Abstract
The product of ferulic acid decarboxylation, 4-vinylguaiacol (4-VG), is an important antioxidant and is reported to have an antioxidant capacity comparable to α-tocopherol. In this study, evaluation on antioxidant capacities of ferulic acid, catechin, and 4-VG was performed when 200 ppm of each compound was added in a 10% O/W emulsion for 50 days. Peroxide value (POV) results of the O/W emulsion containing 4-VG were noteworthy. The POV was 1.9 meq/L of emulsion after 29 days, which was no different to the initial value (day 0). Even when the oxidation was allowed to advance to day 50, the POV remained at 2.2 meq/L of emulsion, representing only a tiny increase relative to the initial value on day 0. 1H-NMR results also showed that the lowest conjugated forms and no aldehydes were detected in emulsion of 4-VG stored for 50 days, proving the excellent antioxidant capacity in the O/W emulsion.
Keywords: Antioxidant capacity, Ferulic acid, O/W emulsion, 4-Vinylguaiacol
Introduction
Hydroxycinnamic acids (e.g. p-coumaric, caffeic, ferulic, and sinapic acid) are compounds containing carboxyl, hydroxyl, or methoxyl groups attached to a phenol structure, and are found mainly in plants, fruits, and vegetables (Nenadis et al., 2003). Among them, ferulic acid (3-methoxy-4-hydroxycinnamic acid) is present in cell wall of plant seeds as either its free form or ester form, and may serve a potential protective function against cell damage by inhibiting free radicals and lipid peroxidation (Srinivasan et al., 2006; Tilay et al., 2008).
As phenolic acids have different solubility according to their functional groups, they exhibit different antioxidant capacities in bulk oils or emulsion systems. Therefore, the antioxidant capacity can be changed by decarboxylation of ferulic acid to remove the carboxyl group to increase its lipid-solubility. The product of ferulic acid decarboxylation, 4-vinylguaiacol (4-VG), is an important antioxidant found in coffee and is reported to have an antioxidant capacity comparable to α-tocopherol. Furthermore, derivatives of ferulic acid are reported to have antioxidant capacities (Chen and Ho, 1997; Fujioka and Shibamoto, 2006; Kikuzaki et al., 2002; Nenadis et al., 2003; Terpinc et al., 2011).
In emulsions, lipid oxidation characteristic is different from that in bulk fats and oils. Particularly, lipid oxidation in oil-in-water (O/W) emulsion is more complex than bulk oil due to its different environmental conditions. For industrial application, antioxidants would be tested in O/W emulsion added a commercial emulsifier such as Tween-20 because the type of used emulsifiers (phospholipids, proteins and small molecule surfactants) may influence lipid oxidation in food emulsions (McClements and Decker, 2000).
In this study, 200 ppm of catechin, ferulic acid, and decarboxylated ferulic acid (4-VG) were added in 10% oil-in-water emulsion prepared with Tween-20, and their antioxidant capacities were compared during 50 days because the maximum usage of the synthetic antioxidant (i.e. 4-VG) in the emulsion products is generally considered up to 200 ppm.
Materials and methods
Materials
Soybean oil was purchased from a local market (Daejeon, Korea). Tween-20, used as an emulsifier, was obtained from Duksan Pharmaceutical Co. (Kyongkido, South Korea). Activated charcoal and silica gels used for the preparation of stripped soybean oil were obtained from Daejung Co. (Incheon, South Korea) and Merck Co. (Darmstadt, Germany), respectively. Ferulic acid, and chloroform-D (CDCl3, 99.8 atom%D) were purchased from Sigma-Aldrich (St. Louis, MO, USA). N,N-Dimethylformamide (DMF) was from Tokyo Chemical Industry Co. (Tokyo, Japan). Catechin with commercial grade was provided from Ilshinwells Co. (Seoul, South Korea). Organic solvents used for high-performance liquid chromatography (HPLC) and 1H-NMR were all of HPLC grade purity.
Preparation of stripped soybean oil (SSBO)
In order to remove natural tocopherols from soybean oil, the stripping process was performed as followings: soybean oil (20 g) and n-hexane (10 mL) were mixed and passed through a column (3.5 cm diameter, 20 cm length) packed sequentially from the bottom with 1 g of anhydrous sodium sulfate followed by 6 g of activated charcoal and another 20 g of silica gel (Wang et al., 2014). The flow valve was closed for the absorption of tocopherols and colorants naturally existed in soybean oil on silica gel. After 30 min, the flow valve was opened and then additional 300 mL of n-hexane was eluted. The solvent in the stripped soybean oils (SSBO) was removed completely using a vacuum rotary evaporator and nitrogen gas. The tocopherol contents (ppm) of soybean oil and the SSBO were determined using a normal-phase HPLC system (Agilent Technologies, Little Falls, DE, USA) with little modification (Yang et al., 2013). LiChrospher® Diol 100 column (25 cm × 4 mm, i.d. 5 μm, Merck KGaA, Darmstadt, Germany) was used. The mobile phase was a mixture of n-hexane and isopropanol (99.4:0.6, v/v) with isocratic elution. Other conditions were the same as a previous study (Yang et al., 2013). Quantification was carried out using an external standard curve.
Preparation and analysis of 4-VG
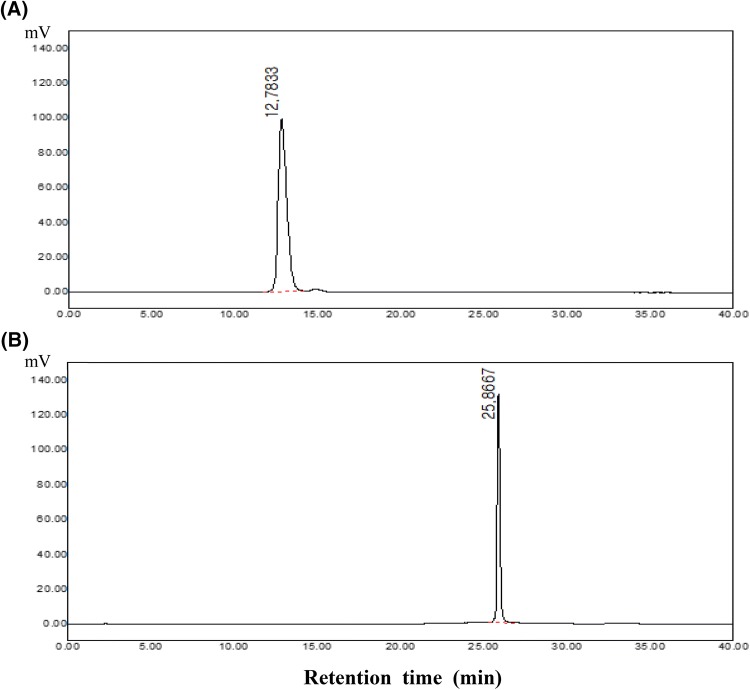
4-VG was synthesized by decarboxylation of ferulic acid with some modifications according to the previous method (Terpinc et al., 2011). Ferulic acid (100 mg) was dissolved in DMF (2 mL), and sodium acetate (20 mg) as a catalyst was added into each screw-capped test tube. The mixtures were reacted at 130 °C for 1 h, respectively. After the reaction, saturated NaCl solution (20 mL) was added and the high-purity 4-VG was extracted with diethyl ether (2 mL × 5 times). The solvents were removed using a vacuum rotary evaporator. Finally, 4-VG was obtained from the decarboxylation of ferulic acid. Reversed-phase HPLC analysis of ferulic acid and 4-VG was performed according to the previous method with slight modifications (Vuorela et al., 2003). The HPLC system (HPLC, Younglin, Anyang, Korea) was equipped with a Younglin SP 930D dual pump, UV/Vis detector (Younglin, Anyang, Korea) and Nova-Pak® C18 column (150 × 3.9 mm, i.d. 4 μm, Waters, Milford, MA, USA). The mobile phase consisted of solvent A (0.02 M pH 2.15 ammonium dihydrogen phosphate buffer/methanol, 75:25, v/v) and solvent B (methanol). The gradient elution was as follows: 5% B solvent (0–15 min), 5–35% B (15–20 min), 35% B (20–35 min), 35–100% B (35–45 min), 100–5% B (45–50 min), 5% B (50–60 min). The flow rate was 0.6 mL/min. Samples were dissolved in the mobile phase and a volume of 10 μL was injected into the HPLC. Detection was performed at 325 nm for ferulic acid and 275 nm for 4-VG, respectively (Fig. 1).
Fig. 1.
HPLC chromatograms of ferulic acid and 4-vinylguaiacol (4-VG). (A) Ferulic acid standard (40 µg/mL) at 325 nm (B) 4-Vinylguaiacol (40 µg/mL) at 275 nm
Preparation of O/W emulsion and their oxidation study
As an antioxidant, ferulic acid, 4-VG, and catechin were respectively used in O/W emulsion. A 10% SSBO O/W emulsion was prepared by adding emulsifier Tween-20 (0.3 g) and SSBO (10 g) slowly into 20 mM bis–tris buffer (89.7 g with 0.02% sodium azide, pH 7). The mixture was pre-mixed with Silverson premixer (L4RT, Silverson Machines Ltd., UK) at 5000 rpm for 2 min, and then the 10% O/W emulsion was prepared using a microfluidizer (M-110Y, Microfluidics, MA, USA) under 3000 psi with two passes. The oxidation stability of antioxidants in O/W emulsion prepared from SSBO was studied by adding ferulic acid, 4-VG, and catechin (each 200 ppm dissolved in methanol 100 μL) with 10% SSBO O/W emulsion in a 25 mL vial with the cap, then placed in a dark at 37 °C for 50 days. Control was prepared by adding 100 μL methanol. All emulsion samples for antioxidant experiments were prepared in duplicate (n = 2). All antioxidant experiments were performed in duplicate.
Measurement of droplet size distribution
Droplet sizes in emulsions were measured with laser particle size analyzer (Mastersizer S, Malvern Instrument, Worcestershire, UK). The particle size measurement was expressed as the volume-surface average emulsion diameter (d3,2, μm). The droplet sizes of emulsion were measured at 0, 10, 30 and 50 days. The data were presented as the average and standard deviation of duplicate measurements.
Peroxide value (POV)
The POV of each treatment in a 10% O/W emulsion was measured according to the previous method (Mei et al., 1999). Absorption was measured using a UV/Vis spectrophotometer (UV 1700, Shimadzu, Kyoto, Japan) at 510 nm. POV (meq/L emulsion) of the emulsion was calculated from an external standard using a hydrogen peroxide solution.
1H-NMR analysis
The conjugated diene groups (Z,E- and E,E-conjugated forms), α-linolenic (C18:3) and linoleic (C18:2) acids, and aldehydes in 10% O/W emulsion were monitored by 1H-NMR (BRUKER AVANCE III 600, Bruker, Germany) (Guillen and Goicoechea, 2009; Guillen and Ruiz, 2005; Knothe and Kenar, 2004). For the oxidized O/W emulsion, the oil within emulsion was extracted with chloroform (2 mL × 3 times). The extracted oil (approximately 50 mg) was dried completely under nitrogen gas and then dissolved in 700 μL of chloroform-d [purity 99.8%, contain 0.1% tetramethylsilane (TMS)]. The mixture was placed into an NMR tube of 5-mm diameter. The acquisition parameters for 1H-NMR analysis were a spectral width of 12,335.5 Hz, scan number of 16 rounds, and acquisition time of 2.656 s. All chemical shifts (δ) were set relative to TMS at δ = 0 ppm during analysis. The assignment of signals for aldehydes (n-alkenals, 4,5-epoxy-(E)-2-alkenals, (E,E)-2,4-alkadienals, and (E)-2-alkenals) and conjugated diene groups (Z,E-conjugated form and E,E-conjugated form) was performed according to previous studies (Guillen and Goicoechea, 2009; Guillen and Ruiz, 2005; Wang et al., 2014). The amount of linolenic acid and linoleic acid in TAG molecules of oxidized oils was calculated as follows (Knothe and Kenar, 2004):
where LnA = linolenic acyl group (CH=CH–CH2–CH3, 0.95 ppm), Acyl = all acyl group except linolenic (CH2CH2CH2–CH3, 0.88 ppm), Bis = bis-allylic group (linolenic and linoleic acyl group, CH=CH–CH2–CH=CH, 2.84 ppm).
Results and discussion
Preparation of SSBO and identification of 4-VG
Tocopherol is widely used as a representative antioxidant to inhibit the oxidation of oils (Jung and Min, 1990). Therefore, tocopherols were removed from soybean oil through silica-gel column, providing the stripped soybean oil (SSBO) for further oxidation experiments. After stripping the oil, tocopherol content in SSBO was around 38 ppm (δ-tocopherol, 30 ppm; α-tocopherol, 5 ppm; others, 3 ppm).
In chromatographic separation of RP-HPLC analysis using a C18 column, polar compounds based on opposite attraction on an octadecyl silica support particle can be separated first with the chromatographic order from polar to non-polar. Moreover, non-polar substances were sequentially detected with long retention time (RT) by their similar attraction on a C18 column. On our HPLC chromatographic results, the RT of ferulic acid was 12.8 min while the RT of 4-VG was 25.9 min (Fig. 1). These results demonstrated that 4-VG, being devoid of the carboxyl group of ferulic acid, was less polar than ferulic acid. Our results were in accordance with the previous observations (Vuorela et al., 2003), who found that sinapic acid, a hydroxycinnamic acid with a similar structure to ferulic acid, had a shorter RT than its decarboxylation product, 4-vinylsyringol.
Droplet size distribution in O/W emulsion
Emulsions added with 4-VG, catechin, ferulic acid and control (without antioxidant) showed similar particle size (d3,2) of fat globule, having 0.41 μm at 0 day. Thereafter, particle size of fat globule did not much change in all emulsions, suggesting that prepared emulsions remained quite stable until 50 days. The particle size (d3,2) of fat globule in emulsions ranged from 0.43 to 0.46 μm at 50 days (Table 1).
Table 1.
Particle size of fat globule (d3,2) in the emulsions added with different antioxidants (200 ppm)
| Days | Droplet size distribution (d3,2, μm) | |||
|---|---|---|---|---|
| Control | Catechin | Ferulic acid | 4-VG | |
| 0 | 0.41 ± 0.02 | 0.41 ± 0.02 | 0.41 ± 0.02 | 0.41 ± 0.02 |
| 10 | 0.37 ± 0.01 | 0.41 ± 0.01 | 0.43 ± 0.05 | 0.39 ± 0.01 |
| 30 | 0.40 ± 0.04 | 0.40 ± 0.03 | 0.43 ± 0.01 | 0.43 ± 0.02 |
| 50 | 0.43 ± 0.04 | 0.43 ± 0.01 | 0.46 ± 0.02 | 0.45 ± 0.01 |
The values are expressed as mean ± standard deviation (n = 2)
O/W emulsions were prepared with 200 ppm of ferulic acid, 4-vinylguaiacol (4-VG), and catechin, respectively
Oxidation stability in O/W emulsion
Ferulic acid, 4-VG, and catechin were added to a 10% O/W emulsion prepared from SSBO, and the POV was measured while oxidation was progressed at 37 °C in the dark for 50 days (Fig. 2). POV in the emulsion without additional antioxidant (control) increased up to day 15, then remained without distinct change before increasing after day 29. After 50 days of oxidation, the POV was about 110 meq/L of emulsion. When ferulic acid and catechin was added, there was no remarkable change of POV until 22 days of oxidation after which POVs increased to 17.3 and 24.6 meq/L on day 29, respectively. When oxidation was progressed more than 29 days, POVs started to increase, showing remarkable changes except for O/W emulsion with 4-VG. When oxidation progressed to day 50, the POVs of the emulsions containing catechin was 89.8 meq/L of emulsion. In contrast, when ferulic acid was added to O/W emulsion, it showed a better antioxidant capacity than catechin (Fig. 2).
Fig. 2.
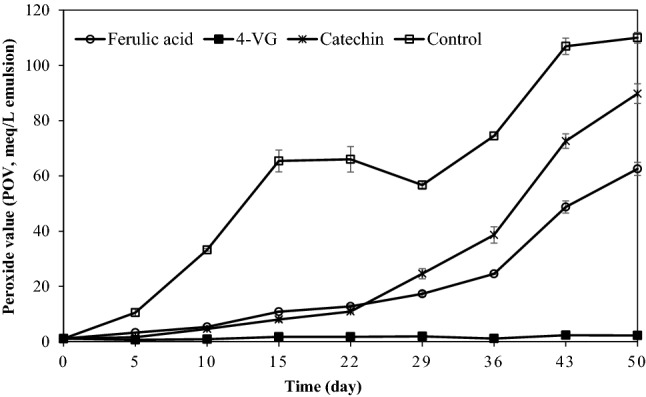
Peroxide values (POV) in 10% O/W emulsions added with different antioxidants. O/W emulsions were prepared with 200 ppm of ferulic acid, 4-vinylguaiacol (4-VG), and catechin, respectively
Moreover, the results of the O/W emulsion containing 4-VG were noteworthy; in this case, the POV was 1.9 meq/L of emulsion after 29 days of oxidation, which was no different to the initial value (day 0). Even when the oxidation was allowed to advance to day 50, the POV remained at 2.2 meq/L of emulsion, representing only a tiny increase relative to the initial value on day 0. Therefore, 200 ppm of 4-VG exhibited excellent antioxidant capacity in the 10% O/W emulsion prepared using SSBO.
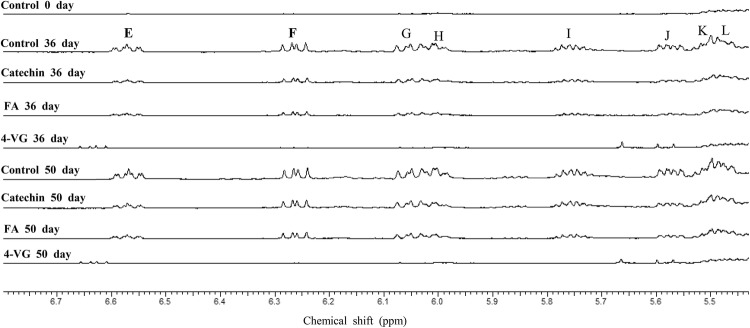
Because signals at the 8–10 ppm region represent oxidation products (i.e. hydroperoxides and aldehydes) (Falch et al., 2004; Sacchi et al., 1996), enlarged spectra with same scale are shown in Fig. 3. In control, new signals were found and gradually increased during prolonged oxidation, while relatively low intensities were observed in O/W emulsions containing ferulic acid and catechin. However, when oxidation was conducted during 50 days in O/W emulsion with 4-VG, those signals were hardly observed, demonstrating that oxidation was not much occurred. Also, conjugated dienes at the region between 5 and 7 ppm showed similar trend (Falch et al., 2004), in which signals of the emulsion containing 4-VG were much lower than those from other treatments until day 50 (Fig. 4). Therefore, it could be concluded from this study that catechin and ferulic acid all effectively delayed oxidation in emulsions. In the case of 4-VG, not only were conjugated diene groups produced in a tiny quantity, but secondary oxidation products (aldehydes) were hardly detected, proving the excellent antioxidant capacity in the O/W emulsion.
Fig. 3.
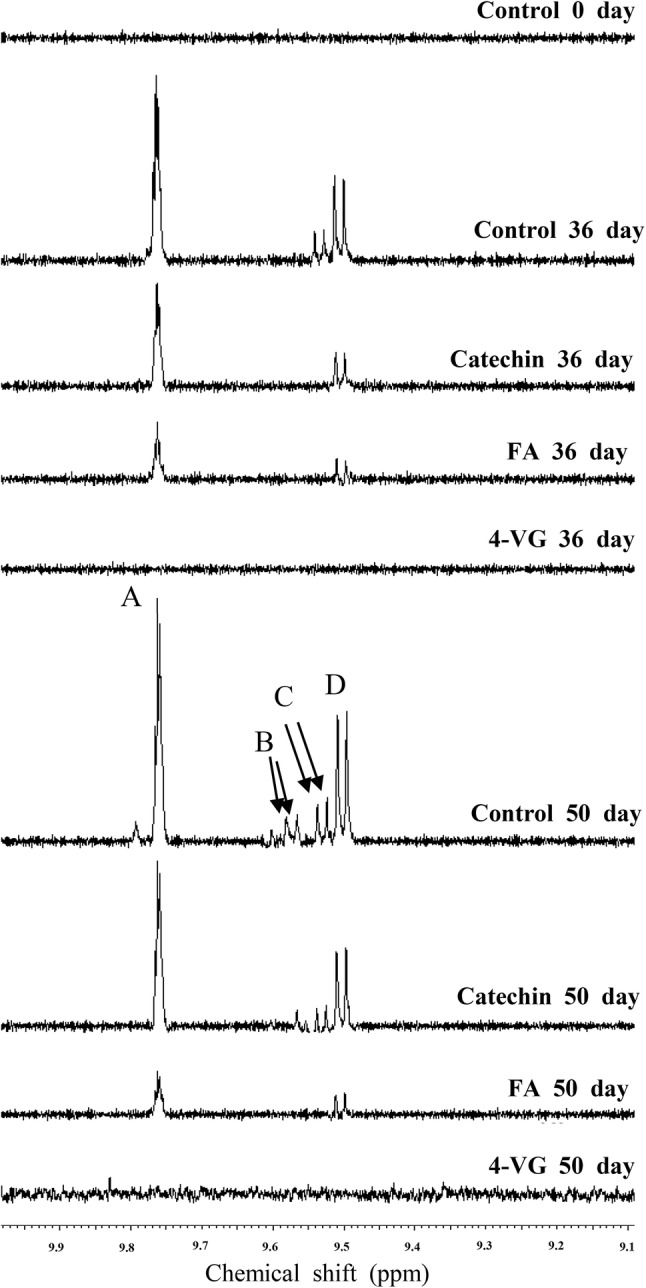
The expanded regions of secondary oxidation products (aldehydes) in the 1H NMR spectra of 10% O/W emulsions added with different antioxidants. O/W emulsions were prepared with 200 ppm of ferulic acid (FA), 4-vinylguaiacol (4-VG), and catechin, respectively and their oxidation study was performed for 50 days at 37 °C in the dark. Chemical shifts (ppm) for signal assignment of aldehydic groups (CHO-) were 9.75 ppm (signal A, triplet, n-alkenals), 9.54 ppm (signal B, doublet, 4,5-epoxy-(E)-2-alkenals), 9.52 ppm (signal C, doublet, (E,E)-2,4-alkadienals), and 9.48 ppm (signal D, doublet, (E)-2-alkenals), respectively
Fig. 4.
The expanded regions of primary oxidation products (conjugated diene groups) in the 1H NMR spectra of 10% O/W emulsions added with different antioxidants. O/W emulsions were prepared with 200 ppm of ferulic acid (FA), 4-vinylguaiacol (4-VG), and catechin, respectively. Oxidation study was performed for 50 days at 37 °C in the dark. Conjugated diene groups were Z,E-conjugated form [multiplet, E = 6.55 ppm (CH = CH − CH = CH), H = 6.00 ppm, J = 5.55 ppm, K = 5.50 ppm] and E,E-conjugated form [multiplet, F = 6.25 ppm (CH = CH − CH = CH), G = 6.05 ppm, I = 5.75 ppm, L = 5.45 ppm), respectively
In the previous study, ferulic acid and sinapic acid showed antioxidative activity in the emulsified methyl linoleate, in which both of them are methoxylated phenolic acid (Pekkarinen et al., 1999). Similarly, ferulic acid improved the oxidation stability in the corn oil-in-water emulsion when Triton X-100 used as an emulsifier. Such antioxidative activity resulted from the hydroxyl and methoxyl groups of molecules, providing electron donation (Chen and Ho, 1997). Along with structure, polarity or solubility of molecule should be considered because location of the molecule in the O/W emulsion system [i.e., at the dispersed (oil) or continues (water) phase or interface] affect its antioxidant activity. When ferulic acid is decarboxylated, its partition coefficient (log P) is changed. The log P value of 4-VG is known as 2.4 while that of ferulic acid is 1.5. Such difference resulted in change of polarity and thus affinity to location in the O/W emulsion system (Nakayama et al., 1998; Terpinc et al., 2011).
In Fig. 5, the emulsions containing any antioxidant (4-VG, ferulic acid, and catechin) slowly decreased in C18:2 and C18:3 levels. After 50 days of oxidation, the content of C18:2 was not changed with 4-VG. However, 3.6, and 9.7% of C18:2 were lost in the emulsions containing ferulic acid and catechin, respectively. Similar trend of decrease in C18:3 was observed. Only 0.2% of C18:3 decreased in the emulsion containing 4-VG, while 1.9 (control), 0.6 (ferulic acid), and 1.2% (catechin) of C18:3 were degraded after 50 days of oxidation. Change of fatty acid composition also showed that 4-VG was more resistant to oxidation of O/W emulsion than other treatments.
Fig. 5.
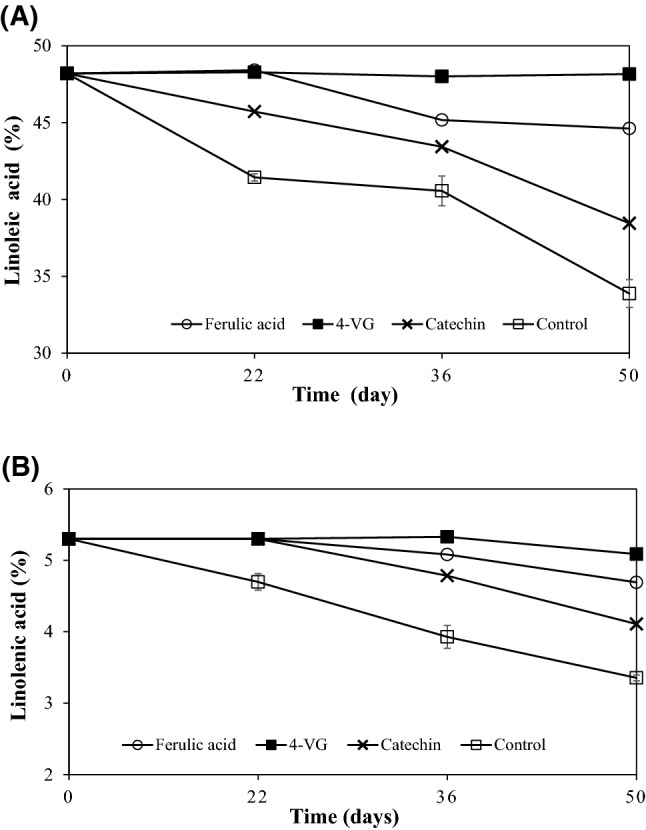
Change of linoleic acid (A) and linolenic acid (B) levels in 10% O/W emulsions added with different antioxidants. O/W emulsions were prepared with 200 ppm of ferulic acid, 4-vinylguaiacol (4-VG), and catechin, respectively
In conclusion, the antioxidant capacity of catechin, ferulic acid, and decarboxylated ferulic acid (4-vinylguaiacol, 4-VG) were investigated, wherein 200 ppm of each was added to a 10% O/W emulsion, respectively. Oxidation was monitored during 50 days. The POV, conjugated form, and aldehyde results showed that the antioxidant capacity of ferulic acid and 4-VG in the emulsion was better than catechin. Particularly, 4-VG showed excellent antioxidant capacity on O/W emulsion. These results could be due to the compound structure and relative solubility in O/W emulsion. Decarboxylated ferulic acid, 4-VG, possessed relatively non-polar characteristics compared to native form of ferulic acid; however, a polar hydroxyl group was still present, and might have been responsible for the high antioxidant capacity in the O/W emulsion.
Acknowledgements
We wish to thank for technical assistance related to 1H NMR in CNU laboratory.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Jung-Ah Shin and Sang-Hwa Jeong are co-first authors, who contribute equally to this study.
References
- Chen JH, Ho CT. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J. Agric. Food Chem. 1997;45:2374–2378. doi: 10.1021/jf970055t. [DOI] [Google Scholar]
- Falch E, Anthonsen HW, Axelson DE, Aursand M. Correlation between 1H NMR and traditional methods for determining lipid oxidation of ethyl docosahexaenoate. J. Am. Oil Chem. Soc. 2004;81:1105–1110. doi: 10.1007/s11746-004-1025-1. [DOI] [Google Scholar]
- Fujioka K, Shibamoto T. Quantitation of volatiles and nonvolatile acids in an extract from coffee beverages: correlation with antioxidant activity. J. Agric. Food Chem. 2006;54:6054–6058. doi: 10.1021/jf060460x. [DOI] [PubMed] [Google Scholar]
- Guillen MD, Goicoechea E. Oxidation of corn oil at room temperature: Primary and secondary oxidation products and determination of their concentration in the oil liquid matrix from 1H nuclear magnetic resonance data. Food Chem. 2009;116:183–192. doi: 10.1016/j.foodchem.2009.02.029. [DOI] [Google Scholar]
- Guillen MD, Ruiz A. Monitoring the oxidation of unsaturated oils and formation of oxygenated aldehydes by proton NMR. Eur. J. Lipid Sci. Technol. 2005;107:36–47. doi: 10.1002/ejlt.200401056. [DOI] [Google Scholar]
- Jung MY, Min DB. Effects of α-, γ-, and δ-tocopherols on oxidative stability of soybean oil. J. Food Sci. 1990;55:1464–1465. doi: 10.1111/j.1365-2621.1990.tb03960.x. [DOI] [Google Scholar]
- Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002;50:2161–2168. doi: 10.1021/jf011348w. [DOI] [PubMed] [Google Scholar]
- Knothe G, Kenar JA. Determination of the fatty acid profile by 1H-NMR spectroscopy. Eur. J. Lipid Sci. Technol. 2004;106:88–96. doi: 10.1002/ejlt.200300880. [DOI] [Google Scholar]
- McClements DJ, Decker EA. Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000;65:1270–1282. doi: 10.1111/j.1365-2621.2000.tb10596.x. [DOI] [Google Scholar]
- Mei L, McClements DJ, Decker EA. Lipid oxidation in emulsions as affected by charge status of antioxidants and emulsion droplets. J. Agric. Food Chem. 1999;47:2267–2273. doi: 10.1021/jf980955p. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Ono K, Hashimoto K. Affinity of antioxidative polyphenols for lipid bilayers evaluated with a liposome system. Biosci. Biotechnol. Biochem. 1998;62:1005–1007. doi: 10.1271/bbb.62.1005. [DOI] [PubMed] [Google Scholar]
- Nenadis N, Zhang HY, Tsimidou MZ. Structure-antioxidant activity relationship of ferulic acid derivatives: effect of carbon side chain characteristic groups. J. Agric. Food Chem. 2003;51:1874–1879. doi: 10.1021/jf0261452. [DOI] [PubMed] [Google Scholar]
- Pekkarinen SS, Stöckmann H, Schwarz K, Heinonen IM, Hopia AI. Antioxidant activity and partitioning of phenolic acids in bulk and emulsified methyl linoleate. J. Agric. Food Chem. 1999;47:3036–3043. doi: 10.1021/jf9813236. [DOI] [PubMed] [Google Scholar]
- Sacchi R, Patumi M, Fotanazza G, Barone P, Fiordiponti P, Mannina L, Rossi E, Segre AL. A high-field 1H nuclear magnetic resonance study of the minor components in virgin olive oils. J. Am. Oil Chem. Soc. 1996;73:747–758. doi: 10.1007/BF02517951. [DOI] [Google Scholar]
- Srinivasan M, Sudheer AR, Pillai KR, Kumar PR, Sudhakaran PR, Menon VP. Influence of ferulic acid on gamma-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture is isolated rat hepatocytes. Toxicology. 2006;228:249–258. doi: 10.1016/j.tox.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Terpinc P, Polak T, Šegatin N, Hanzlowsky A, Ulrih NP, Abramovič H. Antioxidant properties of 4-vinyl derivatives of hydroxycinnamic acids. Food Chem. 2011;128:62–69. doi: 10.1016/j.foodchem.2011.02.077. [DOI] [PubMed] [Google Scholar]
- Tilay A, Bule M, Kishenkumar J, Annapure U. Preparation of ferulic acid from agricultural wastes: its improved extraction and purification. J. Agric. Food Chem. 2008;56:7644–7648. doi: 10.1021/jf801536t. [DOI] [PubMed] [Google Scholar]
- Vuorela S, Meyer AS, Heinonen M. Quantitative analysis of the main phenolics in rapeseed meal and oils processed differently using enzymatic hydrolysis and HPLC. Eur. Food Res. Technol. 2003;217:517–523. doi: 10.1007/s00217-003-0811-3. [DOI] [Google Scholar]
- Wang XY, Yang D, Zhang H, Jia CH, Shin JA, Hong ST, Lee YH, Jang YS, Lee KT. Antioxidant activity of soybean oil containing 4-vinylsyringol obtained from decarboxylated sinapic acid. J. Am. Oil Chem. Soc. 2014;91:1543–1550. doi: 10.1007/s11746-014-2492-4. [DOI] [Google Scholar]
- Yang D, Gan LJ, Shin JA, Kim S, Hong ST, Park SH, Lee JH, Lee KT. Antioxidative activities of Gikgo biloba extract on oil/water emulsion system prepared from an enzymatically modified lipid containing alpha-linolenic acid. J. Food Sci. 2013;78:43–49. doi: 10.1111/j.1750-3841.2012.03010.x. [DOI] [PubMed] [Google Scholar]