Summary
Cellular immunotherapy using allogeneic natural killer (NK) cells may overcome chemotherapy-refractory acute myeloid leukemia (rAML). Our goal was to document NK cell homing/persistence in the bone marrow following adoptive immunotherapy. Our cohort included 109 patients who received NK cell therapy for rAML following lymphodepleting conditioning +/− denileukin diftitox, +/− low-dose total body irradiation. We evaluated the NK cell density in bone marrow core biopsies performed an average of 14 days after NK cell transfer using a CD56 immunohistochemical stain. The NK cell density in core biopsies showed only moderate correlation with NK cell percentage in bone marrow aspirates evaluated by flow cytometry (rs = 0.48) suggesting that distribution of CD56 positive cells in the bone marrow niche offers unique insight into NK cell homing. Better leukemia control was associated with increased NK cell density, such that patients with <5% blasts had a higher NK cell density (p=0.01). As well, NK cell density above the median of reference group was significantly associated with morphologic remission of leukemia (p=0.01). Moreover, the NK cell density varied significantly between conditioning protocols. Our findings suggest that the use of low dose irradiation or CD25-targeting immunocytokine (denileukin diftitox, IL2DT) as part of conditioning results in increased NK cell homing/persistence in the bone marrow. These novel results will help guide future immunotherapy with NK cells.
Keywords: Immunotherapy, NK cells, Acute myeloid leukemia, bone marrow, CD56
Introduction
Natural killer (NK) cells are a part of the innate immune system able to secrete cytokines and kill virally infected or malignant cells without prior sensitization 1, 2. Unlike T-or B-lymphocytes, NK cells do not undergo rearrangement of unique antigen specific receptors; rather variable sets of activating and inhibitory receptors control their activity. Nevertheless, a subset of NK cells can be long lived and show adaptive properties with amplification of recall responses to chemical haptens and viruses 3, 4.
The use of NK cells as cellular immunotherapy for patients with refractory acute myeloid leukemia (AML) is, and has been, an area of active research. The University of Minnesota has conducted clinical trials with adoptive immunotherapy using allogeneic NK cells for over a decade 5. In this approach, allogeneic NK cells are isolated from the donor peripheral blood mononuclear cells, activated overnight in vitro and infused following preparatory lymphodepleting regimen (fludarabine/cyclophosphamide). Following NK cell transfer, stimulatory cytokines such as Interleukin 2 (IL-2) or Interleukin 15 (IL-15) are administered to the recipient to promote NK cell expansion. Such treatment has induced leukemia remission in a subset of patients with chemotherapy-refractory AML 6. In-vivo persistence of donor NK cells in the blood has been observed in some recipients and is associated with increased likelihood of leukemia clearance 5, 6. Less is known about homing and persistence of infused allogeneic NK cells in the bone marrow niche, the primary site of disease burden in leukemia. Bone marrow is the primary site of NK cell development and is the site of expansion and proliferation of NK cells in the setting of viral infection 7, 8. The mechanisms controlling trafficking of NK cells to and from the bone marrow are relatively less well studied and include the CXCR4 – CXCL12 axis as well as CXCR3, CCR1 and CX3CR1 and S1P5 receptors 9. Recent studies in animal model showed that NK cells are actively patrolling the bone marrow and when stimulated they can actively enter the bone marrow parenchyma, where they proliferate and expand 10. Relatively less is known about the number and distribution of NK cells in human bone marrow.
Our study goal was to document NK cell homing/persistence in the bone marrow following adoptive immunotherapy. To this aim, we studied the number and distribution of NK cells in bone marrow biopsies performed routinely two to three weeks after adoptive transfer in patients with chemotherapy-refractory AML. The results show variability in the density of NK cells in the bone marrow, correlating with disease clearance. Moreover, we observed significant differences in the density of NK cells between clinical immunotherapy protocols. Our analysis suggests that the use of total body irradiation (TBI) or CD25-targeting immunocytokine (denileukin diftitox, IL2DT) as part of conditioning result in increased density of CD56 positive cells in the bone marrow. These findings offer unique insight into the clinical factors which promote NK cell homing/persistence in the bone marrow niche after adoptive immunotherapy.
Methods
In this Institutional Review Board approved retrospective study, we identified patients who received NK-cell therapy for relapsed/recurrent acute myeloid leukemia between 2003–2014 including patients treated under seven prospective clinical trials (MT2003–1, MT2003–23, MT2004–25, MT2008–36, MT2010–02, MT2010–10, and MT2011–05). The patients enrolled in the NK cell immunotherapy studies had relapsed or primary refractory AML or other high grade myeloid neoplasm with adequate organ function, who had failed ≥2 prior therapies. The patients had active disease based on hematopathology assessment of bone marrow biopsy findings. All patients received lymphodepleting chemotherapy with fludarabine and cyclophosphamide. Patient characteristics including age, percentage of marrow blasts at time of treatment, and the number of prior therapies have been described in more detail previously 5, 11. There were no restrictions based on karyotype, mutational status or risk group beyond those stated above. A subset received total body irradiation (TBI) and a separate subset received denileukin diftitox, an anti-CD25 antibody coupled with difteria toxin aimed to deplete regulatory T-cells. For the subset of patients who received TBI, dosing was 400cGy. For the subset of patients who received denileukin diftitox, dosing varied (One (n = 11) or 2 doses (n = 4) of IL2DT, 12 (n = 11) or 18 mg/kg (n = 4) IV, at day −1 ± −2). Details are previously published 11. The donors were HLA haploidentical family members, who underwent unstimulated mononuclear cell collections by apheresis, with the product enriched for NK cells using large-scale immunomagnetic selection (CliniMACS, Miltenyi Biotech, Bergisch Gladbach, Germany) by depletion of CD3+ T cells, and CD19+ B cells in some protocols. NK-cellular products were prepared following good manufacturing practice conditions in the University of Minnesota Molecular and Cellular Therapy Facility, as previously described 12. The donor NK cells were activated by incubation with IL-2 (or IL-15 in one protocol) overnight. Patients received activated donor NK cells by intravenous infusion at 1.5–8 × 107/kg body weight. Subcutaneous IL-2 (or, in one protocol, intravenous IL-15) was given for six doses over two weeks to facilitate survival/expansion of infused NK cells. The protocol and consent procedures were approved by the University of Minnesota Institutional Review Board (clinicaltrials.gov NCT00274846 and NCT01106950).
Clinicopathologic characteristics were extracted from the electronic medical record. Hematoxylin and eosin (H&E) stained core sections and Wright-Giemsa stained aspirate and peripheral blood smears were evaluated by study authors (LM, EC, and BG).
CD56 immunohistochemical stain (clone MRQ-42, Cell Marque, Rocklin, CA) was performed on 5 micron thick, acetic zinc formalin fixed, decalcified, paraffin embedded core biopsy sections using a Ventana Ultra automated stainer (Tucson, AZ). CD56 staining was quantified by estimating the percentage of cellularity composed of CD56 positive cells on low power (4X objective) and by manual count of the CD56 positive cells on high power (40× objective), counting 10 non-overlapping fields with the highest density of CD56 positive cells. Study authors BG and EC performed this evaluation independently, with re-review of discordant cases to reach consensus in evaluation technique. An average CD56 positive cell count per HPF was calculated as an average of the 20 combined fields between the two reviewers. The low power CD56 positive percentage was averaged between the two reviewers.
Flow cytometry was performed on marrow aspirate specimens according to the protocols at the time of biopsy and the data were re-analyzed to enumerate total lymphocytes and NK cells. Relevant antibodies used included: CD3 epsilon, CD56, and CD45 (Becton Dickinson, San Jose, CA). The samples were acquired on FACS-Canto instruments (Becton Dickinson) and analyzed using Kaluza software (Beckman Coulter, Indianapolis, IN). Lymphocytes were enumerated by standard gating on CD45 versus side scatter plots and NK cells were defined as CD56 positive, surface CD3 negative lymphocytes.
To evaluate for disease control/clearance, we obtained the marrow blast percentage from the final pathology report. We used fewer than 5% blasts to indicate morphologic remission of leukemia while ≥ 5% blasts was considered residual/persistent disease. Cases with no blast percentage reported due to very low bone marrow cellularity precluding differential count (n=13) were retrospectively reviewed by the study authors to determine disease status.
As a reference cohort, we identified 56 patients with an underlying diagnosis of AML with bone marrow biopsies performed at 14–21 days (average 16 days) after standard induction chemotherapy for AML (Cytarabine and Daunorubicin7+3). Microscopic evaluation of CD56 staining and review of flow cytometry data were performed in the reference cohort as described for the study cases. Six of the reference cases were excluded from the analysis of CD56 staining due to CD56 positivity on leukemic blasts. We also had flow cytometry data available for analysis of NK cell content in the bone marrow aspirates for 46 of the reference cases.
To test the association between CD56 density on core biopsies or NK cell percentage by flow cytometry and variables of interest, Mann – Whitney, Wilcoxon rank-sum, Kruskal-Wallis, and Chi-Square tests were performed, as appropriate. To investigate the relationships between CD56/HPF density, low power CD56 percentage, and NK cell percentages by flow cytometry, Spearman correlation coefficients were obtained. Statistical analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, C), GraphPad Prism version 6 for windows, (GraphPad software, LaJolla, CA), and R version 3.4.0 (http://www.R-project.org/).
Results
We identified 109 patients, 47 females and 62 males, with an average age of 47 years (range 4–75) at the time of NK cell therapy. All patients had a pre-infusion biopsy showing residual/recurrent AML, obtained an average of 20 days prior to infusion (range 7–92), with an average cellularity of 54% (range 5%−100%) and an average blast percentage of 35% (range 2–94%) at that time. The routine first post-infusion biopsies obtained an average of 14 days (range 9–33 days) after adoptive immunotherapy showed a wide range of cellularity with 45 having a cellularity of ≤ 5% and 10 having a cellularity of ≥ 90%. The majority of cases showed leukopenia (104/109, 95%) with a WBC< 4.0 K/ul.
Eighty-nine of the first post-infusion biopsies could be evaluated for CD56 positive cell density. We did not evaluate the CD56 immunohistochemical stain for 14 specimens due to missing biopsy material, very small and/or non-representative biopsy, poor preservation of the sections, or unacceptable immunohistochemical staining. In addition, we did not quantify CD56 positive cell density for six specimens due to evident staining of leukemic cells with CD56, precluding accurate distinction between NK cells and blasts. The CD56 expression by blasts was corroborated by flow cytometry results showing a CD56 positive immunophenotype. Fifty-four of the first post-infusion biopsies had a concurrent marrow aspirate specimen which could be evaluated for NK cells by flow cytometry. For the reference cohort of 56 cases obtained following standard induction chemotherapy for AML, 6 cases showed prominent CD56 positivity on leukemic cells and were excluded from the analysis of CD56 staining. Flow cytometry data was available for analysis of NK cell content in the bone marrow aspirates for 46 of the reference cases.
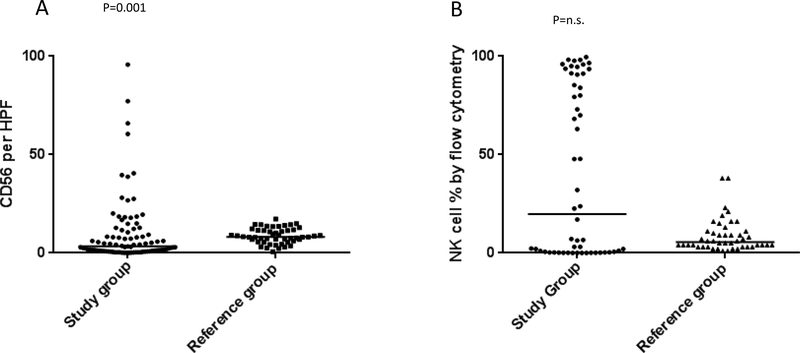
Characteristics of the study and reference cases are presented in Table 1. The distribution of NK cell density ( expressed as average number of CD56 positive cells per microscopic High Power Field,CD56/HPF) and percentage of NK cells in the lymphocyte population by flow cytometry in the study and reference groups are shown in Figure 1A and B, respectively. The distribution of CD56/HPF density differed between the study group and reference group (p=0.001) with the median CD56 positive cell density higher in the reference group (median of 8.2 versus 3.4). Despite the higher median CD56 positive cell density in the reference group, 30% (27/89) of the study patients with first post-infusion biopsies evaluable for CD56 by immunohistochemistry had a CD56 positive cell density above the median CD56 positive cell density of the reference cases.
Table 1:
Characteristics of the study and reference cases
| Study Cases (n=109) | Reference Cases AML post induction chemotherapy (n=56) | P value in comparison with the study group | |
|---|---|---|---|
| Patient gender (M:F) | 62:47 | 34:22 | Non-significant |
| Patient age at therapy, years, median (range) | 49 (4–75) | 60 (19–75) | 0.0004 |
| CD56 positive cell density, cells/HPF, median (range) | 3.4 (0.1–95.8)1 | 8.2 (0.7–17.25)3 | 0.001 |
| Low power CD56 percentage, median (range) | 1.8 (0–42.5)1 | 0 (0 – 3)3 | <0.0001 |
| NK cells as a percentage of lymphocytes by flow cytometry, median (range) | 19.8 (0–99.6)2 | 5.5 (1 – 38)4 | Non-significant |
n=89;
n=54;
n=50;
n=46
Figure 1:
Results of CD56/HPF quantitation (1A) and flow cytometric evaluation of percentage of NK cells in the lymphocyte population (1B) in the study group. (A) Scatter plot showing cumulative results of average number of CD56 positive cells per microscopic High Power Field (CD56/HPF) in the study group (filled circles, left side of the graph) as compared to AML post-induction chemotherapy reference cohort (filled squares, right side of the graph). The horizontal bars represent Medians (P=0.001 Mann Whitney test, Median 3.4 and 8.2 in the study group and reference group, respectively). (B) Scatter plot showing flow cytometry results obtained on bone marrow aspirate shown here as percentage NK cells per lymphocyte population in the study group (filled circles, left side of the graph) as compared to AML post-induction chemotherapy reference cohort (filled triangles, right side of the graph). The horizontal bars represent Medians (non-significant, Mann Whitney test, Median 19.8 and 5.5 in the study group and reference group, respectively).
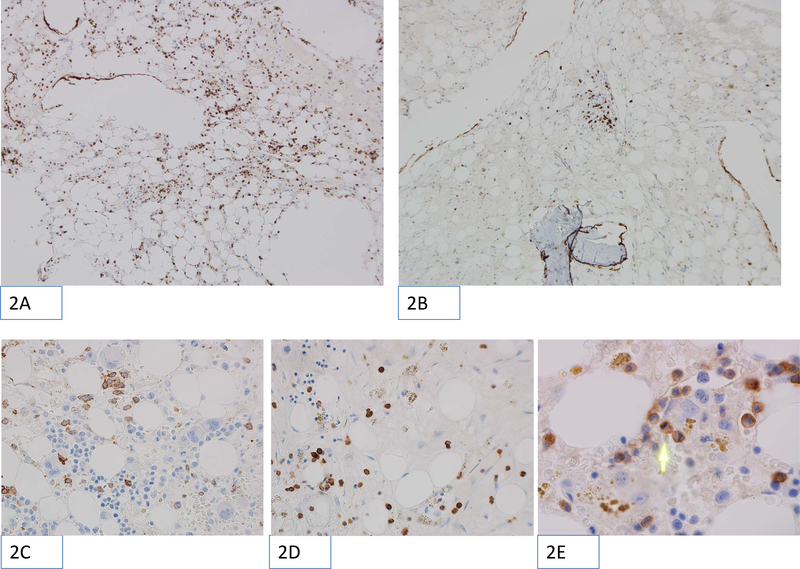
CD56 positive NK cells were either evenly distributed or present in focal clusters (Figure 2, A and B). They were present in both areas of active hematopoiesis and in hypocellular areas (Figure 2, C and D). Frequently the NK cells were seen in the sinusoids, apparently attached to the endothelial wall and rare mitoses of CD56 positive cells within marrow parenchyma were seen (Figure 2E). We did not observe an association of NK cells with large lymphoid aggregates. Thirty-one of the study patients (57%) had an NK cell percentage relative to total lymphocytes by flow cytometry above the median NK cell percentage of reference cases and 23 (43%) had an NK cell percentage by flow cytometry below (Figure 1b).
Figure 2:
Microscopic images showing examples of CD56 immunohistochemical staining performed on trephine core biopsy sections. The positively stained cells appear as brown (chromogenic stain). The images were obtained using Olympus BX46 microscope with ocular, field 22 and magnification 10×10 (panels 2A and 2B), 10×40 (panels 2C and 2D) or 10×100 with oil immersion (panel 2E). The low-power magnification images (100×) show CD56 positive NK cells distributed evenly (2A) or in focal clusters (2B). High-resolution images (400×) show examples of CD56 positive cells found in foci of active hematopoiesis (2C) or in hypocellular areas (2D). Of note, the 400× magnification corresponds to one High Power Field (HPF) in this study. Panel 2E shows very high magnification (1000× with oil immersion), with green arrow pointing to recently divided CD56 positive cell with two daughter cells visible.
Increased bone marrow NK cell density by IHC and as a relative proportion of lymphocytes by flow cytometry correlates with better disease control
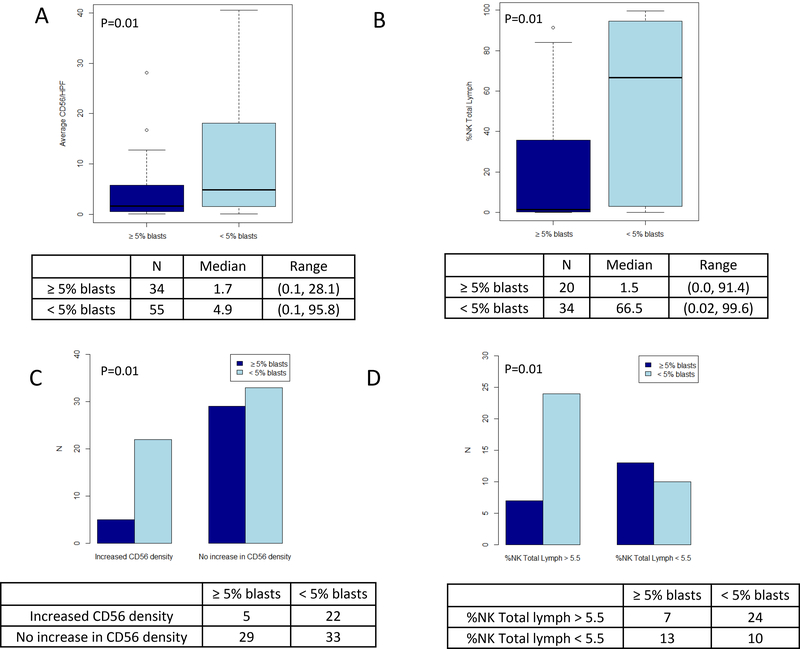
Compared to patients who had persistent disease, patients in morphologic remission following NK cell immunotherapy had a higher density of CD56 positive cells by immunohistochemistry in bone marrow specimens and a higher percentage of NK cells in the lymphocyte gate by flow cytometry (Figure 3, A and B). Patients with a CD56 positive cell density by immunohistochemistry above reference cases showed a higher odds of achieving morphologic remission compared to patients without an increase in CD56 positive cell density relative to reference cases (p=0.01, Figure 3C). Patients with NK cells representing a greater proportion of lymphocytes above median of reference cases, as demonstrated by flow cytometry, showed more frequent morphologic remission (p=0.01, Figure 3D).
Figure 3:
Leukemia control and NK cell density or percentage. (A) Box-and-whisker plots showing the distribution of CD56 positive NK cell density within bone marrow core biopsies, comparing patients with persistent disease (≥5% marrow blasts) and in morphologic remission (<5% marrow blasts). (B) Box-and-whisker plots showing the distribution of the NK cell percentage relative to lymphocytes as determined by flow cytometry performed on marrow aspirate sample, comparing patients with persistent disease and in morphologic remission. (C) Bar charts illustrating the number of patients with CD56 positive cell density above and below the median value of 8.2 from reference cohort and comparing patients with persistent disease and in morphologic remission. (D) Bar charts illustrating the number of patients with NK cell percentage relative to lymphocytes as determined by flow cytometry above- and below the median value of 5.5% from reference cohort and comparing patients with persistent disease and in morphologic remission.
Anti-CD25 and Total Body Irradiation (TBI) increase CD56/HPF NK cell density in the bone marrow
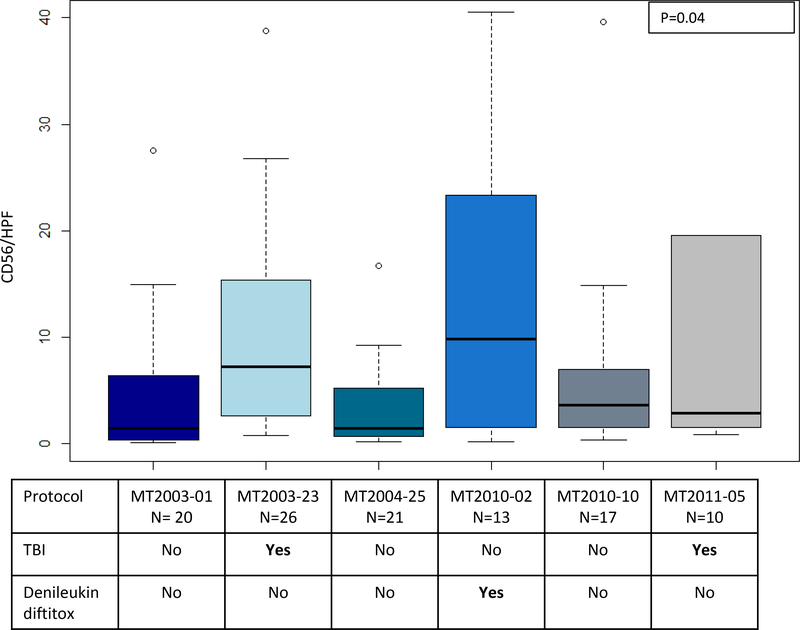
NK cell immunotherapy protocols at the University of Minnesota were revised over the years and our study cohort reflects seven clinical trials; therefore, we investigated the possibility that the CD56/HPF NK cell density in first post-infusion biopsy specimens varied based on lymphodepleting strategy. One clinical protocol (MT2008–36) was excluded from this particular analysis due to low number of participants (only one data point available for this analysis). As can be seen in Figure 4, there was significant variability between the median CD56/HPF count among the clinical protocols (p=0.04, Kruskal-Wallis test).
Figure 4:
Distribution of CD56 positive NK cell density within bone marrow core biopsies, comparing different clinical protocols. Use of total body irradiation (TBI) or CD25-targeting immunocytokine (denileukin diftitox) are delineated below each plot. There was significant variability between the median CD56/HPF count among the clinical protocols (p=0.04, Kruskal-Wallis test). Clinical protocols, MT2003–23, MT2010–02, MT2011–05 showed a relatively higher CD56/HPF coinciding with the use of TBI or denileukin diftitox in the conditioning.
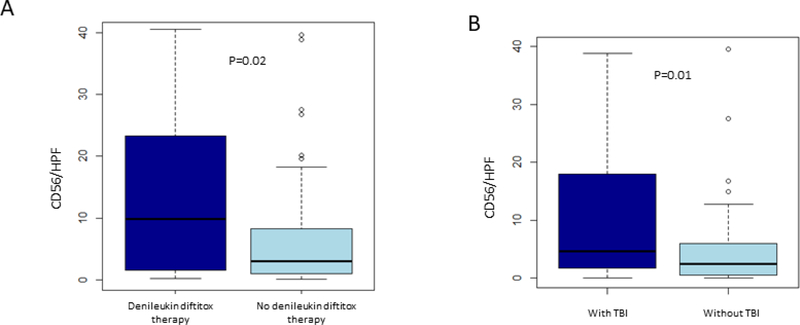
Visual inspection of Figure 4 suggests that the use of denileukin diftitox or the use of TBI may lead to a higher CD56/HPF NK cell density. Therefore, we investigated whether the use of denileukin diftitox affected CD56/HPF NK cell density. Since no patient received both denileukin diftitox and TBI, we were able to analyze them independently. Excluding patients who received TBI, there was a higher CD56/HPF NK cell density in the cohort receiving denileukin diftitox compared to those who did not receive denileukin diftitox (p=0.02, Figure 5A). Similarly, excluding patients who received denileukin diftitox, patients who received TBI had a higher CD56/HPF NK cell density compared to patients who did not receive TBI (p=0.01, Figure 5B). We did not see a difference in CD56/HPF NK cell density depending on the use of IL-2 or IL-15 within our cohort (data not shown).
Figure 5:
NK cell density and use of denileukin diftitox or total body irradiation. A) Box-and-whisker plots showing the distribution of CD56 positive NK cell density within bone marrow core biopsies, comparing patients who received or did not receive CD25-targeting immunocytokine (denileukin diftitox). (B) Box-and-whisker plots showing the distribution of CD56 positive NK cell density within bone marrow core biopsies, comparing patients who received or did not receive total body irradiation (TBI).
CD56/HPF NK cell density in tissue sections has weak correlation with the percentage of NK cells by flow cytometric evaluation
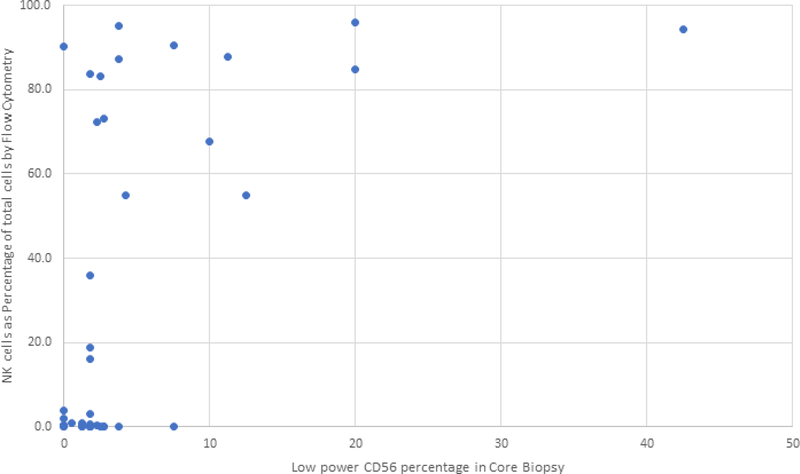
There was excellent correlation between the CD56/HPF count and the low power CD56 positive NK cell percentage, both assessed by microscopic evaluation (Spearman correlation coefficient of 0.87, data not shown). These two variables are differentially affected by the presence of other cells in the specimen: low power density is estimated as a percentage of NK cells out of hematopoietic cellular elements, whereas CD56/HPF is a reflection of number of NK cells per surface area, irrespective of presence or absence of other cellular elements. The correlation between CD56/HPF count and flow cytometric evaluation of percentage NK cells within the total leukocyte population or within the lymphocyte gate was not strong, with Spearman correlation coefficients of 0.48 and 0.54, respectively. Figure 6 shows a scatterplot of the NK cell percentage of total cells by flow cytometry of marrow aspirate sample versus the low power CD56 percentage in the core biopsy. In this plot, it can be seen that a number of cases had a high percentage of NK cells in the aspirate (>50% of total cells) by flow cytometry but showed only a moderate number of CD56 positive cells as a percentage of total hematopoietic elements. These results point to the possibility of peripheral blood contribution to the aspirate sample evaluated by flow cytometry and emphasize the distinct significance of bone marrow in situ findings.
Figure 6:
Correlation of NK cell density in core biopsies with NK cell percentage in bone marrow aspirates evaluated by flow cytometry. Scatterplot of the NK cell percentage relative to total cells as evaluated by flow cytometry from marrow aspirate specimens compared to the low power CD56 percentage as evaluated by CD56 immunohistochemical stain on bone marrow biopsy specimens. Cases in the upper-left of this scatter-plot showed a high percentage of NK cells by flow cytometry but only a moderate number of CD56 positive cells as a percentage of total hematopoietic elements. This discrepancy could be caused by peripheral blood contamination of the marrow aspirate specimen used for flow cytometric evaluation.
Discussion
Adoptive immunotherapy with NK cells has the potential to give some patients with chemotherapy-refractory AML a chance for achieving remission. The use of immunotherapy as a bridge to allogeneic transplantation has gained wide attention world-wide 13. Factors influencing the effectiveness of NK cell immunotherapy in various clinical settings include the alloreactive potential of NK cells determined by sets of Killer Immunoglobulin-like Receptors (KIR) and Human Leukocyte Antigens (HLA) genes, the number of alloreactive NK cells infused 14, 15, the functional status of the NK cells 16, 17 and composition of lymphodepleting chemotherapy (5,18). In this large cohort of patients treated with allogeneic NK cell immunotherapy we focused on the histopathologic determination of NK cell density in the bone marrow.
We used CD56 immunohistochemical staining as a marker of NK cells within the bone marrow niche. CD56 is one of the forms of NCAM (Neural Cell Adhesion Molecule) and is well established as a marker of human NK cells 18. CD56 expression is not unique to NK cells and can be expressed by other hematopoietic cells including monocytes, plasma cells, and leukemic blasts, and can be acquired by activated T cells. Also rare non-hematopoietic cells of neural or neuro-endocrine origin can be positive for CD56 19. A small number of cases with involvement by numerous CD56 positive blasts were excluded from this analysis.
We are not aware of previous studies characterizing the density of NK cells in human bone marrow. Characteristics which may conceivably affect bone marrow density of NK cells include underlying diagnosis, the timing and type of prior chemotherapy, overall marrow cellularity, patient age, gender, other comorbidities, infections and other factors. An optimal control group for the NK cell immunotherapy patients would be composed of individuals of similar age with refractory AML who had received lymphodepleting chemotherapy conditioning regimen 14 days prior (Fludarabine – Cytoxan or similar), but did not receive allogeneic NK cell infusion. Such control group is not available to us. In the current study the median density of NK cells in reference cohort of AML post-induction biopsies is higher than median density in NK cell immunotherapy patients. While the reason for this finding is not clear, we speculate that lymphodepleting conditioning chemotherapy used in the NK cell immunotherapy patients (Fludarabine, Cytoxan) more effectively depletes lymphocytes including NK cells, compared to Daunorubicine and Cytarabine (7+3 induction). Moreover, the study group included heavily pretreated patients with overall low bone marrow cellularity, whereas the reference AML cohort for the most part showed early hematopoietic recovery.
Based on our findings, we hypothesize that CD56 immunohistochemical staining of bone marrow core biopsy specimens is a more accurate representation of the bone marrow NK cell population than flow cytometry of marrow aspirate specimens, due to the likely hemodilution of marrow aspirate specimens. Hemodilution is known to be a factor in bone marrow aspirate specimens particularly in cases with low marrow cellularity and fibrosis. We show that NK cell immunotherapy patients who achieved higher NK cell density and higher percentage NK cells by flow cytometry compared to AML reference cohort had better control of disease.
Studies on animal models have previously shown that NK cell populations can expand in allogeneic hosts 20 and have the ability to home to lymphoid tissues. As well, in a model of NK cell response to viral infection the primary site of NK cell proliferation was shown to be in the bone marrow10, even when the bone marrow was not the primary target of the virus 8. Bone marrow is the primary site of AML involvement and it is conceivable that the homing and persistence of NK cells in the bone marrow could determine to some extent whether or not leukemia can be eradicated. The rules governing trafficking of human NK cells after therapeutic infusion are not well studied and our work describes a unique set of findings. We show that NK cells can persist and expand in the bone marrow within a month post infusion and that an increase in CD56 positive NK cell density in the bone marrow at this time point correlates with leukemia clearance.
By comparing NK cell density between various clinical protocols, we identified two clinical treatment factors which were associated with higher NK cell density: the use of TBI and the use of denileukin diftitox, a therapeutic antibody against CD25 coupled with toxin. We hypothesize that the action of CD25-targeting immunotoxin depends on targeting T regulatory cells (Tregs), a subset of T cells with distinctly higher expression of CD25 and immunosuppressive properties 11. Tregs have been shown to dampen NK cell response 21 and their elimination could result in improved NK cell activity. The mechanism of TBI is less clear; it has been previously proposed that it augments lymphodepletion and improves availability of IL-15 5. More recently irradiation has been shown to increase NK cell trafficking to sarcoma in an animal model, corroborating our observation 22. The additive effect of radiation and immunotherapy can be also explained by upregulation of tumor antigens including NKG2D ligands, as well as chemokines and adhesion molecules increasing recruitment of effector cells.
In summary, our study suggests that increased NK cell homing/persistence in the bone marrow 2–3 weeks after adoptive NK cell immunotherapy was associated with higher likelihood of achieving leukemia remission and appeared to be promoted by TBI and denileukin diftitox. These original findings may be helpful in guiding future immunotherapy with NK cells.
Acknowledgments
Sources of Funding
Supported in part by the Production Assistance for Cellular Therapies (PACT) program from NIH/NHLBI at University of Minnesota Molecular and Cellular Therapeutics Facility (PACT Contract #HHSN268201000008C); NIH P30CA77598 utilizing the Translational Therapy Laboratory Shared Resource of the Masonic Cancer Center, University of Minnesota; Children’s Cancer Research Fund, Leukemia Research Fund, and American Cancer Society, NIH P01 CA111412 and P01 CA65493. Research reported in this publication was supported by NIH grant P30 CA77598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure of Interest
The authors have no conflict of interest.
References
- 1.Knorr DA, Bachanova V, Verneris MR, et al. Clinical utility of natural killer cells in cancer therapy and transplantation. Semin Immunol. 2014;26(2):161–72. doi: 10.1016/j.smim.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grzywacz B, Miller JS, Verneris MR. Use of natural killer cells as immunotherapy for leukaemia. Best Pract Res Clin Haematol. 2008;21(3):467–83. doi: 10.1016/j.beha.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Leary JG, Goodarzi M, Drayton DL, et al. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7(5):507–16. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 4.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–61. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 6.Foley B, Felices M, Cichocki F, et al. The biology of NK cells and their receptors affects clinical outcomes after hematopoietic cell transplantation (HCT). Immunol Rev. 2014;258(1):45–63. doi: 10.1111/imr.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seaman WE, Gindhart TD, Greenspan JS, et al. Natural killer cells, bone, and the bone marrow: studies in estrogen-treated mice and in congenitally osteopetrotic (mi/mi) mice. J Immunol. 1979;122(6):2541–7. [PubMed] [Google Scholar]
- 8.van Helden MJ, de Graaf N, Boog CJ, et al. The bone marrow functions as the central site of proliferation for long-lived NK cells. J Immunol. 2012;189(5):2333–7. doi: 10.4049/jimmunol.1200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernardini G, Benigni G, Antonangeli F, et al. Multiple levels of chemokine receptor regulation in the control of mouse natural killer cell development. Front Immunol. 2014;5:44. doi: 10.3389/fimmu.2014.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milo I, Blecher-Gonen R, Barnett-Itzhaki Z, et al. The bone marrow is patrolled by NK cells that are primed and expand in response to systemic viral activation. Eur J Immunol. 2018;48(7):1137–1152. doi: 10.1002/eji.201747378. [DOI] [PubMed] [Google Scholar]
- 11.Bachanova V, Cooley S, Defor TE, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–63. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams SM, Sumstad D, Kadidlo D, et al. Clinical-scale production of cGMP compliant CD3/CD19 cell-depleted NK cells in the evolution of NK cell immunotherapy at a single institution. Transfusion. 2018. doi: 10.1111/trf.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjorklund AT, Carlsten M, Sohlberg E, et al. Complete Remission with Reduction of High-Risk Clones following Haploidentical NK-Cell Therapy against MDS and AML. Clin Cancer Res. 2018. doi: 10.1158/1078-0432.CCR-17-3196. [DOI] [PubMed] [Google Scholar]
- 14.Curti A, Ruggeri L, Parisi S, et al. Larger Size of Donor Alloreactive NK Cell Repertoire Correlates with Better Response to NK Cell Immunotherapy in Elderly Acute Myeloid Leukemia Patients. Clin Cancer Res. 2016;22(8):1914–21. doi: 10.1158/1078-0432.CCR-15-1604. [DOI] [PubMed] [Google Scholar]
- 15.Parisi S, Lecciso M, Ocadlikova D, et al. The More, The Better: “Do the Right Thing” For Natural Killer Immunotherapy in Acute Myeloid Leukemia. Front Immunol. 2017;8:1330. doi: 10.3389/fimmu.2017.01330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romee R, Rosario M, Berrien-Elliott MM, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8(357):357ra123. doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis ZB, Cooley SA, Cichocki F, et al. Adaptive Natural Killer Cell and Killer Cell Immunoglobulin-Like Receptor-Expressing T Cell Responses are Induced by Cytomegalovirus and Are Associated with Protection against Cytomegalovirus Reactivation after Allogeneic Donor Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2015;21(9):1653–62. doi: 10.1016/j.bbmt.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanier LL, Le AM, Civin CI, et al. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136(12):4480–6. [PubMed] [Google Scholar]
- 19.Niedecken H, Wehrmann W, Bauer R, et al. Anti-Leu 19 monoclonal antibody detects an antigen on autonomic nerves in human skin. J Cutan Pathol. 1988;15(4):212–4. [DOI] [PubMed] [Google Scholar]
- 20.Olson JA, Zeiser R, Beilhack A, et al. Tissue-specific homing and expansion of donor NK cells in allogeneic bone marrow transplantation. J Immunol. 2009;183(5):3219–28. doi: 10.4049/jimmunol.0804268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedroza-Pacheco I, Madrigal A, Saudemont A. Interaction between natural killer cells and regulatory T cells: perspectives for immunotherapy. Cell Mol Immunol. 2013;10(3):222–9. doi: 10.1038/cmi.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canter RJ, Grossenbacher SK, Foltz JA, et al. Radiotherapy enhances natural killer cell cytotoxicity and localization in pre-clinical canine sarcomas and first-in-dog clinical trial. J Immunother Cancer. 2017;5(1):98. doi: 10.1186/s40425-017-0305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]