Abstract
The vulnerability of oils in emulsions to oxidation depends on the structural and physicochemical properties of oil droplet interface. To evaluate the implications of the interfacial characteristics of emulsion droplets on lipid oxidation, particularly lipid hydroperoxide decomposition, emulsions were prepared using emulsifiers with various lengths of polar groups because the length of hydrophilic heads of emulsifiers could be an important factor in determining the thickness of the droplet surface. The decomposition rate constants of cumene hydroperoxide in emulsions showed that the cumene hydroperoxide in emulsions having a thick emulsion droplet interface was decomposed faster than in emulsions having a loosen one. Our findings also showed that the denseness of the droplet interface affected cumene hydroperoxide decomposition in emulsions. Conclusively, this study suggested that the interfacial thickness and denseness of the emulsion droplets influence oxidative stability of emulsions.
Keywords: Emulsions, Interfacial membrane, Iron, Lipid hydroperoxide decomposition, Lipid oxidation
Introduction
When lipids are present in foods, many natural and processed foods exist in the form of emulsions consisting of lipid and water, with oil (or water) dispersed as small droplets in the water (or oil) (Dalgleish, 2004). However, when oil (or water) exists in the dispersion in the water (or oil) as small droplets, the much higher contact area between oil and water phases than their simple mixture causes the thermodynamic instability of emulsions (McClements, 2005). Therefore, the interfacial tension between oil and water has to be reduced by adding emulsifiers before mixing (Friberg et al., 2004). The energy required to disperse oil (or water) into water (or oil) as small droplets could be also reduced by the addition of emulsifiers. Therefore, the stable emulsions are comprised of three distinct regions: lipid and water phases, and interface located between lipid and water phases. As a physical barrier, the interfacial membrane bestows emulsions with the high stability against emulsion instabilization such as flocculation and coalescence, etc. In addition, it could alter the rates of the chemical reactions between compounds in oil and water phases. Therefore, the interfacial membrane could protect the emulsified oils in oil-in-water emulsions from oxidation but lipid oxidation would be also accelerated depending on the properties of emulsion interface formed with emulsifiers. The previous studies show that the structural and physicochemical properties of emulsifiers such as molecular weight and the charge of their hydrophilic groups, etc. are one of important factors affecting the susceptibility of emulsified oils to oxidation (Berton et al., 2011; Berton-Carabin et al., 2014; Chen et al., 2010). Iron further accelerates the oxidation of the emulsified oils in emulsions prepared with anionic emulsifiers than in those prepared with nonionic or cationic emulsifiers (Berton-Carabin et al., 2014; Donnelly et al., 1998; Mei et al., 1998). The interfacial membrane also affects the stability of oil-soluble compounds encapsulated in droplets (McClements, 2013). Degradation of oil-soluble flavors and carotenoids by iron was also fairly faster in emulsions having negatively charged emulsion surfaces than in emulsions having uncharged or positively charged ones (Boon et al., 2008; Choi et al., 2010). Iron could be accumulated around the negatively-charged droplet surfaces due to electrostatic attraction and this would increase the rate and extent of interaction between iron and emulsion droplets.
Several studies, listed above, have shown that the surface charge of emulsion droplets is one of main parameters in the stability of emulsified oils against oxidation. However, the implications of the structures of emulsifiers on the oxidative stability of emulsified oils is not currently unclear. Therefore, in this study, the effect of the polar and nonpolar group sizes of the emulsifier on lipid oxidation in model emulsions was evaluated and the effect of emulsifier packing in the membrane on the rate of lipid hydroperoxide decomposition in emulsions was also determined.
Materials and methods
Materials
Cumene hydroperoxide and Brij emulsifiers (polyethylene glycol 10 stearyl ether (PEG10S), polyethylene glycol 20 stearyl ether (PEG20S), polyethylene glycol 23 lauryl ether (PEG23L), and polyethylene glycol 100 stearyl ether (PEG100S)), nonionic emulsifiers consisting of hydrophilic polyethylene oxide chains and hydrophobic n-alkyl chains, were obtained from Sigma-Aldrich (St. Louis., MO, USA). The molecular structures of the emulsifiers used in this study are present in Fig. 1. Medium chain triglyceride (Delios S), comprised of approximately 30% capric acid and 70% caprylic acid, was purchased from BASF (Ludwigshafen, Germany).
Fig. 1.
Molecular structures of emulsifiers (polyethylene glycol alkyl ethers) in this study
Preparation of emulsions
Aqueous solutions were prepared by dissolving emulsifiers to their minimum emulsifier concentrations (MECs, PEG10S, PEG20S, PEG23L, and PEG100S to 3.165, 2.926, 1.784, and 0.994 mM, respectively) into 10 mM phosphate buffer (pH 7.0). Emulsions were prepared by mixing 5% (w/w) oil phase (medium chain triglyceride; MCT) with 95% (w/w) water phase. Coarse emulsions, prepared by homogenizing the oil and water phases using a high-speed blender for 2 min at room temperature, were homogenized with five at 100 MPa passes using a microfluidizer (MN400BF, Micronox, Seongnam, Korea). Next, the pH of the emulsion sample was adjusted to a predetermined value using 0.1 and 1.0 N HCl solutions. Cumene hydroperoxide (22 mmol/kg emulsion) was added to the emulsions, which were gently stirred at the predetermined pH for 30 min under nitrogen. Then, 10 g of emulsion was transferred into 12 mL of airtight glass vial and stored at 25 °C in the dark in the presence of ferrous sulfate (2 mmol/kg emulsion).
Determination of cumene hydroperoxide concentration
Cumene hydroperoxides were measured using a method adapted from Nuchi et al. (2001). A portion of the emulsion sample (0.3 g) was vigorously mixed with 1.5 mL of isooctane/2-propanol (3:1, v:v) three times for 10 s, followed by centrifugation for 2 min at 1350×g. The upper layer (0.2 mL) was collected and mixed with 2.8 mL of methanol/1-butanol (2:1, v:v) and 30 μL of thiocyanate/ferrous sulfate solution was added to the mixture and vigorously mixed for 10 s. Thiocyanate/ferrous sulfate solution was prepared by mixing equal volumes of 3.94 M thiocyanate solution and 0.072 M ferrous sulfate solution (obtained from the supernatant of a mixture of one part of 0.144 M ferrous sulfate and one part of 0.132 M barium chloride in 0.4 M hydrochloric acid solution). The mixtures were incubated for 20 min at 25 °C, and the absorbance was measured at 510 nm using a UV/visible spectrophotometer.
The decomposition rate constant () of cumene hydroperoxide in emulsions was calculated, assuming the following a 1st-order reaction (Eq. 1):
| 1 |
where and are cumene hydroperoxide concentration (mmol/kg emulsion) remaining at time 0 and (h, ≤ 12 h), respectively. The value was estimated by performing a linear regression on the plot of versus .
Determination of emulsion droplet size
Mean emulsion droplet diameters were measured using a static light scattering instrument (BT-9300BT; Bettersize Instruments Ltd., Dandong, China), with distilled/deionized water as a dispersion medium. A few drops of emulsion were added to water in a sample dispersion unit stirred at 1600 rpm until an appropriate laser obscuration was attained. The refractive indices of MCT and dispersion medium were set to 1.47 and 1.33, respectively. Particle size data are reported as the volume-weighted mean diameter (), where is the number of particles with diameter .
Results and discussion
To minimize the negative impact of micelles on emulsion stability (McClements, 1994; Wulff-Pérez et al., 2009) and their possible influence on lipid oxidation, all emulsions were prepared at their MECs, determined in a previous study (Han et al., 2018). The initial emulsion droplet sizes (d43) of PEG10S-, PEG20S-, PEG23L-, and PEG100S-stabilized emulsions prepared at their MECs were 0.32, 0.33, 0.39, and 0.35 μm, respectively, and they rarely changed (p > 0.05) over storage, regardless of the emulsifier type and pH. Therefore, it suggested that all emulsions in this study had a similar specific surface area.
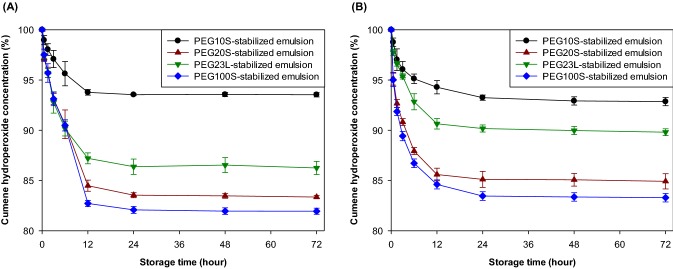
Lipid oxidation by transition metals (e.g. iron species such Fe2+ and Fe3+) present generally in the aqueous phase is one of frequently observed chemical destabilization of emulsions. As noted above, the rate of lipid oxidation occurred by the interaction between iron and oil droplets could be varied depending on characteristics of the droplet surfaces (Silvestre et al., 2000). Here, we study how the interfacial membrane properties impact the rate and degree of oxidation in emulsified oils, particularly the interactions between metal ions and lipid hydroperoxides. Cumene hydroperoxide concentrations in emulsions did not change significantly (p > 0.05) during 72-h storage at 25 °C in the absence of Fe2+ (data not shown), irrespective of the emulsifier type and pH (data not shown). However, all emulsions showed the rapid decrease in cumene hydroperoxide concentration in the presence of ferrous iron (Fig. 2). As shown in Fig. 2, the drastic reduction in cumene hydroperoxide concentration during the first 12–24 h of storage was observed in all emulsions, irrespective of pH and emulsifier type, but cumene hydroperoxide did not decrease any more during subsequent storage. In this study, nonionic emulsifiers were used to prepare emulsions but the data obtained from droplet surface charge measurement told a different story. The droplet surface charges of the emulsions stabilized by PEG10S, PEG20S, PEG23L, and PEG100S were approximately − 6.8, − 5.5., − 9.4, and − 1.7 mV, respectively, at pH 7. When pH of emulsions decreased to 3, the electrical charges went to almost zero (− 1.7, − 1.7, − 1.9, and − 1.3 mV for PEG10S-, PEG20S-, PEG23L-, and PEG100S-stabilized emulsions, respectively). It was expected cumene hydroperoxide rapidly decomposed at pH 7 because iron molecules what were attracted to the negatively-charged surface of emulsion droplets at pH 7 could easily interact with cumene hydroperoxide in oil droplets. However, cumene hydroperoxide decomposition rate at pH 7 was not significantly different from that at pH 3 (p > 0.05) (Table 1). This observation could be due to the increased solubility of ferrous iron at an acidic pH compared with a neutral pH. Interestingly, although there was no difference in droplet surface charge of PEG100S-stabilized emulsion between at pH 3 and 7, the decomposition rate of cumene hydroperoxide in PEG100S-stabilized emulsion at pH 7 was greater than that at pH 3.
Fig. 2.
Change in cumene hydroperoxide (initial concentration of cumene hydroperoxide 22 mmol/kg emulsion) in MCT emulsions stabilized by PEG10S, PEG20S, PEG23L, or PEG100S at pH 7 (A) and 3 (B) and 25 °C in the presence of ferrous iron (2 mmol/kg emulsion)
Table 1.
Decomposition rate constants (k, (h−1)) of cumene hydroperoxide in emulsions calculated assuming a 1st-order reaction
| Emulsifier used for emulsion preparation | ||||||||
|---|---|---|---|---|---|---|---|---|
| PEG10S | PEG20S | PEG23L | PEG100S | |||||
| k | r2 | k | r2 | k | r2 | k | r2 | |
| pH 7 | A0.005c | 0.966 | A0.012ab | 0.992 | A0.009b | 0.924 | A0.014a | 0.994 |
| pH 3 | A0.003b | 0.792 | A0.009a | 0.906 | A0.006ab | 0.956 | B0.009a | 0.854 |
The values with different capital-letter superscripts in a same column are significantly different (p < 0.05) by Chow test
The values with different small-letter superscripts in a same row are significantly different (p < 0.05) by Chow test
In this study, although all emulsions have a similar droplet size, the emulsifier concentrations in emulsions varied, indicating that the mass loading at the interface is higher in emulsions with a higher emulsifier concentration. Although PEG10S- and PEG20S-stabilized emulsions had a similar interfacial denseness, cumene hydroperoxide in PEG20S-stabilized emulsions was decomposed faster in S10-stabilized emulsions, independing on pH. It appeared that the emulsions stabilized by emulsifiers having a small hydrophilic group retarded hydroperoxide decomposition more effectively than those stabilized by emulsifiers having a large hydrophilic group when emulsions had a similar interfacial denseness. Even assuming that the difference in the hydrophilic group size between PEG20S and PEG23L was negligible, although the mass loading at the interface of PEG20S-stabilized emulsion was greater than that of PEG23L-stabilized emulsions, cumene hydroperoxide decomposition rate was not significantly different from each other (p > 0.05). Therefore, when emulsions were stabilized with emulsifiers having a similar size of hydrophilic group, the interfacial denseness of emulsions played a minor role in cumene hydroperoxide decomposition. With this hypothesis, the reason why PEG100S-stabilized emulsion was vulnerable to cumene hydroperoxide decomposition could be easily explained. PEG100S had the largest hydrophilic head among emulsifiers attempted in this study and PEG100S-stabilized emulsion had the most loosely packed interface among emulsions.
In previous study, the faster cumene hydroperoxide decomposition by ferrous iron was observed in emulsions stabilized with emulsifiers containing smaller hydrophilic groups (Silvestre et al., 2000). Antithetically, our results show that cumene hydroperoxide was decomposed rapidly in emulsions stabilized with emulsifiers having a large hydrophilic group than in ones stabilized by emulsifiers having a small hydrophilic group (Fig. 2 and Table 1). The discordance between our observations and those of Silvestre et al. (Silvestre et al., 2000) likely stems from differences in the emulsifier concentrations used for the creation of emulsions. In our study, to create emulsions having little to no micelle, MECs of emulsifiers were used. However, 6 times higher emulsifier concentrations were used to prepare emulsions in the previous study. Although the role of micelles in lipid oxidation is currently unclear, the presence of micelles may contribute to the discrepancy between the findings in this work and previous findings. The emulsion droplet size between this and the previous study also differs, and the difference in the interfacial area of the emulsion droplets may contribute to the conflicting results between the two studies.
Conclusively, the findings of this work suggested lipid hydroperoxide decomposition in oil-in-water emulsions is affected by the thickness of oil droplet interface as well as the denseness of the interfacial membrane of emulsions. Although the effect of the hydrophobic tail length of emulsifiers on lipid hydroperoxide decomposition is currently not clear, it is also one of the various important factors affecting lipid hydroperoxide decomposition in emulsions.
Acknowledgements
This study was supported by the Research Program funded by Seoul National University of Science and Technology (2018-0198).
Compliance with ethical standard
Conflict of interest
The authors declared that they have no conflict of interest.
References
- Berton C, Ropers M-H, Viau M, Genot C. Contribution of the interfacial layer to the protection of emulsified lipids against oxidation. J. Agric. Food Chem. 2011;59:5052–5061. doi: 10.1021/jf200086n. [DOI] [PubMed] [Google Scholar]
- Berton-Carabin CC, Ropers M-H, Genot C. Lipid oxidation in oil-in-water emulsioins: involvement of the interfacial layer. Compr. Rev. Food. Sci. Food Saf. 2014;13:945–977. doi: 10.1111/1541-4337.12097. [DOI] [Google Scholar]
- Boon CS, Xu Z, Yue X, McClements DJ, Weiss J, Decker EA. Factors affecting lycopene oxidation in oil-in-water emulsions. J. Agric. Food Chem. 2008;56:1408–1414. doi: 10.1021/jf072929+. [DOI] [PubMed] [Google Scholar]
- Chen B, McClements DJ, Decker EA. Role of continuous phase anionic polysaccharides on the oxidative stability of Menhaden oil-in-water emulsions. J. Agric. Food Chem. 2010;58:3779–3784. doi: 10.1021/jf9037166. [DOI] [PubMed] [Google Scholar]
- Choi SJ, Decker EA, Henson L, Popplewell LM, McClements DJ. Influence of droplet charge on the chemical stability of citral in oil-in-water emulsions. J. Food Sci. 2010;75:C536–C540. doi: 10.1111/j.1750-3841.2010.01693.x. [DOI] [PubMed] [Google Scholar]
- Dalgleish DG. Food emulsions: their structures and properties. In: Food emulsions. Friberg SE, Larsson K, Sjöblom J (eds). Marcel Dekker, New York, NY (2004)
- Donnelly JL, Decker EA, McClements DJ. Iron-catalyzed oxidation of Menhaden oil as affected by emulsifiers. J. Food Sci. 1998;63:997–1000. doi: 10.1111/j.1365-2621.1998.tb15841.x. [DOI] [Google Scholar]
- Friberg SE, Larsson K, Sjöblom J. Food Emulsions. New York: Marcel Dekker; 2004. [Google Scholar]
- Han SW, Song HY, Moon TW, Choi SJ. Influence of emulsion interfacial membrane characteristics on Ostwald ripening in a model emulsion. Food Chem. 2018;242:91–97. doi: 10.1016/j.foodchem.2017.09.018. [DOI] [PubMed] [Google Scholar]
- McClements DJ. Ultrasonic determination of depletion flocculation in oil-in-water emulsions containing a non-ionic surfactant. Colloid Surf. A-Physicochem. Eng. Asp. 1994;90:24–35. doi: 10.1016/0927-7757(94)02881-8. [DOI] [Google Scholar]
- McClements DJ. Emulsion stability. In: McClements DJ, editor. Food emulsions: principles, practice, and techniques. Boca Raton: CRC Press; 2005. pp. 269–339. [Google Scholar]
- McClements DJ. Nanoemulsion-based oral delivery systems for lipophilic bioactive components: nutraceuticals and pharmaceuticals. Ther. Deliv. 2013;4:841–857. doi: 10.4155/tde.13.46. [DOI] [PubMed] [Google Scholar]
- Mei L, McClements DJ, Wu J, Decker EA. Iron-catalyzed lipid oxidation in emulsion as affected by surfactant, pH and NaCl. Food Chem. 1998;61:307–312. doi: 10.1016/S0308-8146(97)00058-7. [DOI] [Google Scholar]
- Nuchi CD, McClements DJ, Decker EA. Impact of Tween 20 hydroperoxides and iron on the oxidation of methyl linoleate and salmon oil dispersions. J. Agric. Food Chem. 2001;49:4912–4916. doi: 10.1021/jf010370m. [DOI] [PubMed] [Google Scholar]
- Silvestre MPC, Chaiyasit W, Brannan RG, McClements DJ, Decker EA. Ability of surfactant headgroup size to alter lipid and antioxidant oxidation in oil-in-water emulsions. J. Agric. Food Chem. 2000;48:2057–2061. doi: 10.1021/jf991162l. [DOI] [PubMed] [Google Scholar]
- Wulff-Pérez M, Torcello-Gómez A, Gálvez-Ruíz MJ, Martín-Rodríguez A. Stability of emulsions for parenteral feeding: preparation and characterization of o/w nanoemulsions with natural oils and Pluronic f68 as surfactant. Food Hydrocolloids. 2009;23:1096–1102. doi: 10.1016/j.foodhyd.2008.09.017. [DOI] [Google Scholar]