Abstract
Amylosucrases (ASase, EC 2.4.1.4) from Deinococcus geothermalis (DGAS) and Neisseria polysaccharea (NPAS) were heterologously expressed in Bacillus subtilis. While DGAS was successfully expressed, NPAS was not. Instead, NPAS was expressed in Escherichia coli. Recombinant DGAS and NPAS were purified using nickel-charged affinity chromatography and employed to modify daidzin to enhance its water solubility and bioavailability. Analyses by LC/MS revealed that the major products of transglycosylation using DGAS were daidzein diglucoside and daidzein triglucoside, whereas that obtained by NPAS was only daidzein diglucoside. The optimal bioconversion conditions for daidzein triglucoside, which was predicted to have the highest water-solubility among the daidzin derivatives, was determined to be 4% (w/v) sucrose and 250 mg/L daidzin in sodium phosphate pH 7.0, with a reaction time of 12 h. Taken together, we suggest that the yield and product specificity of isoflavone daidzin transglycosylation may be modulated by the source of ASase and reaction conditions.
Keywords: Amylosucrase, Deinococcus geothermalis, Daidzin, Transglycosylation, Bacillus subtilis
Introduction
Phytochemicals, plant chemicals with bio-activities, are being widely examined for their abilities to provide health benefits (Dillard and German, 2000). Among the phytochemicals, flavonoids are the most abundant secondary metabolites in plants and are classified as flavones, flavonols, flavan-3-ols, isoflavones, flavanones, and anthocyanidins (Plaza et al., 2014). The basic structure of flavonoids can have diverse substituents, and most flavonoids exist naturally as glycosides rather than aglycones. The most common flavonoid structures in plants are flavone O/C-glycosides and flavonol O-glycosides (Iwashina, 2014). Isoflavones, a type of polyphenol naturally-occurring plant compounds, are frequently found in the family Fabaceae (i.e. Leguminosae), which includes soybeans (Ko, 2014; Kulkarni et al., 2016; Thrane et al., 2017). They are differentiated by their substituents including methoxy, hydroxyl, and glycoside groups attached to the primary structure (Delmonte et al., 2006). Isoflavones to are known to be beneficial to human health and are well known as phytoestrogens because they have structural similarity to 17-β-estradiol, the major female sex hormone secreted by the premenopausal ovary (Ko, 2014; Lee et al., 2008; Vacek et al., 2008). In addition, isoflavones showed various positive biological activities such as anti-cancer, anti-inflammatory, neuroprotective, and anti-carcinogenic activities, and protective activity against bone loss, cardiovascular diseases, and autoimmune diseases (Shimoda and Hamada, 2010b). However, they have several drawbacks including water-insolubility and low absorbability to be applied in food and pharmaceutical industries (Park et al., 2011; Shimoda et al., 2011). Therefore, the use of isoflavones as functional food ingredients and cosmetic constituents has been limited (Ko, 2014; Shimoda and Hamada, 2010b).
Glycosylation, the reaction of adding a glycosyl group to the functional group of another molecule, is an important method to modify the diverse chemical properties of phenolic phytochemicals (Lundemo, 2015). It has been reported that glycosyl conjugation was much more effective than cyclodextrin complexation to improve the water-solubility of hydrophobic compounds (Shimoda and Hamada, 2010a). Recently, glycosylation has been achieved using various enzymes from microbial sources rather using the high temperatures and pressures required for chemical glycosylation (Park et al., 2011). For example, puerarin transglycosylation reacted with maltogenic amylase from Geobacillus stearothermophilus resulted in the highly water-soluble puerarin derivatives α-d-glucosyl-(1 → 6)-puerarin and α-maltosyl-(1 → 6)-puerarin (Li et al., 2004b). Similarly, glycosyltransferase from B. cereus glycosylated apigenin, genistein, kaempferol, luteolin, naringenin, and quercetin by transferring a glucose unit onto either the 3 or 7 hydroxyl group (Ko et al., 2006).
Amylosucrase (ASase, EC 2.4.1.4) is a glucosyltransferase belonging to GH family 13 (GH13). ASase synthesizes α-1,4-glycosidic linkages to make a linear glucan polymer from sucrose as the sole substrate (Seo et al., 2009). Other than polymerization, ASase has the ability to perform both hydrolysis and isomerization reactions (Kim et al., 2014; Seo et al., 2009), which are well coordinated in a single enzyme. ASase catalyzes sucrose hydrolysis resulting in release of glucose and fructose, polymerization of α-1,4-oligosaccharide with using the released glucose as an acceptor, and production of sucrose isomers such as turanose and trehalulose using the released fructose as an acceptor (Seo et al., 2016). Recently, it was found that ASase could efficiently and selectively transfer the released glucose from sucrose hydrolysis to exogenously added biomolecules. ASase from Alteromonas macleodii was employed to transfer glucose to piceid, which generated glucosyl-α-(1 → 4)-piceid, a compound having 1.14 times higher water-solubility than piceid (Park et al., 2012). ASase from D. geothermalis (DGAS) was used for modifying (+)-catechin to various transformed (+)-catechin glycosides to improve water-solubility (Cho et al., 2011).
In this study, the heterologous expression of DGAS and ASases from N. polysaccharea (NPAS) was performed in B. subtilis, a generally recognized as safe (GRAS) microorganism. The recombinant ASases were employed to modify daidzin, one of the major isoflavones present in soybeans and most non-fermented soy foods. The product specificity of the recombinant DGAS expressed in B. subtilis was compared with NPAS expressed in E. coli. The molecular masses of the reaction products were determined, and the optimal conditions for the glycosylation reaction were examined.
Materials and methods
Chemicals
Sucrose and daidzin were purchased from Duchefa Biochemistry (Haarlem, The Netherlands) and Extrasynthese (Genay, France), respectively. Both dimethyl sulfoxide (DMSO) and methanol used for inactivation of enzyme were obtained from Daejung (Siheung, Korea). All other chemicals used in this study were analytical reagent grade and purchased from Sigma-Aldrich (St. Louis, MO, USA).
Bacterial strains, genes, culture conditions, and enzymes
The ASase genes corresponding to DGAS and NPAS were described previously (De Montalk et al., 1999; Seo et al., 2008; Seo et al., 2016). These genes originated from the genome of D. geothermalis DSM 11300 and N. polysaccharea ATCC 43768, respectively. Bacillus subtilis KCTC3135 was used for the expression of dgas and npas genes. E. coli DH10B and MC1061 were used for gene manipulation. The E. coli BL21-CodonPlus(DE3)-RP strain was selected for heterogeneous gene expression of npas.
Recombinant B. subtilis cells were grown in Luria–Bertani (LB) medium (BD, Franklin Lakes, NJ, USA) containing 1% (w/v) Bacto™ tryptone, 0.5% (w/v) yeast extract, and 0.5% (w/v) NaCl, supplemented with kanamycin (10 mg/mL), whereas recombinant E. coli cells were grown in LB medium supplemented with ampicillin (0.1 mg/mL). Restriction endonuclease, dephosphorylase, and T4 ligase were purchased from New England Biolabs (Beverly, MA, USA).
Construction and transformation of pUBRTAMY-DGAS pUBRTAMY-NPAS into B. subtilis
pUBRTAMY, a B. subtilis-E. coli shuttle vector (Choi et al., 2010), was prepared using the Plus Plasmid Mini Kit (NucleoGen, Seoul, Korea). pHC-DGAS harboring the dgas gene was digested with NdeI/XhoI to obtain the intact dgas gene (Seo et al., 2012). Also, pUBRTAMY was prepared for ligation by digesting with NdeI/XhoI followed by treatment with dephosphorylase. Finally, the dgas gene was ligated into the treated pUBRTAMY to construct the final expression plasmid, pUBRTAMY-DGAS. The procedure for the construction of pUBRTAMY-NPAS was same as that of pUBRTAMY-DGAS except the npas gene was obtained from pRSET-NPAS (Jung et al., 2009).
To transform pUBRTAMY-DGAS and pUBRTAMY-NPAS into B. subtilis, B. subtilis competent cells were prepared by inoculating 3 mL of a B. subtilis culture grown overnight in LB medium into 30 mL SPI medium [chemically defined medium containing 15 mM (NH4)2SO4, 80 mM K2HPO4, 40 mM KH2PO4, 3.5 mM trisodium citrate, 0.8 mM MgSO4, 0.02% (w/v), casamino acids, 0.4% (w/v) yeast extract, 0.5% (w/v) glucose] and growing at 37 °C until the end of logarithmic growth [0.15-0.30 of optical density (OD) at 600 nm]; 3 mL of this culture were added to 27 mL of pre-warmed SPII (SPI medium supplemented with 5.6 mg CaCl2 and. 50.8 mg MgCl2 per liter) medium and incubated at 37 °C for 90 min with slow agitation. After incubation, the culture was kept on ice and harvested by centrifugation (5400 × g for 10 min at 4 °C). Finally, the supernatant was carefully decanted, and approximately 10 mL of the SPII medium was saved. The cell pellet was gently resuspended in the remaining medium, and 1 mL of sterile glycerol was added. Aliquots (0.3 mL) of competent cells were transferred into sterile tubes and stored at − 70 °C prior to use. For transformation, the stored competent cells were warmed at 42 °C. Next, 1.2 mL pre-warmed SPII medium and 1/100 volume of ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid solution (1.9 g/50 mL) were added to the heated culture, and the culture was incubated at 37 °C for 10 min. The cells were centrifuged (5400 × g for 2 min at 4 °C), and the pellet was suspended in 250 μL of the supernatant. The prepared plasmids were added in the culture and incubated for 20 min at 37 °C without shaking. One milliliter of LB was added to the culture, and the culture was incubated with shaking slowly at 37 °C for 90 min. The cells were centrifuged (5400 × g for 2 min at 4 °C) and resuspended in 100 μL of the supernatant. Finally, the cells were spread on an LB agar plate containing 10 mg/mL kanamycin. The plate was incubated at 37 °C for approximately 12 h until colonies appeared. The transformants were selected and confirmed by assaying their ASase activities.
Expression of DGAS and NPAS
Transformed E. coli containing pRSET-NPAS were induced at an OD600 of 0.5 with 1 mM IPTG at 18 °C for 18 h. After induction, cells were harvested by centrifugation (Hanil Combi 514R; Hanil Centrifuge Co., Gimpo, Korea) at 7000 × g for 20 min, and the supernatant was discarded. The pellet was suspended in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, and 10 mM imidazole, pH 7.5) followed by disruption with ultrasonication (Sonifier 450, Branson, Danbury, CT, USA; output 4, 6 × 10 s, constant duty) in an ice bath. The recombinant enzymes in crude cell extract were purified using a nickel-nitrilotriacetic acid (Ni–NTA) affinity column. The columns were Poly-prep chromatography columns (Bio-Rad Laboratories, Inc., Hercules, CA, USA) filled with Ni–NTA Superflow (Qiagen, Hilden, Germany). The expression of transformed B. subtilis harboring pUBRTAMY-DGAS was the same as that of transformed E. coli without the IPTG induction step. Purified enzymes were confirmed using SDS-PAGE (10% acrylamide), and protein concentration was measured using the bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA) with bovine serum albumin as the standard.
SDS-PAGE analysis
Purification of expressed enzymes was confirmed by SDS-PAGE. The resolving gel was prepared as 10% polyacrylamide gel containing 1.9 mL of water, 1.7 mL of acrylamide solution [29.2% (w/v) acrylamide and 0.8% (w/v) N,N′-methylene bis-acrylamide], 1.3 mL of 1.5 M Tris–HCl buffer (pH 8.8), 0.05 mL of 10% (w/v) SDS solution, 0.05 mL of 10% (w/v) ammonium persulfate, and 0.002 mL of N,N,N′,N′-tetramethylethylenediamine (TEMED). The stacking gel was prepared as 5% polyacrylamide gel containing 0.68 mL of water, 0.17 mL of acrylamide solution, 0.13 mL of 1 M Tris–HCl buffer (pH 6.8), 0.01 mL of 10% (w/v) SDS solution, 0.01 mL of 10% (w/v) ammonium persulfate, and 1 μL of TEMED.
Determination of DGAS and NPAS activities
The temperature and pH profiles of the recombinant DGAS in B. subtilis were investigated. The effect of pH was determined at various pH values, from 4.0 to 10.0, using 50 mM sodium acetate (pH 4–5), 50 mM sodium citrate (pH 5–6), 50 mM sodium phosphate (pH 6–8), 50 mM Tris–HCl (pH 7–9), or 50 mM glycine–NaOH (pH 9–10). The optimal temperature was determined in 50 mM Tris–HCl (pH 8.0) at ranging from 30 to 55 °C.
The activities of concentrated enzymes were measured by determining sucrose hydrolysis activity using the 3,5-dinitrosalicylic acid (DNS) solution. The reaction mixture (500 μL) was composed of 50 mM buffer, 5% (w/v) sucrose, and 2 units/mL of the individual enzyme. The reaction conditions for each enzyme were slightly different. The DGAS reaction was performed in Tris–HCl (pH 8.0) buffer at 45 °C, whereas the NPAS reaction was carried out in Tris–HCl (pH 7.0) buffer at 35 °C. After preheating for 5 min, the reaction was started by adding 10% (v/v) concentrated enzymes and incubated for 10 min. To finish the reaction, 500 μL DNS solution was added to the mixture. After boiling for 5 min, the OD of the final reaction mixture was measured at 550 nm using a microplate reader (iMark™ Microplate Absorbance Reader, Bio-Rad Laboratories, Inc.). The measured value was inserted to the fructose standard curve for calculating the reducing sugar concentration. One unit was defined as the amount of enzyme that produced 1 μmol of fructose per min under the assay conditions.
Transglycosylation reaction of daidzin
The transglycosylation reactions for DGAS and NPAS were composed of sucrose as a donor and daidzin as an acceptor in 50 mM buffer and 10% (v/v) DMSO. Sucrose was used at concentrations of 0.5, 1, 2, 4, and 8% (w/v), whereas daidzin was used at 250, 500, and 1000 mg/L. The reaction temperatures of DGAS and NPAS were 45 and 35 °C, respectively. The DGAS reaction was performed in Tris–HCl (pH 8.0) buffer, whereas the NPAS reaction was carried out in Tris–HCl (pH 7.0) buffer. The reaction conditions (temperature, buffer, and pH) for DGAS and NPAS were followed the previous reports (Jung et al., 2009; Kim et al., 2016). The reaction was started by adding one unit of enzyme in the mixture and continued for 24 h. The reaction was stopped by adding the same amount of an inactivation mixture composed of 10% (v/v) DMSO and 90% (v/v) methanol. After vigorous mixing, the mixture was centrifuged (10,000 × g) for 2 min at 4 °C. The supernatant was poured carefully to a new microtube. The final sample was stored in a freezer until analysis was performed.
HPLC analysis
For the HPLC analysis, the reaction mixtures were centrifuged and filtered through a 0.2 μm Nylon 66 syringe filter (Whatman, GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). Samples were analyzed using a Waters alliance 2998 quaternary pump (Waters, Milford, MA, USA) equipped with a ProntoSIL C18 column (120 Å, 5 μm, 4.6 × 250 mm; Bischoff, Leonberg, Germany) and a photo diode array detector (Waters) at 254 nm. Gradient elution was carried out to separate compounds in the reaction mixture.
A linear solvent gradient of binary mobile phase with solvent A [0.1% (v/v) formic acid in deionized water] and solvent B [0.1% (v/v) formic acid in acetonitrile] was applied as follows: 98% A/2% B at 0 min, 98% A/2% B at 0.5 min, 95% A/5% B at 5.5 min, 88% A/12% B at 6.5 min, 75% A/25% B at 21.5 min, 40% A/60% B at 41.5 min, and 98% A/2% B at 42 min. The flow rate of the mobile phase was 1.0 mL/min with an injection volume of 5 μL. All solvents were filtered, degassed, and kept under pressure.
LC/MS analysis
The HPLC conditions for LC/MS were the same as for HPLC analysis described above. An ACQUITY QDa™ Detector (Waters) was used to obtain the MS data. QDa parameters were as follows: capillary voltage, 0.8 kV; cone voltage, 30 V; source temperature, 600 °C; desolvation gas flow, 800 L/h. Full-scan data acquisition was performed, scanning from m/z 100 to 1200 in profile mode. Empower 3 (Waters) was used to control LC-QDa system and analyze data obtained.
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test. Differences were considered significant at p ≤ 0.05.
Results and discussion
DGAS expression in Bacillus subtilis
Bacillus subtilis is a rod-shaped Gram positive bacterium used in the production of many industrial enzymes and fine biological compounds such as vitamin B2 and nucleotides. It is considered as one of the most popular host systems for foreign protein expression since it has a high level of safety with a GRAS status as well as excellent fermentation characteristics and relatively simple genetic operation approaches and tools (Westers et al., 2004).
Two ASase genes (dgas and npas) were heterologously expressed in B. subtilis. The B. subtilis expression system (pUBRTAMY) used in this study contained the promoter from the gene encoding an α-amylase from B. subtilis, NA64 (amyR2), a six-histidine tag sequence, and the T7 transcriptional terminator (Choi et al., 2010). The successful transformation of the two shuttle vectors (pUBRTAMY-DGAS and pUBRTAMY-NPAS) into B. subtilis was confirmed by detecting the dgas and npas genes in the transformed cells. However, the ASase activity was observed only in cell extracts of pUBRTAMY-DGAS transformed cells and not in the cell extracts of pUBRTAMY-NPAS transformed cells. These results indicated that only DGAS could be expressed as a functional protein in B. subtilis while NPAS could not be, even though they exhibit fairly high amino acid sequence similarity (58.7%). There are several possible reasons for the failure of heterologous protein expression such as codon bias, protein toxicity, and incomplete folding. Since DGAS was not harmful to B. subtilis, protein toxicity is likely not the case. To understand the correct cause for unsuccessful expression, further studies are required.
Since the signal peptide was not applied during expression, the recombinant enzymes were found inside of the cells. Thus, recombinant DGAS was homogeneously purified from cell crude extract using a Ni–NTA affinity chromatography (Fig. 1). The recombinant DGAS fused with the 6 × His tag showed a single band with a molecular mass of about 73 kDa, which was in good agreement with the deduced molecular mass of DGAS (Fig. 1). The temperature and pH profiles of the recombinant DGAS in B. subtilis were investigated in the range of 30–55 °C and pH 4.0–10.0 and compared with recombinant DGAS expressed in E. coli (Seo et al., 2008; Seo et al., 2016); the optimal temperature and pH were observed to be 50 °C and pH 8.0, respectively (Fig. 2). This result indicated that optimal temperature and pH of both recombinant DGAS were the same (data not shown). To our knowledge, this is the first report to express ASase in a heterologous host other than E. coli. The recombinant DGAS expressed in B. subtilis and recombinant NPAS expressed in E. coli after Ni–NTA affinity purification were used in subsequent experiments.
Fig. 1.
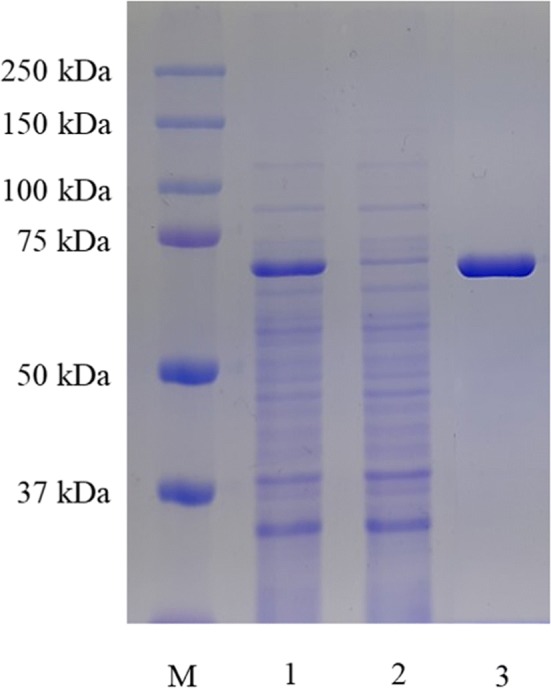
SDS-PAGE analysis of recombinant DGAS expressed in B. subtilis and purified on a Ni–NTA affinity column. Lane M, protein molecular standard marker; Lane 1, crude enzyme; Lane 2, Crude passing through enzyme; Lane 3, purified enzyme
Fig. 2.
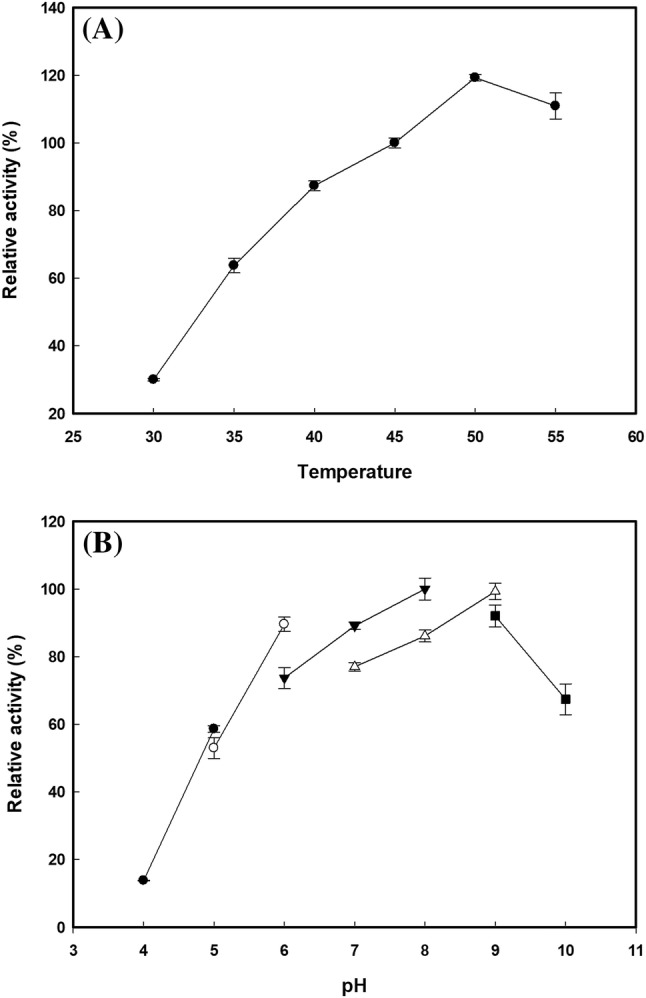
Characterization of the optimal (A) temperature and (B) pH for activity of DGAS expressed in B. subtilis. (A) A temperature range of 30–55 °C was investigated (B) The following buffers and pHs were examined at 50 °C: Filled circle, sodium acetate pH 4.0–5.0; Open circle, sodium citrate pH 5.0–6.0; Filled inverted triangle, sodium phosphate pH 6.0–8.0; Open triangle, Tris–HCl pH 7.0–9.0; and Filled square, glycine–NaOH pH 9.0–10.0
Comparison of daidzin transglycosylation by recombinant DGAS and NPAS
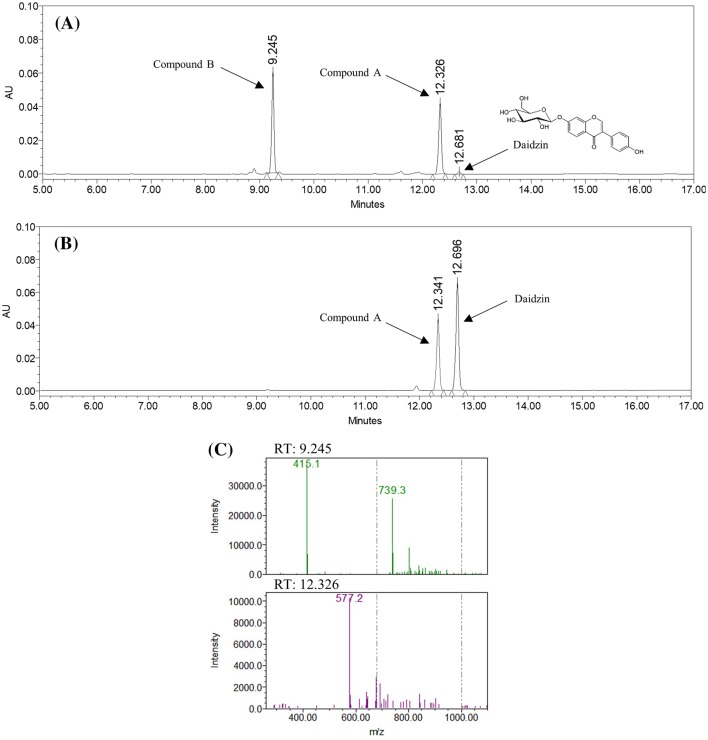
Daidzin, a natural phytochemical shown to be active in the treatment of alcohol dependency (antidipsotropic) (Rezvani et al., 2003), was reacted with both recombinant DGAS and NPAS. HPLC analysis revealed that reaction with NPAS generated a single reaction product, whereas reaction with DGAS created two products (Fig. 3A, B). One of the products [compound A; retention time (RT) = 12.3] that appeared in the NPAS reaction was also observed in the DGAS reaction indicating that this product might be the same product. The other peak (compound B; RT = 9.2) observed in the DGAS reaction had a more hydrophilic nature than compound A, since compound B eluted earlier than compound A in the condition applied in this study.
Fig. 3.
HPLC chromatograms at 254 nm of transglycosylation products: (A) DGAS and (B) NPAS reacted with daidzin (RT 12.7) and sucrose as an acceptor and a donor, respectively. (C) HPLC-QDa Mass analysis of the reaction mixture with DGAS
The conversion yield of DGAS was much higher than that of NPAS. Almost all of the daidzin (99%) was modified to its glucoside derivatives (daidzein diglucoside and triglucoside), whereas only approximately 45% of daidzin was converted to daidzein diglucoside. Even though a direct comparison was unsuitable since the optimal reaction conditions were different, this result suggested that DGAS was more efficient than NPAS in modifying an acceptor molecule under these conditions.
Molecular masses of reaction products were determined by liquid chromatography/mass spectrometry (LC/MS) using ACQUITY QDa detector. Figure 3(C) shows the MS spectra peaks corresponding to the peaks at RT 9.2 and 12.3 min in HPLC analysis. The 577.2 m/z peak at RT 12.3 HPLC peak is [M–H]− in accordance with the characterized m/z values of the anion of daidzein diglucoside [molecular mass (M) = 578). In addition, two peaks (415.1 and 739.3 m/z) at 9.2 min HPLC peak were [M–H]−, corresponding to the calculated molecular masses of the anions daidzin (M = 416) and daidzein triglucoside (M = 740), respectively. This result confirmed compound B as a daidzein triglucoside.
In general, upon glycosylation of the aglycone of hydrophobic biomaterials, the water solubility increases, and, thereby, its industrial applications are broadened. Several previous studies on the daidzein glycosides revealed that the more glucosides were transferred to an acceptor molecule, the more water-soluble synthetic product was produced (Li et al., 2004a; Shimoda et al., 2011). For example, the daidzein trisaccharide, daidzein-7-O-[6-O-(4-O-(β-d-xylopyranosyl))-β-d-xylopyranosyl]-β-d-glucopyranoside synthesized by Catharant husroseus and catalyzed by β-xylosidase from Aspergillus sp. is 850 times more water-soluble than daidzein (Shimoda et al., 2011). Similarly, daidzein-7-O-triglucoside synthesized by Thermotoga maritima maltosyltransferase is 7.5 × 104 more water-soluble than daidzin (Li et al., 2004a). Therefore, we inferred that the modification of daidzin by DGAS and NPAS results in highly soluble daidzin glycosides.
ASase has been applied to modify various bioactive compounds in their aglycone or glycone forms (Kim et al., 2016; Park et al., 2012), and among the ASases, DGAS and NPAS are the most popular enzymes to be used for this purpose (Cho et al., 2011; Jung et al., 2009; Park et al., 2011; Seo et al., 2009). Even though the enzymatic activities of DGAS and NPAS are very similar, their transglycosylation reaction products are somewhat different. When DGAS and NPAS were applied to the synthesis of salicin glycosides, DGAS specifically synthesized only one salicin transglycosylation product (glucosyl salicin), whereas NPAS produced two salicin glycoside transfer products (glucosyl salicin and maltosyl salicin) (Jung et al., 2009). Unlike salicin transglycosylation, DGAS produced more diverse transfer products than NPAS in daidzin transglycosylation, indicating that the donor molecule as well as the enzyme itself are highly involved in the product formation.
Reaction condition for DGAS transglycosylation
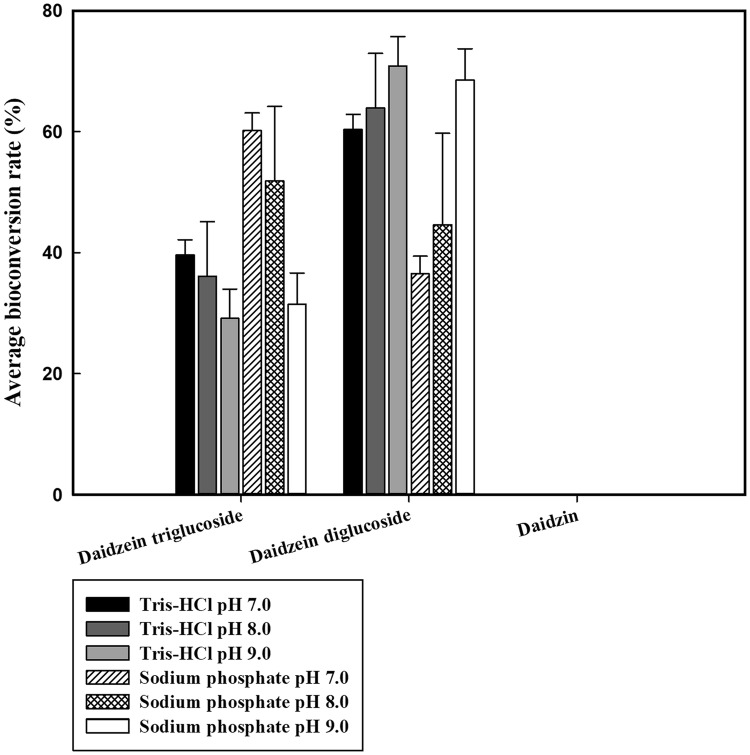
Since the yield of daidzin transglycosylation was exceptionally high in the DGAS reaction, the effect of reaction conditions for DGAS, including pH, buffer, reaction time, and acceptor and donor concentrations, on product formation was investigated. Under the standard conditions (500 mg/L of daidzin, 0.5% (w/v) of sucrose, 45 °C, and 24 h), almost of all substrates were converted to transglycosylation products (data not shown). With the increase of pH from 7.0 to 9.0, the production of compound A (daidzein diglucoside) gradually increased. The yield of compound B (daidzein triglucoside) was higher in sodium phosphate buffers (pH 7.0 and 8.0) (Fig. 4).
Fig. 4.
Effect of Tris–HCl and sodium phosphate on the bioconversion of daidzin. Data are expressed as mean ± standard deviation (SD), n = 3. The different low-case letter superscripts in the same column indicate significant differences in one-way ANOVA followed by Duncan’s multiple range test (p ≤ 0.05)
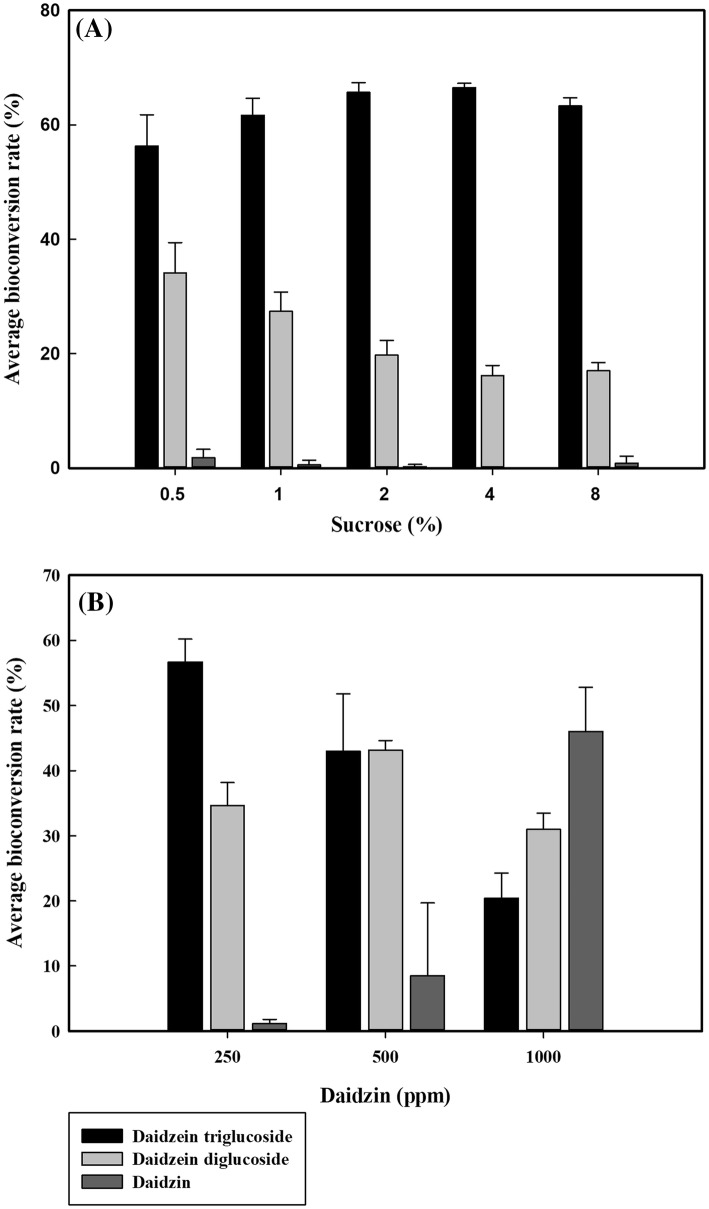
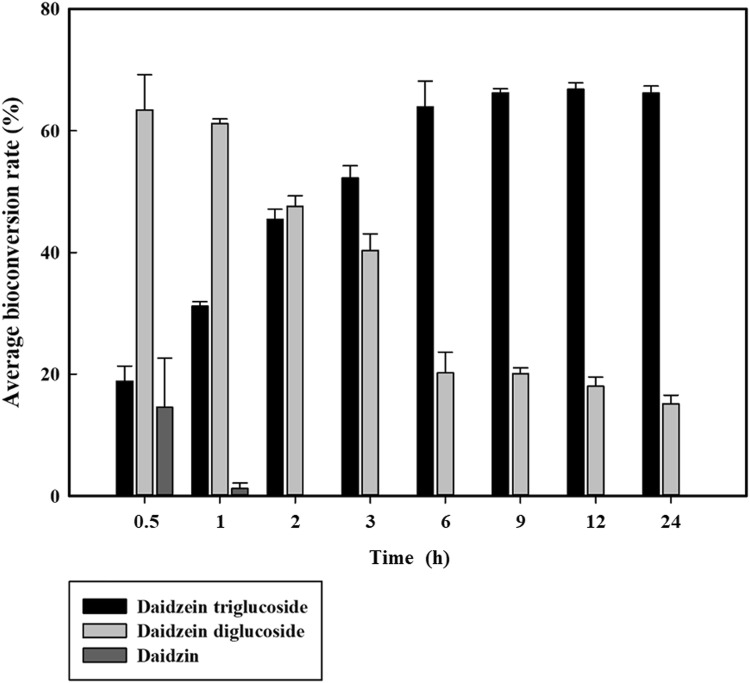
The increase of donor (sucrose) concentration up to 4% decreased the yield of daidzein diglucoside but increased the production of daidzein triglucoside (Fig. 5A). However, the increase of acceptor (daidzin) concentration up to 1000 mg/L reduced the conversion rate from 99 to 54% (Fig. 5B). These results indicated that the high donor/acceptor ratio led to the relatively high yield of daidzin transglycosylation. By 2 h after the start of the reaction, all of the daidzin were converted to either daidzein diglucoside or triglucoside. As the reaction time increased, the portion of daidzein triglucoside gradually increased and the portion of daidzein diglucoside diminished (Fig. 6).
Fig. 5.
Effect of substrate concentration of sucrose (A) and daidzin (B) on the bioconversion of daidzin. Data are expressed as mean ± standard deviation (SD), n = 3. The different low-case letter superscripts in the same column indicate significant differences in different sucrose concentrations with one-way ANOVA followed by Duncan’s multiple range test (P ≤ 0.05). The different capital letter superscripts in the same column indicate significant differences in different daidzin concentrations with one-way ANOVA followed by Duncan’s multiple range test (p ≤ 0.05)
Fig. 6.
Effect of reaction time on the bioconversion of daidzin. Data are expressed as mean ± standard deviation (SD), n = 3. The different low-case letter superscripts in the same column indicate significant differences in different sucrose concentrations using one-way ANOVA followed by Duncan’s multiple range test (p ≤ 0.05)
In general, it is well-known that the reaction conditions including pH and temperature are very important to the enzyme activity and stability (Polizzi et al., 2007). Daidzin transglycosylation performed with DGAS confirmed that the pH, buffer type, and donor/acceptor ratio, could modulate the yield and type of the transglycosylation products. In a study on sucrose isomerase (SI) from Pantoea dispersa UQ68 J and Klebsiella planticola UQ14S, researchers showed that the yield and ratio of sucrose isomer products (isomaltulose and trehalulose) were significantly changed by reaction temperatures and pHs (Wu and Birch, 2004; Wu and Birch, 2005). Several reports revealed that the donor and acceptor concentrations are closely related to the yield and ratio of transglycosylation products in ASase reactions. Typically, the increase of donor/acceptor ratio resulted in relatively high yield and varied product ratios (Cho et al., 2011; Jung et al., 2009; Seo et al., 2012). These results suggested that it is strongly possible to modulate the product ratios in the DGAS transglycosylation reaction and to produce a specific target product by controlling the reaction conditions.
In conclusion, flavonoids such as daidzin and its derivatives have generally been used as active ingredients in commercial products such as functional foods and cosmetics due to their proved functional properties and human bioavailability. Therefore, it is worthwhile to examine both functional properties, such as antioxidant and estrogenic activities, and bioavailability of daidzin glucosides produced by DGAS and NPAS for their possible uses as functional active ingredients. Also, the recombinant DGAS produced by GRAS level microorganisms can broaden the application area of bioactive functional glucosides such as daidzin glucosides.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MEST) (No. 2017R1A2B4004218).
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Choi CH, Kim SH, Jang JH, Park JT, Shim JH, Kim YW, Park KH. Enzymatic synthesis of glycosylated puerarin using maltogenic amylase from Bacillus stearothermophilus expressed in Bacillus subtilis. J. Sci. Food Agric. 2010;90:1179–1184. doi: 10.1002/jsfa.3945. [DOI] [PubMed] [Google Scholar]
- Cho HK, Kim HH, Seo DH, Jung JH, Park JH, Baek NI, Kim MJ, Yoo SH, Cha J, Kim YR. Biosynthesis of (+)-catechin glycosides using recombinant amylosucrase from Deinococcus geothermalis DSM 11300. Enzyme Microb. Technol. 2011;49:246–253. doi: 10.1016/j.enzmictec.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Delmonte P, Perry J, Rader JI. Determination of isoflavones in dietary supplements containing soy, red clover and kudzu: extraction followed by basic or acid hydrolysis. J. Chromatogr. A. 2006;1107:59–69. doi: 10.1016/j.chroma.2005.11.060. [DOI] [PubMed] [Google Scholar]
- De Montalk GP, Remaud-Simeon M, Willemot R, Planchot V, Monsan P. Sequence analysis of the gene encoding amylosucrase from Neisseria polysaccharea and characterization of the recombinant enzyme. J. Bacteriol. 1999;181:375–381. doi: 10.1128/JB.181.2.375-381.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard CJ, German JB. Phytochemicals: nutraceuticals and human health. J. Sci. Food Agric. 2000;80:1744–1756. doi: 10.1002/1097-0010(20000915)80:12<1744::AID-JSFA725>3.0.CO;2-W. [DOI] [Google Scholar]
- Iwashina T. The structure and distribution of the flavonoids in plants. J. Plant Res. 2014;113:287–299. doi: 10.1007/PL00013940. [DOI] [Google Scholar]
- Jung JH, Seo DH, Ha SJ, Song MC, Cha J, Yoo SH, Kim TJ, Baek NI, Baik MY, Park CS. Enzymatic synthesis of salicin glycosides through transglycosylation catalyzed by amylosucrases from Deinococcus geothermalis and Neisseria polysaccharea. Carbohydr. Res. 2009;344:1612–1619. doi: 10.1016/j.carres.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Kim MD, Jung DH, Seo DH, Jung JH, Seo EJ, Baek NI, Yoo SH, Park CS. Acceptor specificity of amylosucrase from Deinococcus radiopugnans and its application for synthesis of rutin derivatives. J. Microbiol. Biotechnol. 2016;26:1845–1854. doi: 10.4014/jmb.1606.06036. [DOI] [PubMed] [Google Scholar]
- Kim MD, Seo DH, Jung JH, Jung DH, Joe MH, Lim S, Lee JH, Park CS. Molecular cloning and expression of amylosucrase from highly radiation-resistant Deinococcus radiopugnans. Food Sci. Biotechnol. 2014;23:2007–2012. doi: 10.1007/s10068-014-0273-3. [DOI] [Google Scholar]
- Ko JH, Kim BG, Ahn JH. Glycosylation of flavonoids with a glycosyltransferase from Bacillus cereus. FEMS Microbiol. Lett. 2006;258:263–268. doi: 10.1111/j.1574-6968.2006.00226.x. [DOI] [PubMed] [Google Scholar]
- Ko KP. Isoflavones: chemistry, analysis, functions and effects on health and cancer. Asian Pac. J. Cancer Prev. 2014;15:7001–7010. doi: 10.7314/APJCP.2014.15.17.7001. [DOI] [PubMed] [Google Scholar]
- Kulkarni YA, Garud MS, Oza MJ, Barve KH, Gaikwad AB. Chapter 5—Diabetes, diabetic complications, and flavonoids A2. In: Watson R, Preedy VR, editors. Fruits, vegetables, and herbs. NY, USA: Academic Press Inc., Elsevier; 2016. pp. 77–104. [Google Scholar]
- Lee JH, Doo EH, Kwon DY, Park JB. Functionalization of isoflavones with enzymes. Food Sci. Biotechnol. 2008;17:228–233. [Google Scholar]
- Li D, Park JH, Park JT, Park CS, Park KH. Biotechnological production of highly soluble daidzein glycosides using Thermotoga maritima maltosyltransferase. J. Agric. Food Chem. 2004;52:2561–2567. doi: 10.1021/jf035109f. [DOI] [PubMed] [Google Scholar]
- Li D, Park SH, Shim JH, Lee HS, Tang SY, Park CS, Park KH. In vitro enzymatic modification of puerarin to puerarin glycosides by maltogenic amylase. Carbohydr. Res. 2004;339:2789–2797. doi: 10.1016/j.carres.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Lundemo P. Transglycosylation by glycoside hydrolases-production and modification of alkyl glycosides. PhD thesis, Lund University, Lund, Scania, Sweden (2015)
- Park HS, Choi KH, Park YD, Park CS, Cha JH. Enzymatic synthesis of polyphenol glycosides by amylosucrase. J. Life Sci. 2011;21:1631–1635. doi: 10.5352/JLS.2011.21.11.1631. [DOI] [Google Scholar]
- Park HS, Kim JE, Park JH, Baek NI, Park CS, Lee HS, Cha JH. Bioconversion of piceid to piceid glucoside using amylosucrase from Alteromonas macleodii deep ecotype. J. Microbiol. Biotechnol. 2012;22:1698–1704. doi: 10.4014/jmb.1208.08014. [DOI] [PubMed] [Google Scholar]
- Plaza M, Pozzo T, Liu J, Gulshan Ara KZ, Turner C, Nordberg Karlsson E. Substituent effects on in vitro antioxidizing properties, stability, and solubility in flavonoids. J. Agric. Food Chem. 2014;62:3321–3333. doi: 10.1021/jf405570u. [DOI] [PubMed] [Google Scholar]
- Polizzi M, Bommarius A, Broering J, Chaparro-Riggers J. Stability of biocatalysts. Curr. Opin. Chem. Biol. 2007;11:220–225. doi: 10.1016/j.cbpa.2007.01.685. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Perfumi M, Massi M. Plant derivatives in the treatment of alcohol dependency. Pharmacol. Biochem. Behav. 2003;75:593–606. doi: 10.1016/S0091-3057(03)00124-2. [DOI] [PubMed] [Google Scholar]
- Seo DH, Jung JH, Ha SJ, Song MC, Cha JH, Yoo SH, Kim TJ, Baek NI, Park CS. Highly selective biotransformation of arbutin to arbutin-α-glucoside using amylosucrase from Deinococcus geothermalis DSM 11300. J. Mol. Catal. B-Enzym. 2009;60:113–118. doi: 10.1016/j.molcatb.2009.04.006. [DOI] [Google Scholar]
- Seo DH, Jung JH, Ha SJ, Yoo SH, Kim TJ, Cha JH, Park CS. Molecular cloning of the amylosucrase gene from a moderate thermophilic bacterium Deinococcus geothermalis and analysis of its dual enzyme activity. In: Park KH, editor. Carbohydrate-active enzymes. NY, USA: Academic Press Inc., Elsevier; 2008. pp. 125–140. [Google Scholar]
- Seo DH, Jung JH, Ha SJ, Cho HK, Jung DH, Kim TJ, Baek NI, Yoo SH, Park CS. High-yield enzymatic bioconversion of hydroquinone to α-arbutin, a powerful skin lightening agnet, by amylosucrase. Appl. Microbiol. Biotechnol. 2012;94:1189–1197. doi: 10.1007/s00253-012-3905-7. [DOI] [PubMed] [Google Scholar]
- Seo DH, Jung JH, Jung DH, Park SY, Yoo SH, Kim YR, Park CS. An unusual chimeric amylosucrase generated by domain-swapping mutagenesis. Enzyme Microb. Technol. 2016;86:7–16. doi: 10.1016/j.enzmictec.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Shimoda K, Hamada H. Production of hesperetin glycosides by Xanthomonas campestris and cyclodextrin glucanotransferase and their anti-allergic activities. Nutrients. 2010;2:171–180. doi: 10.3390/nu2020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda K, Hamada H. Synthesis of β-maltooligosaccharides of glycitein and daidzein and their anti-oxidant and anti-allergic activities. Molecules. 2010;15:5153–5161. doi: 10.3390/molecules15085153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda K, Hamada H, Hamada H. Synthesis of xylooligosaccharides of daidzein and their anti-oxidant and anti-allergic activities. Int. J. Mol. Sci. 2011;12:5616–5625. doi: 10.3390/ijms12095616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrane M, Paulsen PV, Orcutt MW, Krieger TM. Chapter 2—Soy protein: impacts, production, and applications A2. In: Wanasundara JPD, Scanlin L, editors. Sustainable protein sources. NY, USA: Academic Press Inc., Elsevier; 2017. pp. 23–45. [Google Scholar]
- Vacek J, Klejdus B, Lojková L, Kubán V. Current trends in isolation, separation, determination and identification of isoflavones: a review. J. Sep. Sci. 2008;31:2054–2067. doi: 10.1002/jssc.200700569. [DOI] [PubMed] [Google Scholar]
- Westers L, Westers H, Quax WJ. Bacillus subtilis as cell factory for pharmaceutical proteins: a biotechnological approach to optimize the host organism. Biochim. Biophys. Acta-Mol. Cell. 2004;1694:299–310. doi: 10.1016/j.bbamcr.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Wu L, Birch RG. Characterization of Pantoea dispersa UQ68 J: producer of a highly efficient sucrose isomerase for isomaltulose biosynthesis. J. Appl. Microbiol. 2004;97:93–103. doi: 10.1111/j.1365-2672.2004.02274.x. [DOI] [PubMed] [Google Scholar]
- Wu L, Birch RG. Characterization of the highly effieient sucrose isomerase from Pantoea dispersa UQ68 J and cloning of the sucrose isomerase gene. Appl. Environ. Microbiol. 2005;71:1581–1590. doi: 10.1128/AEM.71.3.1581-1590.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]