Abstract
Type 1 diabetes has been associated with alterations in attentional processing and other cognitive functions, and previous studies have found alterations in both brain structure and function in affected patients. However, these previous neuroimaging studies have generally examined older patients, particularly those with major comorbidities known to affect functioning independent of diabetes. The primary aim of the current study was to examine the neural dynamics of selective attention processing in a young group of patients with type 1 diabetes who were otherwise healthy (i.e., without major comorbidities). Our hypothesis was that these patients would exhibit significant aberrations in attention circuitry relative to closely matched controls. The final sample included 69 participants age 19–35 years old, 35 with type 1 diabetes and 34 matched nondiabetic controls, who completed an Eriksen flanker task while undergoing magnetoencephalography. Significant group differences in flanker interference activity were found across a network of brain regions, including the anterior cingulate, inferior parietal cortices, paracentral lobule, and the left precentral gyrus. In addition, neural activity in the anterior cingulate and the paracentral lobule was correlated with disease duration in patients with type 1 diabetes. These findings suggest that alterations in the neural circuitry underlying selective attention emerge early in the disease process and are specifically related to type 1 diabetes and not common comorbidities. These findings highlight the need for longitudinal studies in large cohorts to clarify the clinical implications of type 1 diabetes on cognition and the brain.
Keywords: alpha, conflict monitoring, flanker, magnetoencephalography, theta
1. INTRODUCTION
Type 1 diabetes is a metabolic disorder that is known to be associated with several major complications, including growing recognition of disease‐related structural and functional brain alterations. Both gray matter volume and white matter integrity appear to be affected, with a reduction in both metrics in patients with type 1 diabetes (Bednarik et al., 2017; Biessels & Reijmer, 2014; Musen et al., 2006). Neuropsychological studies have identified the cognitive domains most affected, which include psychomotor speed, intelligence, cognitive flexibility, executive function, and attention (McCrimmon, Ryan, & Frier, 2012; Ryan, van Duinkerken, & Rosano, 2016). Importantly, deficits in these domains have been specifically linked to measures of disease status including glycemic control and disease duration (Desrocher & Rovet, 2004; Tonoli et al., 2014). Such cognitive deficits have been shown to induce a circular pattern of dysfunction, whereby chronic dysglycemia leads to specific cognitive deficits, which in turn lead to impaired ability to monitor and maintain adequate blood sugar levels, and thereby further cognitive decline (Grober, Hall, Hahn, & Lipton, 2011; Hansen et al., 2017).
In cognitive psychology, attention is generally described as the preferential allocation of processing resources to a specific stimulus or set of stimuli. Attention is an essential element in an array of daily activities, including disease care behaviors (i.e., detecting and correcting episodes of dysglycemia), and as mentioned above is known to be affected by key components of the disease (e.g., chronic dysglycemia). Selective attention tasks examine the mechanics of attentional processing in the context of response competition where ignoring distracting information is critical to task performance (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Desimone & Duncan, 1995). In these tasks, functional neuroimaging studies have found recruitment of various components of the frontoparietal network and the anterior cingulate, with much of the work focusing on the latter region for its role in conflict monitoring processes (Botvinick et al., 2001; Botvinick, Cohen, & Carter, 2004; Bunge, Hazeltine, Scanlon, Rosen, & Gabrieli, 2002; Hazeltine, Poldrack, & Gabrieli, 2000). Interestingly, previous functional neuroimaging studies of type 1 diabetes have indicated widespread network activity disruptions across these attention‐related brain areas during a variety of cognitive tasks (Bolo et al., 2011; Gallardo‐Moreno, Gonzalez‐Garrido, Gudayol‐Ferre, & Guardia‐Olmos, 2015; Hwang et al., 2016; Rooijackers, Wiegers, Tack, van der Graaf, & de Galan, 2016; van Duinkerken et al., 2012). These investigations have often examined participants in hypoglycemic states, usually induced through glycemic clamp approaches, which has allowed them to focus on the acute effects of hypoglycemia on brain function during the resting‐state and/or various cognitive tasks (Frier, 2001; Rooijackers et al., 2016; Warren & Frier, 2005). In the present study, we examined a group of young adults with type 1 diabetes during normoglycemic conditions, to more directly examine the longer‐term, sustained effects of type 1 diabetes on neurophysiological responses. Furthermore, to minimize the impact of confounding health factors that are often associated with diabetes (e.g., micro‐ and macro‐vascular damage, obesity, organ damage), we focused on healthy young adults with type 1 diabetes who were free of major comorbidities.
We used high‐density magnetoencephalographic (MEG) imaging to identify how type 1 diabetes affects the neurophysiology of selective attention processing and conflict monitoring during the classic Eriksen flanker paradigm (Eriksen & Eriksen, 1974). Using MEG neuroimaging and the classic flanker task paradigm, we were able to assess the neurophysiology of low versus high attention demand through the flanker interference effect in each group, and determine how type 1 diabetes affected the differing degrees of attention processing. The flanker task is also known to tap processes related to conflict monitoring, which are of major interest in type 1 diabetes. Our primary hypothesis was that participants with type 1 diabetes would exhibit aberrant neural responses during the flanker attention task, particularly during the higher‐attentional demand trials (i.e., flanker interference effect) relative to closely matched controls. In addition, we hypothesized that disease duration would be directly related to these aberrant neural responses.
2. RESEARCH DESIGN AND METHODS
2.1. Participants
A group of 40 patients with type 1 diabetes and no known comorbidities was recruited from the Diabetes Clinic at the University of Nebraska Medical Center (UNMC; age range: 19–35 years, 16 females). A control group (N = 40) matched on age, sex, education, body mass index, ethnicity, and handedness to the patient group was also recruited from the greater Omaha area. Exclusionary criteria included: (a) any medical diagnosis affecting CNS function (e.g., psychiatric and/or neurological disease), (b) known brain neoplasm or lesion, (c) history of significant head trauma, (d) current substance use disorder, (e) pregnancy or lactation, (f) hospitalization within the previous 3 months, (g) any type of cancer, (h) treatment with antipsychotics, antidepressants, and related medications known to affect brain function, with the exception of as‐needed antidepressants following a 24 hr washout period, (i) current or prior treatment with statins, and (j) ferromagnetic implants. Patients were additionally excluded for the presence of (a) micro‐ or macro‐vascular disease defined as a urinary albumin‐to‐creatinine ratio > 30 μg AL/mgCR in the previous 12 months, (b) hypertension (blood pressure > 130/85 mmHg), (c) kidney disease defined by GFR < 60 ml/min/1.73 m2, (d) aspartate transaminase‐to‐alanine transaminase ratio > 2 U/L, (e) a severe hypoglycemic episode within the past three months defined as an event requiring third‐party assistance, (f) untreated thyroid disease, and/or (g) B12 deficiency. Written informed consent was obtained from each participant following the guidelines of the UNMC's Institutional Review Board, who approved the study protocol, in accordance with the Declaration of Helsinki.
2.2. Glucose measurements and health factors
Prior to MEG, a blood panel, including glycated hemoglobin (HbA1c), was completed for participants with type 1 diabetes according to the standards of care described by the American Diabetes Association. In addition, participants were asked about specific demographic factors, their general medical history, and about the number of hypoglycemic episodes experienced per week. Before undergoing MEG, patients' blood glucose level was measured using a point‐of‐care device and had to be within the 70 to 200 mg/dl range. If measurements were between 55 and 70 mg/dl, patients were asked to raise their blood sugar to the normal range, and after 1 hr in the normal range, these participants started their MEG session. Patients whose blood sugars were less than 55 mg/dl or over 200 mg/dl were rescheduled at least 1 week later, as such values equate to clinically significant hypo‐ and hyperglycemia. For full lab results and general characteristics of the patient group (Table 1).
Table 1.
Mean characteristics and lab results
| Type 1 diabetes | |
|---|---|
| Disease duration | 12.2 ± 7.4 years |
| Hypoglycemic episodes per week | 3.02 ± 2.98 episodes |
| Glucose at time of MEG scan | 142.66 ± 32.79 mg/dl |
| A1C | 7.99 ± 1.48% |
| Creatinine | 0.85 ± 0.14 mg/dl |
| GFR | 60 ml/min/1.73 m2 |
| AST | 16.29 ± 8.76 U/L |
| ALT | 14.91 ± 7.27 U/L |
| Albumin/creatinine | 8.0 ± 6.60 μg AL/mgCR |
| TSH | 2.34 ± 1.70 mcIU/ml |
| Vitamin B12 | 512.97 ± 234.51 pg/ml |
Values given in mean ± SD.
2.3. Flanker selective attention task
During the MEG session, participants were seated in a nonmagnetic chair and instructed to fixate on a crosshair presented centrally for 1,450–1,550 ms. Following fixation, a row of five arrows appeared for 2,500 ms and participants were instructed to respond by button press as to the direction the middle arrow. Trials, where the middle arrow was pointing in the same direction as the surrounding (i.e., flanking) arrows, were categorized as congruent, whereas trials where the middle arrow pointed in the opposite direction relative to the flankers were categorized as incongruent (Figure 1). Each trial lasted ~4 s and each participant completed 200 trials; 100 per incongruent and congruent conditions, with both arrow directions presented an equal number of times in each condition.
Figure 1.
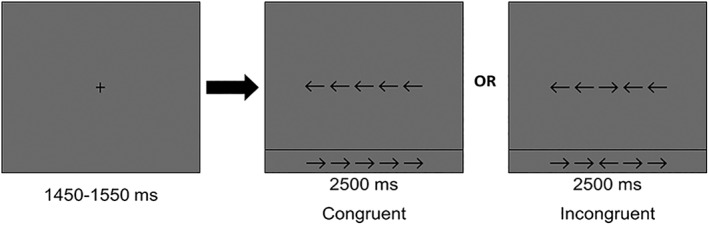
Eriksen flanker attention paradigm. Participants were shown a row of five arrows (duration: 2500 ms) following fixation and were asked to respond as to the direction of the middle arrow while ignoring the surrounding or flanking arrows. In the congruent condition, all five arrows pointed in the same direction. In the incongruent condition, the middle arrow pointed in the opposite direction of the surrounding arrows. The classic finding is an increase in reaction time in the incongruent relative to the congruent condition
2.4. MEG methods and analyses
MEG acquisition and analysis methodology followed standardized pipelines, corresponding to normative studies previously published by our group (Heinrichs‐Graham & Wilson, 2015; McDermott, Wiesman, Proskovec, Heinrichs‐Graham, & Wilson, 2017; Proskovec, Heinrichs‐Graham, Wiesman, McDermott, & Wilson, 2018; Wiesman, Heinrichs‐Graham, Proskovec, McDermott, & Wilson, 2017; Wilson, Heinrichs‐Graham, Proskovec, & McDermott, 2016). Briefly, MEG recordings were conducted within a magnetically shielded room using a 306‐sensor Elekta MEG system (Elekta, Helsinki, Finland). Data were sampled at 1 kHz with an acquisition bandwidth of 0.1–330 Hz. Each participant's data were corrected for head motion and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu & Simola, 2006). Each participant's MEG data were then coregistered with structural T1‐weighted MRI data.
Following artifact rejection (e.g., cardiac artifacts), the continuous MEG time series was divided into 2 s segments (i.e., epochs), with the baseline defined as the −0.45 to −0.05 s window before stimulus onset (0.0 s). Artifact‐free epochs were transformed into the time–frequency domain using complex demodulation, and the resulting spectral power estimations per sensor were averaged over trials to generate time–frequency plots of mean spectral density. These sensor‐level data were normalized using the mean baseline power during the −0.45 to −0.05 s time period. The precise time–frequency windows used for imaging were determined by statistical analysis of the sensor‐level spectrograms across the entire array of gradiometers during the task period. Each data point in the spectrogram was examined using t tests, corrected for multiple comparisons using a cluster‐based nonparametric permutation testing approach (Ernst, 2004; Maris & Oostenveld, 2007). Based on these analyses, the time–frequency windows that contained significant oscillatory events across all participants and conditions (see section 3) were subjected to beamforming.
Significant time–frequency windows were imaged at a 4.0 × 4.0 × 4.0 mm resolution using the dynamic imaging of coherent sources (DICS) beamformer (Gross et al., 2001; Hillebrand, Singh, Holliday, Furlong, & Barnes, 2005), which employs spatial filters in the time–frequency domain to calculate source power for the entire brain volume. Following convention, we computed noise‐normalized source power per voxel in each participant using active (i.e., task) and passive (i.e., baseline) periods of equal duration and bandwidth. Such images are typically referred to as pseudo‐t maps, with units (i.e., pseudo‐t) that reflect noise‐normalized power differences per voxel. All source imaging used the Brain Electrical Source Analysis (BESA) software (Version 6.1; GmbH, Gräfelfing, Germany). Preceding statistical analysis, each participant's functional MEG images were transformed into standardized Montreal Neurological Institute (MNI) space using the transform that was previously applied to the structural images and then spatially resampled (McDermott et al., 2017; Wiesman et al., 2017). After transforming these images into standardized space, we computed maps of the flanker interference effect in each person by subtracting the pseudo‐t map of the congruent condition from that of the incongruent condition per time–frequency window. These maps represent the increased neural recruitment induced by the higher demand incongruent condition relative to the congruent condition. These flanker interference maps were then statistically analyzed for group differences using whole brain independent samples t‐tests. Group difference maps were thresholded at (p < .005) and a spatial extent threshold (cluster threshold: k = 300) was applied to control for multiple comparisons based on the theory of Gaussian random fields (Worsley et al., 1996). Correlations were then computed using the peak voxel value from each significant cluster and disease duration.
3. RESULTS
3.1. Demographic, behavioral and disease status results
Five participants with type 1 diabetes and six controls were excluded at the data analysis phase due to artifactual MEG data. The remaining 35 participants with type 1 diabetes (M age = 25.3 years, SD age = 4.7; 13 females) and 34 controls (M age = 25.3 years, SD age = 3.6; 14 females) did not significantly differ in age, sex, or education level. Average education level was 16.3 years (SD = 1.9) for patients and 16.9 years (SD = 1.4) for controls. The mean disease duration in patients was 12.2 years (SD = 7.4), and the mean HbA1C was 64.0 mmol/mol (SD = 16.2; 7.99%, SD = 1.48).
Both groups performed the flanker task exceptionally well, as was expected, with a mean accuracy rate of 97.65% (SD = 5.03), a mean overall reaction time of 587.6 ms (SD = 119.7), and an average flanker effect—the difference in reaction times between incongruent and congruent condition—of 42.6 ms (SD = 29.9). There were no between‐group differences in these behavioral measures (all ps > .430). Note that the flanker task is relatively easy and equivalent task performance across groups was by design, as accuracy differences would have confounded our MEG results. Basically, if there had been significant behavioral differences, any significant neural effects could have reflected performance disparities rather than the intended neural impact of the disease.
3.2. MEG sensor level results
To identify significant time–frequency windows for anatomical analyses (i.e., beamforming), t‐tests of the spectrograms comparing the active period to the baseline were computed across all participants and conditions, followed by nonparametric testing to correct for multiple comparisons. Time–frequency analyses showed a significant decrease from baseline in the 9–14 Hz (alpha) range from 200–600 ms after stimulus onset and a significant increase from baseline in the 3–7 Hz (theta) range from 250–650 ms (p < .001; Figure 2). These windows are in agreement with previous MEG studies using the flanker task in young adults (McDermott et al., 2017). Both the alpha and theta time–frequency windows were imaged using a beamformer to examine these responses in anatomical space.
Figure 2.
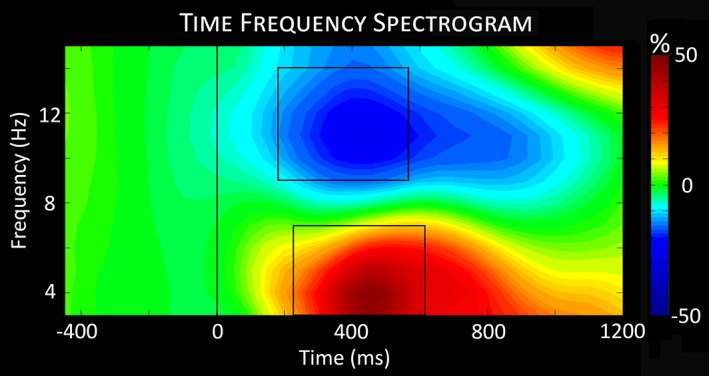
Time–frequency spectrogram. The time–frequency analyses revealed significant oscillatory activity in theta and alpha frequency bands across both conditions (congruent and incongruent) and all participants. The significant windows (p < .001, corrected) were 9–14 Hz from 200 to 600 ms (alpha) and 3–7 Hz from 250 to 650 ms (theta) [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. MEG brain level results
Maps of flanker interference activity were computed by subtracting maps of the congruent condition from that of the incongruent condition in each person separately for the alpha and theta responses. These flanker interference maps were then examined for group differences using independent samples t‐tests. For alpha, group differences in flanker interference activity were found in the right paracentral lobule, left anterior cingulate, and left parietal regions (p < .001), while differences in theta activity were restricted to the left precentral gyrus (p < .001; Figure 3). The differences in the right paracentral lobule, left parietal, and left precentral gyrus reflected stronger flanker interference responses in the patients with type 1 diabetes relative to controls, while the opposite pattern (i.e., stronger responses in controls) was observed in the anterior cingulate.
Figure 3.
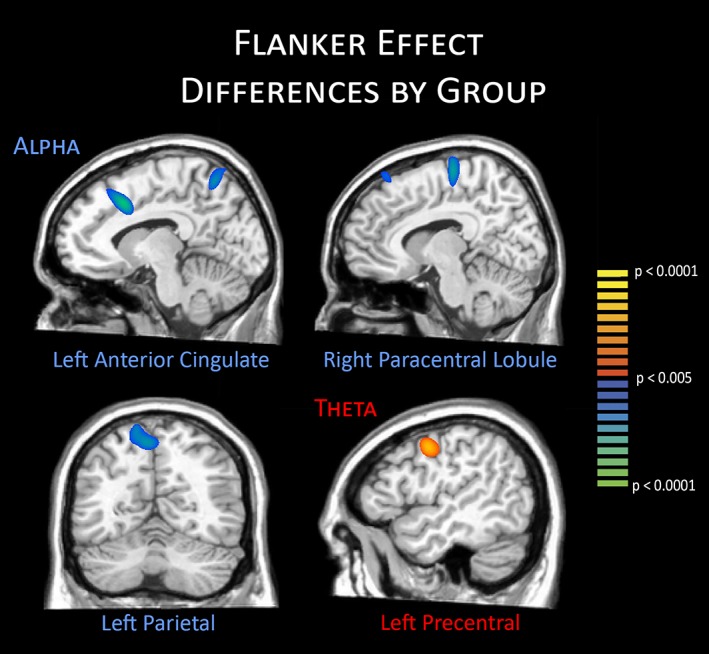
Group differences in the flanker interference effect. Significant group differences in alpha activity were found in the left anterior cingulate, right paracentral lobule, and left parietal regions, whereas significant theta differences were found in the left precentral gyrus. Controls exhibited stronger flanker interference responses in the left anterior cingulate, whereas responses were stronger in patients with type 1 diabetes in all other regions. Data are shown thresholded at p < .005 [Color figure can be viewed at http://wileyonlinelibrary.com]
Finally, we examined how duration of disease correlated with the strength of neural responses in each of the regions identified above in the group‐level analyses. These analyses showed that the overall strength of alpha activity in the left anterior cingulate (r = −.46, p = .006) and right paracentral lobule (r = −.42, p = .013) correlated with disease duration in the patients with type 1 diabetes (Figure 4). Of note, we repeated these correlations using glucose level at the time of scan as a covariate, and this had almost no effect on the relationship between disease duration and neural activity in either the left anterior cingulate, r = −.48, p = .004, or the right paracentral lobule, r = −.43, p = .011. This latter finding suggests that these correlations were not driven by transient glucose levels at the time of scan.
Figure 4.
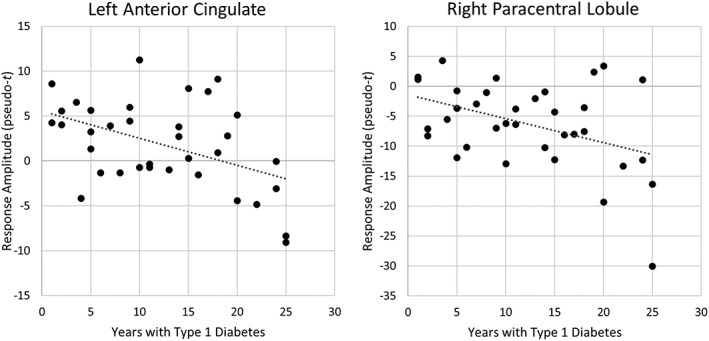
Correlations with disease duration. Years with type 1 diabetes significantly correlated with overall alpha activity levels in the peak flanker interference voxel (peak voxels in Figure 3) of the left anterior cingulate and right paracentral lobule
4. DISCUSSION
Utilizing advanced MEG imaging, we found aberrant neural activity during selective attention processing in young adults with type 1 diabetes, under normoglycemic conditions and in the absence of major comorbidities. Specifically, we found greater flanker interference responses in the right paracentral lobule, left parietal, and left precentral gyrus of patients with type 1 diabetes, whereas controls showed a greater interference effect in the left anterior cingulate. Furthermore, the peak amplitude of the responses in the right paracentral lobule and the left anterior cingulate significantly correlated with disease duration in patients with type 1 diabetes. Previous studies of attention processing have implicated both alpha and theta activity in frontal and parietal regional recruitment, with anterior cingulate engagement associated with response competition and conflict monitoring, and parietal activity associated more with response representation for motor planning (Bunge et al., 2002; Clark, Squire, Merrikhi, & Noudoost, 2015; Fassbender, Foxe, & Garavan, 2006; Hazeltine et al., 2000). Furthermore, the paracentral lobule has previously been linked to shifts of attention (Grosbras, Laird, & Paus, 2005). Below, we discuss the implications of these findings for understanding the impact of type 1 diabetes on neuronal function in attention and response competition circuits. In addition, due to the associations between disease variables and neural activity, we further discuss the clinical implications of these findings and relevance to glycemic control and general disease management in this population.
One of our most interesting findings was the increased recruitment of the left inferior parietal cortices and the right paracentral lobule in patients with type 1 diabetes. Considering that previous studies have linked the paracentral lobule to shifts in attention, the current study may suggest that its role also extends to suppressing local interference in the context of distractors (i.e., the flanking stimuli; Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999). Given the correlation of activity in this region with disease duration, we propose that the increased interference response reflects compensatory processing in the patients with type 1 diabetes, which may enable them to maintain high‐performance levels during the increased interference of the incongruent condition. Interestingly, this pattern was already present in our relatively healthy young adults with type 1 diabetes, which may indicate that eventually these compensatory processes will be exhausted leading to an inferior performance on this task and others that tap attention function. Future aging studies, especially longitudinal studies of type 1 diabetes involving both healthy children and adults, will be needed to map the trajectory of these neural responses and further disentangle the specific health, lifestyle, and disease management factors that contribute to the decline. In regard to the inferior parietal finding, studies have linked this region with updating and maintaining spatial representations and this would also be expected within the current flanker paradigm. Presumably, the increased alpha activity in this brain area reflects stronger recruitment in patients with type 1 diabetes during stimulus processing, which potentially may help offset the decreased anterior cingulate involvement (see below) in these patients. However, this is speculative and future studies will need to clarify the increased role of this region in patients with type 1 diabetes. Of note, activity in the inferior parietal did not correlate with disease duration, which may suggest that this region's increased involvement is less progressive than that observed in other cortices (e.g., right paracentral). Finally, we also observed increased activity in the left precentral gyrus, which almost certainly reflects differences in motor control. Future studies should focus directly on the motor system in patients with type 1 diabetes and determine whether this system is particularly affected early in the disease process, as this could have major implications for disease management across the lifespan.
Another critical finding of this study was the significantly weaker flanker interference responses in the anterior cingulate of patients with type 1 diabetes. The anterior cingulate is a key brain region in the literature on attention, interference, and associated processes. Specifically, a large number of fMRI studies have shown anterior cingulate activation during the flanker and other related tasks (Botvinick et al., 1999, 2001, 2004; Bush, Luu, & Posner, 2000; Carter & van Veen, 2007; Casey et al., 2000; Kiehl, Liddle, & Hopfinger, 2000; van Veen & Carter, 2002; van Veen, Cohen, Botvinick, Stenger, & Carter, 2001). In an early fMRI study by Botvinick et al., the anterior cingulate cortex was implicated in conflict monitoring, and examining and correcting errors over multiple trials rather than in a conditional manner (Botvinick et al., 1999). Using fMRI, Van Veen and colleagues have also identified a response competition role for the anterior cingulate in their studies of flanker interference effects (van Veen et al., 2001; van Veen & Carter, 2002), and a subsequent study associated this role specifically with anterior cingulate cortex rather than distributed across the frontoparietal network involved in attention (Liston, Matalon, Hare, Davidson, & Casey, 2006). More broadly, studies have implicated the anterior cingulate with autonomic regulation, complex motor control, and emotional processing (Bush et al., 2000). Such widespread functional roles can be attributed to the heterogeneity of this brain area and the known sub‐region specificity, with rostral areas associated with error monitoring and autonomic regulation, and dorsal/caudal regions associated with response competition and attention‐related processes (Carter & van Veen, 2007; Casey et al., 2000; Critchley, Tang, Glaser, Butterworth, & Dolan, 2005; Kiehl et al., 2000; Swick & Turken, 2002). Our current findings are within the dorsal anterior cingulate and would overlap with areas previously linked to response competition processes (Critchley et al., 2005; Swick & Turken, 2002).
The well‐studied anterior cingulate is also known to be hypo‐activated in aging populations and those with disorders like attention‐deficit/hyperactivity disorder (ADHD), and these studies have directly linked activity in this region to conflict and error monitoring performance (Dickstein, Bannon, Castellanos, & Milham, 2006; Pardo et al., 2007). In our study, deficits in the anterior cingulate appeared to be progressive, as the decrease in anterior cingulate activity significantly correlated with disease duration. Interestingly, previous studies have shown increased anterior cingulate activation during states of hypoglycemia (Page et al., 2009; Teh et al., 2010; Teves, Videen, Cryer, & Powers, 2004), but even in such states this activity was still weaker in type 1 diabetes relative to control subjects, at least under hypoglycemic clamp conditions (Musen et al., 2008). The current finding extends this to the normoglycemia state, indicating chronic hypo‐activation in the anterior cingulate in patients with type 1 diabetes, and our correlation analyses suggest that the severity of this deficit intensifies with longer disease duration.
Considering the body of literature examining cognitive impairment in patients with type 1 diabetes, deficits in attentional processes may have a unique impact on daily care behaviors and long‐term disease management. The impact of chronic hypoglycemia in insulin‐treated type 1 diabetes is well‐known throughout the literature (Cryer, 2014, 2017; Grober et al., 2011; Hansen et al., 2017; Rooijackers et al., 2016; Seaquist et al., 2013), with these patients experiencing two hypoglycemic episodes on average weekly and an annual incidence of severe hypoglycemia requiring third‐party intervention of 1.0 to 1.7 episodes per patient (Frier, 2008; McCrimmon & Sherwin, 2010). These recurrent episodes appear to lead to impairment in tracking and rectifying dysglycemia, thereby further impairing neural mechanisms in the process. Previous studies examining cognitive deficits and type 1 diabetes have identified a self‐perpetuating loop of disease mismanagement and increased cognitive decline (Grober et al., 2011; Hansen et al., 2017), which may at least partially explain the relationship between attention‐related neural dysfunction and disease duration apparent in the current study. With this progressive inability to manage the condition (i.e., maintain normoglycemia) leading to more rapid cognitive dysfunction, it is imperative that patients manage their glycemic levels carefully from the point of diagnosis to prevent detrimental long‐term outcomes. The long‐term consequences of disease mismanagement also suggest that the costs associated with automated methods (e.g., pumps) may be relatively low for the patient and the health care system given the costs of alternative outcomes.
Before closing, it is important to acknowledge a few limitations with the current study, including the specific focus on the flanker interference paradigm, the young age of the participants, and the absence of major common comorbidities. Although these factors were by design, they may limit the generalizability of these results to the larger population of people living with type 1 diabetes. Future studies should extend these findings to older patients and those with comorbidities. The latter could provide key information about the progressive nature of these neural aberrations and their role in long‐term cognitive health. In addition, the effects of stress and depression in type 1 diabetes were not examined in the current study, although emerging literature suggests these effects should be examined in future studies focusing on differences in cognitive and brain function. In summary, we found abnormal neural activity during a Flanker task in young healthy adults with type 1 diabetes (i.e., without common comorbidities), with particular responses being directly related to disease duration. These findings add to the growing body of literature examining cognitive deficits associated with type 1 diabetes, and in particular provide new data revealing specific neurophysiological differences in multiple brain areas, with some of these deficits progressing with disease duration. Future studies should examine neural and cognitive deficits in relation to complications that commonly arise with type 1 diabetes and in the context of aging. Longitudinal studies will be especially important in discerning the effects of type 1 diabetes on the brain throughout the lifespan.
AUTHOR CONTRIBUTION
E.H.G., A.L.P., K.L.B., A.T.D., C.V.D., and T.W.W. contributed to experimental design. C.M.E., G.H.L., K.L.B., A.T.D., and C.V.D. collected data and assisted in participant recruitment. C.M.E., A.I.W., A.L.P., E.H.G., T.J.M., C.V.D, and T.W.W. contributed to data analysis and interpretation. C.M.E. and T.W.W. drafted the article. C.M.E., E.H.G., A.T.D., C.V.D., and T.W.W. contributed to article revisions. All authors take responsibility for the contents of this article.
CONFLICT OF INTEREST
Authors C.M.E., A.I.W., A.L.P., E.H.G., T.J.M., G.H.L., K.L.B., A.T.D., and T.W.W. have no conflicts of interest to declare. C.V.D. is a consultant with Novo Nordisk and receives research funding from Theracos and Sanofi.
ACKNOWLEDGMENTS
This work was supported by grants R01‐MH103220 (T.W.W.), R01‐MH116782 (T.W.W.), and F31‐AG055332 (A.I.W.) from the National Institutes of Health, grant #1539067 (T.W.W.) from the National Science Foundation, and by a Research Support Fund grant from the Nebraska Health System and the University of Nebraska Medical Center. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Embury CM, Wiesman AI, McDermott TJ, et al. The impact of type 1 diabetes on neural activity serving attention. Hum Brain Mapp. 2019;40:1093–1100. 10.1002/hbm.24431
Funding information National Institute of Mental Health (NIMH), Grant/Award Number: R01‐MH103220 and R01‐MH116782; National Institute on Aging (NIA), Grant/Award Number: F31‐AG055332; National Science Foundation (NSF), Grant/ Award Number: 1539067; Nebraska Health System/UNMC, Grant/Award Number: Research Support Fund
Portions of the results were presented in abstract and poster form at the American Diabetes Association's 78th Scientific Sessions, Orlando, FL, June 22–26, 2018
REFERENCES
- Bednarik, P. , Moheet, A. A. , Grohn, H. , Kumar, A. F. , Eberly, L. E. , Seaquist, E. R. , & Mangia, S. (2017). Type 1 diabetes and impaired awareness of hypoglycemia are associated with reduced brain gray matter volumes. Frontiers in Neuroscience, 11, 529 10.3389/fnins.2017.00529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels, G. J. , & Reijmer, Y. D. (2014). Brain changes underlying cognitive dysfunction in diabetes: What can we learn from MRI? Diabetes, 63(7), 2244–2252. 10.2337/db14-0348 [DOI] [PubMed] [Google Scholar]
- Bolo, N. R. , Musen, G. , Jacobson, A. M. , Weinger, K. , McCartney, R. L. , Flores, V. , … Simonson, D. C. (2011). Brain activation during working memory is altered in patients with type 1 diabetes during hypoglycemia. Diabetes, 60(12), 3256–3264. 10.2337/db11-0506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick, M. , Nystrom, L. E. , Fissell, K. , Carter, C. S. , & Cohen, J. D. (1999). Conflict monitoring versus selection‐for‐action in anterior cingulate cortex. Nature, 402(6758), 179–181. 10.1038/46035 [DOI] [PubMed] [Google Scholar]
- Botvinick, M. M. , Braver, T. S. , Barch, D. M. , Carter, C. S. , & Cohen, J. D. (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–652. [DOI] [PubMed] [Google Scholar]
- Botvinick, M. M. , Cohen, J. D. , & Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences, 8(12), 539–546. 10.1016/j.tics.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Bunge, S. A. , Hazeltine, E. , Scanlon, M. D. , Rosen, A. C. , & Gabrieli, J. D. (2002). Dissociable contributions of prefrontal and parietal cortices to response selection. NeuroImage, 17(3), 1562–1571. [DOI] [PubMed] [Google Scholar]
- Bush, G. , Luu, P. , & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222. [DOI] [PubMed] [Google Scholar]
- Carter, C. S. , & van Veen, V. (2007). Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, & Behavioral Neuroscience, 7(4), 367–379. [DOI] [PubMed] [Google Scholar]
- Casey, B. J. , Thomas, K. M. , Welsh, T. F. , Badgaiyan, R. D. , Eccard, C. H. , Jennings, J. R. , & Crone, E. A. (2000). Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proceedings of the National Academy of Sciences of the United States of America, 97(15), 8728–8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, K. , Squire, R. F. , Merrikhi, Y. , & Noudoost, B. (2015). Visual attention: Linking prefrontal sources to neuronal and behavioral correlates. Progress in Neurobiology, 132, 59–80. 10.1016/j.pneurobio.2015.06.006 [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. , Tang, J. , Glaser, D. , Butterworth, B. , & Dolan, R. J. (2005). Anterior cingulate activity during error and autonomic response. NeuroImage, 27(4), 885–895. 10.1016/j.neuroimage.2005.05.047 [DOI] [PubMed] [Google Scholar]
- Cryer, P. E. (2014). Glycemic goals in diabetes: Trade‐off between glycemic control and iatrogenic hypoglycemia. Diabetes, 63(7), 2188–2195. 10.2337/db14-0059 [DOI] [PubMed] [Google Scholar]
- Cryer, P. E. (2017). Individualized glycemic goals and an expanded classification of severe hypoglycemia in diabetes. Diabetes Care, 40(12), 1641–1643. 10.2337/dc16-1741 [DOI] [PubMed] [Google Scholar]
- Desimone, R. , & Duncan, J. (1995). Neural mechanisms of selective visual attention. Annual Review of Neuroscience, 18, 193–222. 10.1146/annurev.ne.18.030195.001205 [DOI] [PubMed] [Google Scholar]
- Desrocher, M. , & Rovet, J. (2004). Neurocognitive correlates of type 1 diabetes mellitus in childhood. Child Neuropsychology, 10(1), 36–52. 10.1076/chin.10.1.36.26241 [DOI] [PubMed] [Google Scholar]
- Dickstein, S. G. , Bannon, K. , Castellanos, F. X. , & Milham, M. P. (2006). The neural correlates of attention deficit hyperactivity disorder: An ALE meta‐analysis. Journal of Child Psychology and Psychiatry, 47(10), 1051–1062. 10.1111/j.1469-7610.2006.01671.x [DOI] [PubMed] [Google Scholar]
- Eriksen, B. A. , & Eriksen, C. W. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Attention, Perception, & Psychophysics, 16(1), 143–149. [Google Scholar]
- Ernst, M. D. (2004). Permutation methods: A basis for exact inference. Statistical Science, 19(4), 676–685. 10.1214/088342304000000396 [DOI] [Google Scholar]
- Fassbender, C. , Foxe, J. J. , & Garavan, H. (2006). Mapping the functional anatomy of task preparation: Priming task‐appropriate brain networks. Human Brain Mapping, 27(10), 819–827. 10.1002/hbm.20223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frier, B. M. (2001). Hypoglycaemia and cognitive function in diabetes. International Journal of Clinical Practice. Supplement, 123(123), 30–37. [PubMed] [Google Scholar]
- Frier, B. M. (2008). How hypoglycaemia can affect the life of a person with diabetes. Diabetes/Metabolism Research and Reviews, 24(2), 87–92. 10.1002/dmrr.796 [DOI] [PubMed] [Google Scholar]
- Gallardo‐Moreno, G. B. , Gonzalez‐Garrido, A. A. , Gudayol‐Ferre, E. , & Guardia‐Olmos, J. (2015). Type 1 diabetes modifies brain activation in young patients while performing visuospatial working memory tasks. Journal of Diabetes Research, 2015, 703512 10.1155/2015/703512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober, E. , Hall, C. B. , Hahn, S. R. , & Lipton, R. B. (2011). Memory impairment and executive dysfunction are associated with inadequately controlled diabetes in older adults. Journal of Primary Care and Community Health, 2(4), 229–233. 10.1177/2150131911409945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosbras, M. H. , Laird, A. R. , & Paus, T. (2005). Cortical regions involved in eye movements, shifts of attention, and gaze perception. Human Brain Mapping, 25(1), 140–154. 10.1002/hbm.20145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, J. , Kujala, J. , Hamalainen, M. , Timmermann, L. , Schnitzler, A. , & Salmelin, R. (2001). Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 694–699. 10.1073/pnas.98.2.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, T. I. , Olsen, S. E. , Haferstrom, E. C. D. , Sand, T. , Frier, B. M. , Haberg, A. K. , & Bjorgaas, M. R. (2017). Cognitive deficits associated with impaired awareness of hypoglycaemia in type 1 diabetes. Diabetologia, 60(6), 971–979. 10.1007/s00125-017-4233-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeltine, E. , Poldrack, R. , & Gabrieli, J. D. (2000). Neural activation during response competition. Journal of Cognitive Neuroscience, 12(Suppl 2), 118–129. 10.1162/089892900563984 [DOI] [PubMed] [Google Scholar]
- Heinrichs‐Graham, E. , & Wilson, T. W. (2015). Coding complexity in the human motor circuit. Human Brain Mapping, 36(12), 5155–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand, A. , Singh, K. D. , Holliday, I. E. , Furlong, P. L. , & Barnes, G. R. (2005). A new approach to neuroimaging with magnetoencephalography. Human Brain Mapping, 25(2), 199–211. 10.1002/hbm.20102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, M. , Tudorascu, D. L. , Nunley, K. , Karim, H. , Aizenstein, H. J. , Orchard, T. J. , & Rosano, C. (2016). Brain activation and psychomotor speed in middle‐aged patients with type 1 diabetes: Relationships with hyperglycemia and brain small vessel disease. Journal of Diabetes Research, 2016, 9571464 10.1155/2016/9571464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl, K. A. , Liddle, P. F. , & Hopfinger, J. B. (2000). Error processing and the rostral anterior cingulate: An event‐related fMRI study. Psychophysiology, 37(2), 216–223. [PubMed] [Google Scholar]
- Liston, C. , Matalon, S. , Hare, T. A. , Davidson, M. C. , & Casey, B. J. (2006). Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task‐switching paradigm. Neuron, 50(4), 643–653. 10.1016/j.neuron.2006.04.015 [DOI] [PubMed] [Google Scholar]
- Maris, E. , & Oostenveld, R. (2007). Nonparametric statistical testing of EEG‐ and MEG‐data. Journal of Neuroscience Methods, 164(1), 177–190. 10.1016/j.jneumeth.2007.03.024 [DOI] [PubMed] [Google Scholar]
- McCrimmon, R. J. , Ryan, C. M. , & Frier, B. M. (2012). Diabetes and cognitive dysfunction. Lancet, 379(9833), 2291–2299. 10.1016/s0140-6736(12)60360-2 [DOI] [PubMed] [Google Scholar]
- McCrimmon, R. J. , & Sherwin, R. S. (2010). Hypoglycemia in type 1 diabetes. Diabetes, 59(10), 2333–2339. 10.2337/db10-0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott, T. J. , Wiesman, A. I. , Proskovec, A. L. , Heinrichs‐Graham, E. , & Wilson, T. W. (2017). Spatiotemporal oscillatory dynamics of visual selective attention during a flanker task. NeuroImage, 156, 277–285. 10.1016/j.neuroimage.2017.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musen, G. , Lyoo, I. K. , Sparks, C. R. , Weinger, K. , Hwang, J. , Ryan, C. M. , … Jacobson, A. M. (2006). Effects of type 1 diabetes on gray matter density as measured by voxel‐based morphometry. Diabetes, 55(2), 326–333. [DOI] [PubMed] [Google Scholar]
- Musen, G. , Simonson, D. C. , Bolo, N. R. , Driscoll, A. , Weinger, K. , Raji, A. , … Jacobson, A. M. (2008). Regional brain activation during hypoglycemia in type 1 diabetes. The Journal of Clinical Endocrinology and Metabolism, 93(4), 1450–1457. 10.1210/jc.2007-2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, K. A. , Arora, J. , Qiu, M. , Relwani, R. , Constable, R. T. , & Sherwin, R. S. (2009). Small decrements in systemic glucose provoke increases in hypothalamic blood flow prior to the release of counterregulatory hormones. Diabetes, 58(2), 448–452. 10.2337/db08-1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo, J. V. , Lee, J. T. , Sheikh, S. A. , Surerus‐Johnson, C. , Shah, H. , Munch, K. R. , … Dysken, M. W. (2007). Where the brain grows old: Decline in anterior cingulate and medial prefrontal function with normal aging. NeuroImage, 35(3), 1231–1237. 10.1016/j.neuroimage.2006.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskovec, A. L. , Heinrichs‐Graham, E. , Wiesman, A. I. , McDermott, T. J. , & Wilson, T. W. (2018). Oscillatory dynamics in the dorsal and ventral attention networks during the reorienting of attention. Human Brain Mapping, 39(5), 2177–2190. 10.1002/hbm.23997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooijackers, H. M. , Wiegers, E. C. , Tack, C. J. , van der Graaf, M. , & de Galan, B. E. (2016). Brain glucose metabolism during hypoglycemia in type 1 diabetes: Insights from functional and metabolic neuroimaging studies. Cellular and Molecular Life Sciences, 73(4), 705–722. 10.1007/s00018-015-2079-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, C. M. , van Duinkerken, E. , & Rosano, C. (2016). Neurocognitive consequences of diabetes. The American Psychologist, 71(7), 563–576. 10.1037/a0040455 [DOI] [PubMed] [Google Scholar]
- Seaquist, E. R. , Anderson, J. , Childs, B. , Cryer, P. , Dagogo‐Jack, S. , Fish, L. , … Vigersky, R. (2013). Hypoglycemia and diabetes: A report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care, 36(5), 1384–1395. 10.2337/dc12-2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick, D. , & Turken, A. U. (2002). Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America, 99(25), 16354–16359. 10.1073/pnas.252521499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu, S. , & Simola, J. (2006). Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Physics in Medicine and Biology, 51(7), 1759–1768. 10.1088/0031-9155/51/7/008 [DOI] [PubMed] [Google Scholar]
- Teh, M. M. , Dunn, J. T. , Choudhary, P. , Samarasinghe, Y. , Macdonald, I. , O'Doherty, M. , … Amiel, S. A. (2010). Evolution and resolution of human brain perfusion responses to the stress of induced hypoglycemia. NeuroImage, 53(2), 584–592. 10.1016/j.neuroimage.2010.06.033 [DOI] [PubMed] [Google Scholar]
- Teves, D. , Videen, T. O. , Cryer, P. E. , & Powers, W. J. (2004). Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proceedings of the National Academy of Sciences of the United States of America, 101(16), 6217–6221. 10.1073/pnas.0307048101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonoli, C. , Heyman, E. , Roelands, B. , Pattyn, N. , Buyse, L. , Piacentini, M. F. , … Meeusen, R. (2014). Type 1 diabetes‐associated cognitive decline: A meta‐analysis and update of the current literature. Journal of Diabetes, 6(6), 499–513. 10.1111/1753-0407.12193 [DOI] [PubMed] [Google Scholar]
- van Duinkerken, E. , Schoonheim, M. M. , Sanz‐Arigita, E. J. , RG, I. J. , Moll, A. C. , Snoek, F. J. , … Barkhof, F. (2012). Resting‐state brain networks in type 1 diabetic patients with and without microangiopathy and their relation to cognitive functions and disease variables. Diabetes, 61(7), 1814–1821. 10.2337/db11-1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen, V. , & Carter, C. S. (2002). The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior, 77(4–5), 477–482. [DOI] [PubMed] [Google Scholar]
- van Veen, V. , Cohen, J. D. , Botvinick, M. M. , Stenger, V. A. , & Carter, C. S. (2001). Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage, 14(6), 1302–1308. 10.1006/nimg.2001.0923 [DOI] [PubMed] [Google Scholar]
- Warren, R. E. , & Frier, B. M. (2005). Hypoglycaemia and cognitive function. Diabetes, Obesity & Metabolism, 7(5), 493–503. 10.1111/j.1463-1326.2004.00421.x [DOI] [PubMed] [Google Scholar]
- Wiesman, A. I. , Heinrichs‐Graham, E. , Proskovec, A. L. , McDermott, T. J. , & Wilson, T. W. (2017). Oscillations during observations: Dynamic oscillatory networks serving visuospatial attention. Human Brain Mapping, 38(10), 5128–5140. 10.1002/hbm.23720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, T. W. , Heinrichs‐Graham, E. , Proskovec, A. L. , & McDermott, T. J. (2016). Neuroimaging with magnetoencephalography: A dynamic view of brain pathophysiology. Translational Research, 175, 17–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley, K. J. , Marrett, S. , Neelin, P. , Vandal, A. C. , Friston, K. J. , & Evans, A. C. (1996). A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping, 4(1), 58–73. [DOI] [PubMed] [Google Scholar]


