Abstract
Background
Individuals with metabolic syndrome (MetS) and diabetes (DM) are more likely to have decreased lung function and are at greater risk of cardiovascular disease (CVD).
Hypothesis.
Lung‐function measures can predict CVD events in older persons with MetS, DM, and neither condition.
Methods
We followed 4114 participants age ≥ 65 years with and without MetS or DM in the Cardiovascular Health Study. Cox regression examined the association of forced vital capacity (FVC) and 1‐second forced expiratory volume (FEV1; percent of predicted values) with incident coronary heart disease and CVD events over 12.9 years.
Results
DM was present in 537 (13.1%) and MetS in 1277 (31.0%) participants. Comparing fourth vs first quartiles for FVC, risk of CVD events was 16% (HR: 0.84, 95% CI: 0.59–1.18), 23% (HR: 0.77, 95% CI: 0.60–0.99), and 30% (HR: 0.70, 95% CI: 0.58–0.84) lower in DM, MetS, and neither disease groups, respectively. For FEV1, CVD risk was lower by 2% (HR: 0.98, 95% CI: 0.70–1.37), 26% (HR: 0.74, 95% CI: 0.59–0.93), and 31% (HR: 0.69, 95% CI: 0.57–0.82) in DM. Findings were strongest for predicting congestive heart failure (CHF) in all disease groups. C‐statistics increased significantly with addition of FEV1 or FVC over risk factors for CVD and CHF among those with neither MetS nor DM.
Conclusions
FEV1 and FVC are inversely related to CVD in older adults with and without MetS, but not DM (except for CHF); however, their value in incremental risk prediction beyond standard risk factors is limited mainly to metabolically healthier persons.
Keywords: Cardiovascular, Cox Regression, Diabetes, Lung Function, Metabolic Syndrome
1. INTRODUCTION
Previous studies have identified decreased lung function as an independent prognostic predictor for CVD events, and this effect appears to be more pronounced in women than in men.1, 2, 3, 4 In addition, both cross‐sectional and prospective studies show an association of reduced lung function with MetS and DM.5, 6, 7, 8, 9, 10, 11 We have previously shown in the Third National Health and Nutrition Examination Survey (NHANES III) reduced lung function to be associated with total mortality among persons with MetS and DM.12 Also, the spirometric variables of forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) add incremental value in predicting total mortality among intermediate‐risk Framingham Risk Score individuals.13
Those with MetS and DM are also more likely to have subclinical atherosclerosis and are at a greater risk of CVD.14, 15, 16 However, whether reduced lung function in these groups may further refine prediction of CVD events is unclear. Such information would be useful to judge the utility of lung‐function assessment as an independent predictor of future CVD events and mortality in these groups.
This study examined whether spirometric measures of lung function predict CVD events and their components among higher‐risk individuals among those with MetS and DM. We hypothesized that lung‐function measurements are related to the risk of future CVD events in these groups.
2. METHODS
The Cardiovascular Health Study is a prospective National Institutes of Health–sponsored study of adults who were age ≥ 65 years at baseline in 1989–1990.17 An additional African American cohort of 687 persons with measurements of pulmonary function and other risk factors was enrolled in the period from 1992 to 1993, bringing the total cohort to 5888 persons. Participants in the study were recruited from 4 US geographic regions: Sacramento County, California; Washington County, Maryland; Forsyth County, North Carolina; and Pittsburgh, Pennsylvania. Pulmonary‐function test data were available at the baseline examination. Pulmonary‐function testing, which includes FVC and FEV1, was measured with a water‐sealed Collins II spirometer (W.E. Collins, Braintree, MA). Details of quality control and missing data due to unreproducible spirometry tests were previously introduced.18 We also calculated the percent of predicted values using NHANES III reference values.19 Clinical examinations consisted of assessment of medical history, physical examination, and fasting blood analyses. Seated blood pressure (BP) was measured using the auscultatory method, having the mid‐height of the cuff at heart level with the average of 2 measures used. Antihypertensive and lipid treatment data were collected using medication inventory and coded as yes/no in our study.20 Alcohol intake was measured as number of alcoholic beverages per week; smoking status was categorized as never smoker, former smoker, and current smoker; and high‐sensitivity C‐reactive protein measures were also available. Low‐density lipoprotein cholesterol (LDL‐C) was calculated using the Friedewald equation: (LDL‐C = total cholesterol – high‐density lipoprotein cholesterol [HDL‐C] – (1/5) triglycerides [TG]) for TG <400 mg/dL.
Of this sample, we included 4114 participants without a prior history of CVD. Subjects included in the study were stratified by the presence of either DM or MetS. MetS was identified as having any 3 of the following 5 conditions: elevated BP (≥130 mm Hg systolic or ≥ 85 mm Hg diastolic), low HDL‐C (<40 mg/dL in males or < 50 mg/dL in females), elevated TG (≥150 mg/dL), increased waist circumference (>88 cm in females or > 102 cm in males), or impaired fasting glucose (IFG; 100–125 mg/dL). DM was defined as having 1 of the following conditions: baseline glucose ≥126 mg/dL after a 12‐hour fast, use of oral hypoglycemic agents, or the use of insulin. Participants were categorized as having MetS in our study only if they did not also have DM.
Incident CVD was defined as having stroke, myocardial infarction (MI), heart failure, coronary artery angioplasty, coronary artery bypass surgery, claudication, or angina. Incident coronary heart disease (CHD) was identified as the first occurrence of any of the following: angina, MI, coronary artery angioplasty, coronary artery bypass surgery, or death caused by “atherosclerotic CHD.” We also examined the individual CVD components of stroke and congestive heart failure (CHF) as secondary endpoints. Self‐report of physician‐diagnosed CHF was followed by confirmatory review of the participant's medical records. The presence of CHF was determined from both the diagnosis of CHF by a physician and treatment of CHF (ie, a current prescription for a diuretic agent and either digitalis or a vasodilator). In addition, symptoms, signs, and chest x‐ray findings of CHF were reviewed by the CHS Events Committee. The follow‐up time was measured from the baseline pulmonary‐function testing to date of first occurrence of 1 of the CVD events. The CHS events committee adjudicated all primary CHD and CVD events during the follow‐up. Follow‐up for events was available through June 2014.
2.1. Statistical analysis
CHD and CVD events per 1000 person‐years, by percent of predicted FEV1 and FVC quartiles, were calculated and displayed with bar charts. We used multivariate Cox proportional hazards regression to determine hazard ratios (HRs) for CHD and CVD events, adjusted for age, sex, ethnicity, and other non‐MetS risk factors, for quartiles of FVC and FEV1 within MetS and DM, with the lowest quartile as the reference category. In our Cox regression analyses that treated FEV1 and FVC as continuous markers, FEV1 and FVC were rescaled by their respective SDs to make direct comparison of HR for 1‐SD change of FEV1 and 1‐SD change of FVC. We used C‐statistics for survival data to examine whether FVC or FEV1 add incremental predictive value over risk factors for events in subjects with and without MetS and DM. The statistical procedures were done using SAS statistical software, version 9.4 (SAS Institute, Inc., Cary, NC).
3. RESULTS
Participants were followed for incident CHD and CVD events over a mean follow‐up of 12.9 ±4.9 years. Out of the 4241 CVD‐free subjects at baseline, 127 did not have lung‐function data. They showed slightly higher systolic BP (141 mm Hg vs 136 mm Hg) and higher body mass index (28.0 kg/m2 vs 26.5 kg/m2) than those included, but other risk factors were similar. The remaining 4114 participants were included in the study; 1277 (31.0%) had MetS and 537 (13.1%) had DM. Among those with neither MetS nor DM, 24.3% had central obesity (waist circumference > 102 cm for male and > 88 for female), 8.0% had low HDL‐C (<40 mg/dL for male and < 50 mg/dL for female), 9.2% had elevated TG (≥150 mg/dL), 58.1% had elevated BP (≥130 mm Hg systolic or ≥ 85 mm Hg diastolic), and 29.1% had IFG (100–125 mg/dL). Among those with MetS but no DM, 76.0% had central obesity (waist circumference > 102 cm for male and > 88 for female), 50.8% had low HDL‐C (<40 mg/dL for male and < 50 mg/dL for female), 58.8% had elevated TG (≥150 mg/dL), 88.3% had elevated BP (≥130 mm Hg systolic or ≥ 85 mm Hg diastolic), and 77.3% had IFG (100–125 mg/dL). Individuals with MetS and DM had significantly lower HDL‐C values and significantly higher C‐reactive protein and systolic and diastolic BP than participants with neither condition. The cumulative incidences of CVD (64.6% and 54.9% vs 45.9%; P < 0.001), CHD (41.9% and 35.7% vs 28.7%; P < 0.001), CHF (34.6% and 28.3% vs 22.0%; P < 0.001), and stroke (23.3% and 17.5% vs 15.5%) were higher among those with DM and MetS, respectively, than those with neither condition. Individuals with MetS had higher total cholesterol and LDL‐C values than did those with DM. Furthermore, those with MetS and DM had poorer lung function than did those with neither disease. FEV1 for those with IFG was higher than that of those with DM (2.1 L vs 2.0 L, and 87% vs 84% for percent predicted FEV1, both P < 0.05); FVC was comparable between those with IFG and those with DM. The baseline characteristics for the study population can be found in Table 1.
Table 1.
Means and proportions across disease groups in the Cardiovascular Health Study
| Overall, N = 4114 | Neither, n = 2300 (55.9%) | MetS, n = 1277 (31.0%) | DM, n = 537 (13.1%) | P Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Mean age, y | 72.4 ± 5.4 | 72.5 ±5.6 | 72.1 ±5.0 | 72.7 ±5.5 | 0.04 |
| Male sex | 1603 (39.0) | 9356 (40.7) | 418 (32.7) | 250 (46.6) | 0.0001 |
| Race | |||||
| Caucasian | 3581 (87.0) | 2021 (87.9) | 1140 (89.3) | 420 (78.2) | 0.0001 |
| African American | 507 (12.3) | 267 (11.6) | 129 (10.1) | 111 (20.7) | 0.0001 |
| Risk factors | |||||
| FEV1, L | 2.07 ±0.66 | 2.12 ±0.68 | 2.00 ±0.62 | 2.00 ±0.67 | 0.0001 |
| % of predicted FEV1 | 88.5 ±21.8 | 90.3 ±21.9 | 86.9 ±21.4 | 84.7 ±21.4 | 0.0001 |
| FVC, L | 2.13 ±0.69 | 2.18 ±0.71 | 2.05 ±0.64 | 2.10 ±0.70 | 0.0001 |
| % of predicted FVC | 68.2 ±18.7 | 69.4 ±18.4 | 66.8 ±18.4 | 66.4 ±19.8 | 0.0001 |
| TC, mg/dL | 212.7 ±38.8 | 211.5 ±36.6 | 217.7 ±40.3 | 205.8 ±42.6 | 0.0001 |
| HDL‐C, mg/dL | 55.8 ±15.7 | 61.1 ±15.6 | 49.1 ±13.0 | 48.7 ±12.7 | 0.0001 |
| LDL‐C, mg/dL | 129.9 ± 35.5 | 128.0 ±33.8 | 135.1 ±36.8 | 125.5 ±38.0 | 0.0001 |
| SBP, mm Hg | 136.0 ±21.3 | 132.5 ±21.3 | 140.1 ±19.8 | 141.4 ±21.6 | 0.0001 |
| DBP, mm Hg | 71.1 ±11.2 | 70.2 ±11.2 | 72.3 ±10.7 | 72.1 ±11.8 | 0.0001 |
| Current smoker | 493 (12.0) | 286 (12.4) | 148 (11.6) | 59 (11.0) | 0.57 |
| BMI, kg/m2 | 26.5 ±4.54 | 24.8 ±3.74 | 28.7 ±4.49 | 28.5 ±4.73 | 0.0001 |
| Glucose, mg/dL | 108.5 ±32.78 | 96.4 ±8.52 | 105.2 ±9.02 | 168.6 ±58.94 | 0.0001 |
| Family history of CVD | 1391 (33.81) | 748 (32.52) | 452 (35.40) | 191 (35.57) | 0.14 |
| Medications | |||||
| HTN medication | 1580 (38.41) | 618 (26.87) | 669 (52.39) | 293 (54.56) | 0.0001 |
| Lipid‐lowering medication | 179 (4.35) | 81 (3.52) | 68 (5.32) | 30 (5.59) | 0.013 |
| Incident disease | |||||
| CVD | 2104 (51.1) | 1056 (45.9) | 701 (54.9) | 347 (64.6) | 0.0001 |
| CHD | 1340 (32.6) | 659 (28.7) | 456 (35.7) | 225 (41.9) | 0.0001 |
| CHF | 1054 (25.6) | 507 (22.0) | 456 (28.3) | 186 (34.6) | 0.0001 |
| Stroke | 705 (17.1) | 356 (15.5) | 224 (17.5) | 126 (23.3) | 0.0001 |
Abbreviations: BMI, body mass index; CHD, coronary heart disease; CHF, congestive heart failure; CVD, cardiovascular disease; DBP, diastolic blood pressure; DM, diabetes mellitus; FEV1, 1‐second forced expiratory volume; FVC, forced vital capacity; HDL‐C, high‐density lipoprotein cholesterol; HTN, hypertension; LDL‐C, low‐density lipoprotein cholesterol; MetS, metabolic syndrome; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol.
Data are presented as n (%) or mean ± SD.
Analyses of CVD incidence per 1000 person‐years show that the lowest quartiles of percent predicted FEV1 and FVC have the highest risks for CVD, compared with the highest quartiles (Figure 1). Within each quartile, CVD incidence was noticeably higher among individuals with MetS and was also higher among individuals with DM compared with those with no DM/MetS.
Figure 1.
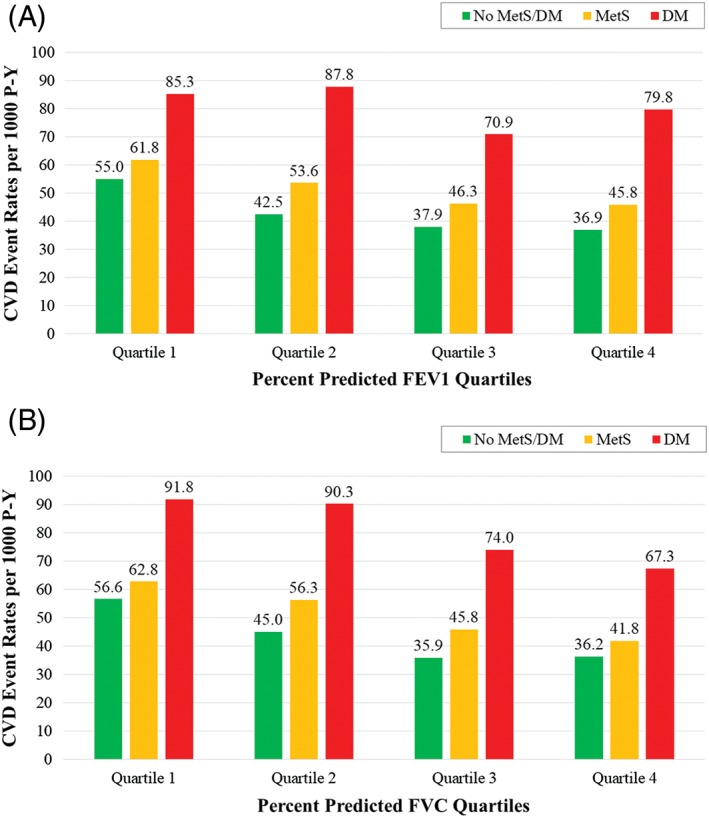
Incident CVD event rates per 1000 P‐Y by quartile of (A) FEV1 or (B) FVC. Abbreviations: CVD, cardiovascular disease; DM, diabetes mellitus; FEV1, 1‐second forced expiratory volume; FVC, forced vital capacity; MetS, metabolic syndrome; P‐Y, person‐years
Comparing the fourth vs first quartiles for FVC, the risk of CVD events was lower by 16% (HR: 0.84, 95% CI: 0.59–1.18), 23% (HR: 0.77, 95% CI: 0.60–0.99), and 30% (HR: 0.70, 0.58–0.84), respectively, in the DM, MetS, and neither disease groups; for FEV1, CVD risk was lower by 2.0% (HR: 0.98, 95% CI: 0.70–1.37), 26% (HR: 0.74, 95% CI: 0.59–0.93), and 31% (HR: 0.69, 95% CI: 0.57–0.82) in DM, respectively (Table 2). Individuals with DM and MetS had attenuated associations of FEV1 and FVC with CHD and CVD events vs those with neither disease. Persons with DM had weaker associations between the pulmonary variables and CHD and CVD events than did those with or without MetS, except for heart failure (HF), which had strong associations with both FEV1 and FVC in all disease groups. No significant relationship was observed between FEV1 or FVC and stroke. Among 1656 subjects with IFG, the HR of CVD is 0.92 (0.84–1.00, P = 0.059) per 1 SD of FEV1 and 0.92 (0.84–1.01, P = 0.079) per 1 SD of FVC; for CHD, corresponding HRs are 1.01 (0.90–1.13, P = 0.858) and 1.02 (0.91–1.14, P = 0.77).
Table 2.
Adjusted Cox proportional hazards regression and 95% CIs for CVD, CHD, CHF, and stroke according to percentage predicted FEV1 and FVCa
| Overall, N = 4036, HR (95% CI) | P Value | Neither, n = 2256 (55.9%), HR (95% CI) | P Value | MetS, n = 1253 (31.0%), HR (95% CI) | P Value | DM, n = 527 (13.1%), HR (95% CI) | P Value | |
|---|---|---|---|---|---|---|---|---|
| CVD | ||||||||
| FEV1 Q4 vs Q1 | 0.73 (0.64–0.83) | <0.0001 | 0.69 (0.57–0.82) | <0.0001 | 0.74 (0.59–0.93) | <0.05 | 0.98 (0.70–1.37) | |
| FVC Q4 vs Q1 | 0.73 (0.64–0.84) | <0.0001 | 0.70 (0.58–0.84) | <0.001 | 0.77 (0.60–0.99) | <0.05 | 0.84 (0.59–1.18) | |
| FEV1 (per SD) | 0.88 (0.84–0.93) | <0.0001 | 0.87 (0.81–0.93) | <0.0001 | 0.89 (0.82–0.97) | <0.01 | 0.96 (0.86–1.08) | |
| FVC (per SD) | 0.89 (0.84–0.93) | <0.0001 | 0.87 (0.81–0.93) | <0.0001 | 0.90 (0.82–0.98) | <0.05 | 0.99 (0.86–1.12) | |
| CHD | ||||||||
| FEV1 Q4 vs Q1 | 0.81 (0.69–0.95) | <0.05 | 0.77 (0.61–0.97) | <0.05 | 0.83 (0.63–1.10) | 0.99 (0.66–1.48) | ||
| FVC Q4 vs Q1 | 0.83 (0.70–0.98) | <0.05 | 0.76 (0.60–0.96) | <0.05 | 0.89 (0.66–1.20) | 0.96 (0.63–1.46) | ||
| FEV1 (per SD) | 0.93 (0.87–0.98) | <0.05 | 0.92 (0.85–1.00) | 0.93 (0.84–1.04) | 0.96 (0.83–1.11) | |||
| FVC (per SD) | 0.93 (0.87–0.99 | <0.05 | 0.92 (0.84–1.01) | 0.93 (0.83–1.04) | 0.97 (0.83–1.13) | |||
| CHF | ||||||||
| FEV1 Q4 vs Q1 | 0.53 (0.44–0.63) | <0.0001 | 0.49 (0.38–0.63) | <0.0001 | 0.59 (0.43–0.83) | <0.01 | 0.48 (0.29–0.78) | <0.01 |
| FVC Q4 vs Q1 | 0.53 (0.44–0.64) | <0.0001 | 0.52 (0.40–0.68) | <0.0001 | 0.53 (0.37–0.76) | <0.001 | 0.46 (0.28–0.74) | <0.01 |
| FEV1 (per SD) | 0.78 (0.73–0.83) | <0.0001 | 0.76 (0.69–0.83) | <0.0001 | 0.80 (0.71–0.90) | <0.001 | 0.80 (0.69–0.94) | <0.01 |
| FVC (per SD) | 0.78 (0.73–0.84) | <0.0001 | 0.76 (0.69–0.84) | <0.0001 | 0.80 (0.70–0.91) | <0.001 | 0.82 (0.70–0.96) | <0.05 |
| Stroke | ||||||||
| FEV1 Q4 vs Q1 | 0.89 (0.71–1.11) | 0.82 (0.61–1.12) | 0.94 (0.63–1.40) | 1.17 (0.65–2.10) | ||||
| FVC Q4 vs Q1 | 0.92 (0.73–1.16) | 0.97 (0.70–1.33) | 0.94 (0.61–1.44) | 0.85 (0.47–1.54) | ||||
| FEV1 (per SD) | 0.95 (0.86–1.03) | 0.94 (0.84–1.05) | 0.96 (0.82–1.11) | 1.05 (0.86–1.29) | ||||
| FVC (per SD) | 0.97 (0.89–1.06) | 0.95 (0.84–1.07) | 0.99 (0.84–1.16) | 1.09 (0.88–1.34) |
Abbreviations: BMI, body mass index; CHD, coronary heart disease; CHF, congestive heart failure; CI, confidence interval; CRP, C‐reactive protein; CVD, cardiovascular disease; DM, diabetes mellitus; FEV1, 1‐second forced expiratory volume; FVC, forced vital capacity; HDL‐C, high‐density lipoprotein cholesterol; HR, hazard ratio; HTN, hypertension; LDL‐C, low‐density lipoprotein cholesterol; MetS, metabolic syndrome; Q, quartile; SBP, systolic blood pressure; SD, standard deviation.
Adjusted for age, sex, race, HTN medication, lipid‐lowering medication, alcohol intake, physical activity, education, smoking status, SBP, BMI, family history of CVD, LDL‐C, HDL‐C, CRP, and glucose level.
We further examined the possible heterogeneous association of lung function and CVD by sex. Females had 22% lower risks of CVD per 1 SD of percent predicted FEV1 (P < 0.0001) and males had a 21% lower risk (P = 0.0006); interaction tests for sex and percent predicted FEV1 were not significant overall as well as in each disease group (interaction test P = 0.977, 0.334, 0.922, and 0.134 for overall, no MetS/DM, MetS, and DM groups). For percent predicted FVC, the interaction with sex was also not significant (interaction test P = 0.763, 0.502, 0.746, and 0.641 for overall, no MetS/DM, MetS, and DM groups).
C‐statistics with only risk factors in the model (Model 1) ranged from 0.611 to 0.658 among the 3 disease groups for CVD events; models with FEV1 or FVC (Model 2) had a significant increase of C‐statistics for CVD events overall and among those with neither disease. For CHD events, C‐statistics of Model 1 and Model 2 remain largely unchanged. For incident CHF, C‐statistics of Model 1 were generally higher than those for other event types (range, 0.647–0.682 for 3 disease groups); and when FEV1 or FVC were added, there was significant improvement in C‐statistics overall and among those with neither disease (Table 3). Given that FVC and FEV1 were not associated with stroke (Table 2), C‐statistics comparing the 2 models would be no different.
Table 3.
C‐statistics for disease groups with percent predictive FEV1 and FVC as added risk factors in predicting events
| Model 1, C‐statistic (95% CI) | Model 2 | Model 2, C‐statistic (95% CI) | P Value of Model 1 vs Model 2 | |
|---|---|---|---|---|
| CVD events | ||||
| Overall, N = 4114 | 0.658 (0.645–0.671) | With FEV1 | 0.661 (0.648–0.674) | 0.032 |
| With FVC | 0.661 (0.648–0.674) | 0.077 | ||
| Neither, n = 2300 | 0.658 (0.641–0.675) | With FEV1 | 0.664 (0.647–0.681) | 0.002 |
| With FVC | 0.663 (0.646–0.680) | 0.003 | ||
| MetS, n = 1277 | 0.632 (0.609–0.654) | With FEV1 | 0.632 (0.610–0.655) | 0.841 |
| With FVC | 0.632 (0.609–0.654) | 0.929 | ||
| DM, n = 537 | 0.611 (0.576–0.645) | With FEV1 | 0.609 (0.573–0.646) | 0.720 |
| With FVC | 0.609 (0.572–0.646) | 0.731 | ||
| CHD events | ||||
| Overall, N = 4114 | 0.654 (0.641–0.668) | With FEV1 | 0.655 (0.642–0.669) | 0.251 |
| With FVC | 0.655 (0.642–0.669) | 0.350 | ||
| Neither, n = 2300 | 0.658 (0.638–0.678) | With FEV1 | 0.659 (0.639–0.680) | 0.418 |
| With FVC | 0.659 (0.639–0.680) | 0.441 | ||
| MetS, n = 1277 | 0.629 (0.601–0.656) | With FEV1 | 0.629 (0.602–0.656) | 0.847 |
| With FVC | 0.629 (0.602–0.656) | 0.710 | ||
| DM, n = 537 | 0.606 (0.570–0.643) | With FEV1 | 0.607 (0.570–0.644) | 0.829 |
| With FVC | 0.607 (0.570–0.643) | 0.992 | ||
| CHF events | ||||
| Overall, N = 4114 | 0.689 (0.669–0.708) | With FEV1 | 0.700 (0.682–0.717) | 0.0003 |
| With FVC | 0.698 (0.680–0.716) | 0.002 | ||
| Neither, n = 2300 | 0.678 (0.653–0.704) | With FEV1 | 0.695 (0.671–0.720) | 0.0008 |
| With FVC | 0.694 (0.669–0.718) | 0.0008 | ||
| MetS, n = 1277 | 0.680 (0.652–0.709) | With FEV1 | 0.686 (0.656–0.715) | 0.328 |
| With FVC | 0.685 (0.655–0.714) | 0.404 | ||
| DM, n = 537 | 0.649 (0.607–0.690) | With FEV1 | 0.656 (0.618–0.694) | 0.468 |
| With FVC | 0.652 (0.613–0.690) | 0.732 |
Abbreviations: CHD, coronary heart disease; CHF, congestive heart failure; CI, confidence interval; CVD, cardiovascular disease; DM, diabetes mellitus; FEV1, 1‐second forced expiratory volume; FVC, forced vital capacity; MetS, metabolic syndrome. Model 1 adjusted for age, sex, race, hypertension medication, lipid‐lowering medication, alcohol intake, physical activity, education, smoking status, SBP, BMI, family history of CVD, LDL‐C, HDL‐C, CRP, and glucose level.
4. DISCUSSION
The main finding of our study is that among our sample of older adults, higher levels of lung function as measured by FVC and FEV1 were generally related to lower risks of incident CVD, especially for HF. These results are consistent with those shown in previous studies with broader age‐range cohorts.15, 16, 21 Although in our study reduced lung function contributed little to CVD risk in DM, recent studies show that reduced lung function may be a precursor of DM.8 Also, people with reduced lung function have greater levels of inflammation,22 and people with DM or MetS,10 including those with elevated C‐reactive protein,23 are at increased risk of CVD. However, the inverse relationships between FEV1 and FVC with CVD were strongest in those with MetS and no disease, and for CHF held in all 3 disease groups. Although these findings were independent of age, sex, and standard CVD risk factors, added incremental prediction over risk factors was mainly limited to CVD and especially CHF in those with neither disease and was not consistently seen in those with MetS or DM. It is possible that the higher burden of disease in those with DM may make it more difficult to show added prognostic value of other measures, such as lung function. This was also the case where we previously showed left ventricular mass not to add to further prediction of CVD outcomes in Cardiovascular Health Study participants with DM.24
Prior studies have been reported on lung function and outcomes in DM or MetS. FEV1 and FVC have an inverse relationship with type 2 DM and fatal CHD events.25 In our previous study using NHANES III data with nonsmoking adults ranging in age from 18 to 79 years, we have shown that those with MetS but not DM had increased risk of overall mortality among those with FVC ≤85% predicted, compared with those with FVC ≥95% predicted.12 Moreover, a smaller community‐based sample of older persons with MetS and DM showed MetS and abdominal obesity, but not DM, to be independently associated with restrictive lung disease.26 Also, although HF risk as a consequence of poor lung function has not previously been reported in MetS and DM patients, others have reported on increased mortality associated with chronic obstructive pulmonary disease (COPD) or restrictive spirometry pattern in patients with HF.27
Additionally, other Cardiovascular Health Study investigations have demonstrated the association of smoking with impaired FEV1/pulmonary function and increased risk of atherosclerosis in the elderly28, 29; however, other Cardiovascular Health Study studies showed a small association of reduced FEV1 and FVC with increased risk of CHD outcomes after excluding cigarette smokers and those with lung diseases known to reduce lung function.30 In our prior NHANES III study, lower FVC did not add to risk of overall mortality among those with only DM12; in our current study, higher FVC or FEV1 was related to lower risk of CHD in those with DM and HF in those with MetS, but not stroke in either condition. Overall, findings between pulmonary function and CHF appeared to be stronger than for other CVD endpoints. This strong relationship between CHF and reduced lung function may be expected, because chronic elevation of atrial pressure due to reduced compliance of left ventricular function is known to cause of elevation of pulmonary artery pressure, which in turn leads to a reduction of FEV1 and FVC; however, no significant relationships were found with stroke. In receiver operating characteristic analyses, however, neither FEV1 nor FVC tended to improve prediction for CVD events over risk factors in MetS or DM. Thus, the utility of FEV1 and FVC measures to improve risk prediction beyond standard risk factors appears limited in those with MetS and DM.
4.1. Study limitations
There are several strengths and limitations to our study. The large sample size of the Cardiovascular Health Study provides a sufficient number of CHD and CVD mortality endpoints and adds high power to our study in predicting such outcomes. In addition, the well‐standardized measurements of pulmonary function and cardiovascular risk factors were an important contributing strength. The older cohort is a useful population for this topic due to the higher prevalence of MetS, DM, CVD, and pulmonary disease, which are all at increased prevalence. Possible confounding factors were taken into account in the analysis. Nevertheless, the ability of pulmonary‐function measures to predict CVD events could be limited by other unmeasured confounders or other comorbidities present in older populations. Previous Cardiovascular Health Study investigations have shown that the most commonly used spirometric reference equations could not create accurate interpretations in the elderly; therefore, some prefer to use actual FVC and FEV1 measures when examining relationships to risk.18 Of interest, the Cardiovascular Health Study has shown that older subjects classified as “normal” using the lower limit of normal approach but who have a FEV1/FVC ratio < 0.70 still have an increased risk of death and COPD‐related hospitalization.31 We only utilized baseline variables both for lung function as well as disease‐group classification. It is likely some participants classified as normal at baseline transitioned to MetS or DM and some classified with MetS at baseline transitioned to DM at follow‐up. Moreover, the Cardiovascular Health Study has previously reported that those with COPD at baseline were more likely not to have follow‐up spirometry tests, and those in the most rapidly declining quartile of FEV1 had a modest increase in risk of hospital admissions and death from COPD.32 Other potentially important measures, including total lung capacity and residual volume, were also not available in our sample, so we were thus unable to examine them in relation to outcomes.
The primarily Caucasian and African American sample of the Cardiovascular Health Study limits the extrapolation of this study's findings to other ethnic groups. Also, as we had an insufficient proportion of subjects in our sample with restrictive (0.3%) or mixed (0.9%) lung disease to examine prognosis, others have previously reported such subjects also to have increased mortality, suggesting they deserve similar attention as those with an obstructive pattern.33 Finally, since the baseline Cardiovascular Health Study examination, the use of preventive therapies such as statins and antihypertensive medication has improved dramatically; it is possible that relationships we observe between lung‐function measures and CVD outcomes may be different if the study were repeated using a more contemporary and better‐treated population.
5. CONCLUSION
Our study supports the use of pulmonary function as an independent predictor of CVD events among our older adult cohort; however, relationships tended to be weaker in those with DM, except for predicting CHF. The predictive ability of pulmonary‐function measures over traditional risk factors, however, is limited mainly to those without MetS nor DM, potentially due to the higher burden of risk factors seen in those with MetS and DM.
Conflicts of interest
The authors declare no potential conflicts of interest.
Lee HM, Zhao Y, Liu MA, et al. Impact of lung‐function measures on cardiovascular disease events in older adults with metabolic syndrome and diabetes. Clin Cardiol. 2018;41:959–965. 10.1002/clc.22985
Funding information This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086, and grants U01HL080295 and U01HL130114, from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by R01AG023629 from the National Institute on Aging. A full list of Cardiovascular Health Study principal investigators and institutions can be found at http://www.CHS-NHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1. Engström G, Hedblad B, Valind S, et al. Increased incidence of myocardial infarction and stroke in hypertensive men with reduced lung function. J Hypertens. 2001;19:295–301. [DOI] [PubMed] [Google Scholar]
- 2. Kannel WB, Hubert H, Lew EA. Vital capacity as a predictor of cardiovascular disease: the Framingham study. Am Heart J. 1983;105:311–315. [DOI] [PubMed] [Google Scholar]
- 3. Marcus EB, Curb JD, MacLean CJ, et al. Pulmonary function as a predictor of coronary heart disease. Am J Epidemiol. 1989;129:97–104. [DOI] [PubMed] [Google Scholar]
- 4. Schroeder EB, Welch VL, Couper D, et al. Lung function and incident coronary heart disease: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2003;158:1171–1181. [DOI] [PubMed] [Google Scholar]
- 5. Lawlor DA, Ebrahim S, Smith GD. Associations of measures of lung function with insulin resistance and type 2 diabetes: findings from the British Women's Heart and Health Study. Diabetologia. 2004;47:195–203. [DOI] [PubMed] [Google Scholar]
- 6. Lazarus R, Sparrow D, Weiss ST. Baseline ventilatory function predicts the development of higher levels of fasting insulin and fasting insulin resistance index: the Normative Aging Study. Eur Respir J. 1998;12:641–645. [DOI] [PubMed] [Google Scholar]
- 7. Ford ES, Mannino DM; National Health and Nutrition Examination Survey Epidemiologic Follow‐up Study . Prospective association between lung function and the incidence of diabetes: findings from the National Health and Nutrition Examination Survey Epidemiologic Follow‐up Study. Diabetes Care. 2004;27:2966–2970. [DOI] [PubMed] [Google Scholar]
- 8. Yeh HC, Punjabi NM, Wang NY, et al. Vital capacity as a predictor of incident type 2 diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2005;28:1472–1479. [DOI] [PubMed] [Google Scholar]
- 9. Engström G, Hedblad B, Nilsson P, et al. Lung function, insulin resistance and incidence of cardiovascular disease: a longitudinal cohort study. J Intern Med. 2003;253:574–581. [DOI] [PubMed] [Google Scholar]
- 10. Lee HM, Le TV, Lopez VA, et al. Association of C‐reactive protein with reduced forced vital capacity in a nonsmoking U.S. population with metabolic syndrome and diabetes. Diabetes Care. 2008;31:2000–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walter RE, Beiser A, Givelber RJ, et al. Association between glycemic state and lung function: the Framingham Heart Study. Am J Respir Crit Care Med. 2003;167:911–916. [DOI] [PubMed] [Google Scholar]
- 12. Lee HM, Chung SJ, Lopez VA, et al. Association of FVC and total mortality in US adults with metabolic syndrome and diabetes. Chest. 2009;136:171–176. [DOI] [PubMed] [Google Scholar]
- 13. Lee HM, Le H, Lee BT, et al. Forced vital capacity paired with Framingham Risk Score for prediction of all‐cause mortality. Eur Respir J. 2010;36:1002–1006. [DOI] [PubMed] [Google Scholar]
- 14. Wong ND. Metabolic syndrome: cardiovascular risk assessment and management. Am J Cardiovasc Drugs. 2007;7:259–272. [DOI] [PubMed] [Google Scholar]
- 15. Ford ES. Risks for all‐cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. [DOI] [PubMed] [Google Scholar]
- 16. Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. [DOI] [PubMed] [Google Scholar]
- 17. Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 18. Enright PL, Kronmal RA, Higgins M, et al. Spirometry reference values for women and men 65 to 85 years of age: Cardiovascular Health Study. Am Rev Respir Dis. 1993;147:125–133. [DOI] [PubMed] [Google Scholar]
- 19. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
- 20. Psaty BM, Lee M, Savage PJ, et al; The Cardiovascular Health Study Collaborative Research Group . Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. J Clin Epidemiol. 1992;45:683–692. [DOI] [PubMed] [Google Scholar]
- 21. Wilson PW, D'Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. [DOI] [PubMed] [Google Scholar]
- 22. Engström G, Lind P, Hedblad B, et al. Lung function and cardiovascular risk: relationship with inflammation‐sensitive plasma proteins. Circulation 2002;106:2555–2560. [DOI] [PubMed] [Google Scholar]
- 23. Ridker PM, Buring JE, Cook NR, et al. C‐reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8‐year follow‐up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. [DOI] [PubMed] [Google Scholar]
- 24. Hoang K, Zhao Y, Gardin JM, et al. LV mass as a predictor of CVD events in older adults with and without metabolic syndrome and diabetes. JACC Cardiovasc Imaging. 2015;8:1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wannamethee SG, Shaper AG, Rumley A, et al. Lung function and risk of type 2 diabetes and fatal and nonfatal major coronary heart disease events: possible associations with inflammation. Diabetes Care. 2010;33:1990–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scalata S, Fimognari FL, Cesari M, et al. Lung function changes in older people with metabolic syndrome and diabetes. Geriatr Gerontol Int. 2013;13:894–900. [DOI] [PubMed] [Google Scholar]
- 27. Plesner LL, Dalsgaard M, Schou M, et al. The prognostic significance of lung function in stable heart failure outpatients. Clin Cardiol. 2017;40:1145–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higgins MW, Enright PL, Kronmal RA, et al. Smoking and lung function in elderly men and women: the Cardiovascular Health Study. JAMA. 1993;269:2741–2748. [PubMed] [Google Scholar]
- 29. Enright PL. Smoking, lung function, and atherosclerosis in the 5000 elderly participants of the Cardiovascular Health Study. Am J Geriatr Cardiol. 1994;3:35–38. [PubMed] [Google Scholar]
- 30. Enright PL, Kronmal RA, Smith VE, et al. Reduced vital capacity in elderly persons with hypertension, coronary heart disease, or left ventricular hypertrophy: the Cardiovascular Health Study. Chest. 1995;107:28–35. [DOI] [PubMed] [Google Scholar]
- 31. Mannino DM, Sonia Buist A, Vollmer VM. Chronic obstructive pulmonary disease in the older adult: what defines abnormal lung function? Thorax. 2007;62:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mannino DM, Davis KJ. Lung function decline and outcomes in an elderly population. Thorax. 2006;61:472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scarlata S, Pedone C, Fimognari FL, et al. Restrictive pulmonary dysfunction at spirometry and mortality in the elderly. Respir Med. 2008;102:1349–1354. [DOI] [PubMed] [Google Scholar]


