CTR1 between these two compartments and the extent to which copper influences this distribution is cell-specific. In the liver, kidney, placenta, and mammary gland, the predominant location of CTR1 is at the basolateral plasma membrane [1,2•,3,4], where it transports copper from circulation by, most probably, retrieving copper from specific carrier(s) [5]. In intestine, CTR1 has an additional role in making dietary copper available for further utilization, presumably, by facilitating its release from intracellular vesicles [6•]. Consistent with this role, a significant fraction of CTR1 in enterocytes is intracellular and is located in the vicinity of the apical membrane.
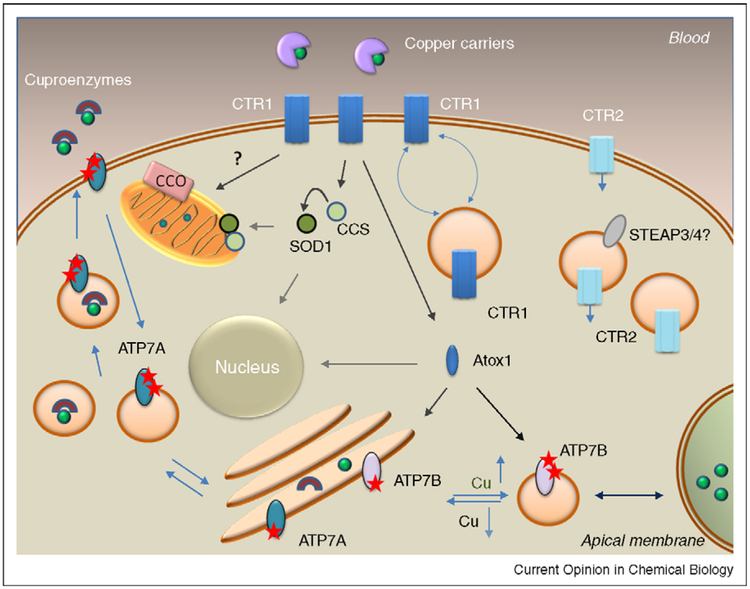
Figure 1.
Copper distribution pathways in a generalized mammalian cell. CTR1 accepts cooper from the extracellular copper carriers and transfers copper into cytosol; changes in copper levels induce reversible trafficking between the plasma membrane and intracellular vesicles. CTR2 is predominantly intracellular but can be found at the plasma membrane. Entering copper is either binds directly or is retrieved from CTR1/CTR2 by copper chaperones that have multiple functions; CCS distributes copper to SOD1 in cytosol and mitochondria, while Atox1 transfers copper to the secretory pathway and nucleus. An ensemble of proteins regulates copper delivery to cytochrome c oxidase (CCO) in mitochindria. Cu-ATPases transport copper to the secretory pathways for incorporation into cuproenzumes and mediate copper excretion by sequestering excess copper in vesicles. The trafficking of Cu-ATPases between these two locations is associated with phosphorylation by a kinase (indicated by stars), which increases in response to copper elevation.
The intracellular distribution of CTR1 is dynamic. Elevation of extracellular copper induces endocytosis of CTR1 to vesicles (thus decreasing copper uptake across the membrane), whereas a decrease in extracellular copper restores the CTR1 levels at the plasma membrane [7]. In addition to acute modulation of plasma membrane levels of CTR1, the total amount of CTR1 is regulated at the mRNA level in response to prolonged changes in copper status [8•]. The homeostatic control of the steady-state levels of CTR1 mRNA is illustrated by the observation that the expression of exogenous hCTR1 is accompanied by the decrease in the amount of mRNA for the endogenous hCTR1 [8•].
Fluctuations of copper levels in a serum are likely to be low keeping the steady-state CTR1 levels (both total and at the plasma membrane) fairly constant. However, physiologic conditions that increase metabolic demands for copper can provide a notable CTR1 upregulation. Larger amounts of CTR1 protein (and an increase in copper uptake) are observed in macrophages in response to hypoxia [9••] or treatment with gamma-interferon [10•], both of which increase the flow of copper towards the secretory pathway. The physiologic signals such as 17-β estradiol may also modulate the response of CTR1 to changes in copper levels [11]. The complex kinetics and tissue specificity of CTR1 regulation is well illustrated by recent studies in fish [12]. The molecular machinery that controls CTR1 abundance is largely unknown; however, recent study suggests a direct role for nuclear protein Sp1 in CTR1 transcription [8•].
Copper enters the cell even if CTR1 is markedly down-regulated, although in this case the amount of entering copper is insufficient to fully support the activity of copper-dependent enzymes [1]. Which protein/process mediates copper uptake in the absence of CTR1 is uncertain. A low-affinity copper transporter CTR2 is largely intracellular [13,14] and may function to release copper from the lysosome or lysosome-like compartments for re-utilization (by analogy with the yeast CTR2 [15]). However, a small fraction of CTR2 is detected at the plasma membrane, and the overexpression of CTR2 is associated with increased copper uptake [14]. Perhaps, under certain conditions, CTR2 can be recruited to the plasma membrane to facilitate copper import. Whether more CTR2 is present at the plasma membrane when CTR1 is downregulated remains to be explored.
Yeast CTR2 works together with a reductase [16], which converts Cu(II) to Cu(I), a form required for translocation. In mammalian cells, the lumen of the lysosome-like compartments is likely to be oxidizing compared with the cytosol and specific reductases might be needed to maintain Cu in the reduced form. STEAP family of reductases are candidates for such a role [17]. STEAP3 and STEAP4 are located in intracellular vesicles and reduce copper in vitro [17]. STEAPs have little effect on copper transport across the plasma membrane, thus it would be interesting to examine if they facilitate the intracellular transport activity of CTR2.
Copper chaperones: an expanding repertoire of functions and mechanisms
The current model for copper homeostasis suggests that upon entry into the cell copper binds to cytosolic copper chaperones, CCS and Atox1, which then transfer copper to specific cellular destinations. CCS activates cytosolic Cu, Zn-dependent superoxide dismutase (SOD1) by inserting copper and a disulfide bond; while Atox1 transfers copper to the copper-transporting ATPases in the secretory pathway (Figure 1). A low molecular weight carrier and/or a yet-to-be characterized protein initiates a series of transfer reactions that result in copper delivery to cytochrome c oxidase (CCO) in mitochondria [18•]. This attractive and widely cited model of ‘one carrier per cell compartment’ needs updating, because recent data paint a more complex picture of chaperone-mediated copper distribution (Figure 1).
Specifically, SOD1 is also found in the inter-membrane space of mitochondria (IMS), where it provides protection from superoxide that cannot be neutralized by the matrix-based MnSOD. The entry, folding, and metallation of SOD1 in the IMS require CCS. In turn, the presence of CCS in mitochondria is influenced by oxygen [19••]. High oxygen concentration promotes oxidative folding of CCSin the cytosol and precludes its import into IMS thus retaining more SOD1 in the cytosol. Lower oxygen concentrations favor CCS mitochondrial localization and SOD1 translocation into IMS. Thus, in addition to its important role as a copper carrier, CCS acts as an oxygen sensor [19••] and a factor regulating SOD1 compartmentalization.
Another novel function for the CCS/SOD1 pair was recently reported in yeast [20]. The CCS-dependent activity of SOD1 and SOD1 entry into the nucleus facilitates the binding of transcription factor Mac1 to its target genes and upregulation of copper uptake [20]. Inactivation of either CCS or SOD1 impairs yeast ability to upregulate the Mac1 target genes and combat copper deficiency [20]. A role in transcription was also proposed for Atox1 [21]. In mouse fibroblasts, the copper-dependent increase in cell proliferation and cyclin D expression require Atox1 [21]. The translocation of Atox1 into the nucleus and direct binding to the cyclin D promoter region suggest that this effect is direct [21]. At the same time, genetic deletion of Atox1 does not prevent copper entry into the nucleus [22] indicating the presence of more than one pathway for copper delivery/action in mammalian nuclei.
The proteins important for copper insertion into cytochrome c oxidase (CCO) in mitochondria (Cox17, Cox11, Sco1 and Sco2) also have multiple roles regulating the stability, redox state, and/or metallation of their partners. In the IMS, copper chaperone Cox17 transfers copper to Sco1 and Cox11, which are implicated in the formation of the Cu(A) and Cu(B) sites of CCO, respectively. A recent study uncovered a fascinating ability of Cox17 to couple the transfer of copper and electrons [23••]. In vitro, copper-bound Cox17 with two intact disulfide bonds simultaneously transfers Cu(I) and two electrons to oxidized Sco1. As a result, Sco1 is reduced and binds copper, while the metal-binding cysteines of Cox17 are oxidized into a third disulfide bond [23••]. Further transfer of copper to CCO requires the formation of a complex with Sco1 and Sco2. In this complex, Sco2 appears to oxidize the copper-coordinating cysteines in Sco1 as a prerequisite step for copper transfer [24•]. The subsequent steps in CCO maturation are still being explored.
A new potential regulator of copper metabolism has recently been described. CutC is a highly conserved cytosolic copper binding protein [25]. The function of this protein in human cells remains unclear; however downregulation of CutC in C. elegans increases sensitivity to high copper [26].
The cross-talk between copper trafficking pathways
It remains unresolved how human cells allocate copper to different intracellular routes. Copper carriers may bind free ion that emerges from a CTR1/CTR2 pore or retrieve copper from a Cu–glutathione complex, which would easily form given high concentrations of glutathione in the cytosol. In this mechanism, the preferential route of copper distribution will be determined by the copper-binding affinity and/or abundance of copper carriers. Alternatively, chaperones may play an ‘active’ role in copper uptake through direct interactions with the transporter. In this case, copper allocation towards a certain route will be established on the basis of preferential interactions between the transporter and a chaperone. In vitro, the exchange of copper between the C-terminus of CTR1 and Atox1 was reported, favoring the above scenario. An ‘active’ role of metallochaperones in copper uptake is also suggested by observation that the human CTR1 expressed in Drosophila has a low transport activity unless it is co-expressed with CCS [27].
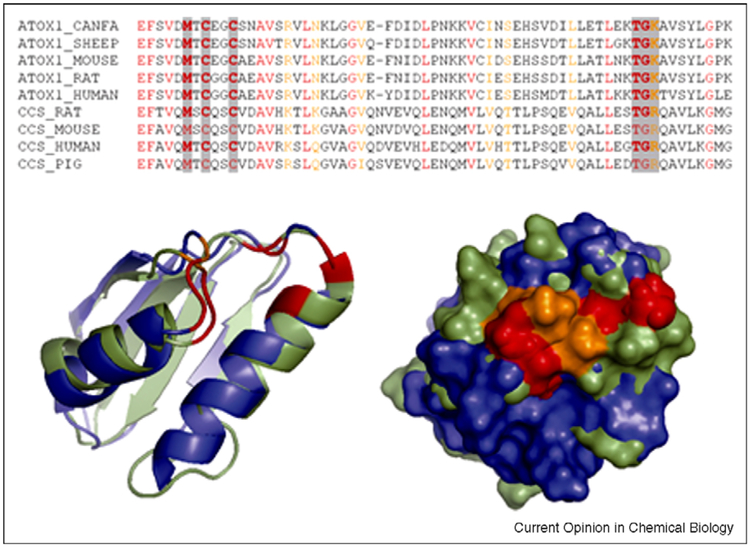
The extramembraneous regions of CTR1 form structural elements that are well positioned to regulate copper access to and from the pore [28•], and it was suggested that metallochaperones can dock to CTR1 (Unger, meeting report). From this perspective, significant structural similarity between Domain I of CCS and Atox1 [29•] is intriguing. Structural alignment illustrates that the identity between these proteins is not limited to the copper-binding CxxC motif and residues involved in protein folding, but also involves several charged residues at the protein surface (Figure 2), which can be involved in protein-protein interactions. Given this similarity, one would expect the Domain 1 of CCS and Atox1 to compete for copper. This prediction finds supports in recent studies on copper distribution in macrophages in hypoxia [9••]. Oxygen deprivation redirects copper flow from mitochondria and the cytosol towards the secretory pathway (demonstrated by the decreased activity of CCO and SOD1 and increased biosynthesis of ceruloplasmin in the secretory pathway [9••]). The mechanism of limited copper delivery to mitochondria is unclear, but in the cytosol hypoxia decreases the CCS levels. Thus, CCS downregulation appears to be necessary to shift copper distribution from the cytosolic targets towards the Atox1-mediated route. A similar effect (re-directing copper for efflux) is caused by mutations in Sco proteins that impair copper incorporation into CCO in mitochondria [18•]. By contrast, simultaneous downregulation of Atox1 and metallothionein leads to the sequestration of copper in vesicles and functional copper deficiency in the cytosol despite overall elevation of copper, and simultaneous upregulation of CTR1 and CCS [30]. Elucidating the mechanisms that coordinate copper distribution pathways in a cell is the next important frontier in studies of copper biology. Better understanding of such a regulon would help to decipher the role of copper homeostasis in resistance of cancer cells to cisplatin (see below).
Figure 2.
Structural comparison of human Atox1 (blue, 1TL5) and Domain I of CCS (green, 2CRL). Sequence alignment illustrates significant similarity of mammalian Atox1 and CCS (identical residues are in red, conserved residues in orange). The conserved residues around the metal-binding site are highlighted by grey (top); their location in the superimposed structures (left) and surface exposure (right) are shown (bottom).
Copper delivery to the secretory pathway and copper efflux
Copper-transporting ATPases (Cu-ATPases) maintain intracellular copper levels. Their activity, post-translational modification, and intracellular localization are modulated by intracellular copper [31]. The Cu-ATPases accept copper from Atox1 and use the energy of ATP hydrolysis to transfer copper into the secretory pathway, where copper is incorporated into copper-dependent enzymes (Figure 1). In response to copper elevation, Cu-ATPases undergo kinase-mediated phosphorylation and relocalization to vesicles in proximity to either basolateral (ATP7A) or apical (ATP7B) membrane. Copper is subsequently exported via vesicle-mediated fusion. Metabolic factors that upregulate copper uptake also trigger trafficking of Cu-ATPases [32,9••,10•]. Recent reports suggest the presence of Cu-ATPase in new cellular locations (ATP7B in tight junctions [32] and late endosomes [33], and ATP7A in the nucleus [34]); further studies are needed to understand the mechanistic significance of these observations.
Similarly to CTR1, regulation of endogenous Cu-ATPases is cell-specific. ATP7B traffics in response to copper in hepatocytes, intestinal, and placental cells, but does not traffic in renal or ovary cells [35,36•]. The lack of trafficking implies that in these cells the role of ATP7B is either to maintain a continuous supply of copper to enzymes in the secretory pathway (when ATP7A traffics away to mediate copper export) or to sequester copper for storage. In many cells, two Cu-ATPases are co-expressed, and dissecting their specific roles is essential for better understanding of human copper homeostasis. Recent studies of hormone-dependent regulation in placental cells revealed a coordinated but different response of two Cu-ATPases to insulin and estrogen. Expression and trafficking of ATP7A to the basolateral membrane is stimulated, while the expression of ATP7B is reduced with a net increase in copper transport to the fetus [37••]. Significant regulation of Cu-ATPases also occurs during development [38,39] and tumorigenesis [40].
Information on the structure and regulation of Cu-ATPases has been steadily accumulating [31,41]. The structure of the ATP-binding domain has been solved [42,43], further illustrating the uniqueness of ATP coordination in Cu-ATPases compared with other P-type pumps. Studies of the archaeal CopA revealed the presence of two copper-binding sites in the transmembrane portion of Cu-ATPases [44•], and demonstrated conformational changes in response to ligand binding [45]. Characterization of CopA has yielded immensely valuable information, however mechanistic differences exist between archaeal and eukaryotic Cu-ATPases and should be considered. In CopA, the chaperone CopZ delivers copper directly to the transmembrane sites [46•]. By contrast, in yeast the transfer of copper by Atx1 to the transmembrane portion of Cu-ATPase Ccc2 requires the N-terminus of the pump [47•]. Also, recent study demonstrates that proteins that accept copper from human Cu-ATPases may influence both the transport activity and trafficking of Cu-ATPases [48•]. Such regulation by acceptor protein(s) can be common for mammalian Cu-ATPases but be absent in archaea and prokaryotes.
Important progress has been made in understanding the directionality of Cu-ATPase trafficking [49•]. The highly conserved sequence, F37AFDNVGYE45 of ATP7B was shown to comprise an apical targeting determinant for trafficking in elevated copper as well as TGN retention under low copper conditions [49•]. This targeting motif may work together with other copper-dependent signals, such as kinase-mediated phosphorylation. Multiple phosphorylation sites have been identified in ATP7A and ATP7B [50,51,52•] perhaps reflecting the variety of metabolic signals to which Cu-ATPases respond. Mutagenesis of candidate Ser residues in ATP7A results in mislocalization in the presence of added copper [51], consistent with the role of these residues in vectorial trafficking. Interestingly, in vitro the membrane-bound ATP7B becomes phosphorylated in the absence of soluble cell extract [50,52•], and an increase in a kinase-mediated phosphorylation parallels an increase in the ATP7B catalytic activity [52•]. These observations raise an intriguing possibility that ATP7B is either tightly associated with a membrane-bound kinase or may have a kinase activity of its own.
Consequences of Cu-ATPases inactivation further illustrate existence of a fine-tuned copper regulon, which involves proteins beyond ‘classic’ copper homeostatic genes. In the liver, ATP7B inactivation leads to copper accumulation in the cytosol and nuclei [53], downregulation of lipid metabolism and increased cell proliferation [53,54•]. Similarly, copper elevation due to ATP7A inhibition in macrophages decreases the promoter activity and protein amounts of cPLA2α, a cytosolic phospholipase [55]. That elevated copper does not simply inhibit transcription is evident from studies showing the upregulation of amyloid precursor protein and prion protein in human fibroblasts lacking ATP7A [56,57]. It is interesting that the transcription factor SP1 appears to contribute to this copper-dependent regulation [56,57]. Thus, SP1 may play a dual role in modulating copper uptake [8•] and regulating the response to copper elevation.
Copper homeostasis and cells’ resistance to cisplatin await integration
Recent years saw an explosion of interest in the role of copper metabolism in resistance of cancer cells to cisplatin-like drugs [58,59•]. The format of this review does not permit detailed discussion of this important and complex subject. It is clear that the components of copper trafficking machinery can directly bind cisplatin [60,36,61]. The structure of the Atox1-cisplatin complex has been solved [59•] illustrating the potential mode of interaction between the drug and copper-binding proteins. The in vivo downregulation of copper efflux pump ATP7B in tumors [36•] was shown to increase tumor sensitivity to cisplatin [36], illustrating clinical potential of modulating copper metabolism. It has also become apparent that cisplatin does not simply mimic copper [61] and that detailed characterization of copper regulon may help to understand how changes in copper balance influence cells resistance to chemotherapeutic drugs.
Acknowledgement
This work has been funded by the NIH grants R01 DK071865 and P01 GM067166 to SL
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Kim H, Son HY, Bailey SM, Lee J: Deletion of hepatic Ctr1 reveals its function in copper acquisition and compensatory mechanisms for copper homeostasis. Am J Physiol Gastrointest Liver Physiol 2009, 296(2):G356–G364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimnicka AM, Maryon EB, Kaplan JH: Human copper transporter hCTR1 mediates basolateral uptake of copper into enterocytes: implications for copper homeostasis. J Biol Chem 2007, 282(36):26471–26480.• By measuring transport of radioactive copper and performing surface biotinylation in polarized cells the authors demonstrate the presence of CTR1 in basolateral membrane of intestinal cells. This observation suggests a general role for CTR1 in copper acquisition from the blood.
- 3.Llanos RM, Michalczyk AA, Freestone DJ, Currie S, Linder MC, Ackland ML, Mercer JF: Copper transport during lactation in transgenic mice expressing the human ATP7A protein. Biochem Biophys Res Commun 2008, 372(4):613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardman B, Manuelpillai U, Wallace E, Monty J, Kramer D, Kuo Y, Mercer J, Ackland M: Expression, localisation and hormone regulation of the human copper transporter hCTR1 in placenta and choriocarcinoma Jeg-3 cells. Placenta 2006, 27(9–10):968–977. [DOI] [PubMed] [Google Scholar]
- 5.Moriya M, Ho YH, Grana A, Nguyen L, Alvarez A, Jamil R, Ackland ML, Michalczyk A, Hamer P, Ramos D et al. : Copper is taken up efficiently from albumin and alpha2-macroglobulin by cultured human cells by more than one mechanism. Am J Physiol Cell Physiol 2008, 295(3):C708–C721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nose Y, Kim BE, Thiele DJ: Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab 2006, 4(3):235–244.• Using tissue-specific knockout mice, the authors provide the first demonstration of the role of CTR1 in dietary copper absorption. The potential second role of CTR1 in the release of from intracellular vesicles was suggested by animals’ phenotype.
- 7.Molloy SA, Kaplan JH: Copper-dependent recycling of hCTR1, the human high affinity copper transporter. J Biol Chem 2009, 284(43):29704–29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song IS, Chen HHW, Aiba I, Hossain A, Liang ZD, Klomp LWJ, Kuo MT: Transcription factor Sp1 plays an important role in the regulation of copper homeostasis in mammalian cells. Mol Pharmacol 2008, 74(3):705–713.• The authors show that the levels of CTR1 mRNA are under tight homeostatic control and suggest the mechanisms for CTR1 regulation.
- 9.White C, Kambe T, Fulcher YG, Sachdev SW, Bush AI, Fritsche K, Lee J, Quinn TP, Petris MJ: Copper transport into the secretory pathway is regulated by oxygen in macrophages. J Cell Sci 2009, 122(Pt 9):1315–1321.•• By measuring levels of proteins involved in copper trafficking and the activity of copper-dependent enzymes in the cytosol and the secretory pathway, the authors provide evidence for redirection of intracellular copper flow in response to hypoxia.
- 10.White C, Lee J, Kambe T, Fritsche K, Petris MJ: A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem 2009, 284(49):33949–33956.• The observed increased of copper uptake and trafficking of ATP7A in response to inflammatory stimuli suggests the role for mammalian Cu-ATPases in response to infection. In bacterial cells the Cu-ATPase appear to play a reciprocal role increasing cell pathogenicity.
- 11.Arredondo M, Nijñez H, López G, Pizarro F, Ayala M, Araya M: Influence of estrogens on copper indicators: in vivo and in vitro studies. Biol Trace Elem Res 2009. http://www.springerlink.com/content/51h5602359q08hx5/. [DOI] [PubMed] [Google Scholar]
- 12.Minghetti M, Leaver MJ, Carpenè E, George SG: Copper transporter 1, metallothionein and glutathione reductase genes are differentially expressed in tissues of sea bream (Sparus aurata) after exposure to dietary or waterborne copper. Comp Biochem Physiol C Toxicol Pharmacol 2008, 147(4):450–459. [DOI] [PubMed] [Google Scholar]
- 13.van den Berghe PV, Folmer DE, Malingré HEM, vanBeurden E, Klomp AEM, vandeSluis B, Merkx M, Berger R, Klomp LWJ: Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake. Biochem J 2007, 407(1):49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertinato J, Swist E, Plouffe LJ, Brooks SPJ, L’abbe MR: Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells. Biochem J 2008, 409(3):731–740. [DOI] [PubMed] [Google Scholar]
- 15.Rees EM, Lee J, Thiele DJ: Mobilization of intracellular copper stores by the ctr2 vacuolar copper transporter. J Biol Chem 2004, 279(52):54221–54229. [DOI] [PubMed] [Google Scholar]
- 16.Rees EM, Thiele DJ: Identification of a vacuole-associated metalloreductase and its role in Ctr2-mediated intracellular copper mobilization. J Biol Chem 2007, 282(30):21629–21638. [DOI] [PubMed] [Google Scholar]
- 17.Ohgami RS, Campagna DR, McDonald A, Fleming MD: The Steap proteins are metalloreductases. Blood 2006, 108(4):1388–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leary SC, Winge DR, Cobine PA: Pulling the plug” on cellular copper: the role of mitochondria in copper export. Biochim Biophys Acta 2009, 1793(1):146–153.• A comprehensive and detailed review on the copper handing machinery in mitochondria and a cross-talk between copper distribution pathways in a cell.
- 19.Kawamata H, Manfredi G: Different regulation of wild-type and mutant Cu, Zn superoxide dismutase localization in mammalian mitochondria. Hum Mol Genet 2008, 17(21):3303–3317.•• A systematic analysis of factors that influence mitochondria localization of SOD1 mitochondrial localization. The authors formulate the idea that CCS couples the function of copper chaperon with oxygen sensing.
- 20.Wood LK, Thiele DJ: Transcriptional activation in yeast in response to copper deficiency involves copper-zinc superoxide dismutase. J Biol Chem 2009, 284(1):404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itoh S, Won Kim H, Nakagawa O, Ozumi K, Lessner SM, Aoki H, Akram K, McKinney RD, Ushio-Fukai M, Fukai T: Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J Biol Chem 2008, 283(14):9157–9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McRae R, Lai B, Fahrni CJ: Copper redistribution in Atox1-deficient mouse fibroblast cells. J Biol Inorg Chem 2009. http://www.springerlink.com/content/k84m26m527232246/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banci L, Bertini I, Ciofi-Baffoni S, Hadjiloi T, Martinelli M, Palumaa P: Mitochondrial copper(I) transfer from Cox17 to Sco1 is coupled to electron transfer. Proc Natl Acad Sci USA 2008, 105(19):6803–6808.•• Using solution NMR, the authors discover simultaneous transfer of copper and electrons; this process may represent a new mechanism of selective copper delivery.
- 24.Leary SC, Sasarman F, Nishimura T, Shoubridge EA: Human SCO2 is required for the synthesis of CO II and as a thioldisulphide oxidoreductase for SCO1. Hum Mol Genet 2009, 18(12):2230–2240.• A comprehensive studies demonstrating that each of two SCO protein has distinct, stage-specific functions during CCO biosynthesis and CuA site maturation.
- 25.Li Y, Du J, Zhang P, Ding J: Crystal structure of human copper homeostasis protein CutC reveals a potential copper-binding site. J Struct Biol 2009. doi: 10.1016/j.jsb.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Calafato S, Swain S, Hughes S, Kille P, Stüirzenbaum SR: Knock down of Caenorhabditis elegans cutc-1 exacerbates the sensitivity toward high levels of copper. Toxicol Sci 2008, 106(2):384–391. [DOI] [PubMed] [Google Scholar]
- 27.Hua H, Georgiev O, Schaffner W, Steiger D: Human copper transporter Ctr1 is functional in Drosophila, revealing a high degree of conservation between mammals and insects. J Biol Inorg Chem 2010, 15(1):107–113. [DOI] [PubMed] [Google Scholar]
- 28.De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM: Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci USA 2009, 106(11):4237–4242.• The structure allows for visualization of copper translocation pathways and suggest the mechanism for copper entry and exit from the transporter. Copper binding to CTR1 is directly demonstrated.
- 29.Boal AK, Rosenzweig AC: Structural biology of copper trafficking. Chem Rev 2009, 109(10):4760–4779.• A comprehensive review summarizing a rapidly growing set of data on the structure of proteins involved in cellular copper delivery and transport in various organisms.
- 30.Miyayama T, Suzuki KT, Ogra Y: Copper accumulation and compartmentalization in mouse fibroblast lacking metallothionein and copper chaperone, Atox1. Toxicol Appl Pharmacol 2009, 237(2):205–213. [DOI] [PubMed] [Google Scholar]
- 31.Veldhuis NA, Gaeth AP, Pearson RB, Gabriel K, Camakaris J: The multi-layered regulation of copper translocating P-type ATPases. Biometals 2009, 22(1):177–190. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez S, Tsuchiya Y, García-Ruiz JP, Lalioti V, Nielsen S, Cassio D, Sandoval IV: ATP7B copper-regulated traffic and association with the tight junctions: copper excretion into the bile. Gastroenterology 2008, 134(4):1215–1223. [DOI] [PubMed] [Google Scholar]
- 33.Yanagimoto C, Harada M, Kumemura H, Koga H, Kawaguchi T, Terada K, Hanada S, Taniguchi E, Koizumi Y, Koyota S et al. : Niemann-Pick C1 protein transports copper to the secretory compartment from late endosomes where ATP7B resides. Exp Cell Res 2009, 315(2):119–126. [DOI] [PubMed] [Google Scholar]
- 34.Collins JF et al. : Alternative Splicing of the Menkes Copper Atpase (Atp7a) Transcript in the Rat Intestinal Epithelium. Am J Physiol Gastrointest Liver Physiol 2009, 297(4):G695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes N et al. : Cell-specific trafficking suggests a new role for renal ATP7B in the intracellular copper storage. Traffic 2009, 10(6):767–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mangala LS et al. : Therapeutic Targeting of ATP7B in Ovarian Carcinoma. Clin Cancer Res 2009, 15(11):3770–3780.• The first in vivo demonstration that the downregulation of ATP7B in animals via a targeted siRNA delivery facilitates the potency of cisplatin treatment and decreases tumor growth.
- 37.Hardman B et al. : Hormonal regulation of the Menkes and·Wilson copper-transporting ATPases in human placental Jeg-3 cells. Biochem J 2007, 402(2):241–250.•• The study provides the first direct demonstration of differential regulation of two Cu-ATPases in same cell types and important functional consequences of such regulation.
- 38.Niciu MJ et al. : Altered ATP7A expression and other compensatory responses in a murine model of Menkes disease. Neurobioi Dis 2007, 27(3):278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linz R et al. : Intracellular targeting of copper-transporting ATPase ATP7A in a normal and Atp7bS−/−-kidney. Am J Physiol Renal Physiol 2008, 294(1):F53–61. [DOI] [PubMed] [Google Scholar]
- 40.Furukawa T et al. : Copper transport systems are involved in multidrug resistance and drug transport. Curr Med Chem 2008, 15(30):3268–3278. [DOI] [PubMed] [Google Scholar]
- 41.Barry AN, Shinde U, Lutsenko S: Structural organization of human Cu-transporting ATPases: learning from building blocks. J Biol Inorg Chem 2010, 15(1):47–59. [DOI] [PubMed] [Google Scholar]
- 42.Tsuda T, Toyoshima C: Nucleotide recognition by CopA, a Cu+-transporting P-type ATPase. EMBO J 2009, 28(12):1782–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banci L et al. : The binding mode of ATP revealed by the solution structure of the N-domain of human ATP7A. J Biol Chem 2010, 285:2537–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonzalez-Guerrero M et al. : Structure of the two transmembrane Cu+ transport sites of the Cu+-ATPases. J Biol Chem 2008, 283(44):29753–29759.• Using site-directed mutagenesis, copper binding measurements and X-ray absorption spectroscopy, the authors provide the first information on potential structure of intramembrane sites in Cu-ATPases.
- 45.Hatori Y et al. : Reaction cycle of Thermotoga maritima copper ATPase and conformational characterization of catalytically deficient mutants. Biochemistry 2009, 48(22):4871–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez-Guerrero M, Hong D, Arguello JM: Chaperone-mediated Cu+ delivery to Cu+ transport ATPases: requirement of nucleotide binding. J Biol Chem 2009, 284(31):20804–20811.• This study offers in vitro evidence for the direct delivery of copper by the chaperone to copper translocation pathway in archeal CopA.
- 47.Morin I et al. : Dissecting the role of the N-terminal metal-binding domains in activating the yeast copper ATPase in vivo. FEBS J 2009, 276(16):4483–4495.• The study provides in vivo evidence that the eucaryotic Cu-ATPase Ccc2 requires its N-terminal domain for the chaperone-mediated delivery of copper to the copper-translocation pathway of Ccc2.
- 48.di Patti MC et al. : Dominant mutants of ceruloplasmin impair the copper loading machinery in aceruloplasminemia. J Biol Chem 2009, 284(7):4545–4554.• The authors provide the first demonstration that the copper-dependent enzymes in the secretory pathway may regulate activity of transporters from which they receive their copper cofactor.
- 49.Braiterman L et al. : Apical targeting and Golgi retention signals reside within a 9-amino acid sequence in the copper-ATPase, ATP7B. Am J Physiol Gastrointest Liver Physiol 2009, 296(2):G433–G444.• This is the first identification of a distinct determinant for TGN retention and polarized apical trafficking of ATP7B.
- 50.Bartee MY, Ralle M, Lutsenko S: The loop connecting metal-binding domains 3 and 4 of ATP7B is a target of a kinase-mediated phosphorylation. Biochemistry 2009, 48(24):5573–5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veldhuis NA et al. : Phosphorylation regulates copper-responsive trafficking of the Menkes copper transporting P-type ATPase. Int J Biochem Cell Biol 2009, 41(12):2403–2412. [DOI] [PubMed] [Google Scholar]
- 52.Pilankatta R et al. : High yield heterologous expression of wild-type and mutant Cu+-ATPase (ATP7B, Wilson disease protein) for functional characterization of catalytic activity and serine residues undergoing copper-dependent phosphorylation. J Biol Chem 2009, 284(32):21307–21316.• By developing a highly efficient adenovirus-mediated expression system, the authors demonstrate a coupling between kinase-mediated phosphorylation and catalytic activity of ATP7B.
- 53.Huster D et al. : Consequences of copper accumulation in the livers of the Atp7b−/− (Wilson disease gene) knockout mice. Am J Pathol 2006, 168(2):423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huster D et al. : High copper selectively alters lipid metabolism and cell cycle machinery in the mouse model of Wilson disease. J Biol Chem 2007, 282(11):8343–8355.• Using mRNA profiling of control and Atp7b−/−mice and metabolic measurements the authors provide evidence for significant and selective effect of copper misbalance on lipid metabolism, in particularly cholesterol biosynthesis.
- 55.Qin Z et al. : Participation of ATP7A in macrophage mediated oxidation of low density lipoprotein. J Lipid Res 2009. http://www.jlr.org/cgi/rapidpdf/jlr.M003426v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bellingham SA et al. : Regulation of prion gene expression by transcription factors SP1 and metal transcription facto r-1. J Biol Chem 2009, 284(2):1291–1301. [DOI] [PubMed] [Google Scholar]
- 57.Qin K et al. : ATM-mediated transcriptional elevation of prion in response to copper-induced oxidative stress. J Biol Chem 2009, 284(7):4582–4593. [DOI] [PubMed] [Google Scholar]
- 58.Safaei R et al. : Effects of the loss of Atox1 on the cellular pharmacology of cisplatin. J Inorg Biochem 2009, 103(3):333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boal AK, Rosenzweig AC: Crystal structures of cisplatin bound to a human copper chaperone. J Am Chem Soc 2009, 131(40):14196–14197.• First direct demonstration of the mode of interaction between cisplain and the copper trafficking molecules.
- 60.Dolgova NV et al. : The soluble metal-binding domain of the copper transporter ATP7B binds and detoxifies cisplatin. Biochem J 2009, 419(1):51–56.By combining a simple bioassay with mass-spectrometry measurements, the authors demonstrate that the recombinant N-terminal domain of ATP7B can bind cisplatin and increase cell survival in the presence of the drug.
- 61.Leonhardt K et al. : Functional interactions of Cu-ATPase ATP7B with cisplatin and the role of ATP7B in the resistance of cells to the drug. J Biol Chem 2009, 284(12):7793–7802. [DOI] [PMC free article] [PubMed] [Google Scholar]