Abstract
This study was conducted to investigate potentially protective and curative effects of Curcuma longa root (turmeric) powder on CCl4-induced hepatotoxicity in rats. Turmeric was administered before (preventive effect) or after (curative effect) treatment with CCl4. Total phenolic and flavonoid levels were 26.35 mg GAE/g and 12.35 mg CE/g, respectively. Using HPLC analysis, turmeric powder was rich in curcumin (62.97%), demethoxycurcumin (20.86%) and bisdemethoxycurcumin (16.17%). Curcuma longa powder showed important in vitro antioxidant activities. Results showed that the activities of aspartate aminotransaminase and alanine aminotransaminase, and the levels of bilirubin and serum lipids were increased in CCl4-treated animals. However, total protein and albumin levels and antioxidant enzyme activities were decreased. Turmeric administration, before or after CCl4 treatment, significantly decreased the activities of marker enzymes and lipid levels in serum. Moreover, total protein and albumin contents were restored to nearly normal levels after turmeric administration accompanied with increase of antioxidant enzymes activities.
Keywords: Curcuma longa root, Liver fibrosis, Hepatic enzymes, Oxidative stress
Introduction
Liver fibrosis results from chronic damage to the liver in conjunction with the accumulation of extracellular matrix proteins, which is a characteristic of most types of chronic liver diseases (Friedman, 2003). The main factors of liver fibrosis are chronic hepatitis C virus infection, alcohol abuse and non-alcoholic steatohepatitis (Ginès et al., 2004). It may result from the over-deposit of extracellular matrix and its component changes (Wang et al., 2002). Oxidative stress has been recently recognized as a fundamental factor in the pathogenic changes observed in liver fibrosis. It plays an important role in pathophysiological changes that progress to liver cirrhosis. However, cirrhosis, the end stage consequence of fibrosis, is generally irreversible. Nevertheless, no curative treatment for liver fibrosis is available until today (Veidal et al., 2011). The damage responses induced by carbon tetrachloride (CCl4) injection in rat model are similar to liver cirrhosis in humans (Weiler-Normann et al., 2007). It leads to the production of free radicals, which causes lipid peroxidation and thus necrosis and cirrhosis Its toxicity is caused by haloalkane metabolites produced during biotransformation and causing oxidative damage to cellular structure and macromolecules (Taniguchi et al., 2004). Reactive oxygen species (ROS) produced under these conditions, lead to membrane lipid peroxidation and consequently to apoptosis and/or necrosis. These ROS can be eliminated by both enzymatic and non-enzymatic antioxidant systems mainly the powerful phenolic antioxidants (Ksouri et al., 2009). Liver damage ranging from subclinical icteric hepatitis to necroinflammatory hepatitis, cirrhosis and carcinoma has been proven to be associated with redox imbalance and oxidative stress (Vrba and Modriansky, 2002). Therefore, compounds that exhibit antioxidant properties, scavenging of free radicals and inhibition of liver enzymes are expected to show hepatoprotective activity (Girish and Pradhan, 2008).
Nowadays, medicinal plants are receiving much importance in several industries mainly food processing ones. They are a dependable source of functional food. Such plants exhibit a high content of bioactive compounds mainly polyphenols, which have been reported to displayed several biological properties. The excellent medicinal properties of these compounds are mainly attributed to their ability to withstand and quench toxic ROS (Dufour et al., 2007). Curcuma longa is a rhizomatous perennial herb, commonly known as turmeric, belonging to the family of Zingiberaceae. It is used as a food spice, additive, flavoring, preservative and as coloring agent in foods. In traditional medicine, it usually serves to treat liver disorders including liver fibrosis. Turmeric possesses antiviral (Kim et al., 2009), anti-inflammatory (Bereswill et al., 2010), antioxidant (Al-Jassabi et al., 2012; Chinedum et al., 2015; Tanvir et al., 2017), antidiabetic (Aziz et al., 2013) and anticancer (Abdel-Lateef et al., 2016) properties. Liver is one of the important organs of the body which plays a major role in the metabolism of proteins, carbohydrates, lipids. Therefore, the condition of liver is important for our safety and health. However, in spite of the therapeutic importance of turmeric, no study about its protective and ameliorative effects when administered as a powder in an experimental model of hepatic fibrosis. Thus, we presumed here that turmeric treatment which started from the early stage of chronic liver disease or after fibrosis onset could effectively attenuate hepatic fibrosis.
Materials and methods
Preparation of plant extract
Curcuma longa roots (turmeric) were purchased from a local market in Tunis (Tunisia), powdered into ground turmeric and then stored under at 4 °C. Methanolic extracts, used to determine the polyphenol content and antioxidant activities, were obtained by magnetic stirring for 30 min of 2.5 g of curcuma powder with 25 ml of 80% methanol. Then extracts were kept at 4 °C for 24 h, and filtered through a Whatman filter paper. This final solution was stored at 4 °C and used to determine total phenolic and flavonoid contents and to estimate antioxidant activities using several tests (total antioxidant activity, scavenging ability of DPPH radical and iron reducing power).
Total phenolic content
Total phenolics were assayed using the Folin-Ciocalteu reagent (Dewanto et al., 2002). Briefly, 125 µl of sample extract were dissolved in 500 µl of distilled water and 125 µl of Folin-Ciocalteu reagent. The mixture was shaken, before addition of 1.25 ml of 7% Na2CO3 adjusting with distilled water to a final volume of 3 ml, and mixed thoroughly. After incubation in the dark for 90 min, the absorbance at 760 nm was measured versus the prepared blank. Total phenolic amounts were expressed as mg of gallic acid equivalents per gram of dry weight (mg GAE/g DW), through a calibration curve with gallic acid.
Total flavonoid content
Total flavonoids were measured using a colorimetric assay (Prieto et al., 1999). An aliquot of diluted sample or standard solution of catechin was added to a 75 μl of NaNO2 solution, and mixed for 6 min, before adding 0.15 ml AlCl3 (100 g/l). After 5 min, 0.5 ml of NaOH was added. The final volume was adjusted to 2.5 ml with distilled water and thoroughly mixed. Absorbance of the mixture was determined at 510 nm against the same mixture, without the sample, as a blank. Total flavonoid content was expressed as mg catechin/g dry weight (mg CE/g DW), through the calibration curve of catechin. The calibration curve range was 50–400 μg/ml (R2 = 0.99). All samples were analyzed in three replications.
DPPH radical-scavenging ability
DPPH· scavenging ability was estimated according to Hatano et al. (1988). The donation capacity of the obtained extracts was measured by bleaching of the purple-colored solution of 1,1-diphenyl-2-picrylhydrazyl radical (DPPH). One ml of the extract at different concentrations was added to 0.5 ml of a DPPH· methanolic solution. The mixture was shaken vigorously and left standing at room temperature for 30 min in the dark. The absorbance of the resulting solution was then measured at 517 nm. The antiradical activity was expressed as IC50 (μg/ml), the antiradical dose required to cause a 50% inhibition. A lower IC50 value corresponds to a higher antioxidant activity of plant extract. The ability to scavenge the DPPH radical was calculated using the following equation:
where A0 is the absorbance of the control at 30 min, and A1 is the absorbance of the sample at 30 min. BHT was used as a positive control. Samples were analysed in triplicate.
Iron reducing power
The iron reductive power was assessed (Yang et al., 2008) and the absorbance was measured at 700 nm. Each extract was mixed with 2.5 ml of sodium phosphate buffer (0.2 mol/l, pH 6.6) and 2.5 ml of potassium ferricyanide (10 g/l), and the mixture was incubated at 50 ◦C for 20 min. 2.5 ml of trichloroacetic acid (100 g/l) were then added, and the mixture was centrifuged at 650 g for 10 min. The upper layer (2.5 ml) was mixed with 2.5 ml of deionised water and 0.5 ml of ferric chloride (0.01 g/l) and thoroughly mixed. The absorbance was measured at 700 nm against a blank in a spectrophotometer. A higher absorbance indicates a higher reducing power. EC50 value (mg/ml) is the effective concentration at which the absorbance was 0.5 for reducing power and was obtained from linear regression analysis. Ascorbic acid was used as control.
Identification of phenolic compounds using HPLC
The identification of compounds was done using HPLC system equipped with a reversed phase C18 analytical column of 4.6 × 100 mm and 3.5 μm particle size (Zorbax Eclipse XDB C18). The DAD detector was set to a scanning range of 425 nm. Temperature of column was maintained at 25 °C. The volume of injected extract was 2 μl and 0.4 ml/min was the mobile phase flow-rate. Mobile phase B was milli-Q water constituted of 0.1% formic acid and mobile phase A was methanol. The optimized chromatographic condition was as follows: 40% A and 60% B. Phenolic compounds identification were obtained by comparing their retention time and the UV spectra with those of pure standards.
Animals
Healthy male Wistar rats weighing (180–220 g) (7–9 week old) were procured from Tunis Pasteur Institute (B.P. 74. 1002 Tunis) and housed in animal cages under standard environmental conditions (temperature 21 ± 1 °C, humidity 60–70%, 12 h light:12 h dark cycle). All animals were fed with standard pellet diet and had free access to drinking water. All experimental procedures were conducted in conformity with institutional guidelines for the care and use of laboratory animals in Tunisia, and the international guidelines on the ethical use of animals (NIH publications No. 80–23).
Animal model of CCl4-induced liver fibrosis
Liver fibrosis was induced by administration of 2 ml of CCl4/olive oil (1:1, V/V) per kg body weight (b.w.) by intraperitoneal injection twice weekly for up to 4 weeks as described by Nakamura et al. (1999).
Study design
Animals were randomly divided into five groups of six rats each as follows:
Group 1: normal control rats administered distilled water daily (control group);
Group 2: rats administered C. longa root powder at 200 mg/kg b.w. twice a week by using intragastric intubation for 4 weeks (C. longa treated group);
Group 3: rats received intraperitoneal injection of CCl4 at 2 ml/Kg b.w. twice weekly for 4 weeks (CCl4 treated group);
Group 4: rats were treated with C. longa root powder (200 mg/kg b.w.) for 4 weeks and then injected with CCl4 (2 ml/Kg b.w.) twice weekly for 4 weeks (preventive group);
Group 5: rats were treated with CCl4 (2 ml/Kg b.w.) twice weekly for 4 weeks and then received C. longa root powder (200 mg/kg b.w.) twice weekly for 4 weeks (curative group).
Sample collection and analytical procedures
At the end of the treatment, food and water were removed 12 h prior to sacrifice. Animals were weighed and then anesthetized by injecting intraperitoneally 100 µg/g of pentobarbital sodium solution. Blood samples were obtained from animals by cardiac puncture and were allowed to clot at room temperature for 30 min and then centrifuged at 3000 × g for 15 min. The livers were quickly excised from the rats and then rinsed in normal cold saline solution (pH 7.4). Following this, the organs were blotted with filter paper and weighed. Gross examination was conducted to check for gross abnormalities of the organs. The liver indices were calculated as the percentage of the body mass. Each liver was excised into two parts. The left lobe was immersed in isotonic 10% buffered formalin for histological assessment and processed for light microscopy while the right lobe was rapidly stored at -80 °C until required for further use in the experiment.
Serum analysis and liver function tests
Activities of serum enzymes aspartate aminotransaminase (AST) and alanine aminotransaminase (ALT) were determined, in addition to the serum levels of total bilirubin, total proteins (TP), total cholesterol (TC) and triglycerides (TG) using commercial assay kits (Abbott Laboratories, USA.).
Assay of enzymatic antioxidants
Superoxide dismutase (SOD) activity was determined in liver homogenate according to the method of Beyer and Fridovich (1987). Catalase (CAT) activity was measured using the method of Aebi (1984) and glutathione peroxidase (GPx) activity was measured according to the method of Flohe and Gunzler (1984).
Histopathological study
Liver tissues were embedded in paraffin and processed for hematoxilin-eosin (H&E) and Masson’s trichrome staining. Four fields were randomly selected from each section and histopathological evaluation was performed twice.
Statistics
Statistical analysis was performed with Statistica TM software, using one-way analysis of variance (ANOVA). Statistical assessments of differences between mean values were performed by Duncan’s multiple range test at p ≤ 0.05.
Results and discussion
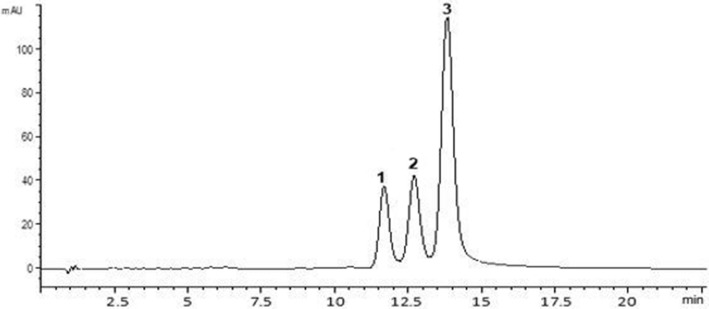
The hepatoprotective effects of plant extracts are mainly related to their richness in phenolic compounds. Phenolic compounds are secondary metabolites widely found in plants, mostly represented by phenolic acids and flavonoids. The growing interest in these bioactive components is principally due to their antioxidant potential and the association between their preventive and curative capacities for some diseases (Serairi Beji et al., 2018). This work was undertaken to determine the phytochemical composition of turmeric powder and to evaluate its preventive and curative effects against CCl4-caused hepatotoxicity in rats. As can be seen in Table 1, total polyphenol and flavonoid contents of turmeric powder were 26.35 mg GAE/g DW and 12.35 mg CE/g DW, respectively. Using HPLC analysis of turmeric powder (Fig. 1), three principle phenolic compounds were detected, belonging essentially to flavonoid class, which were curcumin with 62.97%, demethoxycurcumin with 20.86% and bisdemethoxycurcumin with 16.17%. The antioxidant potential of curcuma longa roots was anticipated by two methods largely used, namely DPPH free radical-scavenging activity and reducing power assays (Table 1). C. longa root powder exhibited a significant ability to quench DPPH radical (IC50 = 35.75 µg/ml) and iron (EC50 = 105 µg/ml). In this study, the contents of total phenolics (26.35 mg GAE/g DW) and flavonoids (12.35 mg CE/g DW) from C. longa root powder were higher than reported by Trinidad et al. (2012) with 1.74 and 1.25 mg GAE/g, respectively. In other study, Tanvir et al. (2017) had determined phenolic and flavonoid contents of ethanolic root extract from different C. longa varieties ranged from 45.2 to 160.7 mg GAE/g and 2.9–54.6 mg CE/g DW, respectively. Akinola et al. (2014) recorded 39.4 mg GAE/g of total phenolic content from methanolic turmeric extract. However, total phenolic content of methanolic extract from C. longa flowers was about 2.10 mg GAE/g (Kumar et al., 2016). In fact, total phenolic and flavonoid contents varied as function of plant organ, variety analyzed and solvent extraction. Tested for activity against DPPH radical, the methanolic extract of C. longa (IC50 = 35.75 µg/ml) is comparable to that of the authentic standard BHT (IC50 = 24 µg/ml). Turmeric powder showed a higher Fe3+ reducing power (EC50 = 105 µg/ml) than BHT (EC50 = 130 µg/ml). A previous study carried out with ethanolic root extract of different C. longa varieties shown that IC50 values for DPPH scavenging were ranged from 1.08 μg/ml to 16.55 μg/ml (Tanvir et al., 2017). This ability to reduce ROS production has been attributed to the presence of natural antioxidants such as phenolic compounds. Earlier studies have claimed that phenolic compounds possess diverse pharmacological effects such as antioxidant, anti-inflammatory and hepatoprotective properties (Ranawat et al., 2010). Curcumin has been reported to be the active principle of C. longa; several recent studies showed the strong antioxidant action of curcumin (Zheng et al., 2018). Previous studies reported that curcumin, the main active compound obtained from C. longa pretreatment prevented oxidative stress and liver damage, and treatment with curcumin attenuated liver injury induced by ethanol, thioacetamide, iron overdose, cholestasis and acute subchronic and chronic CCl4 intoxication (Aziz et al., 2013). The phenolic composition of methanolic turmeric extract can explain its highest biological activity. In fact, the great ability of turmeric powder against CCl4-induced hepatotoxicity was may be correlated to the presence of power antioxidant compounds such bisdemethoxycurcumin (16.17%), demethoxycurcumin (20.86%) and curcumin (62.97%) which were the three abundant phenolic molecules in turmeric powder.
Table 1.
Determination of total polyphenols (mg GAE/g DW), flavonoids (mg CE/g DW), DPPH scavenging activity (IC50, µg/ml) and reducing power (EC50, µg/ml) obtained from turmeric powder
| Total polyphenols (mg GAE/g DW) | Total flavonoids (mg CE/g DW) | DPPH (IC50, µg/ml) | Reducing power (EC50, µg/ml) | |
|---|---|---|---|---|
| Curcuma extract | 26.35 ± 0.51 | 12.35 ± 0.40 | 35.75 ± 0.52 | 105 ± 0.0 |
| BHT | – | – | 24 ± 0.20 | 130 ± 0.0 |
Total polyphenol content was expressed by mg gallic acid equivalent/g; total flavonoid content was expressed by mg catechin equivalent/g; EC50 value is the effective concentration at which the antioxidant activity was 50%; the absorbance was 0.5 for reducing power; IC50 = half-maximal inhibitory concentration;1,1-diphenyl-2-picrylhydrazyl (DPPH) radical was scavenged by 50%; BHT: butylated hydroxytoluene was used as positive control; results were given as mean ± SD from triplicate estimations
DW dry weight
Fig. 1.
Chromatographic profile of curcuminoids obtained from methanolic extract of Curcuma longa monitored at 425 nm: 1. bisdemethoxycurcumin, 2. demethoxycurcumin; 3. curcumin
To evaluate the capacity of turmeric powder to cure liver, we have used CCl4 to induce hepatotoxicity in rats. For the treated rats, Fig. 2 showed that before embarking on the experiment, all groups had no significant difference in body weight (p > 0.05). Results showed that rats administered with turmeric powder (group 2) had similar body mass as the control rats (group 1). However, CCl4-treated rats displayed a significant decrease of body mass in comparison with the control. This reduction could be attributed to the toxic effects of CCl4 commonly used to induce hepatic injury or necrosis. These results are in agreement with previous observations in rats administered with thioacetamide (Kadir et al., 2013). When treated with turmeric powder, rats in group 5 exhibited an increase of their body mass, but this was still significantly lower than control. However, the preventive effect of turmeric powder in group 4 (administered with turmeric and then with CCl4) was more pronounced, in fact, the reduction of body mass was less as compared to group 3 (treated with CCl4) and group 5 (treated with CCl4 and then with turmeric). This reduction of body mass in group 4 could be explained by a great dispense of energy to defend against CCl4 toxicity. The liver plays a major role in protein metabolism and synthesis of amino acids (Kadir et al., 2013). Therefore, levels of total protein and albumin will be affected by CCl4-induced fibrosis. Total protein and albumin concentrations in group 3 (treated with CCl4) were significantly lower in comparison to control group. These alterations are frequently observed in liver diseases and correlated with the severity of fibrosis (Thapa and Walia, 2007). In CCl4-treated group concurrently administered with turmeric as preventive (group 4) or curative (group 5) agent, the levels of total protein and albumin were significantly increased compared to group 3. This improvement is indicative of the beneficial effects of the curcuma administration in protecting the liver. It has been reported that hepatotoxicity was accompanied by a marked increase in total cholesterol and triglycerides levels, revealing the perturbation of membrane stability and function (Pari and Amali, 2005). The results of blood biochemical parameters are given in Table 2. Our findings confirmed these observations and showed that CCl4 treatment caused a significant increase in the levels of serum cholesterol and triglycerides. Oral supplementation with turmeric resulted in a significant decrease in serum lipid, which was indicative of the protective effect of C. longa root powder. These results were similar to those of Pari and Amali (2005), where the treatment of rat with tetrahydrocurcumin inhibited the negative effect of CCl4. Measurements of ALT, AST activities and total bilirubin levels are widely used as markers in evaluating the degree of liver injury since under these conditions high levels of ALT and AST are released from cytosol into the blood. In the present study, CCl4 significantly increased the activity of ALT and AST (3 to 5 times as compared to group 1, respectively) and the total bilirubin level (Table 2). These results were also reported in previous work using chloroquine (Pari and Amali, 2005), quinine (Farver and Lavin, 1999) and amodiaquine (Farombi et al., 2000) as hepatotoxicity-induced agents. In addition, rats injected with CCl4, in the absence of oral administration of turmeric powder (group 3), developed severe liver injury and fibrosis, as evidenced by the prominent steatosis of hepatocytes, pericellular and periportal bridging fibrosis, and distortion of liver architecture. In fact, serum ALT and AST activities were determined for enzymatic evidence of liver injury. However, the pre-treatment with turmeric powder offered hepatoprotection as evidenced by the inhibition of the increase in ALT and AST activities and total bilirubin level. These results are in accordance with the study reported by Al-Jassabi et al. (2012) showing that pretreatment by C. longa showed reduction in bilirubin, AST and ALT activities. This is an indication of stabilization of plasma membranes as well as repair of hepatic tissue damage caused by CCl4. The over-production of ROS induced by CCl4 caused oxidative destruction of cell plasma membrane (Reiter, 1995) and consequently liver diseases.
Fig. 2.
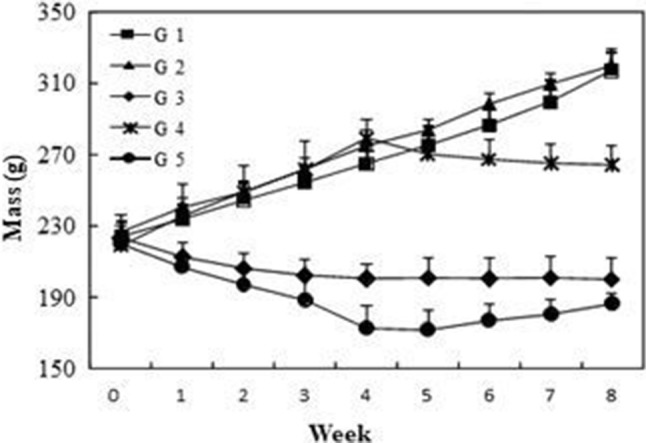
Effects of turmeric powder on body mass evolution of rats with liver fibrosis. Values are the means of 6 replicates ± SD. G1: control group, G2: C. Longa powder administered group, G3: CCl4-treated group, G4: group administered with C. Longa powder and then treated with CCl4 and G5: group treated with CCl4 and then with C. Longa powder
Table 2.
Changes in biochemical parameters of control and treated rats
| Group | AST (IU L−1) | ALT (IU L−1) | Total cholesterol (g L−1) | Triglyceride (g L−1) | Total protein (g L−1) | Albumin (g L−1) | Total bilirubin (g L−1) |
|---|---|---|---|---|---|---|---|
| G1 | 53.6 ± 1.0c | 42.2 ± 0.6d | 0.38 ± 0.4c | 1.01 ± 0.9c | 70.8 ± 0.3a | 12.9 ± 0.2a | 2.1 ± 0.1c |
| G2 | 52.7 ± 1.3c | 41.7 ± 0.3d | 0.34 ± 0.5d | 1.00 ± 0.7c | 70.6 ± 0.3a | 12.5 ± 0.2a | 2.1 ± 0.1c |
| G3 | 266.1 ± 1.9a | 130.4 ± 0.8a | 0.87 ± 0.5a | 1.70 ± 1.3a | 36.8 ± 0.6c | 6.2 ± 0.2c | 7.7 ± 0.7a |
| G4 | 61.0 ± 2.1b | 52.6 ± 0.9c | 0.45 ± 1.8b | 1.15 ± 2.1b | 65.9 ± 1.8b | 10.9 ± 0.1b | 3.5 ± 0.5b |
| G5 | 55.1 ± 1.2c | 58.3 ± 0.7b | 0.42 ± 1.4b | 1.07 ± 0.9c | 68.3 ± 1.4b | 13.1 ± 0.3a | 3.1 ± 0.3b |
G1: control group, G2: C. longa administered group, G3: CCl4-treated group, G4: group administered with C. longa and then treated with CCl4 and G5: group treated with CCl4 and then with C. longa, values are the means of 6 replicates ± SD. For each parameter, different letters indicate significant differences at p ≤ 0.05 as determined by Duncan’s multiple range tests
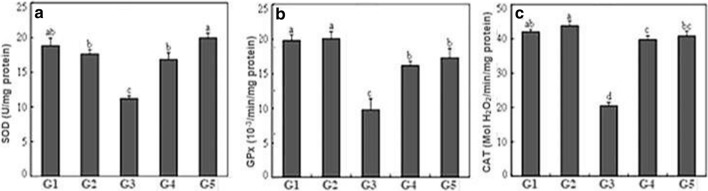
To overcome oxidative damage, enzymatic antioxidant systems, including SOD, CAT and GPx, known as scavengers of superoxide and hydrogen peroxide, are significantly induced during recovery from acute liver injury. These enzymes are located in various cell compartments and represent the main protective mechanisms against oxidative stress. From Fig. 3, it is interesting to note that no significant difference among control group and animals treated with turmeric powder. Compared with the control group, the activities of SOD, CAT and GPx significantly decreased with CCl4 treatment. GPx was the most affected with a 53% reduction followed by CAT with a 51% reduction and SOD was less affected with a 41% reduction. However, both preventive and curative turmeric treatments significantly increased the activity of these enzymes. In fact, turmeric pre-treatment decreased the toxicity of CCl4 showing 50, 40 and 35% amelioration in enzymatic activities of CAT, GPx, and SOD, respectively. In turmeric post-treatment, there was an amelioration of 51% for CAT, 46% for GPx and 45% for SOD. The beneficial effect of turmeric powder was proved by an increase in the activity of these enzymes. This increase reflected the antioxidant efficiency of C. longa root powder to protect liver membrane against lipid peroxidation. So, the free radical scavenging property of turmeric powder is able to donate hydrogen atoms to the free radicals and to convert them into more stable products strongly inhibiting liver damage. Concerning the Fe3+ reduction capacity, it is the reducing ability of antioxidant compounds of turmeric powder from Fe3+ to Fe2+ complex.
Fig. 3.
Effects of turmeric powder on SOD, GPx and CAT activity in rats with liver fibrosis. Values are the means of 6 replicates ± SD. For each parameter, different letters indicate significant differences at p ≤ 0.05 as determined by Duncan’s multiple range tests. G1: control group, G2: C. longa administered group, G3: CCl4-treated group, G4: group administered with C. longa and then treated with CCl4 and G5: group treated with CCl4 and then with C. longa
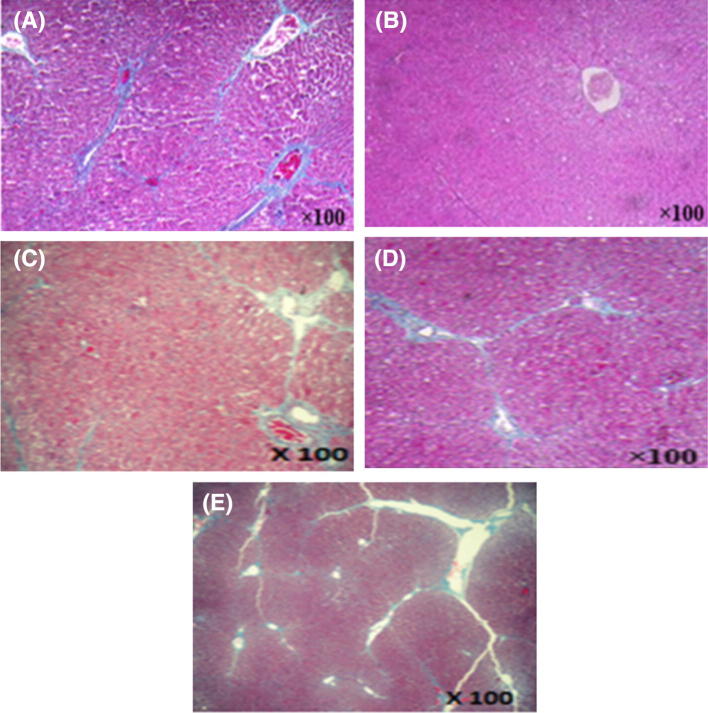
The protective effect of turmeric powder against CCl4-induced oxidative stress in rat liver was also proven with the histopathological observations. Results from the histological studies were in agreement with the measured activities of serum enzymes. As shown in Fig. 4A, there were no abnormalities or histological changes in the livers of normal rats. The treatment with CCl4 caused hepatocyte vacuolization and ballooning degenerations of hepatocytes, associated with neutrophilic infiltration (arrow-heads), which is frequently observed in the case of swelling of the liver cells [Fig. 4(C)]. However, the early hepatic lesions induced by CCl4 were considerably reduced by turmeric powder post-treatment of CCl4-intoxicated rats [Fig. 4(E)]. Turmeric powder pre-treatment before CCl4 intoxication also attenuated the hepatic damage induced by CCl4 [Fig. 4(D)]. The histological pattern was almost normal in rats treated with C. longa root powder [Fig. 4(B)]. These results are in accordance with those obtained by Serairi Beji et al. (2018) which indicated that CCl4 caused histopathological liver changes in rats.
Fig. 4.
Liver histopathology of: (A) control group; (B) C. longa administered group; (C) CCl4-treated group, (D) group administered with C. longa and then with CCl4; (E) group treated with CCl4 and then with C. longa
Acknowledgements
This work was supported by the Tunisian Ministry of Higher Education, Scientific Research and Information and Communication Technologies (LR15CBBC06).
Compliance with ethical standards
Conflict of interest
This research received no grant from any funding agency. Authors declare that there are no conflicts of interest.
References
- Abdel-Lateef E, Mahmoud F, Hammam O, El-Ahwany E, El-Wakil E, Kandil S, Abu Taleb H, El-Sayed M, Hassenein H. Bioactive chemical constituents of Curcuma longa L. rhizomes extract inhibit the growth of human hepatoma cell line (HepG2) Acta Pharmacol. 2016;66:387–398. doi: 10.1515/acph-2016-0028. [DOI] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Akinola A, Ahmad S, Maziah M. Total antioxidant capacity, total phenolic compounds and the effects of solvent concentration on flavonoid content in Curcuma longa and Curcuma xanthorhhiza rhizomes. Med. Aromat. Plants. 2014;3:1–4. doi: 10.4172/2167-0412.1000156. [DOI] [Google Scholar]
- Al-Jassabi S, Ahmed KA, Ameen M. Antioxidant effect of curcumin against microcystin-LR-induced renal oxidative damage in Balb/c mice. Trop. J. Pharm. Res. 2012;11:531–536. doi: 10.4314/tjpr.v11i4.2. [DOI] [Google Scholar]
- Aziz MT, El Ibrashy IN, Mikhailidis DP, Rezq AM, Wassef MA, Fouad HH, Ahmed HH, Sabry DA, Shawky HM, Hussein RE. Signaling mechanisms of a water soluble curcumin derivative in experimental type 1 diabetes with cardiomyopathy. Diabetol. Metab. Syndr. 2013;5:5–13. doi: 10.1186/1758-5996-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereswill S, Munoz M, Fischer A, Plickert R, Haag L, Otto B, Kuhl AA, Loddenkemper C, Gobel UB, Heimesaat MM. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS One. 2010;5:e15099. doi: 10.1371/journal.pone.0015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer WF, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions: Anal. Biochem. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- Chinedum E, Kate E, Sonia C, Ironkwe A, Andrew I. Polyphenolic composition and antioxidant Activities of 6 New turmeric (Curcuma Longa L.) accessions. Recent Pat. Food Nutr. Agric. 2015;7:22–27. doi: 10.2174/2212798407666150401104716. [DOI] [PubMed] [Google Scholar]
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agriculture and Food Chemistry. 2002;50(10):3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- Dufour C, Loonis M, Dangles O. Inhibition of the peroxidation of linoleic acid by the flavonoid quercetin within their complex with human serum albumin. Free Radical Biol. Med. 2007;43:241–252. doi: 10.1016/j.freeradbiomed.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Farombi EO, Olowu BI, Emerole GO. Effect of three structurally related antimalarial drugs on liver microsomal components and lipid peroxidation in rats. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2000;126:217–224. doi: 10.1016/s0742-8413(00)00116-x. [DOI] [PubMed] [Google Scholar]
- Farver DK, Lavin MN. Quinine-induced hepatotoxicity. Ann. Pharmacother. 1999;33:32–34. doi: 10.1345/aph.18172. [DOI] [PubMed] [Google Scholar]
- Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/S0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Liver fibrosis - from bench to bedside. J. Hepatol. 2003;38:S38–S53. doi: 10.1016/S0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Ginès P, Cardenas A, Arroyo V, Rodes J. Management of cirrhosis and ascites. N. Engl. J. Med. 2004;350:1646–1654. doi: 10.1056/NEJMra035021. [DOI] [PubMed] [Google Scholar]
- Girish C, Pradhan SC. Drug development for liver diseases: focus on picroliv, ellagic acid and curcumin. Fundam. Clin. Pharmacol. 2008;22:623–632. doi: 10.1111/j.1472-8206.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- Hatano T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem Pharm Bull. 1988;36:2090–2097. doi: 10.1248/cpb.36.2090. [DOI] [PubMed] [Google Scholar]
- Kadir FA, Kassim NM, Abdulla MA, Yehye WA. Hepatoprotective role of ethanolic extract of Vitex negundo in thioacetamide-induced liver fibrosis in male rats”. Evid Based Compl. Alt. Med. 2013;20:1–9. doi: 10.1155/2013/739850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Yoo HS, Kim JC, Park CS, Choi MS, Kim M, Choi H, Min JS, Kim YS, Yoon SW, Ahn JK. Antiviral effect of Curcuma longa Linn extract against hepatitis B virus replication. J. Ethnopharmacol. 2009;124:189–196. doi: 10.1016/j.jep.2009.04.046. [DOI] [PubMed] [Google Scholar]
- Ksouri R, Falleh H, Megdiche W, Trabelsi N, Mhamdi B, Chaieb K, Bakrouf A, Magné C, Abdelly C. Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents. Food Chem. Toxicol. 2009;47:2083–2091. doi: 10.1016/j.fct.2009.05.040. [DOI] [PubMed] [Google Scholar]
- Kumar A, Monika S, Singh PP, Singh SK, Pratima R, Pandey KD. Antioxidant Efficacy and curcumin content of turmeric (Curcuma longa L.) flower. Int. J. Curr Pharm. Res. 2016;8:112–114. [Google Scholar]
- Nakamura T, Akiyoshi H, Saito I, Sato K. Adenovirus-mediated gene expression in the septal cells of cirrhotic rat livers. J Hepatol. 1999;30:101–106. doi: 10.1016/S0168-8278(99)80013-0. [DOI] [PubMed] [Google Scholar]
- Pari L, Amali DR. Protective role of tetrahydrocurcumin (THC) an active principle of turmeric on chloroquine induced hepatotoxicity in rats. J Pharm Sci. 2005;8:115–123. [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Ranawat L, Bhatt J, Patel J. Hepatoprotective activity of ethanolic extracts of bark of Zanthoxylum armatum DC in CCl4-induced hepatic damage in rats. J. Ethnopharmacol. 2010;127:777–780. doi: 10.1016/j.jep.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB. J. 1995;9:526–533. doi: 10.1096/fasebj.9.7.7737461. [DOI] [PubMed] [Google Scholar]
- Serairi Beji R, Aidi Wannes W, Hamdi A, Tej R, Ksouri R, Saidani Tounsi M, Lachaal M, Karray-Bouraoui N. Antioxidant and hepatoprotective effects of Asparagus albus leaves in carbon tetrachloride-induce liver injury rats. J. Food Biochem. 2018;42:1–11. doi: 10.1111/jfbc.12433. [DOI] [Google Scholar]
- Taniguchi M, Takeuchi T, Nakatsuka R, Watanabe T, Sato K. Molecular process in acute liver injury and regeneration induced by carbon tetrachloride. Life Sci. 2004;75:1539–1549. doi: 10.1016/j.lfs.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Tanvir EM, Hossen MdS, Hossain MdF, Afroz R, Gan SH, Khalil MdI, Karim N. Antioxidant properties of popular turmeric (Curcuma longa) varieties from Bangladesh. J. Food Qual. 2017;2017:1–7. doi: 10.1155/2017/8471785. [DOI] [Google Scholar]
- Thapa BR, Walia A. Liver function tests and their interpretation. Indian J. Pediat. 2007;74:663–671. doi: 10.1007/s12098-007-0118-7. [DOI] [PubMed] [Google Scholar]
- Trinidad TP, Sagum RS, De Leon MP, Mallillin AC, Borlagdan MP. Zingiber officinale and Curcuma longa as potential functional foods/ingredients. Food Public Health. 2012;2:1–4. doi: 10.5923/j.fph.20120202.01. [DOI] [Google Scholar]
- Veidal SS, Karsdal MA, Nawrocki A, Larsen MR, Dai Y, Zheng Q, Hägglund P, Vainer B, Skjot-Arkil H, Leeming DJ. Assessment of proteolytic degradation of the basement membrane: a fragment of type IV collagen as a biochemical marker for liver fibrosis. Fibrogenesis Tissue Repair. 2011;4:2–11. doi: 10.1186/1755-1536-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrba J, Modriansky M. Oxidative burst of Kupffer cells: target for liver injury treatment. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2002;146:15–20. doi: 10.5507/bp.2002.003. [DOI] [PubMed] [Google Scholar]
- Wang JY, Guo JS, Yang CQ. Expression of exogenous rat collagenase in vitro and in a rat model of liver fibrosis. World J. Gastroenterol. 2002;8:901–907. doi: 10.3748/wjg.v8.i5.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler-Normann C, Herkel J, Lohse AW. Mouse models of liver fibrosis. Z. Gastroenterol. 2007;45:43–50. doi: 10.1055/s-2006-927387. [DOI] [PubMed] [Google Scholar]
- Yang J, Guo J, Yuan J. In vitro antioxidant properties of rutin. LWT Food Sci Technol. 2008;41:1060–1066. doi: 10.1016/j.lwt.2007.06.010. [DOI] [Google Scholar]
- Zheng J, Cheng J, Zheng S, Feng Q, Xiao X. Curcumin, a polyphenolic curcuminoid with its protective effects and molecular mechanisms in diabetes and diabetic cardiomyopathy. Front. Pharmacol. 2018;9:472. doi: 10.3389/fphar.2018.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]