Abstract
There has been a recent call for longitudinal imaging studies to better characterize the time course of physiological recovery following sport‐related concussion (SRC) and its relationship with clinical recovery. To address this, we evaluated changes to resting‐state functional connectivity (rs‐FC) of the whole‐brain network following SRC and explored associations between rs‐FC and measures of clinical outcome. High school and collegiate football athletes were enrolled during preseason. Athletes that suffered SRC (N = 62) were assessed across the acute (within 48 hr) and sub‐acute (days 8, 15, and 45) phases. Matched football athletes without concussion served as controls (N = 60) and participated in similar visits. Multi‐band resting‐state fMRI was used to assess whole‐brain rs‐FC at each visit using network‐based statistic and average nodal strength from regions of interest defined using a common whole‐brain parcellation. Concussed athletes had elevated symptoms, psychological distress, and oculomotor, balance, and memory deficits at 48 hr postconcussion relative to controls, with diminished yet significant elevations in symptoms and psychological distress at 8 days. Both rs‐FC analyses showed that concussed athletes had a global increase in connectivity at 8 days postconcussion relative to controls, with no differences at the 48‐hr, 15‐day, or 45‐day visits. Further analysis revealed the group effect at the 8‐day visit was driven by the large minority of concussed athletes still symptomatic at their visit; asymptomatic concussed athletes did not differ from controls. Findings from this large‐scale, prospective study suggest whole‐brain rs‐FC alterations following SRC are delayed in onset but associated with the presence of self‐reported symptoms.
Keywords: athletes, functional MRI, mild traumatic brain injury, postconcussion symptoms
1. INTRODUCTION
Mild traumatic brain injury (mTBI), which includes sport‐related concussion (SRC), is estimated to impact 1.6–3.8 million Americans every year (Langlois, Rutland‐Brown, & Wald, 2006). Presently, injury diagnosis and decisions regarding clinical recovery following SRC are determined using a multi‐pronged clinical evaluation by trained medical professionals based on detailed history, neurological exam, patient‐reported symptoms, and sometimes additional clinical tests (McCrory et al., 2017). Recent evidence has raised the concerning possibility that the time course of physiological recovery may exceed clinical recovery, possibly resulting in premature return‐to‐play (RTP) decisions that could place athletes at increased risk for subsequent, more severe injuries (Kamins et al., 2017). As highlighted in recent systematic reviews, however, the majority of studies to date have used cross‐sectional designs and/or included relatively limited samples (Kamins et al., 2017; McCrea et al., 2017). Thus, there has been a call for large‐scale, longitudinal studies to characterize the time course of pathophysiological recovery and its relationship with clinical recovery following SRC.
Resting‐state functional magnetic resonance imaging (rs‐fMRI) offers the potential to detect subtle physiological changes not detected by conventional clinical testing and/or conventional neuroimaging. Several studies have investigated the sensitivity of resting‐state functional connectivity (rs‐FC), the most common rs‐fMRI technique, to mTBI and SRC during the acute and sub‐acute phase of the injury (Mayer, Bellgowan, & Hanlon, 2015; McCrea et al., 2017). Yet, few studies have evaluated the effects of SRC on rs‐FC longitudinally across the acute (i.e., days) and sub‐acute phases (i.e., weeks), the period in which clinical recovery is observed in most athletes (Churchill et al., 2017; Manning et al., 2017; Meier, Bellgowan, & Mayer, 2017; Zhu et al., 2015). The majority of these studies have focused on specific resting‐state networks or seed‐regions or have included a relatively limited number of injured athletes.
The present study prospectively evaluated the effects of SRC on rs‐FC across multiple time points in the acute (within 48 hr) and sub‐acute phase (days 8, 15, and 45) in high school and college football athletes. Healthy, demographically matched football athletes served as controls and participated in equivalent evaluations at matched time intervals. A whole‐brain network approach was used for rs‐FC analysis to avoid limitations inherently associated with predefining specific regions or networks of interest (e.g., assumption of spatially localized effects). Specifically, we focused on a measure of connectivity strength across the entire network (i.e., average nodal strength) as a simple but fundamental global property that would conceivably be affected by SRC. This hypothesis was based on the known effects of brain injury on central, highly connected, brain structures (e.g., subcortical regions and midbrain), as well as the diffuse nature of SRC on white matter (Bigler & Maxwell, 2012). Moreover, recent work has demonstrated that other global graph theory metrics are biased when groups differ in global connectivity strength, supporting the notion that strength is a fundamental graph property (Hallquist & Hillary, 2018; van den Heuvel et al., 2017). In addition, we also used a nonparametric network analytic method, network‐based statistic, that is sensitive to global effects as well as effects that may be more localized to specific sub‐networks. We hypothesized that concussed athletes would have altered rs‐FC at the acute and early sub‐acute phase, with recovery toward healthy control levels by 45 days postinjury. Furthermore, given the relatively large sample size, subsequent analyses were conducted to determine if observed rs‐FC alterations were present in all concussed athletes or only in those reporting delayed symptom recovery following concussion. Finally, we tested the hypothesis that observed differences in rs‐FC would be associated with clinical measures of injury severity.
2. METHODS
2.1. Participants
High school and collegiate football athletes were recruited following the approval of our institutional review board. The criteria for exclusion from the study were as follows: any contraindication or injury that would prevent participation in the study protocol, current psychotic disorder or narcotic use, or history or suspicion of a clinical condition known to be associated with cognitive impairments (e.g., epilepsy, moderate‐to‐severe TBI). All adult study participants provided written informed consent. Minor participants provided assent in writing, while their parents provided written informed consent.
A total of 857 football athletes completed preseason baseline clinical assessments upon study enrollment prior to the football season. MRI was not included in the baseline visit. A total of 62 athletes sustained concussion and completed clinical assessments and MRI scanning sessions with resting‐state fMRI at 48 hr (32.47 ± 14.19 hr), 8 days (8.20 ± 0.98 days), 15 days (15.42 ± 1.35 days), and 45 days (45.56 ± 3.77 days) postconcussion. The initial identification and clinical diagnosis of concussion were determined by certified athletic trainers and/or team physicians trained in sports medicine at each institution; study investigators screened and triaged all injuries to ensure compliance with the study definition and requirements. The definition of concussion was derived from the Centers for Disease Control and Prevention (CDC) HEADS UP educational initiative: “An injury resulting from a forceful bump, blow, or jolt to the head that results in rapid movement of the head and causes a change in the athlete's behavior, thinking, physical functioning, or the following symptoms: headache, nausea, vomiting, dizziness/balance problems, fatigue, difficulty sleeping, drowsiness, sensitivity to light/noise, blurred vision, memory difficulty, and difficulty concentrating”.
In addition, 60 healthy football athletes matched on demographics and baseline neurocognitive test performance were assessed at similar time points and served as the controls. The demographic details for each group are detailed in Table 1 and the sample size at each time point is detailed in Table 2.
Table 1.
Sample characteristics
| Demographics | Concussed athletes | Control athletes | Statistic |
|---|---|---|---|
| Total N | 62 | 60 | |
| Age at first scan | 18.90 (1.84) | 19.26 (1.70) | t = −1.12, p = .26 |
| Years education | 12.87 (1.60) | 13.20 (1.50) | t = −1.17, p = .24 |
| % college | 75% | 75.8% | X 2 = 0.01, p = .92 |
| WTAR (standard score) | 99.11 (15.24) | 100.25 (13.45) | t = −0.44, p = .66 |
| Years participation | 7.72 (3.12) | 8.03 (2.62) | t = −0.60, p = .55 |
| Median prior concussions [range] | 1 [0–6] | 0 [0–4] | U = 2,187.5, p = .071 |
| Height (inches) | 71.55 (2.96) | 70.83 (2.51) | t = 1.46, p = .15 |
| Weight (lbs) | 211.29 (46.13) | 207.10 (43.10) | t = 0.52, p = .60 |
| Race | X 2 = 2.01, p = .57 | ||
| American Indian or Alaska native (%) | 0 | 1.7 | |
| Black or African‐American (%) | 27.4 | 28.3 | |
| White (%) | 71 | 70 | |
| Unreported (%) | 1.6 | 0 | |
| Ethnicity | X 2 = 2.51, p = .47 | ||
| Hispanic (%) | 3.2 | 5 | |
| Non‐Hispanic (%) | 91.9 | 88.3 | |
| Unknown (%) | 0 | 3.3 | |
| Unreported (%) | 4.8 | 3.3 | |
| ADHD+ (%) | 8.1 | 6.7 | X 2 = 0.09, p = .77 |
| Injury characteristics | |||
| Median days of self‐reported symptoms | 7.0 | NA | |
| Median length return‐to‐play | 12.0 | NA | |
| LOC+ (%) | 4.8 | NA | |
| Median duration LOC (seconds) | 30 | ||
| PTA+ (%) | 14.5 | NA | |
| Median duration PTA (minutes) | 5.0 | ||
| RGA+ (%) | 1.6 | NA | |
| Median duration RGA (minutes) | 90 |
Shown are mean and standard deviation, unless otherwise indicated. WTAR = Wechsler Test of Adult Reading, ADHD = attention deficit hyperactivity disorder, LOC = loss of consciousness, PTA = posttraumatic amnesia, RGA = retrograde amnesia, NA = not applicable.
Table 2.
Available data and rs‐fMRI head motion metrics
| Visit data | Concussed athletes | Control athletes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BL | 48H | 8D | 15D | 45D | BL | 48H | 8D | 15D | 45D | |
| SCAT3 (n) | 62 | 61 | 58 | 54 | 50 | 59 | 60 | 54 | 52 | 49 |
| BESS (n) | 60 | 60 | 58 | 54 | 50 | 60 | 59 | 54 | 52 | 49 |
| BSI‐18 (n) | 62 | 60 | 58 | 55 | 53 | 59 | 60 | 55 | 52 | 49 |
| ImPACT memory (n) | 57 | 60 | 56 | 53 | 53 | 60 | 60 | 55 | 51 | 47 |
| ImPACT speed (n) | 57 | 60 | 56 | 53 | 53 | 60 | 60 | 55 | 51 | 47 |
| King‐Devick (n) | 17 | 26 | 23 | 22 | 22 | 19 | 27 | 23 | 20 | 26 |
| Available rs‐fMRI (n) | NA | 55 | 54 | 49 | 50 | NA | 57 | 55 | 52 | 48 |
| Rs‐fMRI removed due to motion/artifact (n) | NA | 4 | 3 | 4 | 5 | NA | 1 | 2 | 0 | 0 |
| Usable rs‐fMRI (n) | NA | 51 | 51 | 45 | 45 | NA | 56 | 52 | 52 | 48 |
| Average head motion | 0.08 (0.03) | 0.09 (0.03) | 0.08 (0.03) | 0.09 (0.03) | NA | 0.08 (0.03) | 0.08 (0.03) | 0.08 (0.03) | 0.08 (0.04) | |
| % of noncensored volumes | NA | 0.97 (0.05) | 0.97 (0.05) | 0.97 (0.04) | 0.96 (0.06) | NA | 0.97 (0.05) | 0.97 (0.05) | 0.97 (0.04) | 0.97 (0.05) |
Mean and standard deviation are shown for average head motion and % of noncensored volumes. SCAT3 = Sport Concussion Assessment Tool‐3; BESS = Balance Error Scoring System; BSI = Brief Symptom Inventory‐18; ImPACT = Immediate Postconcussion Assessment and Cognitive Testing; NA = not applicable; BL = baseline; 48H = 48‐hr visit; 8D = 8‐day visit; 15D = 15‐day visit; 45D = 45‐day visit.
2.2. Clinical battery
During the preseason assessment, each athlete provided demographic and health history information and completed a battery of questionnaires and neuropsychological tests. The core clinical battery included assessments of self‐report symptoms (Sport Concussion Assessment Tool‐3rd Edition symptom checklist; SCAT3 [McCrory et al., 2013]), balance deficits (Balance Error Scoring System; BESS [Riemann & Guskiewicz, 2000]), oculomotor deficits (King–Devick test [Oride, Marutani, Rouse, & DeLand, 1986]), psychological distress (Brief Symptom Inventory; BSI‐18 [Derogatis, 2001]), and neurocognitive performance (Immediate Postconcussion Assessment and Cognitive Testing battery; ImPACT [Covassin, Elbin 3rd, Stiller‐Ostrowski, & Kontos, 2009]). Composite ImPACT memory and speed scores were calculated (Schatz & Maerlender, 2013). Specifically, ImPACT verbal memory and visual memory scores were z‐transformed using means and standard deviations from the study's larger database of baseline ImPACT testing and averaged to create a composite ImPACT memory score. Reaction time and visual motor speed scores were similarly z‐transformed, reverse coded (reaction time only), and averaged to create a composite ImPACT speed score. The Wechsler Test of Adult Reading was administered during the preseason visit to estimate intellectual function (Wechsler, 2001). Information about concussion management and symptom recovery, including number of days symptomatic and length of return‐to‐play decision, was collected at follow‐up visits in addition to the repetition of the clinical assessment battery.
2.3. Imaging parameters and processing
Imaging data were obtained on a 3 Tesla General Electric MR750 whole‐body MR scanner using a brain‐dedicated 32‐channel receiver coil array optimized for parallel imaging. The study participants were scanned for approximately 6 min using a gradient‐echo echo‐planar image (EPI) sequence to collect 501 volumes of rs‐fMRI data, which had the following parameters: FOV = 210 mm, acquisition matrix = 104 × 104, slice thickness = 2 mm, 72 sagittal slices, TR/TE = 720/30 ms, flip angle = 50°, hyperband acceleration factor = 8. During the resting‐state scanning session, participants were instructed to keep their eyes open and think of nothing in particular. A brief, reverse phase‐encoded scan with the same parameters was collected to allow susceptibility‐induced distortion correction. High‐resolution T1‐weighted structural images were obtained for anatomical reference using a magnetization‐prepared rapid gradient‐echo sequence with the following parameters: FOV = 256 mm, acquisition matrix = 256, slice thickness = 1 mm, 160 slices, TR/TE/TI = 7.592/3.008/900 ms, flip angle = 8°.
MRI data preprocessing was carried out using Analysis of Functional NeuroImages programs (AFNI) unless otherwise noted (Cox, 1996). T1‐weighted anatomical images were skull‐stripped in native space using a union mask of segmented gray matter and white matter from SPM 12. The skull‐stripped brain was registered to the MNI‐152 skull‐stripped template using an affine registration with correlation ratio cost function and trilinear interpolation followed by a nonlinear warp, implemented in FSL (Jenkinson, Bannister, Brady, & Smith, 2002). The first 29 volumes of the resting‐state scan were removed to account for auto‐calibration data and allow for stabilization of longitudinal magnetization. Signal spike artifacts were removed from the time series data sets by interpolation of data from neighboring time points using 3dDespike. Susceptibility‐induced distortion correction was performed using FSL topup (Andersson, Skare, & Ashburner, 2003; Smith et al., 2004). Volumes were registered to the first volume using cubic polynomial interpolation to account for head motion. For spatial normalization, a single transformation matrix was created by concatenating the T1‐weighted to MNI‐152 matrix and the matrix resulting from a 6°‐of‐freedom registration of the first EPI volume to the T1‐weighted scan calculated using FSL's FLIRT with the boundary‐based registration cost‐function (Greve & Fischl, 2009; Jenkinson et al., 2002). The resulting matrix and the nonlinear warp from the T1‐weighted to MNI‐152 brain were applied to the motion corrected image to bring the image in standard space with 2 mm isotropic resolution.
Signals of no‐interest (a total of 18) were regressed from the spatially normalized EPI and included average CSF signal, average white matter signal, the six motion parameters and their derivatives, and the zero‐ through third‐order polynomial trends in all subjects. Average white matter and CSF signals were obtained from MNI‐152 tissue priors available in FSL. Volumes with excessive head motion, defined as Euclidian norm of the six motion parameters >0.30, were censored along with the preceding volume and replaced using interpolation. Finally, a bandpass filter of 0.01 to 0.10 Hz was applied. Averaging voxels within regions of interest (ROI; see below) is effectively spatial smoothing; thus, no additional spatial smoothing was performed. A combination of manual and automated quality assessment was performed for all data. Resting‐state data at multiple processing stages were visually inspected for image artifacts. Resting‐state scans with visually identified artifacts and scans in which the average Euclidian norm of motion parameters was greater than 0.2 were excluded from analyses to minimize potential effects of motion on group analyses. The sample size with usable data at each time point is in Table 2.
2.4. Region of interest selection and extraction
ROIs were identified using the Craddock whole‐brain functional parcellation atlas (Craddock, James, Holtzheimer 3rd, Hu, & Mayberg, 2012). The 200 Craddock ROI atlas using the r t 2‐level parcellation was selected as a compromise between interpretability and anatomical specificity. The AFNI program 3dNetCorr was used to extract the average time course from each ROI and calculate Pearson correlation coefficients between each ROI pair (Taylor & Saad, 2013). The connectivity matrices for each subject at each time point were used as input for network analyses described below. Quality assurance measures ensured that each subject had time series data from at least 20 voxels for every ROI in the atlas.
Correlation matrices based on r values generated for every subject were utilized in the calculation of nodal strength using BRAPH (BRain analysis using GraPH theory; Mijalkov et al., 2017) with the calculation of network metrics based on Brain Connectivity Toolbox (https://sites.google.com/site/bctnet/)). Nodal strength was defined as the sum of weights of all edges (i.e., connections) of a given ROI (Rubinov & Sporns, 2010). Nodal strength was calculated from undirected weighted matrices for each node and average nodal strength was obtained by averaging strength across all nodes for each individual at each time point. Only positive values were used in the calculation of nodal strength (i.e., negative correlations were set to zero).
2.5. Statistical analysis
Statistical analyses were performed using IBM SPSS version 21 (Armonk, NY) and visualized using Prism 7.0 (La Jolla, CA) unless otherwise indicated. The focus of this work was to characterize group differences in clinical data and rs‐FC at each time point. Due to this focus, planned comparisons were conducted at each visit and between each visit without omnibus testing. Two‐tailed Welch's t‐tests, Mann–Whitney tests, and Chi‐square tests were used to assess group differences in demographic information. Two‐tailed Welch's t‐tests were used to assess group differences (i.e., concussed versus healthy) in clinical variables, scanner motion parameters, and graph theory metrics at each visit. Paired t‐tests were used to assess changes in these measures between visits in each group. Spearman correlations were conducted to investigate relationships between clinical variables and rs‐FC at visits in which rs‐FC significantly differed across groups. To account for multiple testing, the Benjamini–Hochberg method was used to control the false discovery rate (FDR) at 0.05 across all hypotheses of interest (i.e., all pairwise comparisons and correlations of rs‐FC and clinical data), implemented in R version 3.4.4. A significance level of p < .05 was used for demographic comparisons, uncorrected.
Finally, network‐based statistic (NBS) was used to identify significant differences in components of the unthresholded, undirected connectivity matrices for the Craddock atlas (Zalesky, Fornito, & Bullmore, 2010). NBS is a nonparametric test that controls for family‐wise error rate (FWER) at the network level using permutation testing. In this work, a primary threshold of t = 2.5 was used at the edge level and 10,000 permutations were used to identify significant clusters of supra‐threshold nodes (i.e., ROI) at a one‐sided FWER p < .025 (equivalent to p < .05 two‐tailed tests). Using this approach, independent samples t tests assessed group differences at each visit and paired t tests compared visits in each group. Due to the stringent FWER correction at the network level, no additional multiple comparison correction was performed for NBS analyses. Additional analyses using alternative edge‐level thresholds and an alternative atlas for defining ROI are reported in the Supporting Information.
3. RESULTS
3.1. Demographic and clinical data
There were no significant differences in demographic variables or any clinical measures at baseline between the concussed and nonconcussed athletes, confirming the detailed matching criteria used for this study (p > .05, Table 1). Concussed athletes had significantly worse performance on the BESS, King‐Devick, and ImPACT memory composite, as well as elevated BSI‐18 and SCAT3 symptom severity scores at the 48‐hr visit following FDR correction (Figure 1a; Table 3). All clinical measures in concussed athletes had returned to or surpassed their own baseline levels by 8 days postconcussion (Table 4). However, BSI‐18 and SCAT3 symptom severity scores were still significantly higher, on average, in concussed athletes than healthy athletes at the 8‐day visit following FDR correction. The median number of days for symptom recovery was 7 and days for clearance to return‐to‐play was 12.
Figure 1.
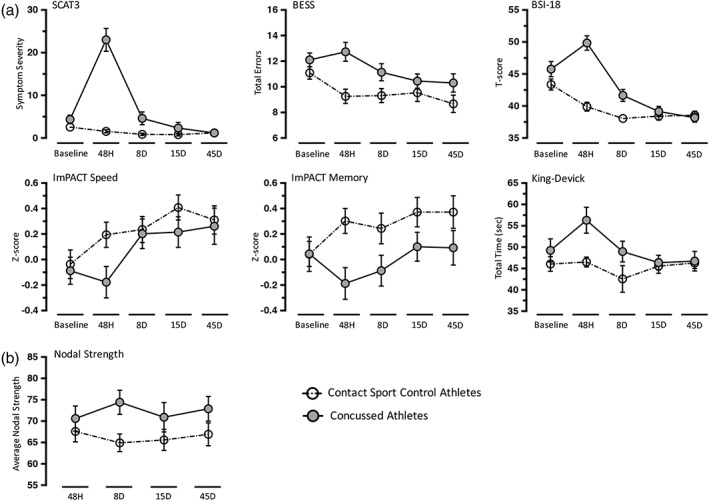
Clinical measures and average nodal strength. Shown are mean scores for core clinical measures (a) and average nodal strength (b) for concussed athletes as well as contact control athletes at baseline, 48 hr (48H), 8 days (8D), 15 days (15D), and 45 days (45D). Error bars represent standard error of the mean. SCAT3 = Sport Concussion Assessment Tool‐3; BESS = Balance Error Scoring System; BSI‐18 = Brief Symptom Inventory‐18 Global Severity Index; ImPACT = Immediate Postconcussion Assessment and Cognitive Testing
Table 3.
Cross‐sectional comparisons
| BL | 48H | 8D | 15D | 45D | ||
|---|---|---|---|---|---|---|
| Average nodal strength | t (p) [d] | NA | 0.79 (0.43) [0.15] | 2.72 (0.008) [0.54] | 1.26 (0.21) [0.26] | 1.52 (0.13) [0.32] |
| SCAT3 (symptom severity) | t (p) [d] | 1.54 (0.13) [0.28] | 7.89 (<0.001) [1.43] | 2.48 (0.02) [0.46] | 1.11 (0.27) [0.21] | −0.07 (0.94) [−0.01] |
| BESS (Total errors) | t (p) [d] | 1.40 (0.17) [0.26] | 3.76 (<0.001) [0.69] | 2.11 (0.04) [0.40] | 1.02 (0.31) [0.20] | 1.66 (0.10) [0.33] |
| BSI‐18 (GSI T‐score) | t (p) [d] | 1.64 (0.10) [0.30] | 7.52 (<0.001) [1.37] | 3.43 (0.001) [0.64] | 0.71 (0.48) [0.14] | −0.41 (0.68) [−0.08] |
| ImPACT memory (Z‐score) | t (p) [d] | 0.01 (0.99) [0.00] | −3.08 (0.003) [−0.56] | −1.94 (0.06) [−0.37] | −1.67 (0.10) [−0.33] | −1.51 (0.13) [−0.30] |
| ImPACT speed (Z‐score) | t (p) [d] | −0.34 (0.74) [−0.06] | −2.36 (0.02) [−0.43] | −0.22 (0.83) [−0.04] | −1.25 (0.22) [−0.24] | −0.28 (0.78) [−0.05] |
| King‐Devick (Total time) | t (p) [d] | 1.0 (0.33) [0.34] | 3.01 (0.005) [0.83] | 1.62 (0.11) [0.48] | 0.33 (0.74) [0.10] | 0.16 (0.87) [0.05] |
NA = not available; SCAT3 = Sport Concussion Assessment Tool‐3; BESS = Balance Error Scoring System; BSI‐18 GSI = Brief Symptom Inventory‐18 Global Severity Index; ImPACT = Immediate Postconcussion Assessment and Cognitive Testing; BL = baseline; 48H = 48‐hr visit; 8D = 8‐day visit; 15D = 15‐day visit; 45D = 45‐day visit. Bold indicates significant differences at p < .05 following FDR correction. Note that p = .01572 corresponded to FDR‐corrected 0.05.
Table 4.
Longitudinal comparisons
| BL‐48H | BL‐8D | BL‐15D | BL‐45D | 48H‐8D | 48H‐15D | 48H‐45D | 8D‐15D | 8D‐45D | 15D‐45D | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average nodal strength | CC | t (p) | NA | NA | NA | NA | 1.27 (0.21) | 0.10 (0.92) | −0.23 (0.82) | −0.39 (0.70) | −0.82 (0.42) | −0.14 (0.89) |
| [d] | NA | NA | NA | NA | [0.18] | [0.01] | [−0.03] | [−0.06] | [−0.13] | [−0.02] | ||
| SRC | t (p) | NA | NA | NA | NA | −1.44 (0.16) | −0.24 (0.81) | 0.25 (0.81) | 1.05 (0.30) | 0.62 (0.54) | −0.01 (0.99) | |
| [d] | NA | NA | NA | NA | [−0.22] | [−0.04] | [0.04] | [0.17] | [0.10] | [0.00] | ||
| SCAT3 (symptom severity) | CC | t (p) | 2.65 (0.01) | 3.26 (0.002) | 2.43 (0.02) | 2.01 (0.05) | 1.92 (0.06) | 1.58 (0.12) | 0.50 (0.62) | 0.25 (0.80) | −0.11 (0.91) | −0.47 (0.64) |
| [d] | [0.34] | [0.45] | [0.34] | [0.29] | [0.26] | [0.22] | [0.07] | [0.04] | [−0.02] | [−0.07] | ||
| SRC | t (p) | −6.72 (<0.001) | −0.13 (0.90) | 1.45 (0.15) | 2.88 (0.006) | 7.70 (<0.001) | 8.22 (<0.001) | 7.69 (<0.001) | 2.43 (0.02) | 2.71 (0.009) | 1.30 (0.20) | |
| [d] | [−0.86] | [−0.02] | [0.20] | [0.41] | [1.02] | [1.13] | [1.10] | [0.34] | [0.39] | [0.19] | ||
| BESS (Total errors) | CC | t (p) | 2.89 (0.005) | 2.76 (0.008) | 1.78 (0.08) | 2.94 (0.005) | 0.28 (0.78) | −0.45 (0.65) | 0.70 (0.49) | −0.48 (0.63) | 0.66 (0.51) | 1.02 (0.32) |
| [d] | [0.38] | [0.38] | [0.25] | [0.42] | [0.04] | [−0.06] | [0.10] | [−0.07] | [0.10] | [0.15] | ||
| SRC | t (p) | −0.79 (0.43) | 2.17 (0.04) | 3.07 (0.003) | 2.94 (0.005) | 2.28 (0.03) | 3.32 (0.002) | 3.27 (0.002) | 0.88 (0.38) | 1.52 (0.13) | 1.58 (0.12) | |
| [d] | [−0.10] | [0.29] | [0.43] | [0.42] | [0.30] | [0.46] | [0.47] | [0.12] | [0.22] | [0.23] | ||
| BSI‐18 (GSI T‐score) | CC | t (p) | 4.01 (<0.001) | 6.51 (<0.001) | 6.08 (<0.001) | 4.82 (<0.001) | 2.80 (0.007) | 2.78 (0.008) | 2.80 (0.007) | −0.25 (0.81) | 0.29 (0.78) | 0.14 (0.89) |
| [d] | [0.52] | [0.89] | [0.85] | [0.70] | [0.38] | [0.39] | [0.40] | [−0.04] | [0.04] | [0.02] | ||
| SRC | t (p) | −2.85 (0.006) | 2.99 (0.004) | 4.92 (<0.001) | 6.33 (<0.001) | 7.33 (<0.001) | 9.92 (<0.001) | 8.99 (<0.001) | 2.59 (0.01) | 3.93 (<0.001) | 1.97 (0.06) | |
| [d] | [−0.37] | [0.39] | [0.66] | [0.87] | [0.98] | [1.36] | [1.26] | [0.36] | [0.54] | [0.28] | ||
| ImPACT memory (Z‐score) | CC | t (p) | −2.14 (0.04) | −1.57 (0.12) | −2.58 (0.01) | −2.59 (0.01) | 0.66 (0.51) | 0.22 (0.82) | −0.66 (0.51) | 0.04 (0.97) | −1.46 (0.15) | −1.14 (0.26) |
| [d] | [−0.28] | [−0.21] | [−0.36] | [−0.38] | [0.09] | [0.03] | [−0.10] | [0.01] | [−0.22] | [−0.17] | ||
| SRC | t (p) | 1.75 (0.09) | 0.71 (0.48) | −0.95 (0.35) | −1.04 (0.30) | −0.62 (0.54) | −2.72 (0.009) | −2.50 (0.02) | −1.41 (0.16) | −1.52 (0.14) | 0.29 (0.77) | |
| [d] | [0.24] | [0.10] | [−0.14] | [−0.15] | [−0.08] | [−0.38] | [−0.35] | [−0.20] | [−0.21] | [0.04] | ||
| ImPACT speed (Z‐score) | CC | t (p) | −3.23 (0.002) | −3.54 (0.001) | −4.43 (<0.001) | −3.64 (0.001) | −0.82 (0.42) | −2.66 (0.01) | −0.59 (0.56) | −2.17 (0.04) | 0.55 (0.59) | 2.87 (0.006) |
| [d] | [−0.42] | [−0.48] | [−0.62] | [−0.53] | [−0.11] | [−0.37] | [−0.09] | [−0.31] | [0.08] | [0.44] | ||
| SRC | t (p) | 0.44 (0.67) | −1.90 (0.06) | −2.80 (0.007) | −2.18 (0.03) | −2.95 (0.005) | −2.71 (0.009) | −2.67 (0.01) | 0.56 (0.58) | −0.62 (0.54) | −0.97 (0.34) | |
| [d] | [0.06] | [−0.27] | [−0.40] | [−0.31] | [−0.39] | [−0.38] | [−0.37] | [0.08] | [−0.09] | [−0.14] | ||
| King‐Devick (Total time) | CC | t (p) | −1.14 (0.28) | 0.90 (0.40) | −1.66 (0.14) | −0.16 (0.87) | −0.27 (0.79) | 0.62 (0.54) | 2.21 (0.04) | 1.31 (0.21) | −1.21 (0.24) | −0.22 (0.83) |
| [d] | [−0.30] | [0.26] | [−0.59] | [−0.05] | [−0.06] | [0.15] | [0.48] | [0.32] | [−0.29] | [−0.05] | ||
| SRC | t (p) | −3.11 (0.008) | −1.16 (0.27) | 0.52 (0.62) | 1.53 (0.16) | 3.36 (0.003) | 4.17 (0.001) | 3.96 (0.001) | 2.53 (0.02) | 3.33 (0.004) | 2.17 (0.05) | |
| [d] | [−0.80] | [−0.35] | [0.16] | [0.48] | [0.72] | [0.93] | [0.89] | [0.58] | [0.76] | [0.53] |
SRC = athletes with sport‐related concussion; CC = contact controls; SCAT3 = Sport Concussion Assessment Tool‐3; BESS = Balance Error Scoring System; BSI‐18 GSI = Brief Symptom Inventory‐18 Global Severity Index; ImPACT = Immediate Postconcussion Assessment and Cognitive Testing; BL = baseline; 48H = 48‐hr visit; 8D = 8‐day visit; 15D = 15‐day visit; 45D = 45‐day visit. Bold indicates significant differences at p < .05 following FDR correction. Note that p < .01572 corresponded to FDR‐corrected 0.05.
3.2. Network metrics: whole‐group analysis
There were no significant differences between groups or visits in head motion or the percentage of uncensored volumes (p > .05; Table 2). Concussed athletes had significantly higher average nodal strength at 8 days postconcussion relative to controls following FDR correction (t[91.82] = 2.72, p = .008, d = 0.54; Figure 1b; Table 3). There were no differences between groups at 48 hr, 15 days, or 45 days postconcussion, and there were no significant differences in average nodal strength across visits within concussed or control athletes following FDR correction (p > .05; Table 4).
NBS also showed significantly stronger connections in concussed athletes relative to control athletes at 8 days postconcussion (Figure 2). A significant group difference was observed in a network consisting of 1,464 connections and 180 nodes (p = .008). There were no significant differences between groups at 48 hr, 15 days, or 45 days postconcussion. Similarly, there were no differences between any two visits in either concussed or control athletes.
Figure 2.
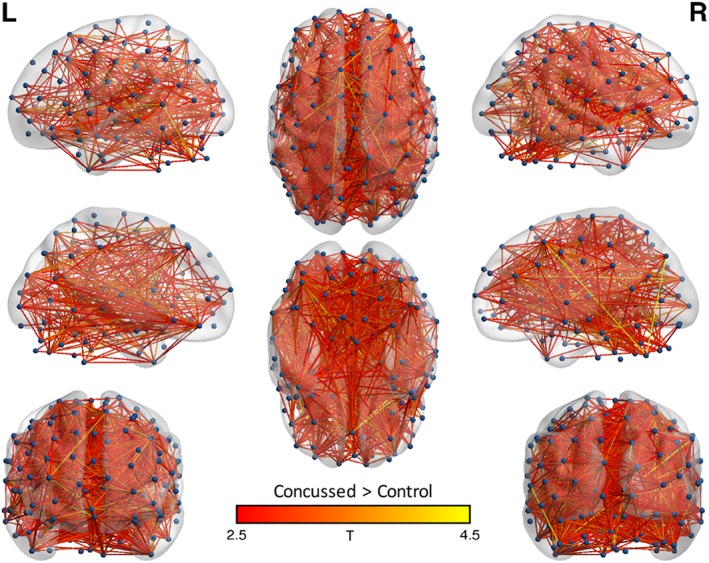
Network‐based statistic results: whole group analysis. Shown are the nodes (i.e., regions of interest) and edges (connections) of networks in which concussed athletes had significantly greater connectivity than controls at the 8‐day visit. Edges are colored based on t‐stat values. Nodes and edges are projected onto the MNI standard template brain using BrainNet Viewer. Nodes are displayed via a sphere at the center of mass for each ROI. L = left; R = right [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Network metrics: sub‐group analysis at 8 days
Additional analyses were performed to determine if rs‐FC abnormalities were present in both symptomatic (n = 20) and asymptomatic (n = 31) concussed athletes at 8 days postconcussion, based on the self‐reported duration of symptoms. Average nodal strength was significantly higher in symptomatic concussed athletes at 8 days postconcussion relative to controls following FDR correction (t[30.10] = 2.92, p = .007, d = 0.79). In contrast, average nodal strength in asymptomatic concussed athletes at 8 days postconcussion did not differ from controls (t[46.91] = 1.66, p = .10, d = 0.39).
Similarly, NBS found differences relative to controls only in symptomatic concussed athletes at 8 days postconcussion (Figure 3). Symptomatic concussed athletes had higher connectivity in a network consisting of 2,312 connections and 192 nodes (p = .001). Asymptomatic concussed athletes at 8 days postconcussion did not differ from controls.
Figure 3.
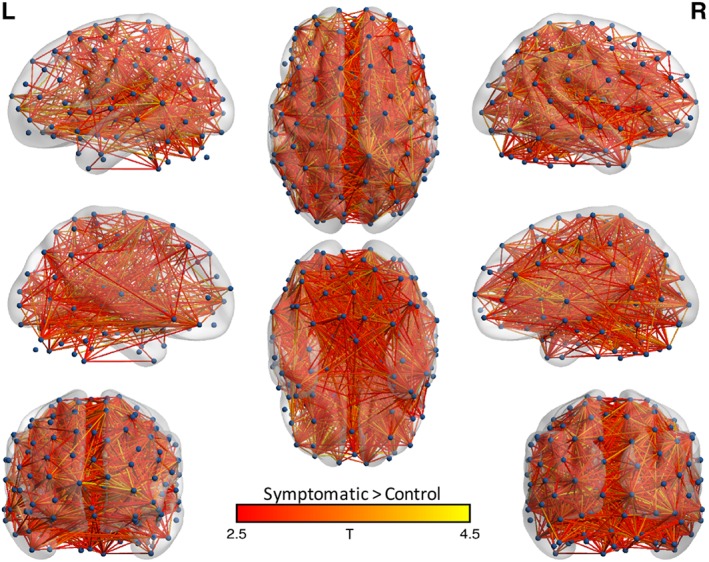
Network‐based statistic results: sub‐group analysis. Shown are the nodes (i.e., regions of interest) and edges (connections) of networks in which symptomatic concussed athletes had significantly greater connectivity than controls at the 8‐day visit. Edges are colored based on t‐stat values. Nodes and edges are projected onto the MNI standard template brain using BrainNet Viewer. Nodes are displayed via a sphere at the center of mass for each ROI. L = left; R = right [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Correlation with clinical metrics
Spearman correlations were conducted to determine if the significant elevations in average nodal strength at 8 days postconcussion were associated with clinical measures at the acute (48‐hr) or 8‐day visit. Change scores relative to baseline measures were used to account for baseline differences in each measure, when possible. No relationships survived FDR correction (See Supporting Information Table S2 for effect sizes).
4. DISCUSSION
To the best of our knowledge, this is the largest study to prospectively assess the effects of SRC on rs‐FC over multiple time points during the acute and the sub‐acute phase, addressing recent calls for prospective, longitudinal studies of the neurophysiological effects of SRC (Kamins et al., 2017). Clinical effects of concussion were most evident at the acute phase (48 hr), with more subtle group differences in postconcussive symptoms and psychological distress at 8 days postconcussion and no group differences at the 15‐ or 45‐day visits. In contrast, a global increase in rs‐FC strength was observed at 8 days postinjury relative to healthy football athletes, with no significant differences at 48 hr, 15 days, or 45 days postinjury. Importantly, sub‐group analyses focused on concussed athletes that were either symptomatic or asymptomatic at the 8‐day visit showed that rs‐FC effects were driven by still‐symptomatic athletes. Effects were observed using both network‐based statistic (NBS) and average nodal strength. These results suggest that the onset of the physiological effects of SRC may be delayed relative to clinical symptoms, but the recovery of rs‐FC abnormalities follows a trajectory similar to clinical recovery.
The fact that physiological recovery assessed using rs‐FC follows a similar time course of recovery as clinical symptoms support current clinical practice, which is dependent on clinical judgment based largely on patient self‐report. There is a concern, based on findings from preclinical literature (Longhi et al., 2005; Prins, Hales, Reger, Giza, & Hovda, 2010), that clearing athletes to RTP before the brain has fully recovered might lead to heightened risk for repeat and potentially more severe injury. The current work, however, does not support the need for major changes in current RTP guidelines (e.g., longer mandatory symptom‐free waiting periods). Furthermore, current results provide objective validation for subjective self‐report measures that inform clinical decisions regarding return to activities.
The absence of evidence is not evidence of absence, and we cannot rule out the possibility that physiological recovery is prolonged relative to clinical recovery even in the current sample. In fact, our observation of similar time course of recovery for physiological effects and clinical symptoms following SRC runs contrary to recent work suggesting physiological effects extend beyond clinical recovery. For example, group differences in rs‐FC have been reported in clinically recovered athletes after clearance for complete RTP (Churchill, Hutchison, Richards, et al., 2017), at approximately 10 days postinjury (Johnson et al., 2012), after 3 months postconcussion (Manning et al., 2017), and even within 6 months postconcussion (Czerniak et al., 2015). As highlighted in a recent systematic review, much of the prior work has included single postinjury visits and/or has included relatively small numbers of injured athletes. The large sample in the current study allowed for additional comparisons of prospectively recruited concussed athletes with and without symptoms that other studies may not have been appropriately powered to address.
Furthermore, most previous studies assessing the effects of SRC on rs‐FC have focused on a priori defined seed‐regions or functional networks of interest (Johnson et al., 2012; Manning et al., 2017; Mayer et al., 2015; Zhu et al., 2015). By modeling the entire brain as a connected network, the current study required no assumptions about specific regions or resting‐state networks that might be affected by SRC. To the best of our knowledge, this is the first study to apply a whole‐brain network, or connectome, analysis approach in the acute to sub‐acute phase of SRC. Current results, however, are largely consistent with previous studies in other mTBI samples that also report increased connectivity at various time points postinjury using NBS or graph theory metrics in mTBI patients relative to controls (Iraji et al., 2016; Yan et al., 2017), in mTBI patients that subsequently develop prolonged symptoms (Messe et al., 2013), and in symptomatic relative to asymptomatic patients (van der Horn et al., 2017).
We observed a relatively global increase in rs‐FC in concussed athletes at 8 days postconcussion relative to controls, rather than a strong localized effect in specific resting‐state networks or regions. This study cannot determine why athletes with SRC have a global increase in connectivity at 8 days postconcussion. However, in regard to the timeline of the observed effect, any delayed effect that was associated with clinical symptoms would only be observed at the 8‐day visit given the current study design, as nearly all concussed athletes had recovered by the 15‐day visit. Regarding the underlying pathophysiology, there are several potential explanations that could conceivably result in increased connectivity strength. First, the global effect seen here could be due to the underlying diffuse impact of mTBI on axons (Johnson, Stewart, & Smith, 2013; Shultz et al., 2017) or microvasculature (Werner & Engelhard, 2007). The acute effects of mTBI also incite a cascade of secondary effects such as neuroinflammation, edema, disruption of blood‐brain barrier, reduced cerebral blood flow, and other biochemical or neurometabolic responses that could also potentially result in delayed global effects on rs‐FC (Giza & Hovda, 2014; Marchi et al., 2013). Ultimately, we are unable to definitively link our observed changes in rs‐FC with specific pathophysiology.
Our observation of hyperconnectivity following SRC, however, is consistent with the hypothesis that hyperconnectivity is a common response to neurological disruption regardless of pathophysiology (Hillary et al., 2015; Hillary & Grafman, 2017). Hyperconnectivity is thought to represent the recruitment of additional neural resources to enable communication following disruption of functional networks. Thus, it is conceivable that such recruitment of additional neural resources is needed only in concussed athletes that do not have rapid recovery (i.e., those still symptomatic at 8 days postinjury). Whether hyperconnectivity is adaptive or maladaptive is difficult to establish, as the relationship between hyperconnectivity and clinical measures has varied across studies of mTBI. Some have associated hyperconnectivity with less severe injury (Manning et al., 2017; Yan et al., 2017). Others have reported hyperconnectivity associated with more symptoms or longer recovery time, consistent with current findings (Churchill, Hutchison, Richards, et al., 2017; van der Horn et al., 2017). The nature of hyperconnectivity observed following mTBI and its relationship to injury severity and outcome likely depend on several factors such as age at injury, injury mechanism, time since injury, and preinjury cognitive function or availability of neural resources (i.e., cognitive reserve theory; Stern, 2002). Moreover, it is uncertain if the observed hyperconnectivity represents reorganization of neural circuitry in response to injury, as proposed by the hyperconnectivity hypothesis, or is simply secondary to the previously described neurometabolic consequences of SRC (e.g., altered glucose metabolism) (Giza & Hovda, 2014). Future work utilizing multimodal neuroimaging is needed to pinpoint the exact neurometabolic consequences of SRC and their effects on intrinsic functional connectivity.
4.1. Limitations and methodological considerations
It is uncertain if results in high school and collegiate football players are generalizable to other populations (e.g., youth, women, and other sports). In addition, obtaining preinjury neuroimaging data was not feasible under the study design and resources, so we are unable to determine changes in rs‐FC relative to preinjury levels. The fact that sub‐group analyses showed that rs‐FC differences were only observed in still‐symptomatic athletes, however, supports the conclusion that effects were due to the recent injury. There was a nonsignificant trend for more prior concussions in the concussed group than in the control group, which could affect rs‐FC (Churchill, Hutchison, Leung, Graham, & Schweizer, 2017; Meier, Lancaster, Mayer, Teague, & Savitz, 2017). Supplementary analyses, however, confirmed that the number of prior concussions had no effect on current results (see Supporting Information). Previous work has demonstrated that the reliability of resting‐state data is improved with scan durations longer than 6 min and it is possible that this study was unable to capture signal dynamics at lower resting‐state frequencies (Birn et al., 2013).
There were several methodological decisions made which could potentially impact the observed effects. First, we made the a priori decision to focus on average nodal strength as our primary graph metric of interest. There are several alternative graph metrics that could have been analyzed; however, recent work has highlighted the difficulty of interpreting group differences in many of these other graph metrics when groups differ in connectivity strength (Hallquist & Hillary, 2018; van den Heuvel et al., 2017). Descriptive statistics for other common graph metrics are presented in the Supporting Information following recent reporting recommendations (Hallquist & Hillary, 2018). Second, negative correlations were not included in our calculation of the average nodal strength, a decision which could potentially affect graph metrics. However, supplementary analyses showed the same group effect at 8 days postconcussion when including absolute values of negative correlations (See Supporting Information). Finally, the choice of parcellation scheme can also potentially affect findings; however, supplementary analyses demonstrated the current findings were independent of the parcellation scheme adopted (see Supporting Information).
5. CONCLUSIONS
At 8 days postinjury, concussed football players showed, on average, a global increase in rs‐FC. Additional analyses showed that the overall group differences in rs‐FC were driven by still‐symptomatic concussed athletes; asymptomatic concussed athletes did not differ from controls. This large‐scale, longitudinal study suggests that the physiological effects of SRC assessed using rs‐FC are delayed in onset but linked with the trajectory of clinical recovery after SRC. These findings support contemporary approaches to injury management and return‐to‐play after SRC, which are based on detailed evaluation of clinical recovery.
Supporting information
Appendix S1: Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Defense Health Program under the Department of Defense Broad Agency Announcement for Extramural Medical Research through Award No. W81XWH‐14‐1‐0561. The REDCap electronic database used for this project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR001436. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript and decision to submit the manuscript for publication. This work was completed in part with computational resources and technical support provided by the Research Computing Center at the Medical College of Wisconsin.
Kaushal M, España LY, Nencka AS, et al. Resting‐state functional connectivity after concussion is associated with clinical recovery. Hum Brain Mapp. 2019;40:1211–1220. 10.1002/hbm.24440
Funding information National Institutes of Health, Grant/Award Number: UL1TR001436; National Center for Advancing Translational Sciences; Department of Defense, Grant/Award Number: W81XWH‐ 14‐1‐0561
REFERENCES
- Andersson, J. L. , Skare, S. , & Ashburner, J. (2003). How to correct susceptibility distortions in spin‐echo echo‐planar images: Application to diffusion tensor imaging. NeuroImage, 20, 870–888. [DOI] [PubMed] [Google Scholar]
- Bigler, E. D. , & Maxwell, W. L. (2012). Neuropathology of mild traumatic brain injury: Relationship to neuroimaging findings. Brain Imaging and Behavior, 6, 108–136. [DOI] [PubMed] [Google Scholar]
- Birn, R. M. , Molloy, E. K. , Patriat, R. , Parker, T. , Meier, T. B. , Kirk, G. R. , … Prabhakaran, V. (2013). The effect of scan length on the reliability of resting‐state fMRI connectivity estimates. NeuroImage, 83, 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill, N. , Hutchison, M. G. , Leung, G. , Graham, S. , & Schweizer, T. A. (2017). Changes in functional connectivity of the brain associated with a history of sport concussion: A preliminary investigation. Brain Injury, 31, 39–48. [DOI] [PubMed] [Google Scholar]
- Churchill, N. W. , Hutchison, M. G. , Richards, D. , Leung, G. , Graham, S. J. , & Schweizer, T. A. (2017). Neuroimaging of sport concussion: Persistent alterations in brain structure and function at medical clearance. Scientific Reports, 7, 8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin, T. , Elbin, R. J., 3rd , Stiller‐Ostrowski, J. L. , & Kontos, A. P. (2009). Immediate post‐concussion assessment and cognitive testing (ImPACT) practices of sports medicine professionals. Journal of Athletic Training, 44, 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Craddock, R. C. , James, G. A. , Holtzheimer, P. E., 3rd , Hu, X. P. , & Mayberg, H. S. (2012). A whole brain fMRI atlas generated via spatially constrained spectral clustering. Human Brain Mapping, 33, 1914–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniak, S. M. , Sikoglu, E. M. , Liso Navarro, A. A. , McCafferty, J. , Eisenstock, J. , Stevenson, J. H. , … Moore, C. M. (2015). A resting state functional magnetic resonance imaging study of concussion in collegiate athletes. Brain Imaging and Behavior, 9, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis, L. R. (2001). Brief symptom inventory 18 (BSI‐18): Administration, scoring, and procedures manual. Minneapolis, MN: Pearson. [Google Scholar]
- Giza, C. C. , & Hovda, D. A. (2014). The new neurometabolic cascade of concussion. Neurosurgery, 75(Suppl 4), S24–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve, D. N. , & Fischl, B. (2009). Accurate and robust brain image alignment using boundary‐based registration. NeuroImage, 48, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist, M.N. , Hillary, F.G. (2018) Graph theory approaches to functional network organization in brain disorders: a critique for a brave new small‐world. Network Neuroscience. bioRxiv. 012041. [DOI] [PMC free article] [PubMed]
- Hillary, F. G. , & Grafman, J. H. (2017). Injured brains and adaptive networks: The benefits and costs of Hyperconnectivity. Trends in Cognitive Sciences, 21, 385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary, F. G. , Roman, C. A. , Venkatesan, U. , Rajtmajer, S. M. , Bajo, R. , & Castellanos, N. D. (2015). Hyperconnectivity is a fundamental response to neurological disruption. Neuropsychology, 29, 59–75. [DOI] [PubMed] [Google Scholar]
- Iraji, A. , Chen, H. , Wiseman, N. , Welch, R. D. , O'Neil, B. J. , Haacke, E. M. , … Kou, Z. (2016). Compensation through functional Hyperconnectivity: A longitudinal connectome assessment of mild traumatic brain injury. Neural Plasticity, 2016, 4072402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson, M. , Bannister, P. , Brady, M. , & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17, 825–841. [DOI] [PubMed] [Google Scholar]
- Johnson, B. , Zhang, K. , Gay, M. , Horovitz, S. , Hallett, M. , Sebastianelli, W. , & Slobounov, S. (2012). Alteration of brain default network in subacute phase of injury in concussed individuals: Resting‐state fMRI study. NeuroImage, 59, 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, V. E. , Stewart, W. , & Smith, D. H. (2013). Axonal pathology in traumatic brain injury. Experimental Neurology, 246, 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamins, J. , Bigler, E. , Covassin, T. , Henry, L. , Kemp, S. , Leddy, J. J. , … Giza, C. C. (2017). What is the physiological time to recovery after concussion? A systematic review. British Journal of Sports Medicine, 51, 935–940. [DOI] [PubMed] [Google Scholar]
- Langlois, J. A. , Rutland‐Brown, W. , & Wald, M. M. (2006). The epidemiology and impact of traumatic brain injury: A brief overview. The Journal of Head Trauma Rehabilitation, 21, 375–378. [DOI] [PubMed] [Google Scholar]
- Longhi, L. , Saatman, K. E. , Fujimoto, S. , Raghupathi, R. , Meaney, D. F. , Davis, J. , … McIntosh, T. K. (2005). Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery, 56, 364–374 discussion 364–374. [DOI] [PubMed] [Google Scholar]
- Manning, K. Y. , Schranz, A. , Bartha, R. , Dekaban, G. A. , Barreira, C. , Brown, A. , … Menon, R. S. (2017). Multiparametric MRI changes persist beyond recovery in concussed adolescent hockey players. Neurology, 89, 2157–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi, N. , Bazarian, J. J. , Puvenna, V. , Janigro, M. , Ghosh, C. , Zhong, J. , … Janigro, D. (2013). Consequences of repeated blood‐brain barrier disruption in football players. PLoS One, 8, e56805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, A. R. , Bellgowan, P. S. , & Hanlon, F. M. (2015). Functional magnetic resonance imaging of mild traumatic brain injury. Neuroscience and Biobehavioral Reviews, 49, 8–18. [DOI] [PubMed] [Google Scholar]
- McCrea, M. , Meier, T. , Huber, D. , Ptito, A. , Bigler, E. , Debert, C. T. , … McAllister, T. (2017). Role of advanced neuroimaging, fluid biomarkers and genetic testing in the assessment of sport‐related concussion: A systematic review. British Journal of Sports Medicine, 51, 919–929. [DOI] [PubMed] [Google Scholar]
- McCrory, P. , Meeuwisse, W. , Dvorak, J. , Aubry, M. , Bailes, J. , Broglio, S. , … Vos, P. E. (2017). Consensus statement on concussion in sport‐the 5(th) international conference on concussion in sport held in Berlin, October 2016. British Journal of Sports Medicine, 51, 838–847. [DOI] [PubMed] [Google Scholar]
- McCrory, P. , Meeuwisse, W. H. , Aubry, M. , Cantu, B. , Dvorak, J. , Echemendia, R. J. , … Turner, M. (2013). Consensus statement on concussion in sport: The 4th international conference on concussion in sport held in Zurich, November 2012. British Journal of Sports Medicine, 47, 250–258. [DOI] [PubMed] [Google Scholar]
- Meier, T. B. , Bellgowan, P. S. F. , & Mayer, A. R. (2017). Longitudinal assessment of local and global functional connectivity following sports‐related concussion. Brain Imaging and Behavior, 11, 129–140. [DOI] [PubMed] [Google Scholar]
- Meier, T. B. , Lancaster, M. A. , Mayer, A. R. , Teague, T. K. , & Savitz, J. (2017). Abnormalities in functional connectivity in collegiate football athletes with and without a concussion history: Implications and role of neuroactive kynurenine pathway metabolites. Journal of Neurotrauma, 34, 824–837. [DOI] [PubMed] [Google Scholar]
- Messe, A. , Caplain, S. , Pelegrini‐Issac, M. , Blancho, S. , Levy, R. , Aghakhani, N. , … Lehericy, S. (2013). Specific and evolving resting‐state network alterations in post‐concussion syndrome following mild traumatic brain injury. PLoS One, 8, e65470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijalkov, M. , Kakaei, E. , Pereira, J. B. , Westman, E. , Volpe, G. , & Alzheimer's Disease Neuroimaging Initiative. (2017). BRAPH: A graph theory software for the analysis of brain connectivity. PLoS One, 12, e0178798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oride, M. K. , Marutani, J. K. , Rouse, M. W. , & DeLand, P. N. (1986). Reliability study of the Pierce and king‐Devick saccade tests. American Journal of Optometry and Physiological Optics, 63, 419–424. [DOI] [PubMed] [Google Scholar]
- Prins, M. L. , Hales, A. , Reger, M. , Giza, C. C. , & Hovda, D. A. (2010). Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Developmental Neuroscience, 32, 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann, B. L. , & Guskiewicz, K. M. (2000). Effects of mild head injury on postural stability as measured through clinical balance testing. Journal of Athletic Training, 35, 19–25. [PMC free article] [PubMed] [Google Scholar]
- Rubinov, M. , & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52, 1059–1069. [DOI] [PubMed] [Google Scholar]
- Schatz, P. , & Maerlender, A. (2013). A two‐factor theory for concussion assessment using ImPACT: Memory and speed. Archives of Clinical Neuropsychology, 28, 791–797. [DOI] [PubMed] [Google Scholar]
- Shultz, S. R. , McDonald, S. J. , Vonder Haar, C. , Meconi, A. , Vink, R. , van Donkelaar, P. , … Christie, B. R. (2017). The potential for animal models to provide insight into mild traumatic brain injury: Translational challenges and strategies. Neuroscience and Biobehavioral Reviews, 76, 396–414. [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Woolrich, M. W. , Beckmann, C. F. , Behrens, T. E. , Johansen‐Berg, H. , … Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(Suppl 1), S208–S219. [DOI] [PubMed] [Google Scholar]
- Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8, 448–460. [PubMed] [Google Scholar]
- Taylor, P. A. , & Saad, Z. S. (2013). FATCAT: (an efficient) functional and Tractographic connectivity analysis toolbox. Brain Connectivity, 3, 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel, M. P. , de Lange, S. C. , Zalesky, A. , Seguin, C. , Yeo, B. T. T. , & Schmidt, R. (2017). Proportional thresholding in resting‐state fMRI functional connectivity networks and consequences for patient‐control connectome studies: Issues and recommendations. NeuroImage, 152, 437–449. [DOI] [PubMed] [Google Scholar]
- van der Horn, H. J. , Liemburg, E. J. , Scheenen, M. E. , de Koning, M. E. , Spikman, J. M. , & van der Naalt, J. (2017). Graph analysis of functional brain networks in patients with mild traumatic brain injury. PLoS One, 12, e0171031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (2001). Wechsler test of adult Reading: WTAR. San Antonio, TX: The Psychological Corp. [Google Scholar]
- Werner, C. , & Engelhard, K. (2007). Pathophysiology of traumatic brain injury. British Journal of Anaesthesia, 99, 4–9. [DOI] [PubMed] [Google Scholar]
- Yan, Y. , Song, J. , Xu, G. , Yao, S. , Cao, C. , Li, C. , … Du, H. (2017). Correlation between standardized assessment of concussion scores and small‐world brain network in mild traumatic brain injury. Journal of Clinical Neuroscience, 44, 114–121. [DOI] [PubMed] [Google Scholar]
- Zalesky, A. , Fornito, A. , & Bullmore, E. T. (2010). Network‐based statistic: Identifying differences in brain networks. NeuroImage, 53, 1197–1207. [DOI] [PubMed] [Google Scholar]
- Zhu, D. C. , Covassin, T. , Nogle, S. , Doyle, S. , Russell, D. , Pearson, R. L. , … Kaufman, D. I. (2015). A potential biomarker in sports‐related concussion: Brain functional connectivity alteration of the default‐mode network measured with longitudinal resting‐state fMRI over thirty days. Journal of Neurotrauma, 32, 327–341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Material


