Abstract
Hydroponic ginseng (HPG) and soil-cultured ginseng (SCG) were extracted in 70% methanol to quantify relative content of 8 ginsenosides and polyphenolic compounds, and flavonoids to compare their antioxidative effects. Level of nitric oxide and inflammatory targets produced in LPS-stimulated RAW 264.7 cells were measured. 2-year-old HPG shoots contained highest levels of ginsenoside Rb2, Rb3, Rd, Re, and F1. Total polyphenol content was highest in shoots of HPG, followed by roots of HPG and SCG. HPG shoots had high radical scavenging activity and an elevated ability to inhibit linoleic acid oxidation. 2-year-old HPG shoots reduced nitric oxide production in RAW 264.7 cells by 47%, whereas 6-year-old SCG roots reduced it by only 21%. HPG also significantly lowered mRNA expression of iNOS, TNF-α, IL-1β, and IL-6, as determined by RT-PCR, compared to SCGs. Therefore, HPG may have potential for utilization as an alternative to SCG, because of superior antioxidant and anti-inflammatory properties.
Keywords: Hydroponic ginseng, Panax ginseng, Flavonoid, Antioxidative effect, Anti-inflammatory effect
Introduction
Oxidative stress accompanied by free radicals leads to peroxidation of proteins, lipids, and DNA, and ultimately to a variety of diseases. In particular, nitric oxide (NO), which is a reactive oxide generated from l-arginine via inducible nitric oxide synthases (iNOS) by oxidative stress, is an inflammatory mediator and activates nuclear factor-κB (NF-κB), the transcription factor for the inflammatory response (Young et al., 2018). Previous studies reported that oxidative stress induces inflammatory responses, resulting in cancer, diabetes, cardiovascular disease, and aging (Lee et al., 2017). Therefore, many researches on various antioxidants is still regularly conducted.
The root of Panax ginseng Meyer has been widely used in East Asia in traditional herbal medicine and has been reported to have anti-hyperglycemic (Chung et al., 2016a; 2016b), anti-obesity (Siraj et al., 2014), and anticancer (Eom et al., 2018) effects, due to its numerous phenolic and other chemical components, including more than 20 kinds of ginsenosides. Additionally, many studies have described the antioxidative and anti-inflammatory effects of ginseng. According to a previous study by Zhang et al. (2018), supercritical water extract of ginseng exhibits strong radical-scavenging activity because of its polyphenol contents. Furthermore, several papers have presented the anti-inflammatory effect of ginseng. Ginsenoside Rb1, Rb2, Rg1, Rc, and Re contained in ginseng inhibit iNOS expression (Baek et al., 2016) and Rg3 suppresses the expression of TNF-α, IL-1β, IL-6, and COX-2 in LPS-stimulated RAW 264.7 cells (Bak et al., 2012).
It takes a minimum of 4–6 years for ginseng cultivated in soil to be ready for commercial utilization (Kim et al., 2018) and there are many obstacles to its maturation, including infectious diseases from soil such as damping-off and root rot (Doumbou et al., 2001) and the accumulation of heavy metals and pesticides (Noh et al., 2016). Currently, the interest in hydroponic ginseng, which is cultivated in mineral nutrient solutions without pesticides, has increased as it provides a means for overcoming these obstacles. Additionally, hydroponics can shorten ginseng’s growth period by 1–2 years, and the productivity per unit area is very high (Kim et al., 2010). The current management cost of a hydroponic cultivation system is 36,069 dollars per acre, while the conventional soil-cultivation method costs 281,560 dollars per acre (Ministry of Agriculture, Food and Rural Affairs, 2017). In addition, it is relatively easy to adjust growth conditions compared to conventional farming. Moreover, unlike conventional cultivation in which only the roots are used, with HPG all plant parts can be utilized, including its leaves, stems, and fruits. However, there are lacks of studies comparing the health functional effects of hydroponic ginseng to those of ginsengs grown in soil.
The objective of this study was to compare the antioxidative activities and anti-inflammatory effects of HPG and SCG, in terms of their biologically active compounds, both by plant age and by plant parts. Based on these findings, HPG is suggested as an alternative to 4- to 6-year old SCG.
Materials and methods
Materials
One-year- and two-year-old ginseng cultivated in a hydroponic system (1YHPG, and 2YHPG, respectively) was purchased from Chungjung-Saessacksam Company (Gwangju, South Korea) as whole plants, including roots and shoots. 1YHPG and 2YHPG were cultivated as 1- and 2-year-old seedlings in a hydroponic cultivation system for 2 months, respectively. Roots of 4-year and 6-year-old ginseng grown in soil were procured from Sangdo-Insamsa Company (Punggi, South Korea). HPLC grade water and acetonitrile were purchased from J.T. Baker (Center Valley, PA, USA). Ginsenoside standards (Rb1, Rb1, Rb3, Rc, Re, Rd, Rf, and F1) were purchased from Biopurify Phytochemicals Ltd. (Chengdu, China), and from Suzhou Star Ocean Ginseng Bio-pharmaceutical Co., Ltd. (Suzhou, China). Gallic acid, 2,2-diphenyl-1-picrylhydrazyl, 2,2-2,2′-Azinobis (2-ethyl benzothiazoline-6-sulfonate), quercetin, and kaempferol were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). RAW 264.7 cells were obtained from Korean Cell Line Bank (Seoul National University, Seoul, Korea).
Sample preparation
One- and two-year-old hydroponic ginseng plants were separated as roots and shoots parts, and then washed and lyophilized. Each lyophilizate (40 g) was extracted with 700 mL of 70% methanol at 80 °C for 1 h in a reflux system. The extraction was repeated twice. Total extracts were concentrated using a rotary evaporator (EYELA N-1000 V, Tokyo, Japan) at 60 °C, after which they were lyophilized and ground into powder. The lyophilizates of roots of 4- and 6-year-old soil-cultured ginseng (4YSCG, and 6YSCG, respectively) were prepared using the same processing method and were stored at − 20 °C until used.
Determination of total flavonoid content measurement (TFC)
The colorimetric method for the determination of TFC was conducted (Padhi et al., 2017). A 500 μL sample was mixed with 100 μL of 10% aluminum chloride in 1.5 mL of ethanol and the tubes were incubated at 25 °C for 30 min. After reaction, the absorbance was measured at 415 nm. Kaempferol was used as a standard for the construction of calibration curves. The concentrations of total flavonoids in samples were calculated as milligrams of kaempferol equivalents (KPE) per g dry weight.
Determination of total polyphenolic compounds content (TPC)
The Folin–Ciocalteu method reported by Padhi et al. (2017) was used for the determination of TPC, with slight modification. A 100 μL sample was added to 2 mL of 2% sodium carbonate and left for 3 min after vortexing. A 50% Folin–Ciocalteu solution (100 μL) was added, and the mixture was incubated for 30 min at 25 °C. The absorbance was measured at a wavelength of 750 nm. Gallic acid was used as a standard for the creation of the calibration curve. The concentration of total polyphenolic compounds in each sample was expressed as milligrams of gallic acid equivalents (GAE) per g dry weight.
HPLC analysis
Sample preparation for HPLC analysis
Preparation of samples was performed according to the method of Hong et al. (2009), with minor modification. Freeze-dried ginseng extracts were dissolved in 25 mL of distilled water. A Sep-Pak® Plus C18 cartridge (Waters, Milfore, MA, USA) was used to prepare sample for HPLC analysis. The cartridge filters were placed on vacuum port in a 10 mL syringe and were cleaned by passing 5 mL of methanol through them. Following this, the cartridges were preconditioned with 20 mL of distilled water, and 5 mL of ginseng extract solutions were then applied onto the cartridges. Next, the cartridges were washed with 20 mL of distilled water, followed by elution with 15 mL of 30% methanol. The cartridge eluent solutions were filtered through 0.45-μm PVDF syringe filters and then prepared for HPLC analysis.
HPLC methods
The compositions of ginsenosides in samples were measured using an 1100 Agilent series HPLC (Agilent Technology, Pal Alto, CA, USA). A reverse phase column (ZORBAX Eclips XDB-C18, 4.6 mm × 150 mm) was used (Agilent Technology, Pal Alto, CA, USA) and the mobile phase was a binary eluent of A (14% acetonitrile in water) and B (acetonitrile) under following gradient program: 0 min (0% B), 0–3 min (6% B), 3–10 min (9% B), 10–20 min (21.6% B), 20–30 min (30.4% B), 30–35 min (0% B). The flow rate was 10 μL/min and the ginsenosides were detected using a UV detector at 203 nm. The concentration ranges and calibration curves of each standard were as follows:
Rb1 (), Rb2 (), Rb3 (), Rc (Y = 2.39255125X + 54.475684, R2 = 0.9982), Rd (), Re (), Rf (), F1 (), where X is the amount of analyte (μg/mL) and Y is peak area.
Determination of antioxidative activity
2,2,-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay
A DPPH radical scavenging assay was conducted according to the method described by Macêdo et al. (2018). A 100 μM 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution was prepared by dissolving in 99.5% ethanol. This solution had an absorbance of 1.2 at a wavelength of 517 nm. A 1.0 mL of DPPH solution was added to 200 μL of sample and the reaction allowed to proceed for 15 min in the dark. Then, the absorbance was measured at 517 nm by spectrophotometer (X-ma 3200PC, Human Corporation, Republic of Korea). The antioxidation rate was calculated as follows:
where Ac and As are the absorbance values for the negative control (distilled water) and sample, respectively.
β-carotene-linoleic acid assay
The inhibition of β-carotene/linoleic acid co-oxidation was determined using the β-carotene-linoleic acid assay described by Eom et al. (2017). Two milligrams of β-carotene, 44 μL linoleic acid, and 200 μL Tween 80 were mixed thoroughly in 10 mL of chloroform in a round-bottomed flask. The mixture was evaporated in a rotary evaporator (EYELA N-1000 V, Tokyo, Japan) at 42 °C. The resultant residue was dissolved in 100 mL of distilled water and mixed to form an emulsion (the absorbance was 1.8–2.0 at 470 nm). Samples (500 μL) were added to 4.5 mL aliquots of the emulsion. The tubes were incubated in a water bath at 50 °C for 3 h, following which the absorbance was measured at 470 nm. The antioxidant activity was calculated using the following equation:
where A0 is the absorbance value at the start of incubation for sample and control, and At is the absorbance at t = 180 min.
Determination of anti-inflammatory activity
Measurement of NO production in RAW 264.7 cells
Analysis of NO produced in RAW 264.7 cells was performed to evaluate anti-inflammatory activity. The RAW 264.7 cells were seeded in a 96-well microplate at a density of 2 × 105 cells/well and incubated for 2 h at 37 °C. The cells were treated with different samples concentrations from HPGs and SCGs with different ages and stimulated with 0.1 μg/mL of LPS for 24 h. Each medium was transferred to a new microplate in 100 μL increments and the presence of nitrite was determined by adding 100 μL of Greiss reagent. Absorbance was measured at a wavelength of 570 nm using an ELISA reader (Emax, Torrance, CA, USA). The amount of nitrite present was calculated from a sodium nitrite (NaNO2) standard curve.
Measurement of inflammation-related cytokines
Total mRNA was extracted from RAW 264.7 cells using a RNeasy® Mini Kit (QIAGEN, Hilden, Germany). The cDNA was synthesized using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, UK) and was followed by a reverse transcription-polymerase chain reaction (RT-PCR) with SensiFAST™ SYBR No-ROX Kit (Bioline, London, UK). The following primers were used: β-actin (forward, 5′-GTC GGC GGC CCT AGG CAC CAG-3′; reverse, 5′-GGA GGA AGA GGA TGC GGC AGT-3′), iNOS (forward, 5′-CCC TTC CGA ACT TTC TGG CAG CAG C-3′; reverse, 5′-GGC TGT CAG AGT CTC GTG GCT TTG G-3′), TNF-α (forward, 5′-GCA GAA GAG GCA CTC CCC CA-3′; reverse, 5′-GAT CCA TGC CGT TGG CCA GG-3′), IL-1β (forward, 5′-CAG GAT GAG GAC ATG AGC ACC-3′; reverse, 5′-CTC TGC AGA CTC AAA CTC CAC-3′), IL-6 (forward, 5′-AGT TGC CTT CTT GGG ACT GA-3′; reverse, 5′-TTC TGC AAG TGC ATC ATC GT-3′).
Statistical analysis
All experiments were conducted using triplicate samples and were repeated three times. The values were expressed as mean ± standard deviation and one-way analysis of variance (ANOVA) was carried out using SPSS software (version 20, SPSS Inc., Chicago, USA). Duncan’s multiple range test was employed to test for significant differences between the treatments at p < 0.05.
Results and discussion
Total polyphenolic compounds and flavonoids contents
The phenolic compounds and flavonoids are strongly related to antioxidant activity and their importance has been previously emphasized. Phenolic compounds have hydroxyl groups (–OH) conjugated to an aromatic ring in their structure and can therefore bind to macromolecules, such as proteins or enzymes, and have metal-ion-chelating and reductive activities (Köksal et al., 2017). In this study, total polyphenolic compounds and flavonoid contents of HPG and SCG were measured, with the results are reported in Table 1. The total polyphenolic contents of ginseng were shown to differ significantly between HPG and SCG, with the 6YSCG roots containing less than 43% that of 1YHPG. The highest levels of GAE were found in shoots of 1YHPG(45.28 ± 0.30 GAE (mg/g)), followed by shoots of 2YHPG(36.49 ± 0.38 GAE (mg/g)), root of 2YHPG(31.80 ± 0.60 GAE (mg/g)), 1YHPG(30.29 ± 0.66 GAE (mg/g)), 6YSCG(17.36 ± 0.52 GAE (mg/g)), and 4YSCG(15.88 ± 0.29 GAE (mg/g)). The GAE level decreased by plant age, as described in previous studies (Shi et al., 2007). The TFC was also significantly higher in HPG shoots than in HPG roots, both of which had higher levels than were found in SCG roots. The 6YSCG (6YSCG, 1.44 ± 0.05 KPE (mg/g)) showed the lowest TFC level. These results suggest that it may be more advantageous to utilize HPG, where the whole plant can be used, than to utilize SCG, which takes 4–6 years to harvest and has a lower phenolic compound content.
Table 1.
Total polyphenol and total phenolic compound contents
| Samples | Total polyphenol content (GAE) | Total flavonoid content (KPE) |
|---|---|---|
| HPG (shoots) | ||
| 1YHPG | 45.28 ± 0.30a | 35.85 ± 0.25a |
| 2YHPG | 36.49 ± 0.38b | 19.51 ± 0.19b |
| HPG (roots) | ||
| 1YHPG | 30.29 ± 0.66d | 2.29 ± 0.10c |
| 2YHPG | 31.80 ± 0.60c | 2.31 ± 0.04c |
| SCG (roots) | ||
| 4YSCG | 15.88 ± 0.29f | 1.77 ± 0.07d |
| 6YSCG | 17.36 ± 0.52e | 1.44 ± 0.05e |
All values were expressed as mean ± SD
GAE mg Gallic acid/g solid content of sample, KPE mg kaempferol/g solid content of sample
a–fValues with different superscripts in same row are significantly different at p < 0.05 (n = 3) by Duncan’s multiple range test. 1YHPG, 1-year-old hydroponic ginseng; 2YHPG, 2-years-old hydroponic ginseng; 4YSCG, 4-years-old soil-cultured ginseng; 6YSCG, 6-years-old soil-cultured ginseng
Ginsenoside content
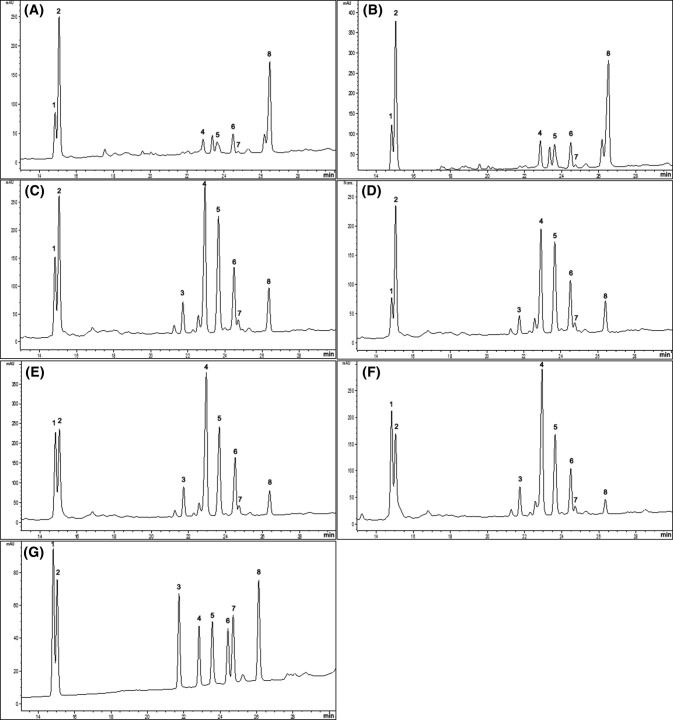
Ginsenoside, a saponin specific to ginseng, is classified according to the number of hydroxyl group bound to structure as either PPD (protopanaxadiol) or PPT (protopanaxatriol). It has been reported that the composition and content of ginsenoside varies depending on a variety of other conditions including the region in which it is cultivated and its growth environment (Lu et al., 2018). The extraction yields of crude saponin from the roots of 1YHPG, 2YHPG, 4YSCG, and 6YSCG, and the shoots of 1YHPG and 2YHPG, were 8.24, 9.67, 8.98, 7.49, 26.36, and 33.33%, respectively (data not shown). The ginsenoside composition was analyzed using HPLC and the detected ginsenosides were labeled as peaks 1–18 (Fig. 1). Each of the ginsenosides were identified by comparison of their retention times with those of the authentic standards. Ginsenoside compositions varied significantly between cultivation systems, plant ages, and plant parts. Rb1, Rb2, Rb3, Rc, Rd, Re, Rf, and F1 were detected in the roots of HPG and SCG (Fig. 1A, B, C, D) but the composition of each was different. Shoots of 1YHPG and 2YHPG also contained ginsenosides Rb1, Rb2, Rb3, Rc, Rd, Re, and F1, but Rf was not detected (Fig. 1E, F). The amount of Rb1, Rc, and Rf was highest in the roots of 4YSCG (1094.69 ± 1.79, 1053.68 ± 1.82, 217.51 ± 2.07 μg/g, respectively) and the highest levels of Rb2, Rb3, Rd, Re, and F1 were detected in the shoots of 2YHPG (1043.66 ± 0.20, 48.85 ± 1.90, 408.20 ± 2.06, 2385.80 ± 3.18, 1638.73 ± 4.89 μg/g, respectively). Ginsenoside Re and F1, particularly, are much higher in HPG shoots than in the roots of SCG and HPG. The ginsenoside Re, reported to be abundant in ginseng berries, has been shown to inhibit overexpression of TNF-α in RAW 264.7 cells damages by doxorubicin (Wang et al., 2017b). The ginsenosides F1 is mostly resent in leaves, and a previous study by Kim et al., (2015) was showed that F1 reduced α-melanocyte-stimulating hormone-induced melanin secretion in B16F10 cell culture media by 60%. As Table 2 suggests, the amount of each ginsenoside in 4YSCG is significantly higher than roots of 1YHPG, with the exception of ginsenoside F1. However, as hydroponic ginseng is consumed whole, including roots, stems, and leaves, ingesting HPG provides more ginsenosides than are provided through consuming only the root of SCG. The changes in the content of ginsenosides are not obviously dependent on plant age. Total ginsenosides content was not increased with aging. The total ginsenoside content of SCG was higher than HPG, but both decreased over time. Normally, the total content of ginsenosides content increases with plant age, however, this tends to correlate with the diameter of ginseng roots. According to a study of Han et al., (2013), as the root thickens by their ages, the xylem/cortical layer thickness ratio increases, and thus the root diameter is negatively correlated with its ginsenoside content. On the other hand, although undetected in the present study, Lee et al. (2017) identified ginsenoside Rh23 in HPG leaves and investigated its inhibitory effect on melanogenesis in zebrafish in vivo. As a result, both the leaves and roots of HPG can be utilized as a source for variety of ginsenosides in place of SCG.
Fig. 1.
HPLC chromatogram of hydroponic ginseng (HPG), soil-cultured ginseng (SCG), and mixed ginsenoside standards. The x-axis is retention time and the y-axis is a intensity of absorbance (mAU milli-Absorbance Units). (A) shoots of 1-year-old HPG (1YHPG), (B) shoots of 2-years-old HPG (2YHPG), (C) roots of 1YHPG, (D) roots of 2YHPG, (E) roots of 4-years-old SCG (4YSCG), (F) roots of 6-years-old SCG (6YSCG), (G) ginsenoside standards. 1, Rd; 2, Re; 3, Rf; 4, Rb1; 5, Rc; 6, Rb2; 7, Rb3; 8, F1
Table 2.
Ginsenoside composition of HPG and SCG by their ages and parts
| Ginsenoside contents | Shoots | Roots | ||||
|---|---|---|---|---|---|---|
| 1-year old HPG | 2-years old HPG | 1-year old HPG | 2-years old HPG | 4-years old SCG | 6-years old SCG | |
| Panaxadiol (PPD) (μg/g) | ||||||
| Rb1 | 222.77 ± 1.50f | 548.08 ± 6.24d | 750.03 ± 3.15c | 482.24 ± 0.50e | 1094.69 ± 1.79a | 772.59 ± 1.83b |
| Rb2 | 551.62 ± 4.09d | 1043.66 ± 0.20a | 565.39 ± 0.75c | 441.84 ± 0.29e | 729.98 ± 5.90b | 436.80 ± 1.25f |
| Rb3 | 42.85 ± 4.76e | 48.85 ± 1.90a | 27.25 ± 0.17c | 25.22 ± 0.34d | 31.55 ± 0.60b | 24.13 ± 0.36d |
| Rc | 297.64 ± 4.82f | 754.85 ± 5.78c | 1002.49 ± 3.56b | 746.46 ± 1.13d | 1053.68 ± 1.82a | 710.77 ± 1.70e |
| Rd | 173.06 ± 0.55d | 408.20 ± 2.06a | 169.78 ± 2.04d | 85.30 ± 1.15e | 285.24 ± 1.57b | 244.55 ± 0.50c |
| Panaxatriol (PPT) (μg/g) | ||||||
| Re | 1243.35 ± 3.64b | 2385.80 ± 3.18a | 534.00 ± 0.47d | 507.15 ± 0.72e | 568.32 ± 1.47c | 392.56 ± 0.48f |
| Rf | ND | ND | 164.51 ± 0.54b | 89.35 ± 0.76d | 217.51 ± 2.07a | 155.30 ± 2.04c |
| F1 | 860.03 ± 3.47b | 1638.73 ± 4.89a | 156.13 ± 1.09c | 105.69 ± 0.74e | 116.46 ± 1.02d | 51.59 ± 0.88f |
| Total (μg/g) | 3391.33 | 6871.02 | 3369.58 | 2483.26 | 4097.44 | 2788.27 |
All values were expressed as mean ± SD
ND Not detected
a–fValues with different superscripts in same row are significantly different at p < 0.05 (n = 3) by Duncan’s multiple range test. 1YHPG, 1-year-old hydroponic ginseng; 2YHPG, 2-years-old hydroponic ginseng; 4YSCG, 4-years-old soil-cultured ginseng; 6YSCG, 6-years-old soil-cultured ginseng
Antioxidant activity of HPG and SCG
In this study, the antioxidative effects of SCG and HPG were measured and compared. The DPPH assay is a standard method for determining the in vitro radical-scavenging activity of samples. There were significant differences between activities in HPGs and SCGs. As the DPPH IC50 value suggested (Table 3), the lowest level was found in shoots of 1YHPG (2.4 2 ± 0.03 mg/mL), followed by shoots of 2YHPG (2.88 ± 0.03 mg/mL), roots of 1YHPG (4.65 ± 0.01 mg/mL), 2YHPG (6.27 ± 0.63 mg/mL), 6YHPG (11.86 ± 0.86 mg/mL), and 4YHPG (12.28 ± 0.49 mg/mL). According to previous studies by Chung et al. (2016a; 2016b) and Park (2012), the antioxidant activity of ginseng leaves was higher than that of the roots and was significantly correlated to the TPC, results which are identical to those found in the present study. The polyphenolic compounds, including flavonoids, found in natural organisms exhibit superior antioxidant properties by scavenging superoxide anion radicals, hydrogen peroxide, and hydroxyl radicals (Gülçin 2012). Korean ginseng was reported to contain various phenolic acids and flavonoids including chlorogenic acid, gentisic acid, p- and m-coumaric acid, and rutin (Chung et al., 2016a; 2016b). The inhibition rate of β-carotene and linoleic acid oxidation (%) was found to be highest in shoots of HPG (1YHPG; 39.35 ± 0.10, 2YHPG; 42.51 ± 0.06%). However, unlike radical scavenging activity, roots of SCG showed higher inhibition rate on linoleic acid oxidation compared to roots of HPGs. Although total ginsenoside content (in Table 2) and antioxidative activity are not necessarily identical, there are studies which indicate that ginsenosides protect cells from oxidative stress by their antioxidant capacity. Wang et al. (2017a) reported that ginsenoside Rb1 suppresses oxidative stress in 3T3-L1 cells and attenuates adipocyte dysfunction. Furthermore, ginsenosides Rg3 and Rh2 reduced oxidative stress in mouse hepatocytes by increasing level of ROS, according to the research of Park et al. (2012). As a result, antioxidant property superior to SCG of HPG inhibits the occurrence of ROS and may prevent progression of several chronic diseases that caused thereby.
Table 3.
Antioxidative activities of HPG and SCG by ages and parts
| Sample | DPPH IC50 (mg/mL) | Inhibition of β-carotene and linoleic acid oxidation (%) |
|---|---|---|
| HPG (shoots) | ||
| 1YHPG | 2.42 ± 0.03c | 60.65 ± 0.10a |
| 2YHPG | 2.88 ± 0.03c | 57.49 ± 0.06b |
| HPG (roots) | ||
| 1YHPG | 4.65 ± 0.01bc | 28.73 ± 0.04f |
| 2YHPG | 6.27 ± 0.63b | 31.79 ± 0.10e |
| SCG (roots) | ||
| 4YSCG | 12.28 ± 0.49a | 39.91 ± 0.09c |
| 6YSCG | 11.86 ± 0.86a | 38.57 ± 0.24d |
All values were expressed as mean ± SD
a–eValues with different superscripts in same row are significantly different at p < 0.05 (n = 3) by Duncan’s multiple range test
Inhibitory effect of HPG on NO production in LPS-stimulated RAW 264.7 murine macrophage cells
Inflammatory mediators such as nitric oxide (NO), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) are released from macrophages following stimulation by proinflammatory mediators. Proinflammatory mediators such as lipopolysaccharide (LPS), interleukin-1β (1L-1β), and interferon-γ (IFN-γ) bind to toll-like receptor-4 (TLR-4), activating the inflammatory signaling pathway nuclear factor-κB (NF-κB) and stimulating macrophages (Huang et al., 2016). Therefore, these biomarkers are important molecular targets for establishing the mechanism by which substances isolated from natural plants exhibiting inflammatory-reaction-attenuating activity. In this study, RAW 264.7 cells were stimulated by LPS (0.1 μg/mL) and treated with HPG and SCG, after which the amount of NO present in the media was quantified. RAW 264.7 cells were treated with HPG and SCG extracts at concentrations of 100, 200, and 400 μg/mL, concentrations which maintain cell viability above 85% in MTT assays (Fig. 2A). As shown in Fig. 2B, significant dose-dependent inhibition of NO production was detected in HPG and SCG. Of all the ginseng extracts, the 400 μg/mL extract from shoots of 2YHPG displayed the strongest inhibition of NO production. From these data, it appeared that shoots of 2YHPG had the highest anti-inflammatory activity. Notably, p-coumaric acids, vanillic acids, and maltol, which are reported to be abundant in ginseng, showed NO radical-scavenging activity (Ekinci-Akdemir et al., 2017), suggesting that polyphenolic compounds in HPG shoots play a more significant role as NO radical scavengers than do ginsenosides. Additionally, panaxcerol D isolated from leaves of HPG had a significantly higher inhibitory effect on NO production than L-NG-monomethyl arginine, a well-known inhibitor, in LPS-induced RAW 264.7 cells in a previous study of Cha et al. (2015).
Fig. 2.
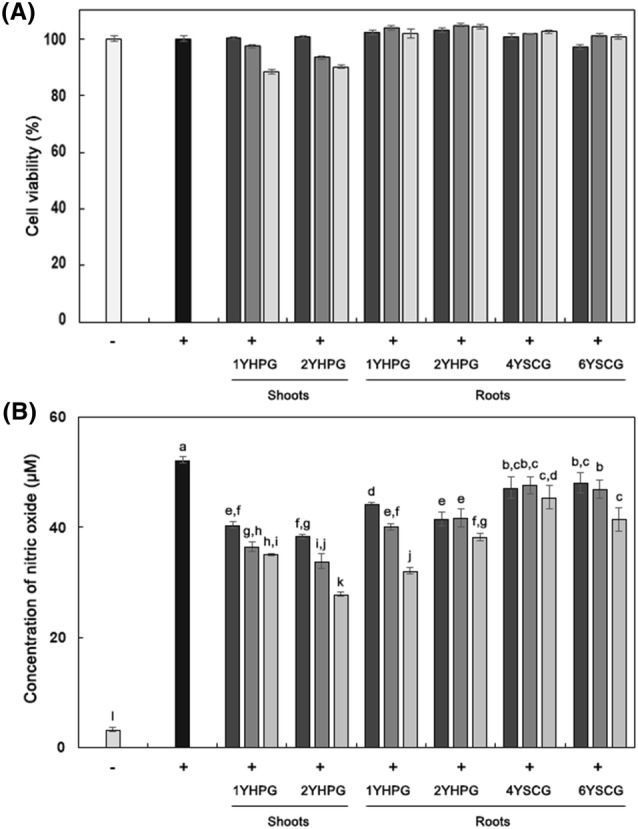
Cell viability and produced nitric oxide concentration in RAW 264.7 murine macrophage cells treated with root extracts from HPG and SCG. (A) Cell viability of RAW 264.7 cells. (B) Concentration of nitric oxide in RAW 264.7 cells (μM). Cell viability was assessed by MTT assay with different sample concentrations (400 μg/mL, filled square; 200 μg/mL, open square; 100 μg/mL, open square). Cell viability (%) = {(absorbance of sample − absorbance of blank)/(absorbance of control − absorbance of blank)} × 100. Concentration of nitrite (μM) = (absorbance of sample at 540 nm + 0.0008)/0.0071, R2 = 0.9999. (−), Non-treated with LPS; (+), treated with LPS (0.1 μg/mL). Alphabet notation represents significant difference among samples based on Duncan’s multiple test (p < 0.05)
Attenuative effect of HPG and SCG on the release of iNOS, TNF-α, IL-1β, and IL-6 in LPS-stimulated RAW 264.7 cells
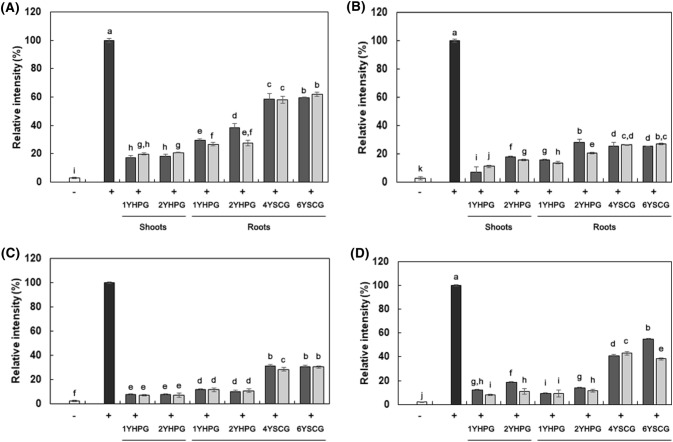
The expression levels of inflammatory mediators (iNOS, TNF-α, IL-1β, and IL-6) in RAW 264.7 cells stimulated with LPS were examined using RT-PCR. HPG and SCG extract samples were administered to RAW 264.7 at concentrations of 200 and 400 μg/mL. The expression level of β-actin, a reference gene, remained constant in all experiments (data not shown). As shown in Fig. 3, LPS-only-treated macrophage showed dramatic induction of iNOS, TNF-α, IL-1β, and IL-6. All of the ginseng extract samples significantly inhibited iNOS, TNF-α, IL-1β, and IL-6 mRNA expression. In addition, ginseng from different cultivation systems showed different inhibitory activities. The expression of iNOS was most strongly inhibited by treatment with root extracts. Expression of iNOS causes excessive production of NO and inflammatory reactions. A 400 μg/mL HPG shoot extract showed the strongest suppressive effect against TNF-α, IL-1β, and IL-6 expression, followed by extracts from HPG roots, and from SCG at the same doses. The results suggested significant correlations between TNF-α, IL-6, and IL-1β mRNA expression, and the radical scavenging activity of HPG and SCG. They also indicated that factors related to the antioxidant activity of HPG and SCG may also play a role in anti-inflammation by suppressing TNF-α, IL-6, or IL-1β mRNA expression. Ginsenoside Rb1, in particular, has been reported to have an anti-arthritic effect in collagen-induced arthritis (CIA) mice. Rb1 inhibits TNF-α induction stimulated by LPS or IL-1β (Wang et al., 2017a). Ginsenoside Rd suppresses the expression of iNOS (Ye et al., 2011).
Fig. 3.
Anti-inflammatory effect based on inhibition of LPS-stimulated inflammation related cytokine expressions in RAW 264.7 cells treated with extracts from shoots of 1YHPG, 2YHPG, and roots of 1YHPG, 2YHPG, 4YSCG, and 6YSCG (400 μg/mL, filled square; 200 μg/mL, filled square). (A) iNOS, (B) TNF-α, (C) IL-6, (D) IL-1β. Alphabet notation represents significant difference among samples based on Duncan’s multiple test (p < 0.05)
In conclusion, HPG showed significantly higher potential for antioxidative and anti-inflammatory properties than SCG. HPG had higher a total ginsenoside content in the whole plant body than the roots of soil-cultured ginseng, and contained high concentrations of Rb2, Rb3, Rd, Re, and F1. Regardless of the plant part examined, HPG’s antioxidant activity, based on its radical scavenging activity and inhibitory effect of lipid oxidation, was superior to that of SCG. This may be attributed to the fact that HPG contains significantly higher concentrations of phenolic compounds, including flavonoids. HPG was also found to reduce iNOS expression, and to inhibit NO production, better than SCG in LPS-stimulated RAW264.7 cells. Moreover, HPG causes down-regulation of IL-6, IL-1β, and TNF-α. This study highlights the need for further clinical and experimental investigations into other health-enhancing effects of hydroponic ginseng, and to expand its utility.
Acknowledgements
This research was supported by the Priority Research Centers Program through the National Research Foundation of Korea (NFR) funded by the Ministry of Education, Science and Technology (Grant Number: 2009-0093824).
Compliance with ethical standards
Conflict of interest
The authors declared that they have no conflict of interest.
Footnotes
The original version of this article was revised: The presentation of the row “Total (μg/g)” in table 2 was incorrect.
Change history
9/28/2018
The original version of this article unfortunately contained a mistake. The presentation of the row “Total (μg/g)” in Table 2 was incorrect.
References
- Baek KS, Yi YS, Son YJ, Yoo S, Sung NY, Kim Y, Hong S, Aravinthan A, Kim JH, Cho JY. In vitro and in vivo anti-inflammatory activities of Korean Red Ginseng-derived components. J. Ginseng Res. 2016;40:437–444. doi: 10.1016/j.jgr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak MJ, Hong SG, Lee JW, Jeong WS. Red ginseng marc oil inhibits iNOS and COX-2 via NFκB and p38 pathways in LPS-stimulated RAW 264.7 macrophages. Molecules. 2012;17:13769–13786. doi: 10.3390/molecules171213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha BJ, Park JH, Shrestha S, Baek NI, Lee SM, Lee TH, Kim J, Kim GS, Kim SY, Lee DY. Glycosyl glycerides from hydroponic Panax ginseng inhibited NO production in lipopolysaccharide-stimulated RAW264.7 cells. J. Ginseng Res. 2015;39:162–168. doi: 10.1016/j.jgr.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung IM, Lim JJ, Ahn MS, Jeong HN, An TJ, Kim SH. Comparative phenolic compound profiles and antioxidative activity of the fruit, leaves, and roots of Korean ginseng (Panax ginseng Meyer) according to cultivation years. J. Ginseng Res. 2016;40:68–75. doi: 10.1016/j.jgr.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SI, Nam SJ, Xu M, Kang MY, Lee SC. Aged ginseng (Panax ginseng Meyer) reduces blood glucose levels and improves lipid metabolism in high fat diet-fed mice. Food Sci. Biotechnol. 2016;25:267–273. doi: 10.1007/s10068-016-0039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Macêdo LAR, de Oliveira Júnior RG, Souza GR, de Oliveira AP, de Lavor ÉM, e Silva MG, Pacheco AGM, de Menezes IRA, Coutinho HDM, do Ó Pessoa C, da Costa MP, da Silva Almeida JRG. Chemical composition, antioxidant and antibacterial activities and evaluation of cytotoxicity of the fractions obtained from Selaginella convoluta (Arn.) Spring (Selaginellaceae) Biotechnol. Biotechnol. Equip. 2018;32(2):506–512. doi: 10.1080/13102818.2018.1431055. [DOI] [Google Scholar]
- Doumbou CL, Hamby Salove MK, Crawford DL, Beaulieu C. Actinomycetes, promising tools to control plant diseases and to promote plant growth. Phytoprotection. 2001;82(3):85–102. doi: 10.7202/706219ar. [DOI] [Google Scholar]
- Ekinci-Akdemir FN, Gülçin I, Gürsul C, Alwasel SH, Bayir Y. Effect of p-coumaric acid against oxidative stress induced by cisplatin in brain tissue of rats. J. Anim. Plant Sci. 2017;27(5):1560–1564. [Google Scholar]
- Eom SJ, Hwang JE, Kim K-T, Paik H-D. Increased antioxidative and nitric oxide scavenging activity of ginseng marc fermented by Pediococcus acidilactici KCCM11614P. Food Sci. Biotechnol. 2017;27:185–191. doi: 10.1007/s10068-017-0207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom SJ, Hwang JE, Kim HS, Kim K-T, Paik H-D. Anti-inflammatory and cytotoxic effects of ginseng extract bioconverted by Leuconostoc mesenteroides KCCM 12010P isolated from kimchi. Int. J. Food Sci. Technol. 2018;53:1331–1337. doi: 10.1111/ijfs.13713. [DOI] [Google Scholar]
- Gulcin I. Antioxidant activity of food constituents: an overview. Arch. Toxicol. 2012;86:345–391. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- Han JS, Tak HS, Lee GS, Kim JS, Choi JE. Comparison of ginsenoside content according to age and diameter in Panax ginseng C. A. Meyer cultivated by direct seeding. Korean Soc. Med. Crop. Sci. 2013;21:184–190. doi: 10.7783/KJMCS.2013.21.3.184. [DOI] [Google Scholar]
- Hong H, Sim EM, Kim K, Rho J, Rhee YK, Cho C. Comparison of preparation methods for the quantification of ginsenosides in raw Korean ginseng. Food. Sci. Biotechnol. 2009;18(2):565–569. [Google Scholar]
- Huang SS, Su SY, Chang JS, Lin HJ, Wu WT, Deng JS, Huang GJ. Antioxidants, anti-inflammatory, and antidiabetic effects of the aqueous extracts from Glycine species and its bioactive compounds. Bot. Stud. 2016;57:38. doi: 10.1186/s40529-016-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Baek EJ, Lee EJ, Yeom MH, Park JS, Lee KW, Kang NJ. Ginsenoside F1 attenuates hyperpigmentation in B16F10 melanoma cells by inducing dendrite retraction and activating Rho signaling. Exp. Dermatol. 2015;24:150–152. doi: 10.1111/exd.12586. [DOI] [PubMed] [Google Scholar]
- Kim GS, Hyun DY, Kim YO, Lee SE, Kwon H, Cha SW, Park CB, Kim YB. Investigation of ginsenosides in different parts of Panax ginseng cultured by hydroponics. Kor. J. Hort. Sci. Technol. 2010;28(2):216–226. [Google Scholar]
- Kim YJ, Joo SC, Shi J, Hu C, Quan S, Hu J, Sukweenadhi J, Mohanan P, Yang DC, Zhang D. Metabolic dynamics and physiological adaptation of Panax ginseng during development. Plant Cell. Rep. 2018;37:393–410. doi: 10.1007/s00299-017-2236-7. [DOI] [PubMed] [Google Scholar]
- Köksal E, Bursal E, Gülçin İ, Korkmaz M, Çağlayan C, Gören AC, Alwasel SH. Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by liquid chromatography and tandem mass spectrometry. Int. J. Food Prop. 2016;20:514–525. doi: 10.1080/10942912.2016.1168438. [DOI] [Google Scholar]
- Lee YM, Yoon H, Park HM, Song BC, Yeum KJ. Implications of red Panax ginseng in oxidative stress associated chronic diseases. J. Ginseng Res. 2017;41:113–119. doi: 10.1016/j.jgr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Li J, Wang S, Yao L, Liang W, Wang J, Gao W. Advances in ginsenoside biosynthesis and metabolic regulation. Biotechnol. Appl. Biochem. 2018;65:514–522. doi: 10.1002/bab.1649. [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture, Food and Rural Affairs. Ginseng statistics report (2017). Available from: http://www.mafra.go.kr/bbs/mafra/131/190029/download.do Accessed 2017.
- Noh HH, Lee JY, Park HK, Jeong HR, Lee JW, Jin MJ, Choi H, Yun SS, Kyung KS. Monitoring and safety assessment of pesticide residues in ginseng (Panax ginseng C.A. Meyer) from traditional markets. Korean J. Pestic. Sci. 2016;20:23–29. doi: 10.7585/kjps.2016.20.1.23. [DOI] [Google Scholar]
- Padhi EMT, Liu R, Hernandez M, Tsao R, Ramdath DD. Total polyphenol content, carotenoid, tocopherol and fatty acid composition of commonly consumed Canadian pulses and their contribution to antioxidant activity. J. Funct. Food. 2017;38:602–611. doi: 10.1016/j.jff.2016.11.006. [DOI] [Google Scholar]
- Park HM, Kim SJ, Mun AR, Go HK, Kim GB, Kim SZ, Jang SI, Lee SJ, Kim JS, Kang HS. Korean red ginseng and its primary ginsenosides inhibit ethanol-induced oxidative injury by suppression of the MAPK pathway in TIB-73 cells. J. Ethnopharmacol. 2012;141(3):1071–1076. doi: 10.1016/j.jep.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Park JH. Antioxidant activities in shoots and roots of hydroponic cultured ginseng. J. App. Ori. Med. 2012;12(2):21–26. [Google Scholar]
- Shi W, Wang Y, Li J, Zhang H, Ding L. Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chem. 2007;102:664–668. doi: 10.1016/j.foodchem.2006.05.053. [DOI] [Google Scholar]
- Siraj FM, Kim YJ, Natarajan S, Jung SK, Yang DU, Yang DC. Ginseng and obesity: observations from assorted perspectives. Food Sci. Biotechnol. 2014;23:1007–1016. doi: 10.1007/s10068-014-0137-x. [DOI] [Google Scholar]
- Wang M, Chen Y, Xion Z, Yu S, Zhou B, Ling Y, Zheng Z, Shi G, Wu Y, Qian X. Ginsenoside Rb1 inhibits free fatty acids-induced oxidative stress and inflammation in 3T3-L1 adipocytes. Mol. Med. Rep. 2017;16:9165–9172. doi: 10.3892/mmr.2017.7710. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu X, Tan W, Liu W, Wang W. GW28-e0602 Protective effects of ginsenoside Re on macrophages RAW 264.7 cells injury induced by doxorubicin. J. Am. Coll. Cardiol. 2017;70(16):C63. doi: 10.1016/j.jacc.2017.07.214. [DOI] [Google Scholar]
- Ye R, Yang Q, Kong X, Han J, Zhang X, Zhang Y, Li P, Liu J, Shi M, Xiong L, Zhao G. Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem. Int. 2011;58:391–398. doi: 10.1016/j.neuint.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Young R, Bush SJ, Lefevre L, McCulloch MEB, Lisowski ZM, Muriuki C, Waddell LA, Sauter KA, Pridans C, Clark EL, Hume DA. Species-specific transcriptional regulation of genes involved in nitric oxide production and arginine metabolism in macrophages. ImmunoHorizons. 2018;2:27–37. doi: 10.4049/immunohorizons.1700073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang Y, Taha AA, Ying Y, Li X, Chen X, Ma C. Subcritical water extraction of bioactive components from ginseng roots (Panax ginseng C.A. Mey) Ind. Crop. Prod. 2018;117:118–127. doi: 10.1016/j.indcrop.2018.02.079. [DOI] [Google Scholar]