Abstract
While there is a controversy regarding the causal relationship between high-density lipoprotein cholesterol (HDL-C) and cardiovascular disease (CVD), recent studies have demonstrated that the cholesterol efflux capacity (CEC) of HDL is associated with the incidence of CVD. However, there are several limitations to current assays of CEC. First, CEC measurements are not instantly applicable in clinical settings, because CEC assay methods require radiolabeled cholesterol and cultured cells, and these procedures are time consuming. Second, techniques to measure CEC are not standardized. Third, the condition of endogenous cholesterol donors would not be accounted for in the CEC assays. Recently, we established a simple, high-throughput, cell-free assay system to evaluate the capacity of HDL to accept additional cholesterol, which is herein referred to as “cholesterol uptake capacity (CUC)”. We demonstrated that CUC represents a residual cardiovascular risk in patients with optimal low-density lipoprotein cholesterol control independently of traditional risk factors, including HDL-C. Establishing reproducible approaches for the cholesterol removal capacity of HDL is required to validate the impact of dysfunctional HDL on cardiovascular risk stratification in the “real world”.
Keywords: High-density lipoprotein (HDL), Cardiovascular disease, HDL cholesterol (HDL-C), Cholesterol efflux capacity (CEC), Cholesterol uptake capacity (CUC)
Introduction
Accumulated epidemiological evidence has demonstrated that low levels of circulating high-density lipoprotein cholesterol (HDL-C) are associated with an increased risk of cardiovascular disease (CVD)1–3). However, there is a controversy regarding the causal relationship between HDL-C and CVD4, 5). In the AIM-HIGH study, the addition of niacin to statin therapy did not reduce cardiovascular events, despite significant improvements in HDL-C levels6). Until recently, trials of cholesterol ester transfer protein (CETP) inhibitors, which have been developed to raise HDL-C levels, have failed to provide evidence of cardiovascular benefit7–9). Finally, the REVEAL trial has demonstrated that the CETP inhibitor anacetrapib reduced cardiovascular events in patients undergoing statin therapy10). However, anacetrapib might work by lowering low-density lipoprotein cholesterol (LDL-C), rather than by increasing HDL-C11). Genetic polymorphisms that are associated with increased HDL-C also do not predict a reduced CVD risk12). Strikingly, the Framingham Offspring Study has demonstrated that an isolated low HDL-C level does not predict risk when LDL-C and triglyceride (TG) levels are completely normal13). Moreover, recent reports have demonstrated that extremely high HDL-C was associated with increased rather than decreased mortality14, 15).
The limitation of HDL-C as a cardiovascular risk is attributable to the fact that HDL-C is anything more than a snapshot of HDL metabolism. For example, the mutation of CETP, which promotes reverse cholesterol transport (RCT) by transferring a cholesterol ester from HDL to the apolipoprotein B (apoB)-containing lipoproteins in exchange for TG, results in an elevation in HDL-C, but does not necessarily reduce cardiovascular events16). The mutation of scavenger receptor class B type I (SR-BI), an HDL receptor expressed abundantly in the liver, also increases HDL-C, but also accelerates atherosclerosis17).
On the contrary, recent large cohort studies have demonstrated that the cellular cholesterol efflux capacity (CEC) of HDL, a dynamic rate of initial step in RCT, is associated with both the prevalence and the incidence of CVD, and is a better predictor than steady-state circulating HDL-C levels18–20). However, at present, CEC measurements are not instantly applicable as a high-throughput assay in clinical settings. In this review, we discuss the limitations of current CEC assays and future directions.
Cellular Cholesterol Efflux
As most types of cells are unable to catabolize cholesterol, RCT is indispensable for homeostasis. The efflux of cholesterol from cells to serum is an initial step in the RCT pathway. HDL is the component of serum responsible for mediating cholesterol efflux 21). Cholesterol-enriched macrophages can release cholesterol to HDL using several pathways22).
First, ATP-binding cassette transporter A1 (ABCA1) is an important player in HDL biogenesis, especially in the setting of cholesterol enrichment. ABCA1 mediates the cellular binding of apoAI, the major structural protein of HDL, and the unidirectional export of cholesterol and phospholipids (PLs) to lipid-free/-poor apoAI, leading to nascent HDL formation23–25).
On the other hand, SR-BI and ATP-binding cassette transporter G1 (ABCG1) contribute to the maturation of HDL. SR-BI mediates bidirectional cholesterol exchange22, 26). ABCG1 does not mediate the cellular binding of HDL, but promotes the transport of free cholesterol from the cell interior to the plasma membrane27). The cholesterol translocated by ABCG1 in the cell membrane is exported to HDL via an aqueous diffusion pathway22). Lecithin: cholesterol acyltransferase (LCAT) on HDL prevents the return of accepted cholesterol to the cell by esterification, because cholesteryl esters are more hydrophobic than free cholesterol and move to the core of HDL28). Mature HDL particles can be remodeled to smaller particles with the release of apoAI by the actions of hepatic lipase, endothelial lipase, and phospholipase A2, which hydrolyze TG and PLs in HDL29–36).
Current Understanding of CEC
HDL is a heterogeneous population of particles, and ranges in size from < 7 to > 14 nm37, 38). Subspecies of HDL are considered to be involved in different pathways of cellular cholesterol efflux39, 40). The smallest apoAI-containing HDL (pre-β-1) and small, dense HDL subfractions (HDL3b and 3c) are principal transporters via the ABCA1 pathway38, 41). On the other hand, PL-rich HDL2 is an efficient acceptor of cholesterol because it provides a larger target for effective collisions with diffusing free cholesterol42–44). The fatty acid composition of PLs in HDL has also been proposed to affect CEC. We have demonstrated that the oral administration of eicosapentaenoic acid (EPA) improved CEC in patients with dyslipidemia45), and confirmed the beneficial effects of EPA-rich HDL on cholesterol efflux using reconstituted HDL46). Increases in unsaturated PL acyl chains in HDL particles have been demonstrated to result in more efficient cholesterol acceptors47). Omega 3 fatty acids have also been shown to increase LCAT activity48).
On the other hand, the oxidation of several specific amino acids in apoA1 could result in a reduction in CEC49–51)(Fig. 1). Granular leukocyte-derived myeloperoxidase (MPO) promotes the oxidation of apoAI, while paraoxonase 1 (PON1) has antioxidant properties for HDL52, 53). We have demonstrated that patients with high MPO levels and low PON1 activity in serum exhibit impaired CEC54).
Fig. 1.
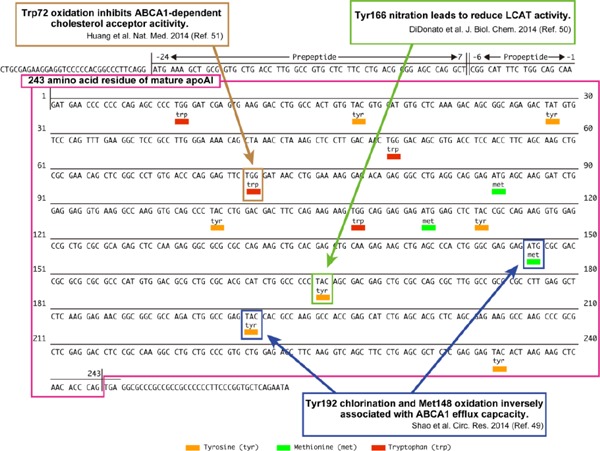
Post-transcriptional modification of apoAI and CEC
Limitations of Conventional CEC Assays
Fig. 2 shows the conventional procedure for a CEC assay. Macrophages are exposed by radiolabeled cholesterol. Then, they are stimulated by HDL, and the excreted cholesterol is measured by a scintillation counter55).
Fig. 2.
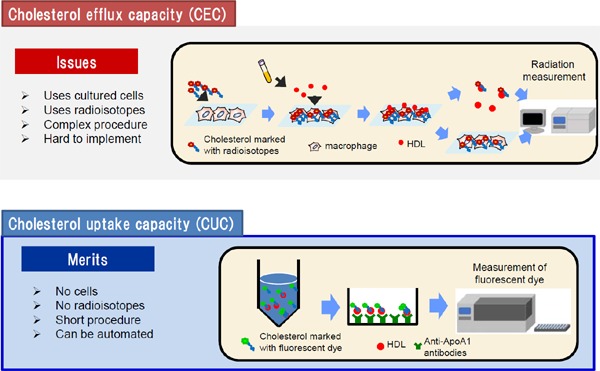
The procedural schema of CEC and CUC
Although CEC has been used as a marker for CVD, there are several limitations to current CEC assays for clinical application56). First, this method requires radiolabeling and cultured cells, and these procedures are time consuming. Second, procedures for CEC measurement are not standardized, which makes it difficult to compare different CEC studies. Varied systems to measure CEC exist, as shown in Fig. 356, 57). Several donor cells are employed for CEC measurements. Fu5AH rat hepatoma cells, which express high levels of SR-BI but lack functional ABCA1, are used to assess SR-BI-mediated efflux58). On the other hand, J774 mouse macrophage cells express low levels of SR-BI, and stimulation with cyclic adenosine monophosphate (cAMP) can upregulate its ABCA1 expression59, 60). In the Dallas Heart Study19) and the European Prospective Investigation of Cancer (EPIC)-Norfolk study20), J774 cells treated with cAMP were used to measure CEC. On the contrary, Ogura, et al. have recently demonstrated that CEC determined using J774 cells without cAMP treatment was also inversely associated with the presence of atherosclerotic CVD in patients with familial hypercholesterolemia61). In cases requiring the assessment of ABCA1-dependent CEC, the basal CEC (without cAMP) is subtracted from the total CEC (with cAMP)62). Because the ultracentrifugation procedure for HDL isolation requires several days, most of the recent reports employed apoB-depleted serum as the cholesterol acceptor. However, apoB-depleted serum has been reported to contain not only HDL and apoA1 but also other components, such as albumin, that can accept the cholesterol released from macrophages63). Moreover, HDL composition and/or size distribution might vary depending on the apoB depletion methods64). Li, et al. even reported that cholesterol efflux to apoB-depleted serum was paradoxically associated with an increased prospective risk of CVD63). While a protocol using radiolabeled cholesterol does not lend itself to the development of a highthroughput assay, fluorescence-labeled cholesterol is alternatively available for CEC measurements. Fractional efflux rates obtained with BODIPY-cholesterol were reported to be greater than those with tritium-labeled cholesterol65).
Fig. 3.
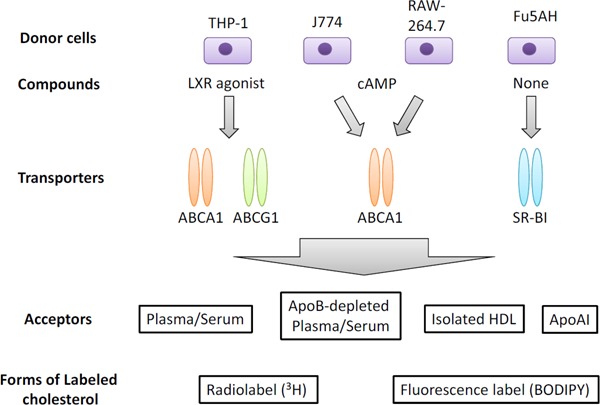
Varied systems to measure CEC
Adapted from Ref. 57 (Progress in Lipid Research 2018; 69: 21–32).
The third limitation is that the status of endogenous cholesterol donors would not be accounted for in in vitro CEC assays. Changes of in vivo macrophage cellular function resulting from various conditions have been reported as follows: phenolic acids increased ABCG1 and SR-BI expression66); on the other hand, xanthine oxidoreductase suppressed ABCA1 and ABCG1 expression in macrophages67); while we have demonstrated that EPA could improve CEC45, 46), another group has reported that EPA might reduce ABCA1 functionality in macrophages68).
Curiously, ABCA-1 dependent CEC was reported to be enhanced rather than impaired in patients with high TG levels69). In those patients, a reduction in large HDL particles and an increase in pre-β-1 particles were observed. Concomitantly, SRBI-dependent efflux, which is mediated mainly by large HDL, decreased. On the other hand, accompanied by an increase in pre-β-1 particles, ABCA-1-dependent efflux was also augmented69). However, ABCA1-dependent efflux was determined using J744 cells as described above69). The lack of the macrophage ability assessment in an individual might cause overestimation.
Cholesterol Uptake Capacity, A New Measure for HDL Functionality
In order to break through this situation, we have recently established a simple, high-throughput, cell-free assay system to evaluate the “cholesterol uptake capacity (CUC)” as a novel concept for HDL functionality70).
The procedural schema of our new assay is shown in Fig. 2. After removing apoB, serum is incubated with fluorescence-labeled cholesterol, HDL is captured by specific antibodies for apoAI coated on a microplate, and then the amount of the labeled cholesterol in the HDL is measured using a plate reader. This assay system does not require radiolabeling and cultured cells, and the procedures are simple, with a short turnaround time. Moreover, the application of the anti-apoAI antibody allows a specific evaluation of the ability of HDL to accept cholesterol.
We revealed that CUC was suppressed by MPO treatment, indicating that CUC has the potential to evaluate the oxidation-induced inactivation of HDL70). Furthermore, we found that CUC correlated inversely with the requirement for revascularization because of the recurrence of coronary lesions in patients with optimal control of LDL-C. A multivariate analysis adjusted for traditional coronary risk factors, including HDL-C, showed that only CUC remained significant70).
Difference between CEC and CUC
Since CUC was determined by a cell-free assay, CUC does not reflect ABCA1-mediated efflux (Fig. 4). On the other hand, we demonstrated that CUC was associated with CEC determined using J774 cells without cAMP (non-ABCA1-mediated, basal CEC)70), which was employed in the study conducted on patients with familial hypercholesterolemia61). As the CUC assay is an aqueous diffusiondependent system, it appears to mainly reflect the contribution of PL-rich, matured HDL to cholesterol efflux (Fig. 4). As expected, HDL particle concentration (HDL-P) measurements using nuclear magnetic resonance spectroscopy demonstrated that large HDL-P showed a more prominent association with CUC than small HDL-P, suggesting that CUC is influenced predominantly by the concentration of matured HDL particles (Fig. 5).
Fig. 4.
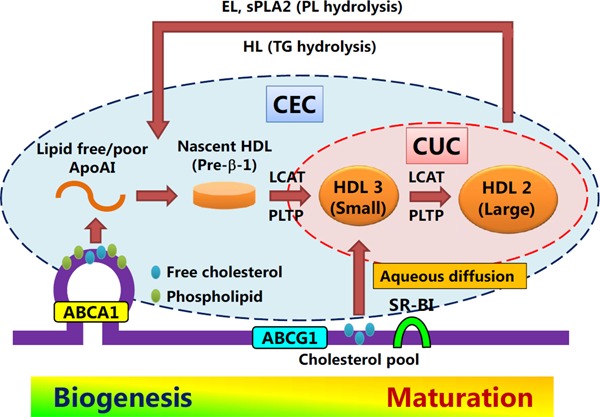
Differences in concept between CEC and CUC
ABCA1: ATP-binding cassette transporter A1; ABCG1: ATP-binding cassette transporter G1; SR-BI: scavenger receptor class B type I; LCAT: Lecithin:cholesterol acyltransferase; PLTP: phospholipid transfer protein; HL: hepatic lipase; EL: endothelial lipase; sPLA2: secreted phospholipase A2.
Fig. 5.
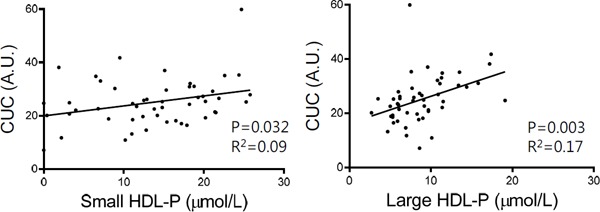
Correlations between CUC and HDL particle concentration (HDL-P)
Small (diameter range: 7.3–8.2 nm) and large (diameter range: 9.4–14 nm) HDL-P were determined at LipoScience/LabCorp (Burlington, NC) using nuclear magnetic resonance spectroscopy and the LipoProfile-3 algorithm.
Since the facilitation of HDL biogenesis is a potential therapeutic approach, small HDL particles that promote cholesterol efflux by the ABCA1 pathway are emphasized as cardioprotective species of HDL38, 41). In such a context, CEC was determined using ABCA1-upregulated cells in recent large cohort studies18–20). However, all patients with Tangier disease, which is caused by null-mutations in ABCA1, may not necessarily be at high risk of CVD despite marked deficiencies in HDL-C and apoAI71). Therefore, the impact of ABCA1-mediated cholesterol efflux on antiatherosclerotic process is still controversial. On the other hand, most evidence suggests that levels of large HDL particles are inversely related with the risk of CVD, whereas levels of small HDL particles are positively correlated with this risk72–74). In dyslipidemic conditions, such as diabetes and insulin resistance, a remodeling of HDL toward smaller particles is assumed to occur due to activity alterations of lipases involved in HDL metabolism and CETP75, 76). However, an increase in small HDL particles might seemingly cause an enhancement of CEC, as described above69). On the contrary, CUC has the potential utility of cardiovascular risk stratification through the monitoring of disturbances in HDL maturation. Further trials are required to compare the clinical usefulness between “cell-based” CEC and “cell-free” CUC assays.
In terms of the reproducibility of CUC, the intra-assay coefficient of variation (within-run precision) was less than 5% and the inter-assay coefficient of variation (between-run precision) was 7%, both of which are comparable to those of CEC reported in the EPIC-Norfolk study20). We are currently developing a completely automated system to measure CUC.
Conclusion
Although CEC as an indicator of HDL function has been used as a marker for CVD, there is no established method for its measurement for use in routine practice. On the other hand, the cell-free assay system to measure CUC will allow a high-throughput characterization of HDL functionality. Establishing reproducible, standardized approaches for HDL-cholesterol removal capacity is required for further validation of its impact on cardiovascular risk stratification in the “real world.”
Acknowledgments
This research was supported by a Grant-in-Aid for Scientific Research (C) JP18K08073 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflicts of Interest
The Division of Evidence-based Laboratory Medicine, Kobe University Graduate School of Medicine, was established by an endowment fund from the Sysmex Corporation.
References
- 1). Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Jr., Bangdiwala S, Tyroler HA: High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation, 1989; 79: 8-15 [DOI] [PubMed] [Google Scholar]
- 2). Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, Patsch W, Atherosclerosis Risk in Communities Study G : Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation, 2001; 104: 1108-1113 [DOI] [PubMed] [Google Scholar]
- 3). Emerging Risk Factors C. Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J: Major lipids, apolipoproteins, and risk of vascular disease. JAMA, 2009; 302: 1993-2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Rosenson RS: The High-Density Lipoprotein Puzzle: Why Classic Epidemiology, Genetic Epidemiology, and Clinical Trials Conflict? Arterioscler Thromb Vasc Biol, 2016; 36: 777-782 [DOI] [PubMed] [Google Scholar]
- 5). Karathanasis SK, Freeman LA, Gordon SM, Remaley AT: The Changing Face of HDL and the Best Way to Measure It. Clin Chem, 2017; 63: 196-210 [DOI] [PubMed] [Google Scholar]
- 6). Investigators A-H, Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W: Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med, 2011; 365: 2255-2267 [DOI] [PubMed] [Google Scholar]
- 7). Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B, Investigators I : Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med, 2007; 357: 2109-2122 [DOI] [PubMed] [Google Scholar]
- 8). Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS, dal OI: Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med, 2012; 367: 2089-2099 [DOI] [PubMed] [Google Scholar]
- 9). Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, Gibson CM, Granger C, Menon V, Montalescot G, Rader D, Tall AR, McErlean E, Wolski K, Ruotolo G, Vangerow B, Weerakkody G, Goodman SG, Conde D, McGuire DK, Nicolau JC, Leiva-Pons JL, Pesant Y, Li W, Kandath D, Kouz S, Tahirkheli N, Mason D, Nissen SE, Investigators A : Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N Engl J Med, 2017; 376: 1933-1942 [DOI] [PubMed] [Google Scholar]
- 10). Group HTRC. Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, Wiviott SD, Cannon CP, Braunwald E, Sammons E, Landray MJ: Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. N Engl J Med, 2017; 377: 1217-1227 [DOI] [PubMed] [Google Scholar]
- 11). Tall AR, Rader DJ: Trials and Tribulations of CETP Inhibitors. Circ Res, 2018; 122: 106-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Holm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, Konig IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schafer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O'Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S: Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet, 2012; 380: 572-580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Bartlett J, Predazzi IM, Williams SM, Bush WS, Kim Y, Havas S, Toth PP, Fazio S, Miller M: Is Isolated Low High-Density Lipoprotein Cholesterol a Cardiovascular Disease Risk Factor? New Insights From the Framingham Offspring Study. Circ Cardiovasc Qual Outcomes, 2016; 9: 206-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Madsen CM, Varbo A, Nordestgaard BG: Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J, 2017; 38: 2478-2486 [DOI] [PubMed] [Google Scholar]
- 15). Hirata A, Sugiyama D, Watanabe M, Tamakoshi A, Iso H, Kotani K, Kiyama M, Yamada M, Ishikawa S, Murakami Y, Miura K, Ueshima H, Okamura T, Evidence for Cardiovascular Prevention from Observational Cohorts in Japan Research G : Association of extremely high levels of high-density lipoprotein cholesterol with cardiovascular mortality in a pooled analysis of 9 cohort studies including 43,407 individuals: The EPOCH-JAPAN study. J Clin Lipidol, 2018; 12: 674-684 e675 [DOI] [PubMed] [Google Scholar]
- 16). Hirano K, Yamashita S, Nakajima N, Arai T, Maruyama T, Yoshida Y, Ishigami M, Sakai N, Kameda-Takemura K, Matsuzawa Y: Genetic cholesteryl ester transfer protein deficiency is extremely frequent in the Omagari area of Japan. Marked hyperalphalipoproteinemia caused by CETP gene mutation is not associated with longevity. Arterioscler Thromb Vasc Biol, 1997; 17: 1053-1059 [DOI] [PubMed] [Google Scholar]
- 17). Zanoni P, Khetarpal SA, Larach DB, Hancock-Cerutti WF, Millar JS, Cuchel M, DerOhannessian S, Kontush A, Surendran P, Saleheen D, Trompet S, Jukema JW, De Craen A, Deloukas P, Sattar N, Ford I, Packard C, Majumder A, Alam DS, Di Angelantonio E, Abecasis G, Chowdhury R, Erdmann J, Nordestgaard BG, Nielsen SF, Tybjaerg-Hansen A, Schmidt RF, Kuulasmaa K, Liu DJ, Perola M, Blankenberg S, Salomaa V, Mannisto S, Amouyel P, Arveiler D, Ferrieres J, Muller-Nurasyid M, Ferrario M, Kee F, Willer CJ, Samani N, Schunkert H, Butterworth AS, Howson JM, Peloso GM, Stitziel NO, Danesh J, Kathiresan S, Rader DJ, Consortium CHDE, Consortium CAE and Global Lipids Genetics C : Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science, 2016; 351: 1166-1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ: Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med, 2011; 364: 127-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW: HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med, 2014; 371: 2383-2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, Kastelein JJ, Boekholdt SM, Khaw KT, Wareham N, Rader DJ: Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol, 2015; 3: 507-513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Rothblat GH, de la Llera-Moya M, Atger V, Kellner-Weibel G, Williams DL, Phillips MC: Cell cholesterol efflux: integration of old and new observations provides new insights. J Lipid Res, 1999; 40: 781-796 [PubMed] [Google Scholar]
- 22). Phillips MC: Molecular mechanisms of cellular cholesterol efflux. J Biol Chem, 2014; 289: 24020-24029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Wang S, Gulshan K, Brubaker G, Hazen SL, Smith JD: ABCA1 mediates unfolding of apolipoprotein AI N terminus on the cell surface before lipidation and release of nascent high-density lipoprotein. Arterioscler Thromb Vasc Biol, 2013; 33: 1197-1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Wang S, Smith JD: ABCA1 and nascent HDL biogenesis. Biofactors, 2014; 40: 547-554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Gulshan K, Brubaker G, Conger H, Wang S, Zhang R, Hazen SL, Smith JD: PI(4,5)P2 Is Translocated by ABCA1 to the Cell Surface Where It Mediates Apolipoprotein A1 Binding and Nascent HDL Assembly. Circ Res, 2016; 119: 827-838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Zannis VI, Chroni A, Krieger M: Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med (Berl), 2006; 84: 276-294 [DOI] [PubMed] [Google Scholar]
- 27). Sankaranarayanan S, Oram JF, Asztalos BF, Vaughan AM, Lund-Katz S, Adorni MP, Phillips MC, Rothblat GH: Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J Lipid Res, 2009; 50: 275-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Czarnecka H, Yokoyama S: Regulation of cellular cholesterol efflux by lecithin:cholesterol acyltransferase reaction through nonspecific lipid exchange. J Biol Chem, 1996; 271: 2023-2028 [DOI] [PubMed] [Google Scholar]
- 29). Perret B, Mabile L, Martinez L, Terce F, Barbaras R, Collet X: Hepatic lipase: structure/function relationship, synthesis, and regulation. J Lipid Res, 2002; 43: 1163-1169 [PubMed] [Google Scholar]
- 30). Jin W, Marchadier D, Rader DJ: Lipases and HDL metabolism. Trends Endocrinol Metab, 2002; 13: 174-178 [DOI] [PubMed] [Google Scholar]
- 31). McCoy MG, Sun GS, Marchadier D, Maugeais C, Glick JM, Rader DJ: Characterization of the lipolytic activity of endothelial lipase. J Lipid Res, 2002; 43: 921-929 [PubMed] [Google Scholar]
- 32). Ishida T, Choi S, Kundu RK, Hirata K, Rubin EM, Cooper AD, Quertermous T: Endothelial lipase is a major determinant of HDL level. J Clin Invest, 2003; 111: 347-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Sun L, Ishida T, Miyashita K, Kinoshita N, Mori K, Yasuda T, Toh R, Nakajima K, Imamura S, Hirata K: Plasma activity of endothelial lipase impacts high-density lipoprotein metabolism and coronary risk factors in humans. J Atheroscler Thromb, 2014; 21: 313-321 [DOI] [PubMed] [Google Scholar]
- 34). de Beer FC, de Beer MC, van der Westhuyzen DR, Castellani LW, Lusis AJ, Swanson ME, Grass DS: Secretory non-pancreatic phospholipase A2: influence on lipoprotein metabolism. J Lipid Res, 1997; 38: 2232-2239 [PubMed] [Google Scholar]
- 35). de Beer FC, Connell PM, Yu J, de Beer MC, Webb NR, van der Westhuyzen DR: HDL modification by secretory phospholipase A(2) promotes scavenger receptor class B type I interaction and accelerates HDL catabolism. J Lipid Res, 2000; 41: 1849-1857 [PubMed] [Google Scholar]
- 36). Tietge UJ, Maugeais C, Cain W, Grass D, Glick JM, de Beer FC, Rader DJ: Overexpression of secretory phospholipase A(2) causes rapid catabolism and altered tissue uptake of high density lipoprotein cholesteryl ester and apolipoprotein A-I. J Biol Chem, 2000; 275: 10077-10084 [DOI] [PubMed] [Google Scholar]
- 37). Asztalos BF, Tani M, Schaefer EJ: Metabolic and functional relevance of HDL subspecies. Curr Opin Lipidol, 2011; 22: 176-185 [DOI] [PubMed] [Google Scholar]
- 38). Heinecke JW: Small HDL promotes cholesterol efflux by the ABCA1 pathway in macrophages: implications for therapies targeted to HDL. Circ Res, 2015; 116: 1101-1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Rothblat GH, Phillips MC: High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol, 2010; 21: 229-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Camont L, Chapman MJ, Kontush A: Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med, 2011; 17: 594-603 [DOI] [PubMed] [Google Scholar]
- 41). Du XM, Kim MJ, Hou L, Le Goff W, Chapman MJ, Van Eck M, Curtiss LK, Burnett JR, Cartland SP, Quinn CM, Kockx M, Kontush A, Rye KA, Kritharides L, Jessup W: HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res, 2015; 116: 1133-1142 [DOI] [PubMed] [Google Scholar]
- 42). Davidson WS, Rodrigueza WV, Lund-Katz S, Johnson WJ, Rothblat GH, Phillips MC: Effects of acceptor particle size on the efflux of cellular free cholesterol. J Biol Chem, 1995; 270: 17106-17113 [DOI] [PubMed] [Google Scholar]
- 43). Fournier N, de la LleraMoya M, Burkey BF, Swaney JB, Paterniti J, Jr., Moatti N, Atger V, Rothblat GH: Role of HDL phospholipid in efflux of cell cholesterol to whole serum: studies with human apoA-I transgenic rats. J Lipid Res, 1996; 37: 1704-1711 [PubMed] [Google Scholar]
- 44). Yancey PG, de la Llera-Moya M, Swarnakar S, Monzo P, Klein SM, Connelly MA, Johnson WJ, Williams DL, Rothblat GH: High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J Biol Chem, 2000; 275: 36596-36604 [DOI] [PubMed] [Google Scholar]
- 45). Tanaka N, Ishida T, Nagao M, Mori T, Monguchi T, Sasaki M, Mori K, Kondo K, Nakajima H, Honjo T, Irino Y, Toh R, Shinohara M, Hirata K: Administration of high dose eicosapentaenoic acid enhances anti-inflammatory properties of high-density lipoprotein in Japanese patients with dyslipidemia. Atherosclerosis, 2014; 237: 577-583 [DOI] [PubMed] [Google Scholar]
- 46). Tanaka N, Irino Y, Shinohara M, Tsuda S, Mori T, Nagao M, Oshita T, Mori K, Hara T, Toh R, Ishida T, Hirata K: Eicosapentaenoic acid-enriched high-density lipoproteins exhibit anti-atherogenic properties. Circ J, 2018; 82: 596-601 [DOI] [PubMed] [Google Scholar]
- 47). Davidson WS, Gillotte KL, Lund-Katz S, Johnson WJ, Rothblat GH, Phillips MC: The effect of high density lipoprotein phospholipid acyl chain composition on the efflux of cellular free cholesterol. J Biol Chem, 1995; 270: 5882-5890 [DOI] [PubMed] [Google Scholar]
- 48). Kasbi Chadli F, Nazih H, Krempf M, Nguyen P, Ouguerram K: Omega 3 fatty acids promote macrophage reverse cholesterol transport in hamster fed high fat diet. PLoS One, 2013; 8: e61109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Shao B, Tang C, Sinha A, Mayer PS, Davenport GD, Brot N, Oda MN, Zhao XQ, Heinecke JW: Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ Res, 2014; 114: 1733-1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). DiDonato JA, Aulak K, Huang Y, Wagner M, Gerstenecker G, Topbas C, Gogonea V, DiDonato AJ, Tang WH, Mehl RA, Fox PL, Plow EF, Smith JD, Fisher EA, Hazen SL: Site-specific nitration of apolipoprotein A-I at tyrosine 166 is both abundant within human atherosclerotic plaque and dysfunctional. J Biol Chem, 2014; 289: 10276-10292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Huang Y, DiDonato JA, Levison BS, Schmitt D, Li L, Wu Y, Buffa J, Kim T, Gerstenecker GS, Gu X, Kadiyala CS, Wang Z, Culley MK, Hazen JE, Didonato AJ, Fu X, Berisha SZ, Peng D, Nguyen TT, Liang S, Chuang CC, Cho L, Plow EF, Fox PL, Gogonea V, Tang WH, Parks JS, Fisher EA, Smith JD, Hazen SL: An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat Med, 2014; 20: 193-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52). Nicholls SJ, Hazen SL: Myeloperoxidase, modified lipoproteins, and atherogenesis. J Lipid Res, 2009; 50 Suppl: S346-351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53). Huang Y, Wu Z, Riwanto M, Gao S, Levison BS, Gu X, Fu X, Wagner MA, Besler C, Gerstenecker G, Zhang R, Li XM, DiDonato AJ, Gogonea V, Tang WH, Smith JD, Plow EF, Fox PL, Shih DM, Lusis AJ, Fisher EA, DiDonato JA, Landmesser U, Hazen SL: Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J Clin Invest, 2013; 123: 3815-3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54). Haraguchi Y, Toh R, Hasokawa M, Nakajima H, Honjo T, Otsui K, Mori K, Miyamoto-Sasaki M, Shinohara M, Nishimura K, Ishida T, Hirata K: Serum myeloperoxidase/paraoxonase 1 ratio as potential indicator of dysfunctional high-density lipoprotein and risk stratification in coronary artery disease. Atherosclerosis, 2014; 234: 288-294 [DOI] [PubMed] [Google Scholar]
- 55). Khera AV, Rader DJ: Cholesterol efflux capacity: full steam ahead or a bump in the road? Arterioscler Thromb Vasc Biol, 2013; 33: 1449-1451 [DOI] [PubMed] [Google Scholar]
- 56). Anastasius M, Kockx M, Jessup W, Sullivan D, Rye KA, Kritharides L: Cholesterol efflux capacity: An introduction for clinicians. Am Heart J, 2016; 180: 54-63 [DOI] [PubMed] [Google Scholar]
- 57). Talbot CPJ, Plat J, Ritsch A, Mensink RP: Determinants of cholesterol efflux capacity in humans. Prog Lipid Res, 2018; 69: 21-32 [DOI] [PubMed] [Google Scholar]
- 58). Miwa K, Inazu A, Kawashiri M, Nohara A, Higashikata T, Kobayashi J, Koizumi J, Nakajima K, Nakano T, Niimi M, Mabuchi H, Yamagishi M: Cholesterol efflux from J774 macrophages and Fu5AH hepatoma cells to serum is preserved in CETP-deficient patients. Clin Chim Acta, 2009; 402: 19-24 [DOI] [PubMed] [Google Scholar]
- 59). Bortnick AE, Rothblat GH, Stoudt G, Hoppe KL, Royer LJ, McNeish J, Francone OL: The correlation of ATP-binding cassette 1 mRNA levels with cholesterol efflux from various cell lines. J Biol Chem, 2000; 275: 28634-28640 [DOI] [PubMed] [Google Scholar]
- 60). Santamarina-Fojo S, Remaley AT, Neufeld EB, Brewer HB, Jr.: Regulation and intracellular trafficking of the ABCA1 transporter. J Lipid Res, 2001; 42: 1339-1345 [PubMed] [Google Scholar]
- 61). Ogura M, Hori M, Harada-Shiba M: Association Between Cholesterol Efflux Capacity and Atherosclerotic Cardiovascular Disease in Patients With Familial Hypercholesterolemia. Arterioscler Thromb Vasc Biol, 2016; 36: 181-188 [DOI] [PubMed] [Google Scholar]
- 62). de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH: The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol, 2010; 30: 796-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63). Li XM, Tang WH, Mosior MK, Huang Y, Wu Y, Matter W, Gao V, Schmitt D, Didonato JA, Fisher EA, Smith JD, Hazen SL: Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol, 2013; 33: 1696-1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64). Davidson WS, Heink A, Sexmith H, Melchior JT, Gordon SM, Kuklenyik Z, Woollett L, Barr JR, Jones JI, Toth CA, Shah AS: The effects of apolipoprotein B depletion on HDL subspecies composition and function. J Lipid Res, 2016; 57: 674-686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65). Sankaranarayanan S, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Asztalos BF, Bittman R, Rothblat GH: A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. J Lipid Res, 2011; 52: 2332-2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66). Uto-Kondo H, Ayaori M, Ogura M, Nakaya K, Ito M, Suzuki A, Takiguchi S, Yakushiji E, Terao Y, Ozasa H, Hisada T, Sasaki M, Ohsuzu F, Ikewaki K: Coffee consumption enhances high-density lipoprotein-mediated cholesterol efflux in macrophages. Circ Res, 2010; 106: 779-787 [DOI] [PubMed] [Google Scholar]
- 67). Kushiyama A, Okubo H, Sakoda H, Kikuchi T, Fujishiro M, Sato H, Kushiyama S, Iwashita M, Nishimura F, Fukushima T, Nakatsu Y, Kamata H, Kawazu S, Higashi Y, Kurihara H, Asano T: Xanthine oxidoreductase is involved in macrophage foam cell formation and atherosclerosis development. Arterioscler Thromb Vasc Biol, 2012; 32: 291-298 [DOI] [PubMed] [Google Scholar]
- 68). Fournier N, Tardivel S, Benoist JF, Vedie B, Rousseau-Ralliard D, Nowak M, Allaoui F, Paul JL: Eicosapentaenoic acid membrane incorporation impairs ABCA1-dependent cholesterol efflux via a protein kinase A signaling pathway in primary human macrophages. Biochim Biophys Acta, 2016; 1861: 331-341 [DOI] [PubMed] [Google Scholar]
- 69). Asztalos BF, Horvath KV, Mehan M, Yokota Y, Schaefer EJ: Influence of HDL particles on cell-cholesterol efflux under various pathological conditions. J Lipid Res, 2017; 58: 1238-1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70). Harada A, Toh R, Murakami K, Kiriyama M, Yoshikawa K, Miwa K, Kubo T, Irino Y, Mori K, Tanaka N, Nishimura K, Ishida T, Hirata K: Cholesterol Uptake Capacity: A New Measure of HDL Functionality for Coronary Risk Assessment. J Appl Lab Med, 2017; 2: 186-200 [DOI] [PubMed] [Google Scholar]
- 71). Serfaty-Lacrosniere C, Civeira F, Lanzberg A, Isaia P, Berg J, Janus ED, Smith MP, Jr., Pritchard PH, Frohlich J, Lees RS, et al. : Homozygous Tangier disease and cardiovascular disease. Atherosclerosis, 1994; 107: 85-98 [DOI] [PubMed] [Google Scholar]
- 72). Maeda S, Nakanishi S, Yoneda M, Awaya T, Yamane K, Hirano T, Kohno N: Associations between small dense LDL, HDL subfractions (HDL2, HDL3) and risk of atherosclerosis in Japanese-Americans. J Atheroscler Thromb, 2012; 19: 444-452 [DOI] [PubMed] [Google Scholar]
- 73). Akinkuolie AO, Paynter NP, Padmanabhan L, Mora S: High-density lipoprotein particle subclass heterogeneity and incident coronary heart disease. Circ Cardiovasc Qual Outcomes, 2014; 7: 55-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74). Kontush A: HDL particle number and size as predictors of cardiovascular disease. Front Pharmacol, 2015; 6:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75). Fukui T, Hirano T: High-density lipoprotein subspecies between patients with type 1 diabetes and type 2 diabetes without / with intensive insulin therapy. Endocr J, 2012; 59: 561-569 [DOI] [PubMed] [Google Scholar]
- 76). Borggreve SE, De Vries R, Dullaart RP: Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: role of lipolytic enzymes, lecithin:cholesterol acyltransferase and lipid transfer proteins. Eur J Clin Invest, 2003; 33: 1051-1069 [DOI] [PubMed] [Google Scholar]


