Abstract
INTRODUCTION:
The National Healthcare Safety Network (NHSN) Hemovigilance Module (HM) collects data on the frequency, severity, and imputability of transfusion-associated adverse events. These events contribute to significant morbidity and mortality among transfusion patients. We report results from the first systematic assessment of eight attributes of the HM.
MATERIALS AND METHODS:
Standard methods were used to assess the HM. Evaluation data included training materials, system modification history, and facility survey information. A concordance analysis was performed using data from the Baystate Medical Center’s (Boston, MA) electronic transfusion reporting system.
RESULTS:
In 2016, system representativeness remained low, with 6% (277 of 4690) of acute care facilities across 43 jurisdictions enrolled in the HM. In 2016, 48% (2147 of 4453) and 89% (3969 of 4,453) of adverse reactions were reported within 30 and 90 days of the reaction date, respectively, compared to 21% (109 of 511) and 56% (284 of 511) in 2010, demonstrating improved reporting timeliness. Data quality from most reactions was adequate, with 10% (45 of 442) misclassified transfusion-associated circulatory overload reactions, and no incomplete transfusion-transmitted infection data reported from 2010 to 2013. When compared to the Baystate system to assess concordance, 43% (24 of 56) of NHSN-reported febrile reactions were captured in both systems (unweighted kappa value, 0.47; confidence interval, 0.33–0.61).
CONCLUSION:
Since the 2010 HM pilot, improvements have led to enhanced simplicity, timeliness, and strengthened data quality. The HM serves an important and unique role despite incomplete adoption nationwide. Facility efforts to track and prevent transfusion-associated adverse events through systems like the NHSN HM are a key step toward improving transfusion safety in the United States.
More than 17 million blood products are transfused to patients annually in the United States.1 Though rare, some transfusions result in adverse reactions that may be life threatening or fatal. Systems to monitor these reactions have been implemented in numerous other countries.2–6 Data collected from these hemovigilance systems have previously helped identify shortcomings in blood safety for which interventions were needed to improve transfusion safety, including efforts to prevent bacterial contamination of blood products and methods to reduce the occurrence of transfusion-associated lung injury.4,7
In 2010, the Centers for Disease Control and Prevention (CDC) began operating the National Healthcare Safety Network (NHSN) Hemovigilance Module (HM) as part of a broad-based, public-private initiative to improve transfusion safety.8–13 The NHSN HM is available for use by US health care facilities where blood components and manufactured blood products are transfused. Briefly, the NHSN HM is a voluntary, passive surveillance system that collects transfusion-related adverse reaction data from participating facilities. The system is used to estimate the frequency of transfusion-related adverse reactions and transfusion-related incidents (i.e., process errors) that result in reactions, monitor trends, and take advantage of the demonstrated benefits of hemovigilance. Participation in the system grew incrementally from 82 facilities in 2010 to 277 facilities in 2016 of the estimated 4,000 acute care facilities in the United States.1 Requirements for reporting transfusion-associated adverse events (TAAEs) varies among states. In Massachusetts, adverse event reporting has been compulsory for all hospital blood banks and transfusion services since 1970.12 In 2014, facilities were required to enroll in, and electronically report, transfusion-related adverse reactions using the HM, replacing the previous paper-based reporting system.14
Public health surveillance systems require regular evaluation to ensure that relevant needs are met and that issues of public health importance are monitored efficiently and effectively.15 Additionally, state-level reporting mandates may affect the quality of the data collected by national surveillance systems. Here, we report results from a collaborative effort among the CDC, the Massachusetts Department of Public Health, and one Massachusetts hospital to examine and evaluate the NHSN HM using previously published CDC guidelines for the evaluation of public health surveillance systems.15
METHODS
Study data
NHSN HM and Baystate Medical Center (BMC) (Springfield, MA) clinical data were used for this study. This medical center has an inpatient capacity of approximately 750 beds, issues approximately 17,000 blood product units, and evaluates approximately 180 suspected transfusion reactions annually. NHSN HM data collected from July 2012 to December 2016 were used to evaluate system attributes. Details of NHSN HM reporting and data collection have been previously described.9 Briefly, facilities conduct monthly surveillance according to the NHSN HM protocol, which includes adverse transfusion reaction case classification criteria, reporting requirements, documents and forms, and reporting timeline. The protocol outlines surveillance criteria for 12 transfusion-related adverse reactions, including allergic, febrile nonhemolytic, acute and delayed hemolytic, delayed serologic, hypotensive, circulatory overload, transfusion-associated acute lung injury, dyspnea, graft-versus-host disease, posttransfusion purpura, transfusion-transmitted infection, and incidents that result in a transfusion-related adverse reaction. The protocol includes multitiered designations for case definition, severity, and imputability (i.e., likelihood that the transfusion caused the reaction) for each reaction.
The total number of transfused units is reported monthly and used to generate rates of transfusion-related adverse reactions. Facility characteristics, including location, bed size, and transfusion services, are reported annually and used to describe facilities that reported reaction and transfusion data. Static data sets are created monthly and include a snapshot of NHSN HM data at that time. Data sets created on July 1, 2017, were used for the analysis of accessibility, representativeness, and timeliness. Data sets generated on or before May 26, 2016, were used for the concordance analysis.
We assessed seven surveillance system attributes, including usefulness, simplicity, flexibility, data quality, acceptability, representativeness, and timeliness. The definitions for these terms for public health surveillance purposes have been previously defined.15 While guidelines for public health surveillance system evaluation recommend assessment of sensitivity, we attempted to assess similar metrics by calculating concordance with another facility-based transfusion reaction recognition system.15 Data for this report were collected for surveillance and program evaluation purposes and were determined not to require Institutional Review Board approval by the CDC Office of the Associate Director of Science and the Institutional Review Board at Baystate Medical Center. Data from these monthly downloads were made available to the CDC for analysis in a deidentified, Health Insurance Portability and Accountability Act–compliant format according to a BMC Institutional Review Board non-research determination approved data sharing quality improvement protocol (BH-15–231).
Usefulness
The number of publications, abstracts, and presentations that present HM data by the CDC, state health department, or other authors and the number of publications that have cited these works were counted to assess system usefulness.
Simplicity
Time estimates for collecting and reporting required data and completing user training were calculated to evaluate simplicity.
Flexibility
A review of system changes in response to routine requests and public health emergencies over the study period was used to assess flexibility.
Data quality
Transfusion-associated circulatory overload reports were reviewed for misclassification of case definition designations to assess protocol compliance. Records of transfusion-transmitted infections reported from January 2010 through November 2015 were reviewed for incomplete reporting (e.g., missing numerator or denominator report) to assess completeness. HM published data were reviewed to identify whether the volume of HM data increased over time.
Acceptability
In 2012, prior to adoption of a statewide reporting mandate to the HM, the MDPH and the American Red Cross North-east Region’s Medical Advisory Committee (MAC) conducted a survey to better understand facility knowledge of the NHSN HM and assess attitudes toward adoption of the system. The results of this survey were previously presented but are included here, as they were reviewed during this evaluation.16 A Web-based survey was distributed to 212 facilities in the New England region (consisting of Massachusetts, Maine, Connecticut, New Hampshire, and Vermont). The survey included 28 questions designed to assess knowledge of, and participation in, the NHSN HM, as well as to evaluate the level of interest in modification of the existing, mandatory, paper-based method of transfusion statistics reporting in Massachusetts. Responses were collected between November 26 and December 29, 2012, and analyzed.17
Representativeness
The number of reporting facilities and the number of components under surveillance in each US state and territory was tallied. Additionally, the number of participating facilities was compared with the number of acute care hospitals in the United States as reported to the NHSN Patient Safety Component.18
Timeliness
The recommended reporting schedule for adverse reactions is within 30 days of the month that the reaction occurred or when the investigation is completed. Monthly totals of transfused units should be reported within 30 days of the end of the reporting month. The proportion of facilities reporting adverse reactions and total transfused units within 30, 60, and 90 days, respectively, was calculated.
Concordance
No transfusion reaction reporting system in the United States was available for comparison to calculate sensitivity. BMC maintains an electronic system that collects data on clinical signs and symptoms throughout the transfusion process and is incorporated into the hospital’s patient electronic health record as part of biovigilance and quality improvement operations.19 Information captured includes, but is not limited to, patient vital sign values at discrete intervals in the peritransfusion period (pre-/15 minutes/ posttransfusion), any adverse reactions identified during the transfusion, the start and stop times for the transfusion episodes, and select patient demographic elements. The data captured in the system are used by the hospital to aid in the identification of unrecognized or unreported transfusion-related adverse events, to monitor for documentation compliance, and to identify opportunities for enhancing hemotherapy safety. This system electronically captures a variety of patient-related clinical and laboratory parameters in a format capable of being viewed in real time during transfusion episodes or retrospectively for any time interval since January 27, 2010, when the system was first activated. Approximately 90% of all transfusion episodes, except for those given intraoperatively, in locales where computer access is not available, or during computer system downtimes, are documented via this electronic format. This hospital has been participating in the HM since August 2011, prior to the implementation of the state reporting mandate.
Febrile nonhemolytic transfusion reactions (FNHTRs) were compared for this concordance assessment. The concordance evaluation was limited to this reaction type because it occurs relatively frequently, and the HM case definition is solely based on vital sign characteristics for which data are readily available in the BMC electronic report (e.g., temperature, weight, and signs/symptoms of a suspected transfusion reaction such as chills). Electronic data collected by the BMC system, representing an approximate 9-day interval of reporting per month from July 2012 to December 2015 (54 discrete weeks), was assessed for concordance with data reported to the HM within the same time periods.
An algorithm was created to query the BMC electronic system for data on signs/symptoms that matched the HM case definition for a FNHTR.20 The concordance of the HM and BMC systems was estimated by comparing the occurrence of potential FNHTRs in a large “semiselected” hemo-transfusion cohort routinely captured by BMC using its quality improvement hemotransfusion electronic documentation system with cases reported to, adjudicated, classified, and subsequently submitted to the HM by BMC. The list of FNHTRs identified by this algorithm was compared to adverse events reported to the HM during the corresponding time frames. Cases from both systems meeting the targeted criteria were matched on date/time, patient age, and blood type to determine concurrence, as personal identifiers were unavailable. A kappa statistic was determined for the two reporting systems (GraphPad QuickCalcs, GraphPad Software) as previously described.21
RESULTS
Usefulness
Transfusion-related adverse reaction data are available to the CDC, participating health care facilities, and organizations operating groups within the NHSN to estimate transfusion-related morbidity and mortality, monitor trends, and better understand the effect of safety interventions. These data have previously been used to better understand transfusion-related adverse reactions, such as higher reaction rates with apheresis components, the emerging infection risk of Babesia spp., and bacterial contamination associated with platelet transfusions.22,23 As of September 2017, nine publications, abstracts, and presentations that present HM data authored by the CDC, state health department(s), or other organizations have been identified. The HM protocol or use of case definitions are cited in 76 publications by non-CDC authors, including seven books chapters, 12 conference proceedings, and 59 peer-reviewed journal articles.
Simplicity
Data reporting includes five “data entry forms” that require between 1 minute and 2 hours to complete per form (Table 1). Transfusion-related adverse reactions are reported on the adverse reaction form and require assessment of clinical evidence to identify the type of reaction and the level of agreement between HM protocol criteria and supporting clinical evidence. Introduction to HM reporting is a 1-hour training session taken by facility personnel prior to reporting data; however, there is no method to monitor whether users complete this training. There are 1 required and 17 optional trainings sessions to assist and guide the user through a range of topics related to HM reporting. Training methods include written (e.g., frequently asked questions), audio and visual (e.g., Quick Learn videos), and written and visual (e.g., Quick Reference Guide) tools (Table 2).16 Efforts to reduce the time required to complete reporting have been implemented following feedback from participating facilities, including changes to exclude nonsevere allergic reactions and incidents that did not result in a reaction from required reporting.
Table 1.
NHSN HM process list by process frequency, number of steps, and required time to completion per year
| Process | Frequency | Number of steps | Time required | Required resources | |
|---|---|---|---|---|---|
| HM reporting | Month reporting plan | 12 | 1 | 1 minute | HM Monthly Reporting Plan HM Monthly Reporting Plan Table of Instructions |
| Annual facility survey | 1 | 1 | 2 hours | HM Annual Facility Survey Form HM Annual Facility Survey Table of Instructions |
|
| Transfusion-related adverse reaction (numerator data) | At least 12 | Per event | 15 minutes | HM Adverse Reaction Form HM Adverse Reaction Form Table of Instructions |
|
| Incidents resulting in a transfusion-related adverse reaction (numerator data) | At least 12 | Per event | 10 minutes | HM Incident Form HM Incident Form Table of Instructions |
|
| Total number of units transfused (denominator data) - Manual reporting NOTE: Only one method of reporting is required - manual or electronic |
12 | 1 | 1 hour and 10 minutes | HM Monthly Reporting Denominators HM Monthly Reporting Denominators Table of Instructions |
|
| Total number of units transfused (denominator data) - Electronic reporting via CDA NOTE: Only one method of reporting is required - manual or electronic |
12 | 3 | 10 minutes | HM Monthly Reporting Denominators HM Monthly Reporting Denominators Table of Instructions |
HM = Hemovigilance Module; NHSN = National Healthcare Safety Network.
Table 2.
NHSN HM trainings
| Type of training | Topic | Length |
|---|---|---|
| On-demand, self-paced, interactive | Introduction to HM Reporting | 1 hour |
| Slide set | Introduction to HM Analysis | 1 hour |
| Quick Learn | HM Annual Facility Survey Form | 6 minutes |
| HM Adverse Reaction Form | 9 minutes | |
| HM Incident Form | 7 minutes | |
| HM Denominator Form | 5 minutes | |
| HM Custom Fields | 6 minutes | |
| Quick Reference Guides | Create a Group | 5 minutes |
| Maintain a Group | 5 minutes | |
| Biovigilance Component Alerts | 5 minutes | |
| HM Customizing Forms | 5 minutes | |
| Location Mapping in NHSN | 5 minutes | |
| Linking Incidents Records to Adverse Reaction Records in the HM | 5 minutes | |
| Reporting Zero Adverse Events | 5 minutes | |
| Joining a group | 5 minutes | |
| SAMS registration steps | 5 minutes | |
| Frequently Asked Questions (FAQ) | Electronic Reporting - Clinical Documentation Architecture (CDA) | 7 minutes |
| General HM | 15 minutes |
HM = Hemovigilance Module; NHSN = National Healthcare Safety Network.
Flexibility
Modifications to the system occur approximately annually. Changes associated with public health emergencies are made as soon as possible based on available resources and the anticipated start date of reporting. For example, modifications to collect information on transfusion transmission of Zika virus and transfusion of blood products subjected to pathogen reduction technology were emergently introduced to capture information relevant to the Zika virus epidemic. Routine changes to the HM reporting schedule and case definitions are implemented on January 1 of each year. For example, reporting nonsevere allergic reactions and incidents not resulting in a transfusion reaction became optional in January 2013 following feedback from participating facilities In 2014, nonsevere allergic reactions represented a smaller proportion of the total number of allergic reactions reported to the module compared to 2013 (90% vs. 91%, respectively). The proportion of nonsevere allergic reactions continued to decline through 2016 (89%). Additionally, 741 incidents were reported to the module in 2013. After the change in required reporting for incidents that do not result in a transfusion reaction, the total number of incidents reported to the module declined to 470 in 2014 and further declined to 182 in 2016. This decline may in part be due to the change in reporting requirements.24
Data quality
From 2010 to 2013, there were 45 (45 of 442, 10%) transfusion-associated circulatory overload reactions reported with a probable case definition designation, though the module protocol did not contain a “probable” designation for this reaction. To prevent further designations that do not conform to protocol guidance, the application was modified in 2014 to only allow for entry of a case definition designation defined by the HM protocol. Another application modification, deployed in December 2017, assigns designations for case definition, severity, and imput-ability, to further limit data entry errors or inconsistent HM case definition interpretations by users. From 2010 to 2016, all transfusion-transmitted infection records (81of 81, 100%) were complete and included both a transfusion-transmitted infection report and corresponding denominator data (i.e., total units transfused for the same month as the reaction). Data quality checks are performed on a monthly basis to identify reporting inconsistencies and discrepancies for transfusion reaction and denominator reports.
Acceptability
The survey results indicated that 81% of surveyed providers anticipated benefits by enrolling in NHSN for transfusion adverse event reporting. Commonly perceived benefits from participating facilities included better comparison of data between facilities (81%) and improvement in collection of TAAE statistics (75%). Frequently mentioned potential barriers to the new system included lack of knowledge for how participation would be initiated (46%) and concerns that the new system would be too complex and labor intensive (37%).
Representativeness
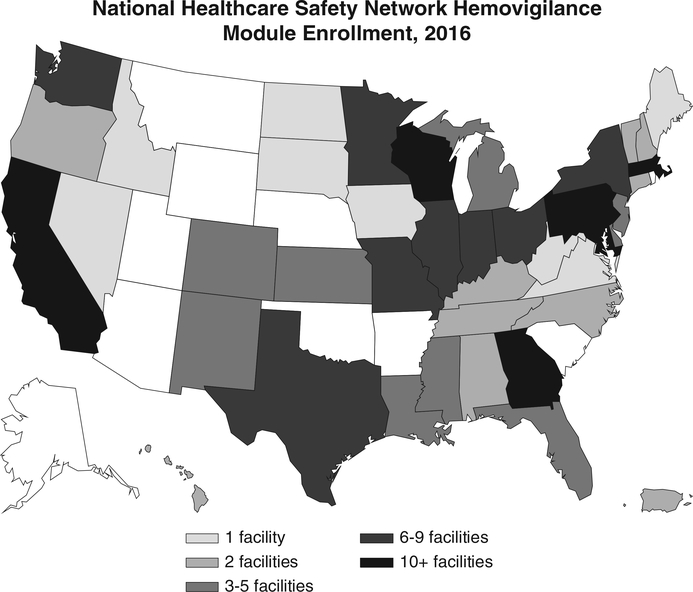
In 2009, nine facilities participated in the HM pilot. National enrollment began in 2010, which resulted in 82 enrolled facilities. Enrollment continued to increase steadily through 2013. In 2014, enrollment spiked to 248 facilities, due in large part to the Massachusetts statewide reporting mandate. As of 2016, 277 of 4690 (6%) acute care facilities were enrolled in the HM, of which 69 (25%) were located in Massachusetts (Table 3).18 Enrolled facilities are located in 42 states and Puerto Rico. States with 10 or more enrolled facilities include California, Maryland, Massachusetts, Georgia, Pennsylvania, and Wisconsin (Fig. 1). In 2011, 56 facilities reported 784,866 total transfused components, which represents approximately 4% of 20,691,000 units transfused in the United States.25–27 In 2013, 75 facilities reported 1,196,598 total transfused components, which represents approximately 6% of 20,180,000 units transfused in the United States.27 In 2015, 142 facilities reported 1,535,414 transfused components, which represents approximately 9% of 17,226,000 units transfused in the United States.1
Table 3.
NHSN HM Enrollment, 2009–2016*
| Year | Total Facilities |
|---|---|
| 2009† | 9 |
| 2010 | 82 |
| 2011 | 120 |
| 2012 | 149 |
| 2013 | 186 |
| 2014 | 248 |
| 2015 | 262 |
| 2016 | 277 |
July 2017 data set used to calculate enrollment.
NHSN HM pilot.
HM = Hemovigilance Module; NHSN = National Healthcare Safety Network.
Fig. 1.
Number of facilities enrolled in the NHSN HM for 2016 by state. July 2017 data set used to calculate enrollment.
Timeliness
Timely reporting of adverse reaction and denominator data improved for each time interval (e.g., 30, 60, and 90 days) by year. In 2016, 2147 of 4453 (48%) of adverse reactions were reported within 30 days of the reaction date compared to 109 of 511 (21%) in 2010. In 2016, 3969 of 4453 (89%) of adverse reactions were reported within 90 days of the reaction date compared to 284 of 511 (56%) in 2010. Since the state of Massachusetts requires all transfusion facilities to report to NHSN, we compared Massachusetts and national-level data to determine if this mandate improved timeliness. Facilities in Massachusetts had more complete reaction data at 60- and 90-day time intervals from 2010 to 2012 and 2014 to 2016 when compared to non-Massachusetts facilities (Table 4). In 2013, Massachusetts facilities (60 days: 222 of 332, 66.9%; 90 days: 238 of 332; 71.7%) and non-Massachusetts facilities (60 days: 1367 of 3130, 69.5%; 90 days: 2496 of 3130, 79.7%) submitted a similar number of reaction reports at 60- and 90-day time intervals (Table 4). Massachusetts facilities also had a higher number of submitted denominator reports at 1-, 2-, and 3-month time intervals for all years except 2012 (Table 5). In 2012, fewer Massachusetts facilities completed denominator reporting than non-Massachusetts facilities at 2 and 3 months before the mandate. At 2 and 3 months after the mandate was implemented, more Massachusetts facilities completed denominator reporting than facilities not in Massachusetts. Complete reporting by non-Massachusetts facilities remained relatively stable from 2012 to 2016. The increased number of Massachusetts facilities that completed reporting at 2 and 3 months from 2013 to 2016 is likely due to anticipation and implementation of the mandate for Massachusetts facilities to report to the module.
Table 4.
Total and proportion of total adverse reactions reported to the NHSN HM, reported at 30, 60, and 90 days following investigation at the facility, United States 2010–2016
| n/N | % of adverse reactions reported within 30 days | n/N | % of adverse reactions reported within 60 days | n/N | % of adverse reactions reported within 90 days | |
|---|---|---|---|---|---|---|
| All facilities | ||||||
| 2010 | 109/511 | 21.3% | 193/511 | 37.8% | 284/511 | 55.6% |
| 2011 | 871/2568 | 33.9% | 1488/2568 | 57.9% | 1824/2568 | 71.0% |
| 2012 | 1300/3363 | 38.7% | 2212/3363 | 65.8% | 2562/3363 | 76.2% |
| 2013 | 1459/3462 | 42.1% | 2397/3462 | 69.2% | 2734/3462 | 79.0% |
| 2014 | 1937/4743 | 40.8% | 3241/4743 | 68.3% | 3750/4743 | 79.1% |
| 2015 | 1952/4733 | 41.2% | 3228/4733 | 68.2% | 3722/4733 | 78.6% |
| 2016 | 2147/4453 | 48.2% | 3331/4453 | 74.8% | 3969/4453 | 89.1% |
| Massachusetts facilities | ||||||
| 2010 | 6/23 | 26.1% | 17/23 | 73.9% | 23/23 | 100.0% |
| 2011 | 47/186 | 25.3% | 178/186 | 95.7% | 185/186 | 99.5% |
| 2012 | 62/280 | 22.1% | 243/280 | 86.8% | 261/280 | 93.2% |
| 2013 | 92/332 | 27.7% | 222/332 | 66.9% | 238/332 | 71.7% |
| 2014 | 413/1021 | 40.5% | 750/1021 | 73.5% | 780/1021 | 76.4% |
| 2015 | 617/1181 | 52.2% | 1006/1181 | 85.2% | 1080/1181 | 91.4% |
| 2016 | 862/1198 | 72.0% | 1170/1198 | 97.7% | 1187/1198 | 99.1% |
| Non-Massachusetts facilities | ||||||
| 2010 | 103/488 | 21.1% | 103/488 | 36.1% | 261/488 | 53.5% |
| 2011 | 824/2382 | 34.6% | 824/2382 | 55.0% | 1639/2382 | 68.8% |
| 2012 | 1238/3083 | 40.2% | 1238/3083 | 63.9% | 2301/3083 | 74.6% |
| 2013 | 1367/3130 | 43.7% | 1367/3130 | 69.5% | 2496/3130 | 79.7% |
| 2014 | 1524/3722 | 40.9% | 1524/3722 | 66.9% | 2970/3722 | 79.8% |
| 2015 | 1335/3552 | 37.6% | 1335/3552 | 62.6% | 2642/3552 | 74.4% |
| 2016 | 1285/3255 | 39.5% | 1285/3255 | 66.4% | 2782/3255 | 85.5% |
HM = Hemovigilance Module; NHSN = National Healthcare Safety Network.
Table 5.
Total and proportion of total denominator records reported to the NHSN HM, records reported at 1, 2, and 3 months following the month under surveillance, United States 2010–2016
| n/N | % of denominator data reported within following month | n/N | % of denominator data reported within following 2 months | n/N | % of denominator data reported within following 3 months | |
|---|---|---|---|---|---|---|
| All facilities | ||||||
| 2010 | 27/226 | 11.9% | 43/226 | 19.0% | 53/226 | 23.5% |
| 2011 | 205/467 | 43.9% | 263/467 | 56.3% | 295/467 | 63.2% |
| 2012 | 370/665 | 55.6% | 460/665 | 69.2% | 506/665 | 76.1% |
| 2013 | 493/787 | 62.6% | 612/787 | 77.8% | 661/787 | 84.0% |
| 2014 | 838/1395 | 60.1% | 1111/1395 | 79.6% | 1204/1395 | 86.3% |
| 2015 | 1057/1550 | 68.2% | 1282/1550 | 82.7% | 1353/1550 | 87.3% |
| 2016 | 987/1641 | 60.1% | 1252/1641 | 76.3% | 1393/1641 | 84.9% |
| Massachusetts facilities | ||||||
| 2010 | 2/10 | 20.0% | 4/10 | 40.0% | 4/10 | 40.0% |
| 2011 | 16/18 | 88.9% | 18/18 | 100.0% | 18/18 | 100.0% |
| 2012 | 21/36 | 58.3% | 24/36 | 66.7% | 26/36 | 72.2% |
| 2013 | 34/45 | 75.6% | 38/45 | 84.4% | 40/45 | 88.9% |
| 2014 | 433/603 | 71.8% | 536/603 | 88.9% | 557/603 | 92.4% |
| 2015 | 709/838 | 84.6% | 809/838 | 96.5% | 822/838 | 98.1% |
| 2016 | 690/840 | 82.1% | 769/840 | 91.5% | 796/840 | 94.8% |
| Non-Massachusetts facilities | ||||||
| 2010 | 25/216 | 11.6% | 39/216 | 18.1% | 49/216 | 22.7% |
| 2011 | 189/449 | 42.1% | 245/449 | 54.6% | 277/449 | 61.7% |
| 2012 | 349/629 | 55.5% | 436/629 | 69.3% | 480/629 | 76.3% |
| 2013 | 459/742 | 61.9% | 574/742 | 77.4% | 621/742 | 83.7% |
| 2014 | 405/792 | 51.1% | 575/792 | 72.6% | 647/792 | 81.7% |
| 2015 | 348/712 | 48.9% | 473/712 | 66.4% | 531/712 | 74.6% |
| 2016 | 297/801 | 37.1% | 483/801 | 60.3% | 597/801 | 74.5% |
HM = Hemovigilance Module; NHSN = National Healthcare Safety Network.
Concordance
The BMC data set included 15,939 discreet transfusion episodes occurring between July 20, 2012, and December 26, 2015 (54 weeks). Of these episodes, 56 events met the NHSN surveillance case definition for an FNHTR. During this same time frame, 46 FNHTRs were reported to the HM by BMC or corresponded to a reaction observed at BMC (identical incident report categorized in NHSN as something other than FNHTR). Additional information on identified reactions not captured by the BMC system was not available. This includes whether the reactions were identified by BMC or if any actions were taken in response. Between the two systems, 24 of the reactions were captured in both systems (unweighted kappa value 0.47; confidence interval, 0.33–0.61) (Table 6).
Table 6.
Febrile nonhemolytic transfusion reactions reported to the NHSN HM and the Baystate system, January 1,2012, to December 31,2015
| Year | NHSN HM Reactions | Baystate System Reactions | Concordance |
|---|---|---|---|
| 2012 | 3 | 5 | 60% |
| 2013 | 7 | 18 | 39% |
| 2014 | 10 | 19 | 53% |
| 2015 | 4 | 14 | 29% |
HM = Hemovigilance Module; NHSN = National Healthcare Safety Network.
DISCUSSION
Since its inception in 2010, the NHSN HM has been the sole system dedicated to nationwide surveillance for transfusion-related adverse events in the United States. Most attributes assessed as part of this evaluation, including system simplicity, timeliness, and data quality, were found to be adequate, and evidence indicates improvement over time as the system matured. Analyses of data reported to the system have identified important areas that could be targeted for further study or interventions to reduce morbidity and mortality among transfusion recipients.22,23,25 A key finding based on data reported to this system was the identification of higher rates of adverse reactions among apheresis platelets compared with whole blood–derived platelets, which was consistent with reports from international hemovigilance systems.28 Unexpected public health challenges, such as the Zika virus response, demonstrate the system’s flexibility and led directly to the implementation of new features and functionalities. We found that while facility participation has improved, overall national enrollment remains low relative to the total number of acute care hospitals in the United States,1,18 thereby limiting the representativeness of the system and generalizability of its findings. At the current rate of facility enrollment and participation, national participation in this system may be limited for the forseeable future. However, based on the Massachusetts experience, state reporting mandates can dramatically increase facility participation and can serve as a model for transfusion surveillance improvements in other states.
We found that experience gained over the first 6 years of HM use led to steady improvements in usability and the quality of data collected. Initial reports often lacked key demographic or denominator data required to accurately stratify and analyze data. This problem has been mitigated by a few key changes in reporting. First, alterations to case definition criteria have streamlined reporting for some reactions. Following feedback from providers, nonsevere allergic reactions and incidents that do not result in transfusion reactions were removed from required reporting, which reduced the number of reportable events and increased data quality. However, conclusions about these events may be affected by fewer reports. Second, the reporting system itself has been enhanced with more checks to prevent submission of erroneous data. This includes a requirement that all transfusion-transmitted infections have evidence of a confirmatory laboratory test. Additional modifications include efforts to track transfused components treated with pathogen reduction technology and to reduce reporting burden by configuring the HM to allow for electronic data upload. Finally, participation in the HM allows for facilities to track TAAE trends over time to better address potential transfusion threats. Participation in this system also enables state and local public health organizations to compare data across facilities, simplifying efforts to assess the impact of changes to transfusion practice.
Public health surveillance system guidelines often recommend an evaluation of sensitivity. This typically includes a statistical comparison to another gold standard system. However, data were not available from another comparable US-based system. The BMC electronic hemotherapy documentation and its subsequent data capture system were designed to simplify the direct reporting of suspected transfusion reactions to the hospital transfusion service and for quality improvement monitoring purposes, respectively. These electronic resources have been used for more than 5 years at three of the medical facilities of the parent Baystate Health network and were therefore considered to be an appropriate surrogate for comparative analysis.
Our limited study of concordance between the two systems suggests a low to moderate level of agreement and may indicate that a significant number of reactions are not being captured in either system. One possible explanation may be that the small sample size examined for the purpose of this study limited the concordance estimate. Other potential explanations include that the reactions are unrecognized by clinical staff and hence unreported, or alternatively that signs and symptoms are being recognized by bedside caregivers but are being attributed to other causes. Such possibilities underscore the importance of bedside surveillance of patients receiving transfusions and the need for ongoing provider education regarding suspected transfusion reaction recognition and reporting to the blood bank. Early results from this analysis have led BMC to investigate methods to improve provider training to ensure timely and complete identification of possible TAAEs. Future, more comprehensive efforts to compare hospital-based hemovigilance systems with the HM may allow for improved statistical estimates of sensitivity and concordance.
The findings of this study are subject to the following limitations. First, facilities report data to the HM following internal investigation procedures, which may vary across hospitals and health systems. The potential effects on the estimates presented here cannot be quantified. The CDC is currently planning validation exercises with some facilities to better ascertain data quality and adherence to protocol definitions. Next, we relied on one hospital-based system and one specific transfusion reaction category to measure concordance, thereby limiting the generalizability of the presented estimates. Data from this hospital-based system did not include information on imputability, which limited our ability to determine why discrepencies between the two systems may have occurred. Additionally, the number of acute care facilities that perform transfusion in the United States is not readily available and in flux as facilities engage in mergers and acquisitions. We used 4690 as a national estimate of the number of acute care facilities based on participation in a separate NHSN component.18 Assessment of whether these facilities perform transfusions was not conducted as part of the study. Finally, some of the attributes were assessed qualitatively, as no quantitative measures were available.
In summary, the NHSN HM is a national system that enables estimation and tracking of transfusion-related adverse reactions in the United States. The system incorporates stakeholder, user, and subject matter expert input to improve data collection and quality. While we found that this system is useful and accepted among users, low participation may limit the generalizability of our evaluation. Despite the currently limited representativeness of the system, the continued steady increase in participating facilities should lessen this issue over time. Results from a small study of concordance demonstrate the need to ensure that adverse events are properly diagnosed at the bedside and subsequently reported to the HM. Data from the state of Massachusetts indicates that state-based requirements are effective at improving facility enrollment and reporting timeliness. Aggregate data from the HM has allowed for identification and monitoring of existing and emerging complications and threats to blood safety, which can inform prevention efforts. Continued and expanded participation in national hemovigilance efforts through the NHSN HM is a key piece of the ongoing efforts to improve the safety of transfusions in the United States.
ABBREVIATIONS:
- BMC
Baystate Medical Center
- CDC
Centers for Disease Control and Prevention
- FNHTRs
febrile nonhemolytic transfusion reactions
- HM
Hemovigilance Module
- NHSN
National Healthcare Safety Network
- TAAEs
transfusion-associated adverse events
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the authors’ affiliated institutions. Use of trade names, commercial sources, or private organizations is for identification only and does not imply endorsement by the US Department of Health and Human Services and/or CDC.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
REFERENCES
- 1.Ellingson KD, Sapiano MRP, Haass KA, et al. Continued decline in blood collection and transfusion in the United States–2015. Transfusion 2017;57:1588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faber JC. Worldwide overview of existing haemovigilance systems. Transfus Apher Sci 2004;31:99–110. [DOI] [PubMed] [Google Scholar]
- 3.Ditomasso J, Liu Y, Heddle NM. The Canadian Transfusion Surveillance System: what is it and how can the data be used? Transfus Apher Sci 2012;46:329–35. [DOI] [PubMed] [Google Scholar]
- 4.Stainsby D, Jones H, Asher D, et al. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev 2006;20:273–82. [DOI] [PubMed] [Google Scholar]
- 5.Giampaolo A, Piccinini V, Catalano L, et al. The first data from the haemovigilance system in Italy. Blood Transfus 2007;5:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mafirakureva N, Khoza S, Mvere DA, et al. Incidence and pattern of 12 years of reported transfusion adverse events in Zimbabwe: a retrospective analysis. Blood Transfus 2014;12:362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez P, Salmi LR, Folléa G, et al. Determinants of transfusion-associated bacterial contamination: results of the French BACTHEM Case-Control Study. Transfusion 2001;41:862–72. [DOI] [PubMed] [Google Scholar]
- 8.US Department of Health Human Services. Biovigilance in the United States: efforts to bridge a critical gap in patient safety and donor health. US Department of Health and Human Services; 2009. [cited 2017 August 1]. Available from https://www.researchgate.net/publication/255703308. [Google Scholar]
- 9.Chung K-W, Harvey A, Basavaraju SV, et al. How is national recipient hemovigilance conducted in the United States? Transfusion 2015;55:703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funk M Benefit of transfusion-related acute lung injury risk-minimization measures–German haemovigilance data (2006–2010). Vox Sang 2012;102:317–23. [DOI] [PubMed] [Google Scholar]
- 11.Okazaki H The benefits of the Japanese haemovigilance system for better patient care. ISBT Science Series 2007;2:104–9. [Google Scholar]
- 12.Bolton-Maggs PH, Cohen H. Serious Hazards of Transfusion (SHOT) haemovigilance and progress is improving transfusion safety. Br J Haematol 2013;163:303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morel P, Hervé P. Surveillance of blood transfusion safety: contribution of the hemovigilance strategy in France. Transfus Med Rev 1998;12:109–27. [DOI] [PubMed] [Google Scholar]
- 14.Chung KW, Cumming M, Osinski A, et al. Reporting of transfusion-related adverse events using the National Healthcare Safety Network: adoption of mandatory statewide reporting in Massachusetts. CSTE Annual Conference; 2014; Nashville, TN. [Google Scholar]
- 15.German RR et al. Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep 2001;50:1–35; quiz CE1–7. [PubMed] [Google Scholar]
- 16.US Centers for Disease Control and Prevention. National Healthcare Safety Network: Blood Safety Surveillance. 2017. [cited 2017 August 28]. Available from https://www.cdc.gov/nhsn/acute-care-hospital/bio-hemo/
- 17.Uhl L, Andrzejewski C, Pisciotto PT, et al. Perceived benefits and barriers to participation in the National Healthcare Safety Network (NHSN) Hemovigilance Program: a survey of New England Region Blood Banks and Transfusion Services (Abstract). Transfusion 2013;53:219–78A. [Google Scholar]
- 18.Vallabhaneni S, Sapiano M, Weiner LM, Lockhart SR, Magill S. Antifungal susceptibility testing practices at acute care hospitals enrolled in the National Healthcare Safety Network, United States, 2011–2015. Open Forum Infect Dis. 2017;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy R et al. Development of electronic medical record charting for hospital-based transfusion and apheresis medicine services: early adoption perspectives. J Pathol Inform 2010;1:8. doi: 10.4103/2153-3539.65345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratcliffe N, Dunbar NM, Brown CI. Enhancing platelet transfusion safety: not a one-size-fits-all approach. Transfusion 2016; 56:1483–4. [DOI] [PubMed] [Google Scholar]
- 21.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22:276–82. [PMC free article] [PubMed] [Google Scholar]
- 22.Kuehnert M, Sapiano MR, Basavaraju S Transfusion-transmitted infections (TTI) reported to the National Healthcare Safety Network (NHSN) Hemovigilance Module (HM), 2010–2015 (Abstract). 2016 CSTE Annual Conference; 2016. [Google Scholar]
- 23.Basavaraju SV, Haass KA, Sapiano MRP, et al. Severe adverse transfusion-related reactions reported to the National Healthcare Safety Network Hemovigilance Module, United States, 2013 to 2015 (Abstract). In 34th International Congress of the International Society of Blood Transfusion; 2016. Dubai, United Arab Emirates [Google Scholar]
- 24.US Centers for Disease Control and Prevention. The National Healthcare Safety Network (NHSN) Manual: Biovigilance Component v2.4 2017. [cited 2017 August 28]. Available from www.cdc.gov/nhsn/PDFs/Biovigilance/BV-HV-protocol-current.pdf
- 25.Harvey AR, Basavaraju SV, Chung KW, et al. Transfusion-related adverse reactions reported to the National Healthcare Safety Network Hemovigilance Module, United States, 2010 to 2012. Transfusion 2015;55:709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitaker B Hinkins S National Blood Collection and Utilization Survey 2011. Report number HHSP23320110008TC, OMB, 2016(0990–0313). [Google Scholar]
- 27.Chung KW, Basavaraju SV, Mu Y, et al. Declining blood collection and utilization in the United States. Transfusion 2016;56: 2184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daurat A, Roger C, Gris JC, et al. Apheresis platelets are more frequently associated with adverse reactions than pooled platelets both in recipients and in donors: a study from French hemovigilance data. Transfusion 2016;56: 1295–303. [DOI] [PubMed] [Google Scholar]