Introduction
Lipoprotein glomerulopathy (LPG) is caused by mutations in APOE, the gene encoding apolipoprotein E. The most commonly encountered mutations are APOE Sendai (p.Arg145Cys), APOE Kyoto (p.Arg25Cys or p.Arg43Cys), and APOE Tokyo (p.Leu141_Lys143del or p.Arg142_Leu144del), whose names correspond to the cities in which the index patients lived.1 Most cases have been reported from East Asian countries; only a few cases from the United States have been described.2 We present a case of LPG that was first diagnosed in a protocol kidney allograft biopsy. Diagnosis was challenging because of the many unusual features, including the patient’s European ancestry, lack of family history or significant proteinuria, very young age at progression to chronic renal failure, which had been erroneously attributed to primary membranoproliferative glomerulonephritis (MPGN), and complex kidney allograft histologic features consisting of donor-derived and subsequent posttransplant complications.
Case Presentation
A 6-year-old Italian-American girl presented to an outside hospital with reported renal dysfunction, proteinuria, microhematuria, and negative serologic workup (detailed information unavailable). The native kidney biopsy report described MPGN with mesangiolysis. Renal function deteriorated gradually, and the patient underwent bilateral nephrectomy at the age of 14 due to uncontrollable hypertension, followed by a deceased-donor kidney transplant 1 month later at an outside institution. The transplant course was complicated by polyomavirus nephropathy and T-cell–mediated rejection leading to graft failure 32 months posttransplantation.
At age 25, the patient received a second deceased-donor renal allograft at our institution and was found to have preformed circulating donor-specific antibodies and mild donor-derived IgA nephropathy, detected in the postreperfusion biopsy. After induction therapy with Thymoglobulin, the patient received i.v. Ig and was maintained on tacrolimus and Myfortic. Seventeen days posttransplantation, serum creatinine increased from 1.3 to 5.9 mg/dl in the setting of fluctuating tacrolimus and increased circulating donor-specific antibody levels. An allograft biopsy showed focal C4d staining in peritubular capillaries and segmental weak subepithelial immunofluorescence staining for IgG consistent with de novo membranous glomerulopathy, likely related to humoral alloreactivity. With supportive care alone, serum creatinine fell to 1.6 mg/dl and circulating donor-specific antibody levels declined gradually. Follow-up biopsies at 1 and 3 months posttransplantation demonstrated negative C4d staining without features of acute rejection. Approximately 4 months posttransplantation, an allograft biopsy showed borderline changes suspicious for acute T-cell–mediated rejection, which was treated with pulse steroids followed by prednisone maintenance. The 6-month protocol biopsy was obtained in the setting of serum creatinine of 1.6 mg/dl, urine protein/creatinine: 0.1 g/g, bland urine sediment, and low-level circulating donor-specific antibody.
Protocol Kidney Biopsy at 6 Months Posttransplantation
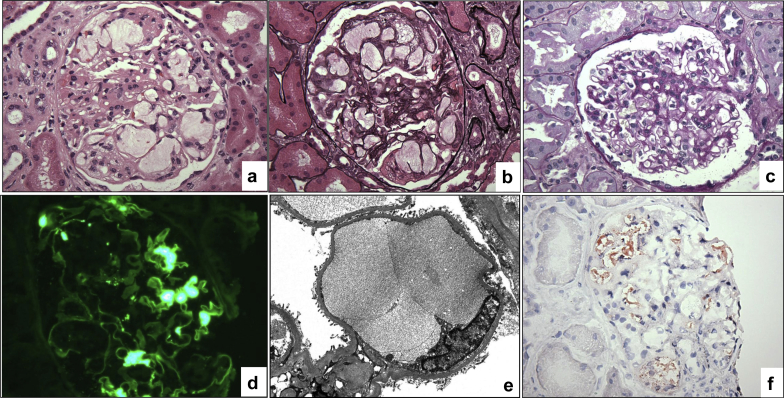
Sampling for light microscopy contained 39 glomeruli, none of which was segmentally or globally sclerotic. Five glomeruli showed capillary dilatation accompanied by segmental double contours and mesangiolysis. The dilated capillaries contained loose acellular material with subtle lamellation (Figure 1a and b) that stained negative with periodic acid–Schiff and pale blue with trichrome stain. Unaffected glomeruli showed mild focal mesangial hypercellularity without glomerulitis or double contours (Figure 1c). There was mild tubulointerstitial scarring, moderate arteriosclerosis, and moderate arteriolosclerosis without significant interstitial inflammation or peritubular capillaritis. The immunofluorescence showed segmental mesangial staining for IgA (Figure 1d), kappa and lambda, with no glomerular capillary wall staining for IgG and negative peritubular capillary staining for C4d. Paraffin tissue was reprocessed for electron microscopy. Despite processing artifacts, ultrastructural evaluation revealed focal occlusion of the glomerular capillaries by weakly dense extracellular material (Figure 1e), scattered mesangial deposits, and focal foot process effacement.
Figure 1.
Histologic findings in the 6-month protocol allograft biopsy. (a) A glomerulus with dilated glomerular capillaries containing acellular material (hematoxylin-eosin, original magnification ×400). (b) Same glomerulus showing vaguely lamellated intracapillary material, segmental mesangiolysis, and rare foamy cells within the mesangium (Jones methenamine silver, original magnification ×400). (c) Nonaffected glomerulus showing mild segmental mesangial hypercellularity (periodic acid–Schiff, original magnification ×400). (d) Mesangial IgA staining reflecting the donor-derived IgA nephropathy (immunofluorescence IgA, original magnification ×400). (e) Ultrastructural examination showing a dilated capillary filled with extracellular material (electron microscopy from paraffin-embedded tissue, original magnification ×6000). (f) Cryosections showing intraglomerular fat droplets (red; Oil red-O, original magnification ×400).
In summary, the 6-month protocol biopsy showed occlusive intracapillary acellular material with membranoproliferative features involving 13% of sampled glomeruli and mild donor-derived IgA nephropathy.
Differential diagnosis for the unusual intraglomerular findings included foreign, mucinous, or lipid material that may have developed in the donor, at implantation, or after transplantation. To determine the temporal sequence, we re-reviewed all allograft biopsies. Similar but more subtle material was evident only in the previous 4-month posttransplantation biopsy, supporting a progressive posttransplant process.
The patient denied drug abuse or injecting any foreign substance. Special stains for mucicarmine and Alcian blue were negative for mucinous material. Immunohistochemical stain for adipophilin, which labels intracytoplasmic fat, was also negative. However, the frozen sample showed diffuse staining for Oil red-O within glomerular capillary lumina (Figure 1f). As the latter stain labels fat, these findings were most consistent with lipidosis, namely LPG.
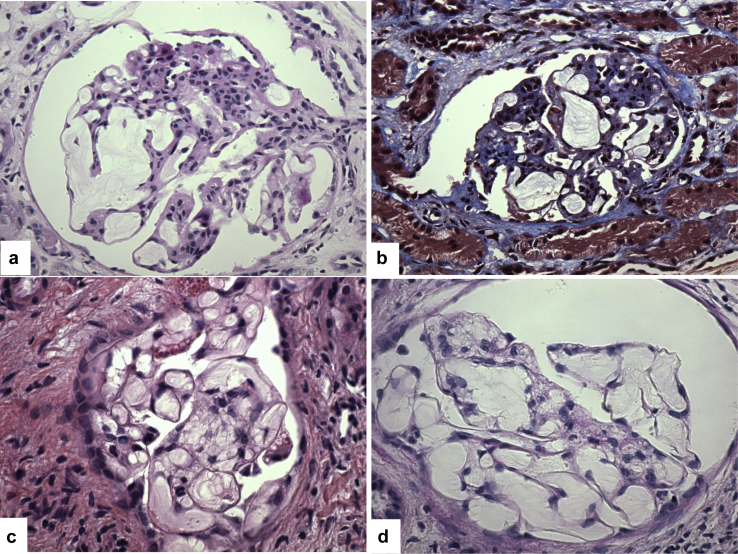
Because the likelihood of de novo LPG is extremely low, we next pursued the possibility of a recurrent disease. The outside pathology reports were reviewed. The native biopsy with MPGN mentioned mesangiolysis, IgM staining, and only scattered electron densities. The last allograft biopsy from the first transplant described abnormal glomerular capillary dilatation. Given these unusual findings, the original slides from these 2 biopsies were requested for independent evaluation. Light microscopic assessment of these biopsies confirmed the presence of findings similar to, but more developed and widespread than those in our biopsies (Figure 2a–d), strongly supporting LPG in both native and allograft biopsies.
Figure 2.
Histologic findings in the native kidney and the last allograft biopsy from the previous transplant. (a,b) In the native biopsy, all glomeruli show massively dilated capillaries containing acellular material that stains negative with periodic acid–Schiff (a) and pale blue with trichrome (b; original magnification ×400). (c,d) In the last allograft biopsy, most of the sampled glomeruli display dilated glomerular capillaries containing lamellated acellular material (c, hematoxylin-eosin; d, periodic acid–Schiff, original magnification ×600).
Follow-up
Laboratory workup revealed normal lipid profile (cholesterol: 150 [<200 mg/dl], triglycerides: 103 [≤149 mg/dl], high-density lipoprotein: 53 [40–60 mg/dl], and low-density lipoprotein: 76 [70–190 mg/dl]). All coding exons of the APOE gene were assessed using Sanger sequencing, as described previously.1 We detected a heterozygous C>T substitution in exon 3, corresponding to the established causal APOE Kyoto variant (p.Arg25Cys).1, 2, 3 The patient's father was negative for the variant. We were unable to contact the mother who, according to the proband and her father, did not have any history of dyslipidemia or kidney disease. Thus, the APOE variant detected in our patient may be a de novo mutation, or, given the incomplete penetrance and variable expressivity of LPG, may have been inherited from her mother.
Given the past history of LPG that led to native renal failure and recurred in the first allograft, the histologic findings that support progressive LPG in the current allograft (no glomerular manifestation in the first 3 months after transplantation, subtle findings in month 4, and 13% affected glomeruli in month 6), and the unexplained renal dysfunction, we elected to treat the patient. Because of the suboptimal allograft function, we started with a low dose of fenofibrate (43 mg p.o. daily) with the initial plan to uptitrate the dose as tolerated. However, fenofibrate was stopped 4 months later because of acute increase in serum creatinine to 2.5 mg/dl and the patient was started on atorvastatin. An allograft biopsy showed focal cortical scarring. The dosage of tacrolimus was then lowered to 1 mg p.o. every 12 hours and belatacept was initiated. One month later (approximately 1-year posttransplantation), the patient had a serum creatinine of 1.56 mg/dl, urine protein/creatinine: 0.1 g/g, and bland urine sediment.
Discussion
LPG is a rare kidney disease that is often associated with dyslipidemia, including elevated levels of APOE, triglycerides, and low-density lipoprotein3 (although some cases, including our patient, have normal lipid profile). Clinically, LPG is typically a renal-limited disease that presents with renal insufficiency and proteinuria (sometimes with nephrotic syndrome), and progresses to end-stage renal disease usually several years after diagnosis, especially in untreated patients.3 LPG has been mainly encountered in Asian populations, but also has been reported rarely in European-Americans.2 Although usually manifesting in adolescents or adults, LPG can affect children, sometimes as young as 3 years of age.4 Our patient was European-American with clinical onset of the disease at 6 years of age and progression to chronic renal failure requiring transplantation at 14 years of age.
LPG is an autosomal dominant disorder with incomplete penetrance and variable expressivity. Thus, family members of affected individuals may harbor the causal APOE mutation, yet be asymptomatic2 or show isolated dyslipidemia.3 Given their filtration function and relatively slow blood flow, the glomerular capillaries offer an optimal microenvironment for accumulation of large macromolecules, including lipoproteins. Importantly, APOE mutations can increase lipid binding to endothelial cells and inhibit mesangial clearance of lipids.1, 5 Macrophage impairment may also contribute by inhibiting lipid clearance from the glomerular capillaries.1
Pathognomonic histologic features include thrombus-like dilatation of the glomerular capillaries without significant infiltration by foamy macrophages. Focal membranoproliferative features, namely mesangiolysis and glomerular basement membrane duplication, can occur secondary to expansile forces exerted by the accumulating lipoprotein and may lead to an erroneous diagnosis of MPGN. Thus, it is important to recognize that MPGN is a pattern of glomerular injury rather than a distinct diagnostic entity. In contrast to immune complex–mediated MPGN, LPG is characterized by glomerular capillary lipid thrombi and lacks hypocomplementemia, significant hematuria, substantial immunofluorescence staining for immunoglobulins, or more than scattered nonspecific densities by electron microscopy.
Recurrence of LPG has been described in a few patients6, 7, 8, 9 (Table 1), including one who progressed to renal failure within the first year after transplantation. Our patient’s first transplant failed within 3 years and graft failure was probably multifactorial related to polyomavirus, rejection, and LPG (because most glomeruli were involved by lipoprotein thrombi). A diagnosis of recurrent and de novo glomerular disease requires knowledge of the original process leading to end-stage renal disease in the native kidney, especially in the setting of complicated intercurrent allograft pathologies. In our case, review of the outside native and first allograft kidney biopsies was critical for establishing the diagnosis of disease recurrence.
Table 1.
Published cases of posttransplant lipoprotein glomerulopathy
| Cases | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
| Authors | Kamada et al. (1989)a | Mourad et al. (1998) | Miyata et al. (1999) | Foster et al. (2005) | Batal et al. (2018) | |
| Age | 19 | 30 | 42 | 42 | 14 or 15 | 25 |
| Gender | Male | Female | Male | Male | Female | |
| Race | Asian | European | Asian | Asian | European | |
| De novo versus recurrent | Recurrent | Recurrent | Recurrent | Recurrent | Recurrent | |
| Allograft source | Living (father) | Deceased | Deceased | Deceased | Deceased | Deceased |
| Time posttransplantation, yr | Within first year | 0.4 | 1.3 | 2 | NA | 0.5 |
| sCr at biopsy, mg/dl | 1.3 | 1.6 | 1.3 (protocol biopsy) | 2 | NA | 1.6 (protocol biopsy) |
| Proteinuria, g/d | 4.0 | 5.7 | 0.3 | 4.9 | NA | 0.1 |
| Abnormal triglyceride | Yes | Yes | Yes | Yes | Probably not | No |
| Treatment | NA | ACE inhibitor | NA | ACE inhibitor | No treatment | Fenofibrate (temporarily) |
| Outcome | Failed within 1 year after transplant | Functioning (4.2 years after transplant) | NA | Functioning (6 years after transplant) | Failed (2.6 years after transplant) | Functioning (1 year after transplant) |
ACE, angiotensin-converting enzyme; NA, not available; sCr, serum creatinine.
See Supplementary Reference list.
Lipid-lowering therapy is the main treatment strategy in LPG, and the use of fibrates has been reported to induce a clinical remission in some patients.3 In the transplant setting, 2 patients with significant proteinuria due to recurrent LPG achieved a reduction in proteinuria with angiotensin-converting enzyme inhibitor therapy.7, 9
In conclusion, LPG is a distinct clinico-pathologic entity caused by mutations in APOE gene (Table 2). We report a rare case of APOE Kyoto LPG in a young European-American patient that recurred in 2 successive renal allografts without dyslipidemia or family history of LPG. Greater familiarity with the defining pathologic features of this entity is needed to ensure timely diagnosis and optimal management, both in the native kidney and the transplant setting. As suggested by this case, protocol biopsies may be beneficial in monitoring LPG recurrence in the allograft.
Table 2.
Key clinical and pathological characteristics of lipoprotein glomerulopathy
| Genetics | APOE gene mutations; recurrent causal mutations include APOE Kyoto (p.Arg25Cys), APOE Sendai (p.Arg145Cys), and APOE Tokyo (p.Leu141_Lys143del) |
| Age | Usually in adolescence or young adulthood, rarely in children |
| Race | Typically Asian (especially Japanese and Chinese), rarely white |
| Laboratory abnormalities | Hyperlipidemia (mainly triglycerides and APOE), proteinuria (sometimes nephrotic syndrome) and/or elevated serum creatinine |
| Pathologic characteristics | Light microscopy:
|
| Treatment | Fibrate and/or angiotensin-converting enzyme inhibitor |
| Occurrence in transplant setting | Universal for recurrent disease, not reported as de novo |
Disclosure
All the authors declared no competing interests.
Footnotes
Supplementary Reference.
Supplementary material is linked to the online version of the paper at http://www.kireports.org/.
Supplementary Material
References
- 1.Saito T., Matsunaga A., Ito K. Topics in lipoprotein glomerulopathy: an overview. Clin Exp Nephrol. 2014;18:214–217. doi: 10.1007/s10157-013-0887-4. [DOI] [PubMed] [Google Scholar]
- 2.Rovin B.H., Roncone D., McKinley A. APOE Kyoto mutation in European Americans with lipoprotein glomerulopathy. N Engl J Med. 2007;357:2522–2524. doi: 10.1056/NEJMc072088. [DOI] [PubMed] [Google Scholar]
- 3.Hu Z., Huang S., Wu Y. Hereditary features, treatment, and prognosis of the lipoprotein glomerulopathy in patients with the APOE Kyoto mutation. Kidney Int. 2014;85:416–424. doi: 10.1038/ki.2013.335. [DOI] [PubMed] [Google Scholar]
- 4.Matsunaga A., Furuyama M., Hashimoto T. Improvement of nephrotic syndrome by intensive lipid-lowering therapy in a patient with lipoprotein glomerulopathy. Clin Exp Nephrol. 2009;13:659–662. doi: 10.1007/s10157-009-0207-1. [DOI] [PubMed] [Google Scholar]
- 5.Murano T., Matsumura R., Misawa Y. Interaction of endothelial cells and triglyceride-rich lipoproteins with apolipoprotein E (Arg-->Cys) from a patient with lipoprotein glomerulopathy. Metabolism. 2002;51:201–205. doi: 10.1053/meta.2002.29990. [DOI] [PubMed] [Google Scholar]
- 6.Saito T., Sato H., Oikawa S. Lipoprotein glomerulopathy. Report of a normolipidemic case and review of the literature. Am J Nephrol. 1993;13:64–68. doi: 10.1159/000168591. [DOI] [PubMed] [Google Scholar]
- 7.Foster K., Matsunaga A., Matalon R. A rare cause of posttransplantation nephrotic syndrome. Am J Kidney Dis. 2005;45:1132–1138. doi: 10.1053/j.ajkd.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 8.Miyata T., Sugiyama S., Nangaku M. Apolipoprotein E2/E5 variants in lipoprotein glomerulopathy recurred in transplanted kidney. J Am Soc Nephrol. 1999;10:1590–1595. doi: 10.1681/ASN.V1071590. [DOI] [PubMed] [Google Scholar]
- 9.Mourad G., Djamali A., Turc-Baron C. Lipoprotein glomerulopathy: a new cause of nephrotic syndrome after renal transplantation. Nephrol Dial Transplant. 1998;13:1292–1294. doi: 10.1093/ndt/13.5.1292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.