Abstract
In this study, it was demonstrated that 1,3-dichloro-2-propanol (1,3-DCP) induced oxidative stress and cell death in HuH7, human hepatocytes. The protective effects of Erythronium japonicum (E. japonicum) and Corylopsis coreana Uyeki (C. coreana Uyeki) extracts against 1,3-DCP-treated cells were also investigated. First, the activities of superoxide dismutase (SOD) and catalase (CAT) were diminished by the treatment of 1,3-DCP. Moreover, 1,3-DCP stimulated the expression and catalytic activity of cytochrome P450 2E1 (CYP2E1), an enzyme that generates reactive oxygen species in the liver. In contrast, co-treatment of 1,3-DCP with the extracts significantly decreased ROS generation and inhibited CYP2E1 activity without affecting its expression. The co-administration of extracts also restored the activities of SOD and CAT reduced by 1,3-DCP and protected against 1,3-DCP-mediated cell death. In conclusion, these results suggest that 1,3-DCP induces oxidative stress through the elevated CYP2E1 level, which is inhibited by the extracts, protecting cells against the effects of 1,3-DCP.
Keywords: Erythronium japonicum; Corylopsis coreana Uyeki; 1,3-Dichloro-2-propanol; Cytochrome P450 2E1; HuH7 cells; Oxidative stress
Introduction
1,3-Dichloro-2-propanol (1,3-DCP) is a widely used pesticide and a well-known food contaminant produced during various food manufacturing, which is suggested to have toxicological properties. Exposure to 1,3-DCP may occur through the diet (from hydrochloric acid-hydrolyzed vegetable protein) and drinking water (from epichlorohydrin polyamine polyelectrolytes used as flocculants and coagulants in water purification) (Nyman et al., 2003). In a previous study in rats, intraperitoneal injection of 1,3-DCP (18–290 mg/kg) induced drowsiness, liver injury, decreased white blood cell and platelet counts, and increased blood clotting time (Katoh et al., 1998). More specifically, it was demonstrated that 1,3-DCP provoked hepatotoxicity through the generation of oxidative stress and inflammatory response (Lee et al., 2016).
Plant and plant-derived products contain significant quantities of bioactive compounds possessing antioxidant activities. Erythronium japonicum is one of those perennial herbs belonging to the Lily family. Heo et al. (2007) reported that E. japonicum extract had free radical scavenging activity and showed anti-proliferative effects in human colorectal carcinoma cells. Corylopsis coreana Uyeki (Korean winter hazel), belonging to the Hamamelidaceae family, is cultivated as an ornamental plant. Some species of the genus Corylopsis, such as Hamamelis virginiana (witch hazel), have long been used in traditional herbal medicine for the treatment of irritated skin and inflammatory disease (Kim et al., 2013). Likewise with E. japonicum, the phenolic compounds from C. coreana Uyeki also showed anti-oxidative and anti-proliferative activities on human prostate cancer cell lines (Kim et al., 2013).
In this study, we expanded the previous suggestions on 1,3-DCP-induced generation of oxidative stress and cell death, and evaluated the effects of extracts from E. japonicum and C. coreana Uyeki on 1,3-DCP-treated human hepatocytes.
Materials and methods
Test chemicals
Powdered extracts of E. japonicum and C. coreana Uyeki were prepared as previously reported (Seo et al., 2016). 1,3-DCP was purchased from Merck KGaA (Darmstadt, Germany).
Cell culture
All cells were obtained from Korean Cell Line Bank (Seoul, South Korea). HuH7 and HepG2 cells were seeded into six-well plates at a density of approximately 4.0 × 105 cells/well in culture medium [RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS)]. To cultivate HeLa cells, the DMEM medium supplemented with 10% FBS was used. All cells were cultured for 1 d at 37 °C before use. After the treatment of cells with the extracts and/or 1,3-DCP, cells were collected by conventional procedures such as centrifugation (200×g for 5 min) and washing with phosphate-buffered saline.
Cell fractionation and CYP2E1 activity assay
Cytosolic and microsomal fractions were obtained as previously described (Yang et al., 2004) and the microsomal fractions were suspended in 100 mM Tris–HCl (pH 7.4, 4 °C). To investigate the catalytic activity of CYP2E1, the p-nitrophenol hydroxylase activity was measured using the microsomal fractions as previously reported (Reinke and Moyer, 1985).
Western blot analysis of microsomal CYP2E1
The microsomal proteins of cells (50 μg/well) were analyzed by a conventional Western blotting method using monoclonal anti-CYP2E1 antibody (BD Biosciences Korea, Seoul, South Korea). The CYP protein band was visualized using the enhanced chemiluminescence method (GE Healthcare, Princeton, NJ, USA).
Measurement of oxidative stress and enzyme activities
The level of ROS was measured by a spectrofluorimetric method with the cytosolic fractions of lysates using 2,7-dichlorofluorescein diacetate (DCF-DA) as a probe according to the method described in Bhagwat et al. (1998). Reduced glutathione (GSH) levels were determined by the protein-free sulfhydryl content using Ellman’s reagent (Buttar et al., 1977). The level of lipid peroxidation (LPO) was estimated by measuring thiobarbituric acid-reactive substances, using malondialdehyde as a standard as described in Ohkawa et al. (1979). Glutathione S-transferase (GST) activity was measured according to the method reported (Habig et al., 1974). The superoxide dismutase (SOD) activity was determined according to the method of Beauchamp and Fridovich (1971). Catalase (CAT) activity was determined using the method of Aebi (1984): a decrease in H2O2 absorbance was measured in 1 mL reaction mixture containing 10 mM H2O2 and 20 μL supernatant in 50 mM potassium phosphate buffer (pH 7.0) at 240 nm. CAT activity was expressed as 1 mol of H2O2 decomposed·mg protein−1 min−1 at pH 7.0 at 25 °C.
MTT assay
Cell viability was assayed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. Briefly, after removal of culture media, each indicated amount of serum-free media and MTT solution was added to cultured cells in 96-well plate. After the plate was incubated at 37 °C for 3 h, the indicated amount of MTT solution was added and then the absorbance at 590 nm was measured.
Statistical analysis
The results are expressed as the mean ± standard deviation (S.D.). Differences between groups were analyzed via one-way analysis of variance followed by Dunnett’s multiple comparison test. p < 0.05 was considered a statistically significant difference.
Results and discussion
It has been previously suggested that 1,3-DCP increased oxidative stress in rat liver (Lee et al., 2015). To obtain more insight into the physiological effects of 1,3-DCP, we measured 1,3-DCP-mediated oxidative stress in HuH7 cells. It was shown that treatment with 10 μM 1,3-DCP to cells for 2 h induced changes in all parameters measured (Table 1); ROS and LPO levels increased by approximately 4.8- and 3.5-fold, respectively, compared to untreated cells. GSH and GST levels also decreased to approximately 43 and 51% of the control levels, respectively. Moreover, SOD and CAT activities were significantly diminished by 1,3-DCP treatment. Taken together, these results indicate that 1,3-DCP induced significant stimulation of oxidative stress in hepatic cells.
Table 1.
Oxidative stress and enzyme activities in 10 μM 1,3-DCP-treated HuH7 cells
| Assay | Group | |
|---|---|---|
| Control | 1,3-DCP | |
| ROS (pmol/mg protein) | 4.2 ± 0.3 | 20.1 ± 2.9* |
| LPO (nmol/mg protein) | 1.4 ± 0.3 | 4.9 ± 0.7* |
| GSH (nmol/mg protein) | 15.5 ± 2.7 | 6.7 ± 1.4* |
| GST (nmol/min/mg protein) | 117.6 ± 25.8 | 60.1 ± 17.2* |
| SOD (units/mg protein) | 87.3 ± 20.2 | 33.9 ± 11.4* |
| CAT (units/mg protein) | 8.6 ± 1.0 | 2.6 ± 0.5* |
ROS production, GSH concentration and the enzyme activities (GST, SOD and CAT) were measured with cytosolic fraction of cells. LPO level was measured with microsomal fraction of cells. 1,3-DCP was treated to HuH7 cells at 37 °C for 2 h and then each parameter was measured after cell fractionations. Values represent the means ± S.D. of five independent experiments
*Significant difference at p < 0.05 compared to the control
To investigate 1,3-DCP-induced oxidative stress in more detail, we measured ROS production in HuH7 (human hepatoma cells), HepG2 (human hepatoma cells), and HeLa (human cervical carcinoma cells) cells with increasing concentrations of 1,3-DCP. As the concentration of 1,3-DCP increased, ROS level was also enhanced in HuH7 and HepG2 cells compared to untreated cells and both cells showed similar dependency in the treatment concentration of 1,3-DCP (Fig. 1). However, ROS were slightly produced in HeLa, a cervical cancer cell when the same concentrations of 1,3-DCP were treated. Therefore, it is likely that 1,3-DCP-induced oxidative stress is different in animal cell types, and the precise mechanism for the ROS production in cells should be further studied.
Fig. 1.
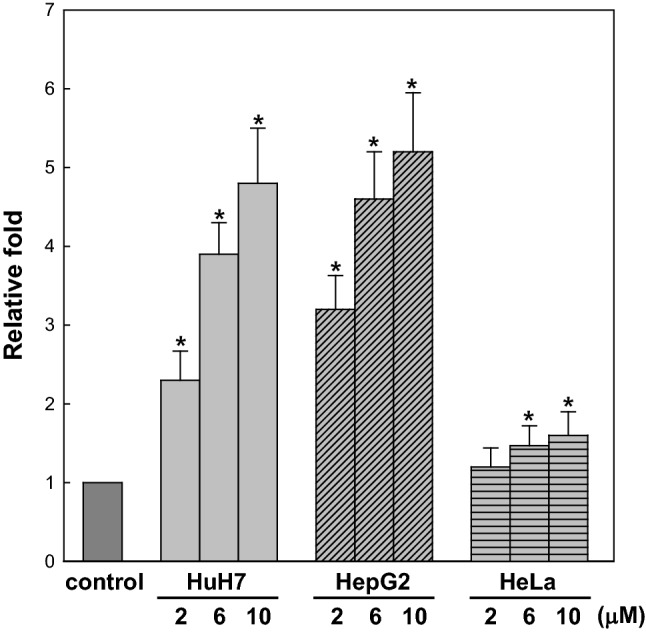
1,3-DCP-induced ROS in HuH7, HepG2 and HeLa cells. Cells were treated with each indicated concentration of 1,3-DCP for 2 h, and ROS levels were measured with the cytosolic fractions of cells using DCF-DA. The y-axis represents the relative fold increase in ROS level, with the level of each untreated cell (control) set to one (1). Values represent the mean ± S.D. of five independent experiments. *p < 0.05, compared to the untreated control
In contrast, the co-treatment with 1,3-DCP and the extracts of E. japonicum or C. coreana Uyeki resulted in a remarkable decrease in ROS, including LPO, as ROS and LPO levels were approximately 2.5- and 2.8-fold of untreated cells when 0.2% (w/v) E. japonicum was co-incubated (Fig. 2). C. coreana Uyeki exerted a more attenuated effect on the ROS production, which approximately 2.1- and 2.3-fold increase in ROS and LPO, respectively, were shown at 0.2% concentration. Protection from 1,3-DCP-induced increase in ROS and LPO was also dependent in the extract concentration, as the 0.5% extracts resulted in even more attenuation in the generation of oxidative stress. In addition, SOD and CAT activities were restored by the extracts (Table 2). However, the co-treatment with extracts caused slight recovery in GSH activity and GST level when those two values were compared with the changes in other parameters.
Fig. 2.

Effects of E. japonicum and C. coreana Uyeki extracts on 1,3-DCP-induced oxidative stress. HuH7 cells were co-treated with 1.3-DCP and the plant extracts (0.2 or 0.5%) for 2 h, and ROS and LPO levels were measured with the cytosolic and microsomal fractions. The y-axis represents the relative fold increase in ROS and LPO levels, with the levels in untreated cells set to one (1). E j and CU represent E. japonicum and C. coreana Uyeki, respectively. *p < 0.05 and #p < 0.05, compared to ROS and LPO, respectively, in cells treated with 1,3-DCP alone. (B)
Table 2.
GSH levels and enzyme activities in HuH7 cells co-treated with 10 μM 1,3-DCP and 0.5% E. japonicum and C. coreana Uyeki extracts
| Assay | Group | ||
|---|---|---|---|
| 1,3-DCP alone | +E. Japonicum | +C. coreana Uyeki | |
| GSH (nmol/mg protein) | 6.7 ± 1.4 | 7.5 ± 1.6 | 8.0 ± 2.1 |
| GST (nmol/min/mg protein) | 60.1 ± 17.2 | 63.4 ± 20.4 | 71.2 ± 19.5 |
| SOD (units/mg protein) | 33.9 ± 11.4 | 68.6 ± 21.3† | 66.2 ± 18.2† |
| CAT (units/mg protein) | 2.6 ± 0.5 | 5.2 ± 1.1† | 6.8 ± 1.9† |
1,3-DCP and the extracts were co-treated to HuH7 cells at 37 °C for 2 h. Values represent the means ± S.D. of five independent experiments
†Significant difference at p < 0.05 compared to the 1,3-DCP-treated sample
It was revealed that 1,3-DCP decreased cell viability of HuH7 in a concentration-dependent manner, and approximately 24% of total cells were viable 24 h after the treatment with 10 μM 1,3-DCP (Fig. 3). Based on the results, it is enticing to speculate that the decrease in cell viability may be directly related with the generation of oxidative stress by 1,3-DCP. In contrast, co-treatment with either extract markedly inhibited cell death, with approximately 83% of total cells viable after 24 h incubation with 10 μM 1,3-DCP and 0.5% C. coreana Uyeki extract. Erythronium japonicum extract showed a similar protective effect. Therefore, these results suggest that the extracts may protect the cells from death caused by oxidative stress. Treatment with extracts alone resulted in similar levels of cell viability to the untreated control.
Fig. 3.

1,3-DCP-induced cell death and the effects of E. japonicum (E j) and C. coreana Uyeki (CU) extracts on cell viability. Cell viability was measured after treatment with 10 μM 1,3-DCP in the presence or absence of 0.2 or 0.5% plant extracts. *p < 0.05 and #p < 0.05, compared to the untreated control and the 10 μM 1,3-DCP-treated sample, respectively. *E j and *CU represent the cell viabilities after treatment with 0.5% extract alone in the absence of 1,3-DCP. Values represent the mean ± S.D. of five independent experiments
The cytochrome P450 enzyme CYP2E1 can produce ROS, including LPO, which is involved in the etiology and pathology of many diseases, especially those involving hepatocellular injury (Leung and Nieto, 2013). Therefore, we investigated CYP2E1 expression and enzymatic activity in cells treated with 1,3-DCP. It was shown that the protein level of CYP2E1 increased by 10 μM 1,3-DCP treatment with increasing the incubation time when analysed via an immunoblotting method using monoclonal anti-CYP2E1 antibody (Fig. 4). CYP2E1 enzyme activity was also enhanced concurrently, with an approximately sevenfold increase at maximum compared to the control. In contrast, when the extracts were co-administrated, CYP2E1 activity was remarkably reduced, with 1.5–4.3-fold increases in the activity, depending on the type and concentration of the extract. However, the enhanced expression of CYP2E1 by 1,3-DCP was little affected by the co-incubation with both extracts regardless of their concentrations, suggesting that the extracts act as inhibitors of CYP2E1, thereby reducing the production of oxidative stress.
Fig. 4.

Analysis of CYP2E1 expression (A and B) and activity (C) in the microsomal fractions of 1,3-DCP-treated HuH7 cells. (A) Microsomal CYP2E1 was analyzed with increasing time after treatment with 10 μM 1,3-DCP using Western blotting. (B) Microsomal CYP2E1 was analysed 24 h after the co-treatment with 10 μM 1,3-DCP and each indicated concentration of extract. (C) The hydroxylation of p-nitrophenol was measured after the indicated treatments. Values represent the mean ± S.D. of five independent experiments. *p < 0.05 and #p < 0.05, compared to the untreated control and the 10 μM 1,3-DCP-treated sample, respectively
Recently, four markers from C. coreana Uyeki have been identified and their extraction was also optimized: bergenin (17.5%, w/w), isosalipurposide (8.6%, w/w), quercitrin (1.6%, w/w) and quercetin (0.05%, w/w) (Seo et al., 2016). Bergenin has several beneficial functions such as protection against d-galactosamine-induced hepatotoxicity in rats (Lim et al., 2001). The chalcone compound isosalipurposide has a cytoprotective effect against oxidative injury via Nrf2 activation (Han et al., 2015). Quercetin and quercitrin also show the hepatoprotective effect in vivo mouse model (Shanmugam et al., 2016; Xie et al., 2016). In relation to E. japonicum, it was found that chlorogenic acid and caffeic acid might be active compounds, which exerts antioxidant effect protecting against oxidative stress-induced hepatotoxicity (Koriem and Soliman, 2014) and is also an immunoregulatory molecule (Sy et al., 2011), respectively. Based on these results, we anticipate that the compounds mentioned above may play an important role in the reduction of 1,3-DCP-induced oxidative stress, although the present experiments were not performed with each purified compound.
In conclusion, this study suggests that elevated CYP2E1 expression and activity may be responsible for the increase in oxidative stress caused by 1,3-DCP treatment, and that E. japonicum and C. coreana Uyeki extracts may attenuate the production of ROS (and LPO) by inhibiting CYP2E1. However, it should be further revealed which component(s) in the extracts are involved in protecting cells from the detrimental effects of 1,3-DCP.
Acknowledgements
This study was financially supported by Chonnam National University (Grant No. 2017-2787).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bhagwat SV, Vijayasarathy C, Raza H, Mullick J, Avadhani NG. Preferential effects of nicotine and 4-(N-methyl-N-nitrosamine)-1-(3-pyridyl)-1-butanone on mitochondrial glutathione S-transferase A4-4 induction and increased oxidative stress in the rat brain. Biochem. Pharmacol. 1998;56:831–839. doi: 10.1016/S0006-2952(98)00228-7. [DOI] [PubMed] [Google Scholar]
- Buttar HS, Chow AYK, Downie RH. Glutathione alterations in rat liver after acute and subacute oral administration of paracetamol. Clin. Exp. Pharmacol. Physiol. 1977;4:1–6. doi: 10.1111/j.1440-1681.1977.tb02371.x. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Han JY, Cho SS, Yang JH, Kim KM, Jang CH, Park DE, Bang JS, Jung YS, Ki SH. The chalcone compound isosalipurposide (ISPP) exerts a cytoprotective effect against oxidative injury via Nrf2 activation. Toxicol. Appl. Pharmacol. 2015;287:77–85. doi: 10.1016/j.taap.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Heo BG, Park YS, Chon SU, Lee SY, Cho JY, Gorinstein S. Antioxidant activity and cytotoxicity of methanol extracts from aerial parts of Korean salad plants. BioFactors. 2007;30:79–89. doi: 10.1002/biof.5520300202. [DOI] [PubMed] [Google Scholar]
- Katoh T, Haratake J, Nakano S, Kikuchi M, Yoshikawa M, Arashidani K. Dose-dependent effects of dichloropropanol on liver histology and lipid peroxidation in rats. Ind. Health. 1998;36:318–323. doi: 10.2486/indhealth.36.318. [DOI] [PubMed] [Google Scholar]
- Kim MH, Ha SY, Oh MH, Kim HH, Kim SR, Lee MW. Anti-oxidative and anti-proliferative activity on human prostate cancer cells lines of the phenolic compounds from Corylopsis coreana Uyeki. Molecules. 2013;18:4876–4886. doi: 10.3390/molecules18054876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koriem KM, Soliman RE. Chlorogenic and caftaric acids in liver toxicity and oxidative stress induced by methamphetamine. J. Toxicol. 2014;2014:583494. doi: 10.1155/2014/583494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IC, Ko JW, Lee SM, Kim SH, Shin IS, Moon OS, Yoon WK, Kim HC, Kim JC. Time-course and molecular mechanism of hepatotoxicity induced by 1,3-dichloro-2-propanol in rats. Environ. Toxicol. Pharmacol. 2015;40:191–198. doi: 10.1016/j.etap.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Lee IC, Lee SM, Ko JW, Park SH, Shin IS, Moon C, Kim SH, Kim JC. Role of mitogen-activated protein kinases and nuclear factor-kappa B in 1,3-dichloro-2-propanol induced hepatic injury. Lab. Anim. Res. 2016;32:24–33. doi: 10.5625/lar.2016.32.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver diseases. J. Hepatol. 2013;58:395–398. doi: 10.1016/j.jhep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Lim HK, Kim HS, Choi HS, Choi J, Kim SH, Chang MJ. Effects of bergenin, the major constituent of Mallotus japonicus against d-galactosamine-induced hepatotoxicity in rats. Pharmacology. 2001;63:71–75. doi: 10.1159/000056115. [DOI] [PubMed] [Google Scholar]
- Nyman P, Diachenko G, Perfetti G. Survey of chloropropanols in soy sauces and related products. Food Addit. Contam. 2003;20:909–915. doi: 10.1080/02652030310001603792. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Reinke LA, Moyer MJ. p-Nitrophenol hydroxylation. A microsomal oxidation which is highly inducible by ethanol. Drug Metab. Dispos. 1985;13:548–552. [PubMed] [Google Scholar]
- Seo JH, Kim JE, Shim JH, Yoon G, Bang MA, Bae CS, Lee KJ, Park DH, Cho SS. HPLC analysis, optimization of extraction conditions and biological evaluation of Corylopsis coreana Uyeki Flos. Molecules. 2016;21:94. doi: 10.3390/molecules21010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam S, Thangaraj P, Lima BDS, Chandran R, de Souzza Araújo AA, Narain N, Serafini MR, Júnior LJQ. Effects of luteolin and quercetin 3-β-d-glucoside identified from Passiflora subpeltata leaves against acetaminophen induced hepatotoxicity in rats. Biomed. Pharmacother. 2016;83:1278–1285. doi: 10.1016/j.biopha.2016.08.044. [DOI] [PubMed] [Google Scholar]
- Sy LB, Yang LK, Chiu CJ, Wu WM. The immunoregulatory effects of caffeic acid phenethyl ester on the cytokine secretion of peripheral blood mononuclear cells from asthmatic children. Pediatr. Neonatol. 2011;52:327–331. doi: 10.1016/j.pedneo.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Xie W, Wang M, Chen C, Zhang X, Melzig MF. Hepatoprotective effect of isoquercitrin against acetaminophen-induced liver injury. Life Sci. 2016;152:180–189. doi: 10.1016/j.lfs.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cao J, Shi Y. Identification and characterization of a gene encoding human LPGAT1, an endoplasmic reticulum-associated lysophosphatidylglycerol acyltransferase. J. Biol. Chem. 2004;279:55866–55874. doi: 10.1074/jbc.M406710200. [DOI] [PubMed] [Google Scholar]


