Abstract
OBJECTIVE:
Magnetic resonance (MR) thermography-guided laser interstitial thermal therapy, or stereotactic laser ablation (SLA), is a minimally invasive alternative to open surgery for focal epilepsy caused by cerebral cavernous malformations (CCMs). We examined the safety and effectiveness of SLA of epileptogenic CCMs.
METHODS:
We retrospectively analyzed 19 consecutive patients who presented with focal seizures associated with a CCM. Each patient underwent SLA of the CCM and adjacent cortex followed by standard clinical and imaging follow-up.
RESULTS:
All but one patient had chronic medically-refractory epilepsy (median duration 8 years, range 0.5–52 years). Lesions were located in temporal (13), frontal (5), and parietal (1) lobes. CCMs induced magnetic susceptibility artifacts during thermometry, but perilesional cortex was easily visualized. Fourteen of 17 (82%) with >12 months follow-up achieved Engel I outcomes, of which 10 (59%) were Engel IA. Two patients who were not seizure free from SLA alone became so following intracranial electrode-guided open resections. Delayed post-surgical imaging validated CCM involution (median 83% volume reduction) and ablation of surrounding cortex. Histopathological examination of one previously ablated CCM following open surgery confirmed obliteration. SLA caused no detectable hemorrhages. Two symptomatic neurological deficits (visual and motor) were predictable, and neither was permanently disabling.
Keywords: Cerebral cavernous malformation, lesional epilepsy, seizure, magnetic resonance thermometry, stereotactic laser ablation, laser interstitial thermal therapy
INTRODUCTION
Cerebral cavernous malformations (CCMs) are abnormal vascular lesions within the central nervous system, the growth or hemorrhage of which may be associated with headaches, seizures, and neurological deficits.1,2 Structurally, CCMs are intertwined, “mulberry-like” clusters of thin-walled vessels lined by endothelial cells, without intervening brain parenchyma, and associated with developmental venous anomalies. On T2-weighted magnetic resonance imaging (MRI), CCMs appear heterogenously hyperintense with a surrounding hypointense rim of hemosiderin-laden parenchyma. CCMs are relatively common (incidence of 0.16%3) and carry a 5-year symptomatic hemorrhage risk of 15.8%.4–7 CCMs cause gliosis and neuronal irritation,8,9 up to 70% of patients with supratentorial CCMs present with seizures, and 40–50% of these cases become medically-refractory.2,10,11
Surgical resection of a CCM and surrounding cortex is considered critical to achieving seizure freedom.4–6 SLA uses using real-time MR thermography to coagulate tissue at 50–90°C while visualizing collateral structures to be protected.12,13 SLA provides an alternative to open epilepsy surgery for a variety of brain pathologies, 13–19 and provides neurocognitive outcomes superior to traditional open resection for medial temporal lobe epilepsy.20–22
We previously described the technical feasibility of SLA of CCM.23 Here we report the outcomes of 19 subjects that underwent SLA of CCMs with respect to safety, clinical effects, imaging features, and an example of post-ablation histopathological findings.
METHODS
Patient Selection
Following our first five successive patients,23 additional patients underwent SLA between July 2012 and September 2018 at a single institution. Indication for surgery was the presence of a CCM causing drug resistant seizures, except for patient 6 who sought early intervention to discontinue anti-epileptic medications.24 Brain MRI in each case showed a characteristic “popcorn” appearance with a rim of hypointensity on T2 weighted sequences and prominent blooming artifact on susceptibility-weighted sequences indicating the presence of hemosiderin. In all but patient 6, additional preoperative evaluation included neuropsychometric testing, long term video-electroencephalography, and 18-fluorodeoxyglucose positron-emission tomography (PET). Additionally, patient 16, with a lesion in the dominant postcentral gyrus/sulcus, underwent functional MRI for language and stereoelectroencephalography (SEEG) with cortical mapping by direct electrical stimulation. Patient 17, with a lesion in the nondominant precentral gyrus/sulcus, underwent functional MRI for motor localization and awake motor testing during ablation.
All patients elected to undergo laser ablation as the primary surgical intervention over open resection and signed informed surgical consent. The first 5 patients were told that application of SLA to CCM was novel, untested, and carried un uncertain risk of bleeding23. Thereafter, all patients were apprised of accruing evidence of safety from our initial experience. All patients were specifically warned that bleeding, unanticipated neurological deficits, or need for additional surgery could occur. This retrospective review of clinical data for research was approved by the Emory University Institutional Review Board.
Stereotactic Surgical Procedure
All patients underwent general anesthesia except for patient 17 who was treated awake. At the beginning of each procedure, patients were administered intravenous antibiotic for infection prophylaxis. Most were also administered intravenous dexamethasone and many received levetiracetam. Ablations were performed by one of two surgeons (JTW or REG) using the Visualase Thermal Therapy® system (Medtronic, Inc., Louisville, CO), which utilizes laser energy (up to 15W, 980nm) delivered through an optical fiber inside a saline-cooled cannula (1.65mm outer diameter) to produce rapid and localized tissue injury.12 For the minority of cases we used a traditional stereotactic frame (Cosman-Roberts-Wells [CRW] stereotactic frame, Integra Neurosciences, Plainsboro, NJ), with the Stealth S7 (Medtronic, Inc., Louisville, CO) Framelink® targeting software, with placement of the stereotactic bolt in the operating room followed by transport to the MR suite for treatment. For the majority of cases we used the ClearPoint® MRI-guided Neuro Navigation Platform (MRI Interventions, Irvine, CA), performing the entire procedure within an interventional MRI suite. The relative advantages of each workflow are detailed elsewhere.13,25
In all cases, we performed final insertion of the device into the body of the CCM only with the patient positioned in the MRI scanner, followed by immediate imaging. The Visualase workstation analyzed real-time thermography to predict cumulative tissue damage during the ablation. Post-ablation MRI, including diffusion weighted, T2 inversion recovery, and contrast-enhanced T1 sequences confirmed total area of ablation. When using the ClearPoint® platform, a second ablation track was made to maximize the treatment area when judged important. Following each procedure, the device was completely removed, the surgical site closed with a dissolvable suture, general anesthesia was reversed, and the patient was extubated. Patients were admitted to a regular hospital ward until discharge. Patients were instructed to remain on antiepileptic medications and weaned at the discretion of a treating neurologist.
Clinical Follow-up and Analysis
At each return visit, patients were queried as to the occurrence of seizures, any adverse effects, and medication status. A small minority provided follow-up by documented phone interviews with clinical staff. Seizure outcomes are reported using the Engel classification scheme.26 All but patients 18 and 19 had >12 months of follow-up after ablation. Patients not rendered free of disabling seizures by ablation alone were considered eligible for additional surgical procedures. Kaplan-Meier survival analysis was performed with respect to postoperative recurrence of any debilitating seizure occurring more than 4 weeks post-ablation (consistent with the Engel classification scheme), and censorship occurred at each subject’s last follow-up.
Image Follow-up and Processing
Volumes were calculated using OsiriX (Pixmeo, Geneva, Switzerland) by tracing diameters in each slice along the dimension of thinnest cut. Pre-ablation CCM volumes were measured using the T2-weighted MRI, tracing hypointense hemosiderin rims. Immediate post-ablation volumes were measured by tracing the enhancing borders of gadolinium-contrasted T1-weighted images (thermal injury zone). At last available follow-up imaging, the residual CCM volume was traced around the hypointense residual nodule on T2-weighted MRI.
Pathological Examination
Patients 3 and 16 underwent open resection after ablation. While the ablation from patient 3 was not identified, a nodular structure corresponding to the ablated CCM from patient 16 was resected, fixed in formalin, embedded in paraffin, sectioned at 4μm, and stained with haematoxylon and eosin (H&E). Immunohistochemistry was performed using antisera directed against glial fibrillary acidic protein (GFAP; Dako, Carpinteria, CA; Polyclonal, no dilution) and MIB1/Ki67 (Dako, Carpinteria, CA; MIB-1 1:80 dilution).
RESULTS
Preoperative Characteristics
We performed SLA on 19 sequential patients (9 female) presenting with focal (motor or cognitive) seizures with or without progression to secondary tonic-clonic activity and a clinically-correlating CCM (Table 1, Figures 1–3). The median pre-operative epilepsy duration was 8 (range 0.5–52) years, and the mean age at surgery was 40.4 (SD ±17.2, range 16–76) years. Lesion locations included temporal (13), frontal (5), and parietal (1) lobes. All but patient 6 (who sought early intervention to discontinue seizure medications) met strict criteria for medically refractory epilepsy.27 Two patients had prior interventions at outside institutions (stereotactic radiosurgery in patient 8, and vagus nerve stimulation for unclear indications in patients 8 and 11). Patient 16 underwent intracranial monitoring (SEEG) at our institution for unsatisfactory concordance of noninvasive studies prior to SLA.
Table 1.
Preoperative patient and lesion characteristics
| Patient # | Sex | Age at surgery (y) | Epilepsy duration (y) | Baseline seizure type (ILAE classification24) | Baseline AEDs | Prior procedure | Location | Associated vascular structures |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 37 | 8 | Focal impaired awareness | LAC, LAM | - | L Temporal (FG) | Tentorial edge |
| 2 | M | 29 | 5 | Focal aware and focal impaired awareness +/− to bilateral tonic-clonic | LEV, OXC, TOP | - | R Temporal (Hc, uncal apex) | PCA (ATA) |
| 3 | F | 67 | 46 | Focal aware and focal impaired awareness +/− to bilateral tonic-clonic | CLOB, LEV, ZON | - | R Frontal (MFG) | Small DVA |
| 4 | M | 66 | 20 | Focal aware cognitive +/− to bilateral tonic-clonic | LEV | - | L Temporal (FG & ITG) | DVA and tentorial edge |
| 5 | F | 76 | 52 | Focal impaired awareness +/− to bilateral tonic-clonic | GAB, PRI | - | L Temporal (posterior ITG) | DVA and v. Labbé |
| 6 | F | 34 | 0.5 | Focal to bilateral tonic-clonic | LAM | - | R Frontal (MOG) | DVA to sphenoparietal sinus |
| 7 | F | 40 | 8 | Focal impaired awareness | LAC | - | R Temporal (Hc, uncal apex) | AChA (choroid fissure) |
| 8 | F | 37 | 5 | Focal impaired awareness +/− to bilateral tonic-clonic | LAC, LAM | SRS, VNS | R Temporal (pole) | DVA to sphenoparietal sinus |
| 9 | M | 21 | 6 | Focal to bilateral tonic-clonic (comorbid PNES) | LAM, LEV | - | R Temporal (pole) | DVA to sphenoparietal sinus |
| 10 | M | 21 | 8 | Focal impaired awareness | CLON, DIV, LAM | - | L Temporal (anterior perirhinal cortex) | Tentorial edge |
| 11 | M | 16 | 5 | Focal impaired awareness | LAM, LEV, OXC | VNS | L Temporal (Hc, posterior body | Large DVA and AChA (choroid fissure) |
| 12 | F | 31 | 13 | Focal impaired awareness +/− to bilateral tonic-clonic | LAC, LAM | - | R Temporal (anterior ITG) | DVA to sphenoparietal sinus |
| 13 | M | 19 | 7 | Focal aware clonic | CLOB, LAM, OXC | - | L Frontal (PrCG) | Small DVA to v. of Trolard |
| 14 | F | 56 | 7 | Focal impaired awareness +/− to bilateral tonic-clonic | LEV, OXC | - | L Temporal (posterior ITG) | DVA and v. of Labbé |
| 15 | M | 33 | 6 | Focal to bilateral tonic-clonic | GAP, TOP | - | R Frontal (MFG) | Small DVA |
| 16 | F | 50 | 21 | Predominantly nocturnal focal impaired awareness | LAC, LEV, ZON | SEEG | L Parietal (PoCG, SMG) | Small DVA to superficial MCV |
| 17 | M | 36 | 11 | Focal aware motor seizure | LAC, LEV | - | R Frontal (PrCG) | Small DVA to v. of Trolard |
| 18 | M | 62 | 14 | Focal impaired awareness | LAC, LEV | - | L Temporal (entorhinal region) + MTS | AChA (choroid fissure) |
| 19 | F | 36 | 2 | Focal impaired awareness +/− to bilateral tonic-clonic | LAC, ZON | - | L Temporal (Hc, posterior body) | DVA to choroid fissure |
ILAE, International League Against Epilepsy operational classification of seizure types.24 PNES, psychogenic non-epileptic seizures; AED, antiepileptic drug; CLOB, clobazem; CLON, lonazepam; DIV, divalproate; GAB, gabapentin; LAC, lacosimide; LAM, lamotrigine; LEV, levetiracetam; OXC, oxcarbazine; PRI, primidone; TOP, topirimate; ZON, zonisimide; SRS, stereotactic radiosurgery; VNS, vagus nerve stimulator; SEEG, stereoelectroencephalography; FG, fusiform g.; Hc, hippocampus; MFG, middle frontal g.; ITG, inferior temporal g.; MOG, medial orbital g.; PrCG, precentral g.; PoCG, postcentral g.; SMG, supramarginal g. PCA, posterior cerebral a.; ATA, anterior temporal a.; DVA, developmental venous anomaly; AChA, anterior choroidal a.; MCV, middle cerebral v.; MTS, mesial temporal sclerosis; PChA, posterior choroidal a.
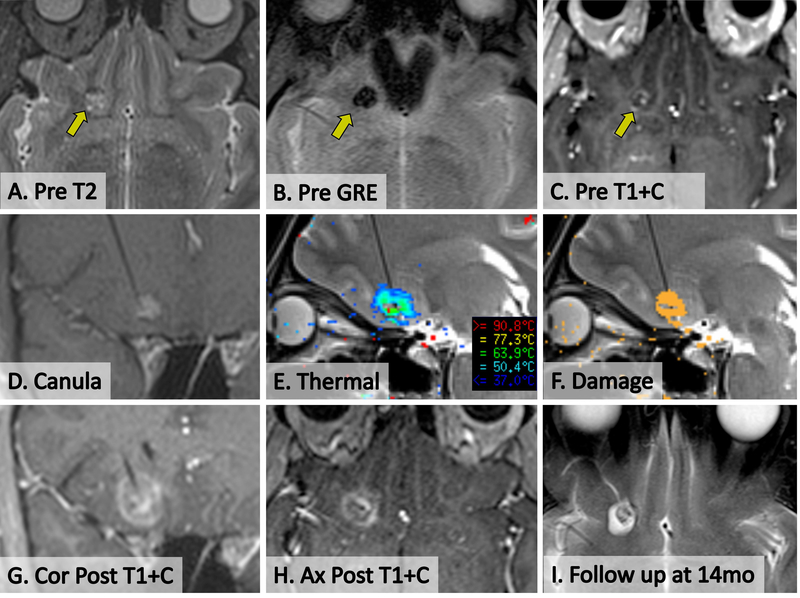
Figure 1. SLA of CCM in patient 6.
(A) Pre-operative axial T2 showing CCM in right medial orbital frontal gyrus (arrow). (B) Pre-operative axial GRE demonstrating blood products within CCM. (C) Pre-operative post-contrast axial T1 showing minimal early enhancement of CCM. (D) Intra-operative delayed-contrast coronal T1 showing cannula placement within CCM, without evidence of new bleeding or mass effect. (E) Intra-operative screenshot from Visualase workstation showing live thermal map overlaid on sagittal T2 image during ablation. Note some central signal dropout of gradient echo-based thermal imaging from static blood products. (F) Intra-operative screenshot from Visulase workstation showing live cumulative irreversible damage estimate (orange pixels) overlaid on sagittal T2 during ablation. Note damage at the brain-lesion interface. (G,H) Immediate post-ablation post-contrast coronal and axial T1 demonstrate actual extent of ablation. (I) Delayed axial T2 at 14 months post-ablation demonstrates a small hypointense involuting CCM surrounded by a hyperintense ablation cavity.
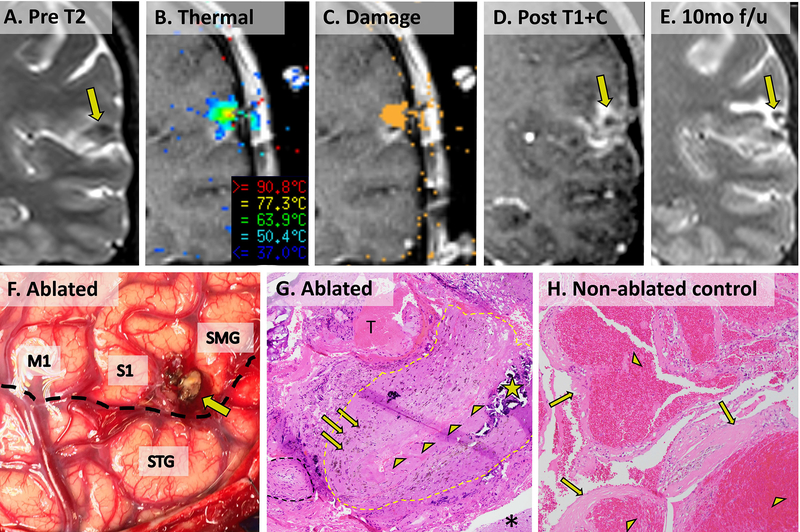
Figure 3. SLA and eventual resection of CCM in patient 16 with pathological comparison to an non-ablated CCM from an unrelated patient.
(A) Preoperative coronal T2 image showing CCM just above the Sylvian fissure in the postcentral sulcus. (B) Visualase ablation screenshot showing thermal temperature map. (C) Visualase ablation screenshot showing cumulative irreversible damage estimate (orange pixels). (D) Contrasted T1 image showing final extent of ablation. (E) Delayed post-ablation T2 image showing smaller hypointense CCM with surrounding hyperintense ablation cavity. (F) Operative photograph of ablated CCM (tan nodule, arrow) posterior to precentral gyrus (M1) between postcentral gyrus (S1) and supramarginal gyrus (SMG) and superior to the Sylvian fissure (dotted black line) and superior temporal gyrus (STG). (G) 100x microscopic appearance of patient 16’s CCM (H&E stain) showing post-ablation reactive type changes surrounding a large collapsed thickened vessel (perimeter approximated by dotted yellow outline and collapsed lumen lined by arrowheads). Scattered prior hemorrhage (arrows) and calcification (star) are evident within the thickened vessel wall. A small sclerotic hyalinized vessel (dotted black outline), an acute extravascular/extraparenchymal thrombus (T, likely a surgical artifact), and reactive brain parenchyma (asterisk) surround the collapsed vessel. (H) For comparison, a surgically resected CCM from an unrelated patient shows multiple engorged, dilated vascular lumina (arrowheads) with thickened, hyalinized walls (arrows) without intervening brain parenchyma.
Stereotactic Laser Ablation
Table 2 details each surgical approach, and Figures 1–3 show each ablation, with Figures 1 (patient 6) and 3 (patient 16) providing additional details. The laser apparatus was placed via CRW frame and stereotactic bolt in 3 cases. The remaining 16 cases were performed entirely within an interventional MRI suite with a disposable MR-guidance frame (ClearPoint®). In all cases, we successfully placed the laser applicator and ablated the tissue volume encompassing the CCM and surrounding parenchyma. For patients 12, 16, a second trajectory was used to extend ablation of hemosiderin-laden cortex. For patient 18 a second trajectory provided additional ablation of associated mesial temporal sclerosis.
Table 2.
Operative approach and results.
| Patient # | Stereotactic method* (surgeon) | Adverse events | LOS | Ablation volume (cm3) | Absolute and relative change lesion volume (cm3 and %)*** | Imaging f/u (m) | Clinical f/u (m) | Seizure outcome after SLA (Engel class) | Seizure outcome after subsequent open surgery (Engel class) | Seizures post-ablation (if present) | AED status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CRW (REG) | None | 1 | 1.0 | 0.3→0.03(−90%) | 22 | 41 | IA | - | - | LAM (2→1) |
| 2 | ClearPoint (REG) | None | 1 | 4.8 | 0.4→0.08(−80%) | 17 | 29 | IB | - | Focal aware seizure at 4 m post ablation with medication withdrawal | LEV, OXC (3→2) |
| 3 | ClearPoint (REG) | None | 1 | 1.9 | 0.2→0(−100%) | 7 | 42 | IVA** | IA | Continued focal aware and focal impaired awareness seizures (less severe) until subsequent open surgery 9 m post ablation. | LEV (3→1) |
| 4 | ClearPoint (REG) | None | 2 | 4.0 | 2.2→0.2(−91%) | 42 | 42 | ID | - | Focal to bilateral tonic-clonic seizure at 9 m with medication withdrawal and alcohol binge | CAR (1→1) |
| 5 | CRW (JTW) | None | 2 | 4.6 | 0.6→0.3(−50%) | 21 | 32 | IB | - | Focal aware seizures (brief nondisabling speech arrests) beginning at 6 m post ablation. | LAM, PRI (2→2) |
| 6 | ClearPoint (REG) | None | 1 | 2.5 | 0.6→0.2(−67%) | 15 | 49 | IA | - | - | None (1→0) |
| 7 | ClearPoint (REG) | None | 1 | 4.8 | 0.5 | - | 44 | IA | - | - | None (1→0) |
| 8 | ClearPoint (JTW) | None | 1 | 2.6 | 1.0 | - | 38 | IIIA | - | Focal impaired awareness +/- to bilateral tonic-clonic seizures ultimately controlled >1 y with medication adjustments | TOP, PHE (2→2) |
| 9 | ClearPoint (REG) | None | 1 | 9.3 | 0.8→0.4(−50%) | 27 | 27 | IA | - | Known comorbid PNES | LAM, LEV (2→2) |
| 10 | ClearPoint (JTW) | None | 1 | 4.4 | 0.1→0.0(−100%) | 13 | 40 | IA | - | - | None (3→0) |
| 11 | ClearPoint (REG) | Superior quadrant-opsia (non-disabling). | 1 | 7.0 | 0.9→0.1(−89%) | 32 | 32 | IC | - | Focal impaired awareness seizure with missed medications at 2 m post-ablation. | None (3→0), VNS removed |
| 12 | ClearPoint2 trajectories (JTW) | None | 1 | 10.0 | 1.2→0.1(−92%) | 14 | 30 | IA | - | - | None (2→0) |
| 13 | ClearPoint (REG) | None | 1 | 2.0 | 0.7→0.1(−86%) | 14 | 40 | IA | - | - | None (3→0) |
| 14 | CRW (REG) | None | 1 | 2.1 | 0.9→0.5(−44%) | 12 | 25 | IA | - | - | LEV (2→1) |
| 15 | ClearPoint (REG) | None | 1 | 1.1 | 0.1 | - | 26 | IA | - | - | None (2→0) |
| 16 | ClearPoint2 trajectories (JTW) | None after laser. Dysaesthesia after open surgery. | 1 | 1.1 | 0.1→0.08(−20%) | 6 | 24 | IID** | IA | Exclusively nocturnal focal impaired awareness seizures (less frequent recurred at 6 m) until subsequent open surgery at 11 m post ablation | LAC, LEV, TOP (3→3) |
| 17 | ClearPoint (REG) | Hand weakness (fully recovered). | 1 | 5.0 | 2.5→1.0(−60%) | 12 | 12 | IA | - | - | LAC, LEV (2→2) |
| 18 | ClearPoint 2 trajectories (JTW) | None | 1 | 3.5 | 0.2 | - | 3 | - | - | Focal impaired awareness seizure at 2 w post ablation | LAC, LEV (2→2) |
| 19 | Clearpoint (REG) | None | 1 | 0.6 | 0.4 | - | 2 | - | - | - | LAC, ZON (2→2) |
Stereotactic method utilized either traditional stereotactic Cosman-Roberts-Wells arc headframe (CRW, Integra, Inc.), or percutaneously mounted direct MRI guidance miniframe (ClearPoint System, MRI Interventions, Inc.). See methods.
In cases of early failure to maintain seizure freedom and transition to open surgery before 12 m, the outcome after laser ablation alone was imputed forward to operationally represent the lower Engel class at 12 m.
Second absolute lesion volume and relative change are presented for the 14/19 cases in follow-up imaging was available. For remaining 5 cases, only preopserative lesion volume is presented.
Engel Classification of epilepsy surgery outcomes: IA, Completely seizure-free since surgery; IB, Non-disabling simple partial seizures only since surgery; IC, Some disabling seizures after surgery, but free of disabling seizures for at least 2 years; ID, Generalized convulsions with antiepileptic drug withdrawal only; IIB, Rare disabling seizures since surgery; IID, Nocturnal seizures only; IIIA, worthwhile seizure reduction, IVA, no worthwhile seizure reduction; SF, seizure free <12 m in which Engel class may not yet be assigned.
LOS, length of stay; f/u, follow-up; AED, antiepileptic drug; PNES, psychogenic non-epileptic seizures; CAR, carbamazepine; CLOB, clobazem; LAC, lacosimide; LAM, lamotrigine; LEV, levetiracetam; OXC, oxcarbazine; PHE, phenytoin; PRI, primidone; TOP, topirimate; VNS, vagus nerve stimulator.
We found that due to the paramagnetic effects of concentrated blood products within CCMs, intraoperative thermal (gradient echo) imaging suffered signal dropout within the boundaries of larger lesions, thus delaying the real-time estimation of irreversible damage (Figure 1E-F and Figure 3B-C). However, since the perilesional cortex surrounding each CCM imaged normally, overall monitoring of the extent of the intended ablation was executed without technical difficulty. Immediate post-ablation T2-fluid-attenuated inversion recovery (FLAIR), diffusion-weighted imaging (DWI), and post-contrast T1-weighted sequences verified intended ablations (Figures 1–3).
Adverse Events
We observed no hemorrhagic complications following initial cannula insertion to target depth, during ablation, or following withdrawal of the laser cannula (Table 2). Moreover, no hemorrhagic complications resulted from additional trajectories in 3 cases (patients 12, 16, and 18). Seventeen of 19 patients were discharged on the first postoperative day, with two (patients 4 and 5) being discharged on the second day.
In patient 11, the ablation of a CCM in the posterior hippocampus also extended into the temporal lobe white matter. This ablation caused a partial right superior quadrantopsia, likely due to injury of the optic radiation (Meyer’s loop). This deficit was not disabling as the patient recovered subjectively and subsequently obtained a license to drive. Patient 17, who underwent awake ablation in order to monitor motor function, began to develop intrinsic weakness of the nondominant hand, and ablation was then discontinued, in a manner analogous to the accepted approach for awake open surgery in eloquent cortex. The patient underwent occupational therapy, returned to work as a practicing dentist within one week, and denied any functional disability at 12 m follow-up. Patient 16 had no subjective deficits following ablation, but did sustain expected peri-oral sensory disturbance from open resection of the lateral post-central gyrus which has been persistent but non-disabling.
There were no readmissions related to surgery or seizures, and no other delayed complications were identified. Neuropsychological outcomes of laser ablation across different brain regions, collected in a subset of patients that chose to participate in a prospective research study with an independent consent process, will be the subject of future investigation.
Seizure Outcomes
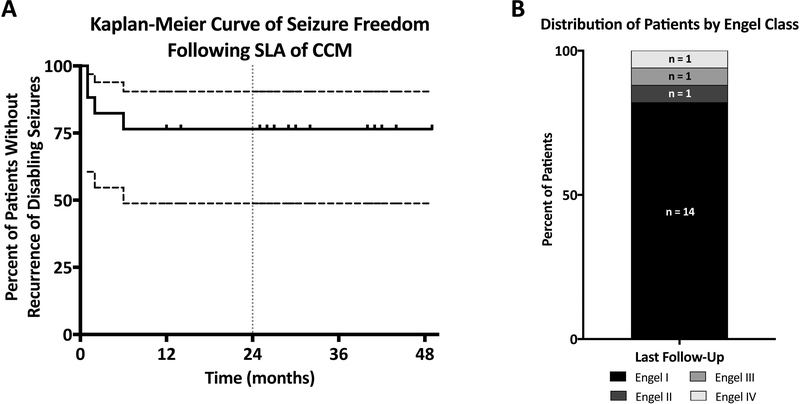
Follow-up was obtained for a mean 30.6 months ± SD 12.6 (median 32, range 2–49, Table 2). Kaplan-Meier analysis revealed no seizure recurrence beyond 6 months after the perioperative period in the first 17 patients having >12 follow-up (Figure 4A). Two patients each had a single seizure (patient 5 at 6 months and patient 11 at 2 months), but ultimately achieved Engel IC status by remaining seizure free for at least 2 years thereafter. At last follow-up, 14 of 17 patients (82%) were free of disabling seizures from ablation alone (Engel I) with 10 (59%) having been completely seizure free from the time of surgery (Engel 1A)(Figure 4B). Additionally, 9 of 16 patients with >24 m follow-up (56%) were prescribed a reduced number of medications and 5 (31%) were off all antiepileptic medications at last follow-up (Tables 1 and 2). Patients 18 and 19 were not assigned an Engel classification due to short follow-up post-ablation.
Figure 4. Long term seizure outcomes.
(A) Kaplan-Meier curve analysis depicts the proportion of patients having >12 m follow-up (patients 1–17) who never experienced a disabling seizure following stereotactic laser ablation. No patient outcome deteriorated after 6 months. Dashed lines indicate the 95% confidence intervals. Tick marks indicate censorship. A vertical grey dotted line indicates the 12-month time point. (B) Histogram bar graph show proportion (and numbers) of patient outcomes resulting from stereotactic laser ablation alone by Engel classification (I-IV). Only the 17 patients with >12 m outcome are presented (median last follow-up 32 m, range 12–49 m).
Patients 3, 8, and 16 failed to achieve freedom from disabling seizures after ablation alone (Table 2). Patient 8 is classified as Engel IIIA, but has since been completely free of seizures for the last year after medication adjustment. Patient 3 (Figure 2, panel 3) had no period of meaningful seizure control and underwent open subdural electrode grid placement and ictal electrocorticography-guided right frontal topectomy at 9 months post-ablation, achieving 2 years of subsequent seizure freedom (Engel IA). Likewise, patient 16 (Figure 3) had recurrent nocturnal seizures at 6 months post-ablation (Table 2) and sought further surgical management. She underwent open subdural electrode grid placement and ictal electrocorticography-guided topectomy of the inferior postcentral gyrus (in which the site of her prior CCM ablation was identified, Figure 3F). This caused continuous peri-oral/tongue dysaesthesia that was not disabling, and she remained completely seizure-free at 12 months post-resection.
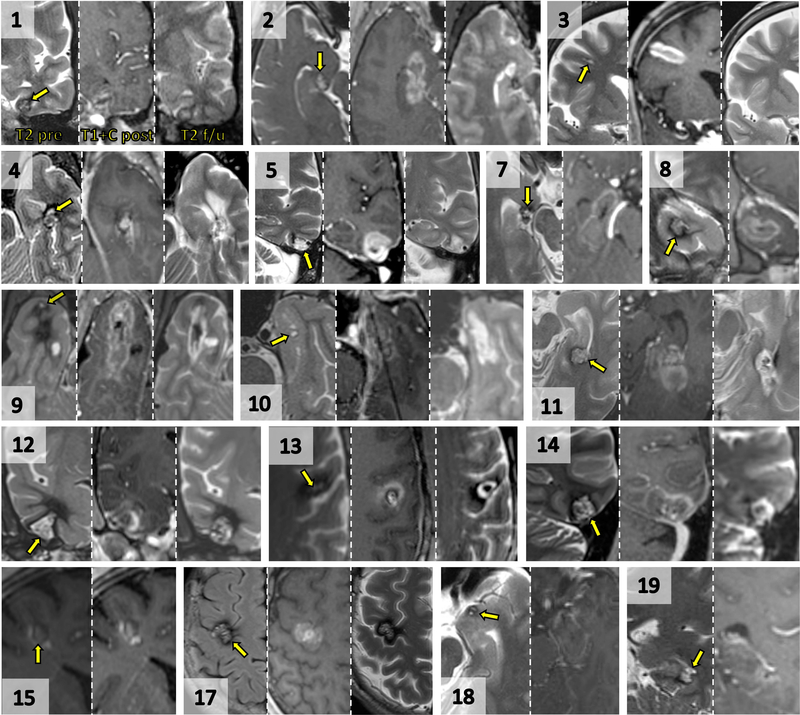
Figure 2. Imaging of CCMs before and after SLA.
For each patient image set, preoperative T2, intraoperative immediate post-ablation post-contrast T1, and delayed post-operative T2 (when available) are shown. Numbers refer to patients in the clinical series (patients 6 and 16 are presented in further detail in Figures 1 and 3, respectively). Yellow arrows point to preoperative lesion locations. (1) Coronal, left fusiform gyrus of temporal lobe. (2) Axial, uncal apex of hippocampus. (3) Coronal, right middle frontal gyrus. (4) Axial, left pes hippocampus. (5) Coronal, left posterior inferior temporal gyrus. (7) Axial, right uncal apex of hippocampus. (8) Coronal, right temporal pole. (9) Axial, right temporal pole. (10) Axial, right anterior perirhinal cortex. (11) Axial, left posterior hippocampus. (12) Coronal, right inferior temporal gyrus. (13) Axial, left precentral gyrus. (14) Coronal, left posterior inferior temporal gyrus. (15) Coronal, right middle frontal gyrus. (17) Axial, right precentral gyrus. (18) Axial, left entorhinal cortex of uncus. (19) Coronal, left posterior hippocampus. In general, delayed post-ablation imaging reveals T2 hypointensity within the involuted CCM and surrounding T2 hyperintensity.
Imaging outcomes
Lesions were in proximity to either an apparent developmental venous anomaly (14/19) or to another contrast-enhancing vessel or pial/dural interface (5/19)(Table 1). The mean preoperative volume of CCMs in this series was 0.7 ± 0.6 cm3 (range 0.1 – 2.5 cm3) while immediate post-ablative contrasted T1-weighted images showed an mean ablation zone of 3.8 ± 2.6 cm3 (range 0.6–10.0 cm3)(Table 2, Figures 1–3). Of the patients with follow-up imaging (14/19) at a mean 18 ± 10 (median 15, range 6–42) months post-ablation, we observed that ablated CCMs took on a nodular T2 hypointense appearance (Figures 1–3) and decreased volumes (Table 2, mean volume 0.2 ± 0.3 cm3). Involuting CCMs were surrounded by T2-hyperintense ablation cavities (encephalomalcia). Patient 17, with a CCM adjacent to primary motor cortex who underwent awake partial ablation, did not show encephalomalacia (Figure 2). We observed no delayed hemorrhages or unexpected post-ablation findings.
Pathology
Patients 3 and 16 underwent additional open resection after laser ablation. In Patient 3, histopathological examination of tissue resected from the epileptogenic zone, which included the prior ablation, revealed cortex with reactive gliosis, but without definitive features of a cavernous malformation. This absence may have resulted from insufficient sampling, or from prior complete obliteration of the relatively small lesion in this case. In patient 16, the location of prior CCM (nodular tan-colored friable structure in Figure 3F, arrow) was grossly verified, dissected, and sent as an isolated specimen. Microscopically, standard H&E staining of the specimen revealed post-surgical reactive type changes, but no definite residual/recurrent cavernous malformation. The sample contained primarily reactive astrogliosis, hemosiderin-laden macrophages (indicating prior hemorrhage), inflammatory infiltration, scattered calcifications, and areas of apparent fibrotic/sclerotic arachnoid mater (Figure 2G). Instead of dilated thin-walled vessels, we observed arachnoid tissue containing collapsed hyalinized vessels. Immunohistochemistry demonstrated both a low MIB1/Ki67 proliferation index (< 1%) and a reactive gliosis, as highlighted by GFAP stain (not shown). For comparison, the typical microscopic features of a resected CCM from an unrelated, non-ablated control patient shows dilated thin-walled vessels (Figure 2H). We interpret our findings to be consistent with obliteration of the presumed CCM.
DISCUSSION
Recent studies have demonstrated the use of SLA in varied locations and pathological entities associated with epilepsy, including medial temporal lobe epilepsy,13,21 focal cortical dysplasia,28 tuberous sclerosis,28 insular epilepsy,14,19 hypothalamic hamartomas,5 tumors,19,29–33 and periventricular nodular heterotopias.34 CCMs, with their inherent vascularity and propensity toward recurrent hemorrhage, could be at greater risk of bleeding during stereotactic probe insertion and ablation. Nevertheless, certain empiric observations regarding CCMs suggest that the clinically significant bleeding risk of manipulating CCMs may be acceptably low. First, CCMs are made up of thin-walled vessels that are occult on arterial phase angiogram due to low vascular pressure. Second, open surgical experience with resection of CCMs indicates that these lesions do not generally bleed if associated vessels are protected. Third, the natural history of CCMs may vary by location and genetic factors,35 and our series selected for subjects presenting with epilepsy rather than symptomatic hemorrhage. Indeed, this series does not include any patient with a deep/brainstem lesion, thunderclap headaches, neurological deficits, or familial cavernous malformations. Our previous technical report demonstrated the feasibility of stereotactic ablation of CCMs 23. The present study of 19 epilepsy patients further supports relative safety of the approach, as hemorrhages were not observed, and neurological deficits induced by ablation were expected for location and ultimately non-disabling. Hospital lengths of stay were brief, and patients achieved a high rate of long-term seizure control.
Comparison of SLA to other interventions
Ictal electrocorticography-guided resection of the CCM and surrounding cortex is considered the gold standard for epilepsy management35 yielding seizure control in 70–80% of patients.4,36–42 Larger resections (i.e. lobectomies) and frontal/insular locations have been associated with the best seizure outcomes.36 However, microsurgery for CCMs requires an incision and craniotomy, risks unintended collateral injury, especially in deep or eloquent regions3, and lobectomies induce wider neurocognitive deficits.20–22
Stereotactic radiosurgery (SRS) is an incisionless alternative to open surgery that yields rates of seizure freedom generally below those of open resection. In one large retrospective cohort, only 53% of patients were seizure-free (n=26/49), and patients suffering more disabling complex partial seizures fared worse with respect to seizure control than did those with simple partial seizures.39 SRS for CCM is also associated with a protracted temporal course and symptomatic radiation necrosis, especially at doses used to treat seizures.3,43–45
By comparison, SLA is minimally invasive and immediately effective in most cases. Our high rate of seizure control (82% Engel I) is comparable to open surgery, and in two patients (3 and 16) not initially rendered seizure free, prior ablation proved no barrier to successful open surgery. Nor did prior interventions prevent ablation, as some patients had already undergone SRS and VNS. While neuropsychological outcomes were not detailed in this report, such results in a large number of ablations across diverse brain locations is the subject of a different study currently in preparation.
While there were no hemorrhages, we observed two symptomatic neurological deficits resulting from ablations, and another from a subsequent open resection. In patient 11, a partial superior quadrantopsia resulted from ablating a posterior hippocampal CCM near the optic radiation. Notably, any standard open approach to this lesion would likely transgress the optic radiation, and superior quadrantopsia is known risk of standard open temporal lobe surgery. In our patient, this partial visual field deficit was non-disabling (noticed only when playing basketball) and he subsequently obtained a license to drive. In patient 17, a neurological deficit (increased nondominant hand weakness) was an expected result of ablating a CCM in the precentral gyrus, and the ablation was carried out with the patient awake in order to monitor and minimize disability. He quickly returned to work as a dentist and reported complete functional recovery by 2 months. Notably, patient 16 underwent awake open surgery and likewise sustained an expected deficit from resection in the lateral post-central gyrus (non-disabling peri-oral sensory disturbance). Epilepsy surgery by any method is by nature destructive to cortex, and when performed in eloquent cortex, the chance for seizure freedom must be weighed against the risks of symptomatic neurological deficits.
Study limitations
While this series includes all presenting cases of medically refractory epilepsy associated with a CCM at a high volume epilepsy center over a 6-year period, it still represents a relatively small retrospective review of a inhomogenous real-world cohort in which the outcome of any one patient will significantly impact group results. Nevertheless, our results align with the expected effectiveness of more extensive open microsurgery. Ideally, a larger series and a cohort design comparing SLA against gold standard open microsurgery would help evaluate the relative risks and benefits of SLA, but studies of this design have been prohibitively difficult to enroll, rendering statistical conclusions impractical44. Despite limitations, this series suggests relative safety and effectiveness of this procedure as a necessary first step toward future studies.
Technical considerations
The Visualase Thermal Therapy system generates an ablation volume that is time- and power-dependent, and an ablation diameter may generally reach >2 cm if unlimited by anatomic boundaries such as pia or ventricles. In 3 patients, however, following completion of the initial ablation trajectory, the surgeon judged intraoperatively that the irregular size and shape of the target with its adjacent hemosiderin-laden cortex called for more extensive therapy. In these cases, use of an MRI guidance platform (ClearPoint®) facilitated redirection and execution of a second ablation trajectory during the same surgical session. SLA performed with other workflows utilizing a stereotactic bolt provides less flexibility. In this setting, the surgeon may consider initial insertion of more than one bolt to support additional ablation trajectories.
Unlike other ablation targets, we observed that static blood products within CCMs can impair gradient echo-based thermography. The resulting signal dropout within portions of a CCM may degrade the thermal-anatomic correlation utilized to optimize safety. Thus, CCMs require additional vigilance, and the surgeon must rely more upon prior empirical experience with spatial-temporal ablation dynamics. As temperatures within a CCM have greater potential for inaccuracy, additional care should be taken to use conservative laser power settings (e.g. <12–13 W). Once ablation volume extends outside the CCM, thermal-anatomic correlation becomes more readily apparent, and thermography is sufficient for monitoring treatment of both perilesional epileptogenic cortex and off-target tissue at risk.
Delayed post-ablation imaging, obtained in the majority of subjects, showed a central T2-hypointense nodule circumscribed by T2 hyperintensity. These findings are consistent with a parenchymal ablation cavity (encephalomalcia) around the central involuting CCM. No subject showed postoperative evidence of CCM growth or hemorrhage. Indeed, histopathological examination of an ablated CCM found no remaining pathognomonic features. Together, these findings support the notion that SLA can obliterate structural and cellular features of CCMs.
Our observations may not necessarily apply to all cavernous malformations. For instance, a CCM can come to clinical attention not from epilepsy, but from a natural history of hemorrhage and growth, causing headaches or neurological deficits. Subcortical, brainstem, and spinal cord cavernous malformations provide additional management challenges with respect to the sensitivity of surrounding neural structures. Also, familial CCMs may present with multiple lesions, varied neurological sequelae, and greater risk of recurrence. None of the subjects in this series had a personal history of symptomatic bleeding. Thus, greater caution before using SLA for a deep-seated CCM, multi-focal or familial CCM, and/or lesions with aggressive natural histories may be warranted, especially since the tissue temperatures achieved during laser interstitial thermal therapy are well below those achieved by direct current electrocautery instruments and unlikely to provide direct hemostasis.46 In patients presenting with acute hemorrhage and neurological deficits related to mass effect, strong consideration should still be given to open microsurgery.
Conclusion
Real-time MR thermography-guided SLA is a minimally invasive alternative to open microsurgery that can definitively ablate both the CCM and associated epileptic cortex while potentially minimizing collateral injury to off-target structures. SLA of CCMs is feasible, and when compared to open surgery, may be as effective for epilepsy and as tolerable or more tolerable. SLA thus holds promise as a first-line, minimally-invasive therapy for management of epilepsy associated with CCMs, but larger case-controlled long-term studies are needed.
SIGNIFICANCE:
In a consecutive retrospective series, MR thermography-guided SLA was an effective alternative to open surgery for epileptogenic CCM. The appoach was free of hemorrhagic complications, and clinically significant neurological deficits were predictable. SLA presents no barrier to subsequent open surgery when needed.
KEY POINTS:
Magenetic susceptibility of sequestered blood products within CCM can compromise MR thermography within the boundaries of these lesions, but perilesional cortex is imaged with relative ease.
MR thermography-guided SLA is a potentially safe and effective surgical alternative for epileptogenic cerebral cavernous malformations.
While we observed no hemorrhages, SLA and open surgery should be used with caution in regions at risk of causing disability.
Interval imaging and pathological examination suggest that SLA causes involution of cerebral cavernous malformations.
SLA presents no barrier to subsequent intracranial monitoring or open resection.
ACKNOWLEDGEMENTS
We thank Gloria Novak for clinical research coordination and Robert Smith for coordinating MRI scan acquisition. This study was funded in part by Medtronic, Inc. to DLD (A1225797BFN:1056035), the NIH/NINDS to DLD (R01NS088748, K02NS070960), and a Shared Instrumentation Grant (S10: Grant 1@10OD016413-01) to the Emory University Center for Systems Imaging Core.
Footnotes
DISCLOSURES
JTW and REG serve as consultants to Medtronic, Inc. and receive compensation for these services. DLD has had an industry-sponsored research grant from Medtronic, Inc. Medtronic, Inc. develops products related to the research described in this paper. The terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict of interest policies. The remaining authors have nothing to disclose.
ETHICAL PUBLICATIONS STATEMENT
All authors confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Raychaudhuri R, Batjer HH, Awad IA. Intracranial cavernous angioma: a practical review of clinical and biological aspects. Surg neurol. 2005;63:319–328. [DOI] [PubMed] [Google Scholar]
- 2.Rosenow F, Alonso-Vanegas MA, Baumgartner C, et al. Cavernoma-related epilepsy: Review and recommendations for management - Report of the Surgical Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2013;54:2025–2035. [DOI] [PubMed] [Google Scholar]
- 3.Poorthuis MHF, Klijn CJM, Algra A, et al. Treatment of cerebral cavernous malformations: a systematic review and meta-regression analysis. J Neurol Neurosurg Psychiatry. 2014;85:1319–1323. [DOI] [PubMed] [Google Scholar]
- 4.Baumann CR, Schuknecht B, Lo Russo G, et al. Seizure outcome after resection of cavernous malformations is better when surrounding hemosiderin-stained brain also is removed. Epilepsia. 2006;47:563–566. [DOI] [PubMed] [Google Scholar]
- 5.von der Brelie C, von Lehe M, Raabe A, et al. Surgical resection can be successful in a large fraction of patients with drug-resistant epilepsy associated with multiple cerebral cavernous malformations. Neurosurgery. 2014;74:147–153; discussion 153. [DOI] [PubMed] [Google Scholar]
- 6.Kim W, Stramotas S, Choy W, et al. Prognostic factors for post-operative seizure outcomes after cavernous malformation treatment. J Clin Neurosci. 2011;18:877–880. [DOI] [PubMed] [Google Scholar]
- 7.Horne MA, Flemming KD, Su IC, et al. Clinical course of untreated cerebral cavernous malformations: a meta-analysis of individual patient data. Lancet Neurol. 2016;15:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steiger HJ, Markwalder TM, Reulen HJ. Clinicopathological relations of cerebral cavernous angiomas - observations in 11 cases. Neurosurgery. 1987;21:879–884. [DOI] [PubMed] [Google Scholar]
- 9.Awad I, Jabbour P. Cerebral cavernous malformations and epilepsy. Neurosurg Focus. 2006;21:e7. [DOI] [PubMed] [Google Scholar]
- 10.Chang EF, Gabriel RA, Potts MB, et al. Supratentorial cavernous malformations in eloquent and deep locations: surgical approaches and outcomes Clinical article. J Neurosurg. 2011;114:814–827. [DOI] [PubMed] [Google Scholar]
- 11.Moran NF, Fish DR, Kitchen N, et al. Supratentorial cavernous haemangiomas and epilepsy: a review of the literature and case series. J Neurol Neurosurg Psychiatry. 1999;66:561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNichols RJ, Gowda A, Kangasniemi M, et al. MR thermometry-based feedback control of laser interstitial thermal therapy at 980 nm. Lasers Surgery Med. 2004;34:48–55. [DOI] [PubMed] [Google Scholar]
- 13.Willie JT, Laxpati NG, Drane DL, et al. Real-Time Magnetic Resonance-Guided Stereotactic Laser Amygdalohippocampotomy for Mesial Temporal Lobe Epilepsy. Neurosurgery. 2014;74:569–584; discussion 584–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curry DJ, Gowda A, McNichols RJ, et al. MR-guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav. 2012;24:408–414. [DOI] [PubMed] [Google Scholar]
- 15.Schwarzmaier H-J, Eickmeyer F, von Tempelhoff W, et al. MR-guided laser-induced interstitial thermotherapy of recurrent glioblastoma multiforme: Preliminary results in 16 patients. Eur J Radiol. 2006;59:208–215. [DOI] [PubMed] [Google Scholar]
- 16.Carpentier A, Chauvet D, Reina V, et al. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg Med. 2012;44:361–368. [DOI] [PubMed] [Google Scholar]
- 17.Tovar-Spinoza Z, Carter D, Ferrone D, et al. The use of MRI-guided laser-induced thermal ablation for epilepsy. Childs Nerv Sys. 2013;29:2089–2094. [DOI] [PubMed] [Google Scholar]
- 18.Rao MS, Hargreaves EL, Khan AJ, et al. Magnetic Resonance-Guided Laser Ablation Improves Local Control for Postradiosurgery Recurrence and/or Radiation Necrosis. Neurosurg. 2014;74:658–667. [DOI] [PubMed] [Google Scholar]
- 19.Hawasli AH, Bagade S, Shimony JS, et al. Magnetic Resonance Imaging-Guided Focused Laser Interstitial Thermal Therapy for Intracranial Lesions: Single-Institution Series. Neurosurg. 2013;73:1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drane D, Loring D, Voets N, et al. Temporal lobe epilepsy surgical patients undergoing MRI-guided sterotactic laser ablation exhibit better episodic memory outcome as compared to standard surgical approaches. Epilepsy Currents. 2014;14:468–469 B.407. [Google Scholar]
- 21.Gross RE, Stern MA, Willie JT, et al. Stereotactic Laser Amygdalohippocampotomy for Mesial Temporal Lobe Epilepsy. Ann Neurol. 2018;83:575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drane DL, Loring DW, Voets NL, et al. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia. 2015;56:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCracken DJ, Willie JT, Fernald BA, et al. Magnetic Resonance Thermometry-Guided Stereotactic Laser Ablation of Cavernous Malformations in Drug-Resistant Epilepsy: Imaging and Clinical Results. Oper Neurosurg. 2016;12:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–530. [DOI] [PubMed] [Google Scholar]
- 25.Willie JT, Tung JK, Gross RE. Chapter 16 - MRI-Guided Stereotactic Laser Ablation A2 - Golby, Alexandra J. Image-Guided Neurosurgery. Boston: Academic Press; 2015:375–403. [Google Scholar]
- 26.Engel J, Van Ness PC, Rasmussen TB, et al. Outcome with Respect to Epileptic Seizures In: Engel J, ed. Surgical Treatment of the Epilepsies. Second ed. New York: Raven Press; 1993:609–621. [Google Scholar]
- 27.Hauser WA, Kurland LT. The Epidemiology of Epilepsy in Rochester, Minnesota, 1935 Through 1967. Epilepsia. 1975;16:1–66. [DOI] [PubMed] [Google Scholar]
- 28.Lewis EC, Weil AG, Duchowny M, et al. MR-guided laser interstitial thermal therapy for pediatric drug-resistant lesional epilepsy. Epilepsia. 2015;56:1590–1598. [DOI] [PubMed] [Google Scholar]
- 29.Jethwa PR, Barrese JC, Gowda A, et al. Magnetic Resonance Thermometry-Guided Laser-Induced Thermal Therapy for Intracranial Neoplasms: Initial Experience. Neurosurgery. 2012;71:133–144. [DOI] [PubMed] [Google Scholar]
- 30.Hawasli AH, Ray WZ, Murphy RKJ, et al. Magnetic Resonance Imaging-Guided Focused Laser Interstitial Thermal Therapy for Subinsular Metastatic Adenocarcinoma: Technical Case Report. Neurosurgery. 2012;70:332–338. [DOI] [PubMed] [Google Scholar]
- 31.Jethwa PR, Lee JH, Assina R, et al. Treatment of a supratentorial primitive neuroectodermal tumor using magnetic resonance-guided laser-induced thermal therapy. J Neurosurg Pediatr. 2011;8:468–475. [DOI] [PubMed] [Google Scholar]
- 32.Sloan AE, Ahluwalia MS, Valerio-Pascua J, et al. Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma Clinical article. J Neurosurg. 2013;118:1202–1219. [DOI] [PubMed] [Google Scholar]
- 33.Leonardi MA, Lumenta CB. Stereotactic guided laser-induced interstitial thermotherapy (SLITT) in gliomas with intraoperative morphologic monitoring in an open MR: Clinical expierence. Minim Invasive Neurosurg. 2002;45:201–207. [DOI] [PubMed] [Google Scholar]
- 34.Esquenazi Y, Kalamangalam GP, Slater JD, et al. Stereotactic laser ablation of epileptogenic periventricular nodular heterotopia. Epilepsy res. 2014;108:547–554. [DOI] [PubMed] [Google Scholar]
- 35.Akers A, Al-Shahi Salman R, I AA, et al. Synopsis of Guidelines for the Clinical Management of Cerebral Cavernous Malformations: Consensus Recommendations Based on Systematic Literature Review by the Angioma Alliance Scientific Advisory Board Clinical Experts Panel. Neurosurgery. 2017;80:665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jehi LE, Palmini A, Aryal U, et al. Cerebral cavernous malformations in the setting of focal epilepsies: pathological findings, clinical characteristics, and surgical treatment principles. Acta neuropathol. 2014;128:55–65. [DOI] [PubMed] [Google Scholar]
- 37.Yeon JY, Kim JS, Choi SJ, et al. Supratentorial cavernous angiomas presenting with seizures: surgical outcomes in 60 consecutive patients. Seizure. 2009;18:14–20. [DOI] [PubMed] [Google Scholar]
- 38.Hsu PW, Chang CN, Tseng CK, et al. Treatment of epileptogenic cavernomas: surgery versus radiosurgery. Cerebrovasc Dis. 2007;24:116–120; discussion 121. [DOI] [PubMed] [Google Scholar]
- 39.Bartolomei F, Regis J, Kida Y, et al. Gamma Knife radiosurgery for epilepsy associated with cavernous hemangiomas: A retrospective study of 49 cases. Stereotact Funct Neurosurg. 1999;72:22–28. [DOI] [PubMed] [Google Scholar]
- 40.Cappabianca P, Alfieri A, Maiuri F, et al. Supratentorial cavernous malformations and epilepsy: seizure outcome after lesionectomy on a series of 35 patients. Clin Neurol Neurosurg. 1997;99:179–183. [DOI] [PubMed] [Google Scholar]
- 41.Zanello M, Wager M, Corns R, et al. Resection of cavernous angioma located in eloquent areas using functional cortical and subcortical mapping under awake conditions. Outcomes in a 50-case multicentre series. Neurochirurgie. 2017;63:219–226. [DOI] [PubMed] [Google Scholar]
- 42.Delev D, Oehl B, Steinhoff BJ, et al. Surgical Treatment of Extratemporal Epilepsy: Results and Prognostic Factors. Neurosurgery. 2018. [DOI] [PubMed] [Google Scholar]
- 43.Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic Radiosurgery for Epilepsy and Functional Disorders. Neurosurg Clin N Am. 2013;24:623–+. [DOI] [PubMed] [Google Scholar]
- 44.Barbaro NM, Quigg M, Ward MM, et al. Radiosurgery versus open surgery for mesial temporal lobe epilepsy: The randomized, controlled ROSE trial. Epilepsia. 2018;59:1198–1207. [DOI] [PubMed] [Google Scholar]
- 45.Quigg M, Harden C. Minimally invasive techniques for epilepsy surgery: stereotactic radiosurgery and other technologies. J Neurosurg. 2014;121 Suppl:232–240. [DOI] [PubMed] [Google Scholar]
- 46.Consiglieri GD, Killory BD, Germain RS, et al. Utility of the CO2 Laser in the Microsurgical Resection of Cavernous Malformations. World Neurosurg. 2013;79:714–718. [DOI] [PubMed] [Google Scholar]