See article vol. 26: 136–144
Understandings from Causal Associations between Biomarkers and Clinical Outcomes
To establish a causal association between a biomarker and an outcome, randomized controlled trials (RCT) are the gold standard. On the other hand, the Mendelian randomization trial is a technique that uses genotypes as instruments to assess causal associations between biomarkers and outcomes1). In a Mendelian randomization trial, a genetic variant associated with a particular biomarker is used as a proxy for the biomarker. Outcomes are compared between the group with the effect allele and a group with the reference allele. This approach can be considered a proxy for an RCT, in which the randomized groups have similar confounding variables. Accordingly, a Mendelian randomization trial can be regarded as a natural RCT. Using this technique, a causal association between LDL cholesterol and atherosclerotic diseases have been firmly confirmed. With respect to triglycerides, they have been associated with atherosclerotic diseases in numerous epidemiological studies2). Establishing triglyceride as a causal factor of atherosclerotic diseases has been difficult because it is difficult to distinguish the effects of triglyceride on atherosclerotic diseases from those of LDL cholesterol and HDL cholesterol by RCT or by Mendelian randomization trials. To overcome this issue, an interesting study creating a framework to observed whether triglyceride causally influences the risk of atherosclerotic diseases was published in 20133). They constructed a model adjusting for the effects of LDL cholesterol and/or HDL cholesterol levels on the risk of atherosclerotic diseases, and found that the genetic impact of single nucleotide polymorphisms (SNPs) on triglyceride levels was independently associated with the risk of atherosclerotic diseases. This study suggested that triglycerides causally affect the risk of atherosclerotic diseases. In this issue, Liu et al. described an extremely rare case of severe hypertriglyceridemia caused by lipase maturation factor 1 (LMF1) mutations exhibiting atherosclerotic changes4). In addition, rare as well as common genetic variations in lipoprotein lipase (LPL) and apolipoprotein C3 (APOC3) genes have been associated with coronary artery disease5, 6). We strongly believe that consistent directions regarding the effects on phenotype between common as well as rare genetic variations definitely ascertain the truth of nature (Fig. 1). Those observations collectively suggest that triglyceride could be regarded as a causal factor for atherosclerosis, rather than a biomarker.
Fig. 1.
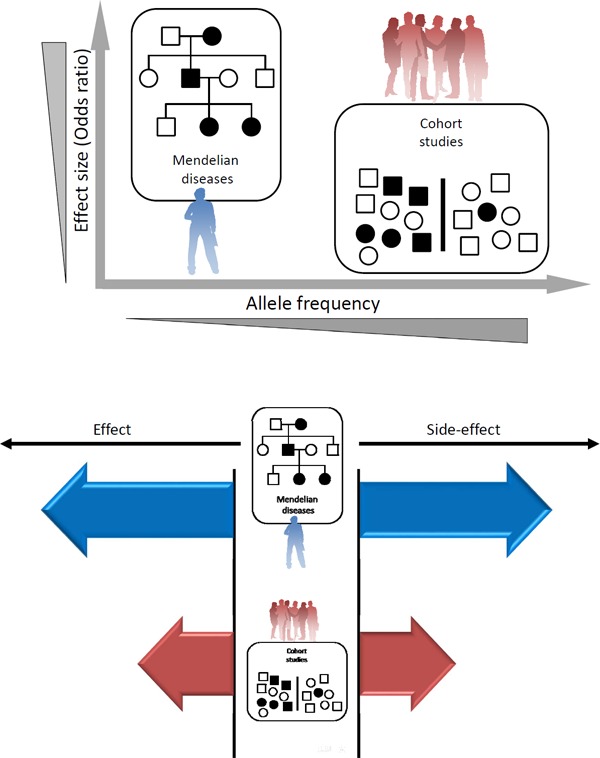
Scheme of human genetic variations and diseases
Upper panel. X-axis represents allele frequency. Y-axis represents effect sizes.
Lower panel. Consistent directions between rare as well as common genetic variations definitely ascertain the truth of nature.
Biology, Genetics, and Outcomes
Although human genetics could potentially illustrate the causal associations between biomarkers and outcomes, underlying biology should also be considered. We have previously shown that rare genetic variations lowering triglyceride were consistently associated with lowering the risk for coronary atherosclerosis, although we could not have enough power to show this favorable association in LMF1 gene (Table 1)7). Interestingly, the effect on the risk of developing diabetes were inconsistent across those genes, suggesting that functions based on biology should be carefully considered when assessing such genetic association studies.
Table 1. Impact of triglyceride lowering genes on CAD and T2DM.
| Gene | Number of variants | Triglyceride | HDL cholesterol | LDL cholesterol | CAD Odds ratio | T2DM Odds ratio |
|---|---|---|---|---|---|---|
| LPL | 7 | −0.138 ± 0.002 (<1.0 × 10−237) |
0.939 ± 0.011 (<1.0 × 10−237) |
0.025 ± 0.012 (0.03) |
0.66 | 0.8 |
| ANGPTL4 | 1 | −0.273 ± 0.01 (4.2 × 10−175) |
0.891 ± 0.035 (4.8 × 10−146) |
−0.014 ± 0.036 (0.7) |
0.6 | 0.67 |
| APOA5 | 7 | −0.227 ± 0.002 (<1.0 × 10−237) |
0.453 ± 0.009 (<1.0 × 10−237) |
−0.145 ± 0.009 (8.4 × 10−59) |
0.76 | 0.88 |
| APOC3 | 3 | −1.069 ± 0.032 (3.2 × 10−237) |
0.695 ± 0.03 (9.0 × 10−120) |
−0.106 ± 0.03 (4.7 × 10−4) |
0.85 | 1.07 |
| ANGPTL3 | 1 | −0.077 ± 0.003 (6.1 × 10−170) |
−0.136 ± 0.035 (1.2 × 10−4) |
−0.588 ± 0.037 (4.8 × 10−58) |
0.89 | 1.18 |
| ANGPTL8 | 1 | −0.353 ± 0.051 (2.8 × 10−12) |
1.221 ± 0.141 (5.0 × 10−18) |
−0.167 ± 0.146 (0.25) |
0.74 | 1.58 |
Value are expressed as effect size ± S.E. (p-value). LPL = lipoprotein lipase; ANGPTL4 = angiopoietin-like 4; APOA5 = apolipoprotein A5; APOC3 = apolipoprotein C3; ANGPTL3 = Angiopoietin-like 3; ANGPTL8 = Angiopoietin-like 8; CAD = coronary artery disease; T2DM = type 2 diabetes mellitus
Conclusion
Dyslipidemias, including hypertriglyceridemia, are important causal factors of atherosclerosis. Genetic analyses in Mendelian dyslipidemias as well as Mendelian randomization trials using common genetic variants have contributed to our understanding of lipids and atherosclerotic diseases. Investigation on extreme cases caused by rare genetic variants can lead to the development of novel drugs for the general population, especially when the on-target/off-target directions of rare variants and common variants exhibit the same directions8). We do hope that such cases could be verified in other genes, including LMF1, based on the accumulations of those extreme cases.
Acknowledgments
None.
Conflict of Interest
None.
Sources of Funding
None.
References
- 1). Tada H, Kawashiri MA, Yamagishi M. Comprehensive genotyping in dyslipidemia: mendelian dyslipidemias caused by rare variants and Mendelian randomization studies using common variants. J Hum Genet, 2017; 62: 453-458 [DOI] [PubMed] [Google Scholar]
- 2). Tada H, Kawashiri MA, Sakata K, Yoneda T, Yasuda K, Yamagishi M, Hayashi K. Renal glucosuria is not associated with atherosclerotic cardiovascular disease outcome in a general Japanese community. Atherosclerosis, 2017; 261: 111-116 [DOI] [PubMed] [Google Scholar]
- 3). Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang HY, Demirkan A, Den Hertog HM, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkilä K, Hyppönen E, Isaacs A, Jackson AU, Johansson A, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikäinen LP, Magnusson PK, Mangino M, Mihailov E, Montasser ME, Müller-Nurasyid M, Nolte IM, O'Connell JR, Palmer CD, Perola M, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney AS, Döring A, Elliott P, Epstein SE, Eyjolfsson GI, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ, Illig T, Jones MR, Kaleebu P, Kastelein JJ, Khaw KT, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimäki T, Lin SY, Lindström J, Loos RJ, Mach F, McArdle WL, Meisinger C, Mitchell BD, Müller G, Nagaraja R, Narisu N, Nieminen TV, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A, Rader DJ, Reilly MP, Ridker PM, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stančáková A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen YD, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB, Ferrières J, Ferrucci L, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE, Hunt SC, Hveem K, Iribarren C, Järvelin MR, Jula A, Kähönen M, Kaprio J, Kesäniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D, Kuusisto J, Kyvik KO, Laakso M, Lakka TA, Lind L, Lindgren CM, Martin NG, März W, McCarthy MI, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njølstad I, Pedersen NL, Power C, Pramstaller PP, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PE, Sheu WH, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BH, Altshuler D, Ordovas JM, Boerwinkle E, Palmer CN, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS, Rich SS, Boehnke M, Deloukas P, Mohlke KL, Ingelsson E, Abecasis GR, Daly MJ, Neale BM, Kathiresan S. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet, 2013; 45: 1345-1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Liu Y, Xu J, Tao W, Yu R, Zhang X. A compound heterozygous mutation lipase maturation factor 1 is responsible for hypertriglyceridemia of a patient. J Atheroscler Thromb, 2019; 26: 136-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Khera AV, Won HH, Peloso GM, O'Dushlaine C, Liu D, Stitziel NO, Natarajan P, Nomura A, Emdin CA, Gupta N, Borecki IB, Asselta R, Duga S, Merlini PA, Correa A, Kessler T, Wilson JG, Bown MJ, Hall AS, Braund PS, Carey DJ, Murray MF, Kirchner HL, Leader JB, Lavage DR, Manus JN, Hartzel DN, Samani NJ, Schunkert H, Marrugat J, Elosua R, McPherson R, Farrall M, Watkins H, Lander ES, Rader DJ, Danesh J, Ardissino D, Gabriel S, Willer C, Abecasis GR, Saleheen D, Dewey FE, Kathiresan S, Myocardial Infarction Genetics Consortium, DiscovEHR Study Group, CARDIoGRAM Exome Consortium, and Global Lipids Genetics Consortium Association of Rare and Common Variation in the Lipoprotein Lipase Gene With Coronary Artery Disease. JAMA, 2017; 317: 937-946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute. Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, Lu Y, Tang ZZ, Zhang H, Hindy G, Masca N, Stirrups K, Kanoni S, Do R, Jun G, Hu Y, Kang HM, Xue C, Goel A, Farrall M, Duga S, Merlini PA, Asselta R, Girelli D, Olivieri O, Martinelli N, Yin W, Reilly D, Speliotes E, Fox CS, Hveem K, Holmen OL, Nikpay M, Farlow DN, Assimes TL, Franceschini N, Robinson J, North KE, Martin LW, DePristo M, Gupta N, Escher SA, Jansson JH, Van Zuydam N, Palmer CN, Wareham N, Koch W, Meitinger T, Peters A, Lieb W, Erbel R, Konig IR, Kruppa J, Degenhardt F, Gottesman O, Bottinger EP, O'Donnell CJ, Psaty BM, Ballantyne CM, Abecasis G, Ordovas JM, Melander O, Watkins H, Orho-Melander M, Ardissino D, Loos RJ, McPherson R, Willer CJ, Erdmann J, Hall AS, Samani NJ, Deloukas P, Schunkert H, Wilson JG, Kooperberg C, Rich SS, Tracy RP, Lin DY, Altshuler D, Gabriel S, Nickerson DA, Jarvik GP, Cupples LA, Reiner AP, Boerwinkle E, Kathiresan S. Loss-offunction mutations in APOC3, triglycerides, and coronary disease. N Engl J Med, 2014; 371: 22-3124941081 [Google Scholar]
- 7). Liu DJ, Peloso GM, Yu H, Butterworth AS, Wang X, Mahajan A, Saleheen D, Emdin C, Alam D, Alves AC, Amouyel P, Di Angelantonio E, Arveiler D, Assimes TL, Auer PL, Baber U, Ballantyne CM, Bang LE, Benn M, Bis JC, Boehnke M, Boerwinkle E, Bork-Jensen J, Bottinger EP, Brandslund I, Brown M, Busonero F, Caulfield MJ, Chambers JC, Chasman DI, Chen YE, Chen YI, Chowdhury R, Christensen C, Chu AY, Connell JM, Cucca F, Cupples LA, Damrauer SM, Davies G, Deary IJ, Dedoussis G, Denny JC, Dominiczak A, Dubé MP, Ebeling T, Eiriksdottir G, Esko T, Farmaki AE, Feitosa MF, Ferrario M, Ferrieres J, Ford I, Fornage M, Franks PW, Frayling TM, Frikke-Schmidt R, Fritsche LG, Frossard P, Fuster V, Ganesh SK, Gao W, Garcia ME, Gieger C, Giulianini F, Goodarzi MO, Grallert H, Grarup N, Groop L, Grove ML, Gudnason V, Hansen T, Harris TB, Hayward C, Hirschhorn JN, Holmen OL, Huffman J, Huo Y, Hveem K, Jabeen S, Jackson AU, Jakobsdottir J, Jarvelin MR, Jensen GB, Jørgensen ME, Jukema JW, Justesen JM, Kamstrup PR, Kanoni S, Karpe F, Kee F, Khera AV, Klarin D, Koistinen HA, Kooner JS, Kooperberg C, Kuulasmaa K, Kuusisto J, Laakso M, Lakka T, Langenberg C, Langsted A, Launer LJ, Lauritzen T, Liewald DCM, Lin LA, Linneberg A, Loos RJF, Lu Y, Lu X, Mägi R, Malarstig A, Manichaikul A, Manning AK, Mäntyselkä P, Marouli E, Masca NGD, Maschio A, Meigs JB, Melander O, Metspalu A, Morris AP, Morrison AC, Mulas A, Müller-Nurasyid M, Munroe PB, Neville MJ, Nielsen JB, Nielsen SF, Nordestgaard BG, Ordovas JM, Mehran R, O'Donnell CJ, Orho-Melander M, Molony CM, Muntendam P, Padmanabhan S, Palmer CNA, Pasko D, Patel AP, Pedersen O, Perola M, Peters A, Pisinger C, Pistis G, Polasek O, Poulter N, Psaty BM, Rader DJ, Rasheed A, Rauramaa R, Reilly DF, Reiner AP, Renström F, Rich SS, Ridker PM, Rioux JD, Robertson NR, Roden DM, Rotter JI, Rudan I, Salomaa V, Samani NJ, Sanna S, Sattar N, Schmidt EM, Scott RA, Sever P, Sevilla RS, Shaffer CM, Sim X, Sivapalaratnam S, Small KS, Smith AV, Smith BH, Somayajula S, Southam L, Spector TD, Speliotes EK, Starr JM, Stirrups KE, Stitziel N, Strauch K, Stringham HM, Surendran P, Tada H, Tall AR, Tang H, Tardif JC, Taylor KD, Trompet S, Tsao PS, Tuomilehto J, Tybjaerg-Hansen A, van Zuydam NR, Varbo A, Varga TV, Virtamo J, Waldenberger M, Wang N, Wareham NJ, Warren HR, Weeke PE, Weinstock J, Wessel J, Wilson JG, Wilson PWF, Xu M, Yaghootkar H, Young R, Zeggini E, Zhang H, Zheng NS, Zhang W, Zhang Y, Zhou W, Zhou Y, Zoledziewska M, Charge Diabetes Working Group; EPIC-InterAct Consortium; EPIC-CVD Consortium; GOLD Consortium; VA Million Veteran Program. Howson JMM, Danesh J, McCarthy MI, Cowan CA, Abecasis G, Deloukas P, Musunuru K, Willer CJ, Kathiresan S. Exome-wide association study of plasma lipids in < 300,000 individuals. Nat Genet, 2017; 49: 1758-1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Tada H, Kawashiri MA, Konno T, Yamagishi M, Hayashi K. Common and Rare Variant Association Study for Plasma Lipids and Coronary Artery Disease. J Atheroscler Thromb, 2016; 23: 241-256 [DOI] [PubMed] [Google Scholar]


