Abstract
Background
Despite careful clinical examination, procurement biopsy and assessment on hypothermic machine perfusion, a significant number of potentially useable deceased donor kidneys will be discarded because they are deemed unsuitable for transplantation. Ex vivo normothermic perfusion (EVNP) may be useful as a means to further assess high-risk kidneys to determine suitability for transplantation.
Methods
From June 2014 to October 2015, 7 kidneys (mean donor age, 54.3 years and Kidney Donor Profile Index, 79%) that were initially procured with the intention to transplant were discarded based on a combination of clinical findings, suboptimal biopsies, long cold ischemia time (CIT) and/or poor hypothermic perfusion parameters. They were subsequently placed on EVNP using oxygenated packed red blood cells and supplemental nutrition for a period of 3 hours. Continuous hemodynamic and functional parameters were assessed.
Results
After a mean CIT of 43.7 hours, all 7 kidneys appeared viable on EVNP with progressively increasing renal blood flow over the 3-hour period of perfusion. Five of the 7 kidneys had excellent macroscopic appearance, rapid increase in blood flow to 200 to 250 mL/min, urine output of 40 to 260 mL/h and increasing creatinine clearance.
Conclusions
Favorable perfusion characteristics and immediate function after a 3-hour course of EVNP suggests that high-risk kidneys subjected to long CIT may have been considered for transplantation. The combined use of ex vivo hypothermic and normothermic perfusion may be a useful strategy to more adequately assess and preserve high-risk kidneys deemed unsuitable for transplantation. A clinical trial will be necessary to validate the usefulness of this approach.
The combined use of ex vivo hypothermic and normothermic perfusion may be a useful strategy to more adequately assess and preserve high-risk kidneys deemed unsuitable for transplantation.
The number of patients on the waiting list for renal transplantation far exceeds the number of organs available for transplantation. Efforts to address the organ shortage have resulted in expansion of the donor pool to include donors with acute kidney injury, increase risk of infection transmission, donation after circulatory death (DCD), and older donors or those with other defined comorbidities, previously termed extended criteria donors (ECD). Nearly 51% of candidates on the waitlist have reported a willingness to accept ECD kidneys1 and a survival advantage has been demonstrated with transplantation of ECD and higher risk kidneys.2-4 Despite the severe organ shortage and survival advantage of transplantation, many kidneys procured with the intent to transplant are discarded.
The decision to transplant a kidney is based on the donor history, renal function, kidney appearance, biopsy findings, and duration of warm and/or cold ischemia. Scoring systems incorporating donor clinical risk factors5-8 and/or procurement biopsy findings9,10 have been developed to better assess organ quality and match appropriate organs to suitable recipients yet the use of these tools may not correlate with graft survival and lead to unnecessary kidney discards.11,12 Indeed, the percentage of procured kidneys discarded in the United States has increased from 5.1% to 19.2% over 2 decades, with 3159 kidneys discarded in 2015.13
As the demand for organs has increased, the use of hypothermic machine perfusion (HMP) has been used to assess and preserve high-risk kidneys.14-17 The use of hypothermic perfusion in the United States has progressively increased in recent years, particularly for high-risk kidneys as defined by Kidney Donor Profile Index (KDPI),18 a quantitative measure of the quality of deceased donor kidneys (DDKs) relative to other kidneys recovered. Hypothermic machine perfusion is associated with a reduced risk of delayed graft function (DGF) and graft failure when compared to cold storage15,16 and flow characteristics on machine perfusion have been used to determine suitability for transplantation.16,17 Hypothermic perfusion is therefore commonly used as an additional tool to assess and preserve high-risk kidneys before transplantation.
Ex vivo normothermic perfusion (EVNP) of organs has recently been shown to be an effective means of assessing, preserving and possibly expanding the number of extrarenal organs available for transplantation.19-22 ENVP may be superior in comparison to hypothermic perfusion when it is used to evaluate suitability for transplantation as it allows assessment of viability and function of the metabolically active organ during perfusion. Presently, Hosgood and Nicholson23-26 in the United Kingdom have the only clinical reports using a brief 1-hour period of EVNP before renal transplantation. Renal EVNP has not been reported in the United States, but its potential to expand the donor pool may be significant, considering the large number of potentially transplantable organs currently being discarded. If this modality is to be used in the United States, differences in practice patterns here need to be considered when compared with the use of EVNP in the United Kingdom where preservation is predominantly with cold storage and preservation times short. Incorporation of EVNP into clinical practice in the United States may need to take into account allocation policy and clinical practice characterized by regional sharing of organs across large geographic areas, longer cold preservation time, and the common use of hypothermic perfusion for high-risk organs. The objective of this study is to determine whether a 3-hour course of EVNP would be useful to assess high-risk DDKs that have been exposed to a prolonged cold ischemia time (CIT) and a period of assessment on HMP.
MATERIALS AND METHODS
Deceased Donor Kidneys
From June 2014 to October 2015, our center conditionally accepted 445 kidneys for transplantation from both our local and distant organ procurement organizations (OPOs). After an initial period of cold static storage at the originating OPO the kidneys were transported to our transplant center and placed on HMP (RM3 System; Waters Medical Systems, Rochester, MN) using Kidney Perfusion Solution-1 (Organ Recovery System, Itaca, IL) with 40 international units of insulin and 19 mL of 20% mannitol with the intention to proceed with transplantation. After assessment, 380 kidneys were transplanted and 65 were determined to be “nontransplantable” and were discarded. Seven of the 65 nontransplantable kidneys had research consent from the originating OPO and were entered in this study.
The study protocol was considered exempt by our institutional review board and subsequently approved by the Human Anatomical Specimen and Tissue Oversight Committee.
Parameters for Suitability for Transplantation Before EVNP
Based on overall assessment after hypothermic perfusion, the transplant surgeon made the ultimate decision to transplant or discard the organ. Generally, kidneys deemed unsuitable for transplantation at our institution have biopsy findings of greater than 20% glomerulosclerosis and moderate-to-severe vascular changes, hypothermic pump flow less than 70 mL/min, and renal resistive index greater than 0.50 mm Hg/mL per minute after a period of 4 to 6 hours on hypothermic perfusion. None of these individual exclusion criteria are necessarily considered absolute but were included along with donor factors, total ischemic time, and appropriateness for the intended recipient when making the decision to proceed with transplantation.
Ex Vivo Normothermic Perfusion System
Our EVNP circuit (Figure 1) is similar to the system described by Hosgood25,26 consisting of a pediatric cardiopulmonary bypass system comprised of a centrifugal blood pump (550 Bio-Console and BP-50 Bio-Pump, Medtronic, Minneapolis, MN), heat exchanger (Bio Cal 370, Medtronic), hollow fiber oxygenator with a hard shell reservoir (CX*RX05RW; Terumo Cardiovascular, Ann Arbor, MI), pressure display box and probe (Model 66000; Medtronic), and flow bio-probe (Model TX50P; Medtronic). The kidneys were housed in a stainless-steel platform and perfused at an arterial pressure between 70 and 80 mm Hg for 3 hours at a temperature of 37°C. Flow was adjusted to keep the arterial pressure between the set points.
FIGURE 1.
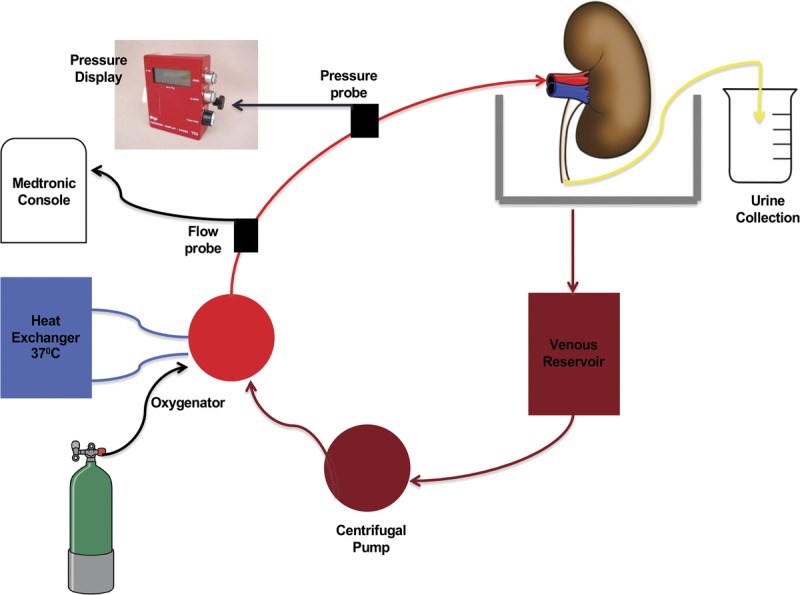
Ex vivo normothermic perfusion circuit. After priming the circuit, the perfusate flows into the kidney via renal arterial cannula. Venous blood passively flows from the renal vein into a reservoir. A centrifugal pump circulates the venous perfusate to the oxygenator and heater/cooler. Flow and pressure probes are used to adjust and maintain mean arterial pressure at 70 to 80 mm Hg. The perfusate returns to the kidney via the renal arterial cannula. Urine is collected into a beaker via a ureteral catheter.
Composition of EVNP Perfusate
Blood type O packed red blood cells obtained by our hospital blood bank was diluted at a 1:1 ratio with Plasma-Lyte A (Baxter Medical) to which was added 2000 international units of heparin sodium (NOVAPLUS) and exogenous anhydrous creatinine (0.06 g; MP Biomedicals, LLC). The EVNP circuit was primed with 500 mL of this perfusate which was oxygenated with 95% O2/5% CO2 and supplemented with parenteral nutrition (Baxter CLINIMIX E 2.75/10) fortified with 100 units of regular insulin (Humulin R), 5 mL of multivitamins (Baxter Infutive Adult), and 26 mL of 8.4% sodium bicarbonate (Hospira, Inc, Lake Forest, IL) per liter of solution to keep perfusate within a 7.3 to 7.4 range, and this solution was added to the perfusate at a rate of 20 mL per hour. During perfusion Plasma-Lyte A was added every 30 minutes to match urine output at a 1:1 ratio.
Kidney Assessment While on EVNP
Kidneys were monitored continuously by observance of global appearance, renal blood flow (RBF)/resistance, and urine output which was recorded every 30 minutes. Measurements of glucose, lactate, and creatinine were done using Stat Sensors (Nova Biomedical Corp.). Arterial blood gas, venous blood gas, and basic metabolic panel were measured at time 0 (immediately at start of perfusion) and at 30-minute intervals during perfusion (Critical Care Xpress, Nova Biomedical Corp.). A wedge biopsy of the renal cortex for hematoxylin and eosin staining was obtained before placement of the kidney on EVNP and at the completion of the 3-hour perfusion period. Urine was analyzed for sodium and creatinine for calculation of creatinine clearance and fractional excretion of sodium (R&D Systems, Minneapolis, MN, and Critical Care Xpress).
The kidneys were divided into 2 groups based on global appearance, RBF, and urine output roughly similar to the criteria previously noted by Hosgood et al.25-27 Kidneys that on initiation of EVNP perfused uniformly and quickly with a pink appearance throughout, attained RBF over 150 mL/min and produced urine greater than 20 mL/30 minutes were designated as the “optimally perfused” group. Kidneys that had a slower reperfusion with patchy coloration and areas of poor perfusion, with blood flow less than 150 mL/min and urine output less than 20 mL/30 minutes were designated “non optimally perfused.”
Statistical Analysis
A retrospective analysis of factors that may have contributed to the decision to discard these kidneys was done to determine potential differences between the non optimally perfused and optimally perfused groups. The Student t test and Mann-Whitney U test were both used to emphasize the validity of the Student t test in assessing group differences involving extremely small sample size with large effect size.28 Effect size and corresponding 95% confidence intervals were provided for these comparisons. We fit linear mixed-effects models to compare changes over time in flow, resistance, urine output, creatinine, lactic acid, and sodium between the optimally and nonoptimally perfused cohorts. These models included random effects for intercept and slope to accommodate correlation of these measures within each case over time and allow for case-specific rates of change. All tests were 2-sided and P values less than 0.05 were concluded statistically significant. Analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Donor Demographics and Characteristics of Kidneys
Seven kidneys that had been discarded after assessment on HMP underwent EVNP. All 7 kidneys were imported from outside of our local organ procurement organization. The mean donor age was 54.3 years (range, 38-65 years; Table 1) and the mean weight was 80.3 kg (range, 56-100 kg). There was 1 DCD and 4 ECD donors with mean KDPI of 79% (range, 53-97%). Most kidneys were discarded because of a combination of clinical factors, CIT, biopsy findings, and pump parameters on hypothermic perfusion. The mean CIT from cross clamp to the start of EVNP was 43.7 hours (range, 28-64 hours). Final hypothermic perfusion parameters were a mean flow of 72.7 mL/min (range, 59-88 mL/min) and resistance of 0.41 mm Hg/mL per minute (range, 0.30-0.52 mm Hg/mL per minute).
TABLE 1.
Donor demographics and characteristics of kidneys placed on ex vivo normothermic perfusion
Assessment on EVNP
All kidneys at baseline were well flushed with a uniform pale tan color. With initiation of EVNP the kidneys gradually warmed up and turned pink, falling into 2 distinct groups based on macroscopic appearance, RBF, and urine output (Figures 2 and 3). Five kidneys termed “optimally perfused” exhibited rapid and uniform perfusion within minutes with a healthy pink color (Figure 2). RBF increased rapidly to a mean rate greater than 200 mL/min by 30 minutes with a continuous increase to over 250 mL/min by 90 minutes where flow plateaued. Correspondingly, renal resistive index of the optimally perfused group fell below 0.40 mm Hg/mL per minute within 60 minutes and remained low. These kidneys began making urine immediately and urine output remained high throughout the course of EVNP with a total mean urine output of 144.3 mL/h (range 42.7-269 mL/h, Figure 3). Two kidneys, termed “nonoptimally perfused,” on initiation of EVNP did not perfuse uniformly, appearing discolored with patchy areas of poor perfusion (Figure 2). Renal blood flow decreased to well below 150 mL/min for the first 60 minutes of perfusion and renal resistance fell more gradually when compared to the optimally perfused group. These kidneys made very little urine throughout the perfusion period (0.47 and 3.75 mL/h, respectively). Interestingly, in these nonoptimally perfused kidneys, blood flow and resistance stabilized at 90 minutes and continued to improve thereafter such that by 120 minutes blood flow and resistance was equal to the optimally perfused group (Figure 3). The optimally perfused kidneys had evidence of superior glomerular and tubular function with a trend toward increased creatinine clearance and lower fractional excretion of sodium throughout perfusion in comparison to the 2 nonoptimally perfused kidneys (Figure 3). Perfusate sodium levels had an increasing trend over time in the optimally perfused group compared the nonoptimally perfused group which had stable perfusate sodium levels throughout the perfusion period. The progressively higher perfusate sodium levels in the optimally perfused group was expected due to the increased urine production in this group requiring equivalent volume perfusate replenishment with relatively hypertonic Plasmalyte solution.
FIGURE 2.
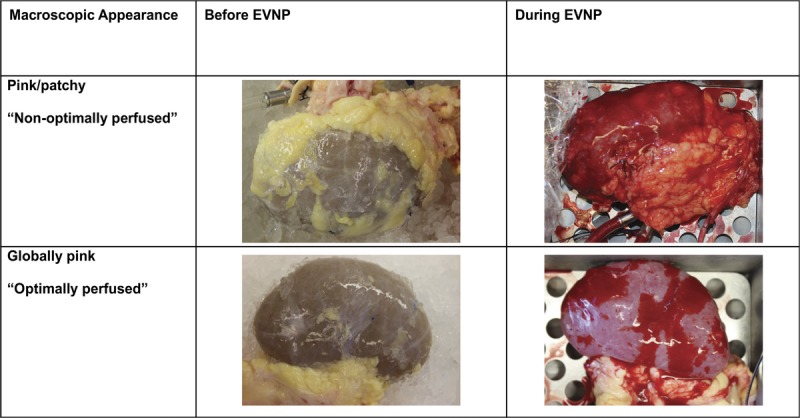
Pictures of kidneys before and during ex vivo normothermic perfusion (EVNP). Although their macroscopic appearance was initially similar before the start of EVNP, the “optimally perfused” kidneys (n = 5) became globally pink during perfusion and the remaining 2 “nonoptimally perfused” kidneys became pink but were also patchy.
FIGURE 3.
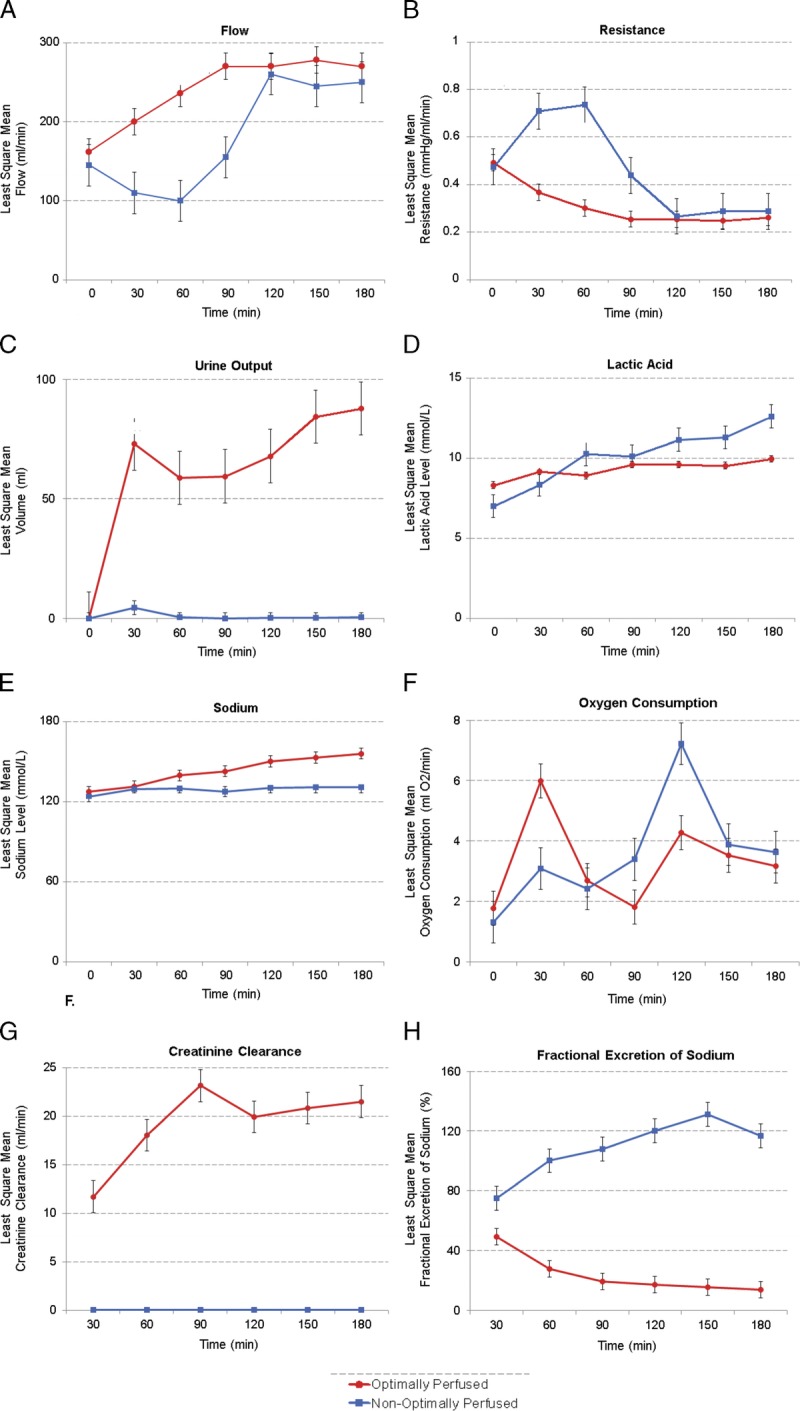
Least square mean of hemodynamic, functional and electrolyte trends on ex vivo normothermic perfusion (EVNP). Overall, for the “optimally perfused” kidneys, red blood flow (RBF) (mL/min) gradually increased and stabilized after 120 minutes (A) whereas Renal Resistive Index (RRI) gradually decreased and stabilized after 120 minutes (B). Both RBF and RRI were variable during the first 60 minutes for the “nonoptimally perfused” kidneys but eventually improved and also stabilized after 120 minutes on EVNP. Urine output (mL/h) remained high in the “optimally perfused” kidneys (C). Perfusate lactate levels steadily increased with time in the nonoptimally perfused kidneys compared to relatively stable lactate levels in the perfusate of the optimally perfused group (D). Perfusate sodium levels steadily increased with time in the optimally perfused kidneys compared to relatively stable sodium levels in the perfusate of the nonoptimally perfused group (E). Oxygen consumption for both kidneys resulted in similar curves with a peak and eventual stable decrease (F). Creatinine clearance (G) and fractional excretion of sodium (H) were more favorable in the optimally perfused kidneys.
Oxygen consumption was calculated as the difference between arterial and venous oxygen content multiplied by RBF.29 The optimally perfused kidneys showed a peak in oxygen consumption within 30 minutes of perfusion after which consumption decreased and stabilized as shown in Figure 3. The nonoptimally perfused kidneys showed a similar oxygen consumption curve but peaked later in the perfusion period at 120 minutes.
We compared the 2 groups with regard to clinical factors that may have contributed to the decision to discard these kidneys as shown in Table 2. Because of the very small sample size, comparison between groups is based on the geometric mean (average of log values transformed) rather than the regular mean.28 No clinical factor that could distinguish between the optimally and nonoptimally perfused kidneys was noted.
TABLE 2.
Retrospective analysis of assessment criteria used to discard the kidneys
Histological analysis revealed that the preperfusion and postperfusion biopsies of both optimally and nonoptimally perfused kidneys showed acute tubular injury characterized by flattening of the tubular epithelial cells with associated mild interstitial edema. Compared with the optimally perfused specimen, the nonoptimal showed more cellular debris, apical cytoplasmic blebbing and scattered oxalate crystals (Figure 4).
FIGURE 4.
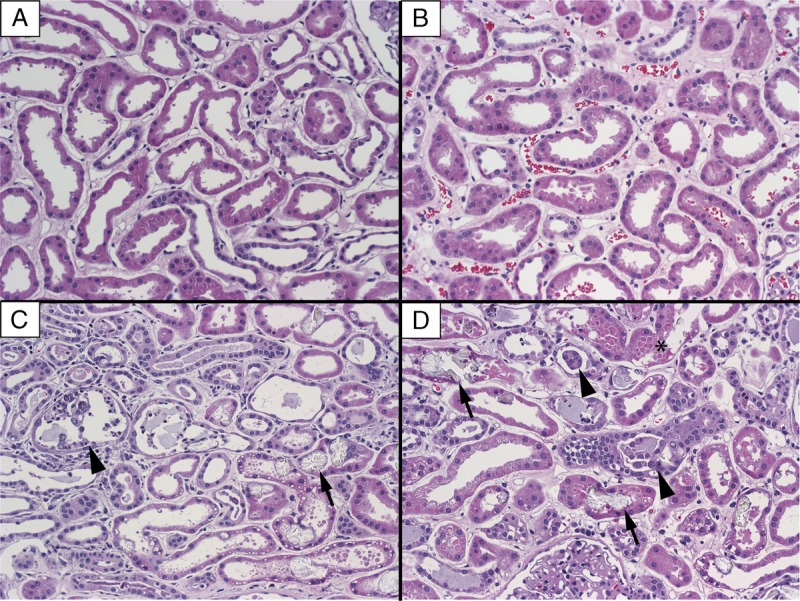
Histology of prebiopsy and postbiopsy. Hematoxylin and eosin, 20×, 2 μm. A, Optimally perfused preperfusion biopsy. B, Optimally perfused postperfusion biopsy. C, Nonoptimally perfused preperfusion biopsy. D, Nonoptimally perfused postperfusion biopsy. Arrows represent examples of oxalate crystals, arrow heads represent sloughed epithelial cells, and asterisk represent cytoplasmic blebbing.
DISCUSSION
The decision to use high-risk DDKs is often difficult and takes into consideration multiple donor and recipient related factors. Despite the use of all the available modalities a significant number of potentially useable kidneys are discarded. We used an EVNP system to assess 7 DDKs that were declined by all centers after comprehensive assessment including the use of hypothermic perfusion. Assessment with EVNP subsequently demonstrated that the majority of these kidneys had favorable perfusion appearance, hemodynamic parameters, urine production and evidence of glomerular and tubular function. Based on these findings, we suggest that the 5 kidneys that had the optimal perfusion characteristics on EVNP could have been considered for transplantation. The potential application of EVNP as an assessment tool to evaluate “high-risk” kidneys currently being discarded will require a clinical trial as is currently being carried out in the United Kingdom.30
The ultimate usefulness of EVNP to assess high-risk kidneys will require the determination of characteristics while on EVNP that will identify which kidneys are “transplantable.” Toward this end, Hosgood assessed 22 discarded kidneys on EVNP and demonstrated that the triad of optimal macroscopic appearance, high blood flow, and high urine output after 1 hour on EVNP were associated with good ex vivo glomerular and tubular function27 and retrospectively applied the same scoring system to DDKs that exhibited improved function after transplantation.25 They also recently used this EVNP-based scoring system prospectively to assess and successfully transplant 2 poorly perfused kidneys from a DCD donor which were declined by all UK centers.26 Interestingly, a recent study from the University of Toronto examined whether an 8-hour period of EVNP would be useful in assessing ischemic injury severity and predicting posttransplant outcomes in a porcine renal auto-transplant model.31 They found that intrarenal resistance during EVNP, perfusate pH, lactate clearance and postperfusion AST levels all correlated with the degree of ischemic injury and posttransplant function. In contrast to the studies by Hosgood, urine output during EVNP was not associated with graft injury or posttransplant outcomes. The discrepancy in the 2 studies regarding the utility of urine output in assessing kidney quality may be explained by differences in the degree of kidney injury, perfusate composition, and other factors related to the different perfusion systems used in the 2 studies. These studies illustrate the need for clinically validated tools that can be used to assess high-risk kidneys on EVNP and identify which ones are transplantable.
Little is known about the optimal duration of EVNP of the kidney. Although the UK clinical series involves EVNP for 1 hour before transplantation, it is possible that a longer period of perfusion may be more beneficial. One potential advantage of a longer period of perfusion is the ability to more adequately assess the quality of the kidney and better differentiate which organs may be safely transplanted. Blum et al29 has demonstrated in a porcine DCD ex vivo transplant simulation model that kidneys preserved with 8 hours of EVNP have equivalent preservation of renal function when compared to HMP. Kaths et al32 showed that porcine kidneys can be successfully transplanted after 8 hours of EVNP. They demonstrated the most significant improvement in RBF, resistance, urine output and lactate clearance occurs over the first 2-3 hours on EVNP, similar to our findings. A subsequent study from the same investigators showed that preservation of porcine kidneys with 16 hours of continuous EVNP resulted in better allograft function when compared with kidneys that were preserved for a brief (1 hour) or intermediate (8 hours) period of EVNP.33 Interestingly, in our study, even the 2 “nonoptimally perfused” kidneys that initially had a patchy, poorly perfused appearance over time showed a steady increase in RBF and were equivalent to the optimally perfused group after 3 hours on EVNP. The optimal duration of EVNP is still to be determined, but assessment of some high-risk kidneys may require longer periods of perfusion.
Another potential benefit of increasing the duration of EVNP is the continuation of reparative processes within the injured kidney before transplantation. Brasile et al34 have studied various strategies for repair of both canine and human kidneys in an ex vivo warm perfusion system. One such strategy is the ex vivo delivery of growth factors to injured kidneys where she demonstrated progressively longer periods of perfusion for 6, 18, and 24 hours resulted in correspondingly time-dependent improvement in cytoskeleton integrity and DNA repair. Similarly, Kaths et al studied the impact of a brief (1 hour), intermediate (8 hours) or prolonged (16 hours) period of EVNP in a porcine DCD autotransplant model. Kidneys that were preserved with intermediate and prolonged periods of EVNP had less tubular injury and better function when compared with a brief 1 hour period of EVNP.35 Interestingly, the kidneys that were on EVNP for only 1 hour showed worse allograft function after transplantation when compared with the kidneys that were preserved with cold storage alone, suggesting that a longer period of EVNP was necessary to observe the presumed benefit of reconditioning and repair. Lastly, another aspect of injury associated with cold ischemia is the loss of renal high energy adenosine triphosphate stores which can fall by over 90% after 4 hours of cold ischemia.36 Animal studies have shown that kidneys depleted of high-energy substrates after cold ischemia are replenished of adenosine triphosphate after 2 to 3 hours of EVNP.37,38 For this reason, we chose to perfuse our kidneys for 3 hours to provide optimal energy replacement and possibly extend the injury repair process. Further studies will need to determine the optimal period of EVNP to balance the potential risks and benefits associated with longer periods of perfusion as is being explored with other organs.39,40
As the possibility of incorporating EVNP into clinical practice draws near the most straightforward practical approach would be implementation after static cold storage as is currently being tested in the United Kingdom.30 This approach has the advantage of simplicity, relatively low cost and ease of transportation to a location where EVNP can then be initiated. If the duration of cold ischemia can be minimized the effectiveness of any intervention aimed at improving outcomes is increased in view of the cumulative risk of renal injury and DGF with longer periods of cold storage.41 Application of EVNP after a short period of static cold storage may be difficult in the United States considering the large geographic donor service areas, allocation complexities, and regional/national sharing of high KDPI kidneys. An alternate approach to implementation of EVNP is to use this modality exclusively after procurement, omitting any cold storage to avoid potential adverse effects of hypothermia. This approach was demonstrated in a porcine transplant model where kidneys that were preserved exclusively with EVNP had less evidence of cell injury and better early graft function compared to those that were subjected to any degree of cold storage combined with EVNP.33 Immediate placement of the organ on EVNP would require the development of a portable platform that could be transported to the donor hospital as is currently practiced with lung, heart, and liver preservation.21,22,42
The application of EVNP after a period of HMP is particularly relevant currently because of the increasing application of hypothermic perfusion to high-risk kidneys.18 Hypothermic machine perfusion has been used to better assess kidney quality of organs that would otherwise be discarded based on donor characteristics alone.43 Clinical studies have also shown reduction in DGF and primary nonfunction in DDKs with greater exposure to ischemic damage.16,44-46 Hypothermic perfusion has also been used to extend the preservation period before transplantation.47 The potential synergistic actions of hypothermic and normothermic perfusion used in combination is an intriguing possibility to assess high-risk organs, optimize function and extend duration of preservation. Rijkmans et al,48 more than 3 decades ago, were able to successfully transplant canine kidneys after 6 days by using hypothermic perfusion combined with an intermediate 3-hour period of normothermic perfusion on day 3 of preservation. The ability to safely extend the preservation period of high-risk kidneys has the benefit of removing time restraints in using these organs and expanding the time to identify appropriate recipients for transplantation. It will be important to determine whether the combination of both ex vivo hypothermic and normothermic perfusion is a useful strategy to expand the number of organs available for transplantation.
This study is limited by several factors including the small sample size, retrospective design, limited number of parameters followed on EVNP, and the unknown clinical impact of EVNP since these kidneys were not transplanted. Additionally, the 3-hour period of EVNP may not have allowed all outcome parameters to reach optimal levels for adequate assessment. The lack of definitive criteria for discarding the kidneys in our study and the possible selection bias of individual surgeons demonstrate the somewhat subjective nature of the decision process in evaluation of high-risk kidneys. Even though all of these kidneys were turned down by other centers before being imported to our center, assessment based on KDPI, hypothermic perfusion, and biopsy characteristics in isolation suggests that these organs were possibly of sufficient quality and potentially transplantable if other donor and recipient factors could have been optimized. Alternatively, the observation that the majority of the kidneys did well on EVNP may suggest that our current protocol for assessment of high-risk kidneys is not adequate.
In conclusion, many kidneys procured with the intention to transplant are discarded based on clinical data, biopsy findings and assessment of the organ on hypothermic perfusion. ENVP may be an additional tool used to assess high-risk kidneys. A prospective clinical study will be necessary to determine the role of EVNP in assessing high-risk kidneys, including the potential use of this modality in combination with HMP.
ACKNOWLEDGMENTS
The authors thank Drs Sarah Hosgood and Michael Nicholson for their assistance in establishing the ex vivo normothermic perfusion system at the University of California, Davis.
Footnotes
S.K.K. and I.P.P. contributed equally to the work presented in this article.
S.K.K., I.P.P., J.S., R.V.P. made substantial contributions to the conception or design of the work. S.K.K., I.P.P., Y.S., I.P., T.B. participated in the acquisition of data for the work. S.K.K., I.P.P., Y.S., J.S., M.N., K.-Y.J., R.V.P. participated in the analysis and interpretation of data. S.K.K. and I.P.P. participated in the drafting of the work. S.K.K., I.P.P., Y.S., J.S., C.T., C.S., J.P.M., R.V.P. participated in revising the work critically for important intellectual content. S.K.K., I.P.P., Y.S., T.B., I.P., J.S., C.T., C.S., J.P.M., M.N., K.-Y.J., R.V.P. made the final approval of the version to be published and the agreement to be accountable for all aspects of the work.
R.V.P. is a member of the clinical advisory board for XOR Laboratories, Toronto, Canada. All the other authors declare no conflicts of interest.
No funding was received to complete this study.
REFERENCES
- 1.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2015 Annual Data Report: kidney. Am J Transplant. 2017;17(Suppl 1):21–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14:1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294:2726–2733. [DOI] [PubMed] [Google Scholar]
- 4.Jay CL, Washburn K, Dean PG, et al. Survival benefit in older patients associated with earlier transplant with high KDPI kidneys. Transplantation. 2017;101:867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nyberg SL, Matas AJ, Rogers M, et al. Donor scoring system for cadaveric renal transplantation. Am J Transplant. 2001;1:162–170. [PubMed] [Google Scholar]
- 6.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88:231–236. [DOI] [PubMed] [Google Scholar]
- 7.Schold JD, Kaplan B, Baliga RS, et al. The broad spectrum of quality in deceased donor kidneys. Am J Transplant. 2005;5(4 Pt 1):757–765. [DOI] [PubMed] [Google Scholar]
- 8.Watson CJ, Johnson RJ, Birch R, et al. A simplified donor risk index for predicting outcome after deceased donor kidney transplantation. Transplantation. 2012;93:314–318. [DOI] [PubMed] [Google Scholar]
- 9.Munivenkatappa RB, Schweitzer EJ, Papadimitriou JC, et al. The Maryland aggregate pathology index: a deceased donor kidney biopsy scoring system for predicting graft failure. Am J Transplant. 2008;8:2316–2324. [DOI] [PubMed] [Google Scholar]
- 10.Remuzzi G, Grinyo J, Ruggenenti P, et al. Early experience with dual kidney transplantation in adults using expanded donor criteria. Double Kidney Transplant Group (DKG). J Am Soc Nephrol. 1999;10:2591–2598. [DOI] [PubMed] [Google Scholar]
- 11.Sung RS, Christensen LL, Leichtman AB, et al. Determinants of discard of expanded criteria donor kidneys: impact of biopsy and machine perfusion. Am J Transplant. 2008;8:783–792. [DOI] [PubMed] [Google Scholar]
- 12.Kasiske BL, Stewart DE, Bista BR, et al. The role of procurement biopsies in acceptance decisions for kidneys retrieved for transplant. Clin J Am Soc Nephrol. 2014;9:562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart DE, Garcia VC, Rosendale JD, et al. Diagnosing the decades-long rise in the deceased donor kidney discard rate in the United States. Transplantation. 2017;101:575–587. [DOI] [PubMed] [Google Scholar]
- 14.Jochmans I, O'Callaghan JM, Pirenne J, et al. Hypothermic machine perfusion of kidneys retrieved from standard and high-risk donors. Transpl Int. 2015;28:665–676. [DOI] [PubMed] [Google Scholar]
- 15.Gallinat A, Moers C, Treckmann J, et al. Machine perfusion versus cold storage for the preservation of kidneys from donors >/= 65 years allocated in the Eurotransplant Senior Programme. Nephrol Dial Transplant. 2012;27:4458–4463. [DOI] [PubMed] [Google Scholar]
- 16.Treckmann J, Moers C, Smits JM, et al. Machine perfusion versus cold storage for preservation of kidneys from expanded criteria donors after brain death. Transpl Int. 2011;24:548–554. [DOI] [PubMed] [Google Scholar]
- 17.de Vries EE, Hoogland ER, Winkens B, et al. Renovascular resistance of machine-perfused DCD kidneys is associated with primary nonfunction. Am J Transplant. 2011;11:2685–2691. [DOI] [PubMed] [Google Scholar]
- 18.Rege A, Irish B, Castleberry A, et al. Trends in usage and outcomes for expanded criteria donor kidney transplantation in the United States characterized by Kidney Donor Profile Index. Cureus. 2016;8:e887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhital KK, Iyer A, Connellan M, et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet. 2015;385:2585–2591. [DOI] [PubMed] [Google Scholar]
- 20.Machuca TN, Mercier O, Collaud S, et al. Lung transplantation with donation after circulatory determination of death donors and the impact of ex vivo lung perfusion. Am J Transplant. 2015;15:993–1002. [DOI] [PubMed] [Google Scholar]
- 21.Ravikumar R, Jassem W, Mergental H, et al. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (first-in-man) clinical trial. Am J Transplant. 2016;16:1779–1787. [DOI] [PubMed] [Google Scholar]
- 22.Ardehali A, Esmailian F, Deng M, et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet. 2015;385:2577–2584. [DOI] [PubMed] [Google Scholar]
- 23.Hosgood SA, Nicholson ML. First in man renal transplantation after ex vivo normothermic perfusion. Transplantation. 2011;92:735–738. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson ML, Hosgood SA. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant. 2013;13:1246–1252. [DOI] [PubMed] [Google Scholar]
- 25.Hosgood SA, Barlow AD, Hunter JP, et al. Ex vivo normothermic perfusion for quality assessment of marginal donor kidney transplants. Br J Surg. 2015;102:1433–1440. [DOI] [PubMed] [Google Scholar]
- 26.Hosgood SA, Saeb-Parsy K, Hamed MO, et al. Successful transplantation of human kidneys deemed untransplantable but resuscitated by ex vivo normothermic machine perfusion. Am J Transplant. 2016;16:3282–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosgood SA, Barlow AD, Dormer J, et al. The use of ex-vivo normothermic perfusion for the resuscitation and assessment of human kidneys discarded because of inadequate in situ perfusion. J Transl Med. 2015;13:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Winter JCF. Using the Student’s t-test with extremely small sample sizes. Practical Assessment, Research & Evaluation. 2013;18:1–12. [Google Scholar]
- 29.Blum MF, Liu Q, Soliman B, et al. Comparison of normothermic and hypothermic perfusion in porcine kidneys donated after cardiac death. J Surg Res. 2017;216:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosgood SA, Saeb-Parsy K, Wilson C, et al. Protocol of a randomised controlled, open-label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open. 2017;7:e012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaths JM, Hamar M, Echeverri J, et al. Normothermic ex vivo kidney perfusion for graft quality assessment prior to transplantation. Am J Transplant. 2018;18:580–589. [DOI] [PubMed] [Google Scholar]
- 32.Kaths JM, Echeverri J, Goldaracena N, et al. Eight-hour continuous normothermic ex vivo kidney perfusion is a safe preservation technique for kidney transplantation: a new opportunity for the storage, assessment, and repair of kidney grafts. Transplantation. 2016;100:1862–1870. [DOI] [PubMed] [Google Scholar]
- 33.Kaths JM, Cen JY, Chun YM, et al. Continuous normothermic ex vivo kidney perfusion is superior to brief normothermic perfusion following static cold storage in donation after circulatory death pig kidney transplantation. Am J Transplant. 2017;17:957–969. [DOI] [PubMed] [Google Scholar]
- 34.Brasile L, Stubenitsky BM, Haisch CE, et al. Repair of damaged organs in vitro. Am J Transplant. 2005;5:300–306. [DOI] [PubMed] [Google Scholar]
- 35.Kaths JM, Echeverri J, Linares I, et al. Normothermic ex vivo kidney perfusion following static cold storage-brief, intermediate, or prolonged perfusion for optimal renal graft reconditioning? Am J Transplant. 2017;17:2580–2590. [DOI] [PubMed] [Google Scholar]
- 36.Marshall V, Ross B, Smith M, et al. Organ and tissue preservation for transplantation: monitoring by 31P nuclear magnetic resonance. Transplant Proc. 1985;17:1693–1696. [PubMed] [Google Scholar]
- 37.Bretan PN, Jr, Vigneron DB, Hricak H, et al. Assessment of clinical renal preservation by phosphorus-31 magnetic resonance spectroscopy. J Urol. 1987;137:146–150. [DOI] [PubMed] [Google Scholar]
- 38.Bagul A, Hosgood SA, Kaushik M, et al. Experimental renal preservation by normothermic resuscitation perfusion with autologous blood. Br J Surg. 2008;95:111–118. [DOI] [PubMed] [Google Scholar]
- 39.Vogel T, Brockmann JG, Quaglia A, et al. The 24-hour normothermic machine perfusion of discarded human liver grafts. Liver Transpl. 2017;23:207–220. [DOI] [PubMed] [Google Scholar]
- 40.Liu Q, Nassar A, Buccini L, et al. Ex situ 86-hour liver perfusion: pushing the boundary of organ preservation. Liver Transpl. 2018;24:557–561. [DOI] [PubMed] [Google Scholar]
- 41.Chapal M, Le Borgne F, Legendre C, et al. A useful scoring system for the prediction and management of delayed graft function following kidney transplantation from cadaveric donors. Kidney Int. 2014;86:1130–1139. [DOI] [PubMed] [Google Scholar]
- 42.Warnecke G, Moradiellos J, Tudorache I, et al. Normothermic perfusion of donor lungs for preservation and assessment with the Organ Care System Lung before bilateral transplantation: a pilot study of 12 patients. Lancet. 2012;380:1851–1858. [DOI] [PubMed] [Google Scholar]
- 43.Patel SK, Pankewycz OG, Nader ND, et al. Prognostic utility of hypothermic machine perfusion in deceased donor renal transplantation. Transplant Proc. 2012;44:2207–2212. [DOI] [PubMed] [Google Scholar]
- 44.Jochmans I, Moers C, Smits JM, et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann Surg. 2010;252:756–764. [DOI] [PubMed] [Google Scholar]
- 45.Moers C, Smits JM, Maathuis MH, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360:7–19. [DOI] [PubMed] [Google Scholar]
- 46.Gallinat A, Amrillaeva V, Hoyer DP, et al. Reconditioning by end-ischemic hypothermic in-house machine perfusion: a promising strategy to improve outcome in expanded criteria donors kidney transplantation. Clin Transplant. 2017;31: e12904. [DOI] [PubMed] [Google Scholar]
- 47.Ciancio G, Gaynor JJ, Sageshima J, et al. Favorable outcomes with machine perfusion and longer pump times in kidney transplantation: a single-center, observational study. Transplantation. 2010;90:882–890. [DOI] [PubMed] [Google Scholar]
- 48.Rijkmans BG, Buurman WA, Kootstra G. Six-day canine kidney preservation. Hypothermic perfusion combined with isolated blood perfusion. Transplantation. 1984;37:130–134. [DOI] [PubMed] [Google Scholar]