Abstract
This study was to establish an integrated process for the co-production of γ-aminobutyric acid (GABA) and live probiotics. Six probiotic bacteria were screened and Bacillus subtilis ATCC 6051 showed the highest GABA-producing capacity. The optimal temperature and initial pH value for GABA production in B. subtilis were found to be 30 °C and 8.0, respectively. A variety of carbon and nitrogen sources were tested, and potato starch and peptone were the preferred carbon and nitrogen sources for GABA production, respectively. The concentrations of carbon source, nitrogen source and substrate (sodium l-glutamate) were then optimized using the response surface methodology. The GABA titer and concentration of viable cells of B. subtilis reached 19.74 g/L and 6.0 × 108 cfu/mL at 120 h. The GABA titer represents the highest production of GABA in B. subtilis. This work thus demonstrates a highly efficient co-production process for GABA and probiotic B. subtilis cells.
Keywords: γ-Aminobutyric acid, Bacillus subtilis ATCC 605, Viable cells, Optimization, Response surface methodology
Introduction
γ-Aminobutyric acid (GABA), a four-carbon non-proteinogenic amino acid, is well-known as its diverse biological functions such as anxiety inhibition, sleep promotion, blood pressure reduction, diabetes treatment, and immune enhancement (Diana et al., 2014; Park and Oh, 2006; Pham et al., 2015). In addition, GABA has also been applied extensively in agriculture for fruit/vegetable cultivation, fruit/vegetables preservation and animal feeds. GABA can alleviate the low-light induced stress via adjusting the antioxidant defense system and improving photochemical efficiency in pepper seedlings (Li et al., 2017). Exogenous GABA treatment may be an effective method to promote growth and production yield of higher plants under soil salinity conditions (Li et al., 2015). GABA can reduce chilling injury in tomato seedlings at low temperatures (Malekzadeh et al., 2014), and it has shown protective effects in preventing freezing damage and maintaining banana fruit quality (Wang et al., 2014b). GABA treatment can decrease the loss of citrate and some important amino acids and thus becomes a very promising way to maintain postharvest quality (Sheng et al., 2017). On the other hand, diets containing GABA for Wenchang chicken can reduce heat stress induced injuries and improve the growth performance under heat stress conditions (Chen et al., 2015). Similarly, GABA as supplements for dairy cows can reduce heat stress by alleviating rectal temperature, increase feed intake and improve the milk production and nutritional quality (Cheng et al., 2014). GABA is also used as a feed additive to improve the productivity and egg quality in layers (Park and Kim, 2016).
In addition to small molecules such as GABA, probiotics, including Bacillus subtilis, are also widely used in plant protection and animal production to produce safe and healthy food. Bacillus subtilis can produce novel antifungal lipopeptide antibiotic. Therefore, B. subtilis has been used to control fruit postharvest disease (Janisiewicz and Korsten, 2002). Bacillus subtilis as a multifunctional probiotic bacterium, has the potential capacity used in functional feeds for aquaculture. Bacillus subtilis can be used in pig farming to improve the growth performance and lipid metabolism in subcutaneous fat (Olmos and Paniagua-Michel, 2014).
Therefore, it is desirable to integrate the biological activities of GABA and probiotics in one production process. The aim of this study was to establish a co-production process of B. subtilis and GABA, and the resulting product will have great potential in agriculture for green and healthy plant or animal production.
Materials and methods
Microbial strains and cultivation conditions
Lactobacillus bulgaricus ATCC 11842, Streptococcus thermophilus ATCC 19258, Lactobacillus casei ATCC 393, Lactobacillus casei NRRL B-441, B. subtilis ATCC 6051 and Bacillus sp. NRRL B-14911 were obtained from the American Type Culture Center (ATCC) or Agricultural Research Service Culture Collection (NRRL). L. bulgaricus ATCC 11842, S. thermophilus ATCC 19258, L. casei ATCC 393 and L. casei NRRL B-441 were cultured in 50-mL centrifuge tube containing 40 mL of MRS medium at 30 °C without shaking for 5 days (three replicates). Bacillus subtilis ATCC 6051 and Bacillus sp. NRRL B-14911 were grown in 250-mL flasks containing 100 mL of LB medium at 30 °C on a rotary shaker at 200 rpm for 5 days (three replicates). Sodium l-glutamate was added as substrate to the cultures at a final concentration of 5 g/L at 48 h for GABA production.
Determination of the titers of GABA
The GABA titer in the fermentation broths were measured using a colorimetric method. Briefly, 1 mL of fermentation broth of each sample was taken and centrifuged at 9391×g for 10 min. Three hundred microliters of supernatant was collected in a test tube, into which 0.2 mL of 0.2 M borate buffer (pH 9.0), 1 mL of 6% phenol and 0.8 mL of 5% sodium hypochlorite were added. The test tube was oscillated intensively and put in a boiling water bath for 10 min, and then placed immediately into an ice bath for 10 min. The tube was shaken vigorously until the blue color appeared. Finally, the reaction mixture was diluted with 2 mL of 60% ethanol, and the optical density of the sample was recorded at 645 nm on a UV–Vis spectrometer (Zhang et al., 2014).
Selection of carbon and nitrogen sources for GABA production
To find out what carbon source works best for GABA production, the broth was inoculated and cultured using the above-described method. A modified LB broth (10 g tryptone/L, 5 g yeast extract/L, 5 g NaCl/L and 2.5 g K2HPO4/L) served as the control medium. The medium composition of the experimental groups contained 10 g tryptone/L, 5 g yeast extract/L, 5 g NaCl/L, 2.5 g K2HPO4/L and the selected carbon source at a final concentration of 2.5 g/L. Nine different carbon sources were tested, including glucose, lactose, sucrose, fructose, glycerol, dextrin, potato starch, soluble starch, and malt extract.
A similar approach was used to identify the best carbon source for GABA production. A modified LB broth (10 g tryptone/L, 5 g yeast extract/L, 5 g NaCl/L, 2.5 g K2HPO4/L and 2.5 g potato starch/L) was used as the control. Tryptone (10 g/L) and yeast extract (5 g/L) in LB medium served as the control nitrogen source. The medium composition of the experimental groups contained 5 g NaCl/L, 2.5 g K2HPO4/L, 2.5 g potato starch/L and the selected nitrogen source at a final concentration of 15 g/L. Seven nitrogen sources were tested, including NaNO3, (NH4)2HPO4, tryptone, peptone, milk power, yeast extract, and soy flour.
Determination of the concentrations of viable cells of B. subtilis
To determine the concentrations of viable cells of B. subtilis in the cultures, five serial dilutions were prepared with fresh LB medium (three replicates). The diluted cultures were spread on LB agar plates, and incubated at 30 °C for 24 h before enumeration (Wang et al., 2014a).
Optimization of the culture medium using the response surface methodology
Response surface methodology (RSM) has been widely used as a statistical tool in the investigation and optimization of several complex processes (Filotheou et al., 2010). In the RSM design, the Box–Behnken experimental design (BBD) needs the fewest runs in the experimental design (Ay et al., 2009). In this work, BBD was used to optimize the culture medium for GABA production. All experiments were performed in triplicate, and the averages of the GABA production were used as the responsive values. ANOVA evaluated the significant variation in GABA production in different culture media (Wang et al., 2015). A second-order polynomial regression model was calculated using BBD analysis. The optimal medium composition for the production of GABA was obtained through Design Expert version 10.
Statistical method
Statistical differences were calculated using a paired Student’s t-test. A paired two-sided Student’s t-test was used to determine the statistical significance of differences in GABA production. A two-tailed p value of < 0.05 was considered to be significant.
Results and discussion
Screening of an efficient GABA-producing strain from six probiotic bacteria
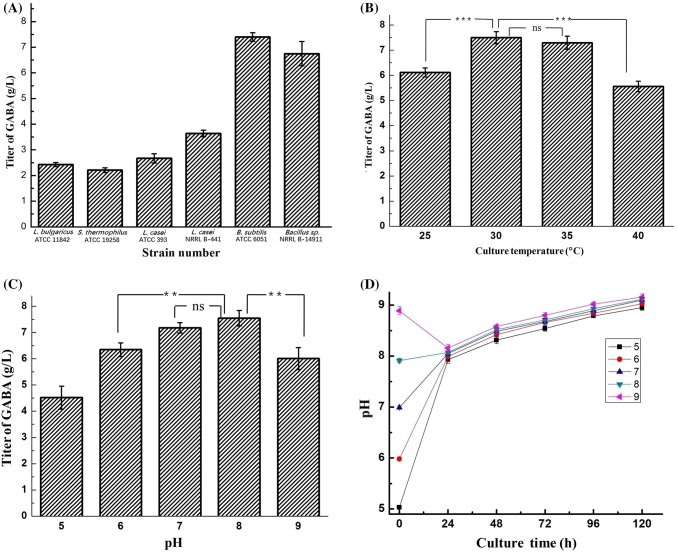
Probiotic bacteria such as lactic acid bacteria are widely used to produce GABA because its well-known ability to produce this compound (Dhakal et al., 2012). Some Bacillus strains were also reported to produce GABA using solid-state fermentation (Suwanmanon and Hsieh, 2014b; Torino et al., 2013). However, there were few studies on GABA production through liquid fermentation. In this study, a total of 4 lactic acid bacteria (Lactobacillus bulgaricus ATCC 11842, Streptococcus thermophilus ATCC 19258, Lactobacillus casei ATCC 393, and Lactobacillus casei NRRL B-441) and 2 Bacillus strains (B. subtilis ATCC 6051 and Bacillus sp. NRRL B-14911) were evaluated for their GABA-producing ability. All of them were able to produce GABA (Fig. 1A). Among these six strains, the two Bacillus strains showed better ability to produce GABA than the four lactic acid bacteria strains. Bacillus subtilis ATCC 6051 showed the highest GABA production titer (7.40 ± 0.17 g/L). Moreover, B. subtilis ATCC 6051 is a food grade probiotic. It was previously used to produce fermented edible seeds containing high levels of bioactive components and B. subtilis cells through solid state fermentation (Gan et al., 2017; Torino et al., 2013). The same strain was also used for microbial biotransformation of a synthetic glucocorticoid named dexamethasone, yielding three metabolites including 6-hydroxydexamethasone, 17-oxodexamethasone, and 6-hydroxy-17-oxodexamethasone. It may be used as an in vitro model to understand the metabolism of similar glucocorticoids (Pervaiz et al., 2015). Bacillus subtilis ATCC 6051 was previously reported to have a weak capacity to produce GABA (2.69 mg/g after 96-h fermentation) with solid state fermentation (Limón et al., 2015). By contrast, our results indicated that it produces a higher amount of GABA in liquid fermentation. Therefore, it will be of interest to combine the benefits of GABA and B. subtilis for agricultural applications.
Fig. 1.
Comparison of the production of GABA by six bacterial strains and the effects of temperature and pH on GABA production. (A) Evaluation of GABA production by six different bacterial strains at 30 °C for 5 days. (B) Effect of culture temperature on GABA production in B. subtilis ATCC 6051. (C) Effect of initial pH on GABA production in B. subtilis ATCC 6051. (D) pH changes of the broths during the 120-h fermentation period
Effect of culture temperature and initial pH on GABA production in B. subtilis ATCC 6051
The effect of the fermentation temperature on GABA production by B. subtilis ATCC 6051 in LB broth was tested. Four different temperatures were tested, including 25, 30, 35 and 40 °C. Figure 1B shows that the titers of GABA were 6.12 ± 0.18, 7.50 ± 0.24, 7.30 ± 0.26 and 5.56 ± 0.21 g/L at these temperatures, respectively, and 30 °C showed the best titer among the four tested temperatures. This is consistent with a previous report in which Ghasemi and Ahmadzadeh (2013) found that B. subtilis UTB96 grew better at 30 °C. The effect of initial pH of the culture medium on GABA production was then examined. Five pH values (5, 6, 7, 8 and 9) were tested. As shown in Fig. 1C, the titer of GABA increases with increasing initial pH in the range of pH 5–8. At pH 8, the titer of GABA reached 7.55 ± 0.29 g/L. The titer decreased when the initial pH was 9 and was determined to be 6.01 ± 0.42 g/L. Therefore, the optimal initial pH was found to be 8 for GABA production in B. subtilis ATCC 6051. In contrast, Suwanmanon and Hsieh (2014a) found that the optimal pH value for GABA production was 7.0 when using a B. subtilis strain isolated from rice straw, suggesting that different B. subtilis strains may have different preferences to the initial pH value of the fermentation medium. The pH change of the fermentation broths during the 120-h period was measured. As shown in Fig. 1D, although the five cultures started at different initial pH values, after 24 h of cell growth, the pH of all the broths changed to about 8 and slightly increased to approximately 9 in a similar pattern during the remaining period of fermentation. Thus, the main pH difference among the five cultures (with initial pH of 5, 6, 7, 8 or 9) was mainly shown in the first 24 h, which might have affected the initial growth rate of the cells.
Effect of carbon and nitrogen sources on the GABA production in B. subtilis ATCC 6051
The effect of various carbon and nitrogen sources on GABA production in B. subtilis ATCC 6051 were studied. The GABA titer for each tested carbon source is shown in Table 1. Table 1 shows that the titers of GABA for above different carbon sources were 8.14 ± 0.50, 7.97 ± 0.50, 8.71 ± 0.45, 8.89 ± 0.44, 8.15 ± 0.29, 8.77 ± 0.34, 9.40 ± 0.49, 8.59 ± 0.43 and 9.15 ± 0.42 g/L, respectively, all of which were higher than 7.84 ± 0.51 g/L in the control. Potato starch showed the best titer among the nine tested carbon sources. The results indicated that the carbon source significantly affects GABA production. A comparison of the titers indicated that the slow-acting carbon sources (potato starch, soluble starch and malt extract) have overall higher GABA production titers than quick-acting carbon sources (glucose, lactose, sucrose and fructose). Among the quick-acting carbon sources, fructose is relatively more conducive to the production of GABA and the titer was 8.89 ± 0.44 g/L. Stülke and Hillen (2000) previously reported that glucose was the most preferred source for carbon and energy for B. subtilis. Suwanmanon and Hsieh (2014a) found that fructose is a better carbon source for GABA production. This is consistent with our result. However, potato starch showed an even better effect on GABA production, and the titer reached 9.40 ± 0.49 g/L. Potato starch is cheaper than glucose and fructose. Considering the cost and productivity, potato starch was chosen as the carbon source for the following optimization studies in this work.
Table 1.
The effects of various carbon and nitrogen sources on GABA production in B. subtilis ATCC 6051
| Nutritional components | Titer of GABA (g/L) |
|---|---|
| Carbon source | |
| Control | 7.84 ± 0.51 |
| Glucose | 8.14 ± 0.50 ns |
| Lactose | 7.97 ± 0.50 ns |
| Sucrose | 8.71 ± 0.45* |
| Fructose | 8.89 ± 0.44* |
| Glycerol | 8.15 ± 0.29 ns |
| Dextrin | 8.77 ± 0.34* |
| Potato starch | 9.40 ± 0.49** |
| Soluble starch | 8.59 ± 0.43 ns |
| Malt extract | 9.15 ± 0.42* |
| Nitrogen source | |
| Control | 8.95 ± 0.30 |
| NaNO3 | 2.24 ± 0.15*** |
| (NH4)2HPO4 | 1.25 ± 0.11*** |
| Tryptone | 8.52 ± 0.13* |
| Peptone | 9.86 ± 0.48** |
| Milk power | 3.69 ± 0.17*** |
| Soy flour | 7.10 ± 0.40** |
| Yeast extract | 9.51 ± 0.28* |
*p < 0.05; **p < 0.01; ***p < 0.001; ns: p > 0.05
The effect of nitrogen source on the production of GABA was also examined. NaNO3, (NH4)2HPO4, tryptone, peptone, milk power, soy flour, and yeast extract were respectively provided as the nitrogen source in the culture medium. A modified LB broth (10 g tryptone/L, 5 g yeast extract/L, 5 g NaCl/L, 2.5 g K2HPO4/L and 2.5 g potato starch/L) as the control. Tryptone (10 g/L) and yeast extract (5 g/L) in LB medium served as the control nitrogen source. The experimental groups contained 5 g NaCl/L, 2.5 g K2HPO4/L, 2.5 g potato starch/L and the selected nitrogen source at a final concentration of 15 g/L. The GABA production for each nitrogen source is shown in Table 1. Among the eight tested nitrogen sources, peptone gave the highest titer of GABA (9.86 ± 0.48 g/L). Yeast extract also showed a great effect on GABA production (9.51 ± 0.28 g/L). Similarly, Suwanmanon and Hsieh (2014a) reported that yeast extract was the most promising nitrogen source for GABA production. Moreover, organic nitrogen sources shown much higher production than inorganic nitrogen sources.
Optimization of the culture medium and substrate concentration using the response surface methodology
Carbon and nitrogen sources are two essential nutrients in the culture media. Based on the above results, potato starch and peptone were chosen as the carbon and nitrogen sources, respectively, in the subsequent experiments. GABA is produced from l-glutamine through decarboxylation. Thus, its concentration will affect the production titer of GABA. Three major factors, including potato starch concentration, peptone concentration and sodium l-glutamate concentration, were then used for optimization. Through single-factor experiments, the appropriate ranges for these three factors were determined: 20–60 g/L for peptone concentration, 5–20 g/L for potato starch concentration, and 5–20 g/L for sodium l-glutamate concentration. The data obtained from the BBD (Table 2) presents the design matrix. The GABA titer represented the response. By using Design Expert version 10, quadratic model (Eq. 1) and their subsequent ANOVA (Table 2) were found to be the best model to explain the correlation between the GABA titer and three variables.
| 1 |
where A is peptone concentration, B is potato starch concentration and C is sodium l-glutamate concentration.
Table 2.
Box–Behnken experimental design for GABA production in B. subtilis ATCC 6051
| Run | Factor A | Factor B | Factor C | Titer of GABA | |
|---|---|---|---|---|---|
| Peptone (g/L) | Potato starch (g/L) | Sodium l-glutamate (g/L) | Observed value (g/L) | Predicted value (g/L) | |
| 1 | 40 | 20 | 5 | 13.12 ± 0.22 | 13.38 |
| 2 | 40 | 5 | 20 | 16.73 ± 1.01 | 16.47 |
| 3 | 20 | 12.5 | 5 | 10.50 ± 0.37 | 9.76 |
| 4 | 40 | 12.5 | 12.5 | 15.59 ± 1.10 | 16.10 |
| 5 | 40 | 12.5 | 12.5 | 16.48 ± 0.73 | 16.10 |
| 6 | 60 | 12.5 | 20 | 16.53 ± 1.06 | 17.27 |
| 7 | 40 | 12.5 | 12.5 | 15.28 ± 1.06 | 16.10 |
| 8 | 20 | 20 | 12.5 | 11.71 ± 1.01 | 12.19 |
| 9 | 40 | 5 | 5 | 11.13 ± 0.53 | 11.29 |
| 10 | 60 | 20 | 12.5 | 19.27 ± 1.75 | 18.69 |
| 11 | 20 | 5 | 12.5 | 13.68 ± 0.88 | 14.26 |
| 12 | 60 | 12.5 | 5 | 18.18 ± 1.79 | 18.50 |
| 13 | 40 | 12.5 | 12.5 | 16.45 ± 1.11 | 16.10 |
| 14 | 60 | 5 | 12.5 | 19.96 ± 1.26 | 19.48 |
| 15 | 40 | 20 | 20 | 11.69 ± 0.38 | 11.53 |
| 16 | 40 | 12.5 | 12.5 | 16.68 ± 1.29 | 16.10 |
| 17 | 20 | 12.5 | 20 | 14.61 ± 0.31 | 14.29 |
The model p value of 0.0002 and “lack of fit” p value of 0.2208 from the analysis of ANOVA (Table 3) showed that Eq. (1) was highly significant to describe the actual relationship between the GABA titer and three factors. The p value of component tests were used to determine the significance of each coefficient. The smaller p value indicates a higher significance for the corresponding coefficient (Zhang et al., 2017). The corresponding p values of each coefficient indicated that peptone concentration (p value < 0.0001), potato starch concentration (p value = 0.0346), and sodium l-glutamate concentration (p value = 0.0189) can significantly affect the production of GABA (Table 3). Moreover, the peptone concentration with F-value of 115.36 and p value of < 0.0001 is one of the most important factor for the GABA production (Table 3).
Table 3.
Analysis of variance (ANOVA) for the regression
| Source | SS | DF | MS | F value | Prob > F |
|---|---|---|---|---|---|
| Model | 123.70 | 9 | 13.74 | 23.09 | 0.0002* |
| A | 68.68 | 1 | 68.68 | 115.36 | < 0.0001 |
| B | 4.08 | 1 | 4.08 | 6.85 | 0.0346 |
| C | 5.49 | 1 | 5.49 | 9.23 | 0.0189 |
| AB | 0.41 | 1 | 0.41 | 0.69 | 0.4342 |
| AC | 8.29 | 1 | 8.29 | 13.93 | 0.0073 |
| BC | 12.36 | 1 | 12.36 | 20.75 | 0.0026 |
| A2 | 3.59 | 1 | 3.59 | 6.03 | 0.0438 |
| B2 | 3.14 | 1 | 3.14 | 5.28 | 0.0551 |
| C2 | 17.94 | 1 | 17.94 | 30.14 | 0.0009 |
| Residual | 4.17 | 7 | 0.60 | ||
| Lack of fit | 2.63 | 3 | 0.88 | 2.28 | 0.2208** |
| Pure error | 1.54 | 4 | 0.38 | ||
| Cor total | 127.86 | 16 |
R2 = 0.967 and adjusted R2 = 0.926, * significant, ** not significant
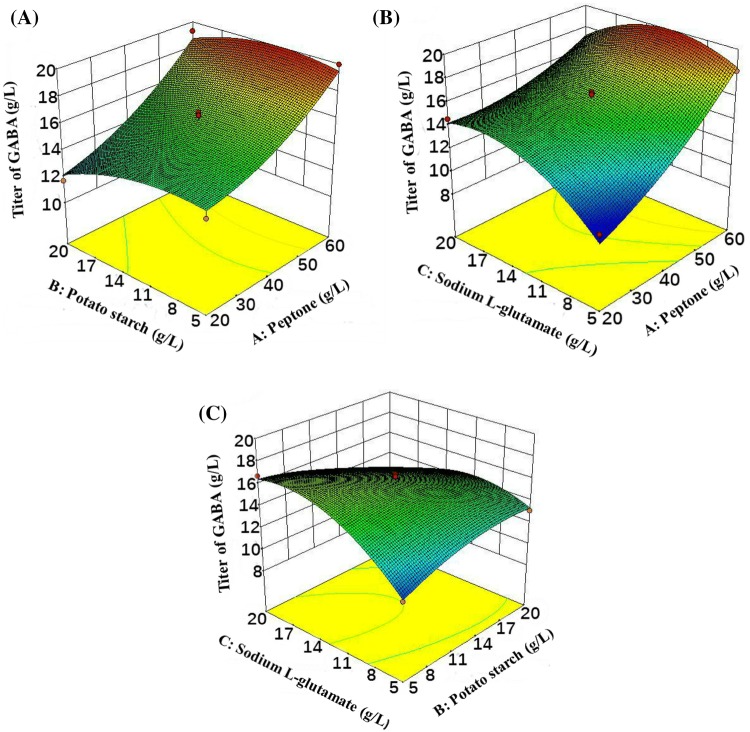
Figure 2A–C showed the effect of GABA production for each pair of factors. The graphs depicted the effects of various factors on GABA production. As shown in this figure, two pairs of the factors (peptone concentration/sodium l-glutamate concentration and potato starch concentration/sodium l-glutamate concentration) exerted a great effect on GABA production.
Fig. 2.
Three-dimensional response surface plots for GABA production in B. subtilis ATCC 6051. (A) Effect of the concentrations of peptone and potato starch on GABA production. (B) Effect of the concentrations of peptone and sodium l-glutamate on GABA production. (C) Effect of the concentrations of potato starch and sodium l-glutamate on GABA production
The optimal composition for GABA production obtained from the maximum point of the model. The optimal conditions for the highest GABA production (20.0 g/L) were obtained with 60 g peptone/L concentration, 11.5 g potato starch/L, and 11.8 g sodium l-glutamate/L.
Co-production of GABA and probiotic B. subtilis with the optimized medium composition
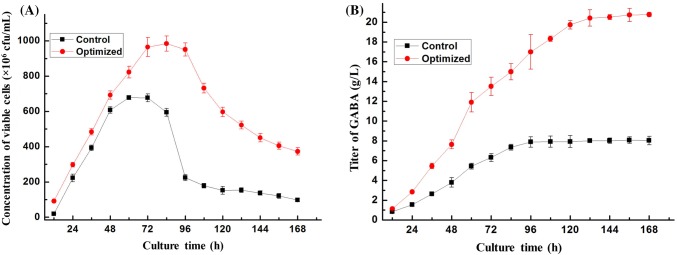
The bacterial growth profile and GABA production from 0 to 168 h were then monitored in the optimized culture medium. The medium without optimization was used as the control. As shown in Fig. 3A, viable cells in the control medium increased rapidly during the first 48 h of incubation and then entered the stationary phase for about 36 h. The maximum concentration of viable cells reached 6.8 × 108 cfu/mL at 60 h. However, in the optimized medium, after about 72 h, the growth of the strain entered the stationary phase, and the highest concentration of viable cells reached 9.9 × 108 cfu/mL at 84 h. The rich nutrients in the optimized medium could support the strain growth for a longer period. However, the stationary phase of the strains in the control and optimized groups just lasted about 36 h and 24 h, respectively, and then the concentration of viable cells rapidly declined. High concentration of GABA may inhibit the growth of the strain and accelerate the aging of the strain.
Fig. 3.
A comparison of the concentration of viable cells of B. subtilis ATCC 6051 and GABA titer before and after optimization. (A) The concentration of viable cells of B. subtilis ATCC 6051 at different time points. (B) The titers of GABA in batch cultures at different time points
Time course analysis of GABA production (Fig. 3B) revealed that the GABA titer in the control group increased rapidly during the first 96 h, and then slowed down and maintained a relative stable level. By contrast, the optimized group had a longer period of active production of GABA and the titer has been increasing steadily in the first 132 h. Accumulation of GABA, death of B. subtilis ATCC 6051 and consumption of the nutrients may contribute to the decreased rate of GABA production in the late stage of the fermentation (Li and Cao, 2010; Tajabadi et al., 2015).
For this co-production process of GABA and live cells of B. subtilis, the GABA titer and concentrations of viable cells in the control group reached 7.89 g/L and 2.3 × 108 cfu/mL at 96 h, respectively. The GABA titer and concentration of viable cells in the optimized group reached 19.74 g/L and 6.0 × 108 cfu/mL at 120 h, respectively. The GABA titer of 19.74 g/L was very close to the predicted value of 20 g/L in the model, indicating that this model is appropriate for optimization of GABA production in B. subtilis. Suwanmanon and Hsieh (2014a) screened a strain of B. subtilis from rice straw, and the titer of GABA reached 15.4 g/L in liquid fermentation. The GABA titer obtained in this study is higher than any other reported production titer by B. subtilis. Optimization of the fermentation conditions increased the GABA production and viable cell concentration by 150.19% and 165.92%, respectively.
In summary, six bacterial strains were tested for GABA production in this work and B. subtilis ATCC 6051 showed the best production ability. The optimal temperature and initial pH value for the biosynthesis of GABA in B. subtilis ATCC 6051 were 30 °C and 8.0, respectively. The optimal medium components for GABA production in B. subtilis ATCC 6051 were 11.481 g potato starch/L, 60 g peptone/L, 5 g NaCl/L, and 2.5 g K2HPO4/L. The optimal concentration of sodium l-glutamate was determined to be 11.825 g/L, which was added into the medium after 48 h. Under the optimized conditions, the GABA titer and concentration of viable cells reached 19.74 g/L and 6.0 × 108 cfu/mL at 120 h, respectively. To conclude, by screening several probiotic strains, our work shows that B. subtilis ATCC 6051 is valuable for producing GABA-rich foods. After rationally optimizing the culture conditions, this research provides a highly efficient co-production process for GABA and probiotic B. subtilis cells. The resulting product may be used in agriculture as health-benefiting plant or animal feed.
Acknowledgements
This work was financially supported by a Grant-In-Aid (16GRNT26430067) from the American Heart Association (USA), the Agricultural and Social Development Program of Hangzhou Science and Technology Bureau of Zhejiang Province (China), the Young College Teachers Studying Abroad fund (Grant No. 3-2016) of Hubei Province (China), Jianghan University Doctoral Research Startup Fund Project (Grant No. 1017-06330003), and Major Technical Innovation Project of Hubei Province (China) (Grant No. 2017ABA147).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
References
- Ay F, Catalkaya EC, Kargi F. A statistical experiment design approach for advanced oxidation of Direct Red azo-dye by photo-Fenton treatment. J. Hazard Mater. 2009;162:230–236. doi: 10.1016/j.jhazmat.2008.05.027. [DOI] [PubMed] [Google Scholar]
- Chen Z, Xie J, Hu MY, Tang J, Shao ZF, Li MH. Protective effects of γ -aminobutyric acid (GABA) on the small intestinal mucosa in heat-stressed Wenchang chicken. J. Anim. Plant Sci. 2015;25:78–87. [Google Scholar]
- Cheng JB, Bu DP, Wang JQ, Sun XZ, Pan L, Zhou LY, Liu W. Effects of rumen-protected gamma-aminobutyric acid on performance and nutrient digestibility in heat-stressed dairy cows. J. Dairy Sci. 2014;97:5599–5607. doi: 10.3168/jds.2013-6797. [DOI] [PubMed] [Google Scholar]
- Dhakal R, Bajpai VK, Baek KH. Production of GABA (γ-aminobutyric acid) by microorganisms: a review. Braz. J. Microbiol. 2012;43:1230–1241. doi: 10.1590/S1517-83822012000400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Quílez J, Rafecas M. Gamma-aminobutyric acid as a bioactive compound in foods: a review. J. Funct Foods. 2014;10:407–420. doi: 10.1016/j.jff.2014.07.004. [DOI] [Google Scholar]
- Filotheou A, Nanou K, Papaioannou E, Roukas T, Kotzekidou P, Liakopoulou-Kyriakides M. Application of response surface methodology to improve carotene production from synthetic medium by Blakeslea trispora in submerged fermentation. Food Bioprocess Tech. 2010;5:1189–1196. doi: 10.1007/s11947-010-0405-6. [DOI] [Google Scholar]
- Gan R-Y, Li H-B, Gunaratne A, Sui Z-Q, Corke H. Effects of fermented edible seeds and their products on human health: bioactive components and bioactivities. Compr. Rev. Food Sci. Food Saf. 2017;16:489–531. doi: 10.1111/1541-4337.12257. [DOI] [PubMed] [Google Scholar]
- Ghasemi S, Ahmadzadeh M. Optimisation of a cost-effective culture medium for the large-scale production of Bacillus subtilis UTB96. Arch. Phytopathol. Plant Protect. 2013;46:1552–1563. doi: 10.1080/03235408.2013.771469. [DOI] [Google Scholar]
- Janisiewicz WJ, Korsten L. Biological control of postharvest diseases of fruits. Annu. Rev. Phytopathol. 2002;40:411–441. doi: 10.1146/annurev.phyto.40.120401.130158. [DOI] [PubMed] [Google Scholar]
- Li H, Cao Y. Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids. 2010;39:1107–1116. doi: 10.1007/s00726-010-0582-7. [DOI] [PubMed] [Google Scholar]
- Li MF, Guo SJ, Yang XH, Meng QW, Wei XJ. Exogenous gamma-aminobutyric acid increases salt tolerance of wheat by improving photosynthesis and enhancing activities of antioxidant enzymes. Biol. Plant. 2015;60:123–131. doi: 10.1007/s10535-015-0559-1. [DOI] [Google Scholar]
- Li Y, Fan Y, Ma Y, Zhang Z, Yue H, Wang L, Li J, Jiao Y. Effects of exogenous γ-aminobutyric acid (GABA) on photosynthesis and antioxidant system in pepper (Capsicum annuum L.) seedlings under low light stress. J. Plant Growth Regul. 2017;36:436–449. doi: 10.1007/s00344-016-9652-8. [DOI] [Google Scholar]
- Limón RI, Peñas E, Torino MI, Martínez-Villaluenga C, Dueñas M, Frias J. Fermentation enhances the content of bioactive compounds in kidney bean extracts. Food Chem. 2015;172:343–352. doi: 10.1016/j.foodchem.2014.09.084. [DOI] [PubMed] [Google Scholar]
- Malekzadeh P, Khara J, Heydari R. Alleviating effects of exogenous Gamma-aminobutiric acid on tomato seedling under chilling stress. Physiol. Mol. Biol. Plants. 2014;20:133–137. doi: 10.1007/s12298-013-0203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos J, Paniagua-Michel J. Bacillus subtilis a potential probiotic bacterium to formulate functional feeds for aquaculture. J. Microb. Biochem. Technol. 2014;6:361–365. doi: 10.4172/1948-5948.1000169. [DOI] [Google Scholar]
- Park JH, Kim IH. Effects of dietary gamma-aminobutyric acid on egg production, egg quality, and blood profiles in layer hens. Vet. Med. 2016;60:629–634. doi: 10.17221/8531-VETMED. [DOI] [Google Scholar]
- Park KB, Oh SH. Enhancement of gamma-aminobutyric acid production in Chungkukjang by applying a Bacillus subtilis strain expressing glutamate decarboxylase from Lactobacillus brevis. Biotechnol. Lett. 2006;28:1459–1463. doi: 10.1007/s10529-006-9112-9. [DOI] [PubMed] [Google Scholar]
- Pervaiz I, Ahmad S, Mukhtar MF, Arshad A, Imran M, Mahmood W. Microbial biotransformation of dexamethasone by Bacillus subtilis (ATCC 6051) Pharm. Chem. J. 2015;49:405–408. doi: 10.1007/s11094-015-1294-9. [DOI] [Google Scholar]
- Pham VD, Lee SH, Park SJ, Hong SH. Production of gamma-aminobutyric acid from glucose by introduction of synthetic scaffolds between isocitrate dehydrogenase, glutamate synthase and glutamate decarboxylase in recombinant Escherichia coli. J. Biotechnol. 2015;207:52–57. doi: 10.1016/j.jbiotec.2015.04.028. [DOI] [PubMed] [Google Scholar]
- Sheng L, Shen D, Luo Y, Sun X, Wang J, Luo T, Zeng Y, Xu J, Deng X, Cheng Y. Exogenous gamma-aminobutyric acid treatment affects citrate and amino acid accumulation to improve fruit quality and storage performance of postharvest citrus fruit. Food Chem. 2017;216:138–145. doi: 10.1016/j.foodchem.2016.08.024. [DOI] [PubMed] [Google Scholar]
- Stülke J, Hillen W. Regulation of carbon catabolism in Bacillus species. Annu. Rev. Microbiol. 2000;54:849–883. doi: 10.1146/annurev.micro.54.1.849. [DOI] [PubMed] [Google Scholar]
- Suwanmanon K, Hsieh PC. Isolating Bacillus subtilis and optimizing its fermentative medium for GABA and nattokinase production. CyTA J. Food. 2014;12:282–290. doi: 10.1080/19476337.2013.848472. [DOI] [Google Scholar]
- Suwanmanon K, Hsieh PC. Effect of gamma-aminobutyric acid and nattokinase-enriched fermented beans on the blood pressure of spontaneously hypertensive and normotensive Wistar-Kyoto rats. J. Food Drug Anal. 2014;22:485–491. doi: 10.1016/j.jfda.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajabadi N, Ebrahimpour A, Baradaran A, Rahim RA, Mahyudin NA, Manap MY, Bakar FA, Saari N. Optimization of gamma-aminobutyric acid production by Lactobacillus plantarum Taj-Apis362 from honeybees. Molecules. 2015;20:6654–6669. doi: 10.3390/molecules20046654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torino MI, Limon RI, Martinez-Villaluenga C, Makinen S, Pihlanto A, Vidal-Valverde C, Frias J. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chem. 2013;136:1030–1037. doi: 10.1016/j.foodchem.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Wang HB, Zhang LW, Luo J, Yu LJ. Rapid and environmentally-friendly extraction of carotenoids from Blakeslea trispora. Biotechnol. Lett. 2015;37:2173–2178. doi: 10.1007/s10529-015-1920-3. [DOI] [PubMed] [Google Scholar]
- Wang L, Li P, Zhang Z, Chen Q, Aguilar ZP, Xu H, Yang L, Xu F, Lai W, Xiong Y, Wei H. Rapid and accurate detection of viable Escherichia coli O157:H7 in milk using a combined IMS, sodium deoxycholate, PMA and real-time quantitative PCR process. Food Control. 2014;36:119–125. doi: 10.1016/j.foodcont.2013.08.011. [DOI] [Google Scholar]
- Wang Y, Luo Z, Huang X, Yang K, Gao S, Du R. Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci. Hort. 2014;168:132–137. doi: 10.1016/j.scienta.2014.01.022. [DOI] [Google Scholar]
- Zhang G, Ren A, Wu F, Yu H, Shi L, Zhao M. Ethylene promotes mycelial growth and ganoderic acid biosynthesis in Ganoderma lucidum. Biotechnol. Lett. 2017;39:269–275. doi: 10.1007/s10529-016-2238-5. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Xiang J, Zhang L, Zhu X, Evers J, van der Werf W, Duan L. Optimizing soaking and germination conditions to improve gamma-aminobutyric acid content in japonica and indica germinated brown rice. J. Funct. Foods. 2014;10:283–291. doi: 10.1016/j.jff.2014.06.009. [DOI] [Google Scholar]