Abstract
Background and objective:
Hypertension can lead to mood disorders that may worsen or ameliorate depending on the type of antihypertensive prescribed. Depression is associated with modifications in basal brain asymmetry particularly that of the frontal cortex, which is involved in blood pressure control. Furthermore, different vasoactive drugs may change the brain's asymmetry in a manner that contributes to cognition status. We studied the bilateral activity of several neuropeptidases in frontal cortex as a reflect of the functional status of certain neuropeptides involved in mood.
Methods:
Using arylamide derivatives as substrates, we fluorometrically analysed the activity of these enzymes in the left and right frontal cortex of control untreated Wistar–Kyoto (WKY) and spontaneously hypertensive rats (SHRs) and compared their activities with WKY or SHR treated with the antihypertensive drugs captopril (CAP) and propranolol (PRO) or with the hypertensive N (G)-nitro-l-arginine methyl ester. SBP was also measured in all WKY and SHR groups.
Results:
Untreated WKY, WKY treated with CAP or PRO and SHR treated with CAP exhibited normotensive values of SBP. However, WKY treated with N (G)-nitro-l-arginine methyl ester as well as untreated SHR and SHR treated with PRO and N(G)-nitro-l-arginine methyl ester demonstrated hypertensive values of SBP. Changes in the bilateral distribution of neuropeptidases were depending on the strain, the enzyme analysed and the drug used. Normotensive WKY groups (WKY, CAP, PRO) revealed intrahemispheric correlations mainly in the left hemisphere. In contrast, WKY treated with N(G)-nitro-l-arginine methyl ester and SHR groups demonstrated intrahemispheric correlations mainly in the right hemisphere. Interhemispheric correlations were mostly observed in WKY as well as in SHR groups with antihypertensive treatments (CAP, PRO).
Conclusion:
Our results suggest specific brain bilateral patterns of neuropeptidase activities in WKY that change in SHR. This observation may be related to the cognitive disorders that have been described in these animals and that change under antihypertensive or hypertensive drug's treatments.
Keywords: antihypertensives, brain asymmetry, hypertension, mood disorders, neuropeptidases, neuropeptides
INTRODUCTION
Hypertension may be responsible for the development of cognitive and mood disorders, including anxiety and depression, and antihypertensive drugs may therefore modulate these disorders [1]. In addition, depression and anxiety are characterized by altered bilateral functioning in the brain, particularly in the frontal cortex [2], which is directly involved in blood pressure control [3]. Thus, changes in cerebral haemodynamics, especially in the frontal cortex, may be involved in mood disorders [4]. It has been suggested that the risk of developing such disorders increases or decreases depending on the type of antihypertensive drugs used for treatments [1]. However, the current available results remain inconclusive with respect to this hypothesis [5,6].
The pathogeny of mood disorders involves alterations in the functional interactions of both the left and right hemispheres, in which the frontal cortex plays a major role [2,7]. Human studies have reported significant correlations between resting heart rate and asymmetry between the left and right frontal lobes as well as between the frontal and parietal lobes [8], suggesting a relative differential association between the left and right frontal and parietal lobes and cardiovascular activity. Similarly, animal studies have confirmed an asymmetrical bidirectional neuroendocrine interaction between the frontal cortex and cardiovascular function [9]. However, functional asymmetries may also be explained by neurochemical lateralizations [10]. Because the functions of neurotransmitters and neuroendocrine factors depend on their specific brain bilateral distribution and on their neurovisceral bilateral connection, deviations in this pattern, such as increases, decreases or changes in the side of dominance, may disrupt the interhemispheric balance and trigger cognitive and peripheral cardiovascular disorders [10,11].
Captopril treatment in hypertensive rats has been found to change the bilateral pattern of neuropeptidase distribution in the frontal cortex as well as the bilateral interaction of neuropeptidase activity between frontal cortex and plasma or ventricles [3,9]. We hypothesized that the use of different antihypertensive or hypertensive vasoactive drugs might result in different patterns of bilateral frontal cortex performance that contribute to the improvement or impairment of the behaviour consequences of hypertension, such as depression and anxiety.
Whereas the neuropeptides oxytocin [12] and enkephalins [13] are regarded as anxiolytic agents, angiotensin (Ang) II is considered as an anxiogenic factor [14]. The functional status of these neuropeptides is regulated by their respective neuropeptidases oxytocinase (cystinyl-aminopeptidase, CysAP, EC 3.4.11.3), enkephalinase (alanyl-aminopeptidase, AlaAP, EC 3.4.11.2,) and angiotensinase A (aminopeptidase A, glutamyl-aminopeptidase, GluAP, EC 3.4.11.7) [15]. We analysed the bilateral behaviour of neuropeptidase activities in the frontal cortex of Wistar–Kyoto (WKY) and spontaneously hypertensive rats (SHRs) treated with the antihypertensive drugs captopril and propranolol. In addition, to evaluate a factor with opposite effects, the influence of the hypertensive N(G)-nitro-l-arginine methyl ester (l-NAME) was also studied.
MATERIALS AND METHODS
Animals and drug treatments
Thirty-two adult male WKY and 32 adult male SHR (Charles River Laboratories, Barcelona, Spain), weighing 100–150 g at the beginning of the experiments, were used in this study. The WKY rats were randomly divided in the following subgroups (n = 8 each): Control WKY (WKY), captopril-treated WKY (CAP), propranolol-treated (PRO) and l-NAME treated. The SHR group was subdivided into similar subgroups (n = 8 each): Control SHR (SHR), captopril-treated SHR (CAP), propranolol treated (PRO) and l-NAME treated. CAP (Sigma-Aldrich, St Louis, Missouri, USA; 100 mg/kg per day), PRO (Sigma-Aldrich; 100 mg/kg per day) and l-NAME treated (Sigma-Aldrich; 70 mg/kg per day) were added to the drinking water of the respective SHR groups for 4 weeks. The dosages and the duration of administration have been reported to be appropriate to achieve the full effect of the drugs [16,17]. To avoid the influence of circadian or seasonal variations, the experiments were conducted between April and July (northern hemisphere) under light conditions between 0900 and 1200 h [10]. The study was performed in accordance with the European Communities Council Directive 86/609/EEC and was approved by the bioethics committee of the University of Jaén.
Blood pressure measurement
SBP was measured using tail-cuff plethysmography in unanaesthetized animals (LE 5001-Pressure Meter; Letica SA, Barcelona, Spain) that were maintained in plastic holders at 37°C. To adapt the animals to the procedure, pressure was measured on several occasions during the treatment period [18].
A minimum of 15 measurements were performed per rat, with the mean values within a range of 5 mmHg representing the recorded SBP level. The first and last determinations of each session were excluded from analyses.
Surgical procedure and tissue collection
At the end of the 4-week drug-treatment periods and after a final SBP recording, tissue samples were obtained under equithensin anesthesia (2 ml/kg body weight) [42.5 g/l chloral hydrate dissolved in 19.76 ml ethanol, 9.72 g/l Nembutal (Sigma-Aldrich) 0.396 l/l propylenglycol and 21.3 g/l magnesium sulfate in distilled water]. Each rat was then perfused with saline, the brain was quickly removed (less than 60 s) and the left and right frontal cortices were dissected according to the stereotaxic Paxinos and Watson atlas [19]. The selected area was between the anterior borders of the frontal lobe up to 13.2 mm anterior to the interaural line.
Enzymatic and protein assays
To obtain the soluble fraction, brain samples were homogenized in a hypoosmolar medium (10 mmol/l HCl-Tris buffer, pH 7.4) and ultracentrifuged at 100 000g for 30 min at 4°C. The supernatants were used for soluble (Sol) protein and enzyme assays. To obtain the particulate fraction, the pellets were rehomogenized in a HCl-Tris buffer (pH 7.4) and 1% Triton X-100 to solubilize membrane proteins. After centrifugation (100 000g, 30 min, 4°C), the protein level and activity of membrane-bound enzymes were measured in triplicate in the supernatants. To ensure complete recovery of activity, the detergent was removed from the medium by adding adsorbent polymeric Bio-Beads SM-2 (Sigma) (100 mg/ml) and shaking the samples for 2 h at 4°C. The activity of Sol and membrane-bound neuropeptidases, measured as glutamyl- (GluAP), alanyl- (AlaAP) and cystinyl-aminopeptidase (CysAP), was determined fluorometrically using the arylamide derivatives, glutamyl- alanyl- and cystinyl-β-naphthylamide, as substrates as previously described [15].
Briefly, GluAP was determined using Glu-β-naphthylamide as a substrate: 10 μl of each supernatant was incubated for 120 min at 37°C with 1 ml of the substrate solution (2.72 mg/100 ml Glu-β-naphthylamide, 10 mg/100 ml bovine serum albumin (BSA), 10 mg/100 ml dithiothreitol (DTT) and 0.555 g/100 ml CaCl2 in 50 mmol/l HCl-Tris, pH 7.4). AlaAP and CysAP were measured using Ala- or Cys-β-naphthylamide as substrates, such that 10 μl of each supernatant and plasma were incubated for 30 min at 25°C with 1 ml of the substrate solution, that is 2.14 mg/100 ml of Ala-β-naphthylamide or 5.53 mg/100 ml of Cys-β-naphthylamide, 10 mg/100 ml BSA and 10 mg/100 ml DTT in 50 mmol/l of phosphate buffer (pH 7.4 for AlaAP) and 50 mmol/l HCl-Tris buffer (pH 6 for CysAP). The reactions were terminated by addition of 1 ml of 0.1 mol/l of acetate buffer, pH 4.2. The amount of β-naphthylamine released as a result of the enzymatic activity was measured fluorometrically at a 412 nm emission wavelength with an excitation wavelength of 345 nm. Proteins were quantified in triplicate using the method advanced Bradford [20] with BSA as a standard. Specific Sol or membrane-bound aminopeptidase activity was expressed as a pmol of the corresponding substrate hydrolyzed per minute per milligram of protein. Fluorogenic assays were linear with respect to time of hydrolysis and protein content.
Statistical analysis
Differences between the groups were evaluated using a two-way analysis of variance. Post hoc comparisons were conducted via LSD tests, and the paired Student's t-test was used for left versus right comparisons. P values below 0.05 were considered significant. To examine the association between aminopeptidases of the left and right sites, Pearson's coefficient of correlation was computed. Computations were performed using SPSS 13.0 (Chicago, Illinois, USA) and STATA 9.0 (STATA Corp, College Station, Texas, USA). P values below 0.05 were considered significant.
RESULTS
Effects of drug treatments on SBP
Wistar–Kyoto rats demonstrated their lowest SBP levels after CAP treatment and the highest with l-NAME treatment. However, the CAP group did not reach statistical significant difference in comparison with control (WKY) or PRO-treated rats. l-NAME-treated group differed significantly (P < 0.001) from the other groups (Fig. 1). SHR rats treated with CAP had significantly (P < 0.001) lower SBP levels than all other groups. In contrast, l-NAME-treated animals exhibited higher SBP levels (P < 0.05 vs. SHR; P < 0.001 vs. CAP; P < 0.01 vs. PRO). The SBP in SHR treated with PRO did not differ from the controls (SHR), but was significantly higher than in the CAP group (P < 0.001) and significantly lower than in the l-NAME-treated group (P < 0.01) (Fig. 1).
FIGURE 1.
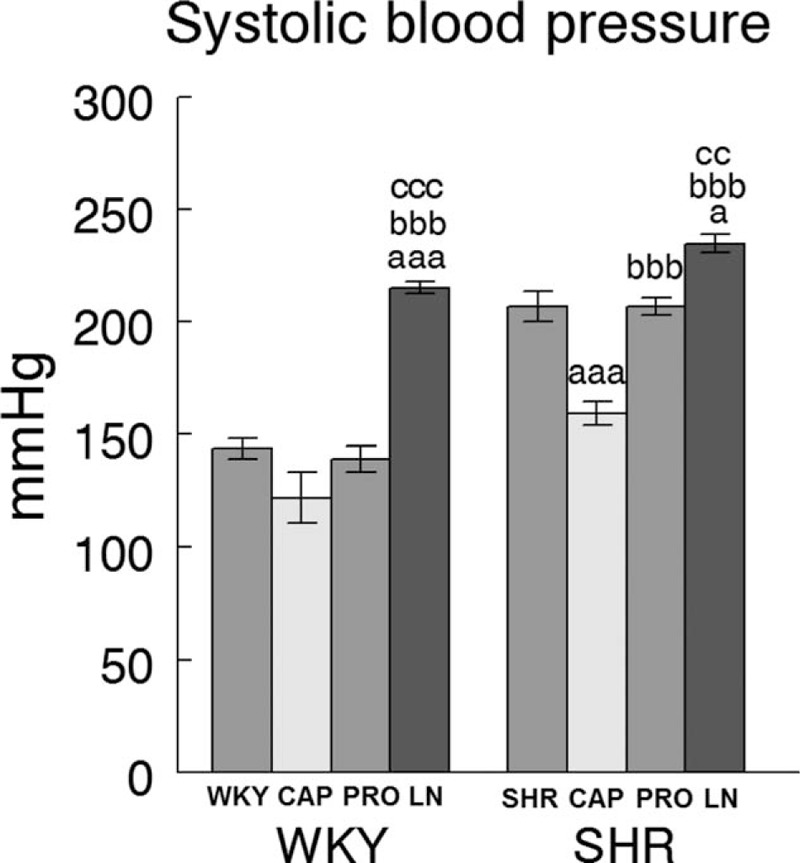
Effects of drug treatments on SBP in WKY and SHR groups. Systolic blood pressure (mmHg) in control (WKY or SHR) (n = 8) vs. WKY or SHR treated during 4 weeks with captopril (CAP) (n = 8), propranolol (PRO) (n = 8) and l-NAME (LN) (n = 8). Values represent the mean ± SEM. (a) Statistical significance vs. control WKY or SHR. (b) Significance vs. CAP. (c) Significance vs. PRO. Single letter (P < 0.05), double letter (P < 0.01), triple letter (P < 0.001).
Bilateral influence of drug treatments on neuropeptidase activities in the frontal cortex of Wistar–Kyoto and spontaneously hypertensive rats
Influence on glutamyl-aminopeptidase activity (angiotensinase)
Soluble activity
In WKY rats, there was a significant (P < 0.001) predominance of the left frontal cortex (LFC) on the right frontal cortex (RFC) one with the exception of the l-NAME-treated subgroup that did not show such left-right differences. Whereas the right side did not differ between groups, the left one decreased significantly in l-NAME treated in comparison to CAP (P < 0.01) and PRO (P < 0.01) groups (Fig. 2). In SHR, with the exception of the PRO group, in which (contrarily to the same group in WKY) the RFC was significantly dominant (P < 0.01) for Sol GluAP over the LFC, there were no left-right differences in the other groups (Fig. 3). Also, in SHR, and in contrast to WKY, the right side was higher for PRO and l-NAME-treated groups than the CAP group (P < 0.05), whereas the left side did not differ among the different groups (Fig. 3). Figures 4 (WKY) and 5 (SHR) represent the percentages of increase or decrease from the mean values of the control WKY or SHR with the different drug treatments and indicates the level of asymmetry, that is the higher (+) or lower (−) level of difference between the left and right hemispheres in comparison with the level of asymmetry in the control SHR. In WKY, there was a clear left dominance in controls (WKY) that increases slightly (+3.7%) in the CAP group. However, the left dominance decreased a little after PRO treatment (−12.4% from controls) and disappeared in the l-NAME-treated group (−57.8%). In SHR, there was a tendency to change the side of dominance from the right side in controls to left one after the CAP treatment. In contrast, a large increase (+35.2% over control) with the PRO treatment and a small decrease (−6.3% over control) with the l-NAME-treated treatment in the control asymmetry of right side dominance was observed. Thus, there was a clear significant right asymmetric difference with the PRO treatment. If we compare the hypertensive SHR groups with untreated WKY rats (Fig. 6), while the left predominance of Sol GluAP in untreated WKY decreased (−50.1%) in the CAP group, this predominance changed to the right one in the rest of the groups. Figures 7 (WKY) and 8 (SHR) represent the individual percentages of left or right predominance for each animal in the different groups analysed. In WKY, while controls (WKY), CAP and PRO groups showed a 100% of (left) predominance, the l-NAME-treated treatment showed a heterogeneous individual side predominance. In SHR, with respect to Sol GluAP, the PRO group showed a right predominance among 87.5% of animals. However, the other groups exhibited a heterogeneous left or right-side predominance among animals.
FIGURE 2.
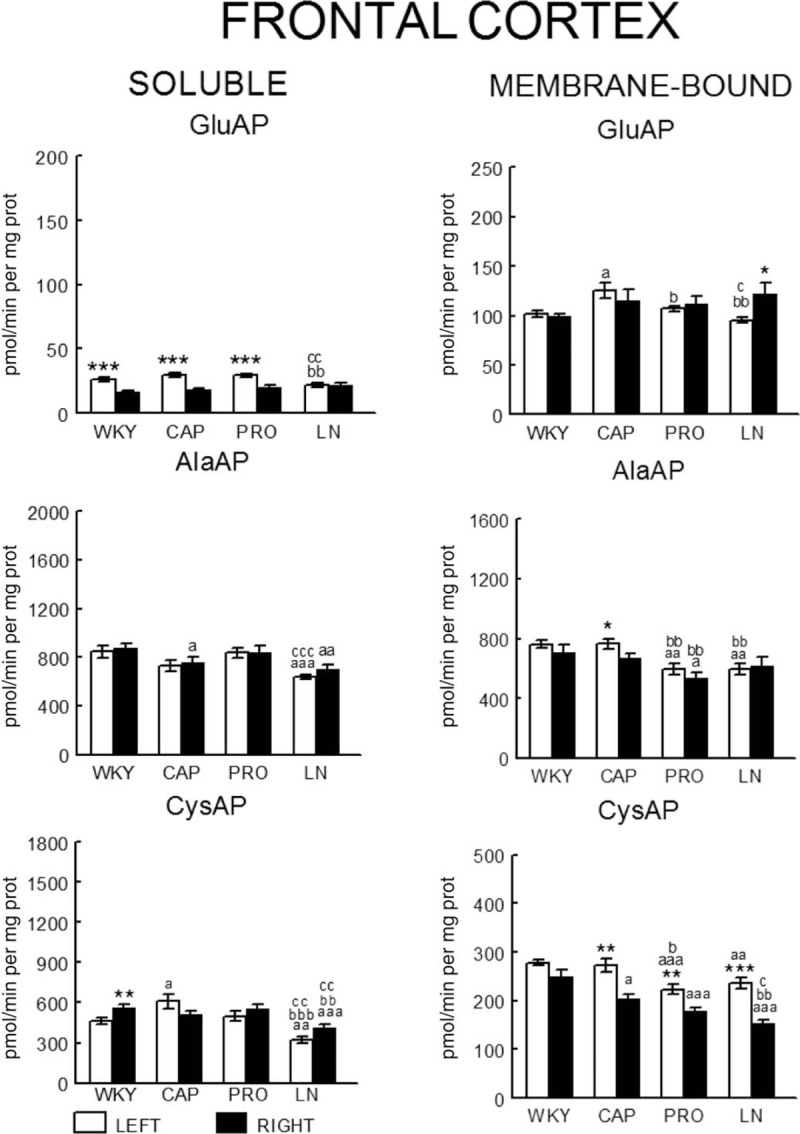
Bilateral influence of drug treatments on neuropeptidase activities in the frontal cortex of WKY rats. Mean ± SEM values of Sol and MB aminopeptidase activity, expressed as pmol/min per mg of proteins, in the left and right FC in control WKY (WKY) and WKY treated with captopril (CAP), propranolol (PRO) and l-NAME (LN). aLeft vs. right statistical significance. (a) Statistical significance vs. same side of WKY. (b) Significance vs. same side of CAP. (c) Significance vs. same side of PRO. Single letter or asterisk (P < 0.05), double letter or asterisk (P < 0.01), triple letter or asterisk (P < 0.001).
FIGURE 3.
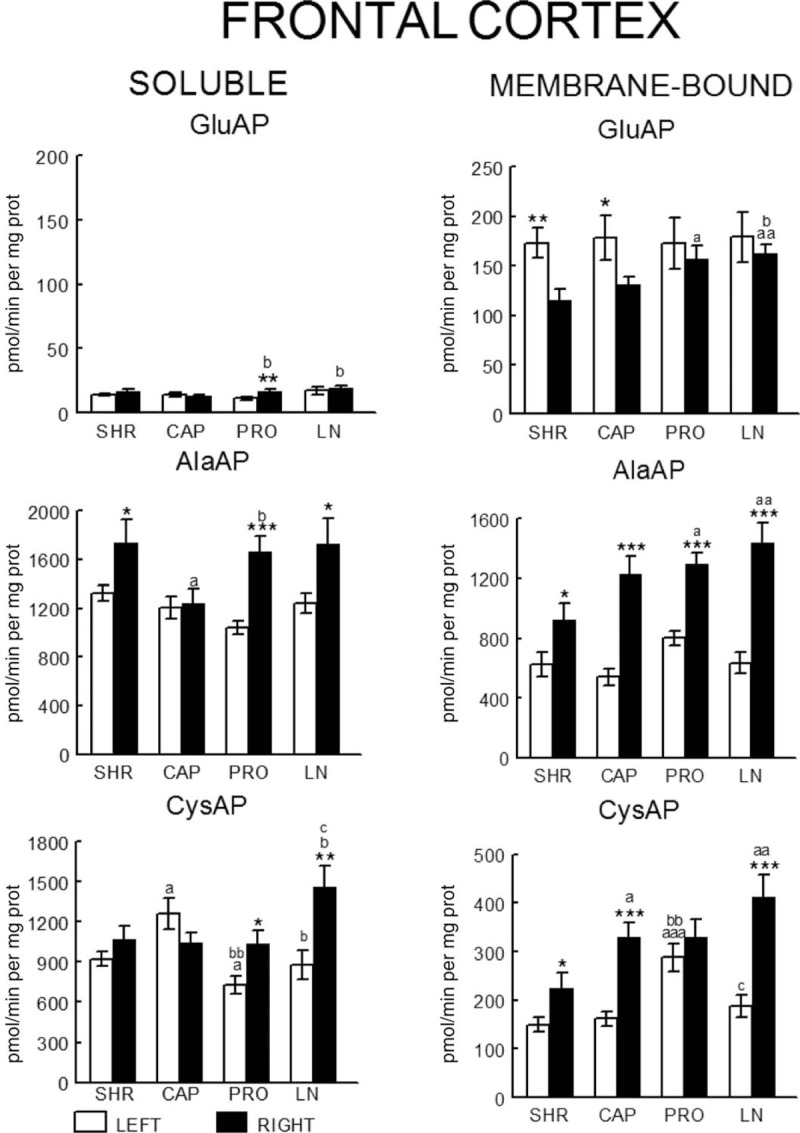
Bilateral influence of drug treatments on neuropeptidase activities in the frontal cortex of SHR rats. Mean ± SEM values of Sol and MB aminopeptidase activity, expressed as pmol/min per mg of proteins, in the left and right FC in control SHR (SHR) and SHR treated with captopril (CAP), propranolol (PRO) and l-NAME (LN). aLeft vs. right statistical significance. (a) Statistical significance vs. same side of SHR. (b) Significance vs. same side of CAP. (c) Significance vs. same side of PRO. Single letter or asterisk (P < 0.05), double letter or asterisk (P < 0.01), triple letter or asterisk (P < 0.001).
FIGURE 4.
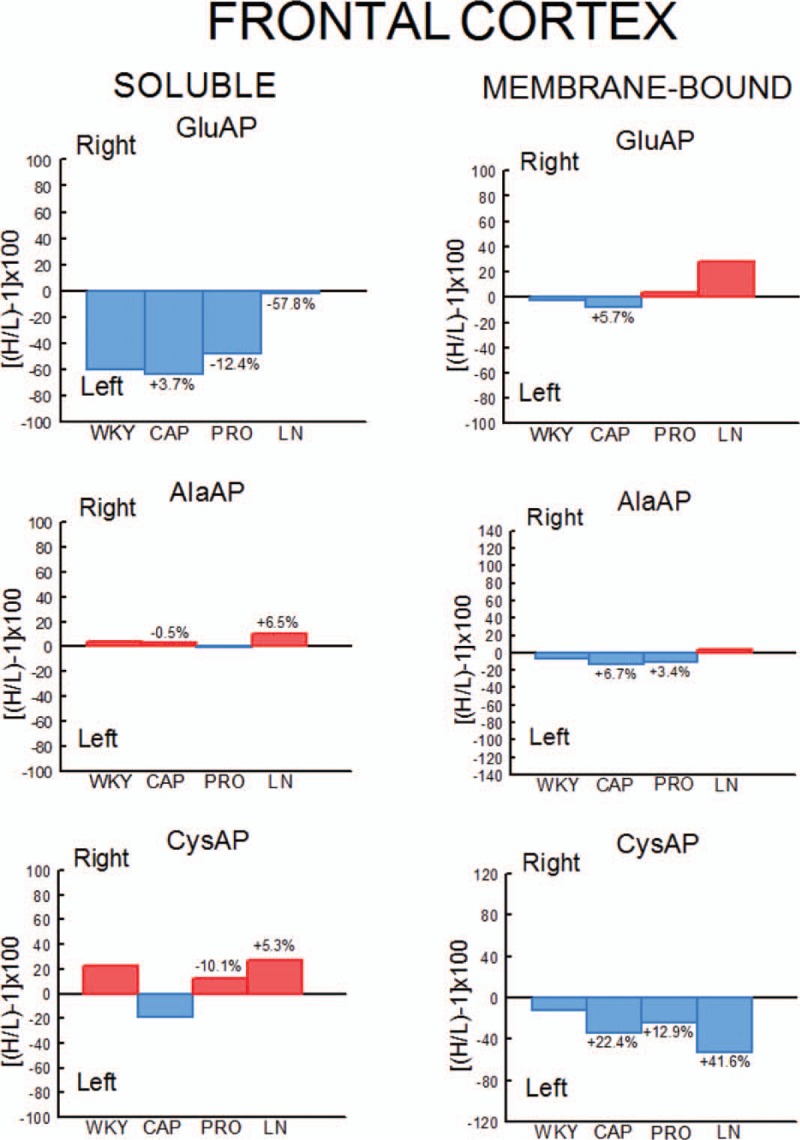
Percentages of left or right predominance from mean values in the four groups studied. Control (WKY) and WKY rats treated with captopril (CAP), propranolol (PRO) and l-NAME (LN). Negative or positive values indicate left or right predominance, respectively. Values above the bars indicate the percentage of increase (+) or decrease (−) of left or right asymmetrical difference versus control (WKY) values. Red bars indicate right predominance; blue bars indicate left predominance. H/L, higher value/lower value.
FIGURE 5.
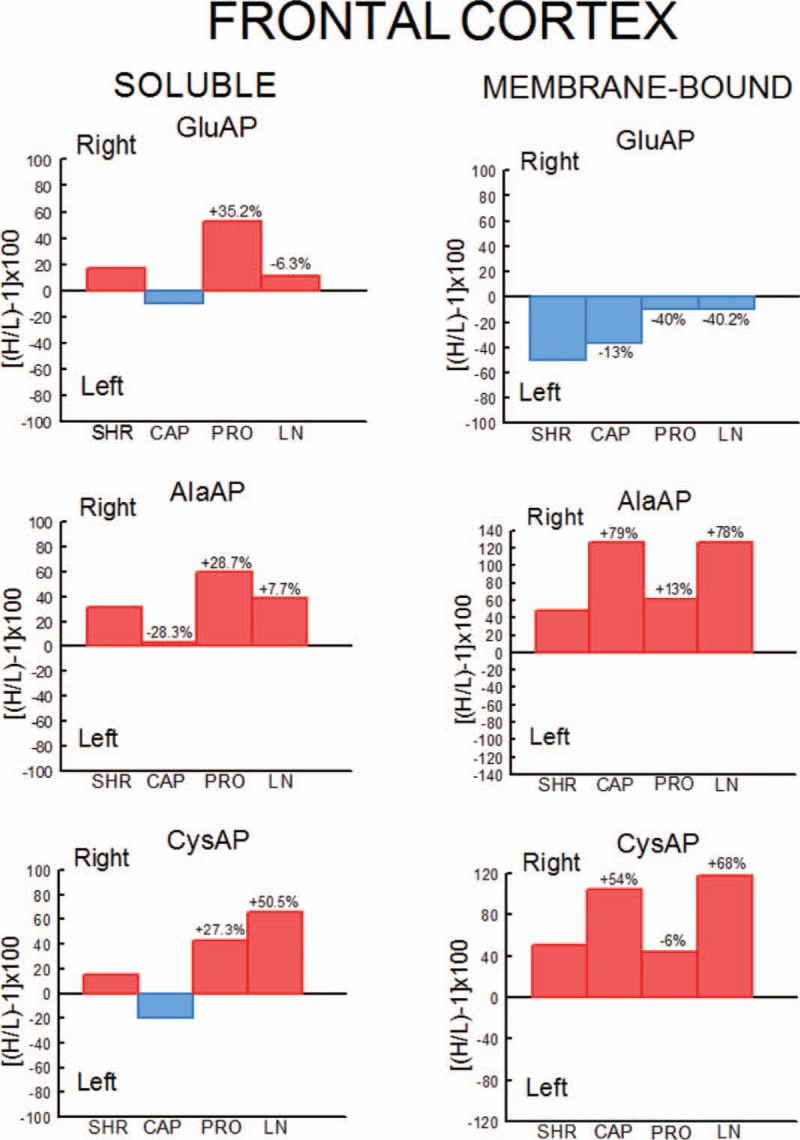
Percentages of left or right predominance from mean values in the four groups studied. Control (SHR) and SHR rats treated with captopril (CAP), propranolol (PRO) and l-NAME (LN). Negative or positive values indicate left or right predominance, respectively. Values above the bars indicate the percentage of increase (+) or decrease (−) of left or right asymmetrical difference versus control (SHR) values. Red bars indicate right predominance; blue bars indicate left predominance. H/L, higher value/lower value.
FIGURE 6.
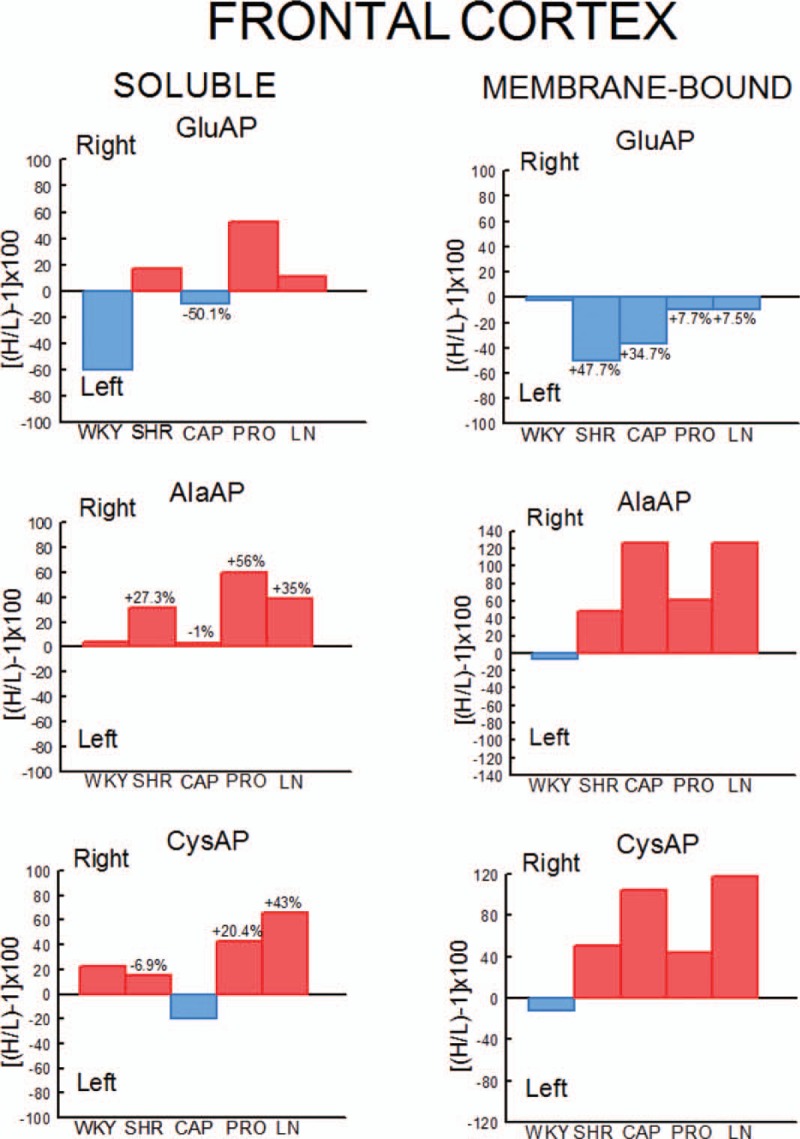
Percentages of left or right predominance from mean values in the four SHR groups in comparison with the group of untreated WKY rats. Controls (WKY and SHR) and SHR rats treated with captopril (CAP), propranolol (PRO) and l-NAME (LN). Negative or positive values indicate left or right predominance, respectively. Values above the bars indicate the percentage of increase (+) or decrease (−) of left or right asymmetrical difference versus controls (WKY or SHR) values. Red bars indicate right predominance; blue bars indicate left predominance. H/L, higher value/lower value.
FIGURE 7.
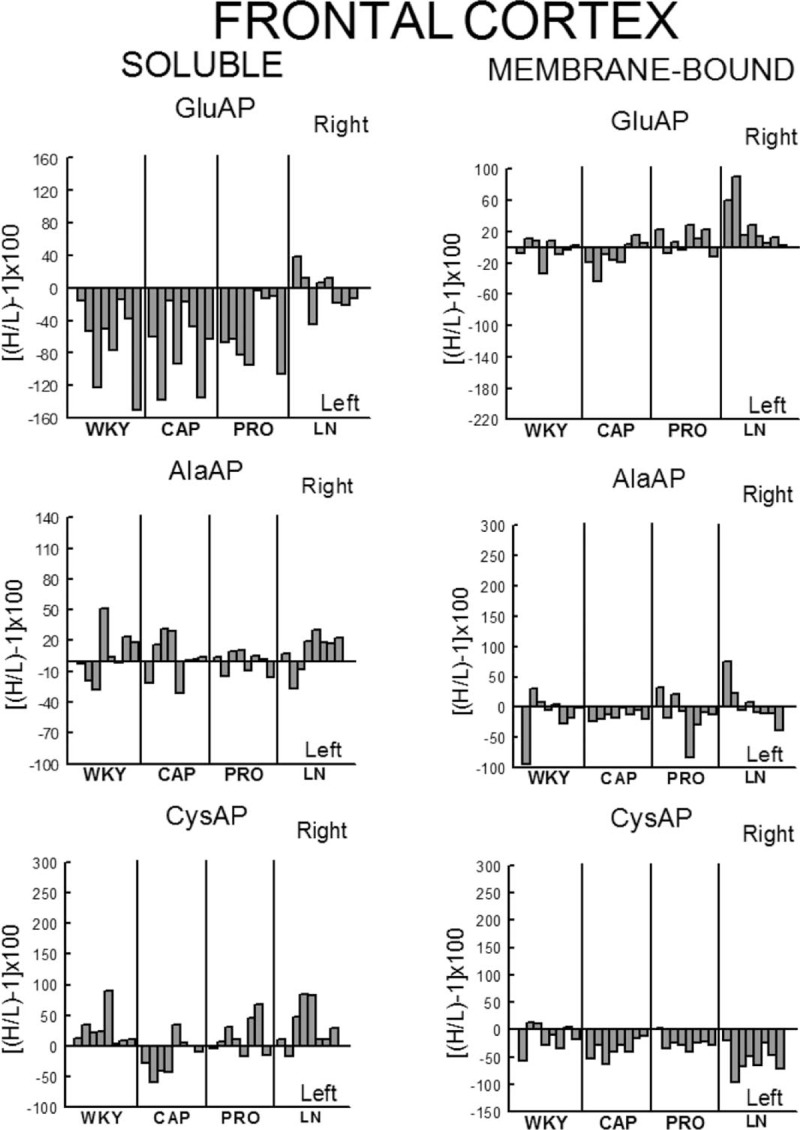
Left or right individual percentage differences in the four groups studied. Control (WKY) and WKY treated with captopril (CAP), propranolol (PRO) and l-NAME (LN). Bars represent the percentage differences in aminopeptidase activity between the left and right sides of the frontal cortex for each of the eight rats studied in the four groups. Negative or positive values indicate left or right predominance, respectively. H/L, higher value/lower value.
FIGURE 8.
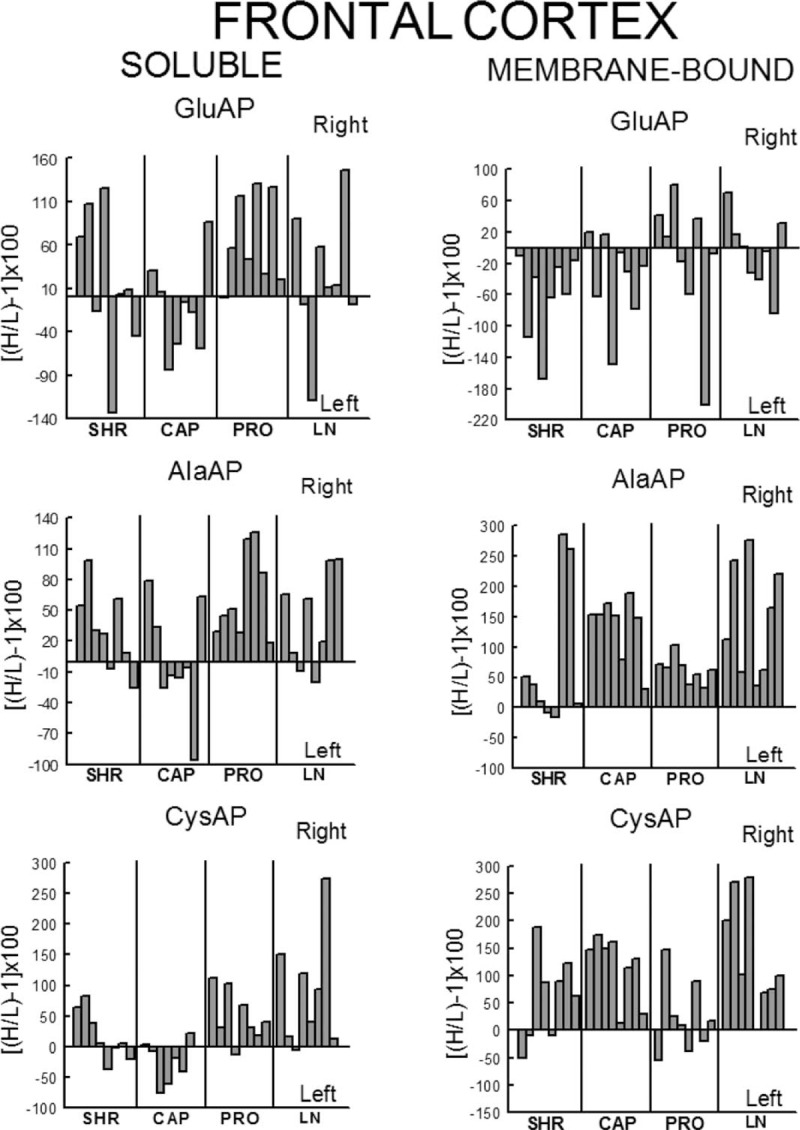
Left or right individual percentage differences in the four groups studied. Control (SHR) and SHR treated with captopril (CAP), propranolol (PRO) and l-NAME (LN). Bars represent the percentage differences in aminopeptidase activity between the left and right sides of the frontal cortex for each of the eight rats studied in the four groups. Negative or positive values indicate left or right predominance, respectively. H/L, higher value/lower value.
Membrane-bound activity
In WKY, there was a significant (P < 0.05) predominance of the right over the LFC in l-NAME-treated group, but the other subgroups did not show any differences between left and right sides. Whereas the right sides did not differ between groups, the left one of the CAP group was higher than control (P < 0.05), PRO (P < 0.05) and l-NAME-treated (P < 0.01) groups. The left side of l-NAME treated was also lower (P < 0.05) than the left side of PRO group (Fig. 2). In SHR, in the control SHR (P < 0.01) and CAP (P < 0.05) groups, MB GluAP was significantly higher in the LFC than in the RFC. No bilateral differences were observed among the other drug treatments. As occurred with Sol GluAP, whereas the levels of membrane-bound GluAP in the LFC did not differ among groups, the levels in the RFC were significantly higher in PRO (versus SHR, P < 0.05) and l-NAME treated (vs. SHR, P < 0.01; vs. CAP, P < 0.05) (Fig. 3). In WKY, although there was a small left predominance in controls that increased slightly after CAP treatment (+5.7%), the side of dominance changed to the right side in the PRO group and especially in the l-NAME-treated group (Fig. 4). In SHR, the percentage of left predominance for mean levels of Glu AP activity in controls was decreased to −13% in the CAP group and to −40% in the PRO and l-NAME treated groups (Fig. 5). The slight left predominance of membrane-bound GluAP in untreated WKY increased remarkably in all SHR groups, especially in controls SHR (+47.7%) and CAP (34.7%) groups (Fig. 6). Regarding the individual percentages of left or right predominance, controls, CAP and PRO-WKY groups showed a heterogeneous individual side predominance, whereas l-NAME-treated WKY rats demonstrated a 100% of right predominance (Fig. 7) in contrast to Sol GluAP. In SHR, whereas 100% of the animals had a left predominance in the control SHR, the rats in other groups presented a varied direction of asymmetry (Fig. 8).
Influence on alanyl-aminopeptidase activity (enkephalinase)
Soluble activity
In WKY, no left-right differences were observed for Sol AlaAP in any group. The left side of l-NAME-treated group decreased from the left side of controls and PRO (P < 0.001) but did not differ from CAP. The right side of controls was higher than the right one of CAP (P < 0.05) and l-NAME treated (P < 0.01) but did not differ from PRO (Fig. 2). In SHR, controls, (P < 0.05), PRO- (P < 0.001) and l-NAME-treated rats (P < 0.05) demonstrated an asymmetric distribution of Sol AlaAP with right predominance, which was particularly significant in the PRO group. However, the CAP treatment exhibited no difference between both sides. In the CAP group, the distribution in the RFC was lower (P < 0.05) than it was on the same side for the SHR and the PRO-treated rats. Again, as occurred with the previous activity, the left side did not differ among groups (Fig. 3). In WKY, only a small tendency to right dominance over controls was observed in l-NAME treated (+6.5%) (Fig. 4). In SHR, the percentage level of asymmetry in comparison with the mean level of controls decreased in the CAP group (−28.3%) but increased in the PRO (+28.7%) and l-NAME-treated (+7.7%) groups (Fig. 5). The slight right predominance of untreated WKY rats decreased only in the CAP group (−1%), whereas it increased clearly in the other SHR groups (Fig. 6). For WKY, all groups showed a heterogeneous individual side-dominance (Fig. 7). In SHR, as observed with Sol GluAP, the greater the increase in asymmetry, the higher the homogeneity in individual predominance, as observed in the PRO group (100% of rats with right predominance) compared with the other groups that displayed more heterogeneous left-right dominance (Fig. 8).
Membrane-bound activity
In WKY rats, membrane-bound AlaAP only demonstrated an asymmetry of left predominance in the CAP group (P < 0.05). The left side in PRO and l-NAME-treated groups was lower (P < 0.01) than the left one in control WKY. The right side in the PRO group was also lower than the right one in control (P < 0.05) and CAP (P < 0.01) groups (Fig. 2). In SHR, membrane-bound AlaAP revealed a right-side predominance in the four subgroups studied, specifically, SHR (P < 0.05), CAP (P < 0.001), PRO (P < 0.001) and l-NAME treated (P < 0.001). Again, whereas the left side did not differ among groups, the right side increased from control SHR in PRO (P < 0.05) and LN (P < 0.01) (Fig. 3). In WKY, although there was a small left dominance in controls, CAP and PRO groups, a little tendency to change the side of dominance to the right side was observed with l-NAME treated treatment (Fig. 4). In SHR, the percentage level of asymmetry after treatment was always higher than the mean level of the control SHR, specifically, CAP (+79%), PRO (+13%) and LN (+78%) (Fig. 5). Whereas untreated WKY rats presented a small left predominance, all SHR subgroups changed the predominance to the right side (Fig. 6). In WKY, although all animals treated with CAP demonstrated a very small left predominance, it was great enough to reach statistical significance. In contrast, the PRO and l-NAME-treated groups showed a heterogeneous bilateral distribution (Fig. 7). In SHR, as previously observed with Sol GluAP and Sol AlaAP, the greater the level of asymmetry (treatments versus control), the greater the degree of homogeneity in individual predominance, that is 100% of rats with right predominance in CAP, PRO and l-NAME treated (Fig. 8).
Influence on cystinyl-aminopeptidase activity (oxytocinase)
Soluble activity
In WKY rats, although the control group demonstrated a right predominance (P < 0.01), the treated groups did not differ between both sides of frontal cortex. The left side showed its highest levels of Sol CysAP in the CAP group, differing from the left side of control (P < 0.05) and l-NAME-treated (P < 0.001) groups. The left side of l-NAME treated was also lower than the left one of control WKY (P < 0.01) and PRO (P < 0.01). The right side of l-NAME treated was significantly lower than the right one of the other groups (Fig. 2). In SHR, the animals treated with PRO (P < 0.05) and l-NAME (P < 0.01) exhibited an asymmetry with a right-side predominance for Sol CysAP. No bilateral differences were observed in the control and CAP-treated rats. The LFC of the CAP group had greater Sol CysAP activity than did the same side of the control SHR (P < 0.05), PRO (P < 0.01) and l-NAME treated (P < 0.05). The RFC of the l-NAME treated group had greater levels of activity than did the RFC of the CAP (P < 0.05) and PRO (P < 0.05) groups (Fig. 3). In WKY rats, there was a tendency after CAP treatment to change the side of the predominance observed in controls from right to left. However, after PRO treatment, there was a decrease (−10.1% from control) but an increase (+5.3% from control) of the right predominance after l-NAME treated (Fig. 4). In SHR, similar to Sol CysAP of WKY and to Sol GluAP of SHR, there was a tendency after CAP treatment to change the side of dominance from right to left, although the results did not reach statistical significance. There was, however, an increase in the asymmetry after PRO (+27.3% over control) and l-NAME (+50.5% over control) treatments (Fig. 5). Although the CAP treatment reversed the side of predominance of Sol CysAP to the left side, the other SHR subgroups maintained the right predominance of untreated WKY, increasing particularly in the l-NAME group (+43%) (Fig. 6). Regarding individual percentages of dominance, although all control WKY rats had a homogeneous right dominance, the side of dominance was heterogeneous for CAP and PRO groups. In the l-NAME-treated group, although the 87.5% of animals had a right predominance, it was not sufficient to reach statistical significance (Fig. 7). In SHR, there was a homogeneous right dominance in PRO and l-NAME-treated groups. However, the bilateral distribution was more heterogeneous in control and CAP groups (Fig. 8).
Membrane-bound activity
In WKY rats, LFC was predominantly higher than the right one in all groups reaching significance in CAP (P < 0.01), PRO (P < 0.01) and l-NAME treated (P < 0.01) without difference in controls. The left side of PRO was lower than the left one of control (P < 0.001) and CAP (P < 0.05) groups. The left side of l-NAME treated was also lower (P < 0.01) than the left one of controls WKY. The right side was lower in CAP (P < 0.05), PRO (P < 0.001) and l-NAME treated (P < 0.001) in comparison with the right side of controls. The right side in LN was significantly lower than the right one of the other subgroups (Fig. 2). In SHR, whereas rats treated with PRO did not display a bilateral difference, the other groups exhibited a significant right predominance of membrane-bound CysAP in the frontal cortex (Fig. 3). The highest levels of membrane-bound CysAP of the left side were observed in the PRO group, differing from control (P < 0.001), CAP (P < 0.01) and l-NAME treated (P < 0.05) groups. Although the right side did not differ between control and PRO, it was higher than controls in CAP (P < 0.05) and l-NAME treated (P < 0.01). In WKY, a left predominance was observed in all groups. Compared with control, the percentage of dominance increased largely in CAP (+22.4%), PRO (+12.9%) and especially in l-NAME treated (+41.6%) (Fig. 4). In SHR, CAP (P < 0.001) (+54%) and l-NAME-treated groups (P < 0.001) (+68%) further increased the difference observed in the control SHR (P < 0.05), whereas the percentage of difference in the PRO group slightly decreased (−6%) in comparison with the percentage of right predominance in the control SHR group (Figs. 3 and 5). All hypertensive groups changed the left side predominance of membrane-bound CysAP observed in untreated WKY to the right side (Fig. 6). Considering individually the percentage of left-right difference in WKY, while control rats showed a heterogeneous side of dominance, 100% of CAP and l-NAME-treated rats and 87.5% of PRO rats demonstrated a left dominance (Fig. 7). In SHR, we found that the control and the PRO group had a more heterogenous left or right preponderance, whereas nearly 100% of the animals in the CAP and l-NAME-treated groups exhibited a right predominance (Fig. 8).
Figure 9 represents the individual left or right predominance obtained in each rat, considering the totality of the soluble, the totality of the membrane-bound and the totality of soluble and membrane-bound (TSM) enzymatic activities analysed in each group studied (Control, CAP, PRO, l-NAME treated) and in all the groups (TOTAL Control + CAP + PRO + l-NAME treated) of WKY and SHR rats. There was a left predominance in WKY when considered totality of the soluble and TSM in CAP (P < 0.05) and TOTAL (P < 0.01). There was a right predominance in SHR when totality of the membrane-bound was considered in controls (P < 0.05), in TSM in CAP (P < 0.01), in TSM in l-NAME (P < 0.05) and in totality of the soluble (P < 0.01), totality of the membrane-bound (P < 0.001) and TSM (P < 0.001) in TOTAL. There were also significant differences when we compared the left and right frontal cortex of WKY with the left and right frontal cortex of SHR groups and in TOTAL (Fig. 9).
FIGURE 9.
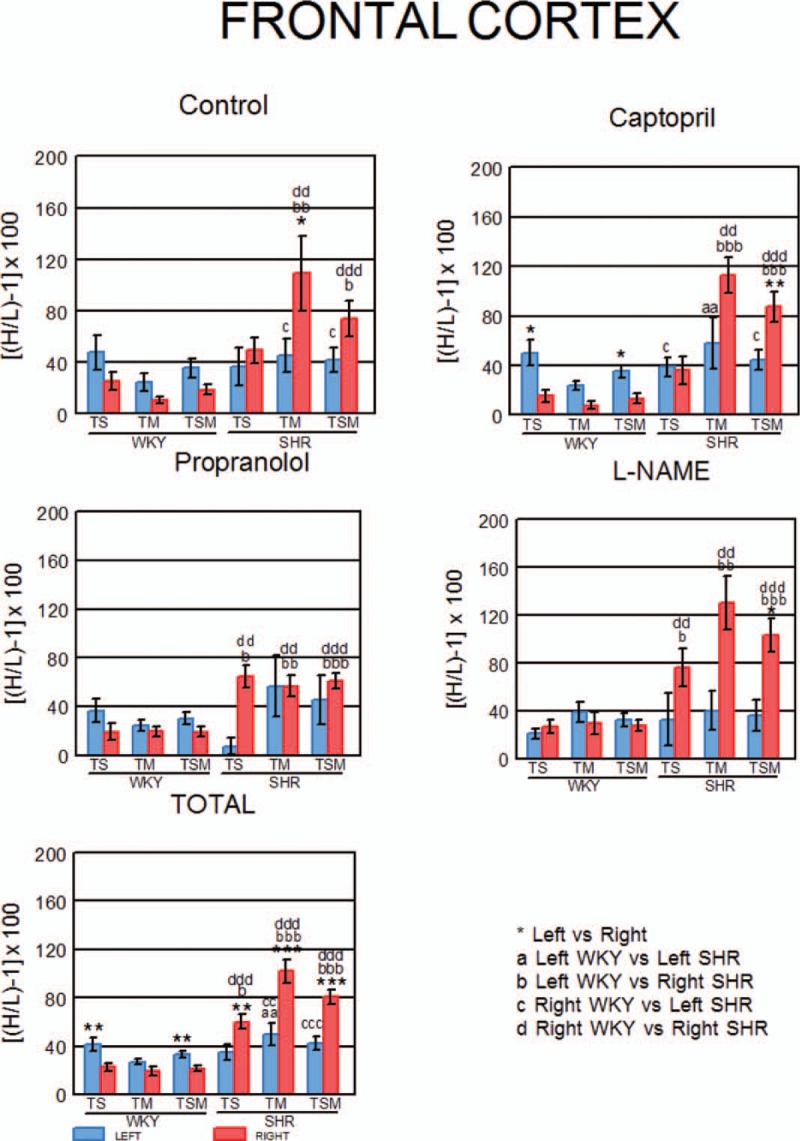
Left or right predominance of total soluble, total membrane bound and total soluble and membrane bound activities in WKY and SHR. Mean ± SEM levels obtained with the individual values of left or right predominance of the total soluble (TS), the total membrane-bound (TM) and the total soluble and membrane-bound (TSM) activities analysed in each group and in all groups (TOTAL) of WKY and SHR. The comparisons between the left and right sides of FC into strains (a) and between strains (a–d) are also indicated.
Correlational study
Whereas a large number of intrahemispheric correlations in the LFC were observed in the CAP-treated WKY rats, most of the correlations of the RFC were in the l-NAME-treated group and essentially with a negative character. The most part of interhemispheric correlations were observed in the CAP and PRO groups of WKY rats (Table 1). In SHR, the intrahemispheric analysis of the correlations among the different enzymatic activities demonstrated a marked prevalence of significant interactions in the RFC compared with the LFC in all groups analysed. A clear reduction of interhemispheric interactions was observed in the SHR groups in comparison with the WKY ones, even disappearing in controls SHR and l-NAME treated (Table 2).
TABLE 1.
Significant left or right intrahemispheric correlations and left vs. right inter-hemispheric correlations between the soluble (Sol) and/or membrane-bound aminopeptidase activities, as measured in the four groups studied of Wistar–Kyoto rats
| Frontal cortex | |||||
| Left | Right | ||||
| Intrahemispheric | Intrahemispheric | ||||
| Correlation | r | P | Correlation | r | P |
| WKY | WKY | ||||
| Sol AlaAP vs. Sol CysAP | −0.803 | 0.01 | No correlations | ||
| Sol AlaAP vs. MB GluAP | −0.715 | 0.04 | |||
| CAP | CAP | ||||
| Sol GluAP vs. Sol AlaAP | −0.757 | 0.02 | MB CysAP vs. Sol AlaAP | −0.751 | 0.03 |
| Sol GluAP vs. MB GluAP | +0.716 | 0.04 | |||
| Sol GluAP vs. MB AlaAP | +0.872 | 0.004 | |||
| Sol GluAP vs. MB CysAP | +0.751 | 0.03 | |||
| Sol AlaAP vs. MB GluAP | −0.747 | 0.03 | |||
| Sol CysAP vs. MB AlaAP | +0.896 | 0.002 | |||
| MB AlaAP vs. MB CysAP | +0.798 | 0.01 | |||
| PRO | PRO | ||||
| Sol GluAP vs. MB AlaAP | +0.777 | 0.02 | Sol AlaAP vs. Sol CysAP | +0.778 | 0.02 |
| MB AlaAP vs. Sol GluAP | −0.743 | 0.03 | |||
| LN | LN | ||||
| MB AlaAP vs. MB CysAP | +0.817 | 0.01 | Sol AlaAP vs. Sol CysAP | +0.883 | 0.003 |
| MB GluAP vs. Sol AlaAP | −0.848 | 0.007 | |||
| MB GluAP vs. Sol CysAP | −0.772 | 0.02 | |||
| MB AlaAP vs. SolAlaAP | −0.868 | 0.005 | |||
| MB AlaAP vs. Sol CysAP | −0.764 | 0.02 | |||
| Interhemispheric | ||
| Correlation | r | P |
| WKY | ||
| Left Sol GluAP vs. Right MB GluAP | +0.745 | 0.03 |
| Left MB CysAP vs. Right MB GluAP | −0.779 | 0.02 |
| CAP | ||
| Left Sol GluAP vs. Right MB AlaAP | +0.799 | 0.01 |
| Left Sol AlaAP vs. Right MB AlaAP | −0.720 | 0.04 |
| Left MB GluAP vs. Right MB GluAP | +0.758 | 0.02 |
| Left MB AlaAP vs. Right MB AlaAP | +0.852 | 0.007 |
| Left MB AlaAP vs. Right MB CysAP | +0.881 | 0.003 |
| Left MB CysAP vs. Right MB GluAP | +0.872 | 0.004 |
| PRO | ||
| Left Sol AlaAP vs. Right Sol GluAP | −0.712 | 0.04 |
| Left Sol AlaAP vs. Right Sol AlaAP | +0.868 | 0.005 |
| Left Sol GluAP vs. Right MB CysAP | +0.898 | 0.002 |
| Left Sol CysAP vs. Right MB CysAP | +0.851 | 0.007 |
| LN | ||
| Left Sol AlaAP vs. Right MB CysAP | −0.889 | 0.002 |
| Left MB CysAP vs. Right MB GluAP | +0.832 | 0.01 |
Pearson's correlation coefficients (r) and P-values are indicated and specify the significance of the differences between these correlations. Negative correlations are in italics.
CAP, captopril; MB, membrane-bound.
TABLE 2.
Significant left or right intrahemispheric correlations and left vs. right interhemispheric correlations between the soluble and/or membrane-bound aminopeptidase activities, as measured in the four groups studied of spontaneously hypertensive rats
| Frontal cortex | |||||
| Left | Right | ||||
| Intrahemispheric | Intrahemispheric | ||||
| Correlation | r | P | Correlation | r | P |
| SHR | SHR | ||||
| Sol AlaAP vs. Sol CysAP | +0.848 | 0.007 | MB AlaAP vs. MB CysAP | +0.788 | 0.02 |
| Sol GluAP vs. Sol AlaAP | +0.881 | 0.003 | |||
| Sol GluAP vs. Sol CysAP | +0.961 | 0.0001 | |||
| Sol AlaAP vs. Sol CysAP | +0.864 | 0.005 | |||
| CAP | CAP | ||||
| Sol GluAP vs. Sol CysAP | +0.843 | 0.008 | Sol GluAP vs. Sol AlaAP | +0.838 | 0.009 |
| Sol GluAP vs. Sol CysAP | +0.791 | 0.01 | |||
| PRO | PRO | ||||
| No correlations | MB AlaAP vs. Sol AlaAP | −0.889 | 0.003 | ||
| MB AlaAP vs. Sol CysAP | −0.732 | 0.03 | |||
| Sol AlaAP vs. Sol CysAP | +0.866 | 0.005 | |||
| LN | LN | ||||
| Sol GluAP vs. Sol CysAP | +0.886 | 0.003 | MB GluAP vs. Sol GluAP | +0.903 | 0.002 |
| MB GluAP vs. Sol AlaAP | +0.829 | 0.01 | |||
| MB GluAP vs. Sol CysAP | +0.924 | 0.001 | |||
| Sol GluAP vs. Sol AlaAP | +0.762 | 0.02 | |||
| Sol GluAP vs. Sol CysAP | +0.978 | <0.0001 | |||
| Sol AlaAP vs. Sol CysAP | +0.741 | 0.03 | |||
| Interhemispheric | ||
| Correlation | r | P |
| SHR | ||
| No correlations | ||
| CAP | ||
| Left MB GluAP vs. Right Sol GluAP | −0.705 | 0.05 |
| Left MB GluAP vs. Right MB CysAP | +0.783 | 0.02 |
| Left MB GluAP vs. Right MB AlaAP | +0.686 | 0.05 |
| PRO | ||
| Left MB AlaAP vs. Right Sol CysAP | −0.714 | 0.04 |
| Left Sol AlaAP vs. Right MB GluAP | +0.772 | 0.02 |
| Left Sol CysAP vs. Right Sol GluA | +0.788 | 0.02 |
| LN | ||
| No correlations | ||
Pearson's correlation coefficients (r) and P-values are indicated and specify the significance of the differences between these correlations. Negative correlations are in italics.
CAP, captopril; LN, LNAME-treated; MB, membrane-bound; SHR, spontaneously hypertensive rats.
DISCUSSION
The present results demonstrated a differential neuropeptidase bilateral behaviour depending on the type of enzymatic activity, the type of drug treatment and the normotensive (WKY) or hypertensive (SHR) strain analysed. A clear tendency to change the side of predominance from left to right or to increase the right predominance is observed in SHR when this strain was compared with WKY (Figs. 4 and 5). This is also obvious when the SHR groups were compared with normotensive untreated WKY rats (Fig. 6). Looking to the individual response of each animals of the different groups, a strong influence of hypertension on the bilateral behaviour of the frontal cortex is clearly apparent (Figs. 7 and 8). In addition, if we consider the individual left predominance observed in each rat with the totality of the enzymatic activities analysed and compare them with the right ones, a differential behaviour between normotensive and hypertensive rats is also revealed, with an increase of the right dominance in SHR (Fig. 9). All these results suggesting a tendency to right predominance in hypertensive individuals are supported by the correlational study between the enzymatic activities. A high number of left intrahemispheric correlations was observed in normotensive animals treated with CAP. In contrast, in the normotensive rats that developed hypertension after treatment with l-NAME, a high number of intrahemispheric correlations were observed on the right side. Regarding interhemispheric correlations, most of them were observed in the CAP group, followed by the PRO in normotensive rats. However, a marked decrease in the interhemispheric correlations was observed in the groups of hypertensive rats, even disappearing in the control-untreated group and in those treated with l-NAME (Tables 1 and 2). Considering the bilateral behaviour between the left and right sides of frontal cortex, all these results clearly suggest a beneficial influence of CAP and a deleterious one of l-NAME treated. In this regard, hypertension has been related to neuroinflammation and particularly the inflammation induced by l-NAME has been reported to be accompanied by microglial activation manifested by microgliosis and proinflammatory cytokine upregulation [21]. Although a direct relationship between neuroinflammation and brain asymmetry has not yet been reported, a strong argument could be provided, as neuroinflammation has been clearly related to human and animal models of Parkinson's disease in which motor and nonmotor functional and neurochemical brain asymmetries are well documented [10,11] and microglial activation with cytokine production has been demonstrated [22,23]. On the contrary, the precise functional meaning of intra and interhemispheric communication between both brain hemispheres is not yet well known [24]. However, several evidences suggested that alterations in intra and interhemispheric interactions may be a characteristic of some mood disorders in humans [25] and animal models [26] as discussed below in more details.
Neuropeptidases, particularly aminopeptidases, regulate neuropeptide function [10]. Although Sol and membrane-bound aminopeptidase activities are able to hydrolyze identical peptidergic substrates, the processes that regulate both forms of the enzyme may be different and therefore exert different functions. It has been suggested that surrounding biochemical conditions induced by drug treatments, pathologic or physiologic circumstances may regulate aminopeptidase activities [27]. Therefore, although the regulation of the enzymatic activity involves essentially the control of the enzyme synthesis at the nuclear level, other mechanisms must also be implicated. It is well known that enzymatic activities are modified by biophysical and biochemical surrounding conditions. This heterogeneity may have a different influence on the interstitial and intracellular Sol or membrane-bound forms of the enzyme [18]. Especially, it was hypothesized that membrane-bound aminopeptidase activities act in a more tissue-specific manner than the Sol ones, which may be more subject to environmental changes and therefore be less specific on its possible endogenous substrates [18]. Nonetheless, study of these enzymes is a valuable tool revealing the functional status of their endogenous substrates [15]. Understanding the functional roles of these enzymes, it is possible to pharmacologically treat certain brain disorders. In fact, inhibitors of these enzymes have already been proposed, such as analgesics and antidepressors [28] or antihypertensives [29,30]. Oxytocin and enkephalins acting in the frontal cortex have been reported to be anxiolytic factors [12,13]. In contrast, because brain Ang II has been suggested to be anxiogenic [14], its blockade has been proposed for the treatment of certain brain disorders [31]. The corresponding neuropeptidases oxytocinase (CysAP), enkephalinase (AlaAP) and angiotensinase (GluAP) may play a major role in regulating the functional status of these neuropeptides [15]. These enzymes and their endogenous substrates participate in cognitive functions, are related to anxiety and depression and exert a major influence in the central and peripheral control of blood pressure [11,32,33].
Hypertension may cause mood alterations, and conversely, mood disorders may underlie the pathogeny of some cardiovascular diseases, thereby suggesting a bidirectional association [1]. The use of antihypertensive drugs may be related to a higher or lower incidence of mood disorders [1]. Furthermore, the brain renin-angiotensin system (RAS) is involved in the control of blood pressure [29,30] and plays a role in brain functions [31,33,34]. It is generally accepted that the use of RAS blocking drugs, such as angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs), induces fewer mood disorders, whereas there is a higher risk of mood disorders associated with beta blockers and calcium channel blockers [1]. However, animal [6] and human studies [5] are unconvincing. If such association existed, asymmetries described in the autonomic nervous system could be partly responsible for asymmetries at the central and peripheral levels [10]. In addition, coexistence and/or interaction between various types of neurotransmitters such as monoamines and neuropeptides are well known [11]. Therefore, sympathetic blockade with a beta-blocker such as propranolol, affecting the release of classical neurotransmitters such as norepinephrine, could influence the neuropeptides with which it interacts or coexists and, consequently, the enzymes that control them in an asymmetrical manner. Similarly, the effect of l-NAME on cognitive functions is conflicting. Fouyas et al.[35], without differentiating left and right hemispheres, have found no differences in local cerebral blood flow in Wistar–Kyoto (WKY) or SHR acutely treated with l-NAME. However, although a beneficial influence of l-NAME on cognition has been observed [36] in normotensive rats, it has also been reported that l-NAME exacerbates cognitive decline in the TgSwDI mouse model of Alzheimer's disease [37]. We speculate that despite the beneficial effects in normotensive rats, increased levels of blood pressure in hypertensive animals due to l-NAME treatment might have deleterious effects on cognition and exacerbate the mood disorders observed in some hypertensive patients.
Neuropeptides and neuropeptidases are asymmetrically distributed in the brain [10,11], and previous results have demonstrated changes in the bilateral distribution of neuropeptidase activity in the frontal cortex after treating SHR with the ACEi captopril [9]. If antihypertensive treatment may influence the mood of hypertensive individuals and if captopril modifies brain asymmetry, other vasoactive drugs may also alter such asymmetry in a potentially different way and influence the mood of hypertensive subjects. In theory, depression is characterized by a functional deficiency of the left and right hemispheres, particularly of the frontal cortex [2]. The physiological overactivation of the right frontal cortex is combined with a decrease in the physiological activity of the left frontal lobe [2,7]. Currently, as the treatment of depression, including mood trouble due to hypertension, is only palliative, new approaches should be explored. If depression is, in part, caused by changes in the bilateral functioning of both hemispheres (right overactivation/left hypofunction), treatments that restore the basal left/right activity may prove beneficial. Therefore, studies that bilaterally analyse brain activity from functional and neurochemical perspectives will be crucial.
The present results reveal a complex picture showing different bilateral behaviours depending on the enzymatic activity and the treatment used, with no obvious pattern. However, we observed some clear and interesting results. Although correlation does not indicate causality, it may be indicative of a functional association. Therefore, considering the groups studied, the marked contrast between the large number of correlations observed in the right frontal cortex and their near absence in the left cortex strongly indicates an asymmetry involving neuropeptidase activity and, consequently, an irregularity in the functions of the enzymes’ respective endogenous substrates (Table 1). Interestingly, the number of correlations in the right hemisphere is directly proportional to the level of SBP (Fig. 1 and Table 1), that is the lower the level and number in the CAP group, the higher the level and number in the l-NAME-treated group. This circumstance may be indicative of a lateralized differential response to the CAP and l-NAME treatment. In contrast, whereas the control and the l-NAME-treated group in WKY and SHR show low or no interhemispheric correlations, respectively, the groups treated with antihypertensive drugs (CAP and PRO) demonstrated significant interhemispheric correlations. These results in WKY and in a lesser extent in SHR again suggest a differential response in these groups that may be applied to restoring the interhemispheric communication absent in hypertensive groups (Tables 1 and 2).
Our results also suggest a tendency for Sol activity to induce left-side predominance exclusively after CAP treatment (Figs. 4 and 5). According to Rotenberg [2], the deep inhibition of left hemispheric activity may be a major factor in the pathogenesis of depression. Therefore, the tendency of Sol activity towards left predominance in the CAP group supports the theory that treatment with an ACEi may be beneficial, as an increase in left hemispheric activity would theoretically mitigate the symptoms of depression [2].
The present results further demonstrate a clear divergence from the bilateral patterns of the membrane-bound activity. Specifically, whereas angiotensinase activity (GluAP) exhibited a tendency towards left predominance (significant in the control SHR and the CAP group), enkephalinase (AlaAP) and oxytocinase (CysAP) activity demonstrated a right predominance in all groups. These results may suggest an opposing asymmetrical distribution of anxiogenic (Ang II) and anxiolytic (enkephalins, oxytocin) neurotransmitters in the SHR.
As SHR is a well recognized animal model for the study of hypertension, it is also a validated model to evaluate attention-deficit hyperactive disorder [38]. Furthermore, because SHR exhibit low anxiety levels in contrast to the high anxiety levels observed in the WKY controls [39], both strains are useful for the study of mood disorders. An interhemispheric pattern of the distribution of dopamine and enkephalinase activity (AlaAP) was recently related to mood disorders and blood pressure [11]. In addition, the dopaminergic depletion of the left hemisphere, but not the right hemisphere, with the neurotoxic 6-hydroxydopamine dramatically increases the SBP in both WKY rats and SHR [40]. These results demonstrate the lateralized brain control of blood pressure in which enkephalins, enkephalinases and dopamine may be involved.
In conclusion, changes in the bilateral pattern of neuropeptidase activities may be part of the pathogeny of some brain disorders. Although the present results demonstrated changes in the bilateral behaviour of neuropeptidases depending on the type of enzyme studied and the drug treatment, the general tendency was that membrane-bound GluAP (angiotensinase) activity revealed a left predominance, whereas oxytocinase and enkephalinase exhibited a right predominance. In addition, there was a clear prevalence of significant intrahemispheric correlations in the RFC compared with the LFC in all groups studied, and interhemispheric significant correlations were observed mainly or exclusively in WKY and SHR groups treated with the antihypertensive drugs CAP and PRO.
In addition, the present data suggest a bilateral differential behaviour between the CAP and the other groups. First, there is direct relationship between the SBP level and the number of significant correlations (which can serve as a support for the previous conclusions): In WKY, although the CAP group had the lowest SBP levels and the highest number of intrahemispheric correlations in the LFC, the l-NAME-treated group had the highest SBP levels and the highest number of intrahemispheric correlations in the RFC. In contrast, in SHR, whereas the CAP group has the lowest SBP levels and the smallest number of intrahemispheric correlations in RFC, the opposite is observed in the l-NAME-treated group. Second, a tendency to left dominance in Sol activity was observed in the CAP group.
The reported results clearly suggest a specific brain bilateral pattern of neuropeptidase activity in hypertensive groups in comparison with the normotensive ones that may be related to the cognitive disorders observed in this animal model.
There are some limitations of the current study and basal behavioural experiments are required to relate imbalance in intrahemispheric neuropeptidase activities with hypertensive-associated mood disorders. Behavioural tests concomitant with bilateral determinations of the susceptible endogenous substrates of the aminopeptidase activities should be performed in left and right frontal cortex of untreated WKY and SHR and treated strain with CAP, PRO and l-NAME treated to support such hypothesis of specific brain bilateral pattern.
ACKNOWLEDGEMENT
This work was supported by the Ministry of Science and Innovation through project no. SAF 2008 04685 C02 01.
Conflict of interest
All authors declare to have no conflict of interest.
Both Isabel Prieto and Ana B. Segarra contributed equally to this work.
Abbreviations: ACEI, angiotensin-converting enzyme inhibitors; AlaAP, alanyl-aminopeptidase; ARBs, angiotensin receptor blockers; BSA, bovine serum albumin; CAP, captopril; CysAP, cystinyl-aminopeptidase; DTT, dithiothreitol; ECAi, angiotensin-converting enzyme inhibitors; GluAP, glutamyl-aminopeptidase; IRAP, insulin-regulated aminopeptidase; LFC, left frontal cortex; LN, l-NAME; l-NAME, N(G)-nitro-l-arginine methyl ester; mPFC, medial prefrontal cortex; PRO, propranolol; RAS, renin-angiotensin system; RFC, right frontal cortex; SHR, spontaneously hypertensive rats; TSM, total soluble and membrane-bound; WKY, Wistar–Kyoto
REFERENCES
- 1.Boal AH, Smith DJ, McCallum L, Muir S, Touyz RM, Dominiczak AF, et al. Monotherapy with major antihypertensive drug classes and risk of hospital admissions for mood disorders. Hypertension 2016; 68:1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rotenberg VS. Functional brain asymmetry as a determinative factor in the treatment of depression: theoretical implications. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1772–1777. [DOI] [PubMed] [Google Scholar]
- 3.Segarra AB, Prieto I, Banegas I, Villarejo AB, Wangensteen R, de Gasparo M, et al. The brain-heart connection: frontal cortex and left ventricle angiotensinase activities in control and captopril-treated hypertensive rats-a bilateral study. Int J Hypertens 2013; 2013:156179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujishima M, Ibayashi S, Fujii K, Mori S. Cerebral blood flow and brain function in hypertension. Hypertens Res 1995; 18:111–117. [DOI] [PubMed] [Google Scholar]
- 5.Yasar S, Schuchman M, Peters J, Anstey KJ, Carlson MC, Peters R. Relationship between antihypertensive medications and cognitive impairment: Part I. Review of human studies and clinical trials. Curr Hypertens Rep 2016; 18:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters R, Schuchman M, Peters J, Carlson MC, Yasar S. Relationship between antihypertensive medications and cognitive impairment: Part II. Review of physiology and animal studies. Curr Hypertens Rep 2016; 18:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. J Abnorm Psychol 1991; 100:535–545. [DOI] [PubMed] [Google Scholar]
- 8.Foster PS, Drago V, Ferguson BJ, Harrison DW. Cerebral moderation of cardiovascular functioning: a functional cerebral systems perspective. Clin Neurophysiol 2008; 119:2846–2854. [DOI] [PubMed] [Google Scholar]
- 9.Segarra AB, Prieto I, Banegas I, Villarejo AB, Wangensteen R, de Gasparo M, et al. Asymmetrical effect of captopril on the angiotensinase activity in frontal cortex and plasma of the spontaneously hypertensive rats: expanding the model of neuroendocrine integration. Behav Brain Res 2012; 230:423–427. [DOI] [PubMed] [Google Scholar]
- 10.Ramírez M, Prieto I, Vives F, de Gasparo M, Alba F. Neuropeptides, neuropeptidases and brain asymmetry. Curr Protein Pept Sci 2004; 5:497–506. [DOI] [PubMed] [Google Scholar]
- 11.Banegas I, Prieto I, Segarra AB, Vives F, de Gasparo M, Duran R, et al. Bilateral distribution of enkephalinase activity in the medial prefrontal cortex differs between WKY and SHR rats unilaterally lesioned with 6-hydroxydopamine. Prog Neuropsychopharmacol Biol Psychiatry 2017; 75:213–218. [DOI] [PubMed] [Google Scholar]
- 12.Neumann ID. Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem Soc Trans 2007; 35:1252–1257. [DOI] [PubMed] [Google Scholar]
- 13.Bilkei-Gorzo A, Racz I, Michel K, Mauer D, Zimmer A, Klingmüller D, et al. Control of hormonal stress reactivity by the endogenous opioid system. Psychoneuroendocrinology 2008; 33:425–436. [DOI] [PubMed] [Google Scholar]
- 14.Saavedra JM, Benicky J. Brain and peripheral angiotensin II play a major role in stress. Stress 2007; 10:185–193. [DOI] [PubMed] [Google Scholar]
- 15.Ramírez M, Prieto I, Banegas I, Segarra AB, Alba F. Neuropeptidases. Methods Mol Biol 2011; 789:287–294. [DOI] [PubMed] [Google Scholar]
- 16.Wang DH, Prewitt RL. Longitudinal effect of captopril on aortic and arteriolar development in normotensive rats. Am J Physiol 1991; 260:H1959–H1965. [DOI] [PubMed] [Google Scholar]
- 17.Priviero FB, Teixeira CE, Claudino MA, De Nucci G, Zanesco A, Antunes E. Vascular effects of long-term propranolol administration after chronic nitric oxide blockade. Eur J Pharmacol 2007; 571:189–196. [DOI] [PubMed] [Google Scholar]
- 18.Prieto I, Villarejo AB, Segarra AB, Wangensteen R, Banegas I, de Gasparo M, et al. Tissue distribution of CysAP activity and its relationship to blood pressure and water balance. Life Sci 2015; 134:73–78. [DOI] [PubMed] [Google Scholar]
- 19.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed.London: Academic Press; 1998. [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 1976; 72:248–254. [DOI] [PubMed] [Google Scholar]
- 21.Shen XZ, Li Y, Li L, Shah KH, Bernstein KE, Lyden P, et al. Microglia participate in neurogenic regulation of hypertension. Hypertension 2015; 66:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pabon MM, Bachstetter AD, Hudson CE, Gemma C, Bickford PC. CX3CL1 reduces neurotoxicity and microglial activation in a rat model of Parkinson's disease. J Neuroinflammation 2011; 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maia S, Arlicot N, Vierron E, Bodard S, Vergote J, Guilloteau D, et al. Longitudinal and parallel monitoring of neuroinflammation and neurodegeneration in a 6-hydroxydopamine rat model of Parkinson's disease. Synapse 2012; 66:573–583. [DOI] [PubMed] [Google Scholar]
- 24.Guo S, Kendrick KM, Zhang J, Broome M, Yu R, Liu Z, et al. Brain-wide functional inter-hemispheric disconnection is a potential biomarker for schizophrenia and distinguishes it from depression. Neuroimage Clin 2013; 2:818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu K, Jiang W, Ren L, Ouyang X, Jiang Y, Wu F, et al. Impaired interhemispheric connectivity in medication-naive patients with major depressive disorder. J Psychiatry Neurosci 2013; 38:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Shimol E, Gass N, Vollmayr B, Sartorius A, Goelman G. Reduced connectivity and inter-hemispheric symmetry of the sensory system in a rat model of vulnerability to developing depression. Neuroscience 2015; 310:742–750. [DOI] [PubMed] [Google Scholar]
- 27.Ramírez M, Prieto I, Alba F, Vives F, Banegas I, de Gasparo M. Role of central and peripheral aminopeptidase activities in the control of blood pressure: a working hypothesis. Heart Fail Rev 2008; 13:339–353. [DOI] [PubMed] [Google Scholar]
- 28.Noble F, Roques BP. Protection of endogenous enkephalin catabolism as natural approach to novel analgesic and antidepressant drugs. Expert Opin Ther Targets 2007; 11:145–159. [DOI] [PubMed] [Google Scholar]
- 29.Bodineau L, Frugière A, Marc Y, Inguimbert N, Fassot C, Balavoine F, et al. Orally active aminopeptidase A inhibitors reduce blood pressure: a new strategy for treating hypertension. Hypertension 2008; 51:1318–1325. [DOI] [PubMed] [Google Scholar]
- 30.Marc Y, Hmazzou R, Balavoine F, Flahault A, Llorens-Cortes C. Central antihypertensive effects of chronic treatment with RB150: an orally active aminopeptidase A inhibitor in deoxycorticosterone acetate-salt rats. J Hypertens 2018; 36:641–650. [DOI] [PubMed] [Google Scholar]
- 31.Saavedra JM. Angiotensin II AT(1) receptor blockers as treatments for inflammatory brain disorders. Clin Sci (Lond) 2012; 123:567–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res 2008; 170:261–276. [DOI] [PubMed] [Google Scholar]
- 33.Wright JW, Harding JW. Brain renin-angiotensin–a new look at an old system. Prog Neurobiol 2011; 95:49–67. [DOI] [PubMed] [Google Scholar]
- 34.Stragier B, De Bundel D, Sarre S, Smolders I, Vauquelin G, Dupont A, et al. Involvement of insulin-regulated aminopeptidase in the effects of the renin-angiotensin fragment angiotensin IV: a review. Heart Fail Rev 2008; 13:321–337. [DOI] [PubMed] [Google Scholar]
- 35.Fouyas IP, Kelly PA, Ritchie IM, Whittle IR. Cerebrovascular effects of nitric oxide manipulation in spontaneously hypertensive rats. Br J Pharmacol 1997; 121:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boultadakis A, Pitsikas N. Effects of the nitric oxide synthase inhibitor L-NAME on recognition and spatial memory deficits produced by different NMDA receptor antagonists in the rat. Neuropsychopharmacology 2010; 35:2357–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kruyer A, Soplop N, Strickland S, Norris EH. Chronic hypertension leads to neurodegeneration in the TgSwDI mouse model of Alzheimer's disease. Hypertension 2015; 66:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan X, Bruno KJ, Hess EJ. Rodent models of ADHD. Curr Top Behav Neurosci 2012; 9:273–300. [DOI] [PubMed] [Google Scholar]
- 39.Durand M, Berton O, Aguerre S, Edno L, Combourieu I, Mormède P, et al. Effects of repeated fluoxetine on anxiety-related behaviours, central serotonergic systems, and the corticotropic axis in SHR andWKY rats. Neuropharmacology 1999; 38:893–907. [DOI] [PubMed] [Google Scholar]
- 40.Banegas I, Prieto I, Segarra AB, Durán R, Vives F, Alba F, et al. Blood pressure increased dramatically in hypertensive rats after left hemisphere lesions with 6-hydroxydopamine. Neurosci Lett 2011; 500:148–150. [DOI] [PubMed] [Google Scholar]


