Abstract
A retrospective analysis of administrative claims data from a large U.S. health insurer was performed to study a potential association between oral antibiotic use during early childhood and occurrence of later gastrointestinal (GI) symptoms in children with autism spectrum disorder (ASD). Among 3253 children with ASD, 37.0% had a GI-related diagnosis during the last two years of their five-year health coverage enrollment period, compared to 20.0% of 278370 children from the general population without an ASD diagnosis. Greater numbers of oral antibiotic fills during the first three years of enrollment were found to significantly increase the hazard rate of having a later GI-related diagnosis (adjusted hazard ratio 1.48; 95% confidence interval 1.34, 1.63) in children both with and without ASD.
Keywords: autism spectrum disorder, gastrointestinal symptoms, oral antibiotics, gut microbiome, administrative claims, retrospective analysis
Autism spectrum disorder (ASD) is a neurodevelopmental condition primarily characterized by social and behavioral deficits. However, individuals diagnosed with the disorder are also often affected by one or more co-occurring conditions (Aldinger, Lane, Veenstra-VanderWeele, & Levitt, 2015; Doshi-Velez, Ge, & Kohane, 2014; Kohane et al., 2012; Muskens, Velders, & Staal, 2017), such as intellectual disability, epilepsy, immune dysfunction, or gastrointestinal (GI) symptoms, that cause the presentation of the disorder to be highly heterogeneous. One recent study of children with ASD from five sites in the United States found that over 95% of the children were affected by at least one co-occurring condition (Soke, Maenner, Christensen, Kurzius-Spencer, & Schieve, 2018). GI symptoms, in particular, are reported to affect individuals with ASD at a disproportionate rate compared to their typically developing peers. Estimates of the prevalence of general GI symptoms among the ASD population vary widely between studies, ranging from 4.1% (Jiang, Matson, Cervantes, Matheis, & Burns, 2017) to 96.8% (Babinská et al., 2014). A review of these studies found the median reported prevalence of GI symptoms in children with ASD to be 46.8%, with variability in the estimates generally arising from differences in methods of reporting, age of study participants, study goals, and overall study design (Holingue, Newill, Lee, Pasricha, & Daniele Fallin, 2018). For comparison, the odds of having a general GI complaint were estimated by one meta-analysis to be 4.4 times greater for children with ASD than typically developing children (McElhanon, McCracken, Karpen, & Sharp, 2014).
Certain GI symptoms have also been found to be correlated with maladaptive behavior (Chaidez, Hansen, & Hertz-Picciotto, 2014) and with subscales of the Autism Treatment Evaluation Checklist, such as speech and physical behavior (J. B. Adams, Johansen, Powell, Quig, & Rubin, 2011), in children with ASD. It is possible that this association is due to a connection between gut and brain activity in which metabolites produced by microbes in the GI tract affect functioning of the central nervous system in addition to influencing local GI activity (Cryan & Dinan, 2012). One current hypothesis states that children with ASD have irregularities in their gut microbiome (i.e. the collective group of microorganisms inhabiting the human GI tract) that cause abnormal regulation of these bacterial metabolites, which in turn contributes to the severity of some ASD-related symptoms (Krajmalnik-Brown, Lozupone, Kang, & Adams, 2015; Vuong & Hsiao, 2017). Several studies investigating potential perturbations in gut homeostasis in children with ASD have found evidence of reduced microbial diversity, increased abundance of pathogenic flora, and decreased abundance of commensal flora compared to typically developing children (Parracho, Bingham, Gibson, & McCartney, 2005; Finegold et al., 2010; Kang et al., 2013; Son et al., 2015; Tomova et al., 2015; Kang et al., 2018), which in turn may influence how the brain is affected by the gut.
Oral antibiotic use during early childhood may be responsible for long-term disruption of the gut microbiome’s composition (Fjalstad, Esaiassen, Juvet, van den Anker, & Klingenberg, 2017) that contributes to the onset of recurring GI symptoms (Krajmalnik-Brown et al., 2015). Young children with ASD are reported to take greater numbers of antibiotics relative to their typically developing peers, perhaps offering an explanation as to why GI abnormalities appear to be more prevalent in ASD. A recent study of outpatient prescription claims in northern New England estimated total antibiotic use to be 20% higher in children with ASD compared to the general pediatric population, with a two-fold difference during the first two years of life (House et al., 2016). This finding supports that of an earlier analysis of children’s medical records from three different sites in the United States, which found children with ASD to take twice as many antibiotics as typically developing children before two years of age (Niehus & Lord, 2006). Similarly, a medical history questionnaire administered to families in Arizona revealed antibiotic usage before age three to be more than twice as high in the ASD group compared to controls (Adams, Romdalvik, Ramanujam, & Legator, 2007). Increased incidence of infections in children with ASD (Adams et al., 2016; Niehus & Lord, 2006) may offer one explanation for the elevated use of antibiotics that has been reported in this population.
The current work performs a retrospective analysis of medical and pharmacy claims data from a U.S. health plan to compare temporal trends in GI symptom occurrence and oral antibiotic use between children with ASD and children from the general population with no ASD diagnosis. These data are then used to investigate the potential association between early oral antibiotic use and later GI symptoms in children both with and without ASD. To the authors’ knowledge, this study uses the largest and most diverse study population to date for examining this association, and is one of few to consider how these factors change throughout early childhood.
Methods
Description of data
This study involves a retrospective analysis of claims data from the OptumLabs® Data Warehouse (OLDW), which includes de-identified claims data for privately insured and Medicare Advantage enrollees in a large, private, U.S. health plan (for this study, only privately insured enrollees were used). The database contains longitudinal health information on enrollees, representing a diverse mixture of ages, ethnicities, and geographical regions across the United States. The health plan provides comprehensive full insurance coverage for physician, hospital, and prescription drug services (OptumLabs, 2018). As all data were pre-existing and de-identified, this study was exempt from Institutional Review Board approval.
Cohort definitions
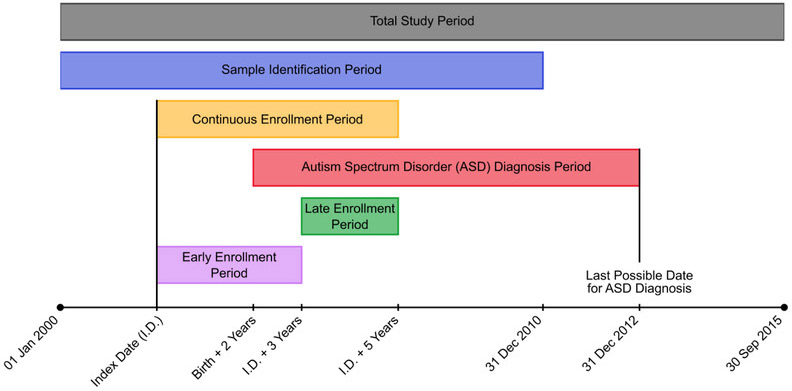
The entire study period spanned from January 1, 2000 to September 30, 2015 and children born between 2000 and 2010 comprised the population of interest for this study (Figure 1). Since only year of birth, and not the exact date of birth, is available in the OLDW, eligible children were required to have their first date of coverage enrollment (index date) be within one year of their birth year. Additionally, included children needed to have at least five years of continuous medical, pharmacy, and mental health coverage beyond their index date; gaps in coverage of 45 days or fewer were bridged to count towards the continuous enrollment period. The five-year continuous enrollment requirement had to be met before September 30, 2015 so as to ensure that all diagnosis codes used in the study adhered to International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coding.
Figure 1.
Timeline of relevant dates in the study’s design. The index date (first date of coverage enrollment) must have been between January 1, 2000 and December 31, 2010 and within one year of a child’s birth year, which needed to be between the years 2000 and 2010. A minimum five years of continuous medical, pharmacy, and behavioral coverage were required to follow the index date for a child to be included in the study.
The ASD cohort in this study consisted of children meeting the above continuous enrollment criteria with at least two medical claims, on separate dates, containing a diagnosis code in any position for autistic disorder (ICD-9-CM code 299.0x), Asperger syndrome (299.8x), or a pervasive developmental disorder not otherwise specified (299.9x), and without any claim containing a diagnosis code for childhood disintegrative disorder (299.1x) or Rett syndrome (330.8x). These criteria have previously been validated against medical charts with a high positive predictive value for ASD (Burke et al., 2013; Coleman et al., 2015). An additional criterion was used in which only ASD diagnoses (both inclusion and exclusion) made at least two years after the birth year were considered in the decision to include or exclude an individual from the ASD cohort. Stable diagnoses of the disorder are typically possible by two years of age (Ozonoff et al., 2015), but any social or behavioral deficits displayed before this age may be part of a child’s normal development and not necessarily ASD-related. Any diagnosis claims made after the minimum two-year cutoff (i.e. not just during the five year continuous enrollment period) were accepted as long as they occurred before the year 2013, so as to ensure that all ASD diagnoses were made according to the common criteria outlined in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (American Psychiatric Association, 2000).
All children meeting the aforementioned continuous enrollment criteria and having no medical claims with any inclusion or exclusion ASD diagnosis codes were assigned to the general population with no ASD diagnosis (POP) cohort. Any individual having just one ASD diagnosis claim was excluded from the study entirely due to the high chance for misclassification in either cohort. Additional conditions for exclusion from the study were having incomplete/conflicting gender or census division information at the first available enrollment period or unavailable race information. Finally, individuals were also excluded if they were diagnosed with a congenital anomaly of the digestive tract (ICD-9-CM 751.xx), such as Hirschsprung’s disease, at any time during the five-year continuous enrollment period.
Key outcomes
The primary outcome for this study was whether a child presented with a GI complaint at any time during the period between three and five years after the index date (i.e. the late enrollment period). A GI complaint was defined as a medical claim containing a diagnosis code, in any position, for constipation (ICD-9-CM code 564.0x), diarrhea (787.91), abdominal pain (789.0x), or noninfectious gastroenteritis/colitis (558.9x). Children presenting with a GI complaint during the late enrollment period were assigned to the “+GI” subcohort of their respective cohort (ASD+GI or POP+GI), while children not presenting with a GI complaint during this period were assigned to the “−no GI” subcohort of their respective cohort (ASD–no GI or POP–no GI). When counting the occurrence of GI symptoms, a unique GI episode was identified by a claim including one or more GI diagnosis codes that was made at least 10 days after a previous GI diagnosis claim was made.
Key indicators
In line with the hypothesis that early oral antibiotic use may influence later GI symptoms, the primary predictor of GI complaints during the late enrollment period was considered to be the number of oral antibiotic prescriptions filled during the first three years of the continuous enrollment period (i.e. the early enrollment period). However, since antibiotic use during the late enrollment period may also result in short-term GI symptoms, the number of oral antibiotic fills during the late enrollment period was also considered. In both cases, fills that overlapped with the days’ supply of a previous fill were excluded from the fill count, as were fills that covered a period of 3 days or less; refills of an existing prescription were considered a separate fill as long as they met these criteria. Other potential indicators of GI complaints during the late enrollment period included gender (Cain et al., 2009) and race/ethnicity (Bhopal, Cezard, Bansal, Ward, & Bhala, 2014; Huerta-Franco, Banderas, & Allsworth, 2018). The presence of a GI symptom during the early enrollment period was also included as a potential predictor of having a GI complaint during the late enrollment period.
Secondary indicators
A secondary analysis considered the medical conditions that oral antibiotics were prescribed for during the early enrollment period. These conditions were indicated by a medical claim with a diagnosis code, in any position, for one of several types of common childhood infections (Alter et al. 2011). Infections were categorized as otitis media (ICD-9-CM code 381.0x, 381.3x, 381.4x, 382.xx), upper respiratory infection (034.0x, 461.xx, 462.xx, 463.xx, 464.xx, 465.9x, 473.xx, 474.xx), pneumonia (481.xx, 482.xx, 486.xx), urinary tract infection (590.0x, 590.1x, 590.8x, 590.9x, 595.0x, 595.1x, 595.2x, 595.9x, 599.0x), and conjunctivitis (372.0x, 372.1x, 372.2x, 372.3x). For the purposes of this analysis, the first oral antibiotic fill made within 7 days of an infection diagnosis was considered to be prescribed to treat that infection; in the event that infection diagnosis claims from separate days were matched to the same oral antibiotic fill, only the first diagnosis claim was used.
Analysis techniques
Demographic and clinical characteristics were gathered for all study participants to compare the compositions of the ASD and POP cohorts as well as the GI subcohorts within each respective cohort. Population-level temporal trends in the key outcomes and indicators over the enrollment period were also investigated. Relationships between the outcomes and indicator variables were quantified with odds ratios (ORs) and their 95% confidence intervals (CIs).
Cox regression was used to estimate unadjusted and adjusted hazard ratios (HRs) for covariates contributing to the time to first GI-related diagnosis (i.e. any diagnosis of constipation, diarrhea, abdominal pain, or noninfectious gastroenteritis/colitis) during the late enrollment period, beginning from the first day of this period (i.e. the start of the fourth year after the index date). This analysis used data from participants in both the ASD and POP cohorts and thus included a binary variable indicating membership in the ASD cohort. The key indicators of GI symptoms, as previously discussed, were also included as model covariates. Oral antibiotic prescriptions filled during the late enrollment period were only counted if they were made prior to an individual’s first GI-related diagnosis, and for individuals who were not diagnosed with a GI symptom, this variable was equal to the total number of fills from this period; the data were also appropriately encoded so as to account for the time-dependent nature of this variable. All aforementioned variables were selected a priori from a large number of claims variables in the OLDW as potential confounders. Due to computational restrictions, the full POP cohort was not modeled with Cox regression and members of the POP cohort were instead randomly gender- and race-matched, without replacement, to members of the ASD cohort at a 10:1 ratio for this analysis.
Aqua Data Studio 15.0 (AquaFold, Inc, Houston, TX) was used for database querying and RStudio 0.98 (RStudio, Inc, Boston, MA) was used for data analysis.
Results
A total of 3278 children with ASD and 279428 children from the general population with no ASD diagnosis were identified. Among these, 25 children with ASD (0.76%) and 1058 children from the general population (0.38%) were diagnosed with a congenital anomaly of the digestive tract and were excluded from further analysis, providing 3253 children in the ASD cohort and 278370 children in the POP cohort. The percentages of children in these cohorts with a claim for each GI symptom during the late enrollment period are presented in Table 1. All symptoms were more commonly diagnosed in children with ASD compared to the general population; overall, a total of 1205 children (37.0%) in the ASD cohort had a GI diagnosis claim during this period, compared to 55697 children (20.0%) in the POP cohort. Based on these numbers, the OR for the occurrence of general GI symptoms during the late enrollment period in children with ASD compared to the general population was 2.35 (95% CI 2.19, 2.53).
Table 1.
Prevalence of gastrointestinal (GI) symptoms during the late enrollment period in children with autism spectrum disorder (ASD) and in children from the general population with no ASD diagnosis (POP cohort).
| GI Symptom | Number in ASD Cohort |
Number in POP Cohort |
Odds Ratio (95% CI) |
|---|---|---|---|
| Constipation | 510 (15.7%) | 18184 (6.5%) | 2.66 (2.42, 2.93) |
| Diarrhea | 407 (12.5%) | 12587 (4.5%) | 3.02 (2.72, 3.36) |
| Abdominal pain | 389 (12.0%) | 25542 (9.2%) | 1.34 (1.21, 1.50) |
| Gastroenteritis/colitis | 386 (11.9%) | 16100 (5.8%) | 2.19 (1.97, 2.44) |
| General GI symptom | 1205 (37.0%) | 55697 (20.0%) | 2.35 (2.19, 2.53) |
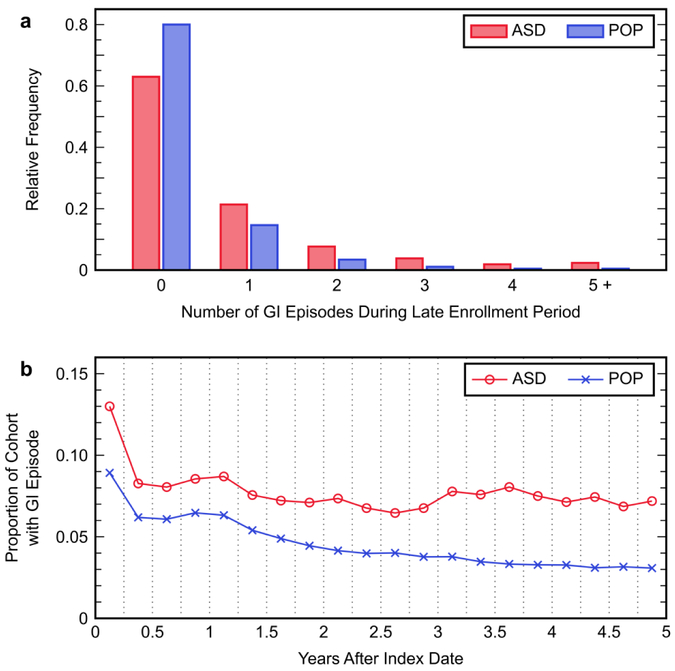
Inspecting the relative frequency distributions for the number of unique GI episodes across the late enrollment period revealed that members of the ASD cohort more frequently experienced a greater number of GI episodes than members of the POP cohort (Figure 2a). Additionally, a small yet notable subset of children had five or more GI episodes during this period, with the proportion of children in this subset being nearly five times larger in the ASD cohort (2.3%) than in the POP cohort (0.5%). Furthermore, the proportion of members in each cohort experiencing a GI episode was analyzed for each three-month interval over the duration of the five-year enrollment period (Figure 2b) to evaluate how patterns of GI diagnosis in each cohort changed with age. The ASD and POP cohorts had relatively similar patterns during the early enrollment period, although with different levels as 53.1% of children with ASD had a GI episode compared to 41.2% of children from the POP cohort throughout this period (OR 1.61; 95% CI 1.50, 1.73). The difference in GI episode prevalence between the two study cohorts became substantially larger during the late enrollment period, as the POP cohort saw a continuing decline in the prevalence of GI symptoms whereas the prevalence stayed relatively constant in the ASD cohort. Throughout the duration of the entire five-year study period, 66.9% of the ASD cohort and 50.2% of the POP cohort were diagnosed with at least one GI symptom (OR 2.00; 95% CI 1.86, 2.15).
Figure 2.
Patterns of gastrointestinal (GI) episode occurrence in the autism spectrum disorder (ASD) cohort and the general population without an ASD diagnosis (POP) cohort: (a) relative frequency distributions for the number of GI episodes that occurred in members of each cohort during the late enrollment period; (b) proportion of the members in each cohort that experienced a GI episode during each three-month interval of the total five-year enrollment period. Each point represents the total proportion of individuals during the indicated three-month period.
Demographic and clinical characteristics of the ASD cohort, POP cohort, and their respective GI subcohorts are presented in Table 2. A much larger percentage of males (81.5%) than females (18.5%) were found to belong to the ASD cohort, whereas the POP cohort contained a nearly even proportion of both genders. The study sample also contained geographic representation from all nine U.S. census divisions, although the composition of the study sample at the state level could not be resolved based upon the data available from the OLDW. It is worth noting that the demographic compositions of the GI subcohorts did not deviate significantly from those of their respective cohorts. Furthermore, a higher percentage of individuals in the ASD cohort were diagnosed with a GI symptom during the early enrollment period compared to the POP cohort. Members of the +GI subcohorts were also more commonly diagnosed with a GI symptom during the early enrollment period compared to the −no GI subcohorts, regardless of ASD/POP cohort membership.
Table 2.
Demographic and clinical characteristics of the autism spectrum disorder (ASD) cohort and the general population with no ASD diagnosis (POP) cohort, including the composition of their subcohorts describing individuals with and without a gastrointestinal (GI) symptom diagnosed during the late enrollment period (+GI and −no GI subcohorts, respectively). Counts are presented with the column-wise percentage of each cohort/sub cohort given in parentheses.
| Characteristic | Total ASD Cohort |
ASD+GI Subcohort |
ASD–no GI Subcohort |
Total POP Cohort |
POP+GI Subcohort |
POP–no GI Subcohort |
|---|---|---|---|---|---|---|
| Overall | 3253 | 1205 | 2048 | 278370 | 55697 | 222673 |
| Gender | ||||||
| Male | 2651 (81.5%) | 966 (80.2%) | 1685 (82.3%) | 141134 (50.7%) | 27504 (49.4%) | 113630 (51.0%) |
| Female | 602 (18.5%) | 239 (19.8%) | 363 (17.7%) | 137236 (49.3%) | 28193 (50.6%) | 109043 (49.0%) |
| Race / Ethnicity | ||||||
| White | 2468 (75.9%) | 903 (74.9%) | 1565 (76.4%) | 210352 (75.6%) | 40449 (72.6%) | 169903 (76.3%) |
| Asian | 234 (7.2%) | 96 (8.0%) | 138 (6.7%) | 17300 (6.2%) | 3833 (6.9%) | 13467 (6.0%) |
| Black | 232 (7.1%) | 70 (5.8%) | 162 (7.9%) | 21730 (7.8%) | 4149 (7.4%) | 17581 (7.9%) |
| Hispanic | 319 (9.8%) | 136 (11.3%) | 183 (8.9%) | 28988 (10.4%) | 7266 (13.0%) | 21722 (9.8%) |
| Census Division | ||||||
| New England | 126 (3.9%) | 47 (3.9%) | 79 (3.9%) | 8656 (3.1%) | 1596 (2.9%) | 7060 (3.2%) |
| Mid Atlantic | 425 (13.1%) | 166 (13.8%) | 259 (12.6%) | 24084 (8.7%) | 5324 (9.6%) | 18760 (8.4%) |
| East North Central | 443 (13.6%) | 152 (12.6%) | 291 (14.2%) | 45029 (16.2%) | 8084 (14.5%) | 36945 (16.6%) |
| West North Central | 450 (13.8%) | 152 (12.6%) | 298 (14.6%) | 37745 (13.6%) | 6716 (12.1%) | 31029 (13.9%) |
| South Atlantic | 903 (27.8%) | 342 (28.4%) | 561 (27.4%) | 69872 (25.1%) | 15364 (27.6%) | 54508 (24.5%) |
| East South Central | 87 (2.7%) | 39 (3.2%) | 48 (2.3%) | 8674 (3.1%) | 1720 (3.1%) | 6954 (3.1%) |
| West South Central | 361 (11.1%) | 144 (12.0%) | 217 (10.6%) | 41765 (15.0%) | 8818 (15.8%) | 32947 (14.8%) |
| Mountain | 202 (6.2%) | 63 (5.2%) | 139 (6.8%) | 23669 (8.5%) | 4361 (7.8%) | 19308 (8.7%) |
| Pacific | 256 (7.9%) | 100 (8.3%) | 156 (7.6%) | 18763 (6.7%) | 3700 (6.6%) | 15063 (6.8%) |
| Other | 0 (0%) | 0 (0%) | 0 (0%) | 113 (<0.1%) | 14 (<0.1%) | 99 (<0.1%) |
| GI episode during early enrollment period |
1726 (53.1%) | 756 (62.7%) | 970 (47.4%) | 114807 (41.2%) | 30703 (55.1%) | 84104 (37.8%) |
In the ASD cohort, 88.9% of children were prescribed an oral antibiotic during the early enrollment period, compared to 86.5% of children from the POP cohort (Table 3). The OR for having any oral antibiotic fill for children with ASD compared to the general population during this period was 1.25 (95% CI 1.12, 1.40). Within the ASD cohort, the OR for belonging to the ASD+GI subcohort compared to the ASD–no GI subcohort, given an oral antibiotic fill during the early enrollment period, was 1.69 (95% CI 1.32, 2.16); similarly, the OR describing the same conditions in the POP cohort was 1.68 (95% CI 1.63, 1.74). The mean number of fills during the early enrollment period was also consistently greater in the ASD cohort compared to the POP cohort and in the +GI subcohorts compared to the −no GI subcohorts, although not by a substantial margin in any case. Differences between the ASD and POP cohorts were slightly larger during the late enrollment period, and greater discrepancies were also seen between each cohort’s +GI and −no GI subcohorts.
Table 3.
Descriptive statistics for oral antibiotic fills during the early and late enrollment periods in the autism spectrum disorder (ASD) cohort and general population with no ASD diagnosis cohort (POP) cohort, and in their subcohorts describing children with and without a gastrointestinal (GI) symptom diagnosed during the late enrollment period (+GI and −no GI subcohorts, respectively). Where counts are reported, the group percentage is given in parentheses. For reported means, the standard deviation is in parentheses.
| Statistic | Total ASD Cohort |
ASD+GI Subcohort |
ASD–no GI Subcohort |
Total POP Cohort |
POP+GI Subcohort |
POP–no GI Subcohort |
|---|---|---|---|---|---|---|
| Number of children with an antibiotic fill during early enrollment |
2892 (88.9%) | 1108 (92.0%) | 1784 (87.1%) | 240764 (86.5%) | 50569 (90.8%) | 190195 (85.4%) |
| Mean number of antibiotic fills per child with a fill per year during early enrollment |
1.95 (1.49) | 2.12 (1.61) | 1.85 (1.41) | 1.83 (1.46) | 2.06 (1.58) | 1.77 (1.42) |
| Mean number of antibiotic fills per child per year during early enrollment |
1.73 (1.53) | 1.95 (1.64) | 1.61 (1.45) | 1.58 (1.49) | 1.87 (1.62) | 1.51 (1.45) |
| Number of children with an antibiotic fill during late enrollment |
2583 (79.4%) | 1032 (85.6%) | 1551 (75.7%) | 204926 (73.6%) | 46753 (83.9%) | 158173 (71.0%) |
| Mean number of antibiotic fills per child with a fill per year during late enrollment |
1.87 (1.57) | 2.18 (1.89) | 1.66 (1.28) | 1.57 (1.27) | 1.89 (1.48) | 1.48 (1.18) |
| Mean number of antibiotic fills per child per year during late enrollment |
1.48 (1.59) | 1.87 (1.91) | 1.26 (1.32) | 1.16 (1.29) | 1.59 (1.53) | 1.05 (1.20) |
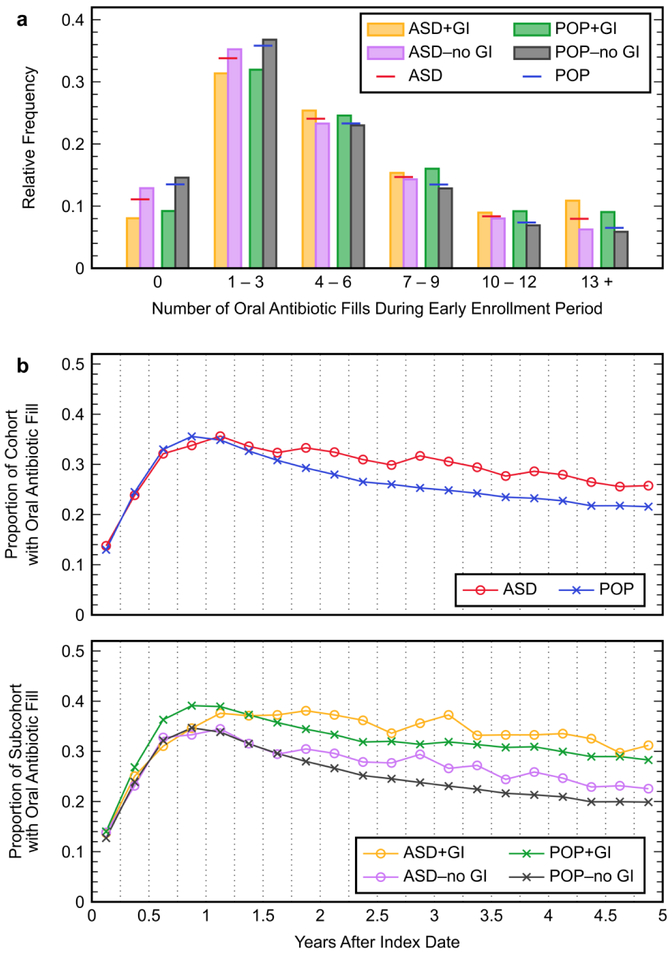
The proportion of children with three or fewer antibiotic fills during the early enrollment period was slightly smaller in the ASD cohort (44.9%) compared to the POP cohort (49.3%), and members of the +GI subcohorts were more frequently prescribed a greater number of antibiotics than members of the respective −no GI subcohorts (Figure 3a). Additionally, 8.0% of the ASD cohort and a comparable 6.5% of the POP cohort had 13 or more fills during the early enrollment period. Over the course of the total enrollment period, the ASD and POP cohorts had similar proportions of individuals being prescribed oral antibiotics during any given three-month interval (Figure 3b), although the percentage was slightly higher in children with ASD during later months of the enrollment period. Oral antibiotic use was also consistently more prevalent in the +GI subcohorts compared to the respective −no GI subcohorts over the course of the entire enrollment period.
Figure 3.
Trends in oral antibiotic use in the autism spectrum disorder (ASD) cohort and general population without an ASD diagnosis (POP) cohort, and in their subcohorts describing children with and without a gastrointestinal (GI) symptom diagnosed during the late enrollment period (+GI and −no GI subcohorts, respectively): (a) relative frequency distributions for the number of oral antibiotic fills during the early enrollment period for members of each GI subcohort and the primary cohorts; (b) proportion of the members in each cohort and GI subcohort that had an oral antibiotic fill during each three-month interval of the total five-year enrollment period. Each point represents the total proportion of individuals during the indicated three-month period.
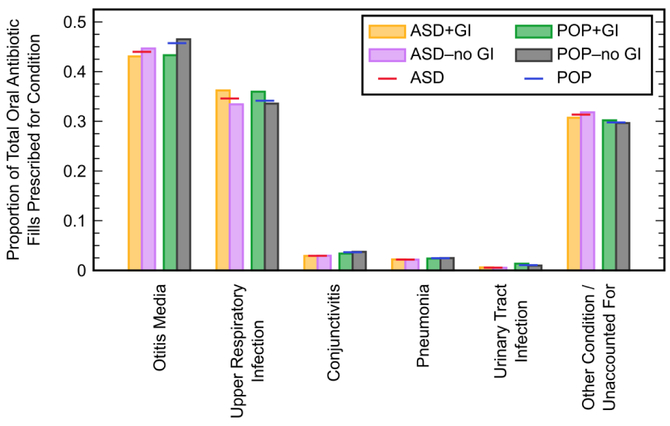
During the early enrollment period, oral antibiotics were found to be most commonly prescribed to treat otitis media, which accounted for 44.0% of fills in the ASD cohort and 45.7% of fills in the POP cohort (Figure 4). Upper respiratory infections, the second most commonly treated type of infection, were responsible for 34.6% and 34.1% of fills in the ASD and POP cohorts, respectively. Conjunctivitis, pneumonia, and urinary tract infections each accounted for less than 4% of total oral antibiotic fills. Approximately 30% of fills were not associated with any of the five studied categories of infections. Prescribing patterns in the GI subcohorts did not deviate greatly from their respective cohorts.
Figure 4.
Proportion of oral antibiotics prescribed for different types of infections during the early enrollment period within the autism spectrum disorder (ASD) cohort and general population with no ASD diagnosis (POP) cohort, and within their subcohorts describing children with and without a gastrointestinal (GI) symptom diagnosed during the late enrollment period (+GI and −no GI subcohorts, respectively). The sum of proportions within each cohort/sub cohort may be greater than unity due to the occasional diagnosis of multiple infection types in one medical claim.
Analysis with Cox regression for the time to first-GI related diagnosis during the late enrollment period (Table 4) using the combined ASD and matched POP cohorts revealed a modest effect of gender, where the adjusted HR for females in comparison to males was 1.18 (95% CI 1.11, 1.25). The effects of race/ethnicity were varied, where the HRs for Asian and Hispanic compared to White were statistically significant, but the HRs for Black (both unadjusted and adjusted) were not. There was an overall increase in HR as the number of oral antibiotic fills during the early enrollment period increased, with the adjusted HR being as large as 1.48 (95% CI 1.34, 1.63) when there were 7–9 fills; the adjusted estimates for this covariate were also notably lower than the unadjusted estimates. Each additional oral antibiotic fill during the late enrollment period increased the hazard rate of being diagnosed with a GI symptom by a factor of approximately 1.10 (95% CI 1.09, 1.11). It was also found that the adjusted HR associated with having a GI episode during the early enrollment period was significant (adjusted HR 1.73; 95% CI 1.65, 1.81). Not unexpectedly, being a member of the ASD cohort nearly doubled the hazard rate for being diagnosed with a GI symptom during the late enrollment period in comparison to the hazard rate for those in the POP cohort. HR estimates did not change substantially when the ASD and POP cohorts were modeled separately, with the exception of race/ethnicity where the HR for Black was less than unity in the ASD cohort, but was greater than unity when the matched POP cohort and both cohorts together were modeled. Due to the matched POP cohort being much larger than the ASD cohort, however, the 95% CIs estimated using the ASD cohort were wider than the 95% CIs obtained using the POP cohort.
Table 4.
Unadjusted and adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) for covariates contributing to the time to first gastrointestinal (GI)-related diagnosis during the late enrollment period. This analysis with Cox regression made use of the entire autism spectrum disorder (ASD) cohort and members of the general population with no ASD diagnosis (POP) cohort that were gender- and race-matched to members of the ASD cohort at a 10:1 ratio, with these cohorts modeled both together and separately.
| ASD and Matched POP Cohorts | ASD Cohort | Matched POP Cohort | ||||
|---|---|---|---|---|---|---|
| Variable | Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
| Gender | ||||||
| Male | Reference | Reference | Reference | Reference | Reference | Reference |
| Female | 1.16 (1.10, 1.23) | 1.18 (1.11, 1.25) | 1.16 (0.97, 1.29) | 1.12 (0.97, 1.29) | 1.17 (1.10, 1.24) | 1.19 (1.12, 1.26) |
| Race / Ethnicity | ||||||
| White | Reference | Reference | Reference | Reference | Reference | Reference |
| Asian | 1.17 (1.08, 1.27) | 1.25 (1.15, 1.36) | 1.19 (0.96, 1.46) | 1.25 (1.02, 1.55) | 1.17 (1.07, 1.29) | 1.25 (1.14, 1.37) |
| Black | 1.04 (0.95, 1.14) | 1.06 (0.97, 1.16) | 0.78 (0.61, 1.00) | 0.80 (0.63, 1.03) | 1.09 (0.99, 1.20) | 1.12 (1.01, 1.23) |
| Hispanic | 1.36 (1.27, 1.46) | 1.32 (1.23, 1.41) | 1.22 (1.02, 1.46) | 1.20 (1.00, 1.44) | 1.39 (1.29, 1.50) | 1.34 (1.25, 1.45) |
|
Antibiotic fill count, early
enrollment |
||||||
| 0 | Reference | Reference | Reference | Reference | Reference | Reference |
| 1–3 | 1.33 (1.22, 1.45) | 1.22 (1.12, 1.33) | 1.37 (1.10, 1.72) | 1.31 (1.05, 1.64) | 1.31 (1.20, 1.44) | 1.20 (1.10, 1.32) |
| 4–6 | 1.64 (1.50, 1.79) | 1.38 (1.27, 1.51) | 1.61 (1.28, 2.02) | 1.43 (1.13, 1.80) | 1.62 (1.48, 1.79) | 1.38 (1.25, 1.52) |
| 7–9 | 1.86 (1.69, 2.04) | 1.48 (1.34, 1.63) | 1.58 (1.24, 2.02) | 1.36 (1.06, 1.75) | 1.88 (1.70, 2.08) | 1.50 (1.35, 1.67) |
| 10–12 | 1.82 (1.64, 2.02) | 1.35 (1.21, 1.50) | 1.62 (1.23, 2.13) | 1.32 (1.00, 1.75) | 1.83 (1.63, 2.05) | 1.36 (1.21, 1.52) |
| 13+ | 2.19 (1.98, 2.44) | 1.47 (1.31, 1.64) | 2.30 (1.77, 2.99) | 1.71 (1.30, 2.26) | 2.14 (1.91, 2.40) | 1.42 (1.26, 1.61) |
| Other clinical factors | ||||||
| Antibiotic fill count, late enrollment (continuous) |
1.13 (1.12, 1.14) | 1.10 (1.09, 1.11) | 1.11 (1.08, 1.15) | 1.08 (1.05, 1.12) | 1.12 (1.11, 1.13) | 1.10 (1.09, 1.11) |
| GI episode during early enrollment |
1.94 (1.85, 2.03) | 1.73 (1.65, 1.81) | 1.68 (1.50, 1.89) | 1.57 (1.39, 1.77) | 1.92 (1.83, 2.01) | 1.76 (1.70, 1.85) |
| ASD cohort membership | 2.10 (1.98, 2.24) | 1.93 (1.82, 2.06) | — | — | — | — |
Discussion
We identified a cohort of 3253 privately insured children diagnosed with ASD in the United States, as well as a control cohort consisting of 278370 children from the general population without an ASD diagnosis. A relatively small number of children were excluded due to a congenital anomaly of the digestive tract, although the proportion of children in the ASD cohort with one of these abnormalities was twice the proportion in the POP cohort. The prevalence of ASD in our study sample is approximately 1.16% for the period ranging between the years 2002 and 2012. This is in agreement with prevalence estimates by the Centers for Disease Control and Prevention for the same time period; the prevalence of ASD in the United States was estimated to be 0.66% in 2002 (Autism and Developmental Disabilities Monitoring Network Surveillance Year 2002 Principal Investigators & Centers for Disease Control and Prevention, 2007) and continuously increased throughout the ten year time span to 1.46% in 2012 (Christensen et al., 2016). ASD was also documented as affecting males disproportionately at a ratio of approximately 4.5:1 compared to females in the year 2012 (Christensen et al., 2016), a statistic that is reinforced by our own study sample’s ratio of 4.4:1. This is noteworthy considering the nearly even distribution of males and females in the POP cohort.
It was found that 37.0% of children in the ASD cohort had at least one medical claim for a GI-related diagnosis (constipation, diarrhea, abdominal pain, noninfectious gastroenteritis/colitis), compared to 20.0% in the POP cohort, during the late enrollment period. These results align exceptionally well with the findings of the recent meta-analysis by Holingue et al., who determined the median prevalence of GI symptoms in individuals with ASD to be 46.8% across all types of studies and 37% in studies using medical records or claims data (Holingue et al., 2018). Holingue et al. also found the median reported prevalence among these types of studies to be 15% for constipation, 18% for diarrhea, and 36% for abdominal pain (Holingue et al., 2018). Our estimates for constipation (15.7%) are again in good agreement, but are much lower for diarrhea (12.5%) and abdominal pain (12.0%). It is possible that our inclusion of noninfectious gastroenteritis/colitis as a GI diagnosis captured some of the symptomatology for diarrhea and/or abdominal pain that would account for our underestimation of the prevalence of these diagnoses. The synthesis of study results in the meta-analysis by McElhanon et al. (McElhanon et al. 2014) found the ORs for the occurrence of GI symptoms in children with ASD compared to controls to be 4.42 for general GI symptoms, 3.86 for constipation, 3.63 for diarrhea, and 2.45 for abdominal pain; minus diarrhea, these are considerably higher than the ORs calculated in this study, although the 95% CIs for our estimates fall entirely within the 95% CIs of their synthesized results. Thus, our estimates of the prevalence of individual GI symptoms in children with ASD relative to healthy controls appear to be underestimated in comparison to the typical findings; this may be because certain patients are unlikely to visit their doctor to report GI symptoms even if they are experiencing these symptoms. It is important to note that inclusion of additional diagnoses (such as irritable bowel syndrome or ulcerative colitis) or GI-related procedure codes (such as those for intestinal endoscopy, sigmoidoscopy, or colonoscopy) contributed minimal additional representation to the GI subcohorts and therefore we limited our indicators of GI disturbances to just the four GI-related diagnosis codes discussed here.
From a temporal perspective, GI symptoms were consistently more prevalent in the ASD cohort than the POP cohort throughout the entire enrollment period. After the first year of enrollment, prevalence in the general population dropped over time, while the prevalence among children with ASD remained relatively constant. The gap between the cohorts was thus largest during the late enrollment period, and given the observed trend it is not unlikely that the gap would continue to grow beyond the studied period. Investigating these temporal profiles is important as regressive forms of ASD are not uncommon (Gadow, Perlman, & Weber, 2017; Ozonoff et al., 2018), and thus patterns in co-occurring conditions may change throughout childhood development. In our case, the patterns in GI symptom occurrence stayed relatively constant in the ASD cohort from 3 to 60 months past the index date, but this represented a significant deviation from the rate of occurrence in the POP cohort. Additionally, our finding that the proportion of children presenting with five or more GI episodes during the late enrollment period was considerably higher in the ASD cohort than in the POP cohort may be indicative of a subpopulation of these children that are pre-disposed to experiencing these types of symptoms more frequently (Doshi-Velez et al., 2014).
Differences in oral antibiotic use were not substantial between the cohorts at any particular time during the five-year enrollment period. During the early enrollment period, we found that children with ASD had 5.20 antibiotic fills on average, compared to 4.75 in the POP cohort (i.e. a 9% increase in ASD) and in the late enrollment period, the mean number of fills for children with ASD was 28% higher compared to the POP cohort; this equates to a 15% greater number of fills in the ASD cohort over the full five-year period. Although there is a difference in means, the large standard deviations associated with these estimates obscure any meaningful difference in antibiotic use. This is in stark contrast to the findings of several previous studies that reported antibiotic use before age two to be approximately twice as high in children with ASD (House et al., 2016; Niehus & Lord, 2006; J. B. Adams et al., 2007), perhaps due to differences in reporting methods between the studies. We also found that children reporting a GI symptom during late enrollment were more likely to have taken greater numbers of oral antibiotics earlier on than those children not reporting a late GI symptom (regardless of ASD or POP status), both in terms of the total number of fills as well as the prevalence of antibiotic use over time. This by itself is suggestive of at least some degree of association between early oral antibiotic use and later-reported GI symptoms. It is worth noting that the OR for having a GI symptom claim in the late enrollment period given an oral antibiotic fill during early enrollment was nearly identical in the ASD and POP cohorts, implying that the risk of later GI symptoms presented by early oral antibiotic use is similar regardless of whether or not a child has ASD. Rates of antibiotic prescription for infections were also in general agreement with the prevalence of common infections in young children, particularly for otitis media and upper respiratory infections (Alter et al. 2011; Niehus and Lord 2006). Possible explanations for the approximately 30% of prescriptions unaccounted for may include antibiotics being prescribed for bacterial infections not encompassed by our five categories, for viral infections (inappropriately), or during scheduled check-ups in which an infection was not explicitly indicated by a diagnosis code.
Our analysis with Cox regression indicated that more frequent oral antibiotic use during early enrollment significantly increased the hazard rate of presenting with a GI symptom during the late enrollment period for children in both the ASD and POP cohorts. This result supports the notion that oral antibiotic use during early childhood may contribute to long-term GI disturbances by perhaps altering the composition of the gut microbiome; furthermore, the similarity of HR estimates obtained when the ASD and POP cohorts were modeled separately suggests that this effect is not exclusive to children with ASD. The effects of gender and race/ethnicity on late GI risk generally support the results put forth by other studies (Bhopal et al., 2014; Cain et al., 2009; Huerta-Franco et al., 2018), although our estimated effects are generally not as significant. Our finding that greater numbers of antibiotic fills during late enrollment increased the hazard rate of having a GI-related diagnosis is not unexpected as oral antibiotics can also have short-term effects on the gut microbiome that may lead to the presentation of GI symptoms. Another interesting finding from our analysis was that the hazard rate of being diagnosed with a GI symptom during the late enrollment period was significantly increased by having a diagnosed GI symptom during the early enrollment period, again suggesting a possible pre-disposition to recurring GI symptoms for a subset of children.
The nearly doubled hazard rate in children with ASD compared to children in the POP cohort closely reflects the prevalence of diagnosed GI symptoms during the late enrollment period, which was almost twice as high in the ASD cohort. Combined with the finding that the risk for later GI symptoms posed by early oral antibiotic use was nearly equal in children with and without ASD (as indicated by the similar HR estimates when the ASD and POP cohorts were modeled separately), this suggests that children with ASD have some predisposition to experiencing GI symptoms that cannot be explained by discrepancies in oral antibiotic use alone. Long-term disorders associated with the immune system may affect (or interact with) gut microbiome homeostasis (Cerf-Bensussan and Gaboriau-Routhiau 2010; Chadwick et al. 2002; Hooper et al. 2012; Vuong and Hsiao 2017), and evidence points to children with ASD being more frequently affected by immune-related conditions than typically developing children (Chen et al. 2013; Kotey et al. 2014; Zerbo et al. 2015); the presence of such disorders could influence observed patterns in GI disturbances and perhaps those of other conditions that co-occur with ASD. It would thus be of interest to study diagnosis patterns for other conditions that co-occur with ASD and how they may be associated with the presentation of GI symptoms.
There are a few points regarding the Cox regression analysis that are worth discussing. For one, the use of the 10:1 matching scheme for the POP cohort, although necessary for computational purposes, reduced the number of potential included samples by nearly 90%; thus only a small portion of the POP cohort was captured in the analysis and the model estimates may not have been as precise as they could have been with all samples included. Secondly, for most covariates the unadjusted HRs did not differ greatly from the adjusted estimates, suggesting minimal confounding among these variables. The estimates for number of early oral antibiotic fills and possibly the diagnosis of a GI episode during early enrollment indicated the most notable effects of confounding, which is not unexpected given that oral antibiotic use may be associated with short-term GI disturbances. Finally, we chose not to include a term for interaction between ASD cohort membership and early oral antibiotic use in the model because the initial univariate analysis did not indicate any considerable interaction between these variables, and inclusion of this interaction term in the regression did not change the estimated HRs by a substantial margin.
Our study has several strengths. For one, its large and diverse sample of children allows a generalized assessment of oral antibiotic use and GI symptoms in children with ASD, at least those privately insured, in the United States. Using an extended period of coverage enrollment for a large number of study participants makes it possible to perform an in-depth analysis of temporal trends in the factors contributing to the pathophysiology of ASD and its related conditions. This is one of few studies that directly investigate the association between oral antibiotic use and GI symptoms in the same sample of children. Furthermore, the use of claims data eliminates the possibility for recall bias that commonly influences the results of studies using questionnaires/surveys to evaluate the prevalence of GI symptoms in children with ASD.
Despite the many strengths of our study, there are also several limitations inherent to the nature of the data that may affect interpretation of the results. Claims for GI-related diagnoses are possibly under-reported in the claims data, which would result in lower estimates of the prevalence of GI complaints in both study cohorts. With pharmacy claims, we cannot be certain that a prescription filled for a certain period was taken for the entirety of that period, or even taken at all; therefore we can only make conclusions based on what quantities of oral antibiotics the children are believed to have taken. Similarly, a claim containing a GI diagnosis does not necessarily indicate the actual presence of a GI symptom, but rather that the symptom is believed to be present; in this case, doctor’s notes could be a valuable resource for ascertaining the presence of these symptoms in studies of smaller scale. Since only year of birth is given for each individual in the data (rather than the exact date of birth), we are unable to realize the ideal scenario of exactly tracking medical histories over the first five years of life. Instead we must track histories after the first date of coverage enrollment, which introduces the potential for significant error in the interpretation of temporal trends. Although the minimum possible difference between the date of birth and index date is zero days, the maximum possible difference (albeit improbable) is two years minus one day even with our inclusion criterion that the index date fall within one year of an individual’s year of birth. We are also unable to make any conclusions of causation and are instead limited to those of a correlative nature.
Several other limitations are introduced by various aspects of our study design. Since we did not adjust for the number of outpatient visits in our analysis, some observed differences between the ASD and POP cohorts may be influenced by ascertainment bias. With regards to this point, it is important to highlight that GI symptoms were more prevalent in children with ASD even during the earliest parts of enrollment when these children had not yet been diagnosed with ASD. This suggests that the increased reporting of GI symptoms in children with ASD cannot be explained solely by more frequent medical observation, and that there may be some actual pathophysiological abnormalities contributing to the higher prevalence. Also, like any retrospective study, this work can only make use of the information available in the data as it is not possible to determine the ground truth. It is possible that the percentage of children with a diagnosis of GI symptoms may not exactly match the percentage of children with GI symptoms in the population. Another limitation arises from allowing an ASD diagnosis to be made at any time during the study period as long as it is made at least two years after the index date; this introduces bias towards identifying individuals with earlier index dates as they are given a longer period of time in which ASD diagnoses may be found. Additionally, when counting incidences of GI symptoms, we used a uniform cut-off of 10 days for determining unique episodes, even though symptoms for particular conditions may actually last for fewer than or more than 10 days. It is also worth mentioning that clinical variables such as maternal antibiotic use during pregnancy and the particular class of oral antibiotic taken by a child may each influence the composition of the gut microbiome, but these were beyond the scope of the current study. Familial socioeconomic variables (e.g. maternal/paternal education or ages at birth) may also serve as confounders in the association between oral antibiotic use and GI symptoms, though these factors were not explored as the information was not explicitly defined for a large number of the records used in this study.
Acknowledgements
The authors gratefully acknowledge partial financial support from the National Institutes of Health (Grant 1R01AI110642–01A1). Additional support for this research was received from the Rensselaer Institute for Data Exploration and Applications. The authors express their gratitude to John Erickson at Rensselaer Polytechnic Institute for supporting the interactions with OptumLabs, and to the staff at OptumLabs for supporting the study design.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Adams DJ, Susi A, Erdie-Lalena CR, Gorman G, Hisle-Gorman E, Rajnik M, … Nylund CM (2016). Otitis media and related complications among children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 46(5), 1636–1642. 10.1007/sl0803-015-2689-x [DOI] [PubMed] [Google Scholar]
- Adams JB, Johansen LJ, Powell LD, Quig D, & Rubin RA (2011). Gastrointestinal flora and gastrointestinal status in children with autism – comparisons to typical children and correlation with autism severity. BMC Gastroenterology, 11, 22 10.1186/1471-230X-11-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JB, Romdalvik J, Ramanujam VMS, & Legator MS (2007). Mercury, lead, and zinc in baby teeth of children with autism versus controls. Journal of Toxicology and Environmental Health, Part A, 70(12), 1046–1051. 10.1080/15287390601172080 [DOI] [PubMed] [Google Scholar]
- Aldinger KA, Lane CJ, Veenstra-VanderWeele J, & Levitt P (2015). Patterns of risk for multiple co-occurring medical conditions replicate across distinct cohorts of children with autism spectrum disorder. Autism Research, 5(6), 771–781. 10.1002/aur.1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter SJ, Vidwan NK, Sobande PO, Omoloja A, & Bennett JS (2011). Common childhood bacterial infections. Current Problems in Pediatric and Adolescent Health Care, 41(10), 256–283. doi: 10.1016/j.cppeds.2011.06.001 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Washington, D.C.: American Psychiatric Publishing. [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2002 Principal Investigators, & Centers for Disease Control and Prevention. (2007). Prevalence of autism spectrum disorders--autism and developmental disabilities monitoring network, 14 sites, United States, 2002. Morbidity and Mortality Weekly Report. Surveillance Summaries; (Washington, D.C.: 2002), 56(1), 12–28. [PubMed] [Google Scholar]
- Babinská K, Bucová M, Ďurmanová V, Lakatošová S, Jánošiková D, Bakoš J, … Ostatníková D (2014). Increased plasma levels of the high mobility group box 1 protein (HMGB1) are associated with a higher score of gastrointestinal dysfunction in individuals with autism. Physiological Research, 63 Suppl 4, S613–618. [DOI] [PubMed] [Google Scholar]
- Bhopal RS, Cezard G, Bansal N, Ward HJT, & Bhala N (2014). Ethnic variations in five lower gastrointestinal diseases: Scottish Health and Ethnicity Linkage Study. BMJ Open, 4(10), e006120 10.1136/bmjopen-2014-006120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JP, Jain A, Yang W, Kelly JP, Kaiser M, Becker L, … Newschaffer CJ (2013). Does a claims diagnosis of autism mean a true case? Autism, 18(3), 321–330. 10.1177/1362361312467709 [DOI] [PubMed] [Google Scholar]
- Cain KC, Jarrett ME, Burr RL, Rosen S, Hertig VL, & Heitkemper MM (2009). Gender differences in gastrointestinal, psychological, and somatic symptoms in irritable bowel syndrome. Digestive Diseases and Sciences, 54(7), 1542 10.1007/s10620-008-0516-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N, & Gaboriau-Routhiau V (2010). The immune system and the gut microbiota: friends or foes? Nature Reviews Immunology, 10(10), 735–744. 10.1038/nri2850 [DOI] [PubMed] [Google Scholar]
- Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, & Wilson I (2002). Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology, 122(7), 1778–1783. 10.1053/gast.2002.33579 [DOI] [PubMed] [Google Scholar]
- Chaidez V, Hansen RL, & Hertz-Picciotto I (2014). Gastrointestinal problems in children with autism, developmental delays or typical development. Journal of Autism and Developmental Disorders, 44(5), 1117–1127. 10.1007/s10803-013-1973-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M-H, Su T-P, Chen Y-S, Hsu J-W, Huang K-L, Chang W-H, et al. (2013). Comorbidity of allergic and autoimmune diseases in patients with autism spectrum disorder: A nationwide population-based study. Research in Autism Spectrum Disorders, 7(2), 205–212. doi: 10.1016/j.rasd.2012.08.008 [DOI] [Google Scholar]
- Christensen DL, Baio J, Braun KVN, Bilder D, Charles J, Constantino JN, … Yeargin-Allsopp M (2016). Prevalence and characteristics of autism spectrum disorder among children aged 8 years — Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. Morbidity and Mortality Weekly Report, 65(3), 1–23. 10.15585/mmwr.ss6503a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman KJ, Lutsky MA, Yau V, Qian Y, Pomichowski ME, Crawford PM, … Croen LA (2015). Validation of autism spectrum disorder diagnoses in large healthcare systems with electronic medical records. Journal of Autism and Developmental Disorders, 45(7), 1989–1996. 10.1007/s10803-015-2358-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, & Dinan TG (2012). Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience, 13(10), 701–712. 10.1038/nrn3346 [DOI] [PubMed] [Google Scholar]
- Doshi-Velez F, Ge Y, & Kohane I (2014). Comorbidity clusters in autism spectrum disorders: an electronic health record time-series analysis. Pediatrics, 133(1), e54–e63. 10.1542/peds.2013-0819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, … Green JA III (2010). Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe, 16(4), 444–453. https://doi.Org/10.1016/j.anaerobe.2010.06.008 [DOI] [PubMed] [Google Scholar]
- Fjalstad JW, Esaiassen E, Juvet LK, van den Anker JN, & Klingenberg C (2017). Antibiotic therapy in neonates and impact on gut microbiota and antibiotic resistance development: a systematic review. Journal of Antimicrobial Chemotherapy. 10.1093/jac/dkx426 [DOI] [PubMed] [Google Scholar]
- Gadow KD, Perlman G, & Weber RJ (2017). Parent-reported developmental regression in autism: epilepsy, IQ, schizophrenia spectrum symptoms, and special education. Journal of Autism and Developmental Disorders, 47(4), 918–926. 10.1007/s10803-016-3004-1 [DOI] [PubMed] [Google Scholar]
- Holingue C, Newill C, Lee L-C, Pasricha PJ, & Daniele Fallin M (2018). Gastrointestinal symptoms in autism spectrum disorder: A review of the literature on ascertainment and prevalence. Autism Research, 11(1), 24–36. 10.1002/aur.1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, & Macpherson AJ (2012). Interactions between the microbiota and the immune system. Science, 336(6086), 1268–1273. 10.1126/science.1223490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- House SA, Goodman DC, Weinstein SJ, Chang C-H, Wasserman JR, & Morden NE (2016). Prescription use among children with autism spectrum disorders in northern New England: intensity and small area variation. The Journal of Pediatrics, 169, 277–283.e2. 10.1016/j.jpeds.2015.10.027 [DOI] [PubMed] [Google Scholar]
- Huerta-Franco M-R, Banderas JW, & Allsworth JE (2018). Ethnic/racial differences in gastrointestinal symptoms and diagnosis associated with the risk of Helicobacter pylori infection in the US. Clinical and Experimental Gastroenterology, 11, 39–49. 10.2147/CEG.S144967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Matson JL, Cervantes PE, Matheis M, & Bums CO (2017). Gastrointestinal issues in infants and children with autism and developmental delays. Journal of Developmental and Physical Disabilities, 29(3), 407–417. 10.1007/sl0882-017-9532-6 [DOI] [Google Scholar]
- Kang D-W, Ilhan ZE, Isern NG, Hoyt DW, Howsmon DP, Shaffer M, … Krajmalnik-Brown R (2018). Differences in fecal microbial metabolites and microbiota of children with autism spectrum disorders. Anaerobe, 49, 121–131. https://doi.Org/10.1016/j.anaerobe.2017.12.007 [DOI] [PubMed] [Google Scholar]
- Kang D-W, Park JG, Ilhan ZE, Wallstrom G, LaBaer J, Adams JB, & Krajmalnik-Brown R (2013). Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLOS ONE, 8(7), e68322 10.1371/journal.pone.0068322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohane IS, McMurry A, Weber G, MacFadden D, Rappaport L, Kunkel L, … Churchill S (2012). The co-morbidity burden of children and young adults with autism spectrum disorders. PLOS ONE, 7(4), e33224 10.1371/journal.pone.0033224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotey S, Ertel K, & Whitcomb B (2014). Co-occurrence of autism and asthma in a nationally-representative sample of children in the United States. Journal of Autism and Developmental Disorders, 44(12), 3083–3088. doi: 10.1007/s10803-014-2174-y [DOI] [PubMed] [Google Scholar]
- Krajmalnik-Brown R, Lozupone C, Kang D-W, & Adams JB (2015). Gut bacteria in children with autism spectrum disorders: challenges and promise of studying how a complex community influences a complex disease. Microbial Ecology in Health and Disease, 26(0). 10.3402/mehd.v26.26914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhanon BO, McCracken C, Karpen S, & Sharp WG (2014). Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics, 133(5), 872–883. 10.1542/peds.2013-3995 [DOI] [PubMed] [Google Scholar]
- Muskens JB, Velders FP, & Staal WG (2017). Medical comorbidities in children and adolescents with autism spectrum disorders and attention deficit hyperactivity disorders: a systematic review. European Child & Adolescent Psychiatry, 26(9), 1093–1103. 10.1007/s00787-017-1020-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehus R, & Lord C (2006). Early medical history of children with autism spectrum disorders. Journal of Developmental and Behavioral Pediatrics, 27(2 Suppl), S120–127. [DOI] [PubMed] [Google Scholar]
- OptumLabs. (2018). OptumLabs and OptumLabs Data Warehouse (OLDW) Descriptions and Citation. Cambridge, MA: n.p. PDF. Reproduced with permission from OptumLabs. [Google Scholar]
- Ozonoff S, Gangi D, Hanzel EP, Hill A, Hill MM, Miller M, … Iosif A-M (2018). Onset patterns in autism: Variation across informants, methods, and timing. Autism Research: Official Journal of the International Society for Autism Research. 10.1002/aur.1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Landa RJ, Brian J, Bryson S, Charman T, … Iosif A-M (2015). Diagnostic stability in young children at risk for autism spectrum disorder: a baby siblings research consortium study. Journal of Child Psychology and Psychiatry, 56(9), 988–998. 10.1111/jcpp.12421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parracho ΗM, Bingham MO, Gibson GR, & McCartney AL (2005). Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. Journal of Medical Microbiology, 54(10), 987–991. https://doi.Org/10.1099/jmm.0.46101-0 [DOI] [PubMed] [Google Scholar]
- Soke GN, Maenner MJ, Christensen D, Kurzius-Spencer M, & Schieve LA (2018). Prevalence of co-occurring medical and behavioral conditions/symptoms among 4- and 8-year-old children with autism spectrum disorder in selected areas of the United States in 2010. Journal of Autism and Developmental Disorders, 1–14. 10.1007/s10803-018-3521-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JS, Zheng LJ, Rowehl LM, Tian X, Zhang Y, Zhu W, … Li E (2015). Comparison of fecal microbiota in children with autism spectrum disorders and neurotypical siblings in the Simons Simplex Collection. PLOS ONE, 10(10), e0137725 10.1371/journal.pone.0137725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, & Ostatnikova D (2015). Gastrointestinal microbiota in children with autism in Slovakia. Physiology & Behavior, 138, 179–187. https://doi.Org/10.1016/j.physbeh.2014.10.033 [DOI] [PubMed] [Google Scholar]
- Vuong ΗE, & Hsiao EY (2017). Emerging roles for the gut microbiome in autism spectrum disorder. Biological Psychiatry, 81(5), 411–423. https://doi.Org/10.1016/j.biopsych.2016.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O, Leong A, Barcellos L, Bernal P, Fireman B, & Croen LA (2015). Immune mediated conditions in autism spectrum disorders. Brain, Behavior, and Immunity, 46, 232–236. doi: 10.1016/j.bbi.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]