Abstract
Research on age‐related memory alterations traditionally targets individuals aged ≥65 years. However, recent studies emphasize the importance of early aging processes. We therefore aimed to characterize variation in brain gray matter structure in early midlife as a function of sex and menopausal status. Subjects included 94 women (33 premenopausal, 29 perimenopausal, and 32 postmenopausal) and 99 demographically comparable men from the New England Family Study. Subjects were scanned with a high‐resolution T1 sequence on a 3 T whole body scanner. Sex and reproductive‐dependent structural differences were evaluated using Box's M test and analysis of covariances (ANCOVAs) for gray matter volumes. Brain regions of interest included dorsolateral prefrontal cortex (DLPFC), inferior parietal lobule (iPAR), anterior cingulate cortex (ACC), hippocampus (HIPP), and parahippocampus. While we observed expected significant sex differences in volume of hippocampus with women of all groups having higher volumes than men relative to cerebrum size, we also found significant differences in the covariance matrices of perimenopausal women compared with postmenopausal women. Associations between ACC and HIPP/iPAR/DLPFC were higher in postmenopausal women and correlated with better memory performance. Findings in this study underscore the importance of sex and reproductive status in early midlife for understanding memory function with aging.
Keywords: anterior cingulate cortex, hippocampus, memory, menopause, sex
1. INTRODUCTION
With the advancing age of our population, age‐related health challenges are rapidly increasing (Koivisto et al., 1995; Ortman, Velkoff, & Hogan, 2014). Approximately 75% of the aging population report memory‐related problems (Koivisto et al., 1995). The decline in cognitive ability is not limited to neurodegenerative diseases, but also part of healthy aging (La Corte et al., 2016; van Geldorp et al., 2015) and has substantial consequences for quality of life (Hannigan, Coen, Lawlor, Robertson, & Brennan, 2015) and mortality (Connors et al., 2015). While most aging studies target individuals 65 years and older (e.g., Boutet, Milgram, & Freedman, 2007; Caselli, Chen, Lee, Alexander, & Reiman, 2008; Metzler‐Baddeley, Jones, Belaroussi, Aggleton, & O'Sullivan, 2011), recent research showed that brain structural and functional abnormalities can precede classical age‐related neurodegeneration decades earlier (Szoeke et al., 2016). Despite outperforming men in most memory‐related cognitive domains during premenopausal years (Bleecker, Bolla‐Wilson, Agnew, & Meyers, 1988; Rentz et al., 2016; Ruff, Light, & Quayhagen, 1989; Trahan & Quintana, 1990; van Hooren et al., 2007; Weiss et al., 2006), women report increased memory problems after menopause (Epperson, Sammel, & Freeman, 2013; Greendale, Derby, & Maki, 2011; Rentz et al., 2016), and some show an early memory decline (Weber, Maki, & McDermott, 2014; Weber, Rubin, & Maki, 2013).
Recent cognitive neuroscience studies demonstrate the impact of menopause, and in particular, decline in 17β estradiol, on working and declarative memory performance (Berent‐Spillson et al., 2012; Boss, Kang, Marcus, & Bergstrom, 2014; Epperson et al., 2013; Jacobs & D'Esposito, 2011; Jacobs et al., 2017; Rentz et al., 2016; Rosenberg & Park, 2002). A review of human cognitive studies among women aged 60 years and older found higher estradiol levels associated with better memory performance across multiple memory domains (Boss et al., 2014). Human cognitive literature is in line with preclinical studies demonstrating the impact of decline in 17β estradiol levels on memory circuitry formation and maintenance of memory performance (Brinton, 2009; Dumas, Kutz, Naylor, Johnson, & Newhouse, 2010a; Dumitriu, Rapp, McEwen, & Morrison, 2010; Liu et al., 2008; Morrison, Brinton, Schmidt, & Gore, 2006; Woolley & McEwen, 1994).
The impact of estradiol on memory function is not surprising given that gonadal hormone receptors are relatively dense in critical brain regions implicated in memory circuitry, brain regions that have historically exhibited sex differences in volumetric size, such as the hippocampus (HIPP), dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), inferior parietal lobule (iPAR), and the parahippocampus (parHIPP; including entorhinal cortex) (Allen, Damasio, Grabowski, Bruss, & Zhang, 2003; Chen, Sachdev, Wen, & Anstey, 2007; Filipek, Richelme, Kennedy, & Caviness, 1994; Giedd, Raznahan, Mills, & Lenroot, 2012; Goldstein et al., 2001; Good et al., 2001; Nopoulos, Flaum, O'Leary, & Andreasen, 2000; Ruigrok et al., 2014; Schlaepfer et al., 1995; Sowell et al., 2007).
We recently demonstrated significant reproductive age‐related changes in regional brain activity and network‐level connectivity during working memory and encoding in early midlife associated with menopausal changes drawn from the cohort presented here (Jacobs et al., 2016, 2017). In the current study, we investigated sex differences and reproductive status on structural brain volumes and their associations within the memory circuit.
Most of the work investigating sex differences in structural brain volumes in memory circuitry focuses on one or few individual brain areas rather than on a network of brain regions. Several methods for structural imaging have been proposed to investigate associations between regions within and between brain networks (Caviness, Lange, Makris, Herbert, & Kennedy, 1999; Kennedy et al., 1998). Among them, techniques based on covariance modeling have been found particularly useful in a few brain disorders (Allen et al., 2003; Chen, He, Rosa‐Neto, Germann, & Evans, 2008; Colibazzi et al., 2008; He, Chen, & Evans, 2008; Lerch et al., 2006; Mechelli, Friston, Frackowiak, & Price, 2005; Mitelman, Buchsbaum, Brickman, & Shihabuddin, 2005a; Mitelman, Shihabuddin, Brickman, & Buchsbaum, 2005b; Seeley, Crawford, Zhou, Miller, & Greicius, 2009). Included in this literature was a recent publication of ours (Abbs et al., 2011) that investigated sex differences in the covariance of regions within the memory circuitry in schizophrenia compared with healthy controls. Covariation between the same regions assessed in that study were examined in the present study, using what is called a “Box's M‐Test” (Box, 1949). An underlying assumption in covariance analyses is that morphometric features of brain regions within the subjects are correlated with each other due to shared neurodevelopmental and functional processes. Inter‐individual variation of gyral morphometry would therefore account for inter‐individual differences in brain function (Kennedy et al., 1998). Further, the approach may have particular relevance to sex differences, given sexual dimorphisms contribute to variance in brain volumes, especially at the gyral level (Giedd et al., 2012; Goldstein et al., 2001; Kennedy et al., 1998). While cortical thickness would be a more sensitive measure to gyral level differences (Winkler et al., 2010), here we studied gray matter volume to allow for examination of subcortical structures, like HIPP or parHIPP.
In the present study, we apply this covariance analytic approach of the memory circuitry (Abbs et al., 2011) to a cross‐sectional cohort of men and women (pre‐, peri‐, and postmenopausal) in early midlife. Based on our previous work on sex differences in memory circuitry, we hypothesized that women will exhibit an advantage compared with men in verbal memory performance that is associated with sex differences in structural variation in relationships between brain regions in memory circuitry. We further predict an attenuation of the sex differences with menopausal transition.
2. MATERIALS AND METHODS
2.1. Subjects
Participants were selected from 17,741 pregnancies that constitute the New England Family Study (NEFS), a Boston‐Providence subsidiary of the national Collaborative Perinatal Project. The NEFS is a prospective study initiated over 50 years ago to investigate prenatal and familial antecedents of pediatric, neurological, and psychological disorders of childhood (Niswander & Gordon, 1972). Pregnant women, recruited between 1959 and 1966, were representative of patients receiving prenatal care in the Boston‐Providence area. In a series of studies over the last 20 years, we followed the offspring of these pregnancies to investigate the fetal programming of adult phenotypes and sex differences therein. The current study investigated the fetal programming of sex differences in memory circuitry aging in early midlife (Gilman et al., 2016; Goldstein et al., 2014; Goldstein, Buka, Seidman, & Tsuang, 2010). Offspring were recruited at the time that they were ages of 46–53 years of age and completed clinical, cognitive, and neuropsychological assessments and functional and structural magnetic resonance imaging (fMRI/sMRI/DTI). Exclusionary criteria included any history of neurologic disease, central nervous system (CNS) damage, head injury with loss of consciousness, endocrine disorders, heart disease, alcohol‐related diseases, current or history of psychosis, current use of hormonal contraceptives, other medical illnesses that may significantly alter CNS function, or any MRI contraindication.
The set of analyses reported here focused on structural MRI (sMRI) and behavioral evaluations of the 202 participants enrolled. One subject was excluded given an incomplete T1 scan. After enrollment, seven women reported current use of a hormone replacement regimen and were therefore excluded from analyses reported here. One additional subject was excluded after quality check of T1 scans. The remaining 193 participants were separated into four groups: premenopausal, perimenopausal, and postmenopausal women and men. Groups were matched on important demographics—such as body mass index, education, parental socioeconomic status, ethnicity, and age (Table 1). The sample presented here differs slightly from our previously published fMRI papers (Jacobs et al., 2016), given that a few subjects had either usable T1 or fMRI scans. Human subjects’ approval was granted by Partners Healthcare and Brown University. All volunteers gave written informed consent and were paid for their participation.
Table 1.
Sample characteristics
| Premenopausal women (n = 33) | Perimenopausal women (n = 29) | Postmenopausal women (n = 32) | Men (n = 99) | |
|---|---|---|---|---|
| Age (years) | 49.24 ± 1.71a | 49.83 ± 1.91a | 50.59 ± 2.23a | 50.08 ± 2.31a |
| BMIb | 28.39 ± 6.08a | 28.59 ± 6.15a | 27.63 ± 5.88a | 29.08 ± 5.29a |
| PSESc | 5.91 ± 2.12a | 5.39 ± 1.86a | 5.87 ± 1.89a | 5.80 ± 1.77a |
| Race/ethnicity | 1× Afro‐American (3.0%) | 3× Afro‐American (10.3%) | 6× Afro‐American (18.8%) | 2× Afro‐American (2.0%) |
| 30× white, non‐Hispanic (90.9%) | 25× white, non‐Hispanic (86.2%) | 26× white, non‐Hispanic (81.3%) | 89× white, non‐Hispanic (89.9%) | |
| 1× other (3.0%) | 1× other (3.4%) | 3× white, Hispanic (3.0%) | ||
| 1 missing value (3.0%) | 4× other (4.%) | |||
| 1 missing value (1.0%) | ||||
| Education level | 6× high school (18.2%) | 7× high school (24.1%) | 8× high school (25.0%) | 2× less than high school (2.0%) |
| 13× some college (39.4%) | 10× some college (34.5%) | 11× some college (34.4%) | 20× high school (20.2%) | |
| 10× 4 years of college (30.3%) | 10× 4 years of college (34.5%) | 9× 4 years of college (28.1%) | 44× some college (44.4%) | |
| 4× graduate school (12.1%) | 2× graduate school (6.9%) | 2× graduate school (6.3%) | 26× 4 years of college (26.3%) | |
| 2 missing values (6.3%) | 6× graduate school (6.1%) | |||
| 1 missing value (1.0%) |
Mean ± SD.
Body mass index.
Parental socioeconomic status was a composite index of family income, education, and occupation and ranged from 1.0 (low) to 9.3 (high).
2.2. Study design
Subjects were seen at Brigham and Women's Hospital Outpatient Clinical Research Center. Pre‐ and perimenopausal women were scheduled in the early follicular phase (Day 3–5) of their menstrual cycle, pursuant to subject report (and validated by serologic evaluation). Subjects fasted for ≥8 hr prior to a morning baseline blood draw. Subjects were offered a light standardized breakfast (excluding caffeine) followed by a 1‐hr MRI scanning session. Following the scan, subjects completed a structured clinical interview, neuropsychological testing, family medical history, and a reproductive/menstrual cycle history administered by an experienced clinical interviewer/clinician.
2.3. Structural magnetic resonance imaging
Structural MR images were acquired using a 3 T whole body Siemens Tim Trio system (Siemens, Erlangen, Germany) with 12‐channel head coil. MRI data were acquired using a high‐resolution 3D MPRAGE sequence with the following parameters: repetition time (TR) 2530 ms, echo time (TE) 3.32 ms T1 1,100 ms, flip angle 7°, matrix size 256 × 256, 174 slices, 1 × 1 × 1 mm isotropic voxel size. Images were checked visually for possible movement artifacts. To correct for head tilt, each MRI scan was realigned, horizontally to the anterior commissure–posterior commissure line, and vertically to the sagittal sulcus. Automatic brain masking was conducted using Multi Atlas Brain Segmentation (Del Re et al., 2016). Segmentation of the scans was executed using FreeSurfer 5.3 (Fischl et al., 2002), and quality of segmentations was determined by visual inspection. Based on visual inspection, all FreeSurfer segmentations were included in further analysis. Gray matter volumes for memory circuitry (ACC, iPAR, parHIPP, HIPP, and DLPFC) were calculated using FreeSurfer segmentation. Regarding the hippocampus, HIPP represents a conservative definition of the hippocampal formation as per Caviness, Meyer, Makris, and Kennedy (1996) and Makris et al. (1999), including cornu amonis, dentate gyrus, subiculum, presubiculum, and parasubiculum (but not the entorhinal cortex), a terminology that has been adopted by the FreeSurfer parcellation system (Fischl et al., 2002).
2.4. Neuropsychological assessment
The neuropsychological tests administered were part of a comprehensive neuropsychological battery that included digit span (Wechsler, 1997), Controlled Oral Association Test for verbal fluency of letters and Categories (Benton, 1968), American National Adult Reading Test (Nelson, 1982), Spielberger State‐Trait Anxiety Inventory, Profile of Mood Questionnaire, and two sleep measures (Pittsburgh Sleep Quality Index, Insomnia Severity Index). In addition, two episodic memory tests—Buschke Selective Reminding Test (Grober, Lipton, Hall, & Crystal, 2000; Lemos, Simoes, Santiago, & Santana, 2015; Masur et al., 1989) and Face Name Associative Memory Task (Rentz et al., 2011; Sperling et al., 2003)—were collected. In multiple previous studies of dementia, the latter two tests were selected because they are particularly challenging and sensitive to working memory and learning deficits associated with early aging and have a high sensitivity for early cognitive decline (Hedden et al., 2012). Z‐score summary scores were created for The Buschke Selective Reminding and the Face Name Associative Memory Task.
2.5. Menopausal staging
The timing of menopausal transition between the first clinical appearance of decreased ovarian function (i.e., shorter intermenstrual time periods) to menstrual irregularity and of final amenorrhea is highly variable and can occur over several years. Most women experience menopause between ages 45 and 60 years, with timing being consistent across societies (Pelosi et al., 2015; Stolk et al., 2012). In our sample, given the age range, some women were already in menopause with permanent amenorrhea, low estradiol levels, and elevated gonadotropins; some exhibited signs of follicular failure (elevated follicle‐stimulating hormone (FSH) and oligomenorrhea); and some showed normal cycling. Reproductive histories and serologic hormonal validation were used to determine the reproductive stage of women in our sample following the Stages of Reproductive Aging Workshop (STRAW)‐10 guidelines (Harlow et al., 2012). Women were categorized as late reproductive (“premenopausal,” n = 33), menopausal transition (“perimenopausal,” n = 29), or early postmenopausal (“postmenopausal,” n = 32).
2.6. Statistical analyses
Statistical analyses were conducted using the Statistical Package for Social Sciences version 24.0 (IBMCorp, 2013) and GraphPad Prism 7 (GraphPadSoftware, 2014). For all analyses, volumes were corrected for total intracranial volume (ICV) by dividing each individual volume by ICV. For visualization of analyses, please see Figure 1.
Figure 1.
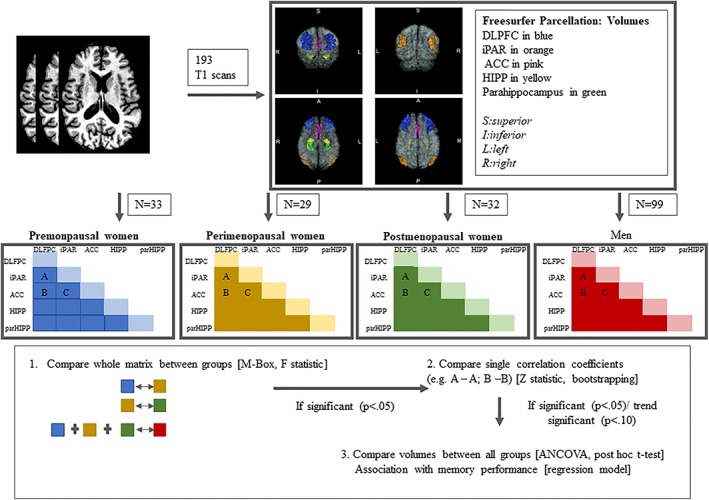
Image processing and statistical analyses. The four panels showing the cortical regions of interest parcellated in this study are three‐dimensional anterior (upper left), posterior (upper right), inferior (lower left), and superior (lower right) views of a representative brain [Color figure can be viewed at http://wileyonlinelibrary.com]
Following Abbs et al. (2011), covariance patterns were analyzed between predefined memory networks (i.e., ACC, iPAR, parHIPP, HIPP, and DLPFC), and covariance structures were compared between groups. We first compared all women against men. Following this, reproductive status among the women was compared to investigate the impact of ovarian decline on memory circuitry covariances (premenopausal women vs. perimenopausal women and perimenopausal women vs. postmenopausal women). For comparison of covariance patterns, we employed the Box's M test (Box, 1949) using the following statistic:
where C i is the variance‐covariance matrix calculated from the group i, T is the number of subgroups for which equality of matrices is tested, and n i is the sample size of each group i.
The Box's M test statistic can be approximated by an F statistic, whereas the rejection of the null hypothesis on a significant level of p < .05 is interpreted as the overall covariance pattern between two groups is different from each other. The Box's M tests allow for the comparison of covariance matrices, rather than looking at single brain volumes or multiple correlations (and hence protects for multiple comparison issues). If the Box's M test results were significant (p < .05) or trended toward significance (p < .10), correlation coefficients for each pair of regions of interest (ROIs) between the two groups were compared. We would argue that the more liberal threshold of p < .10 for trend significance is justified given that the Box's M test already protects against multiple comparison errors. A more liberal threshold also protects against Type II error, likely to occur in correlation comparisons with rather small sample sizes. Correlation coefficients were converted to a normal distribution using Fisher's Z transformation, and Z‐values were used to test for differences between groups.
Additionally, given the relatively low number of participants in each group, bootstrapping (number of samples = 1,000, 95% confidence intervals, bias corrected accelerated, simple sampling) was performed for these correlation coefficients. Bootstrapping is a method to assign accuracy to sample estimates by resampling with replacements from the original data. By looking not only at single value (in this case correlation coefficient), but rather at a confidence interval, one can control and check the stability of original results.
Following this, if significant (p < .05) or trend (p < .1) differences between correlation coefficients were detected, the corresponding volumes were also examined (e.g., correlation coefficients of HIPP and ACC between perimenopausal and postmenopausal women were different, then analysis of covariance models [ANCOVAs] were used to examine volumes of HIPP and ACC). The ANCOVA model was based on structural volume as the dependent variable with independent variables for group status (premenopausal women, perimenopausal women, postmenopausal women, men), controlled for age.
Finally, we investigated in post hoc exploratory analyses if memory performance was associated with structural variation in network connections. Only in the case of significant group differences of correlation coefficients between two groups, we additionally calculated linear regression for the relevant volumes (e.g., if we observed differences between peri‐ and postmenopausal women in correlation coefficients of HIPP and ACC, we then calculated linear regression with hippocampus volume as independent variable and ACC volume as dependent variable). In a second step, we predicted one regional volume conditional on another, based on our regression model (e.g., ACC volume conditional on hippocampus volume). The absolute residual between predicted volume and actual volume is an indicator of how well actual association of two volumes fits the regression model.
Last, we correlated the absolute residual with the memory scores, using Spearman correlation to determine the strength of association. This was followed by Fisher's Exact Score to test whether Spearman correlation coefficients significantly differed from 0.
3. RESULTS
Comparison of covariance matrixes of the memory circuitry using the Box's M test (Box, 1949) showed significant differences between peri‐ and postmenopausal women (Box's M test = 28.94, F = 1.75, df1 = 15, df2 = 13,711, p < .035), but no significant differences between pre‐ and perimenopausal women (Box's M test = 20.83, F = 1.26, df1 = 15, df2 = 13,960, p < .22) and all women and men (Box's M test = 19.06, F = 1.24, df1 = 15, df2 = 144,800, p < .24).
Post hoc analyses of the correlation coefficients comparing peri‐ and postmenopausal women revealed significant differences between HIPP and ACC and a trend toward significant differences between iPAR and ACC and DLPFC and ACC (Table 2). Postmenopausal women showed a positive association between these four regional volumes (Figure 2), while perimenopausal women did not. In order to address the issue of small sample sizes, bootstrapping was conducted. Findings (Table 2, confidence intervals) supported differences of correlation coefficients of ACC and hippocampus, DLPFC, and iPAR between peri‐ and postmenopausal women. Hence, bootstrapping confirmed our initial results demonstrating a higher correlation between ACC and other brain regions among postmenopausal women.
Table 2.
Pearson correlation coefficients between brain regions defining memory circuit for peri‐ and postmenopausal women
| Perimenopausal women | Postmenopausal women | Test statistic | |
|---|---|---|---|
| Pearson correlation and 95% confidence interval | |||
| HIPP‐parHIPP | .58 (.31–.79) | .56 (.19–.80) | Z = .10, p < .46 |
| HIPP‐iPAR | .56 (.23–.82) | .42 (.072–.63) | Z = .72, p < .24 |
| HIPP‐dlPFC | .40 (.065–.66) | .37 (0.66–.62) | Z = .13, p < .45 |
| HIPP‐ACC | −.33 (−.67 to .080) | .57 (.22–.77) | Z = −3.67, p < .0001 |
| parHIPP‐iPAR | .62 (.30–.81) | .58 (.18–.81) | Z = .23, p < .41 |
| parHIPP‐dlPFC | .47 (.15–.71) | .49 (.039–.75) | Z = −0.096, p < .46 |
| parHIPP‐ACC | .14 (−.29 to .51) | .28 (−.12 to .56) | Z = −.54, p < .29 |
| iPAR‐dlPFC | .53 (.23–.76) | .45 (.075–.71) | Z = .39, p < .35 |
| iPAR‐ACC | .087 (−.29 to .41) | .45 (.11–.68) | Z = −1.47, p < .071 |
| PFC‐ACC | .11 (−.25 to .41) | .47 (.17–.67) | Z = −1.48, p < .069 |
ACC = anterior cingulate cortex; DLPFC = dorsolateral prefrontal cortex; HIPP = hippocampus; iPAR = inferior parietal cortex; parHIPP = parahippocampus.
Confidence intervals were created using bootstrapping (number of samples = 1,000, 95% confidence intervals, bias corrected accelerated, simple sampling).
Significant (p < .05) or trend towards significant (p < .1) results are highlighted in bold.
Figure 2.
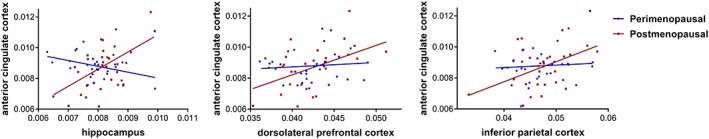
Associations between anterior cingulate cortex volume and hippocampus/ dorsolateral prefrontal cortex/inferior parietal cortex. There are no significant correlations between hippocampus/iPAR/DLPFC and ACC for perimenopausal women, but positive associations between these regions for postmenopausal women [Color figure can be viewed at http://wileyonlinelibrary.com]
Following this, we tested for group differences among HIPP, ACC, iPAR, and DLPFC volumes, controlling for age. Group differences in brain volumes were significant for HIPP (F = 9.94, df = 3, p < .0001) comparing men with pre‐ (p < .0001), peri‐ (p < .001), and postmenopausal women (p < .0001), with women consistently having larger HIPP volumes, relative to intracranial size, than men regardless of reproductive status. Descriptive statistics are provided in Table 3.
Table 3.
Descriptive statistics for hippocampal volume
| Hippocampal volume (adjusted for total intracranial volume) | |
|---|---|
| Premenopausal women | .008159 ± .000914a |
| Perimenopausal women | .008021 ± .000754a |
| Postmenopausal women | .008103 ± .000731a |
| Men | .007464 ± .000788a |
Mean ± SD.
Finally, given that we found significant associations between ACC and HIPP, DLPFC, and iPAR, among postmenopausal women, we predicted ACC volume based on HIPP, DLPFC, iPAR volume of all people using regression models. We afterwards calculated for postmenopausal women the absolute difference between the predicted and observed volume. This is an indicator of how well the actual data meet the predicted linear association. For an individual, high‐absolute differences suggest that the association between the two brain volumes is weaker than for subjects with low‐absolute differences. We predicted that postmenopausal women who had stronger associations between brain volumes (and therefore smaller absolute differences) within the memory circuit would show better memory performance. We therefore—in a post hoc analyses—correlated the absolute differences between predicted and observed volumes of postmenopausal women with memory performance. We observed a negative association between Buschke summary score and association strength between HIPP and ACC (ρ = −0.35), which significantly differed from zero (Fisher's exact score p < .047), indicating that low‐absolute residuals (= higher association between these areas) were correlated with better memory performance in postmenopausal women. Face Name Associative Memory Task was not associated significantly with association strength between HIPP and ACC (rho = 0.20, p < .27).
4. DISCUSSION
In this cross‐sectional study, we examined structural brain volumes in the memory circuitry and the co‐relationships, or covariances, among them in healthy early midlife by sex and reproductive status. Covariance analysis assumes brain areas of a functional network are connected through shared neurodevelopmental processes (Abbs et al., 2011), and thus, suitable to analyze the regions as a network, as distinct from independent regions. In the present study, covariance patterns among memory circuitry regions significantly differed comparing peri‐ with postmenopausal women.
Postmenopausal women showed higher correlations between ACC and HIPP, iPAR, and DLPFC than perimenopausal women. Interestingly, even though we found the expected differences in HIPP volume, with women showing overall larger volumes than men, relative to cerebrum size, the covariance patterns did not significantly differ between men and women overall, controlled for age.
Our findings suggest that, in early midlife, aging of memory circuitry connections in women is revealed with reproductive aging more so than chronologic age.
Perimenopause is a period of substantial fluctuations in gonadal hormone changes. We argue here, as have others in the field, that it is during the profound fluctuations in gonadal hormone changes that one sees the greatest changes in brain function and behavior. However, given the cross‐sectional design of our study, this is speculative and needs to be directly tested.
While menopausal transition may cause structural reorganization, one also needs to consider the possibility of genetic factors that relate to both menopause and altered structural covariance pattern (Alexander‐Bloch, Giedd, & Bullmore, 2013).
While the impact of menstrual cycle and hormone replacement strategies on brain structure and function have been investigated extensively (Berent‐Spillson et al., 2012; Boss et al., 2014; Epperson et al., 2013; Jacobs & D'Esposito, 2011; Protopopescu et al., 2008; Rosenberg & Park, 2002), few studies have examined the impact of ovarian decline on structural brain volumes, and none on the covariance within memory circuitry. Studies that have investigated volumetric outcomes focused on the hippocampus alone, and results were inconsistent among investigations (Fischer, Gleason, & Asthana, 2014). For example, Goto et al. found a reduction in hippocampal volume comparing pre‐ and postmenopausal women (Goto et al., 2011a), and a greater hippocampal volume loss in women compared with men during a similar age span (Goto et al., 2011b). However, one earlier study showed a lack of significant volumetric impact of menopause on hippocampus (Goldman‐Rakic, 1988).
Consistent with Sullivan (2005), we similarly did not observe significant overall volumetric differences in memory circuitry regions among women by menopausal status. We think this lack of volume change is not surprising given that the women in the current study were both healthy and in early postmenopause, and thus, ovarian decline would likely not have a significant impact on brain volumes in this sample. However, covariance analyses may be more sensitive to identifying subtle changes in memory circuitry in early midlife, as in our previous fMRI memory circuitry work on this cohort (Jacobs et al., 2016, 2017), and therefore more suitable to investigate reproductive hormone‐dependent differences in structural associations within the memory circuit in early midlife.
Variation in covariance patterns may represent changes in brain connectivity. Indeed, the memory circuitry regions studied here have direct or indirect anatomical connections (Goldman‐Rakic, 1988; Goldman‐Rakic, Selemon, & Schwartz, 1984). HIPP is connected with iPAR and DLPFC (Seltzer & Van Hoesen, 1979) as well as with ACC (Barbas & Pandya, 1989; Sesack, Deutch, Roth, & Bunney, 1989). Additionally, iPAR is directly connected with DLPFC and ACC (Makris et al., 2005; Petrides & Pandya, 1984). ParHIPP is connected with HIPP and other cortical areas (Petrides & Pandya, 1984). While the latter areas have not been subparcellated herein, they are still considered part of the more broadly defined hippocampal formation (Makris et al., 1999; Petrides & Pandya, 1984; Rosene & Van Hoesen, 1977), which may explain the relatively minor parHIPP associations observed in our study.
Functional findings also suggest associations between brain regions studied here and memory performance (e.g., Addis, Moscovitch, & McAndrews, 2007; Eichenbaum, 2004; Krause et al., 1999; Skinner & Fernandes, 2007; Squire, Stark, & Clark, 2004). A decline in memory performance during menopause (Fuh, Wang, Lee, Lu, & Juang, 2006) was associated with resting state brain activity in the same network as studied here (Maki, 2015), while cognitive impairments in postmenopausal women also showed a disruption in the cortico‐subcortical loop (Huang et al., 2015). Our recent fMRI study of memory encoding in subjects from the same cohort presented here (Jacobs et al., 2016) reported enhanced bilateral hippocampal connectivity in postmenopausal women compared with pre‐ or perimenopausal women. This enhanced functional connectivity was significantly associated with maintaining successful memory performance, further suggesting a recruitment of bilateral brain areas as a potential mechanism to avoid or attenuate memory decline.
Our current findings suggest enhanced associations between the volume of ACC and volumes of other memory circuit regions, namely HIPP, iPAR, and DLPFC, from peri‐ to postmenopause. The ACC is a mid‐brain structure involved in core executive functions, such as selective attention (Cassaday, Nelson, & Pezze, 2014; Smith & Jonides, 1999), motor planning and response functions (Peterson et al., 1999; Weible, 2013), self‐processing (Qin & Northoff, 2011) emotional learning, arousal and control (Allard & Kensinger, 2014; Dolcos, Katsumi, & Dixon, 2014; Vogt, Finch, & Olson, 1992), and working memory. With respect to memory, the ACC seems to be particularly important for long‐term consolidation and plasticity (Insel & Takehara‐Nishiuchi, 2013) as well as executive operations in association with working memory (Hartley & Speer, 2000). Given its role in emotion control and memory consolidation, the importance of ACC for chronic pain (Shyu & Vogt, 2009; Zhuo, 2007), fear (Toyoda et al., 2011), and many psychiatric disorders, such as depression (Devinsky, Morrell, & Vogt, 1995; Spati et al., 2015), has been previously discussed.
Several recent resting state and functional MRI studies investigated age‐related changes in ACC connectivity and its importance for memory performance. While results are not unambiguous (Cao et al., 2014), it appears that healthy older individuals showed increased connectivity between ACC and several other brain areas (Goldstone et al., 2016) and increased activity in ACC (Erb & Obleser, 2013; Salami, Rieckmann, Fischer, & Backman, 2014) when compared with younger individuals. It has been also shown that concentrations of choline, creatine, and N‐acetyl aspartate in ACC increase may represent a compensatory mechanism for higher energy demand and lower blood flow (Vaidya, Paradiso, Boles Ponto, McCormick, & Robinson, 2007), which may lead to neuronal hypertrophy and glial proliferation (Chiu et al., 2014). Interestingly, stronger bilateral ACC connections are associated with better memory performance (Klaassen et al., 2016; Lee, Tan, & Qiu, 2016), suggesting a protective role of ACC in aging. This assumption is further supported by the fact that in older adults who already showed mild cognitive impairments, resting state activity in the ACC was reduced rather than enhanced (Wu et al., 2014). While the association of functional and structural covariance is in general an unsettled issue (Di et al., 2017), our results support functional findings on the ACC. We find that postmenopausal women who showed higher associations between ACC and hippocampus performed better on the Buschke memory test when compared with postmenopausal women with lower associations, additionally suggesting the important role of ACC in maintaining memory performance in aging individuals (Jacobs et al., 2016, 2017).
Aside from reproductive‐dependent differences in associations among regions in the memory circuit, our study showed that women overall have larger hippocampi, relative to cerebrum size, than men. This is consistent with multiple previous studies of sex differences in hippocampal volume in healthy populations (Giedd et al., 2012; Goldstein et al., 2001; Sowell et al., 2007). The hippocampus is one of the most highly sexually dimorphic regions in the brain. Animal and postmortem studies show a high density of estrogen, androgen (Gillies & McArthur, 2010; Simerly, Chang, Muramatsu, & Swanson, 1990), and progesterone (Brinton et al., 2008; Pluchino et al., 2006) receptors in memory‐related brain regions, such as hippocampus and frontal cortex. Sex differences in hippocampus are initiated during ontogeny through interaction of exposure to sex steroids and genotype (Arnold, 2009). Moreover, neurogenesis after birth, prominent in hippocampus (Clelland et al., 2009), and potentiated by puberty (Barha & Galea, 2010; Duarte‐Guterman, Yagi, Chow, & Galea, 2015; Mahmoud, Wainwright, & Galea, 2016), contributes to a larger adult hippocampal volume in females than males (Giedd et al., 1996). Further, healthy women have shown less age‐related decline in hippocampal volume than men (Pruessner et al., 2010). The human literature is consistent with a long history of preclinical studies investigating the impact of sex steroid hormones on hippocampal structure and function, including on synapse density (Hara et al., 2011, 2016; Woolley & McEwen, 1994) and intracellular signaling cascades (Arevalo, Azcoitia, Gonzalez‐Burgos, & Garcia‐Segura, 2015; McEwen, Nasca, & Gray, 2016) impacting neuroplasticity (Brinton, 2009; Cooke & Woolley, 2005; Hao et al., 2006; Liu et al., 2008; Micevych & Christensen, 2012; Shanmugan & Epperson, 2014) and facilitating long‐term memory potentiation (Bayer et al., 2015). In fact, parallel to volumetric differences, sex differences in verbal memory performance also emerge shortly post‐puberty (Epperson et al., 2013; Maki, 2015; Rentz et al., 2016).
While our study and others demonstrate the importance of reproductive status on the structural integrity of the memory circuitry, the exact nature of the impact of sex steroid hormones on aging of memory circuitry structure is still being elucidated (den Heijer et al., 2003), as are the effects of hormonal treatment on brain structure (Wnuk, Korol, & Erickson, 2012), memory performance (Berent‐Spillson et al., 2015; Comasco, Frokjaer, & Sundstrom‐Poromaa, 2014), and neurodegenerative diseases (Coker et al., 2010). Timing of the initiation and type of hormone replacement (Hogervorst, 2013; Maki, 2013), and duration of therapy (Lord, Buss, Lupien, & Pruessner, 2008), in part explain inconsistencies across studies (McClure, Barha, & Galea, 2013). In addition, sex steroid hormones also regulate and interact with the expression of cholinergic (Dumas et al., 2010b), serotonergic (Hall & Steiner, 2013), dopaminergic (Dumas, Filippi, Newhouse, & Naylor, 2016), glucocorticoid (Ycaza Herrera & Mather, 2015), and other neuronal regulatory systems (Gonzalez, Diaz, & Alonso, 2008; Harte‐Hargrove, Maclusky, & Scharfman, 2013), which may contribute to interactions between structure and function of the memory circuitry with ovarian loss.
Overall, our study demonstrated reproductive‐dependent structural differences in early midlife in women, with differences significant between peri‐ and postmenopausal women. Importantly, the covariances in the structural network are accompanied by functional performance differences. Thus, we speculate that differences between peri‐ and postmenopausal women in covariance patterns may indicate the presence of a subtle, ongoing process of reorganization of memory circuitry as a function of menopausal status. Covariance analyses in healthy aging individuals (age > 65) have demonstrated a more localized structural organization in older when compared to younger individuals (Montembeault et al., 2012). However, the differences in associations among memory circuitry regions we observe occur years before one would expect classical aging and neurodegeneration. It is unclear how structural covariance alterations evolve with longer durations of postmenopause and if so, when structural covariance alterations in menopausal transition may be associated with later pathological aging. Further longitudinal studies are needed to explore these questions.
In addition, there are other potential confounding variables which could affect the results. Some previous studies (Apostolova et al., 2006; Isamah et al., 2010) reported a significant impact of race and ethnicity on structural brain volumes. The majority of our participants were Caucasian, thus future studies should include more participants from different ethnic backgrounds to investigate the impact of ethnicity/race on memory circuitry and sex differences therein.
Interestingly, while we observe sex differences for volume analyses, covariance patterns in early midlife between women and men overall did not differ. Further studies are needed to investigate if this can be explained by a lack of power to detect such differences or if overall sex differences in memory circuitry structural covariances may emerge later in life and related to sex differences in the frequency of Alzheimer's disease (Nebel et al., 2018), a disorder of severe memory decline.
5. CONCLUSION
Our results underscore the importance of studying brain systems rather than individual regions and the impact of sex and reproductive status. Further studies are needed to understand the mechanisms underlying these findings and the impact of general health on these processes. Sex hormones impact many systemic processes, such as inflammation, general cognitive and emotional processing (Dedovic, D'Aguiar, & Pruessner, 2009; Frey et al., 2010), and psychiatric disorders, such as depression (Gobinath, Mahmoud, & Galea, 2014; Jacobs et al., 2015; Messay, Lim, & Marsland, 2012; Rosenblat, Cha, Mansur, & McIntyre, 2014; Valkanova, Ebmeier, & Allan, 2013), schizophrenia (Pompili, Arnone, & Gasbarri, 2012), sleep disturbance, and anxiety (Arpels, 1996). Future longitudinal studies are therefore needed to investigate the potential associations between sex and reproductive status on brain volumes and connectivities, how these may develop over time, and the associations with memory performance and sex differences in disorders of brain aging.
CONFLICT OF INTEREST
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
ACKNOWLEDGMENTS
The authors thank Anne Remington, MA for project administration, Harlyn Aizley, MEd for administering structured clinical interviews, Dorene Rentz, PhD for overseeing administration and scoring of the memory tasks, Katie Lancaster, PhD and Julia Longenecker, MA for their initial help with data collection, and Shalendar Bhasin, MD for overseeing mass spectrometry. We also thank all NEFS subjects for their ongoing participation in our studies.
Seitz J, Kubicki M, Jacobs EG, et al. Impact of sex and reproductive status on memory circuitry structure and function in early midlife using structural covariance analysis. Hum Brain Mapp. 2019;40:1221–1233. 10.1002/hbm.24441
Jill M. Goldstein and Nikos Makris shared senior authors.
Funding information National Institute of Mental Health, Grant/Award Number: K24 MH110807 R01 MH090291R01 MH102377; National Institute on Aging, Grant/Award Number: R01 AG04252; Office of Research on Women's Health, Grant/Award Number: K12 HD051959
REFERENCES
- Abbs, B. , Liang, L. , Makris, N. , Tsuang, M. , Seidman, L. J. , & Goldstein, J. M. (2011). Covariance modeling of MRI brain volumes in memory circuitry in schizophrenia: Sex differences are critical. NeuroImage, 56(4), 1865–1874. 10.1016/j.neuroimage.2011.03.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis, D. R. , Moscovitch, M. , & McAndrews, M. P. (2007). Consequences of hippocampal damage across the autobiographical memory network in left temporal lobe epilepsy. Brain, 130(Pt. 9), 2327–2342. 10.1093/brain/awm166 [DOI] [PubMed] [Google Scholar]
- Alexander‐Bloch, A. , Giedd, J. N. , & Bullmore, E. (2013). Imaging structural co‐variance between human brain regions. Nature Reviews. Neuroscience, 14(5), 322–336. 10.1038/nrn3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard, E. S. , & Kensinger, E. A. (2014). Age‐related differences in functional connectivity during cognitive emotion regulation. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 69(6), 852–860. 10.1093/geronb/gbu108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, J. S. , Damasio, H. , Grabowski, T. J. , Bruss, J. , & Zhang, W. (2003). Sexual dimorphism and asymmetries in the gray‐white composition of the human cerebrum. NeuroImage, 18(4), 880–894. [DOI] [PubMed] [Google Scholar]
- Apostolova, L. G. , Dinov, I. D. , Dutton, R. A. , Hayashi, K. M. , Toga, A. W. , Cummings, J. L. , & Thompson, P. M. (2006). 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer's disease. Brain, 129(Pt. 11), 2867–2873. 10.1093/brain/awl274 [DOI] [PubMed] [Google Scholar]
- Arevalo, M. A. , Azcoitia, I. , Gonzalez‐Burgos, I. , & Garcia‐Segura, L. M. (2015). Signaling mechanisms mediating the regulation of synaptic plasticity and memory by estradiol. Hormones and Behavior, 74, 19–27. 10.1016/j.yhbeh.2015.04.016 [DOI] [PubMed] [Google Scholar]
- Arnold, A. P. (2009). The organizational‐activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Hormones and Behavior, 55(5), 570–578. 10.1016/j.yhbeh.2009.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpels, J. C. (1996). The female brain hypoestrogenic continuum from the premenstrual syndrome to menopause. A hypothesis and review of supporting data. The Journal of Reproductive Medicine, 41(9), 633–639. [PubMed] [Google Scholar]
- Barbas, H. , & Pandya, D. N. (1989). Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. The Journal of Comparative Neurology, 286(3), 353–375. 10.1002/cne.902860306 [DOI] [PubMed] [Google Scholar]
- Barha, C. K. , & Galea, L. A. (2010). Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochimica et Biophysica Acta, 1800(10), 1056–1067. 10.1016/j.bbagen.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Bayer, J. , Rune, G. , Schultz, H. , Tobia, M. J. , Mebes, I. , Katzler, O. , & Sommer, T. (2015). The effect of estrogen synthesis inhibition on hippocampal memory. Psychoneuroendocrinology, 56, 213–225. 10.1016/j.psyneuen.2015.03.011 [DOI] [PubMed] [Google Scholar]
- Benton, A. L. (1968). Differential behavioral effects in frontal lobe disease. Neuropsychologia, 6, 53–60. [Google Scholar]
- Berent‐Spillson, A. , Briceno, E. , Pinsky, A. , Simmen, A. , Persad, C. C. , Zubieta, J. K. , & Smith, Y. R. (2015). Distinct cognitive effects of estrogen and progesterone in menopausal women. Psychoneuroendocrinology, 59, 25–36. 10.1016/j.psyneuen.2015.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berent‐Spillson, A. , Persad, C. C. , Love, T. , Sowers, M. , Randolph, J. F. , Zubieta, J. K. , & Smith, Y. R. (2012). Hormonal environment affects cognition independent of age during the menopause transition. The Journal of Clinical Endocrinology and Metabolism, 97(9), E1686–E1694. 10.1210/jc.2012-1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker, M. L. , Bolla‐Wilson, K. , Agnew, J. , & Meyers, D. A. (1988). Age‐related sex differences in verbal memory. Journal of Clinical Psychology, 44(3), 403–411. [DOI] [PubMed] [Google Scholar]
- Boss, L. , Kang, D. H. , Marcus, M. , & Bergstrom, N. (2014). Endogenous sex hormones and cognitive function in older adults: A systematic review. Western Journal of Nursing Research, 36(3), 388–426. 10.1177/0193945913500566 [DOI] [PubMed] [Google Scholar]
- Boutet, I. , Milgram, N. W. , & Freedman, M. (2007). Cognitive decline and human (Homo sapiens) aging: An investigation using a comparative neuropsychological approach. Journal of Comparative Psychology, 121(3), 270–281. 10.1037/0735-7036.121.3.270 [DOI] [PubMed] [Google Scholar]
- Box, G. E. (1949). A general distribution theory for a class of likelihood criteria. Biometrika, 36(3–4), 317–346. [PubMed] [Google Scholar]
- Brinton, R. D. (2009). Estrogen‐induced plasticity from cells to circuits: Predictions for cognitive function. Trends in Pharmacological Sciences, 30(4), 212–222. 10.1016/j.tips.2008.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton, R. D. , Thompson, R. F. , Foy, M. R. , Baudry, M. , Wang, J. , Finch, C. E. , … Nilsen, J. (2008). Progesterone receptors: Form and function in brain. Frontiers in Neuroendocrinology, 29(2), 313–339. 10.1016/j.yfrne.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, W. , Luo, C. , Zhu, B. , Zhang, D. , Dong, L. , Gong, J. , … Yao, D. (2014). Resting‐state functional connectivity in anterior cingulate cortex in normal aging. Frontiers in Aging Neuroscience, 6, 280 10.3389/fnagi.2014.00280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli, R. J. , Chen, K. , Lee, W. , Alexander, G. E. , & Reiman, E. M. (2008). Correlating cerebral hypometabolism with future memory decline in subsequent converters to amnestic pre‐mild cognitive impairment. Archives of Neurology, 65(9), 1231–1236. 10.1001/archneurol.2008.1 [DOI] [PubMed] [Google Scholar]
- Cassaday, H. J. , Nelson, A. J. , & Pezze, M. A. (2014). From attention to memory along the dorsal‐ventral axis of the medial prefrontal cortex: Some methodological considerations. Frontiers in Systems Neuroscience, 8, 160 10.3389/fnsys.2014.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness, V. S., Jr. , Lange, N. T. , Makris, N. , Herbert, M. R. , & Kennedy, D. N. (1999). MRI‐based brain volumetrics: Emergence of a developmental brain science. Brain and Development, 21(5), 289–295. [DOI] [PubMed] [Google Scholar]
- Caviness, V. S., Jr. , Meyer, J. , Makris, N. , & Kennedy, D. N. (1996). MRI‐based topographic parcellation of human neocortex: An anatomically specified method with estimate of reliability. Journal of Cognitive Neuroscience, 8(6), 566–587. 10.1162/jocn.1996.8.6.566 [DOI] [PubMed] [Google Scholar]
- Chen, Z. J. , He, Y. , Rosa‐Neto, P. , Germann, J. , & Evans, A. C. (2008). Revealing modular architecture of human brain structural networks by using cortical thickness from MRI. Cerebral Cortex, 18(10), 2374–2381. 10.1093/cercor/bhn003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Sachdev, P. S. , Wen, W. , & Anstey, K. J. (2007). Sex differences in regional gray matter in healthy individuals aged 44‐48 years: A voxel‐based morphometric study. NeuroImage, 36(3), 691–699. 10.1016/j.neuroimage.2007.03.063 [DOI] [PubMed] [Google Scholar]
- Chiu, P. W. , Mak, H. K. , Yau, K. K. , Chan, Q. , Chang, R. C. , & Chu, L. W. (2014). Metabolic changes in the anterior and posterior cingulate cortices of the normal aging brain: Proton magnetic resonance spectroscopy study at 3 T. Age, 36(1), 251–264. 10.1007/s11357-013-9545-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland, C. D. , Choi, M. , Romberg, C. , Clemenson, G. D., Jr. , Fragniere, A. , Tyers, P. , … Bussey, T. J. (2009). A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science, 325(5937), 210–213. 10.1126/science.1173215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker, L. H. , Espeland, M. A. , Rapp, S. R. , Legault, C. , Resnick, S. M. , Hogan, P. , … Shumaker, S. A. (2010). Postmenopausal hormone therapy and cognitive outcomes: The Women's Health Initiative Memory Study (WHIMS). The Journal of Steroid Biochemistry and Molecular Biology, 118(4–5), 304–310. 10.1016/j.jsbmb.2009.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colibazzi, T. , Zhu, H. , Bansal, R. , Schultz, R. T. , Wang, Z. , & Peterson, B. S. (2008). Latent volumetric structure of the human brain: Exploratory factor analysis and structural equation modeling of gray matter volumes in healthy children and adults. Human Brain Mapping, 29(11), 1302–1312. 10.1002/hbm.20466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comasco, E. , Frokjaer, V. G. , & Sundstrom‐Poromaa, I. (2014). Functional and molecular neuroimaging of menopause and hormone replacement therapy. Frontiers in Neuroscience, 8, 388 10.3389/fnins.2014.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors, M. H. , Sachdev, P. S. , Kochan, N. A. , Xu, J. , Draper, B. , & Brodaty, H. (2015). Cognition and mortality in older people: The Sydney Memory and Ageing Study. Age and Ageing, 44(6), 1049–1054. 10.1093/ageing/afv139 [DOI] [PubMed] [Google Scholar]
- Cooke, B. M. , & Woolley, C. S. (2005). Gonadal hormone modulation of dendrites in the mammalian CNS. Journal of Neurobiology, 64(1), 34–46. 10.1002/neu.20143 [DOI] [PubMed] [Google Scholar]
- Dedovic, K. , D'Aguiar, C. , & Pruessner, J. C. (2009). What stress does to your brain: A review of neuroimaging studies. Canadian Journal of Psychiatry, 54(1), 6–15. [DOI] [PubMed] [Google Scholar]
- Del Re, E. C. , Gao, Y. , Eckbo, R. , Petryshen, T. L. , Blokland, G. A. , Seidman, L. J. , … Bouix, S. (2016). A new MRI masking technique based on multi‐atlas brain segmentation in controls and schizophrenia: A rapid and viable alternative to manual masking. Journal of Neuroimaging, 26(1), 28–36. 10.1111/jon.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer, T. , Geerlings, M. I. , Hofman, A. , de Jong, F. H. , Launer, L. J. , Pols, H. A. , & Breteler, M. M. (2003). Higher estrogen levels are not associated with larger hippocampi and better memory performance. Archives of Neurology, 60(2), 213–220. [DOI] [PubMed] [Google Scholar]
- Devinsky, O. , Morrell, M. J. , & Vogt, B. A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain, 118(Pt. 1), 279–306. [DOI] [PubMed] [Google Scholar]
- Di, X. , Gohel, S. , Thielcke, A. , Wehrl, H. F. , Biswal, B. B. , & Alzheimer's Disease Neuroimaging Initiative . (2017). Do all roads lead to Rome? A comparison of brain networks derived from inter‐subject volumetric and metabolic covariance and moment‐to‐moment hemodynamic correlations in old individuals. Brain Structure & Function, 222(8), 3833–3845. 10.1007/s00429-017-1438-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos, S. , Katsumi, Y. , & Dixon, R. A. (2014). The role of arousal in the spontaneous regulation of emotions in healthy aging: A fMRI investigation. Frontiers in Psychology, 5, 681 10.3389/fpsyg.2014.00681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte‐Guterman, P. , Yagi, S. , Chow, C. , & Galea, L. A. (2015). Hippocampal learning, memory, and neurogenesis: Effects of sex and estrogens across the lifespan in adults. Hormones and Behavior, 74, 37–52. 10.1016/j.yhbeh.2015.05.024 [DOI] [PubMed] [Google Scholar]
- Dumas, J. A. , Filippi, C. G. , Newhouse, P. A. , & Naylor, M. R. (2016). Dopaminergic contributions to working memory‐related brain activation in postmenopausal women. Menopause, 24, 163–170. 10.1097/GME.0000000000000738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas, J. A. , Kutz, A. M. , Naylor, M. R. , Johnson, J. V. , & Newhouse, P. A. (2010a). Increased memory load‐related frontal activation after estradiol treatment in postmenopausal women. Hormones and Behavior, 58(5), 929–935. 10.1016/j.yhbeh.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas, J. A. , McDonald, B. C. , Saykin, A. J. , McAllister, T. W. , Hynes, M. L. , West, J. D. , & Newhouse, P. A. (2010b). Cholinergic modulation of hippocampal activity during episodic memory encoding in postmenopausal women: A pilot study. Menopause, 17(4), 852–859. 10.1097/gme.0b013e3181e04db9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu, D. , Rapp, P. R. , McEwen, B. S. , & Morrison, J. H. (2010). Estrogen and the aging brain: An elixir for the weary cortical network. Annals of the New York Academy of Sciences, 1204, 104–112. 10.1111/j.1749-6632.2010.05529.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum, H. (2004). Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron, 44(1), 109–120. 10.1016/j.neuron.2004.08.028 [DOI] [PubMed] [Google Scholar]
- Epperson, C. N. , Sammel, M. D. , & Freeman, E. W. (2013). Menopause effects on verbal memory: Findings from a longitudinal community cohort. The Journal of Clinical Endocrinology and Metabolism, 98(9), 3829–3838. 10.1210/jc.2013-1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb, J. , & Obleser, J. (2013). Upregulation of cognitive control networks in older adults’ speech comprehension. Frontiers in Systems Neuroscience, 7, 116 10.3389/fnsys.2013.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek, P. A. , Richelme, C. , Kennedy, D. N. , & Caviness, V. S., Jr. (1994). The young adult human brain: An MRI‐based morphometric analysis. Cerebral Cortex, 4(4), 344–360. [DOI] [PubMed] [Google Scholar]
- Fischer, B. , Gleason, C. , & Asthana, S. (2014). Effects of hormone therapy on cognition and mood. Fertility and Sterility, 101(4), 898–904. 10.1016/j.fertnstert.2014.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , Salat, D. H. , Busa, E. , Albert, M. , Dieterich, M. , Haselgrove, C. , … Dale, A. M. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Frey, B. N. , Hall, G. B. , Attard, S. , Yucel, K. , Skelin, I. , Steiner, M. , & Soares, C. N. (2010). Shift in the brain network of emotional regulation in midlife women: Is the menopausal transition the turning point? Menopause, 17(4), 840–845. 10.1097/gme.0b013e3181df840f [DOI] [PubMed] [Google Scholar]
- Fuh, J. L. , Wang, S. J. , Lee, S. J. , Lu, S. R. , & Juang, K. D. (2006). A longitudinal study of cognition change during early menopausal transition in a rural community. Maturitas, 53(4), 447–453. 10.1016/j.maturitas.2005.07.009 [DOI] [PubMed] [Google Scholar]
- Giedd, J. N. , Raznahan, A. , Mills, K. L. , & Lenroot, R. K. (2012). Review: Magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biology of Sex Differences, 3(1), 19 10.1186/2042-6410-3-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd, J. N. , Vaituzis, A. C. , Hamburger, S. D. , Lange, N. , Rajapakse, J. C. , Kaysen, D. , … Rapoport, J. L. (1996). Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: Ages 4‐18 years. The Journal of Comparative Neurology, 366(2), 223–230. [DOI] [PubMed] [Google Scholar]
- Gillies, G. E. , & McArthur, S. (2010). Estrogen actions in the brain and the basis for differential action in men and women: A case for sex‐specific medicines. Pharmacological Reviews, 62(2), 155–198. 10.1124/pr.109.002071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman, S. E. , Cherkerzian, S. , Buka, S. L. , Hahn, J. , Hornig, M. , & Goldstein, J. M. (2016). Prenatal immune programming of the sex‐dependent risk for major depression. Translational Psychiatry, 6(5), e822 10.1038/tp.2016.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobinath, A. R. , Mahmoud, R. , & Galea, L. A. (2014). Influence of sex and stress exposure across the lifespan on endophenotypes of depression: Focus on behavior, glucocorticoids, and hippocampus. Frontiers in Neuroscience, 8, 420 10.3389/fnins.2014.00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman‐Rakic, P. S. (1988). Topography of cognition: Parallel distributed networks in primate association cortex. Annual Review of Neuroscience, 11, 137–156. 10.1146/annurev.ne.11.030188.001033 [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic, P. S. , Selemon, L. D. , & Schwartz, M. L. (1984). Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience, 12(3), 719–743. [DOI] [PubMed] [Google Scholar]
- Goldstein, J. M. , Buka, S. L. , Seidman, L. J. , & Tsuang, M. T. (2010). Specificity of familial transmission of schizophrenia psychosis spectrum and affective psychoses in the New England Family Study's high‐risk design. Archives of General Psychiatry, 67(5), 458–467. 10.1001/archgenpsychiatry.2010.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, J. M. , Cherkerzian, S. , Seidman, L. J. , Donatelli, J. A. , Remington, A. G. , Tsuang, M. T. , … Buka, S. L. (2014). Prenatal maternal immune disruption and sex‐dependent risk for psychoses. Psychological Medicine, 44(15), 3249–3261. 10.1017/S0033291714000683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, J. M. , Seidman, L. J. , Horton, N. J. , Makris, N. , Kennedy, D. N. , Caviness, V. S., Jr. , … Tsuang, M. T. (2001). Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex, 11(6), 490–497. [DOI] [PubMed] [Google Scholar]
- Goldstone, A. , Mayhew, S. D. , Przezdzik, I. , Wilson, R. S. , Hale, J. R. , & Bagshaw, A. P. (2016). Gender specific re‐organization of resting‐state networks in older age. Frontiers in Aging Neuroscience, 8, 285 10.3389/fnagi.2016.00285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, C. , Diaz, F. , & Alonso, A. (2008). Neuroprotective effects of estrogens: Cross‐talk between estrogen and intracellular insulin signalling. Infectious Disorders Drug Targets, 8(1), 65–67. [DOI] [PubMed] [Google Scholar]
- Good, C. D. , Johnsrude, I. , Ashburner, J. , Henson, R. N. , Friston, K. J. , & Frackowiak, R. S. (2001). Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel‐based morphometric analysis of 465 normal adult human brains. NeuroImage, 14(3), 685–700. 10.1006/nimg.2001.0857 [DOI] [PubMed] [Google Scholar]
- Goto, M. , Abe, O. , Miyati, T. , Inano, S. , Hayashi, N. , Aoki, S. , … Ohtomo, K. (2011a). 3 Tesla MRI detects accelerated hippocampal volume reduction in postmenopausal women. Journal of Magnetic Resonance Imaging, 33(1), 48–53. 10.1002/jmri.22328 [DOI] [PubMed] [Google Scholar]
- Goto, M. , Abe, O. , Miyati, T. , Inano, S. , Hayashi, N. , Aoki, S. , … Ohtomo, K. (2011b). Accelerated hippocampal volume reduction in post‐menopausal women: An additional study with Atlas‐based method. Radiological Physics and Technology, 4(2), 185–188. 10.1007/s12194-011-0120-7 [DOI] [PubMed] [Google Scholar]
- GraphPadSoftware . (2014). GraphPad Prism version 6.00 for Windows San Diego, CA: GraphPadSoftware. Retrieved from http://www.graphpad.com
- Greendale, G. A. , Derby, C. A. , & Maki, P. M. (2011). Perimenopause and cognition. Obstetrics and Gynecology Clinics of North America, 38(3), 519–535. 10.1016/j.ogc.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober, E. , Lipton, R. B. , Hall, C. , & Crystal, H. (2000). Memory impairment on free and cued selective reminding predicts dementia. Neurology, 54(4), 827–832. [DOI] [PubMed] [Google Scholar]
- Hall, E. , & Steiner, M. (2013). Serotonin and female psychopathology. Womens Health, 9(1), 85–97. 10.2217/whe.12.64 [DOI] [PubMed] [Google Scholar]
- Hannigan, C. , Coen, R. F. , Lawlor, B. A. , Robertson, I. H. , & Brennan, S. (2015). The NEIL Memory Research Unit: Psychosocial, biological, physiological and lifestyle factors associated with healthy ageing: Study protocol. BMC Psychology, 3(1), 20 10.1186/s40359-015-0079-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao, J. , Rapp, P. R. , Leffler, A. E. , Leffler, S. R. , Janssen, W. G. , Lou, W. , … Morrison, J. H. (2006). Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. The Journal of Neuroscience, 26(9), 2571–2578. 10.1523/JNEUROSCI.3440-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara, Y. , Park, C. S. , Janssen, W. G. , Punsoni, M. , Rapp, P. R. , & Morrison, J. H. (2011). Synaptic characteristics of dentate gyrus axonal boutons and their relationships with aging, menopause, and memory in female rhesus monkeys. The Journal of Neuroscience, 31(21), 7737–7744. 10.1523/JNEUROSCI.0822-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara, Y. , Yuk, F. , Puri, R. , Janssen, W. G. , Rapp, P. R. , & Morrison, J. H. (2016). Estrogen restores multisynaptic boutons in the dorsolateral prefrontal cortex while promoting working memory in aged rhesus monkeys. The Journal of Neuroscience, 36(3), 901–910. 10.1523/JNEUROSCI.3480-13.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, S. D. , Gass, M. , Hall, J. E. , Lobo, R. , Maki, P. , Rebar, R. W. , … For the STRAW + 10 Collaborative Group . (2012). Executive summary of the stages of reproductive aging workshop + 10: Addressing the unfinished agenda of staging reproductive aging. The Journal of Clinical Endocrinology and Metabolism, 97(4), 1159–1168. 10.1210/jc.2011-3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte‐Hargrove, L. C. , Maclusky, N. J. , & Scharfman, H. E. (2013). Brain‐derived neurotrophic factor‐estrogen interactions in the hippocampal mossy fiber pathway: Implications for normal brain function and disease. Neuroscience, 239, 46–66. 10.1016/j.neuroscience.2012.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley, A. A. , & Speer, N. K. (2000). Locating and fractionating working memory using functional neuroimaging: Storage, maintenance, and executive functions. Microscopy Research and Technique, 51(1), 45–53. [DOI] [PubMed] [Google Scholar]
- He, Y. , Chen, Z. , & Evans, A. (2008). Structural insights into aberrant topological patterns of large‐scale cortical networks in Alzheimer's disease. The Journal of Neuroscience, 28(18), 4756–4766. 10.1523/JNEUROSCI.0141-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden, T. , Mormino, E. C. , Amariglio, R. E. , Younger, A. P. , Schultz, A. P. , Becker, J. A. , … Rentz, D. M. (2012). Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. The Journal of Neuroscience, 32(46), 16233–16242. 10.1523/JNEUROSCI.2462-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst, E. (2013). Effects of gonadal hormones on cognitive behaviour in elderly men and women. Journal of Neuroendocrinology, 25(11), 1182–1195. 10.1111/jne.12080 [DOI] [PubMed] [Google Scholar]
- Huang, J. , Bai, F. , Yang, X. , Chen, C. , Bao, X. , & Zhang, Y. (2015). Identifying brain functional alterations in postmenopausal women with cognitive impairment. Maturitas, 81(3), 371–376. 10.1016/j.maturitas.2015.04.006 [DOI] [PubMed] [Google Scholar]
- IBMCorp . (2013). IBM SPSS statistics for Windows, version 22.0. Armonk, NY: IBMCorp. [Google Scholar]
- Insel, N. , & Takehara‐Nishiuchi, K. (2013). The cortical structure of consolidated memory: A hypothesis on the role of the cingulate‐entorhinal cortical connection. Neurobiology of Learning and Memory, 106, 343–350. 10.1016/j.nlm.2013.07.019 [DOI] [PubMed] [Google Scholar]
- Isamah, N. , Faison, W. , Payne, M. E. , MacFall, J. , Steffens, D. C. , Beyer, J. L. , … Taylor, W. D. (2010). Variability in frontotemporal brain structure: The importance of recruitment of African Americans in neuroscience research. PLoS One, 5(10), e13642 10.1371/journal.pone.0013642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, E. , & D'Esposito, M. (2011). Estrogen shapes dopamine‐dependent cognitive processes: Implications for women's health. The Journal of Neuroscience, 31(14), 5286–5293. 10.1523/JNEUROSCI.6394-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, E. G. , Holsen, L. M. , Lancaster, K. , Makris, N. , Whitfield‐Gabrieli, S. , Remington, A. , … Goldstein, J. M. (2015). 17β‐estradiol differentially regulates stress circuitry activity in healthy and depressed women. Neuropsychopharmacology, 40(3), 566–576. 10.1038/npp.2014.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, E. G. , Weiss, B. K. , Makris, N. , Whitfield‐Gabrieli, S. , Buka, S. L. , Klibanski, A. , & Goldstein, J. M. (2016). Impact of sex and menopausal status on episodic memory circuitry in early midlife. The Journal of Neuroscience, 36(39), 10163–10173. 10.1523/JNEUROSCI.0951-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, E. G. , Weiss, B. , Makris, N. , Whitfield‐Gabrieli, S. , Buka, S. L. , Klibanski, A. , & Goldstein, J. M. (2017). Reorganization of functional networks in verbal working memory circuitry in early midlife: The impact of sex and menopausal status. Cerebral Cortex, 27(5), 2857–2870. 10.1093/cercor/bhw127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, D. N. , Lange, N. , Makris, N. , Bates, J. , Meyer, J. , & Caviness, V. S., Jr. (1998). Gyri of the human neocortex: An MRI‐based analysis of volume and variance. Cerebral Cortex, 8(4), 372–384. [DOI] [PubMed] [Google Scholar]
- Klaassen, E. B. , Plukaard, S. , Evers, E. A. , de Groot, R. H. , Backes, W. H. , Veltman, D. J. , & Jolles, J. (2016). Young and middle‐aged schoolteachers differ in the neural correlates of memory encoding and cognitive fatigue: A functional MRI study. Frontiers in Human Neuroscience, 10, 148 10.3389/fnhum.2016.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto, K. , Reinikainen, K. J. , Hanninen, T. , Vanhanen, M. , Helkala, E. L. , Mykkanen, L. , … Riekkinen, P. J., Sr. (1995). Prevalence of age‐associated memory impairment in a randomly selected population from eastern Finland. Neurology, 45(4), 741–747. [DOI] [PubMed] [Google Scholar]
- Krause, B. J. , Horwitz, B. , Taylor, J. G. , Schmidt, D. , Mottaghy, F. M. , Herzog, H. , … Muller‐Gartner, H. (1999). Network analysis in episodic encoding and retrieval of word‐pair associates: A PET study. The European Journal of Neuroscience, 11(9), 3293–3301. [DOI] [PubMed] [Google Scholar]
- La Corte, V. , Sperduti, M. , Malherbe, C. , Vialatte, F. , Lion, S. , Gallarda, T. , … Piolino, P. (2016). Cognitive decline and reorganization of functional connectivity in healthy aging: The pivotal role of the salience network in the prediction of age and cognitive performances. Frontiers in Aging Neuroscience, 8, 204 10.3389/fnagi.2016.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, A. , Tan, M. , & Qiu, A. (2016). Distinct aging effects on functional networks in good and poor cognitive performers. Frontiers in Aging Neuroscience, 8, 215 10.3389/fnagi.2016.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos, R. , Simoes, M. R. , Santiago, B. , & Santana, I. (2015). The free and cued selective reminding test: Validation for mild cognitive impairment and Alzheimer's disease. Journal of Neuropsychology, 9(2), 242–257. 10.1111/jnp.12048 [DOI] [PubMed] [Google Scholar]
- Lerch, J. P. , Worsley, K. , Shaw, W. P. , Greenstein, D. K. , Lenroot, R. K. , Giedd, J. , & Evans, A. C. (2006). Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. NeuroImage, 31(3), 993–1003. 10.1016/j.neuroimage.2006.01.042 [DOI] [PubMed] [Google Scholar]
- Liu, F. , Day, M. , Muniz, L. C. , Bitran, D. , Arias, R. , Revilla‐Sanchez, R. , … Brandon, N. J. (2008). Activation of estrogen receptor‐beta regulates hippocampal synaptic plasticity and improves memory. Nature Neuroscience, 11(3), 334–343. 10.1038/nn2057 [DOI] [PubMed] [Google Scholar]
- Lord, C. , Buss, C. , Lupien, S. J. , & Pruessner, J. C. (2008). Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: A possible window of opportunity effect. Neurobiology of Aging, 29(1), 95–101. 10.1016/j.neurobiolaging.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Mahmoud, R. , Wainwright, S. R. , & Galea, L. A. (2016). Sex hormones and adult hippocampal neurogenesis: Regulation, implications, and potential mechanisms. Frontiers in Neuroendocrinology, 41, 129–152. 10.1016/j.yfrne.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Maki, P. M. (2013). Critical window hypothesis of hormone therapy and cognition: A scientific update on clinical studies. Menopause, 20(6), 695–709. 10.1097/GME.0b013e3182960cf8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki, P. M. (2015). Verbal memory and menopause. Maturitas, 82(3), 288–290. 10.1016/j.maturitas.2015.07.023 [DOI] [PubMed] [Google Scholar]
- Makris, N. , Kennedy, D. N. , McInerney, S. , Sorensen, A. G. , Wang, R. , Caviness, V. S., Jr. , & Pandya, D. N. (2005). Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Cerebral Cortex, 15(6), 854–869. 10.1093/cercor/bhh186 [DOI] [PubMed] [Google Scholar]
- Makris, N. , Meyer, J. W. , Bates, J. F. , Yeterian, E. H. , Kennedy, D. N. , & Caviness, V. S. (1999). MRI‐based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. NeuroImage, 9(1), 18–45. 10.1006/nimg.1998.0384 [DOI] [PubMed] [Google Scholar]
- Masur, D. M. , Fuld, P. A. , Blau, A. D. , Thal, L. J. , Levin, H. S. , & Aronson, M. K. (1989). Distinguishing normal and demented elderly with the selective reminding test. Journal of Clinical and Experimental Neuropsychology, 11(5), 615–630. 10.1080/01688638908400920 [DOI] [PubMed] [Google Scholar]
- McClure, R. E. , Barha, C. K. , & Galea, L. A. (2013). 17β‐estradiol, but not estrone, increases the survival and activation of new neurons in the hippocampus in response to spatial memory in adult female rats. Hormones and Behavior, 63(1), 144–157. 10.1016/j.yhbeh.2012.09.011 [DOI] [PubMed] [Google Scholar]
- McEwen, B. S. , Nasca, C. , & Gray, J. D. (2016). Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology, 41(1), 3–23. 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli, A. , Friston, K. J. , Frackowiak, R. S. , & Price, C. J. (2005). Structural covariance in the human cortex. The Journal of Neuroscience, 25(36), 8303–8310. 10.1523/JNEUROSCI.0357-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messay, B. , Lim, A. , & Marsland, A. L. (2012). Current understanding of the bi‐directional relationship of major depression with inflammation. Biology of Mood & Anxiety Disorders, 2, 4 10.1186/2045-5380-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler‐Baddeley, C. , Jones, D. K. , Belaroussi, B. , Aggleton, J. P. , & O'Sullivan, M. J. (2011). Frontotemporal connections in episodic memory and aging: A diffusion MRI tractography study. The Journal of Neuroscience, 31(37), 13236–13245. 10.1523/JNEUROSCI.2317-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych, P. , & Christensen, A. (2012). Membrane‐initiated estradiol actions mediate structural plasticity and reproduction. Frontiers in Neuroendocrinology, 33(4), 331–341. 10.1016/j.yfrne.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman, S. A. , Buchsbaum, M. S. , Brickman, A. M. , & Shihabuddin, L. (2005a). Cortical intercorrelations of frontal area volumes in schizophrenia. NeuroImage, 27(4), 753–770. 10.1016/j.neuroimage.2005.05.024 [DOI] [PubMed] [Google Scholar]
- Mitelman, S. A. , Shihabuddin, L. , Brickman, A. M. , & Buchsbaum, M. S. (2005b). Cortical intercorrelations of temporal area volumes in schizophrenia. Schizophrenia Research, 76(2–3), 207–229. 10.1016/j.schres.2005.01.010 [DOI] [PubMed] [Google Scholar]
- Montembeault, M. , Joubert, S. , Doyon, J. , Carrier, J. , Gagnon, J. F. , Monchi, O. , … Brambati, S. M. (2012). The impact of aging on gray matter structural covariance networks. NeuroImage, 63(2), 754–759. 10.1016/j.neuroimage.2012.06.052 [DOI] [PubMed] [Google Scholar]
- Morrison, J. H. , Brinton, R. D. , Schmidt, P. J. , & Gore, A. C. (2006). Estrogen, menopause, and the aging brain: How basic neuroscience can inform hormone therapy in women. The Journal of Neuroscience, 26(41), 10332–10348. 10.1523/JNEUROSCI.3369-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel, R. A. , Aggarwal, N. T. , Barnes, L. L. , Gallagher, A. , Goldstein, J. M. , Kantarci, K. , … Mielke, M. M. (2018). Understanding the impact of sex and gender in Alzheimer's disease: A call to action. Alzheimers Dement, 14(9), 1171–1183. 10.1016/j.jalz.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, H. E. (1982). National Adult Reading Test (NART) test manual. Windsor, UK: NFER‐Nelson. [Google Scholar]
- Niswander, K. R. , & Gordon, M. J. (1972). The women and their pregnancies: The Collaborative Perinatal Study of the NAtional Institue of Neurological Diseases and Stroke. Philadelphia: W.B. Saunders Company, National Institute for Health. [Google Scholar]
- Nopoulos, P. , Flaum, M. , O'Leary, D. , & Andreasen, N. C. (2000). Sexual dimorphism in the human brain: Evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Research, 98(1), 1–13. [DOI] [PubMed] [Google Scholar]
- Ortman, J. , Velkoff, V. A. , & Hogan, H. (2014). An aging nation: The older population in the United States. U.S. Census Bureau.
- Pelosi, E. , Simonsick, E. , Forabosco, A. , Garcia‐Ortiz, J. E. , & Schlessinger, D. (2015). Dynamics of the ovarian reserve and impact of genetic and epidemiological factors on age of menopause. Biology of Reproduction, 92(5), 130 10.1095/biolreprod.114.127381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, B. S. , Skudlarski, P. , Gatenby, J. C. , Zhang, H. , Anderson, A. W. , & Gore, J. C. (1999). An fMRI study of Stroop word‐color interference: Evidence for cingulate subregions subserving multiple distributed attentional systems. Biological Psychiatry, 45(10), 1237–1258. [DOI] [PubMed] [Google Scholar]
- Petrides, M. , & Pandya, D. N. (1984). Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. The Journal of Comparative Neurology, 228(1), 105–116. 10.1002/cne.902280110 [DOI] [PubMed] [Google Scholar]
- Pluchino, N. , Luisi, M. , Lenzi, E. , Centofanti, M. , Begliuomini, S. , Freschi, L. , … Genazzani, A. R. (2006). Progesterone and progestins: Effects on brain, allopregnanolone and beta‐endorphin. The Journal of Steroid Biochemistry and Molecular Biology, 102(1–5), 205–213. 10.1016/j.jsbmb.2006.09.023 [DOI] [PubMed] [Google Scholar]
- Pompili, A. , Arnone, B. , & Gasbarri, A. (2012). Estrogens and memory in physiological and neuropathological conditions. Psychoneuroendocrinology, 37(9), 1379–1396. 10.1016/j.psyneuen.2012.01.007 [DOI] [PubMed] [Google Scholar]
- Protopopescu, X. , Butler, T. , Pan, H. , Root, J. , Altemus, M. , Polanecsky, M. , … Stern, E. (2008). Hippocampal structural changes across the menstrual cycle. Hippocampus, 18(10), 985–988. 10.1002/hipo.20468 [DOI] [PubMed] [Google Scholar]
- Pruessner, J. C. , Dedovic, K. , Pruessner, M. , Lord, C. , Buss, C. , Collins, L. , … Lupien, S. J. (2010). Stress regulation in the central nervous system: Evidence from structural and functional neuroimaging studies in human populations ‐ 2008 Curt Richter Award Winner. Psychoneuroendocrinology, 35(1), 179–191. 10.1016/j.psyneuen.2009.02.016 [DOI] [PubMed] [Google Scholar]
- Qin, P. , & Northoff, G. (2011). How is our self related to midline regions and the default‐mode network? NeuroImage, 57(3), 1221–1233. 10.1016/j.neuroimage.2011.05.028 [DOI] [PubMed] [Google Scholar]
- Rentz, D. M. , Amariglio, R. E. , Becker, J. A. , Frey, M. , Olson, L. E. , Frishe, K. , … Sperling, R. A. (2011). Face‐name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia, 49(9), 2776–2783. 10.1016/j.neuropsychologia.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz, D. M. , Weiss, B. K. , Jacobs, E. G. , Cherkerzian, S. , Klibanski, A. , Remington, A. , … Goldstein, J. M. (2016). Sex differences in episodic memory in early midlife: Impact of reproductive aging. Menopause, 24(4), 400–408. 10.1097/GME.0000000000000771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, L. , & Park, S. (2002). Verbal and spatial functions across the menstrual cycle in healthy young women. Psychoneuroendocrinology, 27(7), 835–841. [DOI] [PubMed] [Google Scholar]
- Rosenblat, J. D. , Cha, D. S. , Mansur, R. B. , & McIntyre, R. S. (2014). Inflamed moods: A review of the interactions between inflammation and mood disorders. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 53, 23–34. 10.1016/j.pnpbp.2014.01.013 [DOI] [PubMed] [Google Scholar]
- Rosene, D. L. , & Van Hoesen, G. W. (1977). Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science, 198(4314), 315–317. [DOI] [PubMed] [Google Scholar]
- Ruff, R. M. , Light, R. H. , & Quayhagen, M. (1989). Selective reminding tests: A normative study of verbal learning in adults. Journal of Clinical and Experimental Neuropsychology, 11(4), 539–550. 10.1080/01688638908400912 [DOI] [PubMed] [Google Scholar]
- Ruigrok, A. N. , Salimi‐Khorshidi, G. , Lai, M. C. , Baron‐Cohen, S. , Lombardo, M. V. , Tait, R. J. , & Suckling, J. (2014). A meta‐analysis of sex differences in human brain structure. Neuroscience and Biobehavioral Reviews, 39, 34–50. 10.1016/j.neubiorev.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami, A. , Rieckmann, A. , Fischer, H. , & Backman, L. (2014). A multivariate analysis of age‐related differences in functional networks supporting conflict resolution. NeuroImage, 86, 150–163. 10.1016/j.neuroimage.2013.08.002 [DOI] [PubMed] [Google Scholar]
- Schlaepfer, T. E. , Harris, G. J. , Tien, A. Y. , Peng, L. , Lee, S. , & Pearlson, G. D. (1995). Structural differences in the cerebral cortex of healthy female and male subjects: A magnetic resonance imaging study. Psychiatry Research, 61(3), 129–135. [DOI] [PubMed] [Google Scholar]
- Seeley, W. W. , Crawford, R. K. , Zhou, J. , Miller, B. L. , & Greicius, M. D. (2009). Neurodegenerative diseases target large‐scale human brain networks. Neuron, 62(1), 42–52. 10.1016/j.neuron.2009.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer, B. , & Van Hoesen, G. W. (1979). A direct inferior parietal lobule projection to the presubiculum in the rhesus monkey. Brain Research, 179(1), 157–161. [DOI] [PubMed] [Google Scholar]
- Sesack, S. R. , Deutch, A. Y. , Roth, R. H. , & Bunney, B. S. (1989). Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: An anterograde tract‐tracing study with Phaseolus vulgaris leucoagglutinin. The Journal of Comparative Neurology, 290(2), 213–242. 10.1002/cne.902900205 [DOI] [PubMed] [Google Scholar]
- Shanmugan, S. , & Epperson, C. N. (2014). Estrogen and the prefrontal cortex: Towards a new understanding of estrogen's effects on executive functions in the menopause transition. Human Brain Mapping, 35(3), 847–865. 10.1002/hbm.22218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu, B. C. , & Vogt, B. A. (2009). Short‐term synaptic plasticity in the nociceptive thalamic‐anterior cingulate pathway. Molecular Pain, 5, 51 10.1186/1744-8069-5-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly, R. B. , Chang, C. , Muramatsu, M. , & Swanson, L. W. (1990). Distribution of androgen and estrogen receptor mRNA‐containing cells in the rat brain: An in situ hybridization study. The Journal of Comparative Neurology, 294(1), 76–95. 10.1002/cne.902940107 [DOI] [PubMed] [Google Scholar]
- Skinner, E. I. , & Fernandes, M. A. (2007). Neural correlates of recollection and familiarity: A review of neuroimaging and patient data. Neuropsychologia, 45(10), 2163–2179. 10.1016/j.neuropsychologia.2007.03.007 [DOI] [PubMed] [Google Scholar]
- Smith, E. E. , & Jonides, J. (1999). Storage and executive processes in the frontal lobes. Science, 283(5408), 1657–1661. [DOI] [PubMed] [Google Scholar]
- Sowell, E. R. , Peterson, B. S. , Kan, E. , Woods, R. P. , Yoshii, J. , Bansal, R. , … Toga, A. W. (2007). Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cerebral Cortex, 17(7), 1550–1560. 10.1093/cercor/bhl066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spati, J. , Hanggi, J. , Doerig, N. , Ernst, J. , Sambataro, F. , Brakowski, J. , … Spinelli, S. (2015). Prefrontal thinning affects functional connectivity and regional homogeneity of the anterior cingulate cortex in depression. Neuropsychopharmacology, 40(7), 1640–1648. 10.1038/npp.2015.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling, R. A. , Bates, J. F. , Chua, E. F. , Cocchiarella, A. J. , Rentz, D. M. , Rosen, B. R. , … Albert, M. S. (2003). fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. Journal of Neurology, Neurosurgery, and Psychiatry, 74(1), 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire, L. R. , Stark, C. E. , & Clark, R. E. (2004). The medial temporal lobe. Annual Review of Neuroscience, 27, 279–306. 10.1146/annurev.neuro.27.070203.144130 [DOI] [PubMed] [Google Scholar]
- Stolk, L. , Perry, J. R. , Chasman, D. I. , He, C. , Mangino, M. , Sulem, P. , … Lunetta, K. L. (2012). Meta‐analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nature Genetics, 44(3), 260–268. 10.1038/ng.1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoeke, C. , Lehert, P. , Henderson, V. W. , Dennerstein, L. , Desmond, P. , & Campbell, S. (2016). Predictive factors for verbal memory performance over decades of aging: Data from the Women's Healthy Ageing Project. The American Journal of Geriatric Psychiatry, 24(10), 857–867. 10.1016/j.jagp.2016.05.008 [DOI] [PubMed] [Google Scholar]
- Toyoda, H. , Li, X. Y. , Wu, L. J. , Zhao, M. G. , Descalzi, G. , Chen, T. , … Zhuo, M. (2011). Interplay of amygdala and cingulate plasticity in emotional fear. Neural Plasticity, 2011, 813749 10.1155/2011/813749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trahan, D. E. , & Quintana, J. W. (1990). Analysis of gender effects upon verbal and visual memory performance in adults. Archives of Clinical Neuropsychology, 5(4), 325–334. [PubMed] [Google Scholar]
- Vaidya, J. G. , Paradiso, S. , Boles Ponto, L. L. , McCormick, L. M. , & Robinson, R. G. (2007). Aging, grey matter, and blood flow in the anterior cingulate cortex. NeuroImage, 37(4), 1346–1353. 10.1016/j.neuroimage.2007.06.015 [DOI] [PubMed] [Google Scholar]
- Valkanova, V. , Ebmeier, K. P. , & Allan, C. L. (2013). CRP, IL‐6 and depression: A systematic review and meta‐analysis of longitudinal studies. Journal of Affective Disorders, 150(3), 736–744. 10.1016/j.jad.2013.06.004 [DOI] [PubMed] [Google Scholar]
- van Geldorp, B. , Heringa, S. M. , van den Berg, E. , Olde Rikkert, M. G. , Biessels, G. J. , & Kessels, R. P. (2015). Working memory binding and episodic memory formation in aging, mild cognitive impairment, and Alzheimer's dementia. Journal of Clinical and Experimental Neuropsychology, 37(5), 538–548. 10.1080/13803395.2015.1037722 [DOI] [PubMed] [Google Scholar]
- van Hooren, S. A. , Valentijn, A. M. , Bosma, H. , Ponds, R. W. , van Boxtel, M. P. , & Jolles, J. (2007). Cognitive functioning in healthy older adults aged 64‐81: A cohort study into the effects of age, sex, and education. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 14(1), 40–54. 10.1080/138255890969483 [DOI] [PubMed] [Google Scholar]
- Vogt, B. A. , Finch, D. M. , & Olson, C. R. (1992). Functional heterogeneity in cingulate cortex: The anterior executive and posterior evaluative regions. Cerebral Cortex, 2(6), 435–443. [DOI] [PubMed] [Google Scholar]
- Weber, M. T. , Maki, P. M. , & McDermott, M. P. (2014). Cognition and mood in perimenopause: A systematic review and meta‐analysis. The Journal of Steroid Biochemistry and Molecular Biology, 142, 90–98. 10.1016/j.jsbmb.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, M. T. , Rubin, L. H. , & Maki, P. M. (2013). Cognition in perimenopause: The effect of transition stage. Menopause, 20(5), 511–517. 10.1097/GME.0b013e31827655e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (1997). wais‐iii Administration and scoring manual. San Antonio, TX: The Psychological Association. [Google Scholar]
- Weible, A. P. (2013). Remembering to attend: The anterior cingulate cortex and remote memory. Behavioural Brain Research, 245, 63–75. 10.1016/j.bbr.2013.02.010 [DOI] [PubMed] [Google Scholar]
- Weiss, E. M. , Ragland, J. D. , Brensinger, C. M. , Bilker, W. B. , Deisenhammer, E. A. , & Delazer, M. (2006). Sex differences in clustering and switching in verbal fluency tasks. Journal of the International Neuropsychological Society, 12(4), 502–509. [DOI] [PubMed] [Google Scholar]
- Winkler, A. M. , Kochunov, P. , Blangero, J. , Almasy, L. , Zilles, K. , Fox, P. T. , … Glahn, D. C. (2010). Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage, 53(3), 1135–1146. 10.1016/j.neuroimage.2009.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wnuk, A. , Korol, D. L. , & Erickson, K. I. (2012). Estrogens, hormone therapy, and hippocampal volume in postmenopausal women. Maturitas, 73(3), 186–190. 10.1016/j.maturitas.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley, C. S. , & McEwen, B. S. (1994). Estradiol regulates hippocampal dendritic spine density via an N‐methyl‐D‐aspartate receptor‐dependent mechanism. The Journal of Neuroscience, 14(12), 7680–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L. , Soder, R. B. , Schoemaker, D. , Carbonnell, F. , Sziklas, V. , Rowley, J. , … Rosa‐Neto, P. (2014). Resting state executive control network adaptations in amnestic mild cognitive impairment. Journal of Alzheimer's Disease, 40(4), 993–1004. 10.3233/JAD-131574 [DOI] [PubMed] [Google Scholar]
- Ycaza Herrera, A. , & Mather, M. (2015). Actions and interactions of estradiol and glucocorticoids in cognition and the brain: Implications for aging women. Neuroscience and Biobehavioral Reviews, 55, 36–52. 10.1016/j.neubiorev.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo, M. (2007). A synaptic model for pain: Long‐term potentiation in the anterior cingulate cortex. Molecules and Cells, 23(3), 259–271. [PubMed] [Google Scholar]


