Abstract
In this study, we examined the effects of the water extract of Neolentinus lepideus (WENL), an edible mushroom, on ethanol-induced hepatic lipid accumulation. Ethanol-induced oil red O-positive spots on AML-12 hepatocytes were attenuated by WENL treatment. Furthermore, the oral administration of WENL in acute and chronic ethanol-fed mouse models resulted in the decrease in blood triglyceride and the accumulation of lipid droplets in the liver. Interestingly, the transcriptional expression related to lipid metabolisms, such as sterol regulatory element-binding protein 1, and cytochrome P450 2E1, was decreased by WENL treatment in both ethanol-induced AML-12 hepatocytes and our chronic ethanol-fed mouse models. In addition, WENL effectively attenuated the ethanol induced activation of MAP kinases and NF-κB in AML-12 hepatocytes. Taken together, our results suggested that WENL can be effective in alleviating alcohol-induced hepatic lipid accumulation and may be used as potential candidate for the prevention of alcoholic fatty liver disease.
Keywords: Liver steatosis, Neolentinus lepideus, Hepatocytes cells, Fatty liver
Introduction
Alcohol-induced liver disease (ALD) is one of the leading causes of mortality. In the early phase of ALD, fat accumulation usually occurs in the liver and causes hepatic steatosis. Although a fatty liver is considered a benign histological state, if excessive alcohol intake continues, a fatty liver can develop into a severe liver disease, such as fibrosis or cirrhosis. Therefore, preventing the alcohol-induced fat accumulation in the liver may be important in reducing the progression of alcoholic liver diseases. Although the exact pathway for alcoholic fatty liver has not been clearly elucidated, stimulation of lipogenic transcription factors (Chen et al., 2016), mitochondrial dysfunction (Song et al., 2013), and oxidative stress (Leung and Nieto, 2013; Li et al., 2017) seem to be involved in the processing of alcoholic fatty liver. Moreover, chronic alcohol exposure has been reported to induce triglyceride degradation in adipose tissue and increase hepatic fatty acid uptake (Kang et al., 2007; Tan et al., 2012).
As natural compounds have minimal side effects as well as multi-function effects, they have been given much focus for the development of anti-ALD drugs. The aqueous extracts of Penthorum chinense Pursh were reported to have a hepatoprotective effect against acute ALD (Cao et al., 2015), and cinnamon extract was also found to have protective effects against alcohol-induced liver steatosis (Kanuri et al., 2009). Cannabidiol, a natural antioxidant, was reported to have anti-ALD effects by attenuating oxidative stress (Yang et al., 2014). Mushrooms are one of the well-investigated natural sources that contain rich dietary fiber, vitamins, and minerals (Mattila et al., 2001). In addition, several important compounds, such as β-glucan, α-tocopherol, and ergosterol, have been identified from mushrooms, and their medicinal functions, such as anti-tumor and anti-diabetic effects, have been proposed (Friedman, 2016).
Neolentinus lepideus is an edible mushroom that is widely distributed in East Asia, specifically in China and Korea. N. lepideus seems to have a strong anti-hyperlipidemic activity (Yoon et al., 2011b), and fruiting bodies of Lentinus lepideus were reported to have several natural antioxidants (Yoon et al., 2011a). The functional components of N. lepideus are mainly benzoquinone derivatives, benzofuran, and cinnamic acid derivatives that can be used as inhibitors against nitro oxide (NO) production (Doskocil et al., 2016). However, the suppressive effects of N. lepideus on alcohol-induced fatty liver formation have never been studied.
In this study, we investigated whether the administration of the water extract of N. lepideus (WENL) could effectively alleviate hepatic lipid accumulation and its action of mechanism.
Materials and methods
Preparation of WENL
Neolentinus lepideus was obtained from Gyeonggido Agriculture Research and Extension Services (Gyeonggi, Korea). Exactly 300 g N. lepideus was extracted with 2 L of hot water in 6 h. The supernatant was separated by using whatman filter paper (2 μm pore, Sigma-Aldrich, St. Louis, MO, USA) and concentrated by using a vacuum evaporator (Heidolph Instruments GmbH & Co., Schwabach, Germany) and then lyophilized by using freeze dryer (TFD, ilShinBioBase, Seoul, Korea). The WENL yield was 12.7% (w/w, dry weight 38.1 g).
Animal experiments and plasma analysis
Six-week-old male C57BL/6N mice were purchased from Orient Bio (Gyeonggi, Korea). The mice were adapted for 1 week in a pathogen-free environment under a temperature of 23 ± 2 °C, humidity of 50 ± 10%, and a controlled photoperiod (12 h light/dark cycles). The mice were provided with standard mouse food (Orient Bio, Gyeonggi, Korea) and water ad libitum. All experiments were performed in accordance with the standards of the Animal Research Committee of Sungkyunkwan University (SKKUIACUC-17-6-11-2). Acute and chronic alcoholic liver mouse models were constructed according to the protocol of National Institute on Alcohol Abuse and Alcoholism (NIAAA) (Bertola et al., 2013). The mice (n = 32) were divided into four groups (control, EtOH, EtOH + WENL 0.5, and EtOH + WENL 1). EtOH was obtained from Sigma-Aldrich (St. Louis, MO, USA). For the acute alcoholic liver mouse model, the control mouse group was administrated a normal diet (Lieber-DeCarli #710027, Dyets Inc., Bethlehem, PA, USA,) and the others were fed a 5% ethanol diet (Lieber-DeCarli Ethanol diet #710260, Dyets Inc., Bethlehem, PA, USA) with 0.5 or 1 g/kg body weight/day of WENL for 9 days. On day 10, mice in the EtOH, EtOH + WENL 0.5, and EtOH + WENL 1 groups were gavaged a single dose of ethanol (5 g/kg body weight), and the control mouse group was fed an isocaloric amount of dextrin maltose. For the chronic alcoholic liver mouse model, the entire experiment continued for 4 weeks. After 5 days of adaptation to a liquid diet, normal and ethanol diets with 0.5 or 1 g/kg body weight/day of WENL were administrated to each mouse group. Ethanol was introduced by increasing its concentration to 3 (7 days), 4 (7 days), 5 (7 days), and 5 g/kg body weight (7 days). Mice were laparotomized under sevoflurane (Sigma-Aldrich, St. Louis, MO, USA) anesthesia and blood samples were collected by cardiac puncture. Serum was obtained by centrifuging the blood at 3000 g for 20 min at 4 °C and stored in a − 80 °C refrigerator until analysis. The concentrations of plasma triglyceride (TG), total cholesterol (TC), serum glutamic–oxaloacetic transaminase (AST), and serum glutamic–pyruvic transaminase (ALT) were estimated by using a commercially available kit (Spotchem SP-4410, Arkray Inc., Kyoto, Japan).
Liver tissue histopathology
Liver tissue was fixed with a paraformaldehyde solution (10%, w/v) and embedded in paraffin. Sections (4 μm thick) were stained with a hematoxylin and eosin solution (Sigma-Aldrich, St. Louis, MO, USA). Images of the liver lipid droplets were taken with an optical microscope (Axioplan 2 imaging; Zeiss, Jena, Germany).
Cell culture
AML-12 cells were obtained from ATCC and cultured in DME/F-12 media (Hyclone, UT, USA) containing 10% fetal bovine serum (HyClone, Logan, UT, USA), 1% penicillin–streptomycin (BioWhittaker Inc., Walkersville, MD, USA), 1 × insulin–transferrin–sodium selenite (ITS) (ITS liquid media supplement premix 100 × , Sigma-Aldrich, St. Louis, MO, USA), and 40 ng/mL dexamethasone (Sigma-Aldrich, St. Louis, MO, USA). AML-12 cells of 5 × 105 were seeded on a six-well plate and incubated for 24 h at 37 °C in a CO2 incubator. WENL (50 and 100 µg/mL) and ethanol (100 mM) were treated and further incubated for 3 days. Media was changed once a day with same media. Cells were stained with an Oil red O solution (Sigma-Aldrich, St. Louis, MO, USA), and Oil red O-positive spots were counted under an optical microscope (Axioplan 2 imaging; Zeiss, Jena, Germany).
Western blotting
AML-12 cells of 5 × 105 were seeded on a six-well plate and incubated for 24 h at 37 °C in a CO2 incubator. After serum starvation for 12 h, WENL (50 and 100 µg/mL) were pre-incubated for 1 h and then ethanol (100 mM) were added, and further incubated for 72 h at 37 °C in CO2 incubator. Following incubation with ethanol, AML-12 cells were washed with ice-cold PBS and lysis with protein extraction buffer containing 20 mM Tris–HCl (pH 7.4), 70 μM EDTA, 150 mM NaCl, Nonidet P-40 (1%, w/v), and phosphatase inhibitor cocktails (Boston inc., Danvers, MA, USA) on ice for 1 h. After centrifuge at 13,000×g at 4 °C for 10 min, superna-tants were separated, used to determine the concentration of proteins. Protein samples (20 μg) were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electro-phoresis (SDS-PAGE). Gels were transferred to nitrocellulose membranes, blocked with 5% non-fat-milk and then probed with fist antibody for 12 h at 4 °C. Rabbit anti-phospho-ERK (c9102S), rabbit anti-ERK (c9101S), rabbit anti-phospho-p38 (c9212S), rabbit anti-p38 (c9211S), rabbit anti-phospho-JNK (c9251S), rabbit anti-JNK (c9252S), rabbit anti-phospho-NFκB (c3031S), and rabbit anti-NFκB (c6956S) antibodies were obtained from cell signaling (Danvers, MA, USA). Following incubation with a rabbit horseradish peroxidase–conjugated secondary antibody (Abcam, Cambridge, UK) at room temperature for 1 h, expression of each bands were visualized using an electrochemiluminescence (ECL) detection reagent (Biorad, Hercules, CA, USA) according to the manufacturer’s protocols.
RNA isolation and real-time polymerase chain reaction (RT-PCR)
The total RNA of AML-12 cells and mouse liver was isolated by using TRIzol reagent (Invitrogen, Waltham, CA, USA). Oligo (dT) primed total RNA was used for cDNA synthesis, and a quantitative analysis was performed in a CFX connect real-time system (BioRad, Hercules, CA, USA) by using the SYBR Green real time master mix (Toyobo Co., Tokyo, Japan). Relative expression of each gene was determined by using the comparative Ct method and normalized to the expression of GAPDH. The sequences of the sterol regulatory element-binding protein 1 (SREBP1) primers are 5′-TTGTACCTACTGTGGCTAAATGAGA-3′ (forward) and 5′-CTTGTTTTGAACATTTCTGCTT-3′ (reverse); FASN primers, 5′-CCATGCCCAGAGGGTGGTTG-3′ (forward) and 5′-AACGTCACTTCCAGCTAGAC-3′ (reverse); ACC primers, 5′-CAGGCACACACGATGGAC-3′ (forward) and 5′-CGGAGTGAATCTGGGTTGAT-3′ (reverse); cytochrome P450 2E1 (CYP2E1) primers, 5′-ACAGGGTTATTGGGCCAA-3′ (forward) and 5′-TCTCATGCACTACAGCGT-3′ (reverse); GAPDH primers, 5′-CCACCCAGAAGACTGTGGAT-3′ (forward) and 5′-CACATTGGGGGTAGGAACAC-3′ (reverse).
Estimation of total phenolic contents and total flavonoids
The total phenolic content of WENL was determined by using Folin-Cioalteu method (Abdullah et al., 2012). Initially, 100 µL of WENL solution (5 mg/mL) was mixed with 100 μL of 10% (w/v) Folin-Ciocalteu reagent (Sigma-Aldrich, St. Louis, MO, USA). Mixture was incubated at room temperature for 2 min and then 200 μL of 10% (w/v) sodium carbonate solution (Sigma-Aldrich, St. Louis, MO, USA) was added and incubated for 1 h at room temperature in dark room. Finally, the absorbance of the solution was measured at 750 nm in a 96 well plate by using microplate absorbance reader (Biorad, Hercules, CA, USA). Gallic acid (Sigma-Aldrich, St. Louis, MO, USA) was used to determine standard curve (50–100 μg/mL, R2 = 0.999). The total phenolic contents were expressed as mg of gallic acid equivalents (GAE)/g of WENL. The total flavonoid contents of WENL were determined according to the methods of Moreno et al. (2000) with some modification. Briefly, 110 μL of WENL solution (10 mg/mL), 44 μL of 10% (w/v) aluminium nitrate (Sigma-Aldrich, St. Louis, MO, USA), 44 μL of 1 M potassium acetate (Sigma-Aldrich, St. Louis, MO, USA), 616 μL distilled water, 330 μL of 80% ethanol were mixed and incubated for 40 min at room temperature in the dark room. Finally, the absorbance of the solution was measured at 415 nm by using Optizen POP UV spectrophotometer (MECASYS, Daejeon, Korea). Quercetin (Sigma-Aldrich, St. Louis, MO, USA) was used to determine standard curve (50–400 μg/mL, R2 = 0.999). The total flavonoid contents were expressed as mg of quercetin equivalents (QE)/g of WENL.
Statistical analysis
Statistical analysis was performed by one-way ANOVA and two-tailed unpaired Student’s t test using SPSS 19.0 for Windows. The data were presented as the mean ± standard deviation. Statistical significant was considered at p < 0.05.
Results and discussion
WENL attenuated the ethanol-mediated lipid accumulation on AML-12 cells
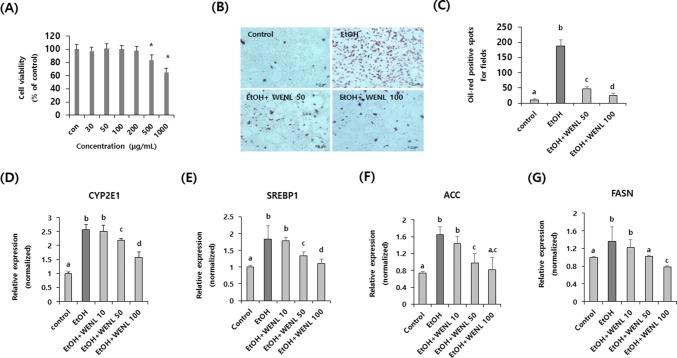
We first determined the cytotoxicity of WENL on AML-12 cells. Administration of WENL up to a concentration of 200 μg/mL did not show cytotoxicity (Fig. 1A). Based on this results, to check the inhibitory effects of WENL on lipid accumulation, WENL (50 and 100 μg/mL) was added to the ethanol-induced mouse hepatocytes cell line (AML-12). Lipid accumulation was calculated by counting the Oil Red O-positive spots. As shown in Fig. 1B, the number of Oil Red O-positive spots dramatically increased in ethanol-treated cells compared with the control cells. However, the number of Oil Red O-positive spots dose-dependently decreased in the WENL-treated AML-12 cells (Fig. 1C) (p < 0.05). In addition, we checked the transcriptional expression of fatty acid synthesis-related genes, such as sterol regulatory element-binding protein 1 (SREBP1) (Fig. 1E), acetyl-coA carboxylase (ACC) (Fig. 1F), fatty acid synthase (FASN) (Fig. 1G). In addition, we also checked the transcriptional expression of cytochrome P450 2E1 (CYP2E1) (Fig. 1D) which is related with ethanol induced fatty liver. As expected, the administration of ethanol to AML-12 hepatocytes significantly increased the expression of these genes. However, the administration of WENL dose-dependently attenuated the effects of ethanol on the transcriptional expression of these genes (Fig. 1) (p < 0.05). These data suggest that WENL has suppressive effects on lipid accumulation induced by ethanol in hepatocytes by attenuating the expression of SREBP1, ACC, FASN and CYP2E1 genes.
Fig. 1.
Alcohol-induced lipogenesis on AML-12 hepatocyte was attenuated by administration of WENL. (A) Various concentration of WENL (0–1000 µg/mL) was treated to AML-12 cells for 24 h. Cell viability was calculated by using the WST-assay kit. Each data value is expressed as the mean ± SE of three wells. Asterisks (*) indicate significant (p < 0.05) differences compared to the control. (B) AML-12 hepatocytes were treated with ethanol with and without WENL (50–100 µg/mL) for 24 h. After fixation, lipid droplets were stained with the Oil red O solution. Scale bar represents 5.0 μm. (C) Oil red O-positive spots are counted and tabled. The transcriptional expressions of CYP2E1 (D), SREBP1 (E), ACC (F), and FASN (G) were examined by real-time RT-PCR. Each data value is expressed as the mean ± SE for at least three independent experiments. Different letters indicate significant (p < 0.05) differences between the groups. WENL, Water extract of Neolentinus lepideus; CYP2E1, cytochrome P450 2E1; SREBP1, sterol regulatory element-binding protein 1; ACC, acetyl-coA carboxylase; FASN, fatty acid synthase
Administration of WENL attenuated the concentration of blood TG in ethanol feeding mouse
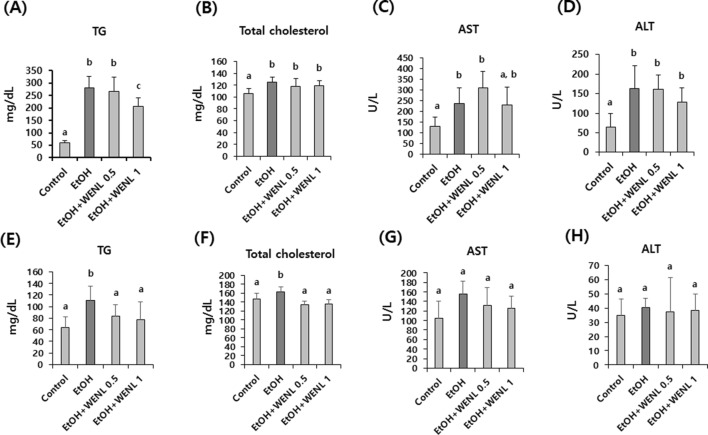
To further estimate the effects of WENL on ethanol-induced fatty liver formation, WENL was administrated to both acute and chronic ethanol-fed mouse models (Fig. 2). In the acute ethanol-fed mouse liver model, the concentration of blood TG and total cholesterol was significantly (p < 0.05) increased in the ethanol-fed mouse groups compared with the control mouse group. In addition, increased levels of AST and ALT were detected in the acute ethanol-fed mouse group compared with the control mouse group (p < 0.05). Although the administration of WENL did not effectively decrease the ethanol-induced AST and ALT levels (Fig. 2C, D), the bloods content of TG significantly (p < 0.05) decreased in the WENL-administrated mouse group (EtOH + WENL 1) compared with the control mouse group (Fig. 2A). Interestingly, in our chronic ethanol-fed mouse liver model, the increased concentration of blood TG and total cholesterol in the ethanol-fed mouse groups was significantly (p < 0.05) attenuated by WENL administration (Fig. 2E, F). These results suggest that the administration of WENL could effectively attenuate ethanol-induced blood lipids, especially the concentration of TG.
Fig. 2.
Administration of WENL alleviated the blood TG levels in acute alcohol liver injury mouse models Mouse was fed with EtOH with and without WENL (0.5–1 g/kg body weight) for 10 days (acute alcohol liver injury model), and the levels of TG (A), Total cholesterol (B), AST (C), and ALT (D) were compared with those in the control mouse group (n = 8). The increased levels of TG in the EtOH-administrated mouse group (n = 8) were significantly attenuated in the WENL-administrated mouse group (EtOH + WENL 1). To investigate the effect of WENL on chronic alcohol liver injury model, mouse was fed with EtOH with and without WENL (0.5–1 g/kg body weight) for four weeks, and the levels of TG (E), Total cholesterol (F), AST (G), and ALT (H) were compared with those in the control mouse group (n = 8). The increased levels of TG and Total cholesterol in the EtOH mouse group (n = 8) were significantly attenuated in the WENL administrated mouse groups (EtOH + WENL 0.5 and EtOH + WENL 1). Each data value is expressed as the mean ± SE, and different letters indicate the significant differences between the groups (p < 0.05). WENL, water extracts of Neolentinus lepideus; TG, triacylglycerol; AST, glutamic-oxaloacetic transaminase; ALT, glutamic-pyruvic transaminase
Ethanol-induced hepatic lipid droplets decreased in WENL-administrated mouse liver
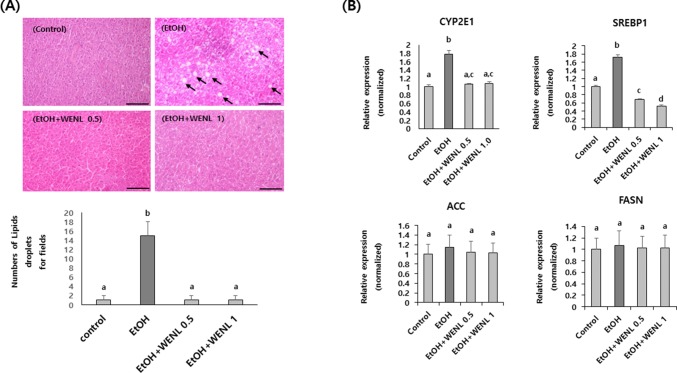
To further confirm the effects of WENL on ethanol-induced hepatic lipid accumulation, we compared the number of lipid droplets in the liver between the control and the chronic ethanol-fed mouse groups. As shown in Fig. 3A, the number of lipid droplets dramatically increased in the ethanol-fed mouse groups compared with the control mouse group. However, the lipid droplets almost disappeared in the WENL-administrated mouse liver (Fig. 3A). Furthermore, the increased transcriptional expression levels of liver CYP2E1 and SREBP1, but not of ACC and FASN, in the ethanol-fed mouse groups were significantly attenuated by WENL administration (Fig. 3B). These results indicate that the administration of WENL could effectively alleviate the ethanol-induced lipid accumulation in the liver by mainly attenuating the transcriptional expression of the CYP2E1 and SREBP1.
Fig. 3.
Accumulation of lipid droplets in mouse liver was attenuated by WENL administration. The representative areas of the liver tissue of each mouse group (n = 8) of the chronic ethanol-fed mouse liver injury model are shown (A) and transcriptional expressions of CYP2E1, SREBP1, ACC, and FASN were examined by real-time RT-PCR (B). Mouse liver tissues were isolated from each mouse group, and paraffin-embedded sections were stained with a hematoxylin and eosin solution. The numbers of lipid droplets dramatically increased in the EtOH mouse group (EtOH) compared with the control mouse group (Control). However, the lipid droplets were disappeared in the WENL-administrated mouse groups (EtOH + WENL 0.5 and EtOH + WENL 1) compared with the EtOH mouse group (EtOH). Arrows indicate lipid droplets. Lipid droplets exceed 5μm diameter were counted and tabulated. Scale bar represents 100 μm. The expression of CYP2E1 and SREBP1, but not of ACC and FASN, was significantly (p < 0.05) increased in the EtOH mouse groups compared with the control group. The administration of WENL significantly (p < 0.05) attenuated the ethanol-induced CYP2E1 and SREBP1 expression in the liver. Each data value is expressed as the mean ± SE, and different letters indicate the significant differences between the groups (p < 0.05). WENL, water extracts of Neolentinus lepideus; CYP2E1, cytochrome P450 2E1; SREBP1, sterol regulatory element-binding protein 1; ACC, acetyl-coA carboxylase; FASN, fatty acid synthase
Fatty liver is considered a single disease that is associated with other diseases related to fat metabolism (Ferre and Foufelle, 2010). Fatty liver is closely related to excessive intake of alcohol and obesity, and it can develop to severe ALD, such as inflammatory liver (steatosis). Recent studies have suggested that daily alcohol intake (24 g/d) can activate the hepatic lipogenic pathway (Siler et al., 1999), and several molecular and biochemical mechanisms in developing ALD have been suggested (Gramenzi et al., 2006; Han et al., 2015). Given that complex mechanisms underlie ALD, developing agents that can completely treat ALD seems a difficult task. However, developing agents than can reduce fat accumulation in the liver is useful in alleviating alcoholic liver injury.
Mushrooms have been used in oriental medicine for the treatment many diseases. Several edible mushrooms have been reported to have neutraceutical effects in the immune system, hyperlipidemia, and tumor progressions (Chang, 1996; Ukawa et al., 2002; Ukawa et al., 2007; Wasser, 2002; Zaidman et al., 2005). Some mushrooms can suppress hepatic fat accumulation. For examples, Mukitake mushroom was found to have suppressive effects on hepatic lipogenesis, and Pleurotus eryngii cellulose was also reported to have suppressive effects on high-fat-induced hepatic liver formation in rat. N. lepideus, which was used in this study, is an edible mushroom that belongs to the family of Tricholomaceteae and order of Agaricales. Although the anti-hyperlipidemic activities of N. lepideus was already reported (Yoon et al., 2011b), to our knowledge, this study is the first to investigate the inhibitory activity of N. lepideus in alcohol-induced hepatic fat accumulation. Alcohol-induced lipid accumulation in the hepatocyte and mouse liver was dramatically alleviated by the administration of WENL (Figs. 1, 3). Although the daily administration of WENL up to 1 g/kg body weight did not appear to be sufficient for reducing the levels of blood AST and ALT in the acute alcoholic liver mouse model (Fig. 2C, D), the alcohol-induced increase in blood TG concentration was significantly attenuated by the administration of WENL (Fig. 2A). Moreover, the administration of WENL was effective in reducing the blood contents of TG in the chronic alcoholic liver mouse model (Fig. 2E). These results strongly indicate that WENL could be developed as natural agents for preventing alcohol-induced lipid accumulation.
Generally, fatty liver is a result of the accumulation of TG and other fats in liver cells. Although several mechanisms underlying the process of alcohol-mediated fatty liver have been suggested, the imbalance of alcohol-mediated lipogenesis is one of the critical reasons in the progression of alcoholic fatty liver (Wada et al., 2008). To determine the regulatory role of WENL in alcohol-induced fatty liver formation, we investigated the effects of WENL on the transcriptional expression of lipogenesis-related genes, such as ACC, FASN, and SREBP1. ACC is a well-known lipogenesis-related gene that provides the malonyl-CoA for the biosynthesis of fatty acids (Tong, 2005), and FASN plays an important role in lipogenesis by catalyzing the synthesis of palmitate from acetyl-CoA and malonyl-CoA (Alberts et al., 1975). SREBP1 is a transcription factor that is required for cholesterol and fatty acid biosynthesis (Brown and Goldstein, 1997). Many groups reported that alcohol exposure enhances lipogenesis-related genes, which improve hepatic lipogenesis, thereby resulting in fatty liver. Interestingly, the increased levels of ACC, FASN, SREBP1, and CYP2E1 in ethanol-treated hepatocytes were significantly attenuated by WENL administration (Fig. 1). However, only the transcriptional expression of the SREBP1 and CYP2E1genes were attenuated by the administration of WENL in our chronic alcoholic liver mouse model (Fig. 3). We thought that the dose of WENL which used in animal study was not sufficient to suppress the expression of ACC and FASN. Considering the fact that SREBP1 is a transcription factor of ACC and FASN genes (Ma et al., 2008; Macfarlane et al., 2008), the use of higher dose of WENL in further our animal study may result in decrease of ethanol induced expression of lipogenesis related genes including ACC and FASN genes.
CYP2E1 is a member of the cytochrome P450 and is responsible for the breakdown of a large number of toxic chemicals in human body (Rendic and Di Carlo, 1997). CYP2E1 have been reported to be a major regulator to ethanol mediated oxidative stress (Leung and Nieto, 2013; Lu and Cederbaum, 2008). In addition, in the chronic ethanol induced fatty liver, CYP2E1 seems to have critical roles in pathogenic regulation. For examples, the expression of CYP2E1 is closely related with the degree of steatosis in rat (Jarvelainen et al., 2000). Inhibiting the expression of CYP2E1 by using specific inhibitor of CYP2E1, chlormethiazole, attenuated the ethanol induced liver injury (Gouillon et al., 2000). Ethanol-induced steatosis was not occurred in CYP2E1 deficient mice (Lu et al., 2008). Therefore, isolation of natural agents which can attenuate the expression of CYP2E1 may be effective to develop anti-steatosis drug. In our study, administration of WENL significantly attenuated the ethanol expression of CYP2E1 in hepatocyte and chronic alcoholic liver mouse model. So, if we determined the active single compound from WENL, WENL could be developed as anti-steatosis agents.
WENL attenuated alcohol induced intracellular signaling pathway
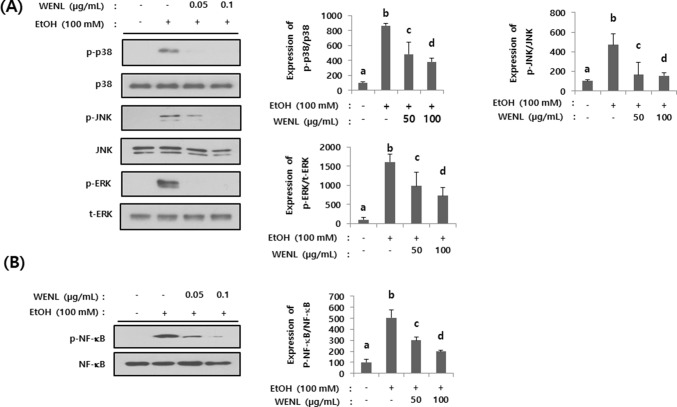
To further investigate the underlying mechanisms of WENL mediated hepatic changes, we investigated the expressional changes of mitogen activated protein kinases (MAPKs) such as ERK, p38 and JNK. Administration of ethanol to AML-12 cells dramatically increased the phosphorylation of ERK, p38 and JNK. Such elevation was abolished by the treatment of WENL (Fig. 4A). Furthermore, we found that WENL can be effective in attenuating the alcohol induced phosphorylation of NF-κB (Fig. 4B). Since it has been suggested that MAPKs/NF-κB pathway are involved in ethanol induced hepatic injury (Xiao et al., 2014), the inhibitive effects of WENL on ethanol induced MAPKs/NF-κB activation could be a mechanism on WENL mediated inhibitive effects on alcohol induced hepatic lipids accumulations.
Fig. 4.
Administration of WENL attenuated the alcohol-induced intracellular signaling pathway. AML-12 cells were pre-treated with WENL (50–100 µg/mL) and then further incubated with and without 100 mM ethanol. AML-12 cells were harvested, and the expression of p38, phosphor p38 (p-p38), total JNK (JNK), phosphor JNK (p-JNK), total ERK (t-ERK) and phosphor ERK (p-ERK) was examined by western blotting (A). Ethanol-induced expression of NF-κB and phosphor-NF-κB (p-NF-κB) were measured by western blotting (B). Each band was quantified by using image J program. Each data value is expressed as the mean ± SE (n = 3), and different letters indicate the significant differences between the groups (p < 0.05)
Total phenolic acids and total flavonoids of WENL
Finally, to determine the active component of WENL, we first measured the total amount of phenolic acids and flavonoids of WENL. Gallic acid and quercetin were used for preparing standard curve for total phenolic acids and total flavonoids respectively. The total amount of phenolic acids and flavonoids of WENL was 20.9 ± 0.55 mg GAE/g and 6.5 ± 1.62 QE/g respectively. Only a few active components of Neolentinus lepideus such as lepidepyrone, a gamma-pyrone derivative, and novel tyrosinase inhibitors have been reported as active components of Neolentinus lepideus (Hosoe et al., 2007; Ishihara et al., 2018). However, there were no reports about functional study of active phenolic acids and flavonoids from Neolentinus lepideus. Since relatively high amount of phenolic acids was detected in WENL (20.9 ± 0.55 mg GAE/g) when compared with other mushrooms (Abdullah et al., 2012), we think that one of the phenolic acids could be an active component for WENL mediated inhibitive effects on alcohol induced hepatic lipids accumulation.
In the present study, we provided the first evidence that the administration of WENL effectively attenuates the alcohol-induced lipid accumulation in the hepatocyte and mouse liver. Although the active components of WENL was not determined in this study, it clearly showed the effects on lowering blood TG and hepatic fat accumulation of alcohol-administrated hepatocyte and mouse liver. In addition MAPKs/NF-κB pathway has been proposed as a possible mechanism for inhibitive effects of WENL on alcohol induced hepatic fat accumulation. Further studies will be performed to determine the chemical identity of WENL that regulates alcohol-mediated fat accumulation in the liver.
Acknowledgements
This work was supported by the Rural Development Administration (PJ01022310), Korea.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abdullah N, Ismail SM, Aminudin N, Shuib AS, Lau BF. Evaluation of Selected Culinary-Medicinal Mushrooms for Antioxidant and ACE Inhibitory Activities. Evid. Based Complement Alternat. Med. 2012;2012:464238. doi: 10.1155/2012/464238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts AW, Strauss AW, Hennessy S, Vagelos PR. Regulation of synthesis of hepatic fatty acid synthetase: binding of fatty acid synthetase antibodies to polysomes. Proc. Natl. Acad. Sci. USA. 1975;72:3956–3960. doi: 10.1073/pnas.72.10.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat. Protoc. 2013;8:627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/S0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- Cao YW, Jiang Y, Zhang DY, Zhang XJ, Hu YJ, Li P, Su H, Wan JB. The hepatoprotective effect of aqueous extracts of Penthorum chinense Pursh against acute alcohol-induced liver injury is associated with ameliorating hepatic steatosis and reducing oxidative stress. Food Funct. 2015;6:1510–1517. doi: 10.1039/C5FO00098J. [DOI] [PubMed] [Google Scholar]
- Chang R. Functional properties of edible mushrooms. Nutr. Rev. 1996;54:S91–S93. doi: 10.1111/j.1753-4887.1996.tb03825.x. [DOI] [PubMed] [Google Scholar]
- Chen LY, Chen Q, Cheng YF, Jin HH, Kong DS, Zhang F, Wu L, Shao JJ, Zheng SZ. Diallyl trisulfide attenuates ethanol-induced hepatic steatosis by inhibiting oxidative stress and apoptosis. Biomed. Pharmacother. 2016;79:35–43. doi: 10.1016/j.biopha.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Doskocil I, Havlik J, Verlotta R, Tauchen J, Vesela L, Macakova K, Opletal L, Kokoska L, Rada V. In vitro immunomodulatory activity, cytotoxicity and chemistry of some central European polypores. Pharm. Biol. 2016;54:2369–2376. doi: 10.3109/13880209.2016.1156708. [DOI] [PubMed] [Google Scholar]
- Ferre P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 2010;12(Suppl. 2):83–92. doi: 10.1111/j.1463-1326.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- Friedman Mendel. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods. 2016;5(4):80. doi: 10.3390/foods5040080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouillon Z, Lucas D, Li J, Hagbjork AL, French BA, Fu P, Fang C, Ingelman-Sundberg M, Donohue TM, Jr, French SW. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc. Soc. Exp. Biol. Med. 2000;224:302–308. doi: 10.1046/j.1525-1373.2000.22435.x. [DOI] [PubMed] [Google Scholar]
- Gramenzi A, Caputo F, Biselli M, Kuria F, Loggi E, Andreone P, Bernardi M. Review article: alcoholic liver disease–pathophysiological aspects and risk factors. Aliment. Pharmacol. Ther. 2006;24:1151–1161. doi: 10.1111/j.1365-2036.2006.03110.x. [DOI] [PubMed] [Google Scholar]
- Han JY, Lee S, Yang JH, Kim S, Sim J, Kim MG, Jeong TC, Ku SK, Cho IJ, Ki SH. Korean Red Ginseng attenuates ethanol-induced steatosis and oxidative stress via AMPK/Sirt1 activation. J. Ginseng Res. 2015;39:105–115. doi: 10.1016/j.jgr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoe T, Sakai H, Ichikawa M, Itabashi T, Ishizaki T, Kawai K. Lepidepyrone, a new gamma-pyrone derivative, from Neolentinus lepideus, inhibits hyaluronidase. J. Antibiot (Tokyo) 2007;60:388–390. doi: 10.1038/ja.2007.53. [DOI] [PubMed] [Google Scholar]
- Ishihara A, Ide Y, Bito T, Ube N, Endo N, Sotome K, Maekawa N, Ueno K, Nakagiri A. Novel tyrosinase inhibitors from liquid culture of Neolentinus lepideus. Biosci. Biotechnol. Biochem. 2018;82:22–30. doi: 10.1080/09168451.2017.1415125. [DOI] [PubMed] [Google Scholar]
- Jarvelainen HA, Fang C, Ingelman-Sundberg M, Lukkari TA, Sippel H, Lindros KO. Kupffer cell inactivation alleviates ethanol-induced steatosis and CYP2E1 induction but not inflammatory responses in rat liver. J. Hepatol. 2000;32:900–910. doi: 10.1016/S0168-8278(00)80094-X. [DOI] [PubMed] [Google Scholar]
- Kang L, Chen X, Sebastian BM, Pratt BT, Bederman IR, Alexander JC, Previs SF, Nagy LE. Chronic ethanol and triglyceride turnover in white adipose tissue in rats: inhibition of the anti-lipolytic action of insulin after chronic ethanol contributes to increased triglyceride degradation. J. Biol. Chem. 2007;282:28465–28473. doi: 10.1074/jbc.M705503200. [DOI] [PubMed] [Google Scholar]
- Kanuri G, Weber S, Volynets V, Spruss A, Bischoff SC, Bergheim I. Cinnamon extract protects against acute alcohol-induced liver steatosis in mice. J. Nutr. 2009;139:482–487. doi: 10.3945/jn.108.100495. [DOI] [PubMed] [Google Scholar]
- Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J. Hepatol. 2013;58:395–398. doi: 10.1016/j.jhep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Li M, He Y, Zhou Z, Ramirez T, Gao Y, Gao Y, Ross RA, Cao H, Cai Y, Xu M, Feng D, Zhang P, Liangpunsakul S, Gao B. MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6-p47phox-oxidative stress pathway in neutrophils. Gut. 2017;66:705–715. doi: 10.1136/gutjnl-2016-311861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- Ma Y, Xu L, Rodriguez-Agudo D, Li X, Heuman DM, Hylemon PB, Pandak WM, Ren S. 25-Hydroxycholesterol-3-sulfate regulates macrophage lipid metabolism via the LXR/SREBP-1 signaling pathway. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1369–E1379. doi: 10.1152/ajpendo.90555.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane DP, Forbes S, Walker BR. Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J. Endocrinol. 2008;197:189–204. doi: 10.1677/JOE-08-0054. [DOI] [PubMed] [Google Scholar]
- Mattila P, Konko K, Eurola M, Pihlava JM, Astola J, Vahteristo L, Hietaniemi V, Kumpulainen J, Valtonen M, Piironen V. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J. Agric. Food Chem. 2001;49:2343–2348. doi: 10.1021/jf001525d. [DOI] [PubMed] [Google Scholar]
- Moreno MI, Isla MI, Sampietro AR, Vattuone MA. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J. Ethnopharmacol. 2000;71:109–114. doi: 10.1016/S0378-8741(99)00189-0. [DOI] [PubMed] [Google Scholar]
- Rendic S, Di Carlo FJ. Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab. Rev. 1997;29:413–580. doi: 10.3109/03602539709037591. [DOI] [PubMed] [Google Scholar]
- Siler SQ, Neese RA, Hellerstein MK. De novo lipogenesis, lipid kinetics, and whole-body lipid balances in humans after acute alcohol consumption. Am. J. Clin. Nutr. 1999;70:928–936. doi: 10.1093/ajcn/70.5.928. [DOI] [PubMed] [Google Scholar]
- Song BJ, Abdelmegeed MA, Henderson LE, Yoo SH, Wan J, Purohit V, Hardwick JP, Moon KH. Increased nitroxidative stress promotes mitochondrial dysfunction in alcoholic and nonalcoholic fatty liver disease. Oxid. Med. Cell Longev. 2013;2013:781050. doi: 10.1155/2013/781050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Sun X, Li Q, Zhao Y, Zhong W, Sun X, Jia W, McClain CJ, Zhou Z. Leptin deficiency contributes to the pathogenesis of alcoholic fatty liver disease in mice. Am. J. Pathol. 2012;181:1279–1286. doi: 10.1016/j.ajpath.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L. Acetyl-coenzyme A carboxylase: crucial metabolic enzyme and attractive target for drug discovery. Cell. Mol. Life Sci. 2005;62:1784–1803. doi: 10.1007/s00018-005-5121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukawa Y, Furuichi Y, Kokean Y, Nishii T, Hisamatsu M. Effect of Hatakeshimeji (Lyophyllum decastes Sing.) Mushroom on serum lipid levels in rats. J. Nutr. Sci. Vitaminol (Tokyo) 2002;48:73–76. doi: 10.3177/jnsv.48.73. [DOI] [PubMed] [Google Scholar]
- Ukawa Y, Izumi Y, Ohbuchi T, Takahashi T, Ikemizu S, Kojima Y. Oral administration of the extract from Hatakeshimeji (Lyophyllum decastes Sing.) mushroom inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. J. Nutr. Sci. Vitaminol (Tokyo) 2007;53:293–296. doi: 10.3177/jnsv.53.293. [DOI] [PubMed] [Google Scholar]
- Wada S, Yamazaki T, Kawano Y, Miura S, Ezaki O. Fish oil fed prior to ethanol administration prevents acute ethanol-induced fatty liver in mice. J. Hepatol. 2008;49:441–450. doi: 10.1016/j.jhep.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002;60:258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- Xiao J, Wang J, Xing F, Han T, Jiao R, Liong EC, Fung ML, So KF, Tipoe GL. Zeaxanthin dipalmitate therapeutically improves hepatic functions in an alcoholic fatty liver disease model through modulating MAPK pathway. PLoS One. 2014;9:e95214. doi: 10.1371/journal.pone.0095214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Rozenfeld R, Wu D, Devi LA, Zhang Z, Cederbaum A. Cannabidiol protects liver from binge alcohol-induced steatosis by mechanisms including inhibition of oxidative stress and increase in autophagy. Free Radic. Biol. Med. 2014;68:260–267. doi: 10.1016/j.freeradbiomed.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KN, Alam N, Lee KR, Shin PG, Cheong JC, Yoo YB, Lee TS. Antioxidant and antityrosinase activities of various extracts from the fruiting bodies of Lentinus lepideus. Molecules. 2011;16:2334–2347. doi: 10.3390/molecules16032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KN, Lee JS, Kim HY, Lee KR, Shin PG, Cheong JC, Yoo YB, Alam N, Ha TM, Lee TS. Appraisal of Antihyperlipidemic Activities of Lentinus lepideus in Hypercholesterolemic Rats. Mycobiology. 2011;39:283–289. doi: 10.5941/MYCO.2011.39.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidman BZ, Yassin M, Mahajna J, Wasser SP. Medicinal mushroom modulators of molecular targets as cancer therapeutics. Appl. Microbiol. Biotechnol. 2005;67:453–468. doi: 10.1007/s00253-004-1787-z. [DOI] [PubMed] [Google Scholar]