Abstract
Objectives:
Greater physical activity is associated with lower risk of mortality in persons with kidney disease however little is known about the appropriate dose of physical activity among hemodialysis patients. Here detected the minimum level of habitual physical activity to help inform interventions aimed at improving outcomes in the dialysis population.
Design:
Prospective cohort study.
Setting and Subjects:
Clinically stable outpatients in a hemodialysis unit from October 2002 to March 2014 were assessed for their eligibility to be included in this 7-year prospective cohort study. We used the Youden index to determine the optimal cut-off points for physical activity. The prognostic effect of physical activity on survival was estimated by Cox proportional hazards regression analysis.
Predictor:
The number of steps per non-dialysis day was recorded by accelerometer at study entry.
Main outcome:
All-cause mortality.
Results:
There were 282 participants who had a mean age of 65±11 years and 45% were female. A total of 56 deaths occurred during the follow-up period (56 months, [interquartile range: 29-84 months]). The cutoff value for the physical activity discriminating those at high risk of mortality was 3,752 steps. After adjustment for the effect of confounders, the hazard ratio in the group of <4,000 steps was 2.37 (95%CI: 1.22-4.60, P=0.01) compared with the others.
Conclusions:
Engaging in physical activity is associated with decreased mortality risk among hemodialysis patients. Our findings of a substantial mortality benefit among those who engage in at least 4,000 steps provide a basis for as a minimum initial recommendation kidney health providers can provide for mobility disability free hemodialysis patients.
Keywords: dialysis, chronic renal failure, ESRD, physical activity, frailty
Introduction
The end stage renal disease (ESRD) population has a high prevalence of physical frailty characterized by physical inactivity1, 2. Low physical activity, assessed by questionnaire1-5 or accelerometer6 based methods, is strongly associated with poor prognosis in chronic kidney disease patients on hemodialysis. Despite evidence suggesting increased physical activity among dialysis patients improves performance-based outcomes, little is known about the appropriate dose of habitual physical activity from epidemiologic studies to help guide recommendations for minimum daily physical activity in this population.
Goal-setting of physical activity is well-known as one of the most popular techniques to promote it in elderly people or people with chronic illnesses7-9. A meta-analysis, which investigated the effectiveness of pedometer used intervention on physical activity in community dwelling people, concluded that setting a physical activity goal is a key motivational factor for increasing physical activity and is absolutely essential for successful intervention10.
Hemodialysis patients confront many challenges to improving their physical activity including time constraint caused by the 4-hour hemodialysis procedure, post-dialysis fatigue, and poor physical function. These challenges make it difficult for these patients to meet the goal of at least 30 minutes of moderate-to-vigorous activity11, or 7,000-10,000 steps per a day12. Therefore, more realistic and specific goal of physical activity for hemodialysis patients is needed. However, it is unclear whether how much physical activity hemodialysis patients need in their daily livings.
The purpose of this study was to detect the minimum level of habitual physical activity to help inform interventions aimed at improving outcomes in the dialysis population.
Methods
Study Population
Clinically stable outpatients in a hemodialysis unit from October 2002 to March 2014 were assessed for their eligibility to be included in this prospective cohort study. Patients were undergoing maintenance hemodialysis therapy at 3 times a week, which is most common in Japan according to the data of the Japanese Society for Dialysis Therapy. Patients were excluded from our study if they had been hospitalized within 3 months prior to the study, suffered from a recent myocardial infarction or angina pectoris, had uncontrolled cardiac arrhythmias, hemodynamic instabilities, uncontrolled hypertension, or renal osteodystrophy with severe arthralgia, or needed assistance in walking from another person. This study was approved by the Research Ethics Committee.
Patients Characteristics
Information on demographic factors (age, sex, time on hemodialysis), physical constitution [body mass index (BMI)], primary kidney disease, and comorbid conditions were collected at the time of the patients’ entry into the study. The following laboratory parameter was extracted from patient hospital charts: serum albumin level. A comorbidity index, which was developed for dialysis patients and composed of end-stage renal disease primary causes, atherosclerotic heart disease, congestive heart failure, cerebrovascular accident/transient ischemic attack, peripheral vascular disease, dysrhythmia, other cardiac diseases, chronic obstructive pulmonary disease, gastrointestinal bleeding, liver disease, cancer, and diabetes, was used to quantify comorbid illnesses in this study. This score was calculated using the method previously described and performed well in analysis for survival in hemodialysis patients13. The geriatric nutritional risk index14 was also calculated based on serum albumin level and BMI as an index of nutritional condition.
Exposure Measurement
Habitual physical activity (Lifecorder; Suzuken Co., Ltd., Nagoya, Japan) was measured using an accelerometer. The device obtains objective information on physical activity patterns because it can continuously measure the intensity, duration, and frequency of activities. The accuracy and reliability of the instrument have been reported in previous studies15, 16. The vector magnitude in the vertical direction that was recorded for every 2-min period reflects the intensity of the physical activity, as described elsewhere 17 The number of steps and energy expenditure of physical activity were recorded by the accelerometer.
The instrument was worn around the waist, and it measured motion as the acceleration of the body. Patients were instructed to wear the accelerometer continuously during their waking hours for 7 days and to avoid getting it wet, such as during bathing. Patients were asked to maintain their typical weekly schedules. In order to ensure that the measurement periods were typical of their weekly activity patterns, data was excluded when patients traveled or manifested as acute illness.
Prior to the analysis, the accelerometer data was inspected in order to ensure that there were no obvious errors, such as a failure to acquire data or if the patient forgot to wear the accelerometer. The measurements from 4 non-dialysis days during the week were analyzed.
Outcome
All-cause mortality was assessed by death registry at the clinic. Recruitment started on October 2002, and date of death was determined on February 2016. We truncated the data for the follow-up to 7 years.
Statistical Methods
Data were presented as mean ± standard deviation (SD) or number (%). Baseline patient characteristics and physical activity were compared by unpaired t-test, one-way analysis of variance, or chi-square test. The Pearson product moment correlation was used to explore the correlation between number of steps and energy expenditure of physical activity. To calculate the area under the curves (AUCs) in number of steps and energy expenditure of physical activity, receiver-operating characteristic (ROC) curve analysis was performed. We compared AUCs between number of steps and energy expenditure of physical activity. We used the Youden index to determine the optimal cut-off points for physical activity18, 19. Youden index is used as a measure of the overall combined specificity and sensitivity of prognostic factor, and is defined as the maximum vertical distance between the ROC curve and the diagonal of chance line and is calculated as: maximum [sensitivity + specificity – 1]. The best Youden index is used to determine the best cut-off point of physical activity. To calculate the discrimination ability of combined use of physical activity, ROC analysis, net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were also performed for the logistic regression models of patient characteristics only and patient characteristics plus physical activity index. NRI and IDI have been developed as more sensitive statistical methods to quantify model improvement with the addition of a new variable to an existing clinical model20.
For the Kaplan-Meier estimate of the survival curves, we truncated the data for the follow-up period of 7 years in order to avoid the number at risk being too small. We adopted the index of the number of steps, because it was easily generalizable to daily practice and its’ discrimination ability was not differ other physical activity indexes. Patients were categorized into 2 physical activity groups by cut-off value of number of steps, and differences of survival curves between groups were tested using a log-rank test. The 7-year cumulative survival probability was estimated using the life table method with the interval length set at 1 month. The independent prognostic effect of physical activity on survival was estimated by Cox proportional hazards regression analysis after adjustment for confounders, including age, sex, time on hemodialysis, BMI, diabetes, peripheral vascular disease, cerebrovascular accident/transient ischemic attack, geriatric nutritional risk index, and comorbidity score. In a sensitivity analysis, we censored those participants who died in the first 6 months at the time of their death in order to exclude those at imminent risk of death. Furthermore, we modeled the non-linear relation between number of steps as a continuous variable and mortality. We adopted four knots for the model as the 5th, 35th, 65th, and 95th centiles, and took the number of steps with the minimum risk as the reference value and compared mortality with the value of other knots to assess the risk of death and construct the hazard ratio graph. Statistical analyses were performed using IBM SPSS software, version 22.0 (IBM Corp, Armonk, New York), R version 3.3.0 (R Foundation for Statistical Computing, Vienna, Austria) and SAS 3.5 (SAS Institute, Inc, Cary, NC). In all analyses, P values of 0.05 or less were used to determine statistical significance.
Results
Patient Characteristics and Habitual Physical activity
Five hundred and fifty outpatients were assessed for their eligibility for inclusion. One hundred and ten patients not satisfying the inclusion criteria were excluded; 157 patients declined to participate in this study. As a result, a total of 282 hemodialysis patients were recruited (Supplemental Figure 1).
Patient characteristics of the patients are summarized in Table 1. Participants had a mean age of 65±11 years and 45% were female. Average time on hemodialysis was 7.0±7.8 years at baseline. The most common underlying kidney disease in the study patients was glomerulonephritis / cystic kidney disease (33.3%), and the next most common was diabetes mellitus (33.0%). The comorbidity score was 4.9±3.1. Those participants with the lower physical activity at baseline were more likely older, have a greater prevalence of diabetes, have greater comorbidity score and lower geriatric nutritional risk index score.
Table 1.
Baseline Characteristics Stratified by Physical Activity Level
| Physical activity level (number of steps on non-dialysis day) |
|||||
|---|---|---|---|---|---|
| All (n=282) |
Tertile 1st < 2800 (n=94) | Tertile 2nd 2800 ≤, < 4854 (n=94) |
Tertile 3rd 4854 ≤ (n=94) |
P value | |
| Age, years | 64.8 ± 10.6 | 69.5 ± 9.7 | 64.2 ± 10.1 | 60.7 ± 10.2 | <0.001 |
| ≥ 65 years, % | 142 (50.4%) | 65 (69.1%) | 45 (47.9%) | 32 (34.0%) | <0.001 |
| Men, % | 154 (54.6%) | 54 (57.4%) | 50 (53.2%) | 50 (53.2%) | 0.795 |
| Height, m | 1.59 ± 0.09 | 1.59 ± 0.09 | 1.59 ± 0.08 | 1.60 ± 0.09 | 0.428 |
| Dry weight, kg | 54.3 ± 10.9 | 53.6 ± 11.0 | 54.8 ± 10.9 | 54.4 ± 10.9 | 0.742 |
| Body mass index, kg/m2 | 21.3 ± 3.3 | 21.2 ± 3.3 | 21.6 ± 3.4 | 21.1 ± 3.1 | 0.529 |
| Time on hemodialysis, years | 7.0 ± 7.8 | 5.7 ± 7.4 | 7.9 ± 8.3 | 7.4 ± 7.4 | 0.117 |
| Nutritional status | |||||
| Serum albumin, g/dL | 3.9 ± 0.3 | 3.8 ± 0.3 | 3.9 ± 0.3 | 3.9 ± 0.3 | 0.005 |
| GNRI | 96.2 ± 6.2 | 94.8 ± 6.2 | 96.7 ± 6.2 | 97.1 ± 6.0 | 0.029 |
| GNRI < 91 (%) | 51 (19.3%) | 22 (25.0%) | 15 (17.9%) | 14 (15.2%) | 0.231 |
| ESRD primary cause (%) | |||||
| Diabetes | 93 (33.0%) | 42 (44.7%) | 28 (29.8%) | 23 (24.5%) | 0.028 |
| Hypertension | 24 (8.5%) | 9 (9.6%) | 8 (8.5%) | 7 (7.4%) | |
| GN/cystic kidney disease | 94 (33.3%) | 22 (23.4%) | 30 (31.9%) | 42 (44.7%) | |
| Other | 71 (25.2%) | 21 (22.3%) | 28 (29.8%) | 22 (23.4%) | |
| Comorbid conditions (%) | |||||
| ASHD | 65 (23.0%) | 23 (24.5%) | 17 (18.1%) | 25 (26.6%) | 0.354 |
| CHF | 33 (11.7%) | 15 (16.0%) | 11 (11.7%) | 7 (7.4%) | 0.193 |
| CVA/TIA | 53 (18.8%) | 25 (26.6%) | 17 (18.1%) | 11 (11.7%) | 0.032 |
| PVD | 41 (14.5%) | 22 (23.4%) | 9 (9.6%) | 10 (10.6%) | 0.011 |
| Other cardiac diseases | 48 (17.0%) | 18 (19.1%) | 16 (17.0%) | 14 (14.9%) | 0.74 |
| COPD | 14 (5.0%) | 7 (7.4%) | 4 (4.3%) | 3 (3.2%) | 0.376 |
| GI | 13 (4.6%) | 2 (2.1%) | 3 (3.2%) | 8 (8.5%) | 0.082 |
| Liver disease | 25 (8.9%) | 10 (10.6%) | 10 (10.6%) | 5 (5.3%) | 0.334 |
| Dysrhythmia | 48 (17.0%) | 22 (23.4%) | 11 (11.7%) | 15 (16.0%) | 0.097 |
| Cancer | 35 (12.4%) | 12 (12.8%) | 10 (10.6%) | 13 (13.8%) | 0.796 |
| Diabetes | 113 (40.1%) | 45 (47.9%) | 35 (37.2%) | 33 (35.1%) | 0.16 |
| Comorbidity score | 4.9 ± 3.1 | 5.9 ± 2.9 | 4.6 ± 3.0 | 4.1 ± 3.0 | <0.001 |
| Number of steps (steps) | |||||
| 7-day | 3920 ± 2797 | 1497 ± 817 | 3381 ± 723 | 6882 ± 2713 | <0.001 |
| Non-dialysis day | 4337 ± 3160 | 1409 ± 751 | 3741 ± 607 | 7862 ± 2768 | <0.001 |
| Dialysis day | 3099 ± 4337 | 1639 ± 1372 | 2718 ± 1399 | 4940 ± 3553 | <0.001 |
| Energy expenditure of physical activity (kcal) | |||||
| 7-day | 83.8 ± 75.7 | 29.5 ± 18.7 | 68.7 ± 23.7 | 153.3 ± 91.3 | <0.001 |
| Non-dialysis day | 95.2 ± 84.7 | 27.5 ± 17.1 | 77.9 ± 23.2 | 180.1 ± 92.8 | <0.001 |
| Dialysis day | 68.7 ± 76.0 | 32.2 ± 29.7 | 56.6 ± 35.1 | 117.5 ± 107.0 | <0.001 |
Values are expressed as mean ± SD or number (percentage) of patients.
Abbreviations: GNRI, geriatric nutritional risk index; ESRD, end-stage renal disease; GN, glomerulonephritis; ASHD, atherosclerotic heart disease; CHF, congestive heart failure; CVA/TIA, cerebrovascular accident/transient ischemic attack; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; GI, gastrointestinal bleeding.
At total of 56 (20%) deaths occurred over a median follow-up was 56 months (interquartile range, 29-84 months). Those who died during this period were older (P<0.001), had lower dry weight (P=0.008), lower BMI (P=0.015), and lower geriatric nutritional risk index (P=0.004). In addition those who died had a greater prevalence of glomerulonephritis / cystic kidney disease as primary kidney disease, atherosclerotic heart disease, and congestive heart failure (P=0.023, P=0.035, and P=0.019, respectively), and had greater comorbidity score compared to survivors (P<0.001). Those who died had a lower number of steps and energy expenditure of physical activity compared to survivors. In addition, number of steps and energy expenditure of physical activity on dialysis day were lower than those on non-dialysis day, respectively. Number of steps was significantly correlated with energy expenditure of physical activity (r=0.93, P<0.001).
Cutoff Value of Physical Activity
We compared AUCs of number of steps and energy expenditure of physical activity using ROC analysis. Discrimination abilities were not significantly different between number of steps and energy expenditure of physical activity (Supplemental Figure 2). The AUCs were increased by adding the number of steps to patient characteristics including age, sex, time on hemodialysis, BMI, geriatric nutritional risk index, and comorbidity score (from 0.75 [95%CI: 0.68-0.82] to 0.78 [95%CI: 0.72-0.84]). Both NRI and IDI suggested that the ability of adding number of steps to patient characteristics improved discrimination of those at high risk of mortality (NRI, 0.60 [95%CI: 0.32 to 0.86], P<0.001; IDI, 0.03 [95%CI: 0.00 to 0.01], P=0.04).
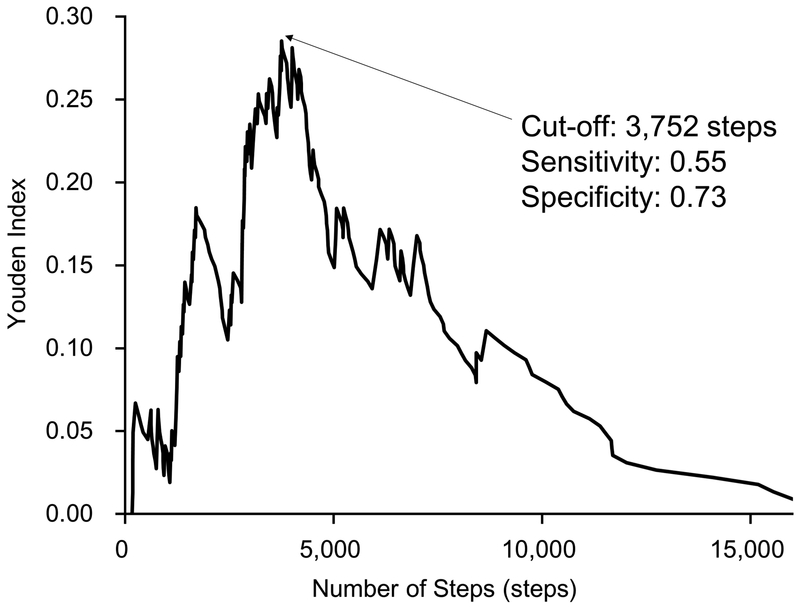
The cutoff value for the number of steps discriminating those at high risk of mortality based on the Youden index was 3,752 steps per non-dialysis day (Figure 1).
Figure 1.
The Cutoff of Number of Steps on Non-dialysis Day Detected by Youden Index.
Kaplan–Meier Estimate of Patient Survival
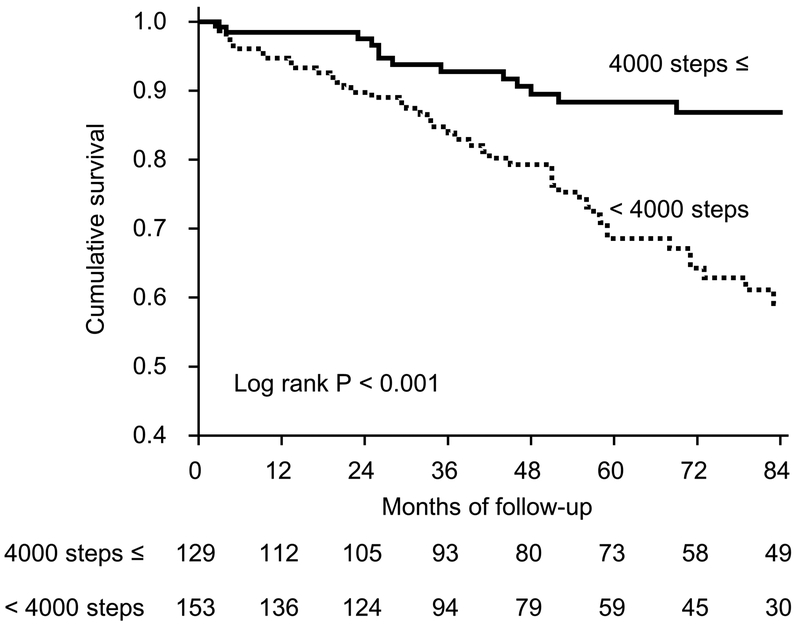
Median follow-up of participants was 56 months (interquartile range, 29-84 months) during which 56 (19.9%) deaths were reported. We divided patients to 2 groups according to number of steps with good discriminatory ability. The 7-year cumulative survival rate were 87.0% in the group of ≤4,000 steps of physical activity and 60.0% in the <4,000 steps. More than half of patients in each group were alive at the end of follow-up. Twenty-five percent of the patients with lower physical activity died after 55 months. On the other hand, the mortality rate of patients with greater physical activity at end of the follow-up was less than 25%. This finding indicates superior survival in patients with greater physical activity (P<0.001) (Figure 2).
Figure 2.
Kaplan-Meier Analysis of Survival for 282 Patients Undergoing Hemodialysis. Patients with physical activity above the 4,000 steps per a non-dialysis (thick dark line) at baseline had significantly better survival than those with lower values (dotted line) (P < 0.001 by log-rank test).
Effects of Habitual Physical Activity on Survival in Cox Proportional Hazards Regression Analysis
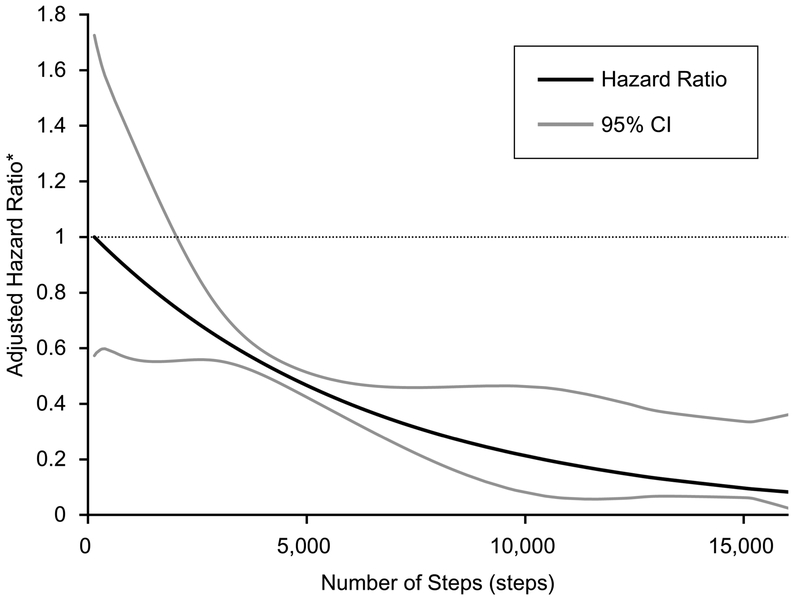
After adjustment for the effect of age, sex, time on hemodialysis, geriatric nutritional risk index, and comorbidity score, the hazard ratio in the group of <4,000 steps of physical activity was 2.37 (95%CI: 1.22 to 4.60, P=0.01) compared with that in the group of 4,000 steps≤, and the hazard ratio of physical activity increased per 1000 steps per non-dialysis day was 0.84 (95%CI: 0.74 to 0.96, P=0.01) (Table 3). Sensitivity analysis censoring those who died in the first 6 months of follow-up did not noticeably impact the estimates of association. Furthermore, there was a continuous association of greater physical activity with reduction in all-cause mortality (Figure 3).
Table 3.
Association between Number of Steps on a Non-dialysis Day and Mortality in Multivariate Cox Proportional Hazards Regression Models
| Number of Steps |
||||
|---|---|---|---|---|
| 4,000 steps ≤ | < 4,000 steps | Per 1,000 steps increase | ||
| Model 1 | HR (95% CI) | Reference | 2.92 (1.51 to 5.63) | 0.82 (0.72 to 0.93) |
| P value | - | 0.001 | 0.002 | |
| Model 2 | HR (95% CI) | Reference | 2.72 (1.41 to 5.30) | 0.83 (0.73 to 0.94) |
| P value | - | 0.003 | 0.004 | |
| Model 3 | HR (95% CI) | Reference | 2.58 (1.32 to 5.03) | 0.84 (0.74 to 0.95) |
| P value | - | 0.006 | 0.007 | |
| Model 4 | HR (95% CI) | Reference | 2.62 (1.35 to 5.08) | 0.83 (0.73 to 0.94) |
| P value | - | 0.004 | 0.003 | |
| Model 5 | HR (95% CI) | Reference | 2.37 (1.22 to 4.60) | 0.84 (0.74 to 0.96) |
| P value | - | 0.01 | 0.01 | |
Analyses were performed using Cox proportional hazards regression. HR, hazard ratio; CI, confidence interval. Model 1 included age, sex, time on hemodialysis, and body mass index. Model 2 added diabetes. Model 3 added peripheral vascular disease. Model 4 added cerebrovascular accident/transient ischemic attack. Model 5 added geriatric nutritional risk index and comorbidity score.
Figure 3.
Graphical Visualization of The Cox Proportional Hazard Model Using The Restricted Cubic Spline Conversion. *Predicted hazard ratios were estimated from Cox proportional hazards regression analysis after adjustment for confounders, including age, sex, time on hemodialysis, body mass index, geriatric nutritional risk index, and comorbidity score. Four knots for the model were placed at the 5th, 35th, 65th, and 95th centiles, and a highest point of mortality risk was taken as the reference value.
Discussion
To our knowledge, this is the first long-term cohort study of hemodialysis patients investigating a simple and suitable recommendation of physical activity for hemodialysis patients in the clinical settings. Our study findings demonstrate a continuous association of increasing objective physical activity measured by accelerometry and reduced mortality among dialysis patients with long-term follow-up. Importantly, objective physical activity level was significantly and meaningfully associated with all-cause mortality in hemodialysis patients, independent of age, sex, time on hemodialysis, BMI, diabetes, geriatric nutritional risk index, and comorbidity score. On the basis of these findings, hemodialysis patients spending more time on ambulatory physical activity on non-dialysis day have a lower mortality risk. Furthermore, our findings support 4,000 steps per non-dialysis day as an initial minimum recommendation of physical activity level for hemodialysis patients.
Panaye M et al. had recently conducted a national epidemiological study, and measured number of steps in 1,163 dialysis patients using a pedometer21. The number of steps was 3,688 steps per a day in the study populations, and this result was similar to that of our study. In addition, physical activity levels were significantly lower on dialysis days when compared non-dialysis days as with that of previous study22. Thus agreements with past findings might well enhance general versatility of our study.
Patients with kidney disease have a high prevalence of frailty23-28 associated with higher risks of injurious falls or fractures28, hospitalization27 and mortality25. Frailty among hemodialysis patients is primary characterized by poor physical performance and physical inactivity. Approximately half of the participants experienced serious deterioration in the muscle function, and decreased muscle strength was associated with walking ability and postural balance function in patients on hemodialysis29. Chen JL et al. had evaluated the effect of supervised exercise training for elderly patients undergoing hemodialysis, and leisure-time physical activity level of the participants was significantly improved with exercise training30. In elderly or frail patients on hemodialysis, supervised exercise training such as intra-dialytic or multiple low-intensity exercise30, 31, chair stand exercise32 or electrostimulation of skeletal muscles33 would be better to increase their physical activity safely and effectively than a motivational approach such as goal settings or pedometer use.
Physical inactivity is the most modifiable component of the frailty phenotype. However, in spite of the evidence in support of the health benefits from engaging in physical activity2, 5, 6 patients on hemodialysis remain substantially less active than the general healthy sedentary population34-36. Expert clinical guidelines from Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group37 or European Renal Best Practice (ERBP) guideline development group38 recommend that kidney health providers regularly encourage patients with kidney disease to undertake regular physical activity. These recommendations have been supported by a recent multicenter clinical trial of walking exercise in the dialysis population demonstrated meaningful physical performance benefits and suggestion of a decreased hospitalization among those who exercise39. Until now, there has been no data to support a minimum objective and practical physical activity goal tailored to the hemodialysis population.
Our findings contribute to the foundation of literature indicating the beneficial effects of ambulatory physical activity and provide a basis for minimal recommended ambulatory physical activity among hemodialysis patients. To engage at least 4000 steps on non-dialysis day for hemodialysis patients was associated with the better outcome from this study. This is consistent with recommendations from the American College of Sports Medicine previously reported 4000 steps per a day as an activity goal for elderly40, and other previous studies also reported same activity goal 41, 42. Our findings agree with those of these studies and will help further bolster the evidence for initial exercise recommendations specifically targeting sedentary patients on hemodialysis. The PACE-Lift trial and some reviews concluded that goal setting of physical activity is one of the most import key enablers to enhanced and sustained physical activity for elderly populations8, 9, 43. In addition to the technique of activity goal settings, wearing pedometer, use of a step diary (monitoring by self) and feedback from medical staff were associated with significant increases in physical activity—a magnitude of approximately 2000-2500 steps per a day in community dwelling people10. This pedometer technique motivated to increase physical activity of hemodialysis patients44.
This study had some limitations. First, because we recruited clinically stable and adequately dialyzed patients, their physical activity was assumed not to fluctuate dramatically. Thus, we evaluated physical activity of hemodialysis patients only at baseline, and could not evaluate fluctuation of physical activity over time. However, physical activity could be affected by not only time constrains during dialysis treatment but also by aging, physical function decline, exacerbation of dialysis-related symptoms or comorbidities, depression and other social factors in hemodialysis patients. Further studies are needed to assess the effects of deterioration in physical activity on prognosis in hemodialysis patients. Second, we excluded patients who had overt mobility limitation indicated by their need for assistance with walking. However, restriction of our study to those free of overt mobility limitation may suggest the estimates of our association of physical activity with mortality may be conservative. Third, our study was limited to a single center, thereby limiting the generalizability of our findings to the broader international hemodialysis population. Further large-scale prospective cohort studies of are needed. Finally, although we reported that patients with shorter physical activity time experienced a higher mortality risk compared with the others, the underlying mechanisms remain to be elucidated.
In conclusion, engaging in walking-based physical activity is associated with decreased mortality risk among hemodialysis patients. Our findings of a substantial mortality benefit among those who engage in at least 4,000 steps per a non-dialysis day provide a basis for as a minimum initial recommendation kidney health providers can provide for mobility disability free hemodialysis patients. Our findings provide additional support for regular patient engagement by kidney health providers to facilitate improvements in physical activity. Such improvements may address the physical frailty phenotype and its associated adverse patient-centered outcomes among hemodialysis patients. Further investigations of high-risk hemodialysis patients with mobility limitation are necessary to validate our findings among the most vulnerable hemodialysis patients.
Practical Application
Engaging in walking-based physical activity is associated with decreased mortality risk among hemodialysis patients. Our findings of a substantial mortality benefit among those who engage in at least 4,000 steps per a non-dialysis day provide a basis for as a minimum initial recommendation kidney health providers can provide for mobility disability free hemodialysis patients.
Supplementary Material
Flow Diagram of Patient Selection and Exclusion Process.
Receiver Operating Characteristics Curves Discriminating for All-cause Mortality between Number of Steps and Energy Expenditure of Physical Activity Evaluated Using Accelerometer on Non-dialysis Day. PA, physical activity; AUC, area under the curve; CI, confidence interval.
Table 2.
Baseline Characteristics in Survivors and Non-survivors
| Survivors (n = 226) |
Non-survivors (n = 56) |
P value | |
|---|---|---|---|
| Age, years | 63.6 ± 10.5 | 69.7 ± 9.8 | < 0.001 |
| ≥ 65 years, % | 103 (45.6%) | 39 (69.6%) | 0.002 |
| Men, % | 121 (53.5%) | 33 (58.9%) | 0.549 |
| Height, m | 1.60 ± 0.09 | 1.58 ± 0.08 | 0.070 |
| Dry weight, kg | 55.2 ± 10.9 | 50.9 ± 10.6 | 0.008 |
| Body mass index, kg/m2 | 21.6 ± 3.2 | 20.4 ± 3.4 | 0.015 |
| Time on hemodialysis, years | 7.1 ± 8.0 | 6.6 ± 6.6 | 0.694 |
| Nutritional status | |||
| Serum albumin, g/dL | 3.9 ± 0.3 | 3.8 ± 0.3 | 0.283 |
| GNRI | 96.8 ± 6.0 | 94.1 ± 6.6 | 0.004 |
| GNRI < 91 (%) | 36 (17.3%) | 15 (26.8%) | 0.128 |
| ESRD primary cause (%) | |||
| Diabetes | 71 (31.4%) | 22 (39.3%) | 0.023 |
| Hypertension | 16 (7.1%) | 8 (14.3%) | |
| GN/cystic kidney disease | 84 (37.2%) | 10 (17.9%) | |
| Other | 55 (24.3%) | 16 (28.6%) | |
| Comorbid conditions (%) | |||
| ASHD | 46 (20.4%) | 19 (33.9%) | 0.035 |
| CHF | 21 (9.3%) | 12 (21.4%) | 0.019 |
| CVA/TIA | 46 (20.4%) | 7 (12.5%) | 0.251 |
| PVD | 28 (12.4%) | 13 (23.2%) | 0.055 |
| Other cardiac diseases | 34 (15.0%) | 14 (25.0%) | 0.110 |
| COPD | 9 (4.0%) | 5 (8.9%) | 0.163 |
| GI | 8 (3.5%) | 5 (8.9%) | 0.144 |
| Liver disease | 18 (8.0%) | 7 (12.5%) | 0.296 |
| Dysrhythmia | 36 (15.9%) | 12 (21.4%) | 0.326 |
| Cancer | 27 (11.9%) | 8 (14.3%) | 0.652 |
| Diabetes | 87 (38.5%) | 26 (46.4%) | 0.290 |
| Comorbidity score | 4.5 ± 3.0 | 6.3 ± 3.0 | < 0.001 |
| Number of steps (steps) | |||
| 7-day | 4,207 ± 2,937 | 2,762 ± 1,731 | < 0.001 |
| Non-dialysis day | 4,656 ± 3,298 | 3,051 ± 2,101 | 0.001 |
| Dialysis day | 3,332 ± 2,820 | 2,162 ± 1,967 | 0.004 |
| Energy expenditure of physical activity (kcal) | |||
| 7-day | 91.9 ± 80.7 | 51.2 ± 36.5 | < 0.001 |
| Non-dialysis day | 104.2 ± 89.7 | 58.9 ± 45.7 | < 0.001 |
| Dialysis day | 75.6 ± 81.0 | 40.9 ± 41.3 | 0.002 |
Values are expressed as mean ± SD or number (percentage) of patients.
Abbreviations: GNRI, geriatric nutritional risk index; ESRD, end-stage renal disease; GN, glomerulonephritis; ASHD, atherosclerotic heart disease; CHF, congestive heart failure; CVA/TIA, cerebrovascular accident/transient ischemic attack; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; GI, gastrointestinal bleeding.
Acknowledgments:
The authors thank the renal staff for their support and the patients for giving their time to complete the research protocol. This work was supported by grants by the NIDDK [1K23DK099442 to BR] and the JSPS KAKENHI [16K16466 to RM].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Support and financial disclosure: Baback Roshanravan reports having received consultancy fees from Stealth Biotherapeutics. Other authors have not declared any conflict of interest. The content is solely the responsibility of the authors. The results presented in this article have not been published previously, in whole or part, except in abstract format.
References
- 1.O'Hare AM, Tawney K, Bacchetti P, Johansen KL. Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis. 2003;41:447–454. [DOI] [PubMed] [Google Scholar]
- 2.Johansen KL, Kaysen GA, Dalrymple LS, et al. Association of physical activity with survival among ambulatory patients on dialysis: the Comprehensive Dialysis Study. Clin J Am Soc Nephrol. 2013;8:248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stack AG, Molony DA, Rives T, Tyson J, Murthy BV. Association of physical activity with mortality in the US dialysis population. Am J Kidney Dis. 2005;45:690–701. [DOI] [PubMed] [Google Scholar]
- 4.Tentori F, Elder SJ, Thumma J, et al. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): correlates and associated outcomes. Nephrol Dial Transplant. 2010;25:3050–3062. [DOI] [PubMed] [Google Scholar]
- 5.Lopes AA, Lantz B, Morgenstern H, et al. Associations of self-reported physical activity types and levels with quality of life, depression symptoms, and mortality in hemodialysis patients: the DOPPS. Clin J Am Soc Nephrol. 2014;9:1702–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuzawa R, Matsunaga A, Wang G, et al. Habitual physical activity measured by accelerometer and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2012;7:2010–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farholm A, Sorensen M. Motivation for physical activity and exercise in severe mental illness: A systematic review of intervention studies. Int J Ment Health Nurs. 2016;25:194–205. [DOI] [PubMed] [Google Scholar]
- 8.McEwan D, Harden SM, Zumbo BD, et al. The effectiveness of multi-component goal setting interventions for changing physical activity behaviour: a systematic review and meta-analysis. Health Psychol Rev. 2016;10:67–88. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien N, McDonald S, Araujo-Soares V, et al. The features of interventions associated with long-term effectiveness of physical activity interventions in adults aged 55–70 years: a systematic review and meta-analysis. Health Psychol Rev. 2015;9:417–433. [DOI] [PubMed] [Google Scholar]
- 10.Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296–2304. [DOI] [PubMed] [Google Scholar]
- 11.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–99. [DOI] [PubMed] [Google Scholar]
- 12.Tudor-Locke C, Craig CL, Aoyagi Y, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2010;77:141–151. [DOI] [PubMed] [Google Scholar]
- 14.Bouillanne O, Morineau G, Dupont C, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82:777–783. [DOI] [PubMed] [Google Scholar]
- 15.Crouter SE, Schneider PL, Karabulut M, Bassett DR Jr. Validity of 10 electronic pedometers for measuring steps, distance, and energy cost. Med Sci Sports Exerc. 2003;35:1455–1460. [DOI] [PubMed] [Google Scholar]
- 16.Schneider PL, Crouter SE, Lukajic O, Bassett DR Jr. Accuracy and reliability of 10 pedometers for measuring steps over a 400-m walk. Med Sci Sports Exerc. 2003;35:1779–1784. [DOI] [PubMed] [Google Scholar]
- 17.Kumahara H, Schutz Y, Ayabe M, et al. The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: a validation study against whole-body indirect calorimetry. Br J Nutr. 2004;91:235–243. [DOI] [PubMed] [Google Scholar]
- 18.Perkins NJ, Schisterman EF. The inconsistency of "optimal" cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163:670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458–472. [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, D'Agostino RB Sr., D'Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 21.Panaye M, Kolko-Labadens A, Lasseur C, et al. Phenotypes influencing low physical activity in maintenance dialysis. J Ren Nutr. 2015;25:31–39. [DOI] [PubMed] [Google Scholar]
- 22.Majchrzak KM, Pupim LB, Chen K, et al. Physical activity patterns in chronic hemodialysis patients: comparison of dialysis and nondialysis days. J Ren Nutr. 2005;15:217–224. [DOI] [PubMed] [Google Scholar]
- 23.Roshanravan B, Khatri M, Robinson-Cohen C, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60:912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roshanravan B, Robinson-Cohen C, Patel KV, et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol. 2013;24:822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansen KL, Dalrymple LS, Glidden D, et al. Association of Performance-Based and Self-Reported Function-Based Definitions of Frailty with Mortality among Patients Receiving Hemodialysis. Clin J Am Soc Nephrol. 2016;11:626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansen KL, Dalrymple LS, Delgado C, et al. Comparison of self-report-based and physical performance-based frailty definitions among patients receiving maintenance hemodialysis. Am J Kidney Dis. 2014;64:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61:896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delgado C, Shieh S, Grimes B, et al. Association of Self-Reported Frailty with Falls and Fractures among Patients New to Dialysis. Am J Nephrol. 2015;42:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuzawa R, Matsunaga A, Wang G, et al. Relationship between lower extremity muscle strength and all-cause mortality in Japanese patients undergoing dialysis. Phys Ther. 2014;94:947–956. [DOI] [PubMed] [Google Scholar]
- 30.Chen JL, Godfrey S, Ng TT, et al. Effect of intra-dialytic, low-intensity strength training on functional capacity in adult haemodialysis patients: a randomized pilot trial. Nephrol Dial Transplant. 2010;25:1936–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esteve Simo V, Junque Jimenez A, Moreno Guzman F, et al. Benefits of a low intensity exercise programme during haemodialysis sessions in elderly patients. Nefrologia. 2015;35:385–394. [DOI] [PubMed] [Google Scholar]
- 32.Matsufuji S, Shoji T, Yano Y, et al. Effect of chair stand exercise on activity of daily living: a randomized controlled trial in hemodialysis patients. J Ren Nutr. 2015;25:17–24. [DOI] [PubMed] [Google Scholar]
- 33.Dobsak P, Homolka P, Svojanovsky J, et al. Intra-dialytic electrostimulation of leg extensors may improve exercise tolerance and quality of life in hemodialyzed patients. Artif Organs. 2012;36:71–78. [DOI] [PubMed] [Google Scholar]
- 34.Johansen KL, Chertow GM, Kutner NG, Dalrymple LS, Grimes BA, Kaysen GA. Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int. 2010;78:1164–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johansen KL, Chertow GM, Ng AV, et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000;57:2564–2570. [DOI] [PubMed] [Google Scholar]
- 36.Zamojska S, Szklarek M, Niewodniczy M, Nowicki M. Correlates of habitual physical activity in chronic haemodialysis patients. Nephrol Dial Transplant. 2006;21:1323–1327. [DOI] [PubMed] [Google Scholar]
- 37.Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney inter., Suppl 2013;3:1–150. [Google Scholar]
- 38.Farrington K, Covic A, Aucella F, et al. Clinical Practice Guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR <45 mL/min/1.73 m2). Nephrol Dial Transplant. 2016;31:ii1–ii66. [DOI] [PubMed] [Google Scholar]
- 39.Manfredini F, Mallamaci F, D'Arrigo G, et al. Exercise in Patients on Dialysis: A Multicenter, Randomized Clinical Trial. J Am Soc Nephrol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WJ C-Z. ACSM's exercise for older asults. 1st ed. . Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2014. 2014. [Google Scholar]
- 41.Aoyagi Y, Shephard RJ. Habitual physical activity and health in the elderly: the Nakanojo Study. Geriatr Gerontol Int. 2010;10 Suppl 1:S236–243. [DOI] [PubMed] [Google Scholar]
- 42.Inoue S, Ohya Y, Tudor-Locke C, Tanaka S, Yoshiike N, Shimomitsu T. Time trends for step-determined physical activity among Japanese adults. Med Sci Sports Exerc. 2011;43: 1913–1919. [DOI] [PubMed] [Google Scholar]
- 43.Victor CR, Rogers A, Woodcock A, et al. What factors support older people to increase their physical activity levels? An exploratory analysis of the experiences of PACE-Lift trial participants. Arch Gerontol Geriatr. 2016;67:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nowicki M, Murlikiewicz K, Jagodzinska M. Pedometers as a means to increase spontaneous physical activity in chronic hemodialysis patients. J Nephrol. 2010;23:297–305. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow Diagram of Patient Selection and Exclusion Process.
Receiver Operating Characteristics Curves Discriminating for All-cause Mortality between Number of Steps and Energy Expenditure of Physical Activity Evaluated Using Accelerometer on Non-dialysis Day. PA, physical activity; AUC, area under the curve; CI, confidence interval.