Abstract
Aims: Recently, incretin therapy has attracted increasing attention because of its potential use in tissue-protective therapy. Neuron-derived orphan receptor 1 (NOR1) is a nuclear orphan receptor that regulates vascular smooth muscle cell (VSMC) proliferation. In the present study, we investigated the vascular-protective effect of Exendin-4 (Ex-4), a glucagon-like peptide-1 receptor agonist, by inhibiting NOR1 expression in VSMCs.
Methods: We classified 7-week-old male 129X1/SvJ mice into control group and Ex-4 low- and high-dose-treated groups fed normal or high-fat diets, respectively. Endothelial denudation injuries were induced in the femoral artery at 8 weeks of age, followed by the evaluation of neointima formation at 12 weeks of age. To evaluate VSMC proliferation, bromodeoxyuridine incorporation assay and cell cycle distribution analysis were performed. NOR1 and cell cycle regulators were detected using immunohistochemistry, western blotting, quantitative reverse-transcription polymerase chain reaction, and luciferase assays.
Results: Ex-4 treatment reduced vascular injury-induced neointima formation compared with controls. In terms of VSMCs occupying the neointima area, VSMC numbers and NOR1-expressing proliferative cells were significantly decreased by Ex-4 in a dose-dependent manner in both diabetic and non-diabetic mice. In vitro experiments using primary cultured VSMCs revealed that Ex-4 attenuated NOR1 expression by reducing extracellular signal-regulated kinase-mitogen-activated protein kinase and cAMP-responsive element-binding protein phosphorylations. Furthermore, in the cell cycle distribution analysis, serum-induced G1–S phase entry was significantly attenuated by Ex-4 treatment of VSMCs by inhibiting the induction of S-phase kinase-associated protein 2.
Conclusion: Ex-4 attenuates neointima formation after vascular injury and VSMC proliferation possibly by inhibiting NOR1 expression.
Keywords: ERK-MAPK; GLP-1 receptor agonist; NOR1, Neointima formation; VSMC proliferation
Background
Individuals with diabetes mellitus have a greater risk of cardiovascular events than those without1). Patients with diabetes frequently experience restenosis after coronary angioplasty, even after intervention with currently available drug-eluting stents2). Consequently, the aim of glycemic control is not only the lowering of the blood glucose level but also improving the quality of life and mortality by preventing the occurrence and progression of vascular complications. Therefore, it is important to investigate the vascular-protective effects of anti-diabetic agents.
Incretin therapy, which includes glucagon-like peptide-1 receptor (GLP-1R) agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors, has been a focus because of its tissue-protective effects beyond lowering blood glucose levels3), such as cardiovascular protection4), anti-hepatic steatosis5), and anti-Alzheimer's disease6). Previously, we have reported the vascular-protective effects of the GLP-1R agonist Exendin-4 (Ex-4), including the attenuation of atheroma formation in apoE-deficient mice by inhibiting NFκB activation in macrophages7) and the reduction of neointima formation after vascular injury by inhibiting vascular smooth muscle cell (VSMC) proliferation8). Furthermore, we have recently demonstrated the anti-cancer effect of Ex-4 using a prostate cancer model9, 10). In addition, we reported that the DPP-4 inhibitor linagliptin attenuates neointima formation by not only incretin effects but also anti-oxidative stress effects11).
The nuclear receptor superfamily has been recognized as one of key regulators of the inflammatory response and VSMC proliferation in atherosclerosis and vascular diseases12). Although much attention is focused on peroxisome proliferator-activated receptors (PPARs)13) and liver X receptors (LXRs)14) as therapeutic targets, we have investigated the role of NR4A orphan nuclear receptors, particularly NR4A3 neuron-derived orphan receptor 1 (NOR1), in atherosclerosis and vascular diseases15). NOR1 was identified in primary cultured rat fetal forebrain cells undergoing apoptosis16). In human coronary arteries, NOR1 is expressed in VSMCs migrating into atherosclerotic lesions, but not in normal vessels, and mitogenic stimulation induces NOR1 expression via extracellular signal-regulated kinase (ERK)-mitogen-activated protein kinase (MAPK) and cAMP-responsive element-binding protein (CREB) phosphorylations17). NOR1 has a pivotal role in VSMC proliferation, and NOR1 deficiency reduces neointima formation after vascular injury by inhibiting G1–S phase entry of cell cycle progression in VSMCs, as we reported previously18, 19). However, currently, there are no reports that have elucidated the interaction between GLP-1 and NOR1 in VSMCs. In the present study, we demonstrate that Ex-4 attenuates NOR1 expression both in vivo and in vitro in VSMCs.
Methods
Animals
The study protocol was reviewed and approved by the Animal Care and Use Committee of Fukuoka University. Six-week-old male 129X1/SvJ mice were purchased from Japan SLC, Inc. (Sizuoka, Japan). All mice were housed in a polycarbonate cage with a wooden chip mat on the floor, and water was available ad libitum. All mice were fed normal chow (20% protein, 70% carbohydrate, and 10% fat; D12450B, Research Diet, New Brunswick, NJ). The animal room was kept in a 12-h light/dark cycle at a constant temperature (22°C ± 1°C) and relative humidity of 55% ± 5% throughout the experimental period. Mice were divided into the saline-control (n = 9) group, Ex-4 low-dose (300 pmol/kg body weight/day, n = 10)-treated group, and Ex-4 high-dose (24 nmol/kg body weight/day, n = 10)-treated group. At 7 weeks of age, a miniosmotic pump (ALZEST, model 1004; DURECT, Cupertino, CA) was implanted under the skin of the back of each mouse after local anesthesia. Saline or Ex-4 (Sigma-Aldrich, Tokyo) was infused via the osmotic pump that continuously delivered the solution for up to 4 weeks. Endothelial denudation injuries were induced in the femoral artery at 8 weeks of age, followed by the evaluation of neointimal formation at 12 weeks of age.
Animals Fed High-Fat Diet
Seven-week-old male 129X1/SvJ mice were fed high-fat diet (20% protein, 20% carbohydrate, and 60% fat, D12492, Research Diet) and divided into the saline-control (n = 5) group, Ex-4 low-dose (300 pmol/kg body weight/day, n = 5)-treated group, and Ex-4 high-dose (24 nmol/kg body weight/day, n = 5)-treated group. Endothelial denudation injuries were induced in the femoral artery at 8 weeks of age. Mice were euthanized at 12 weeks of age and femoral arteries were isolated for tissue analysis.
Guidewire-Induced Endothelial Denudation Injury
Mouse femoral artery endothelial denudation injuries were established, as we previously reported8, 11, 18), in 129X1/SvJ mice treated as control (saline, n = 9), Ex-4 low-dose (300 pmol/kg/day, n = 10), and Ex-4 high-dose (24 nmol/kg/day, n = 10) groups at 8 weeks of age, as described previously8). Briefly, endovascular injuries were induced by four passages of a 0.25-mm SilverSpeed-10 hydrophilic guidewire (Micro Therapeutics Inc., Irvine, CA, USA) into the left femoral artery. Mice were euthanized at 4 weeks after injury, and femoral arteries were isolated for tissue analysis.
Tissue Preparation and Morphometry
Following sacrifice, mice were perfused via a cannula in the left ventricle with phosphate-buffered saline for 5 min, followed by 4% paraformaldehyde for 30 min at 100 cm H2O. The femoral arteries were embedded in paraffin, cut into 5-µm sections, and prepared for Elastica van Gieson and immunofluorescence staining. Serial sections of the 0.5-mm proximal region from the incision site of the wire insertion were evaluated using Elastica van Gieson stain kit (ab150667, Abcam, Cambridge, UK) to visualize the internal elastic lamina, as described previously11). Specimens were viewed under a microscope (BZ9000; Keyence, Tokyo, Japan) connected to a computer. Intimal and medial areas were measured by computerized morphometry using BZ-II analyzer software (Keyence, Tokyo, Japan). Intimal hyperplasia was defined as neointimal layer formation medial to the internal elastic lamina. The medial area represents the area between external and internal elastic laminae. The intima-to-media ratio was calculated as the intimal area divided by the media area, as described previously8, 11).
Immunohistochemistry
Paraffin sections were incubated with a Cy-3-conjugated α-smooth muscle actin antibody (C6198, Sigma-Aldrich). Serial sections were incubated with anti-NOR1 (HPA043360, Sigma-Aldrich) and anti-PCNA (sc-9857; Santa Cruz, CA) antibodies. Sections analyzed for NOR1 were subsequently incubated with Alexa Fluor 647 goat anti-rabbit IgG (A21246, Life technologies), and sections analyzed for PCNA were subsequently incubated with Alexa Fluor 488 donkey anti-goat IgG (A11055, Life technologies). Sections were counterstained with DAPI and visualized using confocal microscopy.
Insulin Measurements
Insulin concentrations in mouse serum were measured using Ultra-Sensitive Mouse Insulin ELISA Kit (Morinaga Institute of Biological Science, Inc. Kanagawa, Japan), according to the manufacturer's protocol.
Cell Culture
Human aortic smooth muscle cells were purchased from Lonza (Allendale, NJ) and maintained in smooth muscle basal medium supplemented with 5% fetal bovine serum (FBS), hEGF, insulin, hFGF-B, and gentamicin/amphotericin-B (SmGM-2 SingleQuots; Lonza), as per the manufacturer's instructions, and rat aortic smooth muscle cells were purchased from Lonza and maintained in Dulbecco's modified Eagle medium supplemented with 10% FBS. Cells were grown to 60%–70% confluence, serum-deprived for at least 12 h, pretreated with 0.1–10 nM Ex-4 or saline for 12 h, and stimulated with FBS at a final concentration of 10%. Cells were used between passages three and six for the experiments, and individual experiments were repeated at least three times with different preparations of cells.
Western Blot Analysis
Western blotting was performed, as described previously9, 10). The following primary antibodies were used: NOR1 (TA804872, ORIGENE), phosphop44/42 MAPK (Thr202/Tyr204) (#9101; Cell Signaling), p44/42 MAPK (#9102; Cell Signaling), phospho-CREB (Ser133) (#9198; Cell Signaling), CREB (#9197; Cell Signaling), phospho-Akt (Ser473) (#4058; Cell Signaling), Protein kinase B (Akt) (#9272; Cell Signaling), phospho-mTOR (Ser2448) (#2971; Cell Signaling), mTOR (#2983; Cell Signaling), p27 Kip1 (#3686; Cell Signaling), and GAPDH (sc-20357; Santa Cruz).
Reverse Transcription (RT) and Quantitative Real-Time PCR
RT and quantitative real-time PCR were performed, as described previously9, 10). mRNA from human aortic SMCs was isolated using RNeasy Mini Kits (Qiagen, Venlo, the Netherlands) and reverse transcribed into cDNA. PCRs were performed using Light Cycler 2.0 (Roche, Basel, Switzerland) and SYBR Premix Ex Taq™ II (Takara, Otsu, Japan). Each sample was analyzed in triplicate and normalized against TATA binding protein (TBP) mRNA expression. The following primer sequences were used: human TBP, 5′-TGCTGCGGTAATCATGAGGATA-3′ (forward), 5′-TGAAGTCCAAGAACTTAGCTGGAA-3′ (reverse); human NOR1, 5′-CCCCTCCAGGTTCCAGTTAT-3′ (forward), 5′-ATTTGGTACACGCAGGAAGG-3′ (reverse); human Skp2, 5′-ACACTGCAAAAGCCCAGTTG-3′ (forward), 5′-TGCAGAATGAAGGCAAAGGG-3′ (reverse).
Plasmids, Transient Transfections, and Luciferase Assay
To evaluate NOR1 transcriptional activity, the luciferase reporter assay was performed in rat aortic SMCs (Lonza) transiently transfected with pGL3-MMTV or NOR1-LUC reporter constructs. Briefly, rat aortic SMCs were transfected for 24 h with 0.5 µg of reporter DNA using FuGENE HD Transfection Reagent (Roche). Next, cells were maintained under serum deprivation for 36 h, pretreated with or without 10 nM Ex-4 for 12 h, and then stimulated with FBS for 12 h. Luciferase activity was assayed using Dual-Luciferase Reporter Assay (Promega, Madison, WI, USA). Transfection efficiency was normalized to Renilla luciferase activity generated by co-transfection of cells with 10 ng/well pRL-SV40 (Promega). NOR1 promoter constructs were provided by Dr. Naganari Ohkura (Osaka University, Osaka, Japan).
Cell Cycle Analysis Using Flow Cytometry
Human aortic SMCs were seeded in 60-mm dishes at a density of 1 × 105 cells/ml. Cells were grown to 60%–70% confluence, serum-deprived for 36 h, pretreated with 10 nM Ex-4 or saline for 12 h, stimulated with FBS for 30 h. Cell cycle analysis was performed using Cycletest™ Plus DNA reagent kit (BD Biosciences), as per the manufacturer's instructions, and a BD FACSVerse (BD Biosciences, Franklin Lakes, NJ, USA). FlowJo (Tree Star, Inc., OR, USA) was used to analyze the flow cytometry data.
Statistical Analysis
Results are expressed as mean ± SEM. All statistical analyses were performed using GraphPad Prism software (version 7.0; GraphPad Software, SD, USA). Unpaired t -tests and one- or two-way ANOVA were performed for statistical analysis, as appropriate. P-values < 0.05 were considered statistically significant.
Results
Exendin-4 Attenuates Neointima Formation After Vascular Injury in Non-Diabetic Mice
129X1/SvJ mice were treated with the control (saline), low-dose Ex-4, or high-dose Ex-4 from 7 to 12 weeks of age. Mouse femoral artery endothelial denudation injuries were established at 8 weeks of age in all groups, and neointima formation was evaluated at 12 weeks of age. Endothelial denudation injury in control mice resulted in considerable neointima formation. As depicted in Fig. 1A, elastic staining revealed the reduction of neointima formation after vascular injury. Quantitative analysis by measuring squares of the intima, media, and intima-to-media (I/M) ratio suggested that Ex-4 reduced neointima formation after vascular injury in a dose-dependent manner (Fig. 1B). However, statistical significances were not achieved (Table 1). As shown in Table 2, although high-dose Ex-4 significantly decreased mouse body weight, the blood glucose level and serum insulin concentration were not changed by Ex-4 treatment because the mice were not diabetic, suggesting that the reduction of neointima formation by Ex-4 was independent of the Ex-4 effect on glucose metabolism.
Fig. 1.
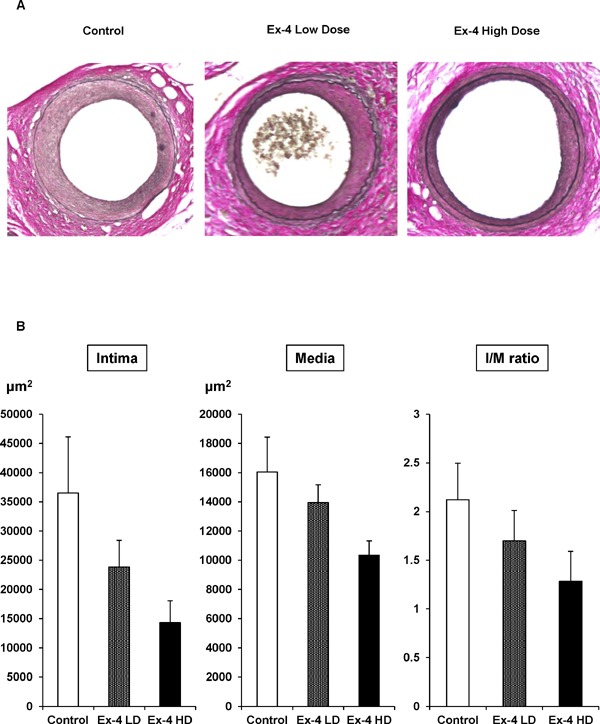
Neointima formation after vascular injury in control and Ex-4-treated mice.
Endothelial denudation injuries were induced in the left femoral artery of control (n = 9) mice, Ex-4 low-dose (n = 9)-treated mice, and Ex-4 high-dose (n = 10)-treated mice. (A) Tissues were evaluated by staining with Elastica van Gieson to visualize the internal elastic lamina (magnification, ×200). (B) The area of the intima, media, and intima/media was calculated for each group. One-way ANOVA was performed to calculate statistical significance. Data are mean ± SEM.
Table 1. Neointima formation in exendin-4 (Ex-4)-treated mice at 12 weeks of age following guidewire-induced endothelial denudation injury.
| Control | Ex-4 Low dose |
Ex-4 High dose |
|
|---|---|---|---|
| Intima (µm2) | 36511.8 ± 9621.7 | 23830.0 ± 4583.6 (P = 0.24) |
14284.1 ± 3801.2 (P = 0.07) |
| Media (µm2) | 16049.2 ± 2385.1 | 13948.9 ± 1235.0 (P = 0.48) |
10338.5 ± 985.9 (P = 0.06) |
| I/M ratio | 2.12 ± 0.37 | 1.70 ± 0.42 (P = 0.42) |
1.28 ± 0.31 (P = 0.12) |
One-way ANOVA was performed to calculate statistical significance. Data are expressed as the mean ± SEM.
P-values refer to comparisons with the control.
Table 2. Characteristics of exendin-4 (Ex-4)-treated mice at 12 weeks of age following guidewire-induced endothelial denudation injury.
| Control | Ex-4 Low dose |
Ex-4 High dose |
|
|---|---|---|---|
| Body weight (g) | 28.1 ± 0.6 | 28.5 ± 0.3 | 26.3 ± 0.5*, ## |
| Plasma glucose (mg/dl) | 150.4 ± 4.0 | 150.0 ± 3.4 | 137.1 ± 8.2 |
| Serum insulin (ng/ml) | 0.64 ± 0.07 | 0.73 ± 0.08 | 0.47 ± 0.09 |
One-way ANOVA was performed to calculate statistical significance. Data are expressed as the mean ± SEM.
P < 0.05 compared with the control.
P < 0.01 compared with low-dose Ex-4.
Exendin-4 Decreases VSMC Proliferation and NOR1 Expression in the Neointima
To confirm that cells occupying the neointima after vascular injury were VSMCs, we next performed immunohistochemistry using VSMC marker α-smooth muscle cell actin. As depicted in Fig. 2, all cells located in the neointima were VSMC (Fig. 2A) in all groups. To examine whether Ex-4 decreased the number of cells occupying neointima formation, we counted cells in the intima and media areas. As shown in Fig. 2B, Ex-4 decreased the cell number in the neointima and the I/M ratio in a dose-dependent manner. Compared with the control, significant reduction in the cell number was achieved in the high-dose Ex-4 group. To further examine whether Ex-4 decreased NOR1 expression in migrating VSMCs after vascular injury, we next conducted NOR1 immunohistochemistry using injured mouse vessels. As depicted in Fig. 2C, profound NOR1 expression with nuclear localization was detected in non-Ex-4-treated control vessels. However, NOR1 expression was dramatically decreased in Ex-4-treated mouse vessels. Finding NOR1-positive cells in the neointima suggested that Ex-4 significantly and dose-dependently decreased NOR1-positive VSMCs (Fig. 2D). Interestingly, in parallel with the reduction of NOR1 expression by Ex-4 in VSMCs, Ex-4 significantly and dose-dependently decreased cell proliferation marker PCNA-positive cells (Fig. 2C, E). These data suggest that Ex-4 decreased proliferating VSMCs in the neointima after vascular injury, which was associated with the reduction of NOR1 expression in vivo.
Fig. 2.
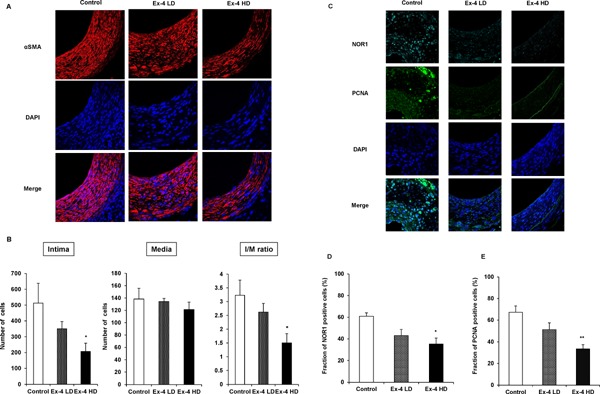
Ex-4 suppresses cell growth after vascular injury and expression of NOR1 and PCNA in VSMCs.
(A) Sections were subjected to immunohistochemistry for αSMA (red) and counterstained with DAPI to visualize nuclei (blue). Magnification, ×630. (B) Total cell count of the intima and media. One-way ANOVA was performed to calculate statistical significance. Data are mean ± SEM. *P < 0.05 vs. control (C) Representative immunofluorescence image of injured femoral arteries showing NOR1 (cyan) and PCNA (green). Magnification, ×630. (D) (E) NOR1- and PCNA-positive cells were quantified by analyzing the fraction of stained cells to the total number of nuclei (Control, n = 7; Ex-4 LD, n = 8; and Ex-4 HD, n = 6). Values are expressed as the percentage of positive cells. One-way ANOVA was performed to calculate statistical significance. *P < 0.05 vs. control, **P < 0.01 vs. control.
Exendin-4 Decreases Serum-Induced NOR1 Expression Via GLP-1R
Next, we examined whether Ex-4 inhibited NOR1 expression in vitro using primary cultured VSMCs. Compatible with our previous reports using rat17) and mouse18) VSMCs, NOR1 expression was also negligible in serum-deprived quiescent human VSMCs, and serum stimulation dramatically and promptly induced NOR1 protein (Fig. 3A) and mRNA (Fig. 3B) expression. Interestingly, after pretreatment with Ex-4, serum-induced NOR1 expression was significantly decreased in a dose-dependent manner (Fig. 3A, B). To elucidate whether Ex-4 decreased NOR1 expression by inhibiting NOR1 promoter activity, we next conducted a luciferase assay using NOR1 promoter-Luc construct transfected into rat VSMCs. As shown in Fig. 3C shows, serum-induced NOR1 promoter activity was significantly reduced by 10 nM Ex-4 pretreatment, suggesting that Ex-4 decreased NOR1 expression by inhibiting NOR1 promoter activity. However, the canonical pathway of GLP-1R is mediated via cAMP-protein kinase A (PKA) activation. As shown in Fig. 3D, co-incubation with GLP-1R antagonist Exendin 9–39 (Ex9–39) and PKA inhibitor PKI cancelled the reduction of NOR1 gene expression by Ex-4. These data suggest that Ex-4 decreased serum-induced NOR1 promoter activation and gene expression via GLP-1R activation and its downstream canonical signaling pathway.
Fig. 3.
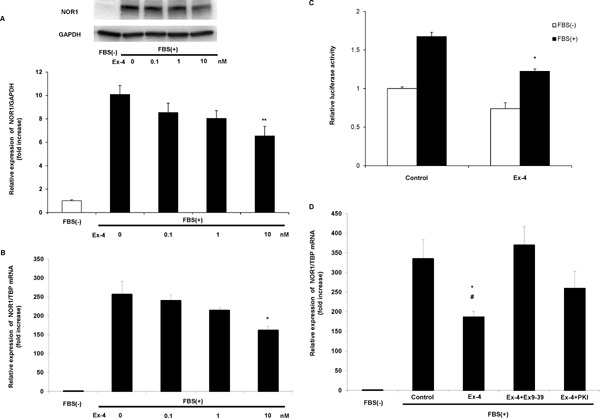
FBS-induced NOR1 expression is suppressed by Ex-4 pretreatment in vitro.
(A) Serum-deprived human aortic SMCs were stimulated with 10% FBS with 0–10 nM Ex-4 pretreatment. NOR1 protein expression was analyzed at 6 h after FBS stimulation by western blotting. (B) FBS-induced NOR1 mRNA expression was analyzed at 2 h after FBS stimulation with 0–10 nM Ex-4 pretreatment by qRT-PCR. One-way ANOVA was performed to calculate statistical significance. Data are mean ± SEM. *P < 0.05 vs. FBS(+) 0 nM Ex-4, **P < 0.01 vs. FBS(+) 0 nM Ex-4. (C) Rat aortic SMCs were transiently transfected with 1.7 kb NOR1 promoter constructs. Serum-deprived cells were pretreated with or without 10 nM Ex-4 and then stimulated with 10% FBS for 12 h. Following stimulation, cells were harvested and luciferase activities were analyzed. Statistical significance was calculated using unpaired t -tests. Data are mean ± SEM. *P < 0.05 vs. Control FBS(+). (D) Serum-deprived SMCs were treated with 10 nM Ex-4 or PBS and pretreated for 30 min with or without 100 nM Exendin (9–39) or 10 µM PKI and subsequently stimulated with FBS at a final concentration of 10%. After 2 h of stimulation, NOR1 mRNA expression was determined. One-way ANOVA was performed to calculate statistical significance. Data are mean ± SEM. *P < 0.05 vs. FBS(+) control, #P < 0.05 vs. FBS(+) Exendin (9–39).
Exendin-4 Inhibits Serum-Induced ERK-MAPK and CREB Phosphorylations
In our previous report, mitogenic stimulation induced NOR1 expression via ERK-MAPK and CREB phosphorylations17). Next, we examined the effect of Ex-4 on ERK-MAPK and CREB phosphorylations in human VSMCs by western blotting. As shown in Fig. 4A, pretreatment with 10 nM Ex-4 significantly decreased serum-induced phosphorylation of ERKMAPK. Furthermore, serum-induced CREB phosphorylation was decreased by Ex-4 pretreatment (Fig. 4B). These data suggest that Ex-4 decreased NOR1 expression via the inhibition of ERK-MAPK-CREB signals in VSMCs. In addition, we examined other VSMC growth signals, protein kinase B (Akt) and mammalian target of rapamycin (mTOR). As shown in Fig. 4C, Ex-4 did not attenuate serum-induced Akt phosphorylation. However, small but significant attenuation of serum-induced mTOR phosphorylation was observed after Ex-4 treatment (Fig. 4C).
Fig. 4.
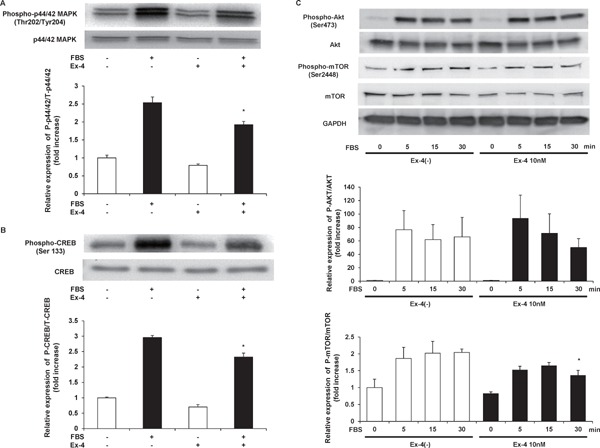
Ex-4 inhibits FBS-induced phosphorylations of p44/42 MAPK, CREB, and mTOR.
Serum-deprived human aortic SMCs were preincubated with vehicle or 10 nM Ex-4 for 12 h and stimulated with 10% FBS for 15 min. Whole cell lysates were analyzed for phosphorylated p44/42 MAPK (A) and Ser-133-phosphorylated CREB (B). Cohybridization for total p44/42 MAPK and total CREB was used as control. Statistical significance was calculated using unpaired t-tests. Data are mean ± SEM. *P < 0.05 vs. FBS(+) Ex-4(−). (C) Serum-deprived human aortic SMCs were preincubated with vehicle or 10 nM Ex-4 for 12 h and stimulated with 10% FBS for the indicated times. Whole cell lysates were analyzed for phosphorylated Akt and mTOR. Two-way ANOVA was performed to calculate statistical significance. Data are mean ± SEM. *P < 0.05 vs. FBS(+) 30 min Ex-4(−).
Exendin-4 Attenuates Cell Cycle Progression in VSMCs
Because NOR1 regulates VSMC proliferation by accelerating G1–S phase during cell cycle progression, as we reported previously18), we next examined whether Ex-4 attenuates cell cycle progression in human VSMCs. As shown in Fig. 5, G1–S phase and G2/M phase were induced by serum stimulation in human VSMCs. Furthermore, 10 nM Ex-4 pretreatment significantly attenuated cell cycle progression induced by serum (Table 3), suggesting that Ex-4 inhibits VSMC proliferation by attenuating cell cycle progression. Next, we examined whether Ex-4 regulated cell cycle regulators. As shown in Fig. 4C, serum-induced degradation of p27 Kip, a major negative regulator of G1–S phase entry, was significantly inhibited by Ex-4 treatment. In addition, S-phase kinase-associated protein 2 (Skp2), which is the ubiquitin ligase of p27 Kip, was induced by serum stimulation and resulted in p27 kip degradation. Interestingly, Ex-4 significantly decreased Skp2 gene expression after serum stimulation (Fig. 5D).
Fig. 5.
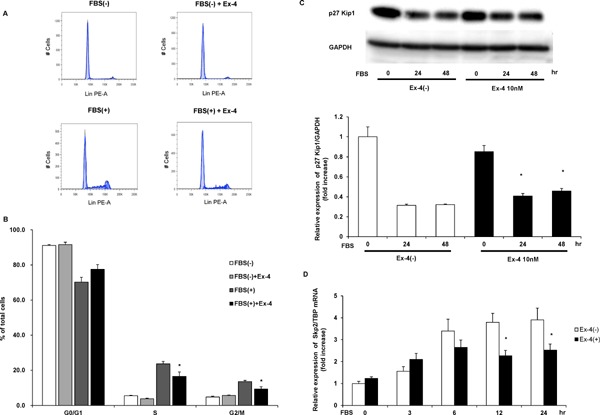
Effect of Ex-4 on FBS-induced cell cycle progression in human aortic SMCs evaluated by flow cytometry.
Serum-deprived human aortic SMCs were preincubated with vehicle or 10 nM Ex-4 for 12 h and stimulated with 10% FBS for 30 h. (A) Representative cell cycle distribution. (B) Values are expressed as the percentage of cells in each phase to the total cells. Statistical significance was calculated using unpaired t-tests. Data are mean ± SEM. *P < 0.05 vs. FBS(+) Ex-4(−). (C) Serum-deprived human aortic SMCs were preincubated with vehicle or 10 nM Ex-4 for 12 h and stimulated with 10% FBS for the indicated times. p27 Kip1 protein expression was analyzed by western blotting. Two-way ANOVA was performed to calculate statistical significance. Data are mean ± SEM. *P < 0.05 vs. FBS(+) Ex-4(−) (D) FBS-induced Skp2 mRNA expression was analyzed at the indicated times after FBS stimulation with vehicle or 10 nM Ex-4 pretreatment by qRT-PCR. Two-way ANOVA was performed to calculate statistical significance. Data are mean ± SEM. *P < 0.05 vs. FBS(+) Ex-4(−)
Table 3. Cell cycle distribution of human vascular smooth muscle cells with or without exendin-4 (Ex-4) treatment.
| Control | Ex-4 | Control | Ex-4 | |
|---|---|---|---|---|
| FBS(−) | FBS(−) | FBS(+) | FBS(+) | |
| G0/G1 (%) | 91.2 ± 0.5 | 91.6 ± 1.3 | 70.3 ± 2.8 | 77.5 ± 2.7 |
| S (%) | 5.6 ± 0.3 | 3.9 ± 0.2 | 23.7 ± 1.4 | 16.5 ± 2.5* |
| G2/M (%) | 4.9 ± 0.5 | 5.8 ± 0.2 | 13.5 ± 0.7 | 9.4 ± 1.3* |
One-way ANOVA was performed to calculate statistical significance. Data are expressed as the mean ± SEM.
FBS, fetal bovine serum.
P < 0.05 compared with the Control FBS(+).
Exendin-4 Attenuates Neointima Formation After Vascular Injury in Diabetic Mice
Because Ex-4 is used as a medication for patients with type 2 diabetes mellitus (T2DM), we next examined whether Ex-4 decreased neointima formation after vascular injury in mice fed high-fat diet, a model for insulin-resistant T2DM. As depicted in Fig. 6A, elastic staining revealed the reduction of neointima formation after vascular injury. Quantitative analysis by measuring squares of the intima, media, and I/M ratio suggested that Ex-4 reduced neointima formation after vascular injury in a dose-dependent manner (Fig. 6B). However, statistical significance was not observed (Table 4). As shown in Table 5, body weight and the plasma glucose level were significantly decreased by Ex-4 in a dose-dependent manner, suggesting that Ex-4 affected glucose metabolism.
Fig. 6.
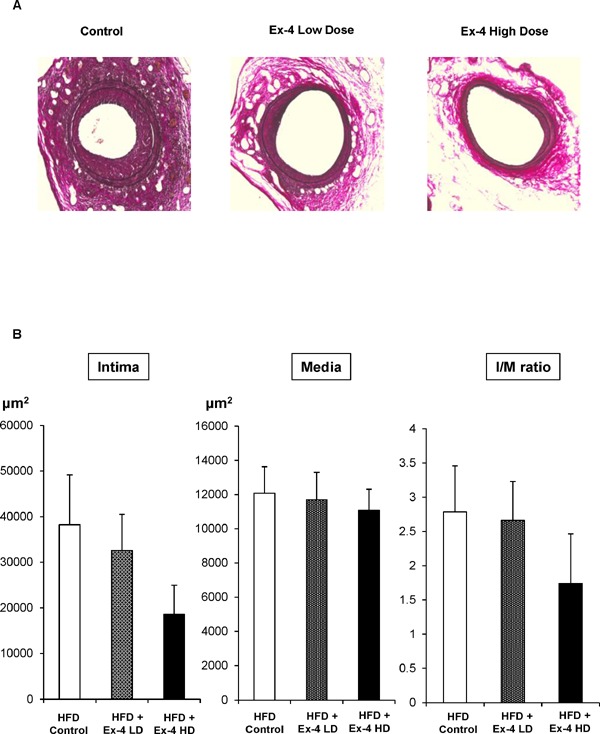
Neointima formation after vascular injury in control and Ex-4-treated mice fed high-fat diet.
Endothelial denudation injuries were induced in the left femoral artery of control (n = 5) mice, Ex-4 low-dose (n = 5)-treated mice, and Ex-4 high-dose (n = 5)-treated mice. (A) Tissues were evaluated by staining with Elastica van Gieson to visualize the internal elastic lamina (magnification, ×200). (B) The area of the intima, media, and intima/media was calculated for each group. One-way ANOVA was performed to calculate statistical significance. Data are mean ± SEM.
Table 4. Neointima formation in exendin-4 (Ex-4)-treated mice fed a high-fat diet at 12 weeks of age following guidewire-induced endothelial denudation injury.
| HFD | HFD + Ex-4 | HFD + Ex-4 | |
|---|---|---|---|
| Control | Low dose | High dose | |
| Intima (µm2) | 38212.6 ± 10921.1 | 32594.0 ± 7907.7 (P = 0.91) |
18532.2 ± 6446.3 (P = 0.35) |
| Media (µm2) | 12079.2 ± 1549.1 | 11684.0 ± 1615.7 (P = 0.90) |
11072.0 ± 1235.9 (P = 0.96) |
| I/M ratio | 2.78 ± 0.67 | 2.66 ± 0.57 (P = 0.99) |
2.66 ± 0.57 (P = 0.99) |
Data are expressed as the mean ± SEM.
P-values refer to comparisons with the control.
Table 5. Characteristics of exendin-4 (Ex-4)-treated mice fed a high-fat diet at 12 weeks of age following guidewire-induced endothelial denudation injury.
| HFD | HFD + Ex-4 | HFD + Ex-4 | |
|---|---|---|---|
| Control | Low dose | High dose | |
| Body weight (g) | 29.1 ± 0.4 | 28.9 ± 0.5 | 25.7 ± 0.8*, # |
| Plasma glucose (mg/dl) | 158.2 ± 4.2 | 126.4 ± 12.3 | 119.4 ± 4.2* |
| Serum insulin (ng/ml) | 1.07 ± 0.21 | 0.60 ± 0.14 | 0.56 ± 0.09 |
One-way ANOVA was performed to calculate statistical significance. Data are expressed as the mean ± SEM.
P < 0.05 compared with the control.
P < 0.05 compared with low-dose Ex-4.
Following experiments using non-diabetic normal mice, we counted cells in the neointima and media areas. As shown in Fig. 7A shows, the neointima was completely occupied by VSMCs, and the cell number in the neointima and I/M ratio were decreased dose-dependently, although statistical significance was not observed. For further investigation, we performed immunohistochemistry of NOR1 and PCNA. As shown in Fig. 7C, Ex-4 decreased NOR1 expression and PCNA-positive proliferating cells. Furthermore, cell counting revealed that the Ex-4-induced reductions of NOR1 expression and PCNA-positive proliferating cells were significant and dose dependent (Fig. 7D, E). These data suggested that Ex-4 decreased NOR1 expression and VSMC proliferation in vivo, even in diabetic model mice.
Fig. 7.
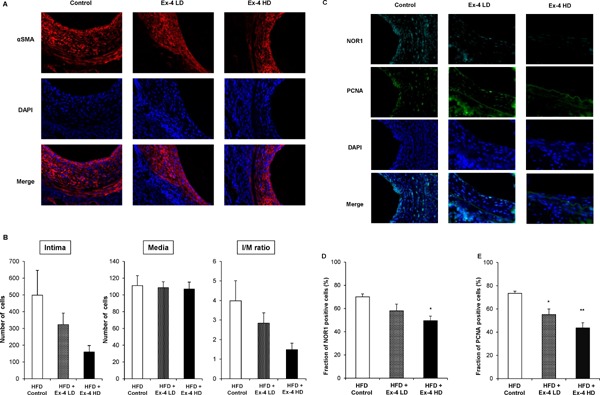
Ex-4 suppresses cell growth after vascular injury and expression of NOR1 and PCNA in VSMCs under high-fat diet feeding.
(A) Sections were subjected to immunohistochemistry for αSMA (red) and counterstained with DAPI to visualize nuclei (blue). Magnification, ×630. (B) Total cell count of the intima and media. One-way ANOVA was performed to calculate statistical significance. Data are mean ± SEM. (C) Representative immunofluorescence image of injured femoral arteries showing NOR1 (cyan) and PCNA (green). Magnification, ×630. (D) (E) NOR1- and PCNA-positive cells were quantified by analyzing the fraction of stained cells to the total number of nuclei (Control, n = 5; Ex-4 LD, n = 5; and Ex-4 HD, n = 5). Values are expressed as the percentage of positive cells. One-way ANOVA was performed to calculate statistical significance. *P < 0.05 vs. control, **P < 0.01 vs. control.
Discussion
The present study suggested that GLP-1R agonist Ex-4 attenuates neointima formation after vascular injury and VSMC proliferation associated with the reduction of NOR1 expression in both diabetic and non-diabetic model mice. Furthermore, the molecular mechanism by which Ex-4 attenuates VSMC proliferation was the reduction of serum-induced Skp2 gene expression, resulting in inhibition of cell cycle progression. As we reported previously7, 8), the direct vascular-protective effect of incretin has been a recent focus. Reports from other groups confirmed the hypothesis that incretins, particularly GLP-1, may have direct anti-atherosclerotic and vascular-protective effects independent of their glucose-lowering effect20, 21). Furthermore, current evidence based on large scale clinical trials in patients with type 2 diabetes using GLP-1R agonists suggest vascular-protective effects of GLP-1 in animal models as well as patients with type 2 diabetes22, 23). VSMC proliferation contributes to not only primary atherosclerotic lesions but also vascular stenosis after vascular injury and coronary angioplasty. In addition, several pathological backgrounds in the diabetic state may accelerate VSMC pathogenesis directly and indirectly24). Therefore, the inhibition of smooth muscle cell proliferation by anti-diabetic agents could be beneficial to patients with type 2 diabetes. Among vascular cells, VSMCs abundantly express GLP-1R8, 21) and important targets for vascular-protective effects by GLP-1. A GLP-1R agonist attenuates neointima formation after vascular injury8, 21), which has a direct effect on VSMCs but not the activation of re-endothelialization after endothelial denudation25), suggesting that the vascular-protective effect of GLP-1 mainly occurs in VSMCs. Some mechanisms by which GLP-1 attenuates VSMC pathogenesis have been reported, such as inhibition of RAS-related C3 botulinus toxin substrate 1 (Rac1) activation26) and enhancement of endoplasmic reticulum-mitochondria coupling27). Furthermore, some data suggest that adiponectin is a molecular target through which incretin protects against vascular diseases28, 29). In addition to these mechanisms, we provide a novel mechanism by which GLP-1 attenuates NOR1 expression in VSMCs in the present study.
NOR1 is a well-elucidated orphan nuclear receptor that regulates VSMC proliferation15). A recent study suggested that NOR1 expression in VSMCs is regulated via epigenetic modification30) and microRNA-638 expression31). Furthermore, NOR1 phosphorylation by DNA-dependent protein kinase reportedly modulates VSMC proliferation32). Despite several known regulation mechanisms of NOR1 expression and its activation, there has been no report suggesting NOR1 regulation in VSMCs by clinical medications, such as antihypertensive drugs, anti-diabetic agents, and anticoagulants. This is the first report suggesting that an antidiabetic agent and a GLP-1R agonist, attenuates NOR1 expression in VSMCs. In our previous reports using mouse VSMCs, we investigated two main targets of NOR1 that accelerate VSMC proliferation: Cyclin D118) and Skp219). In the present study using human VSMCs, Cyclin D1 expression was not changed by Ex-4-induced NOR1 reduction (data not shown). However, Skp2 gene expression was significantly decreased (Fig. 5D), resulting in the upregulation of p27 Kip protein expression (Fig. 5C) and attenuation of cell cycle progression (Fig. 5A, B). These data suggest that the main target of NOR1 related to cell cycle progression is Skp2 in human VSMCs.
In the present study, we found that Ex-4 inhibited serum-induced ERK-MAPK phosphorylation (Fig. 4A). Interestingly, we previously reported the same Ex-4 effect in the prostate cancer cell line LNCaP9). Both VSMCs and cancer cells are pathologically proliferating and growing cells. Ex-4 may regulate pathological cell proliferation and growth as a tissue-protective effect. In the present study, we investigated Ex-4 actions in VSMCs. However, the main clinical target of Ex-4 as an incretin therapy for patients with type 2 diabetes is pancreatic β cells to stimulate glucose-dependent insulin secretion33). Interestingly, a report has suggested an interaction between incretin and NOR1 in pancreatic β cells34). Therefore, further elucidation of the interaction between incretin and NOR1 is required.
In the present study, all our experiments were conducted using Ex-4 as a GLP-1R agonist, which is clinically available as Exenatide. Although the present study and our previous studies7, 8) demonstrated the vascular-protective effect of Ex-4, a recent large scale randomized control trial using Exenatide, the EXSCEL study, could not demonstrate significant reduction compared with the placebo control35), although other GLP-1R agonists did improve cardiovascular outcome22, 23). The EXSCEL study has a shorter study period and higher baseline HbA1c than the LEADER trial using liraglutide22) (3.8 years vs. 3.2 years and 8.0% vs. 8.7%, respectively). In addition, sulfonylureas and insulin use were profoundly increased in the placebo group of the LEADER trial, although GLP-R agonists, sodium-glucose cotransporter 2 inhibitors, and DPP-4 inhibitors use were increased in the placebo group of the EXSCEL trial35). These clinical differences may contribute to the different cardiovascular outcomes between the LEADER trial and EXSCEL study. In fact, the low dose of Ex-4 in the present study is the pharmacological dose, as discussed in our previous reports7, 9, 36), suggesting that some beneficial clinical cardiovascular effect could be expected from the present study. However, different individual effects on cardiovascular systems by GLP-1R agonists are also suggested37). Further clinical trials and basic experiments are required.
Conclusion
Ex-4 attenuates neointima formation after vascular injury and VSMC proliferation possibly by inhibiting NOR1 and Skp2 expression. In addition, Ex-4 attenuates ERK-MAPK and CREB phosphorylations as well as G1–S phase entry during cell cycle progression in VSMCs. The reduction of NOR1 expression could be key in preventing cardiovascular events and atherosclerosis in patients with type 2 diabetes using incretin therapy.
Acknowledgements
We thank Dr. Naganari Ohkura (Osaka University, Osaka, Japan) for providing NOR1 promoter constructs. We also thank Mitchell Arico from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
List of Abbreviations
Akt, Protein kinase B; CREB, cAMP-responsive element-binding protein; DPP-4, dipeptidyl peptidase-4; ERK-MAPK, extracellular signal-regulated kinase-mitogen-activated protein kinase; Ex-4, exendin-4; Ex9-39, Exendin 9-39; GIP, glucose-dependent insulin-otropic polypeptide; GLP-1, glucagon-like peptide-1; GLP-1R, glucagon-like peptide-1 receptor; I/M, intima-media; NOR1, neuron-derived orphan receptor 1; PKA, protein kinase A; PPAR, peroxisome proliferator-activated receptor; LXR, liver X receptor; VSMCs, vascular smooth muscle cells; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; Skp2, S-phase kinase-associated protein 2.
Competing Interests
The present study was supported by a grant from Novartis Pharma K.K. and MSD K.K. to TN. The other authors have no competing interests.
Authors' Contributions
HT performed experiments and wrote the manuscript; TN performed experiments, wrote the manuscript, and conceived the research hypothesis; TK and YH performed the experiments; YT, TT, MT, and DB reviewed and edited the manuscript and assisted in patient recruitment; and TY assisted in the conception of the research hypothesis and reviewed and edited the manuscript. All authors have read and approved the final manuscript. TN is the guarantor of this work, has full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of data analysis.
References
- 1). Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M: Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Eng J Med 1998, 339: 229-234 [DOI] [PubMed] [Google Scholar]
- 2). Scheen AJ, Warzee F: Diabetes is still a risk factor for restenosis after drug-eluting stent in coronary arteries. Diabetes Care 2004, 27: 1840-1841 [DOI] [PubMed] [Google Scholar]
- 3). Pratley Richard E., Gilbert Matthew: Targeting Incretins in Type 2 Diabetes: Role of GLP-1 Receptor Agonists and DPP-4 Inhibitors. Rev Diabet Stud 2008, 5: 73-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Pujadas G, Drucker DJ: Vascular biology of glucagon receptor superfamily peptide: Mechanistic and clinical relevance. Endocr Rev 2016, 37: 554-583 [DOI] [PubMed] [Google Scholar]
- 5). Ding X, Saxena NK, Lin S, Gupta NA, Anania FA: Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonists, reverses hepatic steatosis in ob/ob micec. Hepatology 2006, 43: 173-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG: An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease-associated Aβ oligomers. J Clin Invest 2012, 122: 1339-1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R, Watada H: Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes 2010, 59: 1030-1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Goto H, Nomiyama T, Mita T, Yasunari E, Azuma K, Komiya K, Arakawa M, Jin WL, Kanazawa A, Kawamori R, Fujitani Y, Hirose T, Watada T: Exendin-4, a glucagonlike peptide-1 receptor agonist, reduces intimal thickening after vascular injury. Biochem Biophys Res Commun 2011, 405: 79-84 [DOI] [PubMed] [Google Scholar]
- 9). Nomiyama T, Kawanami T, Irie S, Hamaguchi Y, Terawaki Y, Murase K, Tsutsumi Y, Nagaishi R, Tanabe M, Hidetaka Morinaga, Tanaka T, Mizoguchi M, Nabeshima K, Tanaka M, Yanase T: Exendin-4, a glicagon-like peptide-1 receptor agonist, attenuates prostate cancer growth. Diabetes 2014, 63: 3891-3905 [DOI] [PubMed] [Google Scholar]
- 10). Tsutsumi Y, Nomiyama T, Kawanami T, Hamaguchi Y, Terawaki Y, Tanaka T, Murase K, Motonaga R, Tanabe M, Yanase T, Combined treatment with Exendin-4 and metformin attenuates prostate cancer growth. PLoS ONE 2015, 10: e0139709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Terawaki Y, Nomiyama T, Kawanami T, Hamaguchi Y, Takahashi H, Tanaka T, Murase K, Nagaishi R, Tanabe M, Yanase T. Dipeptidyl peptidase-4 inhibitor linagliptin attenuates neointima formation after vascular injury. Cardiovasc Diabetol 2014, 13: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Kurakura K, Hamers AA, deWaard V, de Vries CJ. Nuclear receptors in atherosclerosis: a superfamily with many ‘Goodfellas’. Mol Cell Endocrinol 2013, 368: 71-84 [DOI] [PubMed] [Google Scholar]
- 13). Cheang WS, Tian XY, Wong WT, Huang Y. The peroxisome proliferator-activated receptorors in cardiovascular diseases: experimental benefits and clinical challenges. Br J Pharmacol 2015, 172: 5512-5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Lee SD, Tontonoz P. Liver X receptors at the intersection of lipid metabolism and atherogenesis. Atherosclerosis 2015, 242: 29-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Zhao Y, Bruemmer D. NR4A orphan nuclear receptors: transcriptional regulators of gene expression in metabolism and vascular biology. Arterioscler Thromb Vasc Biol 2010, 30: 1535-1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Ohkura N, Hijikuro M, Yamamoto A, Miki K: Molecular cloning of a novel thyroid/steroid receptor superfamily gene from cultured rat neuronal cells. Biocem Biophys Res Commun 1994, 205: 1959-1965 [DOI] [PubMed] [Google Scholar]
- 17). Nomiyama T, Nakamachi T, Gizard F, Heywood EB, Jones KL, Ohkura N, Kawamori R, Conneely OM, Bruemmer D: The NR4A orphan nuclear receptor NOR1 is induced by platelet-derived growth factor and mediates vascular smooth muscle cell proliferation. J Biol Chem 2006, 281: 33467-33476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Nomiyama T, Zhao Y, Gizard F, Findeisen HM, Heywood EB, Jones KL, Conneely OM, Bruemmer D: Deficiency of the NR4A neuron-derived orphan receptor-1 attenuates neointima formation after vascular injury. Circulation 2009, 119: 577-586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Gizard F, Zhao Y, Findeisen HM, Qing H, Cohn D, Heywood EB, Jones KL, Nomiyama T, Bruemmer D: Transcriptional regulation of S phase kinase-associated protein 2 by NR4A orphan nuclear receptor NOR1 in vascular smooth muscle cells. J Biol Chem 2011, 286: 35485-35493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Nagashima M, Watanabe T, Terasaki M, Tomoyasu M, Nohtomi K, Kim-Kaneyama J, Miyazaki A, Hirano T: Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia 2011, 54: 2649-2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Hirata Y, Kurobe H, Nishio C, Tanaka K, Fukuda D, Uematsu E, Nishimoto S, Soeki T, Harada N, Sakaue H, Kitagawa T, Shimabukuro M, Nakaya Y, Sata M: Exendin-4, a glucagon-like peptide-1 receptor agonist, attenuates neointimal hyperplasia after vascular injury. Eur J Pharmacol 2013, 699: 106-111 [DOI] [PubMed] [Google Scholar]
- 22). Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Bise JB, LEADER Steering Committee; LEADER Trial Investigators : Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016, 375: 311-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Petterson J, Visvoll T, SUSTAIN-6 Investigators : Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016, 375: 1834-1844 [DOI] [PubMed] [Google Scholar]
- 24). Bruemmer D: C-peptide in insulinresistance and vascular complications: teaching an old dog new tricks. Circ Res 2006, 99: 1149-1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Eriksson L, Saxelin R, Rohl S, Roy J, Caidahl K, Nystrom T, Hedin U, Razuvaev A: Glucagon-like peptide-1 receptor activation does not affect re-endothelialization bur reduces intimal hyperplasia via direct effects on smooth muscle cells in a nondiabetic model of artery injury. J Vas Res 2015, 52: 41-52 [DOI] [PubMed] [Google Scholar]
- 26). Zhao L, Li AQ, Zhou TF, Zhang MQ, Qin XM: Exendin-4 alleviates angiotensin II-induced senescence in vascular smooth muscle cells by inhibiting Rac1 activation via a cAMP/PKA-dependent pathway. Am J Physiol Cell Physiol 2014, 307: C1130-C1141 [DOI] [PubMed] [Google Scholar]
- 27). Morales PE, Torres G, Sotomayor-Flores C, Pena-Oyarzun D, Pivera-Mejias P, Paredes F, Chiong M: GLP-1 Promotes mitochondrial metabolism in vascular smooth muscle cells by enhancing endoplastic reticulum-mitochondria cpoupling. Biochem Biophys Res Commun 2014, 446: 410-416 [DOI] [PubMed] [Google Scholar]
- 28). Yang G, Lei Y, Inoue A, Piao L, Hu L, Jiang H, Sasaki T, Wu H, Xu W, Yu C, Zhao G, Ogasawara S, Okumura K, Kuzuya M, Cheng XW: Exenatide mitigated diet-induced vascular aging and atherosclerotic plaque growth in ApoEdeficient mice under chronic stress. Atherosclerosis 2017, 264: 1-10 [DOI] [PubMed] [Google Scholar]
- 29). Piao L, Zhao G, Zhu E, Inoue A, Shibata R, Lei Y, Hu L, Yu C, Yang G, Wu H, Xu W, Okumura K, Ouchi N, Murohara T, Kuzuya M, Chen XW: Chronic phychological stress accerelates vascular senescence and impairs ischemia-induced neovascularization: the role of dipeptidyl peptidase-4/glucagon-loke peptide-1-adiponectin axis. J Am Heart Assoc 2017, 6: e006421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Zhao Y, Nomiyama T, Findeinsen HM, Qing H, Aono J, Jones KL, Heywood EB, Bruemmer D: Epigenetic regulation of the NR4A orphan nuclear receptor NOR1 by histon acetylation. FEBS Lett 2014, 588: 4825-4830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Li P, Liu Y, Yi B, Wang G, You X, Zhao X, Summer R, Qin Y, Sun J: MicroRNA-638 is highly expressed in human vascular smooth muscle cells and inhibits PDGFBB-induced cell proliferation and migration through targeting orphan nuclear receptor NOR1. Crdiovasc Res 2013, 99: 185-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Medunjanin S, Danisl JM, Weinert S, Dutzmann J, Burgbacher F, Brecht S, Bruemmer D, Kahne T, Naumann M, Sedding DG, Zuschratter W, Braun-Dullaeus RC: DNA-dependent protein kinase (DNA-PK) permits vascular smooth muscle cell proliferation through phosphorylation of the orphan nuclear receptor NOR1. Cardiovasc Res 2015, 106: 488-497 [DOI] [PubMed] [Google Scholar]
- 33). Meier JJ: GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012, 8: 728-742 [DOI] [PubMed] [Google Scholar]
- 34). Ordelheide AM, Gerst F, Rothfuss O, Heni M, Haas C, Thielker I, Herzberg-Schafer S, Bohm A, Machicao F, Ullrich S, Stefan N, Fritsche A, Haring HU, Staiger H: Nor-1, a novel incretin-responsive regulator of insulin genes and insulin secretion. Mol Metab 2013, 2: 243-255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygia Y, Buse JB, Chan JC, Choi J, Gustavson SM, Igbal N, Maggioni AP, Marso SP, Ohman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hemandez AF, EXSCEL Study Group : Effect of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017, 377: 1228-1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Iwaya C, Nomiyama T, Komatsu S, Kawanami T, Tsutsumi Y, Hamaguchi Y, Horikawa T, Yoshinaga Y, Yamashita S, Tanaka T, Terawaki Y, Tanaba M, Nabeshima K, Iwasaki A, Yanase T: Exendin-4, a glucagonlike peptide-1 receptor agonist, attenuates breast cancer growth by inhibiting NF-κB activation. Endocrinology 2017, 158: 4218-4232 [DOI] [PubMed] [Google Scholar]
- 37). Lim S, Kim KM, Nauck MA. Glucagon-like peptide-1 receptor agonists and cardiovascular events: class effects versus individual patterns. Trends Endocrinol Metab 2018, 29: 238-248 [DOI] [PubMed] [Google Scholar]


