Abstract
Objective:
Reduction in capillary density or rarefaction is a hallmark of essential hypertension. We measured the retinal capillary density using noninvasive optical coherence tomographic angiography (OCT-A) in adults with treated systemic hypertension and determined possible correlations with ambulatory blood pressure (BP) and renal parameters.
Methods:
This observational cross-sectional study consisted of 153 normal eyes from 77 nondiabetic hypertensive adults [mean (SD) age, 58 (9) years; 49% women; 23% poorly controlled BP]. Data on 24-h ambulatory BP monitoring, serum creatinine, and urine microalbumin/creatinine ratio (MCR) were collected. Estimated glomerular filtration rate (eGFR) was calculated based on CKD-EPI Creatinine Equation. Retinal capillary density measured with the OCT-A (AngioVue) at superficial (SVP) and deep vascular plexuses (DVP). Linear regression was used to investigate the association of risk factors with capillary density.
Results:
Retinal capillary density (percentage) at DVP was reduced in patients with poorly controlled BP (SBP = 148 ± 8 mmHg; 27.2 ± 13.0) compared with those with well controlled BP (SBP = 125 ± 9 mmHg; 34.7 ± 11.3). In the multivariable analysis, poorly controlled BP [β = −6.49, 95% confidence interval (CI), −12.39 to −0.59], higher SBP (β = −0.23, 95% CI −0.44 to −0.02) and lower eGFR (β = 6.42, 95% CI 1.25–11.60) were associated with sparser retinal capillary density. Systemic factors were not associated with capillary density at SVP (all P > 0.05).
Conclusion:
In adults with treated systemic hypertension, retinal capillary density reduced with higher BP and poorer eGFR. These findings highlight the potential role of OCT-A to study early microvascular changes because of systemic hypertension.
Keywords: ambulatory blood pressure monitoring, blood pressure, hypertension, optical coherence tomographic angiography, renal function, retina
INTRODUCTION
Systemic hypertension has profound effects on both the structure and function of the microvasculature [1]. The presence of microvascular disease, specifically microvascular rarefaction, is thought to be a key pathological characteristic of hypertension [2]. Microvascular rarefaction in turn have been linked to cardiovascular disease [3], stroke, left ventricular failure, and nephropathy [4] seen in hypertension [5], and being also a possible target for treatment [6]. In addition, hypertension is a risk factor for major eye disease such as diabetic retinopathy [7], age-related macular degeneration [8] and glaucoma [9]. Despite the importance of the microcirculation in hypertension, it is technically challenging to conduct in-vivo clinical studies on the microcirculation [10].
The retinal vasculature, measuring 100–300 μm in diameter [11], offer a unique and easily accessible ‘window’ to study the health and disease of the human microcirculation. Previous studies have used computer algorithms to measure retinal arteriolar and venular diameter from digital fundus photographs [12]. Epidemiological data of more than 20 000 individuals has demonstrated that retinal arteriolar narrows, as blood pressure (BP) levels increase [13]. Studies have also reported relationship of retinal arteriolar narrowing with stroke [14–16], kidney disease [17,18] and cardiovascular mortality [19,20]. However, images obtained from this technology do not provide any information about the retinal capillary circulation, which is more representative of the entire microvascular network and includes capillaries that are 3.5–6 μm in diameter [11].
Recent advances in optical coherence tomographic angiography (OCT-A) [21–23] has allowed for fast, noninvasive assessment of the capillaries, and have been used to evaluate eye diseases such as glaucoma [24–26] and diabetic retinopathy [27,28]. Microvascular imaging technology such as the OCT-A can provide information about the architectural organization of capillaries, whose changes in structures may be the earliest markers of ischemia/hypoxia, before arteriolar and venular changes. In diabetic patients, reduced retinal capillary density was associated with hyperlipidemia, smoking and renal impairment [27].
Given that the reduction of capillary density (also known as capillary rarefaction) is a hallmark of essential hypertension, we measured the retinal capillary density using OCT-A in adults with treated systemic hypertension and evaluated possible correlations with ambulatory BP and renal parameters. We hypothesized that individuals with higher BP or poorer kidney function will have reduced retinal capillary density.
METHODS
Study participants
We conducted a prospectively planned observational cross-sectional study including 153 eyes from 77 from participants with essential hypertension enrolled in the Response of the Myocardium to Hypertrophic Conditions in the Adult Population (REMODEL; Response of the myocardium to hypertrophic conditions in the adult population; NCT02670031) [29]. Briefly, Asians with essential hypertension on antihypertensive medications, aged 18 years and older, were recruited from a tertiary cardiac centre and primary care clinics in Singapore, from January 2017 to October 2017. Participants with secondary causes of hypertension, any on-going unstable medical conditions, previously diagnosed significant coronary artery disease (defined as previous myocardial infarction, more than 70% coronary stenosis on invasive coronary angiography or positive cardiac stress tests), strokes, atrial fibrillation and women who are pregnant or breast feeding were excluded from the study.
Study was approved by the SingHealth Centralized Institutional Review Board, and conducted in accordance to the Declaration of Helsinki. Written informed consent was obtained from participants.
Examination procedures
Detailed interviewer-administered questionnaire was used to collect demographic data, lifestyle risk factors, medical history and medication use. Ethnicities were set by the Singapore census [30]. 24-h ambulatory BP (SBP and DBP) were measured in all participants. Blood pressure was monitored using ambulatory blood pressure monitors (SpaceLabs 90217A; SpaceLabs Medical, Dee Why, Sydney, Australia) for 24 h. The device was put on by a trained research officer, average of 14 days before the eye examination. The monitors are programmed to take measurements at 20 min intervals from 0600 to 2200 h and the frequency is reduced to every 30 min after 2300 h. An appropriately sized blood pressure cuff, based on the circumference of the patient's upper arm, will be provided to the patient. Calibration is then performed by measuring at least one manual reading with the monitor to ensure the monitor is working. Of note, the ambulatory blood pressure monitors have been validated according to the British Hypertension Society [31]. Hypertensive patients were stratified into two groups: well controlled BP defined as SBP less than 140 mmHg and/or DBP less than 90 mmHg and poorly controlled BP defined as SBP at least 140 mmHg and/or DBP at least 90 mmHg. Hyperlipidemia was defined based on clinical history of elevated cholesterol or lipid-lowering medications. Participants’ height was measured using a wall-mounted measuring tape, and weight was measured using a digital scale (SECA, model 782 2321009, Germany). BMI was calculated as body weight (in kilograms) divided by body height (in meters) squared. Smoking status was defined as those never smoked, current smokers and past smokers.
Blood and mid-stream urine samples were collected for analysis of serum creatinine and urine microalbumin/creatinine ratio (MCR). Bio-specimens were processed in an accredited laboratory at the Singapore General Hospital. eGFR (in l/min per 1.73 m2)was calculated from plasma creatinine using the recently developed Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [32]. MCR was measured using immunoassay. Normal MCR range was 0.2–3.3 mg/mmol creatinine whilst values greater than 33.9 mg/mmol creatinine implied clinical albuminuria.
Ocular examinations
Participants underwent a questionnaire regarding their ocular history (e.g. intraocular surgery or glaucoma). Intraocular pressure was measured using noncontact tonometry (Auto Non-Contact Tonometer, NT-3000; Nidek, Gamagori, Japan). Fundus photography and OCT-A were performed approximately 30 min after topical instillation of two drops of 1% tropicamide, given 5 min apart. Fundus photography was then performed using a retinal camera (Canon CR-DGi with a 10-DSLR back; Canon, Tokyo, Japan). Patients with eye diseases (e.g. glaucoma, vascular or nonvascular retinopathies, age-related macular degeneration) were not included in the study.
Optical coherence tomographic angiography
The OCT-A imaging system provides a noninvasive method for visualizing the retinal vasculature (AngioVue; Optovue, Inc., Fremont, California, USA). The AngioVue OCT-A captures the dynamic motion of moving particles, such as red blood cells flowing in a blood vessel, and allows a high-resolution three-dimensional visualization of perfused vasculature [24]. The OCT-A characterizes vascular information at each retinal layers as an en face angiogram, a capillary density map (Fig. 1), and quantitatively as capillary density (percentage), calculated as the percentage area occupied by blood vessels with flowing erythrocytes in the selected region.
FIGURE 1.
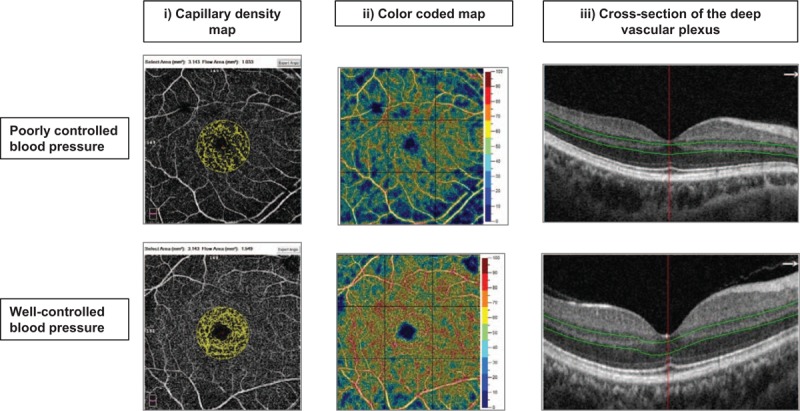
Capillary density map of the macular region showing retinal microvasculature of participants with (a) poorly controlled blood pressure (less dense) and (b) well controlled blood pressure (more dense). (i) Capillary density extracted map with a circular (diameter of 1.0 mm centered on the fovea) measurement region defined. (ii) Capillary density color-coded map. (iii) Deep vascular plexus (slab boundary of 15–70 μm below the inner plexiform layer).
For this study, we used capillary density measurements within the macula, in scans with a 6.0 × 6.0 mm2 field of view centered on the fovea [24]. En face OCT angiograms with 304 × 304-pixel dimensions were produced by maximum decorrelation (i.e. flow) projection between the segmentation lines. Each scan was automatically segmented by the AngioVue software (version 2016.2.0.35) so as to visualize the superficial (SVP) and deep vascular plexuses (DVP). Capillary density was calculated in a circular region with a diameter of 1.0 mm centered on the fovea.
A trained grader masked to the participant characteristics reviewed the quality of all OCT-A scans. Poor quality scans were excluded from the analysis if one of the following criteria were met: poor clarity images; local weak signal caused by artifacts such as floaters; residual motion artifacts visible as irregular vessel patterns on the en face angiogram and scans with segmentation failure.
Of the 95 participants, we excluded participants with diabetes (n = 15) and eye disease (n = 1) and missing or poor quality OCT-A images (n = 2), leaving 77 participants for analysis (Fig. 2).
FIGURE 2.
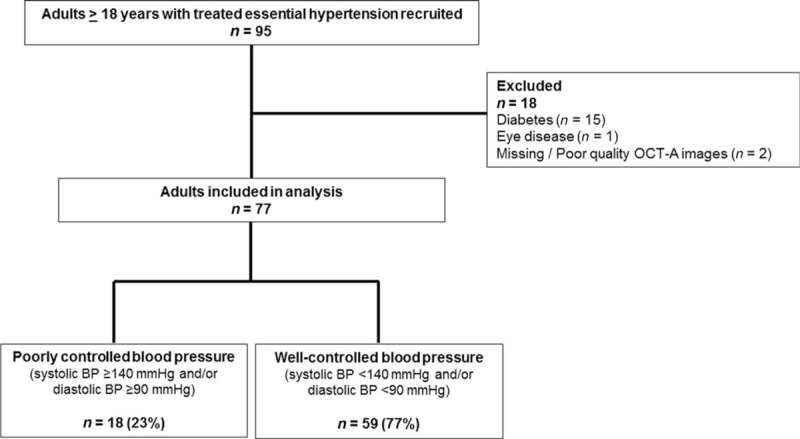
Identification of study participants. Of the 77 participants with systemic hypertension, 18 (23%) had poorly controlled blood pressure despite use of antihypertensive medications.
Statistical analyses
Primary outcome was retinal capillary density at SVP and DVP. Shapiro–Wilk test was used to assess the normality of the distribution of the continuous variables. To compare continuous variables between groups, an independent t-test was performed for normally distributed variables, whereas Kruskal–Wallis test was used for nonnormally distributed variables. Continuous variables that were normally distributed are presented as mean ± standard deviation whereas nonnormally distributed variables are presented as median [interquartile range (IQR)]. Chi-square test or Fisher's exact test were used for categorical variables. There is a moderate positive linear correlation between the right and left eyes for capillary density in the superficial (Pearson correlation coefficient, r = 0.43; P value <0.001) as well as the deeper (r = 0.45; P value <0.001) plexus of the fovea, however, they are far from being perfectly correlated. Hence, there are differences between both eyes within one patient. Using data from summary or one eye of each participant gives reduced power, whereas model considering eye as the unit of analysis with consideration of correlation between paired eyes have greater statistical power and allow maximal use of available data [33]. Hence, we have applied the generalized estimating equation approach, which gives us increased precision of estimation results while accounting for the correlation between fellow eyes [34,35]. Associations between systemic factors (independent variables) with retinal capillary density (dependent variable) were assessed using linear regression models with generalized estimating equations. Data were analyzed with statistical software (STATA, version 13.1; StataCorp LP, College Station, Texas, USA).
RESULTS
The clinical characteristics of the adults with systemic hypertension by BP levels are shown in Table 1. The mean ± SD age of participants was 58 ± 9 years and 49% were women (n = 38). Persons with well controlled BP had lower levels of urine MCR and higher retinal capillary density at DVP than those with poorly controlled BP despite use of antihypertensive medications (P < 0.05 each). Of note, there was no difference in the number and type of antihypertensive medications for both groups.
TABLE 1.
Clinical characteristics of treated hypertensive participants, stratified by blood pressure control
| Participants with poorly controlled blood pressure | Participants with well controlled blood pressure | P value | |
| Number of participants | 18 | 59 | |
| Number of eyes | 36 | 117 | |
| Sex, female (%) | 10 (56%) | 28 (47%) | 0.547a |
| Ethnicity, Chinese (%) | 16 (89%) | 50 (85%) | 0.597a |
| Smoking status, never (%) | 18 (100%) | 55 (93%) | 0.525a |
| Hyperlipidemia (%) | 7 (39%) | 27 (46%) | 0.607a |
| Chronic kidney disease (%) | 1 (6%) | 4 (7%) | 0.668a |
| Type of antihypertensive medications | |||
| Beta blockers | 3 (17%) | 22 (37%) | 0.102a |
| Calcium channel blockers | 12 (67%) | 29 (49%) | 0.192a |
| Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers | 7 (39%) | 32 (54%) | 0.254a |
| Others (diuretics, alpha 2 adrenergic agonist) | 2 (11%) | 9 (15%) | 0.660a |
| Numbers of antihypertensive medications | |||
| One | 12 (67%) | 33 (56%) | |
| Two | 5 (28%) | 18 (30%) | 0.767a |
| Three or more | 1 (5%) | 8 (14%) | |
| Mean ± SD / median (IQR) | |||
| Age (years) | 59 ± 11 | 57 ± 8 | 0.445a |
| BMI (kg/m2) | 25.0 (23.1–27.6) | 26.2 (23.8–28.6) | 0.219a |
| Ambulatory BP (mmHg) | |||
| SBP | 148 ± 8 | 125 ± 9 | <0.001a |
| DBP | 87 ± 7 | 77 ± 7 | <0.001a |
| Mean arterial pressure | 107 ± 5 | 93 ± 7 | <0.001a |
| eGFR (ml/min per 1.73 m2) | 90.2 (86.7–95.7) | 92.4 (76.2–102.2) | 0.945a |
| Creatinine (pmol/l) | 73 (58–86) | 70 (60–79) | 0.346a |
| Urine MCR (mg/g) | 1.6 (1.1–3.7) | 0.75 (0–2.7) | 0.042a |
| Retinal capillary density (%) | |||
| Superficial vascular plexus | Both eyes: 37.2 ± 5.6 Right eyes: 37.4 ± 5.5 Left eyes: 37.6 ± 5.5 | Both eyes: 39.6 ± 5.3 Right eyes: 38.7 ± 5.8 Left eyes: 37.8 ± 6.1 | 0.355b |
| Deep vascular plexus | Both eyes: 27.2 ± 13.0 Right eyes: 26.3 ± 14.2 Left eyes: 25.4 ± 14.0 | Both eyes: 34.7 ± 11.3 Right eyes: 32.7 ± 11.0 Left eyes: 33.9 ± 10.6 | 0.039b |
BP, blood pressure; CI, confidence interval; eGFR, estimated glomerular filtration rate; MCR, microalbumin-to-creatinine ratio.
Data are number (%) or mean ± standard deviation (SD) or median (interquartile range), as appropriate.
aFor individual-level analysis, test for differences between groups was based on independent t test for normally distributed continuous variables or Kruskal–Wallis for nonnormally distributed continuous variables and with chi-square tests or Fisher's exact test for categorical variables.
bFor eye-level analysis, test for differences between groups was based on generalized estimating equation. Bold face indicates statistically significant P value.
The association between BP and other systemic factors and retinal capillary density at DVP is shown in Table 2. Univariate linear regression analysis showed that BP control, SBP, MAP and eGFR were associated with retinal capillary density (Model 1; Table 2). We next adjusted for age and sex because age was significantly associated with retinal capillary density [univariate model: β = −0.45; 95% confidence interval (CI) −0.70 to −0.20; P < 0.001). Even though sex was not significantly associated with retinal capillary density (univariate model: β = −1.37; 95% CI −5.96 to 3.21; P = 0.557), it is plausible that sex may have a biological effect on retinal capillary density. Age and sex-adjusted showed that persons with poorly controlled BP had lower retinal capillary density (β = −6.03; 95% CI −11.63 to −0.43; P = 0.035; Model 2; Table 2) compared with those with well controlled BP. Figure 1 further illustrates the relation of BP control and retinal capillary density. In the age and sex-adjusted model, higher SBP (β = −0.22; 95% CI −0.40 to −0.03; P = 0.021), higher DBP (β = −0.31; 95% CI −0.56 to −0.06; P = 0.016) and higher MAP (β = −0.32; 95% CI −0.58 to −0.07; P = 0.012) were associated with lower retinal capillary density whereas eGFR (β = 1.24; 95% CI −7.04 to 9.53; P = 0.769; Model 2; Table 2) was not associated with retinal capillary density. This is because CKD-EPI Creatinine Equation considered age in their equation when estimating GFR. Both creatinine and urine MCR were not associated with retinal capillary density, in either univariate or age-adjusted models.
TABLE 2.
Associations of systemic factors with retinal capillary density at deep vascular plexus (dependent variable) in treated hypertensive participants (n = 153 eyes)
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||||||||
| β | 95% CI | P value | β | 95% CI | P value | β | 95% CI | P value | β | 95% CI | P value | β | 95% CI | P value | |
| BP control status | |||||||||||||||
| Well controlled BP | Reference | Reference | Reference | ||||||||||||
| Poorly controlled BP | −6.36 | −12.38 to −0.33 | 0.039 | −6.03 | −11.63 to −0.43 | 0.035 | −6.49 | −12.39 to −0.59 | 0.031 | – | – | – | – | – | – |
| Ambulatory BP (mmHg) | |||||||||||||||
| SBP | −0.24 | −0.45 to −0.03 | 0.024 | −0.22 | −0.40 to −0.03 | 0.021 | −0.23 | −0.44 to −0.02 | 0.031 | ||||||
| DBP | −0.21 | −0.47 to 0.06 | 0.124 | −0.31 | −0.56 to −0.06 | 0.016 | – | – | – | −0.25 | −0.52 to 0.03 | 0.080 | |||
| Mean arterial pressure | −0.29 | −0.57 to −0.01 | 0.039 | −0.32 | −0.58 to −0.07 | 0.012 | |||||||||
| eGFR (ml/min per 1.73 m2) | 7.37 | 1.14–13.60 | 0.020 | 1.24 | −7.04 to 9.53 | 0.769 | 7.64 | 2.45–12.85 | 0.004 | 6.42 | 1.25–11.60 | 0.015 | 8.44 | 2.13–14.75 | 0.009 |
| Creatinine | −4.07 | −10.58 to 2.43 | 0.220 | −1.45 | −10.22 to 7.33 | 0.746 | – | – | – | – | – | – | – | – | – |
| Urine MCR (mg/g) | 0.77 | −1.63 to 3.17 | 0.529 | 0.80 | −1.09 to 2.69 | 0.406 | – | – | – | – | – | – | – | – | – |
BP, blood pressure; CI, confidence interval; eGFR, estimated glomerular filtration rate; MCR, microalbumin-to-creatinine ratio. Data were analyzed by the use of linear regression model with generalized estimating equation and presented as mean ± standard deviation or number (%), as appropriate. Bold face indicates statistically significant P value. Model 1: univariate; Model 2: adjusted for age and sex; Model 3: adjusted for eGFR and BP control status; model 4: adjusted for eGFR and SBP; Model 5: adjusted for eGFR DBP.
In separate models that considered only for BP (either for BP control, SBP, DBP or MAP) and eGFR (Table 2), retinal capillary density was related to BP control (β = −6.49, 95% CI −12.39 to −0.59; P = 0.031; Model 3), SBP (β = −0.23, 95% CI −0.44 to −0.02; P = 0.031; Model 4) and MAP (β = −0.30, 95% CI −0.59 to −0.02; P = 0.036) but not with DBP (β = −0.25, 95% CI −0.52 to 0.03; P = 0.080; Model 5). eGFR was associated with retinal capillary density (P < 0.05; Models 3–5; Table 2). In other words, persons with poorly controlled BP, higher SBP, higher MAP and lower eGFR had a sparser retinal capillary density. Figure 3 further illustrates the association observed between higher SBP and lower eGFR with sparser retinal capillary density. There were significant associations between 24-h ambulatory SBP (r = −0.27; P = 0.001) and eGFR (r = 0.26; P = 0.002) with retinal capillary density with among participants with systemic hypertension at deep vascular plexus. Systemic factors were, however, not associated with retinal capillary density at SVP (all P > 0.05; Table 3).
FIGURE 3.
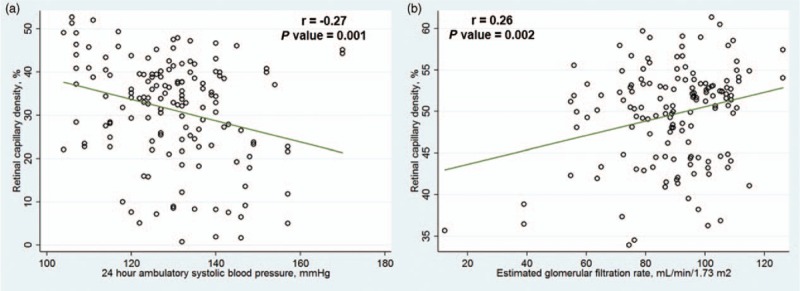
Scatterplot showing (a) negative correlation of retinal capillary density with 24-h ambulatory SBP and (b) positive correlation of retinal capillary density with estimated glomerular filtration rate among participants with systemic hypertension at deep vascular plexus. Pearson's correlation coefficients and P values shown indicate the strengths of the linear relationship between the variables.
TABLE 3.
Associations of systemic factors with retinal capillary density at superficial vascular plexus (dependent variable) in treated hypertensive participants (n = 153 eyes)
| Univariate model | |||
| β | 95% CI | P value | |
| BP control status | |||
| Well controlled BP | Reference | ||
| Poorly controlled BP | −1.13 | −3.54 to 1.27 | 0.355 |
| Ambulatory BP (mmHg) | |||
| SBP | −0.06 | −0.16 to 0.03 | 0.193 |
| DBP | −0.01 | −0.12 to 0.11 | 0.895 |
| Mean arterial pressure | −0.05 | −0.18 to 0.07 | 0.419 |
| eGFR (ml/min per 1.73 m2) | 0.05 | −0.01 to 0.11 | 0.064 |
| Urine MCR (mg/g) | 0.32 | −0.53 to 1.17 | 0.463 |
BP, blood pressure; CI, confidence interval; eGFR, estimated glomerular filtration rate; MCR, microalbumin-to-creatinine ratio. Data were analyzed by the use of linear regression model with generalized estimating equation and presented as mean ± standard deviation or number (%), as appropriate.
DISCUSSION
In this study, we describe the use of OCT-A on adults with treated systemic hypertension and examine the correlation of OCT-A measured capillary density with systemic vascular risk factors. We document changes in the retinal capillary microvasculature that are associated with ambulatory BP as well as eGFR. This highlights the potential role of OCT-A in detecting early microvascular changes for hypertension.
We report several significant findings that are important in advancing our understanding of the changes at the retinal capillary level with ambulatory BP and eGFR. We show that having poorly controlled BP, higher ambulatory BP and lower eGFR were associated with reduced DVP capillary density, suggesting that systemic risk factors could lead to retinal microcirculatory changes in the capillary network of hypertensive patients. This is in keeping with what had been published previously on larger retinal vessels: a correlation between the retinal arteriolar narrowing with increasing levels of BP [36–39] and renal dysfunction [40,41]. Also, people with early diabetes have shown capillary rarefaction compared with healthy persons [42].
The mechanism underlying the relation between retinal capillary density and systemic hypertension is unclear based on the present data. However, it may be those with chronic hypertension develop atherosclerosis over time, where vessels become narrow with age [36–39], leading to increased resistance to blood flow as well as breakdown of retinal vascular autoregulation [43]. Studies using scanning laser Doppler flowmetry have indicated an increased retinal vascular resistance [44,45] and reduction in retinal capillary density [46] in hypertensive patients compared with normotensive individuals [44,45]. An increased resistance of the vasculature may impair blood flow. This slow blood flow may be reflected as nonperfusion on OCT-A. As OCT-A relies on change between consecutive retinal scans, it will detect flow only above a minimum threshold [47]. Regions that have flow below the slowest detectable flow would, therefore, be considered as nonperfusion using the OCT-A imaging technique. Therefore, microvascular rarefaction might present an interesting target for treatment. Indeed, retinal capillary rarefaction was shown to be reversible after antihypertensive therapy in hypertensive patients [48]. Although we have provided evidence to support retinal capillary nonperfusion as the cause of hypertension, this proposed mechanism has not yet been proven. Showing causality would require animal models of chronic hypertension and subsequent OCT-A imaging.
The presence of microvascular changes in the eye (reduced retinal capillary density using OCT-A) was independently associated with kidney function (lower eGFR levels). Such significance of changes in larger retinal vessels, that is, hypertensive retinopathy has long been seen in patients with renal disease [49]. This is in keeping with what had been published previously on larger retinal vessels: a correlation between the retinal arteriolar narrowing with renal dysfunction [40,41], and chronic kidney disease [17,18]. In addition, a recent study using scanning laser Doppler flowmetry reported patients with moderately severe chronic kidney disease showed retinal signs such as arteriolar hypertrophic remodeling and capillary rarefaction [50]. The retinal and renal circulations share similarities in terms of its anatomic and physiologic characteristics [51]. We can speculate that a sparser network of retinal capillaries, expression of microvascular rarefaction, may mirror similar alterations in the renal microcirculation. Future studies can harness the unique noninvasive OCT-A imaging of the retinal microvasculature and explore if it can truly provide an insight into hypertension status and its related damage in the kidney.
What is the immediate significance of these findings? Variables such as BP and eGFR can influence the retinal capillary density and should be accounted for in studies that characterize patients with eye diseases. Most eye-related studies have defined their control groups as individuals without eye diseases [24–28,52,53], except for one study, which further excluded participants if they had systemic vascular conditions, that is, diabetes, hypertension [26]. Clinical investigators when using the OCT-A to investigate the role of altered retinal microvasculature in eye diseases should either exclude individuals having systemic hypertension from the control cohort or statistically account for systemic vascular risks. Failure to account for systemic risk factors as potential confounders can bias study results and lead to erroneous conclusions.
Our data may explain the inconsistencies among studies examining the use of OCT in glaucoma detection [54,55]. Some studies [55] have reported that the OCT-A had a superior diagnostic capability than conventional imaging tools at identifying individuals with glaucoma, whereas others failed to do so [54]. Discrepancies among studies are likely attributed to the systemic vascular profiles of patients included in the analysis, particularly among individuals with systemic hypertension, because higher BP could also reduce the retinal capillary density. As such, further studies with well defined controls are required and systemic blood pressure needs to be reported.
Our study may help shed new light on the role of systemic hypertension in glaucoma. There may be at least two reasons why patients with hypertension have a higher risk of glaucoma [56]. Firstly, we have previously reported in a prospective population-based study that the increase in BP was associated with an increase in eye pressure [57], a key risk factor in glaucoma. Secondly, persons with poorly controlled BP lose their retinal capillaries as a result of high BP, as supported by the current study. This is compatible with the hypothesis of a vascular component being involved in the pathogenesis of glaucoma [58]. As such microvascular retinal damage may be a contributing factor to the progressive loss of retinal ganglion cells with the disease. Although our findings do not directly indicate a beneficial effect of reduced BP on the risk of glaucoma, they are compatible with that possibility.
There are two main levels of capillary networks within the retina, which spread like a net of cobweb throughout the retina [11]. Changes of capillary density were found exclusively within the deeper layer and not the superficial layer, suggesting that systemic hypertension could affect one retinal vascular layer differently from the other. Other systemic vascular conditions such as diabetes demonstrated widespread changes in both vascular plexuses [27]. Bonnin et al.[59] have used OCT-A in normal individuals and reported different topographic organizations of the SVP and DVP. They have speculated that the different structural patterns of these two capillary plexuses may be related to their flow resistance and perfusion.
Study strengths and limitations
Strengths of this study included the use of ambulatory BP monitoring as well as the availability of urine MCR and eGFR for objective measurements of renal function. Some limitations of this investigation warrant discussion. First, the reduced capillary density that we observed could be the result of either capillary dropout or very slow rates of blood flow within the perfused capillaries. The current OCT-A device visualizes capillaries by detecting motion contrast from blood flow. A vessel with very slow or absent flow below the detection threshold of the instrument will not be detected [47]. For this reason, the term ‘capillary density’ is used as a quantitative summary measure of the vascular structures detected that reflects the proportion of area occupied by flowing vessels. Also, the OCT-A technology is unable to determine the true extension of a vessel. An interesting approach may be the combination of OCT-A and adaptive optics [60]. Adaptive optics is an optoelectronic technology that enables in-vivo ultra-high resolution imaging of retinal small arteries in humans [61]. It provides ultra-high resolution of vessel morphology of a small region (1.2 × 1.2 mm). Using adaptive optics-OCT-A, the vessels of the entire retinal vasculature can be better differentiated, providing crucial information on vessel morphology as well as vessel dropout. Second, even though there was reduced capillary density among those with poorly controlled BP in this study using OCT-A, this was only a cross-sectional study. OCT-A was introduced recently and the follow-up study is ongoing. Another limitation of this study was the relatively small sample size, which limited the power for evaluating the effect of some possible confounding variables on the retinal capillary density. In our results, some persons with higher SBP showed reduced retinal capillary density, whereas others seemed to have normal retinal capillary density (Fig. 3). Our small sample size does not allow us to perform sub-group analysis to study the pharmacological effect of differing classes of BP-lowering drugs on changes in retinal capillary density. Prediabetes is known to affect the microvasculature/macrovasculature [62]. As all our participants did not have diabetes, recent HbA1c or fasting glucose measurements was not available in the electronic medical records. In future work, it will be interesting to examine their HbA1c or fasting glucose in relation to OCT-A parameters. Finally, we did not assess the repeatability of our measurements. However, the repeatability for the retinal capillary density measurements has been published previously and reported to be excellent [63].
In conclusion, we have demonstrated a relationship between retinal capillary density with ambulatory BP and eGFR in adults with treated systemic hypertension. Measurement of retinal microvasculature at capillary level, using the OCT-A, is a unique noninvasive tool that has the potential to study early microvascular changes because of hypertension. Further studies are warranted to evaluate the efficacy of retinal capillary density to be a novel biomarker in prognosticating the incidence and progression of microvascular complications associated with hypertension.
ACKNOWLEDGEMENTS
Authors’ contributions: J.C. and L.S. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: T.Y.W., L.S. and J.C.
Acquisition, analysis, or interpretation of data: all authors.
Drafting of the manuscript: J.C., C.W.L.C. and L.S.
Critical revision of the manuscript for important intellectual content: all authors.
Statistical analysis: J.C. and M.L.C.
Obtained funding: L.S.
Administrative, technical, or material support: all authors.
Study supervision: L.S.Funding received for this work from National Medical Research Council (NMRC/CG/C010A/2017), Singapore.
Conflict of interest
There are no conflicts of interest.
Footnotes
Abbreviations: BP, blood pressure; eGFR, estimated glomerular filtration rate; MCR, microalbumin/creatinine ratio; OCT-A, optical coherence tomographic angiography
REFERENCES
- 1.Mulvany MJ, Aalkjaer C. Structure and function of small arteries. Physiol Rev 1990; 70:921–961. [DOI] [PubMed] [Google Scholar]
- 2.Struijker Boudier H, le Noble JL, Messing MW, Huijberts MS, le Noble FA, van Essen H. The microcirculation and hypertension. J Hypertens Suppl 1992; 10:S147–S156. [PubMed] [Google Scholar]
- 3.Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, et al. Prognostic significance of small-artery structure in hypertension. Circulation 2003; 108:2230–2235. [DOI] [PubMed] [Google Scholar]
- 4.Kida Y, Tchao BN, Yamaguchi I. Peritubular capillary rarefaction: a new therapeutic target in chronic kidney disease. Pediatr Nephrol 2014; 29:333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agabiti-Rosei E, Rizzoni D. Microvascular structure as a prognostically relevant endpoint. J hypertens 2017; 35:914–921. [DOI] [PubMed] [Google Scholar]
- 6.Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation 2001; 104:735–740. [DOI] [PubMed] [Google Scholar]
- 7.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012; 35:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology 2000; 107:2224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong TY, Mitchell P. The eye in hypertension. Lancet 2007; 369:425–435. [DOI] [PubMed] [Google Scholar]
- 10.Struijker-Boudier HA, Heijnen BF, Liu YP, Staessen JA. Phenotyping the microcirculation. Hypertension 2012; 60:523–527. [DOI] [PubMed] [Google Scholar]
- 11.Forrester JV, Dick AD, McMenamin PG, Roberts F, Pearlman E. The eye: basic sciences in practice: chapter 1: anatomy of the eye and orbit. 2016; New York: Elsevier, pp. 1–102. [Google Scholar]
- 12.Cheung CY, Ikram MK, Sabanayagam C, Wong TY. Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension 2012; 60:1094–1103. [DOI] [PubMed] [Google Scholar]
- 13.Chew SK, Xie J, Wang JJ. Retinal arteriolar diameter and the prevalence and incidence of hypertension: a systematic review and meta-analysis of their association. Curr Hypertens Rep 2012; 14:144–151. [DOI] [PubMed] [Google Scholar]
- 14.Wong TY, Kamineni A, Klein R, Sharrett AR, Klein BE, Siscovick DS, et al. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the cardiovascular health study. Arch Intern Med 2006; 166:2388–2394. [DOI] [PubMed] [Google Scholar]
- 15.McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, et al. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am J Epidemiol 2009; 170:1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yatsuya H, Folsom AR, Wong TY, Klein R, Klein BE, Sharrett AR, et al. Retinal microvascular abnormalities and risk of lacunar stroke: Atherosclerosis Risk in Communities Study. Stroke 2010; 41:1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yau JW, Xie J, Kawasaki R, Kramer H, Shlipak M, Klein R, et al. Retinal arteriolar narrowing and subsequent development of CKD Stage 3: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 2011; 58:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yip W, Ong PG, Teo BW, Cheung CY, Tai ES, Cheng CY, et al. Retinal vascular imaging markers and incident chronic kidney disease: a prospective cohort study. Sci Rep 2017; 7:9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liew G, Wong TY, Mitchell P, Cheung N, Wang JJ. Retinopathy predicts coronary heart disease mortality. Heart 2009; 95:391–394. [DOI] [PubMed] [Google Scholar]
- 20.Wong TY, Klein R, Nieto FJ, Klein BE, Sharrett AR, Meuer SM, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology 2003; 110:933–940. [DOI] [PubMed] [Google Scholar]
- 21.Kashani AH, Chen CL, Gahm JK, Zheng F, Richter GM, Rosenfeld PJ, et al. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res 2017; 60:66–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res 2018; 64:1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wylegala A, Teper S, Dobrowolski D, Wylegala E. Optical coherence angiography: a review. Medicine 2016; 95:e4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shoji T, Zangwill LM, Akagi T, Saunders LJ, Yarmohammadi A, Manalastas PIC, et al. Progressive macula vessel density loss in primary open angle glaucoma: a longitudinal study. Am J Ophthalmol 2017; 182:107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee EJ, Lee KM, Lee SH, Kim TW. OCT angiography of the peripapillary retina in primary open-angle glaucoma. Invest Ophthalmol Vis Sci 2016; 57:6265–6270. [DOI] [PubMed] [Google Scholar]
- 26.Scripsema NK, Garcia PM, Bavier RD, Chui TY, Krawitz BD, Mo S, et al. Optical coherence tomography angiography analysis of perfused peripapillary capillaries in primary open-angle glaucoma and normal-tension glaucoma. Invest Ophthalmol Vis Sci 2016; 57:OCT611–OCT620. [DOI] [PubMed] [Google Scholar]
- 27.Ting DSW, Tan GSW, Agrawal R, Yanagi Y, Sie NM, Wong CW, et al. Optical coherence tomographic angiography in type 2 diabetes and diabetic retinopathy. JAMA Ophthalmol 2017; 135:306–312. [DOI] [PubMed] [Google Scholar]
- 28.Agemy SA, Scripsema NK, Shah CM, Chui T, Garcia PM, Lee JG, et al. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina 2015; 35:2353–2363. [DOI] [PubMed] [Google Scholar]
- 29.Goh VJ, Le TT, Bryant J, Wong JI, Su B, Lee CH, et al. Novel index of maladaptive myocardial remodeling in hypertension. Circulation Cardiovasc Imag 2017; 10: pii: e006840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Department of Statistics MoTaI, Republic of Singapore. Census of Population 2000 Statistical Release 1: demographic characteristics. Statistics Do editor Singapore. 2001. [Google Scholar]
- 31.O’Brien E, Waeber B, Parati G, Staessen J, Myers MG. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ 2001; 322:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sainani K. The importance of accounting for correlated observations. PM R 2010; 2:858–861. [DOI] [PubMed] [Google Scholar]
- 34.Ying GS, Maguire MG, Glynn R, Rosner B. Tutorial on biostatistics: linear regression analysis of continuous correlated eye data. Ophthalmic Epidemiol 2017; 24:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glynn RJ, Rosner B. Regression methods when the eye is the unit of analysis. Ophthalmic Epidemiol 2012; 19:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhargava M, Cheung CY, Sabanayagam C, Huang L, Lamoureux EL, Wang JJ, et al. Prevalence and risk factors for retinopathy in persons without diabetes: the Singapore Indian Eye Study. Acta Ophthalmol 2014; 92:e602–e609. [DOI] [PubMed] [Google Scholar]
- 37.Jeganathan VS, Cheung N, Tay WT, Wang JJ, Mitchell P, Wong TY. Prevalence and risk factors of retinopathy in an Asian population without diabetes: the Singapore Malay Eye Study. Arch Ophthalmol 2010; 128:40–45. [DOI] [PubMed] [Google Scholar]
- 38.Leung H, Wang JJ, Rochtchina E, Wong TY, Klein R, Mitchell P. Impact of current and past blood pressure on retinal arteriolar diameter in an older population. J Hypertens 2004; 22:1543–1549. [DOI] [PubMed] [Google Scholar]
- 39.Wong TY, Klein R, Klein BE, Meuer SM, Hubbard LD. Retinal vessel diameters and their associations with age and blood pressure. Investig Ophthalmol Vis Sci 2003; 44:4644–4650. [DOI] [PubMed] [Google Scholar]
- 40.Edwards MS, Wilson DB, Craven TE, Stafford J, Fried LF, Wong TY, et al. Associations between retinal microvascular abnormalities and declining renal function in the elderly population: the Cardiovascular Health Study. Am J Kidney Dis 2005; 46:214–224. [DOI] [PubMed] [Google Scholar]
- 41.Wong TY, Coresh J, Klein R, Muntner P, Couper DJ, Sharrett AR, et al. Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J Am Soc Nephrol 2004; 15:2469–2476. [DOI] [PubMed] [Google Scholar]
- 42.Jumar A, Harazny JM, Ott C, Friedrich S, Kistner I, Striepe K, et al. Retinal capillary rarefaction in patients with type 2 diabetes mellitus. PloS One 2016; 11:e0162608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidl D, Garhofer G, Schmetterer L. The complex interaction between ocular perfusion pressure and ocular blood flow - relevance for glaucoma. Exp Eye Res 2011; 93:141–155. [DOI] [PubMed] [Google Scholar]
- 44.Lehmann MV, Schmieder RE. Remodeling of retinal small arteries in hypertension. Am J Hypertens 2011; 24:1267–1273. [DOI] [PubMed] [Google Scholar]
- 45.Kannenkeril D, Harazny JM, Bosch A, Ott C, Michelson G, Schmieder RE, et al. Retinal vascular resistance in arterial hypertension. Blood Press 2018; 27:82–87. [DOI] [PubMed] [Google Scholar]
- 46.Bosch AJ, Harazny JM, Kistner I, Friedrich S, Wojtkiewicz J, Schmieder RE. Retinal capillary rarefaction in patients with untreated mild-moderate hypertension. BMC Cardiovasc Disord 2017; 17:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous 2015; 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jumar A, Harazny JM, Ott C, Kistner I, Friedrich S, Schmieder RE. Improvement in retinal capillary rarefaction after valsartan treatment in hypertensive patients. J Clin Hypertens (Greenwich) 2016; 18:1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunn RM. Ophthalmic evidence of (1) arterial changes associated with chronic renal disease and (2) of increased arterial tension. Trans Ophthalmol Soc UK 1892; 12:124–125. [Google Scholar]
- 50.Bosch A, Scheppach JB, Harazny JM, Raff U, Eckardt KU, Schmieder RE, et al. Retinal capillary and arteriolar changes in patients with chronic kidney disease. Microvasc Res 2018; 118:121–127. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz MM, Lewis EJ, Leonard-Martin T, Lewis JB, Batlle D. Renal pathology patterns in type II diabetes mellitus: relationship with retinopathy. The Collaborative Study Group. Nephrol Dial Transplant 1998; 13:2547–2552. [DOI] [PubMed] [Google Scholar]
- 52.Yarmohammadi A, Zangwill LM, Diniz-Filho A, Suh MH, Manalastas PI, Fatehee N, et al. Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Investig Ophthalmol Vis Sci 2016; 57:OCT451–OCT459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lupidi M, Coscas F, Cagini C, Fiore T, Spaccini E, Fruttini D, et al. Automated quantitative analysis of retinal microvasculature in normal eyes on optical coherence tomography angiography. Am J Ophthalmol 2016; 169:9–23. [DOI] [PubMed] [Google Scholar]
- 54.Rao HL, Kadambi SV, Weinreb RN, Puttaiah NK, Pradhan ZS, Rao DAS, et al. Diagnostic ability of peripapillary vessel density measurements of optical coherence tomography angiography in primary open-angle and angle-closure glaucoma. Br J Ophthalmol 2017; 101:1066–1070. [DOI] [PubMed] [Google Scholar]
- 55.Chen CL, Zhang A, Bojikian KD, Wen JC, Zhang Q, Xin C, et al. Peripapillary retinal nerve fiber layer vascular microcirculation in glaucoma using optical coherence tomography-based microangiography. Invest Ophthalmol Vis Sci 2016; 57:OCT475–OCT485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao D, Cho J, Kim MH, Guallar E. The association of blood pressure and primary open-angle glaucoma: a meta-analysis. Am J Ophthalmol 2014; 158:615–627. e619. [DOI] [PubMed] [Google Scholar]
- 57.Chua J, Chee ML, Chin CWL, Tham YC, Tan N, Lim SH, et al. Inter-relationship between ageing, body mass index, diabetes, systemic blood pressure and intraocular pressure in Asians: 6-year longitudinal study. Br J Ophthalmol 2018; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cherecheanu AP, Garhofer G, Schmidl D, Werkmeister R, Schmetterer L. Ocular perfusion pressure and ocular blood flow in glaucoma. Curr Opin Pharmacol 2013; 13:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonnin S, Mane V, Couturier A, Julien M, Paques M, Tadayoni R, et al. New insight into the macular deep vascular plexus imaged by optical coherence tomography angiography. Retina 2015; 35:2347–2352. [DOI] [PubMed] [Google Scholar]
- 60.Salas M, Augustin M, Ginner L, Kumar A, Baumann B, Leitgeb R, et al. Visualization of micro-capillaries using optical coherence tomography angiography with and without adaptive optics. Biomed Opt Express 2017; 8:207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koch E, Rosenbaum D, Brolly A, Sahel JA, Chaumet-Riffaud P, Girerd X, et al. Morphometric analysis of small arteries in the human retina using adaptive optics imaging: relationship with blood pressure and focal vascular changes. J Hypertens 2014; 32:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguyen TT, Wang JJ, Wong TY. Retinal vascular changes in prediabetes and prehypertension: new findings and their research and clinical implications. Diabetes Care 2007; 30:2708–2715. [DOI] [PubMed] [Google Scholar]
- 63.Coscas F, Sellam A, Glacet-Bernard A, Jung C, Goudot M, Miere A, et al. Normative data for vascular density in superficial and deep capillary plexuses of healthy adults assessed by optical coherence tomography angiography. Investig Ophthalmol Vis Sci 2016; 57:OCT211–OCT223. [DOI] [PubMed] [Google Scholar]


