Abstract
The hippocampus (HPC) and prefrontal cortex (PFC) are both necessary for learning and memory-guided behavior. Multiple direct and indirect anatomical projections connect the two regions, and HPC – PFC functional interactions are mediated by diverse physiological network patterns, thought to sub serve various memory processes. Disconnection experiments using contralateral inactivation approaches have established the role of direct, ipsilateral projections from ventral and intermediate HPC (vHPC and iHPC) to PFC in spatial memory. However, numerous studies have also prominently implicated physiological interactions between dorsal HPC (dHPC) and PFC regions in spatial memory tasks, and recent reports have identified direct dHPC – PFC connections. Whether dHPC – PFC interactions are necessary for spatial learning and memory has yet to be tested. Here, we used a chemogenetic inactivation approach using virally-expressed DREADDs (designer receptors exclusively activated by designer drugs) in rats to investigate the role of dHPC – PFC interactions in learning a hippocampal – dependent spatial alternation task. We implemented a rapid learning paradigm for a continuous W-track spatial alternation task comprising two components: an outbound, working memory component, and an inbound, spatial reference memory component. We investigated the effect of contralateral inactivation of dHPC and PFC on learning this task as compared with naïve and vehicle injection controls, as well as ipsilateral inactivation of the same regions. Contralateral dHPC – PFC inactivation selectively led to a significant impairment in learning the spatial working memory task compared to control groups, but did not impair learning of the spatial reference memory task. Ipsilateral inactivation animals showed similar learning rates as animals in the control groups. In a separate experiment, we confirmed that bilateral inactivation of PFC also leads to an impairment in learning the spatial working memory task. Our results thus demonstrate that dHPC – PFC interactions are necessary for spatial alternation learning in novel tasks. In addition, they provide crucial evidence to support the view that physiological interactions between dHPC and PFC play a key role in spatial learning and memory.
Keywords: Dorsal hippocampus, Prefrontal cortex, DREADDs, Spatial learning, Spatial alternation task, Spatial working memory
1. Introduction
Animals need to form, maintain and retrieve memories of their experiences in novel environments for survival. This capacity to utilize internal representations of the external environment to guide behavior depends upon functional networks distributed across multiple brain regions. The hippocampus (HPC) and medial prefrontal cortex (PFC), anatomically and functionally connected brain regions, both play key roles in our ability to learn, form and use memories to guide behavior (Eichenbaum, 2017; Preston & Eichenbaum, 2013; Shin & Jadhav, 2016). These regions have complementary and overlapping roles in memory processes, with the hippocampus critical for encoding, storage and retrieval of new memories (Day, Langston, & Morris, 2003; Eichenbaum & Cohen, 2001; Moser & Moser, 1998; Riedel et al., 1999); and PFC playing an integral role in long-term memory storage and retrieval, as well as executive functions such as working memory and decision making (Euston, Gruber, & McNaughton, 2012; Frankland, Bontempi, Talton, Kaczmarek, & Silva, 2004; Jung, Baeg, Kim, Kim, & Kim, 2008; Miller & Cohen, 2001; Takehara-Nishiuchi & McNaughton, 2008; Tse et al., 2007).
Inactivation studies in rodents have established the role of both regions in spatial memory formation and retrieval. It is known that bilateral inactivation of either HPC or PFC impairs the ability of rats to perform spatial tasks that require working memory (Churchwell, Morris, Musso, & Kesner, 2010; Floresco, Seamans, & Phillips, 1997; Riedel et al., 1999). In addition, functional interactions between these regions have been shown to be involved in these tasks (Churchwell et al., 2010; Floresco et al., 1997). Anatomically, the HPC and PFC are strongly connected via multiple direct and indirect projections in the rodent brain (Cenquizca & Swanson, 2007; Delatour & Witter, 2002; Shin & Jadhav, 2016; Vertes, 2004; Vertes, Hoover, Szigeti-Buck, & Leranth, 2007). Here, PFC is used to denote the prelimbic (PrL) and infra-limbic (IL) regions of the medial prefrontal cortex. A prominent monosynaptic, ipsilateral and unidirectional projection arises from the ventral and intermediate CA1 and subicular regions of the hippocampus (vHPC, iHPC respectively), and terminates across various regions of PFC (Cenquizca & Swanson, 2007; Swanson, 1981). Previous functional disconnection studies have reported impairments in the ability of rodents to perform spatial tasks upon disruption of vHPC – PFC and iHPC – PFC interactions mediated by these projections (Churchwell et al., 2010; Floresco et al., 1997; Wang & Cai, 2006, 2008). These studies used a contralateral inactivation approach where the vHPC/ iHPC and PFC regions are inactivated in different hemispheres, thereby disrupting interactions mediated by the ipsilateral projections. Using this method, iHPC – PFC interactions have been shown to play a role in for encoding and retrieval in a spatial maze task (Churchwell et al., 2010). In addition, vHPC – PFC interactions are important in spatial working memory performance in a delayed T-maze alternation task (Wang & Cai, 2006) and delayed radial-arm maze performance task (Floresco et al., 1997), as well as in spatial navigation learning in a Morris water maze task (Wang & Cai, 2008). Other indirect anatomical connections between these regions also play a role in spatial working memory; for example, disrupting indirect projections from PFC to HPC via the nucleus reuniens also leads to memory impairments and disruption of hippocampal representations (Hallock, Arreola, Shaw, & Griffin, 2013; Ito, Zhang, Witter, Moser, & Moser, 2015; Layfield, Patel, Hallock, & Griffin, 2015; Viena, Linley, & Vertes, 2018).
Physiologically, multiple network patterns have been shown to mediate the coordination of hippocampal – prefrontal activity, which could sub serve the functional interactions indicated by the inactivation studies. Interestingly, these physiological interactions are seen both with respect to dorsal HPC (dHPC) as well as vHPC. Network patterns that mediate these interactions include phase-locking and coherence during theta oscillations (6–12Hz) (Benchenane et al., 2010; Gordon, 2011; Hyman, Zilli, Paley, & Hasselmo, 2005; Jones & Wilson, 2005; Siapas, Lubenov, & Wilson, 2005), coordinated reactivation during sharp wave ripples (150–250Hz) (Jadhav, Rothschild, Roumis, & Frank, 2016; Peyrache, Khamassi, Benchenane, Wiener, & Battaglia, 2009; Tang & Jadhav, 2018; Tang, Shin, Frank, & Jadhav, 2017), and cross-regional theta-gamma coupling (Spellman et al., 2015; Tamura, Spellman, Rosen, Gogos, & Gordon, 2017), all of which have been implicated in spatial working memory. Numerous studies have thus established that theta oscillations and SWRs mediate dHPC – PFC interactions during learning and performance of spatial memory tasks. Despite the abundance of electrophysiological studies suggesting the importance of dHPC – PFC interactions in spatial alternation behavior, to our knowledge, whether disruption of interactions between these two regions leads to spatial learning impairments has yet to be tested.
Several lines of evidence suggest the possibility that that dHPC – PFC interactions play a role in spatial learning and memory. Recently, direct connections that arise from dHPC and project unilaterally to the PFC have been reported (DeNardo, Berns, DeLoach, & Luo, 2015; Hoover & Vertes, 2007; Xu & Sudhof, 2013; Ye, Kapeller-Libermann, Travaglia, Inda, & Alberini, 2017), and these projections have been shown to mediate contextual fear memory (Ye et al., 2017). Indeed, it is known that dHPC place cells represent spatial information with the highest precision compared to iHPC and vHPC regions, and therefore encode spatial contextual information with high fidelity (Kjelstrup et al., 2008). Further, our previous studies have reported that SWRs in dHPC mediate coordinated reactivation of spatial information in the dHPC – PFC network (Jadhav et al., 2016; Tang et al., 2017). Coordinated dHPC – PFC reactivation is especially strong during initial learning of spatial alternation behavior (Tang & Jadhav, 2018; Tang et al., 2017), suggesting that dHPC – PFC interactions may play a role in novel task learning. In the current study, we therefore investigated whether disruption of dHPC – PFC interactions using a contralateral inactivation approach impairs spatial alternation learning.
We implemented a rapid, single-day learning paradigm in a hippocampal-dependent W-track alternation task comprising two components, a spatial reference memory component, and a spatial working memory component. Using a chemogenetic inactivation approach, we tested if dHPC – PFC interactions contribute to learning in a novel W-track maze. Virally introduced DREADDs were used for precise targeting of excitatory circuits in dHPC and PFC, with systemic Clozapine N-oxide (CNO) injections for inactivating circuits in these animals (Roth, 2016; Urban & Roth, 2015). Learning was assessed in multiple groups of animals: contralateral inactivation of dHPC and PFC, ipsilateral inactivation of these regions, systemic vehicle-injected controls, and naïve controls. We found that contralateral inactivation led to a specific impairment in learning the spatial working memory component of the task. Ipsilateral inactivation, which accounts for effects of unilateral inactivation as well any non-specific effects of systemic CNO injections, showed no deficits in learning. In addition, we also performed experiments to confirm that bilateral PFC inactivation impairs learning in this task, as established in other spatial working memory tasks (Churchwell et al., 2010; Wang & Cai, 2006, 2008). Our results demonstrate that dHPC – PFC interactions are necessary for rapid spatial alternation learning, and provide crucial supporting evidence for the hypothesis that physiological interactions between the dHPC and PFC play an important role in spatial learning and memory.
2. Materials and methods
2.1. Animals
Adult Long Evans rats (n = 46, 6–8 months old, 450–600g) obtained from Charles River Laboratories were used for all experiments. All procedures were conducted in accordance with the guidelines of the US National Institutes of Health and approved by the Institutional Animal Care and Use Committee at Brandeis University. All animals used were individually housed in temperature and humidity regulated cages and kept in a facility maintained in a 12-hour light-dark cycle. Ad libitum food and water were provided to the animal subjects before they were food deprived in preparation for the experiments.
2.2. Handling and linear track pre-training
Animals were handled for 3–6 weeks in order to habituate them to human interaction, and then food deprived until their weight reached 85–90% of their baseline weight. During the food deprivation phase, the animals were also habituated to the sleep box. Thereafter, the animals were pre-trained to run on a linear track interleaved with rest sessions in the sleep box, as previously described (Jadhav, Kemere, German, & Frank, 2012; Jadhav et al., 2016). The animals earned evaporated milk (upon triggering IR beams on the reward wells) at each end of the track for each successful alternation. Repeated visits to recently visited reward wells were not rewarded. On each pre-training day, the animals were first placed in the sleep box for 15–20 mins. The animals were then allowed to freely run on the linear track for two 15– minute training sessions interleaved by a 15–20 minute sleep box session. The animals were trained for 3 – 6 days to reach the behavioral criterion threshold of 50 rewards per 15–minute run session.
2.3. Injection of DREADDs (designer receptors exclusively activated by designer drugs)
2.3.1. Viral vectors
The recombinant adeno-associated viral vector constructs expressing inhibitory DREADDs (hM4Di / rAAV8-CaMKIIα-hM4D(Gi)-mCherry, 3.3×1012 Virus Molecules/ml) used in this study were purchased from the University of North Carolina vector core.
2.3.2. Clozapine N-oxide (CNO) preparation
Clozapine N-oxide (CNO, 3–5 mg/kg, obtained from Tocris Bioscience) was dissolved in DMSO and then diluted with sterile saline (0.9%). During experimental sessions (see 2.4.3 below), this CNO solution was injected intraperitoneally (i.p.) for activation of hM4Di DREADDs (Roth, 2016; Urban & Roth, 2015) 30 minutes before exposure to the W-track.
2.3.3. Surgery
Surgical procedures were as described previously (Jadhav et al., 2012; Jadhav et al., 2016). Briefly, anesthesia was induced using a ketamine-xylazine-atropine mixture (ketamine:100mg/ml, xylazine: 20mg/ml, atropine: 0.54mg/ml, saline: 0.9%) via i.p. injections. Anesthetized state was maintained throughout the surgery with isoflurane (0.8%−2.5% isoflurane by volume in oxygen at a flow rate of 2L/min). For contralateral inactivation, 3µl of the viral vector constructs were microinjected into the medial prefrontal cortex (PFC) in the right hemisphere, with prelimbic (PrL) and infralimbic (IL) cortical regions as primary targets (1.5µl injected: AP = +3.0mm,ML = +0.7mm, DV = −4.0mm from bregma; 1.5µl injected: AP = +3.0mm, ML = +0.7mm, DV = −4.5mm from bregma), and the CA1 region of the dorsal hippocampus in the opposite (left) hemisphere (1.5µl injected: AP = −3.6mm, ML = −2.2mm, DV = −2.4mm from bregma; 1.5µl injected: AP = −3.6mm, ML = −2.2mm, DV = −2.2mm from bregma) (co-ordinate references from (Paxinos & Watson, 2004) using a Nanoject II injector. Ipsilateral inactivation animals received dHPC and PFC virus injections in the same (right) hemisphere, and bilateral PFC inactivation animals received virus injections in PFC in both hemispheres. Administration of postoperative analgesics (Buprenorphine, 0.3mg/kg; Meloxicam, 5mg/kg) was maintained for at least two days post-surgery. After the surgery, the animals were provided with ad libitum food and water for at least a week before being food deprived in preparation for experimental sessions. In order to ensure optimal viral expression, all animals injected with viral constructs of hM4Di were tested on the behavioral task 21 – 24 days after the day of the viral injection.
2.4. Experimental procedure
2.4.1. Retraining
After recovery from surgery, the animals were retrained for at least three days on the linear track, similar to the pre-training procedure. Retraining was done to ensure that the surgery procedure did not impair the animals behaviorally, and to prepare them for the W-track experimental sessions. Animals were required to meet the criterion level of earning at least 50 reward well visits per 15–minute session before they were used for W-track behavioral experiments (Jadhav et al., 2016; Tang et al., 2017).
2.4.2. W-track spatial alternation behavior
The W-track continuous spatial alternation behavioral task has been described in our previous studies (Jadhav et al., 2016; Tang et al., 2017). Briefly, animals were allowed to run freely on a ‘W’ shaped track (Dimensions: 76 × 81 cm). Reward wells that automatically dispensed evaporated milk were placed at the ends of each of the three arms of the W -maze. The reward was only delivered when the animal triggered the infrared beams on the reward wells while adhering to the following rules (see Fig. 1A):
Center well visits were rewarded if either the left or right reward well was previously visited (Inbound task).
Visits to the outer arm wells were rewarded if the center well was previously visited, and the animals visited the reward well on the opposite arm in the prior trial (Outbound task).
Repeated visits to the same reward well were not rewarded.
The first inbound and outbound trajectories were rewarded.
The W-track task is hippocampal – dependent (Kim & Frank, 2009), and further, we have shown that disruption of hippocampal sharp-wave ripples (SWRs) impairs learning in the outbound, spatial working memory component of the task (Jadhav et al., 2012). The outbound component requires animals to remember the previous outer arm visited in order to choose the opposite side arm as the next correct choice. Outbound learning is therefore a spatial working memory task, requiring integration of information across multiple trails. On the other hand, the inbound component is a spatial reference memory task, requiring knowledge of current location and implementation of a return-to-center rule (Jadhav et al., 2012).
Figure 1. W-track behavior and experimental design.
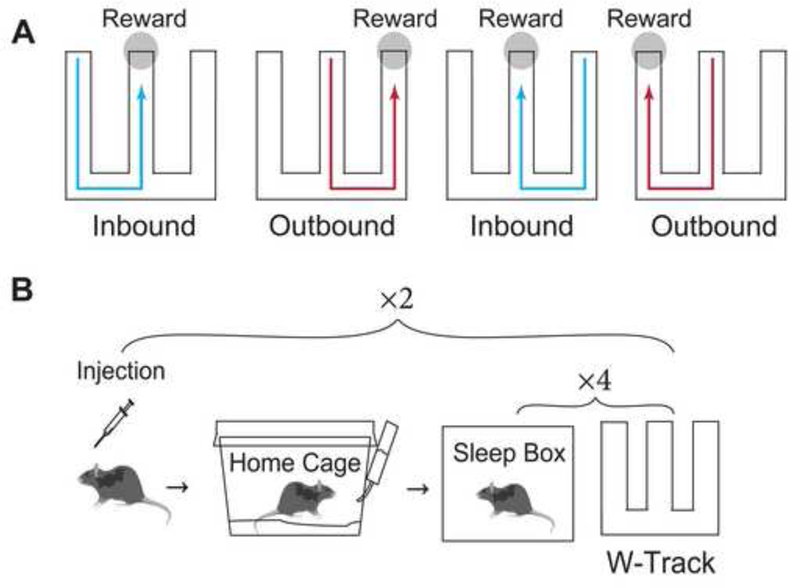
(A) Schematic illustrating the inbound and outbound rules of the W-track task. Animals learn the sequence of rewarded locations in a novel W-track environment by trial and error. Animals must return to the center when in an outside arm (inbound trajectory) and move from the center to alternate outside arms (outbound trajectories) for reward
(B) Schematic illustrating the sequence of rest and run sessions in the rapid learning W-track task. Animals were injected with CNO or a vehicle mixture 30 minutes before the first and fifth W-track session and placed in their home cages for 30 minutes post-injection. Each 15-minute W-track session was preceded by a 15–20 minute rest session in the sleep box. Four run-sleep combinations at ~2 hours marked the mid-point of the experiment. Animals in the naïve group were tested using the same experimental design but received no injections.
In the current experiment, animals in all groups were placed in the sleep box for 20 minutes before being exposed to the novel W-maze environments in the first behavior session. We established a rapid learning paradigm where animals learned the alternation task in a novel W-track environment in a single day with interleaved run and sleep sessions. In this single-day learning paradigm, we thus used a standardized behavioral protocol across all the groups, in which animals ran eight 15–minute run sessions interleaved with 15–20 minute rest box sessions (Fig. 1). Naïve control animals with no manipulations successfully learned both components of the task in these 8 behavioral sessions (Figs. 3–4).
Figure 3. Contralateral Inactivation of dorsal HPC – PFC interactions impairs learning of the spatial working memory component of the W-track task.
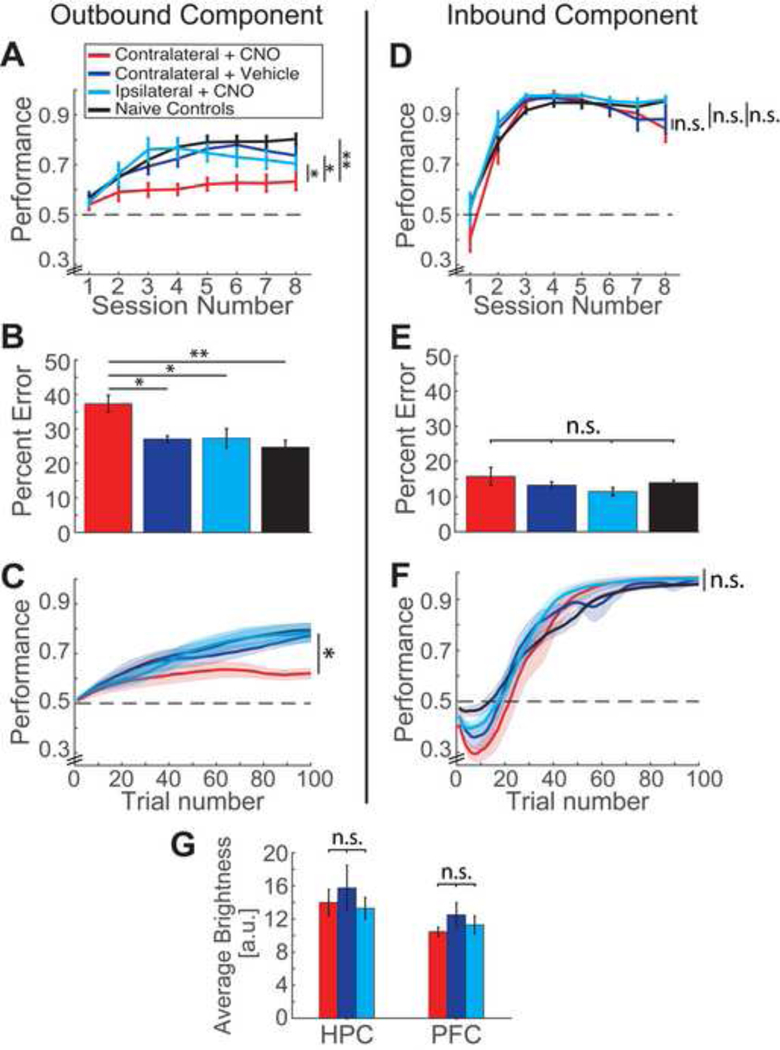
(A) Performance per session for outbound trials for the four groups: Contralateral + CNO/ experimental (red, n = 9), Contralateral + Vehicle/ vehicle controls (blue, n = 8), Ipsilateral + CNO/ ipsilateral controls (cyan, n = 7) and Naïve controls (black, n = 10). Horizontal dotted line represents chance level performance of 0.5. Significance values indicate comparison of learning rate across sessions for the four groups of animals. Animals in the experimental groups were significantly impaired in learning the outbound component as compared to all other groups. *P < 0.05, **P < 0.01, ***P < 0.001.
(B) Percentage of outbound errors made in the W-track task. Significance values indicate comparison of error rate for the entire learning period of eight sessions. Experimental group animals made significantly more errors compared to the other groups.
(C) Outbound learning curves for the first 100 trials. Shaded areas represent SEM. Significance values indicate comparison of error rate for the first 100 trials. Experimental group animals made significantly more errors during the initial learning period compared to the other groups.
(D) Performance per session for inbound trials for the four groups. There were no significant differences across groups in learning the inbound component of the task. n.s – not significant.
(E) Percentage of inbound errors made in the W-track task. The fraction of inbound errors for all eight run sessions were similar across groups.
(F) Inbound learning curves for the first 100 trials. The error rate was similar across groups during the initial learning period of 100 trials. Shaded areas represent SEM.
(G) Quantification of fluorescence intensity levels for the three groups injected with DREADDs in the dHPC and PFC: experimental, vehicle control and ipsilateral control groups.
Color legends are similar across all panels. Error bars represent standard error of mean (SEM). *P < 0.05, **P < 0.01, ***P < 0.001, n.s – not significant (detailed statistics are reported in text).
Figure 4. Bilateral inactivation of PFC impairs spatial alternation learning.
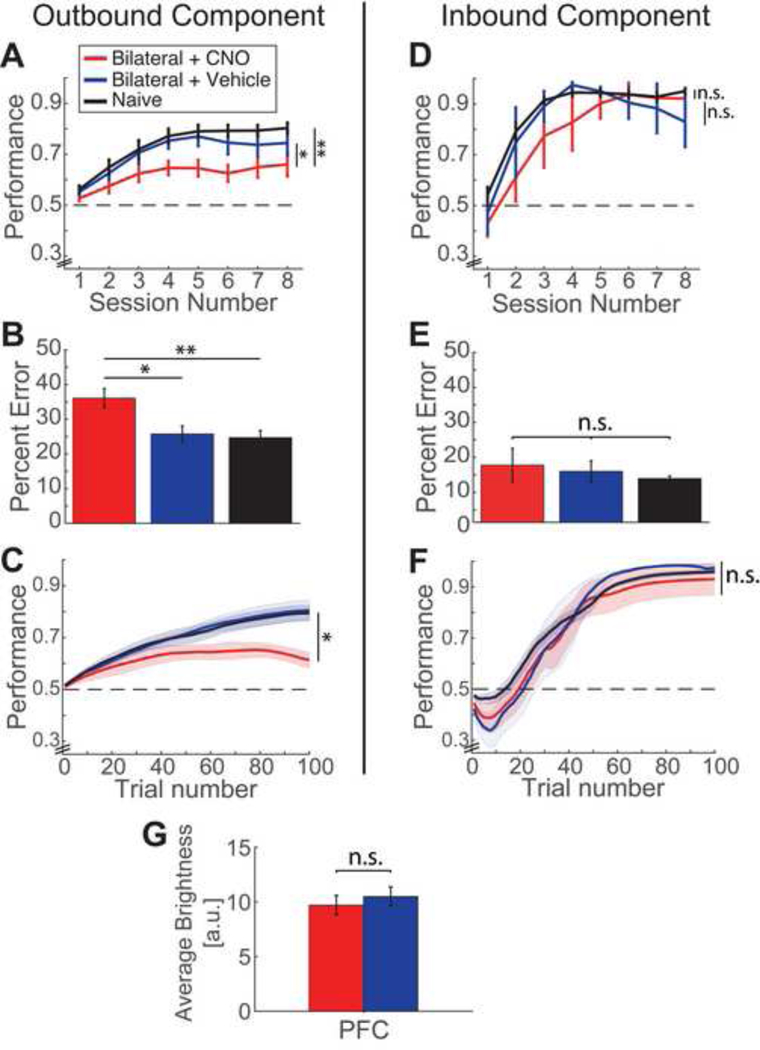
(A) Performance per session for outbound trials for three groups: Bilateral + CNO/ experimental (red, n = 7), Bilateral + Vehicle/ vehicle controls (blue, n = 5), and Naïve controls (black, n = 10) groups of rats. Horizontal dotted line represents chance level performance at the level of chance. Significance values indicate comparison of learning rate across sessions for the three groups of animals. Bilateral inactivation animals are significantly impaired in learning the outbound component compared to the control groups. *P < 0.05, **P < 0.01.
(B) Percentage of outbound errors made in W-track task. Significance values indicate comparison of error rate for the entire learning period of eight sessions. Bilateral PFC inactivation animals had significantly more errors compared to the other groups.
(C) Outbound learning curves for the first 100 trials. Shaded areas represent SEM. Significance values indicate comparison of error rate for the first 100 trials. Bilateral PFC inactivation animals had significantly more errors during the initial learning period.
(D) Performance per session for inbound trials for the three groups. There were no significant differences across groups in learning the inbound component of the task.
(E) Percentage of inbound errors made in W-track task. The fraction of inbound errors across all eight run sessions were similar across groups.
(F) Inbound learning curves for the first 100 trials. The error rate was similar across groups during the initial learning period of 100 trials. Shaded areas represent SEM.
(G) Quantification of fluorescence intensity expression levels for the groups injected with DREADDs bilaterally in PFC: Bilateral + CNO and Bilateral + Vehicle.
Color legends are similar across all panels. Error bars represent standard error of mean (SEM). *P < 0.05, **P < 0.01, n.s – not significant (detailed statistics are reported in text)
2.4.3. Behavior experiments
Animals were tested on their ability to learn and perform the rules of the W-maze task within the eight run sessions on the experimental day. In Experiment 1, we used 4 groups of animals (n = 34 animals): (a) Contralateral + CNO (n = 9): a contralateral inactivation group (experimental group) with virus injections in dHPC and PFC in contralateral hemispheres; (b) Contralateral + Vehicle (n = 8): a group (vehicle control group) with similar virus injections but with i.p injections of vehicle (DMSO and saline mixture) instead of CNO; (c) Ipsilateral + CNO (n = 7): a ipsilateral inactivation group (ipsilateral control group) with virus injections in dHPC and PFC in the same hemispheres; and (d) Naïve control (n = 10): a naïve control group with no manipulations. In Experiment 2, two additional groups were used (n = 12 animals): (a) Bilateral + CNO (n = 7): a bilateral PFC inactivation group (experimental group) with i.p CNO injections; and (b) Bilateral + Vehicle (n = 5): a group (vehicle control group) with similar virus injections bilaterally in PFC, but with i.p. vehicle injections during the experiment.
Animals in all groups were placed in the sleep box for 20 minutes before being exposed to the novel W-maze environments on the first session of the behavioral paradigm. Animals that underwent viral injection surgery were injected i.p with either the CNO preparation or Vehicle (DMSO/Saline mixture) 30 minutes before the start of the first session on the track. Since CNO-based inactivation of DREADDs is known to be effective for at least a period of ~2 hours in vivo, with ~75% reduction in activity of targeted neurons (Roth, 2016; Twining, Vantrease, Love, Padival, & Rosenkranz, 2017), we re-injected animals in the CNO and vehicle groups after four run-sleep session combinations (Experimental Design in Fig. 1B, each run-sleep session combination took ~30 minutes, and four run-sleep combinations at ~2 hours marked the mid-point of the experiment). This was followed by a 30–minute rest session in their home cage, and animals in the naïve control group were also allowed to rest in their home cage for 30 minutes after four sessions for similarity across groups. The behavioral protocol was then re-continued for a further four run-sleep session combinations.
2.5. Histology
2.5.1. Tissue preparation
Following the conclusion of the experiments, animals were anesthetized with isoflurane, injected with Euthanasol, and perfused intracardially with isotonic sucrose and 4% formaldehyde. The brains were harvested and stored in 30% sucrose / 4% formaldehyde before being sectioned at a thickness of 50 μm using a microtome. The sliced brain tissues were mounted onto slides with DAPI Fluoromount. Expression of the hM4Di viral construct in the regions of interest was confirmed and quantified using a Keyence BZ-X700 microscope.
2.5.2. Immuno-histology
The representative images shown in Fig. 2 were obtained using immunostaining. Each slice was washed overnight at 4°C with a block solution containing 10% normal donkey serum, 0.2% Triton-X100 and PBS. The slices were then incubated in a solution containing the primary antibody at a dilution of 1:1000 before being incubated with the secondary antibody at a dilution of 1:500. The tissues were washed with PBS for three 5 minute durations after each incubation step. The antibodies used were rabbit polyclonal anti-mCherry (Novus Biologicals, catalog # NBP2–251157) and polyclonal donkey anti-rabbit Alexa 488 (Invitrogen, catalog #A-21206).
Figure 2. Histological validation of DREADDs expression in dorsal HPC and PFC.
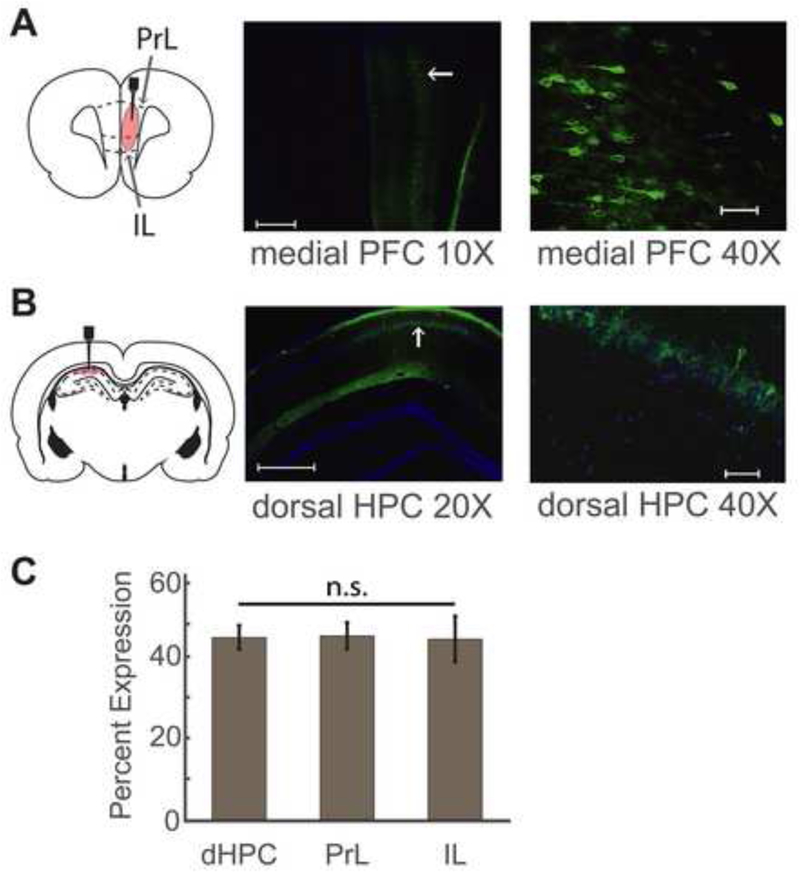
(A) Schematic illustration of targeted viral injection sites in PFC for contralateral dorsal HPC – PFC inactivation (left). Prelimbic (PrL) and infralimbic (IL) cortical regions within PFC are indicated in the schematic. Representative images showing hM4Di (green) and DAPI (blue) expression at 10X magnification (middle) and 40X magnification (right) of the prefrontal cortex region. Scale bars represent 500μm in the 10X image and 50μm in the 40X image. Region in 40X magnification image (right) shows expression in deep layers of PrL, indicated by the white arrow in the lower magnification image (middle).
(B) Schematic illustration of targeted DREADD injection sites in dorsal HPC (dHPC) for contralateral dHPC – PFC inactivation (left). Shaded area in schematic illustrates CA1 region. Representative images showing hM4Di (green) and DAPI (blue) expression at 20X magnification (middle) and 40X magnification (right) of the dHPC region. Scale bars represent 500μm in the 20X image and 50μm in the 40X image. Region in 40X magnification image (right) shows expression in CA1 region, indicated by the white arrow in the lower magnification image (middle). Note that the 40X image is at an angle compared to the lower magnification image.
(C) Quantification of viral infection in target regions. Percentage of cells positive for both hM4Di and DAPI in the dorsal CA1 region (dHPC), prelimbic cortex (PrL) and infralimbic cortex (IL). n.s – not significant, one-way ANOVA, n = 9 sections per regions, p = 0.99.
2.5.3. Quantification methods for virus expression
To compare the expression of the hM4Di viral constructs in PFC and dHPC across the different groups of animals, we used the Keyence BZ-X700 Analyze software to quantify mCherry expression using representative sections from each virus injected animal. PFC expression was quantified in the targeted PrL and IL regions, and CA1 region of dHPC. Average pixel brightness (in arbitrary units) was calculated for manually selected regions of interest and normalized by the average pixel intensity obtained from a second baseline area (Figs. 3G, 4G).
We also quantified estimated infection levels to confirm targeting. Average percentage of infection of hM4Di in PrL, IL and dorsal CA1 regions was calculated using the hybrid cell counting tool in the Keyence BZ-X700 Analyze software. For each region, the total number of cells showing co-expression of mCherry and DAPI in a field of view were calculated and then divided by the total number of DAPI expressing cells observed to get the percent expression (Fig. 2).
2.6. Data Analysis
Behavioral Analysis: Some animals in initial experiments did not show any fluorescence expression due to failure of the virus or injection equipment (expression was categorized as all-or-none), and were not included in any data analysis. Fluorescence expression for all animals used in the study is quantified in Figs. 2C, 3G, 4G. For behavioral analysis, the movement of each animal on the W-track task was recorded using a Mako G-125C camera (30 frames/sec, 0.12 cm/pixel resolution). Behavioral and video data were acquired using a system from SpikeGadgets Inc., and position of the animal on the track was tracked using SpikeGadgets Inc. software applying a semi-automated method. The pixel intensity of the white fur on the back of the animal was used to determine the position of the animal at any given time. The positions were concatenated, smoothed and then classified into distinguishable inbound and outbound trials, as described before (Jadhav et al., 2012; Jadhav et al., 2016). Trials where the animal’s position originated from either the left or right reward well were determined to be inbound trials. Similarly, trials where the animal’s position originated from the middle reward well were determined to be outbound trials (Fig. 1A). We used a state-space model of learning (Jadhav et al., 2012; Jadhav et al., 2016; Smith et al., 2004) to estimate the probability and respective confidence bounds of an individual animal making the correct choice during each trial of the behavior. In contrast to using a moving average analysis to assess learning, using this model enables assessing changes related to learning with greater sensitivity. Task performance per session and learning curves were obtained using the state-space model.
The learning performance of animals was compared with a mixed two-factor analysis of variance analysis with repeated measures, with the groups as the between-subjects factor and session number as the within-subjects factor. If a significant effect was noted, post-hoc analysis with Bonferroni correction was used to analyze performance across groups. Both Bonferroni correction and Tukey post-hoc tests were used, and the more conservative Bonferroni measures are reported here. Average measures of performance for each animal were compared with a one-way analysis of variance, with post-hoc comparisons between groups using Bonferroni correction.
3. Results
3.1. Histology
In order to perturb the activity of dHPC and PFC during learning of the W-track task, we injected adeno-associated viral constructs of an evolved human muscarinic receptor (hM4Di DREADDs) targeting excitatory neurons in the specified regions using a CaMKIIα promoter (Fig. 2). In Experiment 1, we investigated the effects of functionally disconnecting dHPC and PFC during spatial learning by targeting these regions in contralateral hemispheres (contralateral inactivation group). Fig. 2A, B show representative images for PFC and dHPC respectively. Animals in the ipsilateral inactivation group received virus injections in the same hemisphere. Animals in the vehicle control group that received vehicle i.p. injections rather than CNO during the experiment were also injected with virus in contralateral hemispheres. Naïve control animals did not receive any manipulation. In Experiment 2, virus injections were targeted bilaterally in PFC in both the CNO experimental group and the vehicle control group. In order to confirm viral targeting of hippocampal and prefrontal regions, we quantified viral infection levels in dHPC, PrL and IL cortical regions as the percentage of DAPI positive cells that showed hM4Di expression, and saw similar levels of expression in these regions (Fig. 2C; one section each from 9 animals for each region; one-way ANOVA; F(2,24) = 0.01; p = 0.99).
3.2. Experiment 1: Contralateral Inactivation of PFC and dHPC impairs learning of the spatial working memory component of the W-track task.
Behavioral learning on the W-track maze was assessed 21 – 24 days after viral injection to ensure optimal DREADDs expression. CNO was injected 30 minutes before the animals were introduced to the W-track to allow the hM4Di constructs to inactivate the infected regions. Chemogenic inactivation offers the advantage of precisely targeting excitatory circuits in virally targeted regions. Further, systemic CNO injections can inactivate circuits for in vivo for up to ~2 hours (Roth, 2016; Twining et al., 2017). Thus, our experimental design (Fig. 1B) allowed us to test the role of dHPC – PFC interactions in a rapid learning paradigm over repeated run-sleep sessions in a single day.
The effect of functionally disconnecting PFC and dHPC was investigated by comparing learning of the W-track task across four groups: Contralateral + CNO, Contralateral + Vehicle, Ipsilateral + CNO, and Naïve (n = 9, 8, 7 and 10 respectively). (a) The animals in the Contralateral + CNO group (experimental group) received injections of hM4Di viral constructs in the PFC in the right hemisphere of the brain, and dHPC in the left hemisphere of the brain. They were injected with CNO i.p. prior to the start of the first and fifth behavioral session (Fig. 1B) as described in the Materials and Methods section. The contralateral inactivation approach ensures that functional PFC and dHPC regions are preserved in one hemisphere each, while disrupting their ipsilateral interactions within the same hemisphere during learning of the task. (b) The animals in the Contralateral + Vehicle group (vehicle control group) were injected with hM4Di constructs contra-laterally similar to the Contralateral + CNO group. However, they were injected i.p. with a vehicle (DMSO and saline mixture) instead of CNO on the experiment day. (c) In the Ipsilateral + CNO group (ipsilateral control group), the viral hM4Di constructs were injected into the PFC and dHPC regions ipsilaterally, with i.p. CNO injections similar to the Contralateral + CNO group. In addition to accounting for any effects of unilateral inactivation of dHPC and PFC regions, this group also provides a control for non-specific effects of CNO. (d) The final group of Naïve control animals (naïve control group) did not receive any manipulations.
We examined learning performance in the groups separately for the outbound (Fig. 3A–C) and inbound components (Fig. 3D–F) of the task. The outbound component originates at the center well, and requires animals to remember the outer/ side arm visited in the previous trial in order to choose the opposite side arm as the next correct choice. Animals commit outbound errors when they incorrectly choose the same side arm that was visited previously. In contrast, the inbound component originates at the side wells, and requires animals to recognize their current location in a side arm and then return to the center to get reward. Animals commit inbound errors when they incorrectly choose to run from one side arm to another without visiting the center well, a form of perseverative error (Kim and Frank, 2009; Jadhav et al., 2012).
For the outbound component (Fig. 3A), a two factor ANOVA with repeated measures showed a significant main effect of group (n = 9, 8, 7, and 10 animals, F(3,30) = 7.33, p = 0.001), and significant interaction between group and session number (F(21,210) = 2.39, p = 0.001). Posthoc tests with Bonferroni correction revealed that the experimental group (Contralateral + CNO) showed a significant difference in learning performance from all the other groups (p = 0.012 vs. vehicle control group; p = 0.024 vs. ipsilateral control group; p = 0.001 vs. naïve controls). The three control groups showed similar learning performance (p > 0.776 for posthoc comparisons between the control groups). Since a significant interaction between group and session number was observed, a subsequent analysis of simple main effects revealed that significant differences between the experimental and control groups emerged by Session 3 (p = 0.038, p = 0.004, p = 0.005 for experimental vs. vehicle control, ipsilateral control and naïve control groups respectively for Session 3). We also examined average performance of animals over the entire learning period (all 8 sessions) by comparing the fraction of error trials across all eight behavioral sessions (Fig. 3B). The experimental animals had significantly more error trials than the control groups (one-way ANOVA, main effect of group: F(3,30) = 7.34, p = 0.001; posthoc tests with Bonferroni correction: p = 0.012, p = 0.02, p = 0.001 for experimental vs. vehicle control, ipsilateral control and naïve control groups respectively).
We used control analyses to confirm that the observed differences in learning performance by animals across groups were not simply explained by a possible difference in behavioral variables leading to a difference in number of trials. First, total number of outbound trials across the four groups for all sessions was similar (experimental group: 191.4 ± 18.2, vehicle control group: 219.0 ± 10.5, ipsilateral control group: 243.7 ± 13.2, naïve control group: 228.7 ± 10.2 outbound trials respectively; one-way ANOVA, F(3,30) = 1.05; p = 0.38). The number of trials performed per session was also similar across groups (two factor ANOVA with repeated measures, main effect of group, F(3,30) = 1.05, p = 0.38, no significant interaction between group and session number F(21,210) = 0.88, p = 0.63). Further, we confirmed that significant differences across groups were seen during initial learning by examining the first 100 trials across sessions for each animal (Fig. 3C; all animals performed at least 100 outbound trials across the eight behavioral sessions). Similar to the entire 8-session period, the experimental group also had significantly higher the fraction of errors for the first 100 trials compared to the control groups (one-way ANOVA, main effect of group: F(3,30) = 5.24, p = 0.005; posthoc tests with Bonferroni correction: p = 0.035, p = 0.028, p = 0.006 for experimental vs. vehicle control, ipsilateral control and naïve control groups respectively).
In contrast, for the inbound component of the task, all groups were similar in learning performance (Fig. 3D–F). For learning over the eight behavioral sessions (Fig. 3D), a two factor ANOVA with repeated measures showed no significant differences between groups (F(3,30) = 1.77, p = 0.174), and no significant interactions between group and session number (F(21,210) = 1.06, p = 0.384). The average performance as quantified by the number of error trials across all eight behavioral sessions was also similar across groups (Fig. 3E, one-way ANOVA, main effect of group: F(3,30) = 1.22, p = 0.319). In addition, for learning over the first 100 trials, there were no significant differences in fraction of error trials (Fig. 3F, one-way ANOVA, main effect of group: F(3,30) = 0.38, p = 0.77). Similar to the outbound component, the number of inbound trials per session across the four groups was similar (two factor ANOVA with repeated measures, main effect of group, F(3,30) = 1.23, p = 0.32, no significant interaction between group and session number F(21,210) = 0.83, p = 0.68).
Although overall learning performance was similar across groups for the inbound component, an increase in inbound perseverative error was apparent in all groups for early trials when performance in plotted against trajectories (Fig. 3F, note the early dip in performance). It has been previously reported that during early trials on a novel W-track, animals pre-trained on a linear track have a tendency to perseverate in running between the two outer arms back and forth without visiting the center arm (Kim and Frank, 2009; Jadhav et al., 2012). It is also known that hippocampal lesions lead to a significant increase in inbound perseverative error for several sessions (Kim and Frank, 2009). Although we did not find any difference in inbound error rate across the groups for Session 1 (one-way ANOVA for the four groups for Session 1; F(3,30) = 1.87; p = 0.16), we further quantified error rate for the “early trials” in Session 1 (the mean number of inbound trials in Session 1 across all the groups was 30.0 ± 1.6 trials, and we quantified error rate for a subset of “early trials” till the average mid-point of Session 1, i.e. the first 15 trials). We observed that the error rate was indeed significantly different across groups for these early trials (one-way ANOVA for the four groups in Experiment 1; F(3,30) = 5.35; p = 0.005). Post-hoc comparisons showed that this difference was significant only for experimental vs. naïve control group, p = 0.007 (p > 0.20 for all other posthoc comparisons). The partial inactivation of hippocampus in the experimental group may potentially lead to an early, transient increase in this inbound error. However, since this difference was significant only with respect to the naïve control group and not the ipsilateral control or vehicle control groups, we cannot rule out that other factors, such as surgical intervention and i.p. injections, could have contributed to this difference.
Finally, we confirmed that the observed behavioral differences in outbound learning were not due to difference in viral expression by comparing the intensity of fluorescence (Materials and Methods) across the three groups that received hM4Di injections - experimental, vehicle control, and ipsilateral control groups (Fig. 3G, n = 9, 7, and 8 sections respectively in the 3 groups for both HPC and mPFC, one section per animal; one-way ANOVA, HPC: F(2,21) = 0.39; p = 0.68; mPFC: F(2,21) = 0.98; p = 0.39).
3.3. Experiment 2: Bilateral Inactivation of mPFC impairs learning of W-track task.
Since PFC inactivation is known to affect spatial working memory tasks (Churchwell et al., 2010; Euston et al., 2012; Wang & Cai, 2006, 2008), we also investigated the effects of disrupting PFC activity by comparing learning across the following three groups: Bilateral + CNO (bilateral PFC experimental group/ bilateral PFC) with PFC targeted bilaterally for hM4Di injections, Bilateral + Vehicle (bilateral PFC vehicle control group/ vehicle controls) and the Naïve control group (n = 7, 5 and 10 respectively). We found that bilateral PFC inactivation group was significantly impaired in learning the outbound component of the W-track task across sessions as compared to controls (Fig. 4A; n = 7, 5 and 10 animals, repeated-measures ANOVA, main effect of group: F(2,19) = 7.80, p = 0.003, no significant interaction between group and session number: F(14,133) = 1.18, p = 0.298; posthoc tests with Bonferroni correction: p = 0.036 for bilateral PFC vs. vehicle control group; p = 0.003 for bilateral PFC vs. naive control group). Average performance quantified as percent error across all sessions also showed significantly higher error for the bilateral PFC inactivation group (Fig. 4B, one-way ANOVA, main effect of group: F(2,19) = 7.01, p = 0.005; post-hoc tests with Bonferroni correction: p = 0.04, p = 0.006 for bilateral PFC vs. vehicle control and vs. naïve control groups respectively). Similar to Experiment 1, comparison of learning across the first 100 trials (Fig. 4C) also confirmed significant differences in fraction of error trials between the experimental and control groups (one-way ANOVA, main effect of group: F(2,19) = 6.51, p = 0.007; posthoc tests with Bonferroni correction: p = 0.019, p = 0.014, p = 0.94 for bilateral PFC vs. vehicle control, bilateral PFC vs. naïve control, and vehicle control vs. naïve control groups respectively). Control analyses showed that the number of outbound trials per session across the three groups was similar (two factor ANOVA with repeated measures, main effect of group, F(2,19) = 0.45; p = 0.64, no significant interaction between group and session number F(14,133) = 1.65, p = 0.08), ruling out the possibility that the observed effects can be explained simply due to different number of trials across groups.
In contrast, learning in the inbound component was similar across the three groups (Fig. 4D-F; n = 7, 5 and 10 animals). Fig. 4D shows learning performance across sessions (repeated-measures ANOVA, main effect of group: F(2,19) = 1.49, p = 0.251, no significant interaction between group and session number: F(14,133) = 1.03, p = 0.431), Fig. 4E shows fraction of error trials across all sessions (one-way ANOVA, main effect of group: F(2,19) = 0.494, p = 0.618), and Fig. 4F shows learning across the first 100 trials (one-way ANOVA for fraction of error trials, main effect of group: F(2,19) = 0.35, p = 0.61). Inbound perseverative error for early trials (first 15 trials) was also similar across groups (one-way ANOVA, main effect of group: F(2,19) = 1.54, p = 0.24). The number of inbound trials per session across the three groups was similar (two factor ANOVA with repeated measures, main effect of group, F(2,19) = 0.43; p = 0.66, no significant interaction between group and session number F(14,133) = 1.41, p = 0.16). The observed behavioral differences in outbound learning were not due to difference in viral expression (intensity of fluorescence expression across the bilateral PFC and the vehicle group in Fig. 4G, n = 14 and 10 sections in each area respectively for the 2 groups, two bilateral sections per animal; t-test: t = −0.62, p = 0.54).
4. Discussion
Our results establish that dorsal hippocampal – prefrontal interactions are required for rapid spatial alternation learning in novel environments, and specifically, learning of spatial working memory tasks. We used a chemogenetic method to inactivate dHPC and PFC regions in contralateral hemispheres during acquisition of spatial alternation learning in a novel W-track maze. This contralateral inactivation strategy leaves intact the dHPC and PFC in one hemisphere, but disrupts functional interactions mediated by ipsilateral connections. Specific inactivation of these circuits in a single-day learning paradigm was implemented using i.p. CNO injections to activate hM4Di DREADDs expressed in target regions via viral vectors. Control groups included a vehicle control with similar viral expression of hM4Di DREADDs, but with vehicle i.p injections, in order to account for non-specific effects of viral expression. We also used an ipsilateral inactivation group in which hM4Di DREADDs were targeted to hippocampal and prefrontal regions in the same hemisphere, and these animals received i.p. CNO injections similar to the contralateral inactivation group. This group controls for effects of inactivating regions unilaterally, and any effects of contralateral interactions. Further, it accounts for any non-specific effects of CNO injections. This is an especially important control in light of recent reports about CNO dosages required for activating DREADDs in neural circuits (Gomez et al., 2017). Finally, a naïve control group was used to provide a baseline measure of learning and performance in the single day learning paradigm. We found that contralateral inactivation of dHPC and PFC led to a selective impairment in learning the outbound, spatial working memory component of the task relative to all the other control groups. This deficit in outbound learning was not simply a result of differences in behavioral parameters or viral expression. In contrast, learning of the inbound, spatial reference memory component was not affected. Ipsilateral inactivation animals learned similarly to the other control groups. Our results thus indicate that contralateral inactivation animals have a specific deficit in learning the spatial working memory task but not the spatial reference memory task, and this deficit can be attributed to impaired dHPC – PFC ipsilateral interactions. Interestingly, bilateral inactivation of PFC also led to a similar specific impairment in outbound, but not inbound, learning.
The role of hippocampal-prefrontal interactions in spatial learning, working memory, and memory-guided behavior is of great interest. The two regions have complementary roles in memory formation and retrieval, and further, it is thought that communication between the hippocampal episodic memory system and the prefrontal executive system is necessary for memory-guided behavior (Eichenbaum, 2017; Euston et al., 2012; Gordon, 2011; Preston & Eichenbaum, 2013; Shin & Jadhav, 2016). Multiple direct and indirect anatomical pathways between the two regions can support these interactions. These include prominent projections from ventral and intermediate CA1 and subicular regions (vHPC) to deep layers of PFC, indirect projections from hippocampus to PFC via entorhinal cortex, and indirect projections from PFC to hippocampus via the nucleus reuniens (NR) (Cenquizca & Swanson, 2007; Delatour & Witter, 2002; Vertes, 2004; Vertes et al., 2007). The direct connections from vHPC provide a possible pathway to communicate spatial and mnemonic information, which is known to be rapidly encoded in hippocampal circuits. Indeed, functional disconnection of these regions leads to deficits in spatial navigation learning and spatial working memory performance (Churchwell et al., 2010; Floresco et al., 1997; Wang & Cai, 2006, 2008). Disrupting indirect PFC to HPC projections via NR also leads to memory performance impairments (Hallock et al., 2013; Layfield et al., 2015), and this pathway can potentially provide contextual input (Ito et al., 2015) and support memory flexibility (Viena et al., 2018).
Multiple network patterns have been identified as the physiological substrates of these interactions, with hypothesized roles in memory processes (Benchenane, Tiesinga, & Battaglia, 2011; Gordon, 2011; Shin & Jadhav, 2016; Tang & Jadhav, 2018; Zielinski, Tang, & Jadhav, 2017). Prominently, theta and theta-gamma mediated interactions are important for spatial working memory performance (Gordon, 2011; Tamura et al., 2017), and SWR-mediated interactions have potentially an important role in initial learning and memory formation (Tang & Jadhav, 2018; Tang et al., 2017). These interactions have preferential hippocampus leading PFC directionality (Gordon, 2011; Tang & Jadhav, 2018). Interestingly, although these interactions have been observed between dHPC and PFC, the focus of inactivation studies for communication of hippocampal activity to PFC has been on the direct projection from vHPC to PFC (Churchwell et al., 2010; Floresco et al., 1997; Wang & Cai, 2006, 2008). It has been reported that theta oscillation mediated interactions between dHPC and PFC regions can at least partially be mediated through vHPC areas, given the dense interconnectivity along the dorsal – ventral hippocampus axis (O’Neill, Gordon, & Sigurdsson, 2013). However, this still leaves open the possibility that dHPC has a key role in these interactions, and also interactions mediated by other patterns such as SWRs.
Multiple lines of evidence suggest that even with an intact vHPC, loss of dHPC – PFC interactions may lead to memory deficits. a) First, direct connections from dHPC to PFC have been recently reported (DeNardo et al., 2015; Hoover & Vertes, 2007; Rajasethupathy et al., 2015; Xu & Sudhof, 2013; Ye et al., 2017), which play a crucial role in context-retrieval mediated fear memory (Ye et al., 2017). b) Next, since dHPC encodes spatial information with higher precision than vHPC (Kjelstrup et al., 2008), communication of this spatial information to PFC may be important for spatial context-dependent learning. c) Our recent studies have indicated that SWR-mediated interactions between dHPC and PFC are especially strong during initial spatial learning, suggesting a role in novel task learning (Jadhav et al., 2016; Tang & Jadhav, 2018; Tang et al., 2017). Further, it is important to point out that SWRs are not coherent between dHPC and vHPC regions (Patel, Schomburg, Berenyi, Fujisawa, & Buzsaki, 2013). dHPC SWRs can thus mediate PFC interactions for communication of information that is independent of vHPC SWRs. All these lines of evidence point towards a potential key role of dHPC – PFC interactions in spatial memory, possibly by providing access to the hippocampal spatial cognitive map. SWR-mediated dHPC – PFC interactions may especially be crucial for rapid learning in novel mazes, by communicating hippocampal replay to prefrontal networks (Tang & Jadhav, 2018). In the current study, we therefore tested whether disrupting dHPC – PFC interactions using a contralateral inactivation strategy can lead to impairment in spatial alternation learning in a novel maze. The experimental design emphasized novel task learning, in contrast to previous inactivation experiments (Churchwell et al., 2010; Floresco et al., 1997; Wang & Cai, 2006), and our results indeed demonstrate that functional interactions between dHPC and PFC are important for learning a spatial working memory task.
Interestingly, we observed a selective impairment in outbound, but not inbound learning. This selective deficit in learning the spatial working memory task is also observed as a result of disruption of awake hippocampal SWRs (Jadhav et al., 2012), hinting at the possibility of a relationship between SWR-mediated reactivation and dHPC – PFC interactions. Since inbound learning has been shown to be impaired by hippocampal inactivation (Kim & Frank, 2009), it indicates that the hippocampus alone, together with other regions that support simple action-outcome associations, is sufficient to support this component of the task. It is also possible that the inbound, reference memory component is a simpler task than the outbound, working memory component. Indeed, animals achieve higher task performance in the inbound component (Fig. 3, 4). The DREADDs-based chemogenetic inactivation method provides more specific targeting, but will result in more spared circuitry in the target regions as compared to pharmacological inactivation methods (Churchwell et al., 2010; Floresco et al., 1997; Hallock et al., 2013; Wang & Cai, 2006). These residual circuits may be able to support simpler inbound learning, but not outbound learning, which requires working memory and integration of information across space and time (requiring integration across multiple trials) (Jadhav et al., 2012).
The results of this study establish the importance of functional interactions between dHPC and PFC in spatial learning, and specifically, spatial working memory. Additionally, they provide crucial supporting evidence for studies examining the physiological substrates of dHPC –PFC interactions (Benchenane et al., 2011; Gordon, 2011; Shin & Jadhav, 2016; Tang & Jadhav, 2018; Zielinski et al., 2017). The similarity of specific impairments for this inactivation, and that for awake SWR disruption (Jadhav et al., 2012), makes it tempting to speculate that dHPC – PFC connections are the anatomical substrates of this interaction. However, additional studies are needed to dissect the relative contributions of multiple direct and indirect connections arising from dHPC and vHPC regions. By establishing the importance of dHPC – PFC interactions in novel task learning, this study also opens up the possibility that these interactions play a role in flexible task switching, which requires detection of changes in the external environment and changes in task contingencies to learn and implement new rules (Guise & Shapiro, 2017; Viena et al., 2018). Future studies that combine regional and pathway-specific inactivation with multisite physiology in different behavioral paradigms will be able to address these questions.
Highlights.
Chemogenetic inactivation of dorsal hippocampal (dHPC) – prefrontal (PFC) regions.
Contralateral dHPC – PFC inactivation impairs novel spatial alternation learning.
Ipsilateral dHPC – PFC inactivation animals are not impaired.
Bilateral inactivation of PFC also impairs spatial alternation learning.
Acknowledgements:
We thank all members of the Jadhav lab for comments on the manuscript.
Funding Sources:
This work was supported by the National Institute of Health [grant number R01 MH112661]; a Sloan Research Fellowship in Neuroscience (Alfred P. Sloan Foundation), a NARSAD Young Investigator grant (Brain and Behavior Foundation), and Whitehall Foundation award to SPJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: none.
REFERENCES
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, & Wiener SI (2010). Coherent theta oscillations and reorganization of spike timing in the hippocampal-prefrontal network upon learning. Neuron, 66(6), 921–936. doi: 10.1016/j.neuron.2010.05.013 [DOI] [PubMed] [Google Scholar]
- Benchenane K, Tiesinga PH, & Battaglia FP (2011). Oscillations in the prefrontal cortex: a gateway to memory and attention. Curr Opin Neurobiol, 21(3), 475–485. doi: 10.1016/j.conb.2011.01.004 [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, & Swanson LW (2007). Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev, 56(1), 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Musso ND, & Kesner RP (2010). Prefrontal and hippocampal contributions to encoding and retrieval of spatial memory. Neurobiol Learn Mem, 93(3), 415–421. doi: 10.1016/j.nlm.2009.12.008 [DOI] [PubMed] [Google Scholar]
- Day M, Langston R, & Morris RG (2003). Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature, 424(6945), 205–209. [DOI] [PubMed] [Google Scholar]
- Delatour B, & Witter MP (2002). Projections from the parahippocampal region to the prefrontal cortex in the rat: evidence of multiple pathways. Eur J Neurosci, 15(8), 1400–1407. [DOI] [PubMed] [Google Scholar]
- DeNardo LA, Berns DS, DeLoach K, & Luo L (2015). Connectivity of mouse somatosensory and prefrontal cortex examined with trans-synaptic tracing. Nat Neurosci, 18(11), 1687–1697. doi: 10.1038/nn.4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2017). Prefrontal-hippocampal interactions in episodic memory. Nat Rev Neurosci, 18(9), 547–558. doi: 10.1038/nrn.2017.74 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, & Cohen NJ (2001). From conditioning to conscious recollection New York: Oxford University Press. [Google Scholar]
- Euston DR, Gruber AJ, & McNaughton BL (2012). The role of medial prefrontal cortex in memory and decision making. Neuron, 76(6), 1057–1070. doi: 10.1016/j.neuron.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, & Phillips AG (1997). Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci, 17(5), 1880–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, & Silva AJ (2004). The involvement of the anterior cingulate cortex in remote contextual fear memory. Science, 304(5672), 881–883. doi: 10.1126/science.1094804 [DOI] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Michaelides M (2017). Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science, 357(6350), 503–507. doi: 10.1126/science.aan2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA (2011). Oscillations and hippocampal-prefrontal synchrony. Curr Opin Neurobiol, 21(3), 486–491. doi: 10.1016/j.conb.2011.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guise KG, & Shapiro ML (2017). Medial prefrontal cortex reduces memory interference by modifying hippocampal encoding. Neuron, 94(1), 183–192 10.1016/j.neuron.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Arreola AC, Shaw CL, & Griffin AL (2013). Dissociable roles of the dorsal striatum and dorsal hippocampus in conditional discrimination and spatial alternation T-maze tasks. Neurobiol Learn Mem, 100, 108–116. doi: 10.1016/j.nlm.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Hoover WB, & Vertes RP (2007). Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct, 212(2), 149–179. doi: 10.1007/s00429-007-0150-4 [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, & Hasselmo ME (2005). Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus, 15(6), 739–749. doi: 10.1002/hipo.20106 [DOI] [PubMed] [Google Scholar]
- Ito HT, Zhang SJ, Witter MP, Moser EI, & Moser MB (2015). A prefrontal-thalamo-hippocampal circuit for goal-directed spatial navigation. Nature, 522(7554), 50–55. doi: 10.1038/nature14396 [DOI] [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW, & Frank LM (2012). Awake hippocampal sharp-wave ripples support spatial memory. Science, 336(6087), 1454–1458. doi: 10.1126/science.1217230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Rothschild G, Roumis DK, & Frank LM (2016). Coordinated excitation and inhibition of prefrontal ensembles during awake hippocampal sharp-wave ripple events. Neuron, 90(1), 113–127. doi: 10.1016/j.neuron.2016.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, & Wilson MA (2005). Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS.Biol, 3(12), e402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, Baeg EH, Kim MJ, Kim YB, & Kim JJ (2008). Plasticity and memory in the prefrontal cortex. Rev Neurosci, 19(1), 29–46. doi: 10.1515/REVNEURO.2008.19.1.29 [DOI] [PubMed] [Google Scholar]
- Kim SM, & Frank LM (2009). Hippocampal lesions impair rapid learning of a continuous spatial alternation task. PLoS One, 4(5), e5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser MB (2008). Finite scale of spatial representation in the hippocampus. Science, 321(5885), 140–143. [DOI] [PubMed] [Google Scholar]
- Layfield DM, Patel M, Hallock H, & Griffin AL (2015). Inactivation of the nucleus reuniens/rhomboid causes a delay-dependent impairment of spatial working memory. Neurobiol Learn Mem, 125, 163–167. doi: 10.1016/j.nlm.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, & Cohen JD (2001). An integrative theory of prefrontal cortex function. Annu Rev Neurosci, 24, 167–202. doi: 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Moser MB, & Moser EI (1998). Distributed encoding and retrieval of spatial memory in the hippocampus. J Neurosci, 18(18), 7535–7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill PK, Gordon JA, & Sigurdsson T (2013). Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion. J Neurosci, 33(35), 14211–14224. doi: 10.1523/JNEUROSCI.2378-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J, Schomburg EW, Berenyi A, Fujisawa S, & Buzsaki G (2013). Local generation and propagation of ripples along the septotemporal axis of the hippocampus. J Neurosci, 33(43), 17029–17041. doi: 10.1523/JNEUROSCI.2036-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, & Watson C (2004). The Rat Brain in Stereotaxic Coordinates: Academic Press. [DOI] [PubMed] [Google Scholar]
- Peyrache A, Khamassi M, Benchenane K, Wiener SI, & Battaglia FP (2009). Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat Neurosci, 12(7), 919–926. doi: 10.1038/nn.2337 [DOI] [PubMed] [Google Scholar]
- Preston AR, & Eichenbaum H (2013). Interplay of hippocampus and prefrontal cortex in memory. Curr Biol, 23(17), R764–773. doi: 10.1016/j.cub.2013.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasethupathy P, Sankaran S, Marshel JH, Kim CK, Ferenczi E, Lee SY, … Deisseroth K (2015). Projections from neocortex mediate top-down control of memory retrieval. Nature, 526(7575), 653–659. doi: 10.1038/nature15389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G, Micheau J, Lam AG, Roloff EL, Martin SJ, Bridge H, … Morris RG (1999). Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat.Neurosci, 2(10), 898–905. [DOI] [PubMed] [Google Scholar]
- Roth BL (2016). DREADDs for Neuroscientists. Neuron, 89(4), 683–694. doi: 10.1016/j.neuron.2016.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JD, & Jadhav SP (2016). Multiple modes of hippocampal-prefrontal interactions in memory-guided behavior. Curr. Opin. Neurobiol, 40, 161–169. doi: 10.1016/j.conb.2016.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, & Wilson MA (2005). Prefrontal phase locking to hippocampal theta oscillations. Neuron, 46(1), 141–151. doi: 10.1016/j.neuron.2005.02.028 [DOI] [PubMed] [Google Scholar]
- Smith AC, Frank LM, Wirth S, Yanike M, Hu D, Kubota Y, … Brown EN (2004). Dynamic analysis of learning in behavioral experiments. Journal of Neuroscience, 24(2), 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, & Gordon JA (2015). Hippocampal-prefrontal input supports spatial encoding in working memory. Nature, 522(7556), 309–314. doi: 10.1038/nature14445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW (1981). A direct projection from Ammon’s horn to prefrontal cortex in the rat. Brain Res, 217(1), 150–154. doi:0006-8993(81)90192-X [pii] [DOI] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, & McNaughton BL (2008). Spontaneous changes of neocortical code for associative memory during consolidation. Science, 322(5903), 960–963. doi: 10.1126/science.1161299 [DOI] [PubMed] [Google Scholar]
- Tamura M, Spellman TJ, Rosen AM, Gogos JA, & Gordon JA (2017). Hippocampal-prefrontal theta-gamma coupling during performance of a spatial working memory task. Nat Commun, 8(1), 2182. doi: 10.1038/s41467-017-02108-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, & Jadhav SP (2018). Sharp-wave ripples as a signature of hippocampal-prefrontal reactivation for memory during sleep and waking states. Neurobiol Learn Mem doi: 10.1016/j.nlm.2018.01.002 [DOI] [PMC free article] [PubMed]
- Tang W, Shin JD, Frank LM, & Jadhav SP (2017). Hippocampal-prefrontal reactivation during learning is stronger in awake compared with sleep states. J Neurosci, 37(49), 11789–11805. doi: 10.1523/JNEUROSCI.2291-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, … Morris RG (2007). Schemas and memory consolidation. Science, 316(5821), 76–82. doi: 10.1126/science.1135935 [DOI] [PubMed] [Google Scholar]
- Twining RC, Vantrease JE, Love S, Padival M, & Rosenkranz JA (2017). An intra-amygdala circuit specifically regulates social fear learning. Nat Neurosci, 20(3), 459–469. doi: 10.1038/nn.4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban DJ, & Roth BL (2015). DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu Rev Pharmacol Toxicol, 55, 399–417. doi: 10.1146/annurev-pharmtox-010814-124803 [DOI] [PubMed] [Google Scholar]
- Vertes RP (2004). Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse, 51(1), 32–58. doi: 10.1002/syn.10279 [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck K, & Leranth C (2007). Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull, 71(6), 601–609. doi: 10.1016/j.brainresbull.2006.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viena TD, Linley SB, & Vertes RP (2018). Inactivation of nucleus reuniens impairs spatial working memory and behavioral flexibility in the rat. Hippocampus, 28(4), 297–311. doi: 10.1002/hipo.22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GW, & Cai JX (2006). Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behav Brain Res, 175(2), 329–336. doi: 10.1016/j.bbr.2006.09.002 [DOI] [PubMed] [Google Scholar]
- Wang GW, & Cai JX (2008). Reversible disconnection of the hippocampal-prelimbic cortical circuit impairs spatial learning but not passive avoidance learning in rats. Neurobiol Learn Mem, 90(2), 365–373. doi: 10.1016/j.nlm.2008.05.009 [DOI] [PubMed] [Google Scholar]
- Xu W, & Sudhof TC (2013). A neural circuit for memory specificity and generalization. Science, 339(6125), 1290–1295. doi: 10.1126/science.1229534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Kapeller-Libermann D, Travaglia A, Inda MC, & Alberini CM (2017). Direct dorsal hippocampal-prelimbic cortex connections strengthen fear memories. Nat Neurosci, 20(1), 52–61. doi: 10.1038/nn.4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski MC, Tang W, & Jadhav SP (2017). The role of replay and theta sequences in mediating hippocampal-prefrontal interactions for memory and cognition. Hippocampus doi: 10.1002/hipo.22821 [DOI] [PMC free article] [PubMed]


