Abstract
Indonesian Lampung Robusta coffee green beans were roasted at eight roasting levels (green bean, early yellow, brown, 1st crack done, very light, light, medium, and dark), followed by grinding and brewing. The physical properties of ground coffee and chemical properties of brewed coffee were analyzed. The resulting data were mapped in order to investigate the critical roasting level. It was observed that major alterations for physicochemical properties of coffee happened after “first crack” roasting level (when water activity (Aw) of bean decreased from 0.22 to 0.15). This cracking is defined as popping sound of the bean during roasting. Continuous formation of melanoidins under low Aw (< 0.15) was followed by slow degradation of chlorogenic acid (5-CQA) and total phenolic compounds. Caffeine was stable during roasting, while antioxidant activity slightly decreased. The “first crack” was determined to be the critical roasting level to produce roasted coffee beans containing high concentrations of phenolics.
Keywords: Roasted coffee, Roasting, Water activity, Bioactive compounds, Antioxidant activity
Introduction
Indonesia is the 4th largest coffee producer in the world after Brazil (Van der Werf et al., 2014), Vietnam (Amarasinghe et al., 2015), and Colombia (ICO, 2017). Coffee plants grow in areas surrounding Indonesia, and most plantations are commercial with a yield of 660,000 metric tons of green bean per year (ICO, 2017). Lampung province, located on South Sumatera, is the most recognized coffee producer in this region, especially for Robusta coffee (Coffea canephora).
Lampung Robusta coffee bean has a unique character that gives coffee brew with a very strong and persistently bitter taste. Bitterness is frequently perceived in beverages rich in bioactive compounds from alkaloid and phenolic groups. However, there is no information regarding the composition of Lampung Robusta coffee. A study of Lampung Robusta coffee bioactive compounds will provide valuable information for seeking the health benefit of the coffee consumption in addition to having a pleasant flavor.
Coffee brew contains at least four groups of bioactive compounds: phenolics; alkaloids; Maillard reaction products (MPRs); and terpenoids (Ding et al., 2014; Hečimović et al., 2011; Ludwig et al., 2014; Van der Werf et al., 2014; Zanin et al., 2016). These bioactive compounds exhibit an antioxidant activity that may be involved in the prevention of certain degenerative diseases. This property is consistent with epidemiological studies showing a significant role for a coffee brew in the prevention of type 2 diabetes mellitus (Zhang et al., 2011), Parkinson’s and Alzheimer’s (Bae et al., 2014), and certain types of cancer (Li et al., 2011; Yu et al., 2011).
Bioactive compound composition in coffee beans and coffee brew are highly dependent on the processing steps, and roasting is the most crucial of these steps. High temperature and low water activity (Aw) in the roasting process facilitate molecular degradation and the formation of new compounds (Gloess et al., 2014; Liu and Kitts, 2011). During the roasting process, thermal degradation and the Maillard reaction are heavily investigated as the greatest contributor not only to changes in physical properties but also to alterations in the chemical composition of the coffee bean (Gloess et al., 2014; Wang and Lim, 2017). During roasting, water evaporation creates gas pressure that causes cell expansion in the coffee bean. Under this condition is reached, destruction of the inter- and intracellular matrix easily occurs, resulting in the porous structure of roasted coffee beans (Fadai et al., 2017). At the same time, coffee bean color gradually turns darker due to the development of melanoidins via the Maillard reaction (Van der Werf et al., 2014; Vignoli et al., 2014). As a consequence of melanoidins formation, several coffee constituents are degraded or incorporated into a melanoidins molecule (Bartel et al., 2015). Phenolic compounds, especially chlorogenic acid (5-CQA), are a noticeable coffee bioactive compound that is decomposed by that mechanism in addition to thermal degradation (Liu and Kitts, 2011; Vignoli et al. 2011).
Mapping of roasted beans color and bioactive compounds in their coffee brews is described by previous method (Vignoli et al., 2014). In addition, other researchers evaluated the effect of the roasting conditions for the coffee bean such as time and temperature on the bioactive compounds of the coffee brew (Liang et al., 2016). However, the information regarding to the behavior of the physicochemical alteration in correlation with water activity (Aw) or moisture content (MC) change of the coffee beans roasted in different levels is lacking. Since Aw plays an important role in facilitating roasting reactions, this information will provide a better understanding of coffee bean physicochemical changes during roasting as a function of Aw. In this study, the bioactive compounds composition in the coffee beans were represented by the composition of their coffee brew in order to provide the data of the bioactive compounds available for consumption. Therefore, this study elucidates the changes of Aw and other physicochemical properties during roasting to evaluate the critical roasting level for the Robusta coffee bean. It is expected that the coffee industry will be able to use the result from this study as a reference for choosing the roasting level of the coffee bean.
Materials and methods
Materials
These chemicals were purchased from Sigma-Aldrich (Germany): gallic acid; chlorogenic acid (5-O-caffeoylquinic acid/5-CQA); caffeine; ascorbic acid; 2,2-diphenyl-1-picrylhydrazyl (DPPH); 2,4,6-Tri(2-pyridyl)-1,3,5-triazine (TPTZ); and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox). Unless listed otherwise, the chemicals were purchased from Merck (Germany).
Sample preparation
Dried processed Robusta Lampung green bean from Eerste Kwaliteit Grade 1 (EK1) was provided by the Aneka Coffee Industry, Surabaya Indonesia. Green coffee (100 g) was fed to a micro-roaster coffee machine (BRZ 2; PROBAT-WERKE Emmerich am, Rhein, Germany) at an initial temperature 200 °C. The beans were roasted to eight levels: green bean (GB), early yellow (EY), brown (BR), 1st crack done (CD), very light (CR), light (LG), medium (MED), and dark (DR), with end roasting temperature ranges of 185–190, 190–195, 195–200, 200–205, 205–210, 210–215, and 215–220 °C, respectively. The roasting levels for early yellow, brown, and 1st crack done samples were determined by these following specification since the commercial bean at those levels were not available. The beans were roasted at 2 min for early yellow, 4 min for brown, and 5 min for 1st crack done, corresponding to the lightness color (L*) listed at Table 1. The 1st crack done roasting level was also determined by hearing the popping sound. Meanwhile, the duration for the others were 6–7 min, and the roasting levels were visually determined based on the targeted lightness color (L*) of the commercial roasted beans as presented in Table 1. Roasted coffee beans were transferred to convection coolers for 5 min and air coolers for 15 min. The beans (15 g) were packed in aluminum foil for individual analysis.
Table 1.
Lightness color (L*) of roasted beans
| Roasting level | Lightness color (L*) |
|---|---|
| Early yellow | 47–49 |
| Brown | 38–40 |
| 1st crack done | 31–33 |
| Very light | 21–23** |
| Light | 20–21** |
| Medium | 18–19** |
| Dark | 17–18** |
**Lightness color (L*) of commercial roasted beans
Roasted coffee bean (15 g) was ground in a grinder machine (Eureka Mignon MK 2; Eureka, Florence, Italy) for 30 s at 4.0 grade. Five grams of ground coffee was brewed with 100 mL of boiling water, heated at 95 °C, and stirred using a magnetic stirrer for 1 min. The sample was stirred in the ice bath for 2 min and filtered through filter paper (Whatman no 1). The total soluble solid content in filtered coffee brew was measured using a SCM-1000 refractometer (HM Digital, Korea).
Measurement of physical properties for ground coffee
Determination of Aw
The water activity of ground coffee was determined using an Aw meter (Rotronic-HygroLab C1; Yokohama, Japan) based on the instruction manual.
Moisture content (MC) measurement
Moisture content of ground coffee was measured using AOAC method (AOAC, 2012). MC was expressed on a g/100 g dry basis (DB).
Color analysis
The color of ground coffee was analyzed by a chromameter (Minolta CR-310; Minolta Camera Co., Ltd., Osaka, Japan). The sample was loaded into a sample holder, and the color was detected according to the manual instruction. The critical color parameter for the coffee sample was lightness, which was represented by L* value (0 for black and 100 for white).
Particle size measurement
Particle size distribution was measured using a sieving method. A multistage American Society for Testing and Materials (ASTM) vibrating screen (Fisher Scientific, Singapore) with a mesh size of 425 µm, 300 µm, and 250 µm was used to distribute the coffee grounds by particle size. Samples (15 g) were shifted for 10 min, and the particle size distribution was calculated based on the percentage of the particles passing through the shifter.
Analysis of chemical composition and AA of coffee brew
Total phenolic compounds (TPC) analysis
TPC analysis was conducted using a spectrometry method (Shetty et al., 1995). The TPC concentrations were quantified by a gallic acid standard curve (triplicate with 6 concentrations range of 20–140 µg/mL). TPC was expressed by g gallic acid equivalent (GAE)/100 g soluble solid.
Chlorogenic acid (5-CQA) determination
Reverse phase-high performance liquid chromatography (RP-HPLC) methods was used for 5-CQA determination (Li et al., 2015), with some modifications. The coffee brew was filtered through a 0.45 µm cellulose acetate membrane (Sartorius; Germany). The filtrate (10 μL) was separated with a Zorbax C18 column (4.6 × 150 mm, 5 µm) (Agilent Technologies, Santa Clara, USA) with a RP-HPLC system equipped with ultra violet-visible (UV–VIS) detector (Agilent Technologies). HPLC grade acetonitrile (A) and 0.02% phosphoric acid in HPLC grade H2O (B) were used as a mobile phase and delivered at 1 mL/min. Sample was eluted in a gradient system: 0 min (5% A), 0–10 min (20% A), 10–15 min (25% A), 15–20 min (25% A), and 20–25 min (5% A). The component was detected at 320 nm. The quantification was performed by a 7-point calibration curve with concentrations range of 10–150 mg/L (triplicate, LoD = 1 mg/L, and R2 = 0.995). The 5-CQA content was expressed as g/100 g soluble solid.
Caffeine analysis
Determination of caffeine was conducted using an RP-HPLC method (DiNunzio, 1985). The coffee extract was passed through a 0.45 µm membrane filter (PTFE; Sigma-Aldrich, Germany), and 10 µL of sample was injected into an HPLC coupled to a UV–VIS detector (Agilent Technologies). HPLC grade methanol and HPLC grade H2O (20:80 v/v) as a mobile phase were delivered at 1 mL/min to a Zorbax C18 (4.6 × 150 mm, 5 µm) column (Agilent Technologies). The component was detected at 254 nm. The concentration of caffeine was calculated by a 6-point standard curve with concentrations range of 6.25–200 mg/L (triplicate, LoD = 0.7 mg/L, and R2 = 0.999). The caffeine concentration was expressed as g/100 g soluble solid.
Melanoidins analysis
The content of melanoidins was determined using a gravimetric method developed by Vignoli et al. (2011), with several modifications. The coffee brew was dialyzed using a Regenerated Cellulose Dialysis Membrane with a cut off of 12–14 kDa (Spectra/por® 2; USA). The coffee brew was transferred to the membrane, placed in a Baker glass containing 650 mL deionized water, and agitated in a shaker (Innova-platform shaker; New Brunswick Scientific, USA) at 150 rpm. Deionized water was changed after 16, 20 and 24 h of agitation until it was colorless, and the used water was scanned using a spectrophotometer at λ 200–800 nm. The retained sample was dried using a freeze dryer (Labconco, USA) at − 47 °C and a pressure of 0.05–0.1 kPa. Dried sample was weighed, and the content of melanoidins was expressed as g/100 g soluble solid.
Antioxidant activity (AA) analysis
The AA analysis of coffee brew using a DPPH method was conducted according to the method developed by Vignoli et al. (2011). The DPPH value was stated by the concentration of compound that provide 50% reduction of DPPH free radicals concentration (IC50). Ferric reducing ability of plasma (FRAP) analysis developed by Vignoli et al. (2011) was also used for determining the AA of the coffee brew. A trolox standard curve (triplicate with 8 concentrations range of 100–450 mg/L) was used for the quantification. The FRAP value was express as g Trolox equivalent antioxidant capacity (TEAC)/100 g soluble solid.
Data analysis
Data for each parameter was analyzed using multivariate one-way analysis of variance (ANOVA) followed by Duncan test for multiple comparisons (P < 0.05). Physicochemical properties profiling was analyzed by biplot principal component analysis (PCA) with R Statistical software.
Results and discussion
Physical properties of ground coffee
Lampung Robusta green bean had a moisture content (MC) of approximately 10.02 g/100 g on a wet basis (WB) or 11.13 g/100 g on a dry basis (DB) (Table 2). This MC value meets the requirement for good green coffee beans (8–12 g/100 g WB) stated by previous study (Gloess et al., 2014). Initial MC value is an important parameter for green coffee beans, since it significantly affects the characteristics of roasted beans. Lower MC causes limited water mobility in the bean and generates an underdeveloped roasted bean. Conversely, higher MC produces slower evaporation, leading easily to case hardening on the bean surface.
Table 2.
Moisture, Aw, color, and particle size of coffee beans at different roasting level
| Level of roasting | Moisture content (g/100 g)* | Aw* | Color lightness (L)** | Particle size (g/100 g)* | |||
|---|---|---|---|---|---|---|---|
| >425 µm | 300–425 µm | 250–300 µm | <250 µm | ||||
| Green bean | 11.13 ± 0.01e | 0.65 ± 0.01e | 46.35 ± 0.03f | 84.17 ± 0.78f | 5.42 ± 0.40a | 7.38 ± 1.6a | 3.03 ± 0.39a |
| Early yellow | 6.76 ± 0.17d | 0.56 ± 0.01d | 47.37 ± 1.04f | 64.57 ± 3.26e | 18.20 ± 1.63b | 11.13 ± 0.95b | 6.10 ± 2.58a |
| Brown | 3.48 ± 0.15c | 0.37 ± 0.01c | 39.61 ± 0.26e | 47.87 ± 0.74d | 31.57 ± 1.29c | 19.13 ± 0.54c | 1.44 ± 0.01a |
| 1st crack done | 1.68 ± 0.09b | 0.22 ± 0.02b | 32.02 ± 1.35d | 35.02 ± 0.67c | 40.00 ± 0.23d | 22.70 ± 0.94d | 2.28 ± 0.04a |
| Very light | 0.94 ± 0.07a | 0.15 ± 0.01a | 22.33 ± 0.21c | 23.04 ± 0.25b | 39.75 ± 5.00cd | 28.72 ± 1.00e | 8.49 ± 5.75a |
| Light | 1.15 ± 0.37a | 0.17 ± 0.02a | 20.28 ± 0.09b | 19.19 ± 0.76ab | 39.49 ± 5.65cd | 31.65 ± 1.33f | 9.66 ± 6.22a |
| Medium | 0.68 ± 0.37a | 0.14 ± 0.03a | 18.49 ± 0.12a | 16.92 ± 2.82a | 39.51 ± 4.66cd | 35.42 ± 0.82g | 8.15 ± 2.65a |
| Dark | 0.75 ± 0.11a | 0.15 ± 0.01a | 17.21 ± 0.49a | 15.76 ± 3.66a | 37.97 ± 2.66cd | 36.91 ± 0.47g | 9.35 ± 6.78a |
Values with different superscript in the same column are significantly different (P < 0.05)
*Average of four data ± standard deviation
**Average of ten data ± standard deviation
During roasting, MC declined from 11.13 g/100 g (DB) for the green bean to 0.75 g/100 g (DB) for the bean roasted to the dark level (Table 2). A significant change in MC only occurred until the first crack done level, when the roasting duration reached 5 min. Continued roasting to the very light level only slightly decreased the MC; after this roasting, there was no significant change in MC. At the initial roasting stage, water in the green coffee bean matrix evaporates and reduces the MC by up to several percent (Gloess et al., 2014). This water evaporation creates high internal pressure in the matrix and generates bean expansion (Fadai et al., 2017). This condition makes the cellular and intercellular matrix of coffee beans susceptible to degradation. Pyrolysis and other degradation pathways with further heating produce CO2, resulting in porosity and enabling the water vapor and other gases to evaporate more easily. However, water evaporation will stop at a certain roasting level when no more free water is left in the coffee bean matrix. Fadai et al. (2017) stated that the evaporation of water from the bean brings the moisture content decreases from an initial value of 12% to approximately 2%. In our study, this phenomenon occurred after the very light level, which is when further roasting has no significant effect on MC.
In addition to MC, the water activity (Aw) of the beans decreased with the degree of roasting (Table 2). The significant change in Aw only occurred until the very light level, and further roasting did not affect the value. The Aw of green bean is 0.65 and the level reaches 0.1 after roasting to a dark level (Iaccheri et al., 2015). Monitoring of Aw during roasting is important because each reaction has a certain optimal Aw condition. Water content and water activity (Aw) determine the quality of green coffee bean. A certain amount of water in the coffee bean is needed for creating water vapor resulting high gas pressure causes bean to expand and facilitates the reaction during roasting (Fadai et al., 2017). However, the availability of water to support the reactions is represented by Aw, rather than water content. Water activity (Aw) parameter has more significant effect than water content because it considers water availability, which depends on the interactions between the aqueous phase and the biopolymeric matrix in the coffee bean (Iaccheri et al., 2015).
Following a reduction in the MC and Aw of coffee bean, the other parameters also changed during roasting, as presented in Table 2. Coffee bean color lightness level decreased when a more intensive roasting level was applied. The largest decline in lightness was observed from the 1st crack done to very light level. After this point, the color lightness reduction still occurred in a slower rate. According to lightness reduction, the greenish brown color of green beans turned to dark brown during roasting. Color alteration in green beans is caused by the formation of brown pigment (melanoidins) due to the Maillard reaction (Liu and Kitts, 2011; Vignoli et al., 2014). Maillard reaction is browning reaction between reducing sugars and amino acids (BeMiller and Huber, 2008). In the beginning, the reducing sugar reacts with amino acid to form a schiff-base (glycosylamine). Then, Amadori rearrangement will change unstable glycosylamine into intermediate products. The intermediate products will involve in a complex reaction to form volatile compounds and melanoidins.
In addition to the color parameter, ground coffee beans from different roasting levels also had different size distributions, as shown in Table 2. Sieving distributes particle size based on the particle passing through the shifter. High levels of roasting increased the number of small particles (< 300 µm) and decreased the number of large particles (> 425 µm). Ground coffee with smaller particle size came from more fragile, porous coffee beans because these beans are easier to grind. Thus, roasting increases porosity, and rapid increases occur from the initial time to the very light level.
During heating, the evaporation of water in coffee beans increases the internal pressure and the matrix volume, resulting in the formation of a porous, homogenous coffee microstructure (Schenker et al., 2000). This microstructure is primarily composed of polysaccharides developing a permeable 3-dimensional structure (Massini et al., 1990).
Chemical properties of coffee brew
Bioactive content of coffee brew
The bioactive compound composition of coffee brew from a series of roasting levels is presented in Table 3. Roasting significantly reduced the level of TPC, and the largest reduction was from the bean roasted at the 1st crack done level to the one roasted at the very light level.
Table 3.
Soluble solid, chlorogenic acid (5-CQA), total phenolic (TPC), caffeine, and melanoidins content of coffee brew from beans roasted at different levels
| Level of roasting | Soluble solid (g/100 mL) | 5-CQA (g/100 g) | TPC (g GAE/100 g) | Caffeine (g/100 g) | Melanoidins (g/100 g) |
|---|---|---|---|---|---|
| Green bean | 1.47 ± 0.21a | 9.84 ± 0.53f | 14.31 ± 0.93bc | 6.54 ± 0.38b | 8.74 ± 1.36a |
| Early yellow | 1.53 ± 0.19a | 9.68 ± 0.34f | 14.39 ± 0.32c | 5.49 ± 0.28a | 6.48 ± 0.72a |
| Brown | 1.90 ± 0.07b | 6.95 ± 0.42e | 13.38 ± 0.44bc | 5.98 ± 0.70ab | 7.81 ± 1.16a |
| 1st crack done | 1.98 ± 0.08bc | 5.24 ± 0.55d | 12.84 ± 0.04b | 5.83 ± 0.56ab | 12.04 ± 0.05b |
| Very light | 2.06 ± 0.07cd | 2.54 ± 0.08c | 10.30 ± 0.03a | 5.74 ± 0.14ab | 18.31 ± 0.82c |
| Light | 2.10 ± 0.07cd | 1.68 ± 0.17b | 9.65 ± 0.04a | 6.01 ± 0.14ab | 18.45 ± 1.83c |
| Medium | 2.15 ± 0.05d | 0.90 ± 0.01ab | 9.35 ± 0.16a | 5.32 ± 0.04a | 20.03 ± 1.20c |
| Dark | 2.06 ± 0.08d | 0.54 ± 0.04a | 9.59 ± 0.36a | 5.11 ± 0.05a | 23.26 ± 1.40d |
Values with different superscript in the same column are significantly different (P < 0.05)
Average of four data ± standard deviation
Further observations showed that 5-CQA (5-O-caffeoylquinic acid) was the largest phenolic compound found in the coffee brew from Lampung Robusta coffee bean. The 5-CQA concentration in the green bean reached 68% of the TPC.
Roasting dramatically reduced the 5-CQA concentration. The largest change in 5-CQA was between coffee brew from the 1st crack done level to the very light level. The decline in 5-CQA was considerably higher than the overall reduction of TPC, indicating that 5-CQA partly transformed into new phenolic compounds. 5-CQA, an ester of caffeic acid and quinic acid, may be degraded into its derivatives (Hečimović et al., 2011; Van der Werf et al., 2014; Vignoli et al. 2011).
Caffeine was the main alkaloid compound found in the coffee brew (Rodrigues and Bragagnolo, 2013). The caffeine content of coffee brew from Robusta Lampung green bean reached 6.54 g/100 g and slightly decreased to 5.11 g/100 g in the coffee brew from the dark roasted bean. This result was similar with the previous study for the Robusta coffee bean roasted in dark roasting level (Vignoli et al. 2011).
Roasting increased the melanoidins content in Lampung Robusta coffee brew (Table 3). The highest increase was observed in coffee brew prepared from the 1st crack done level to the very light level. After that point, melanoidins content showed continuous increasing during roasting. Brown pigment, melanoidins are considered as the final products of Maillard reaction (Andriot et al., 2004). Therefore, they will be accumulated during normal roasting condition.
Antioxidant activity (AA)
The AA of Lampung Robusta coffee brew measured by DPPH IC50 and FRAP method slightly decreased when the roasting level increased (Table 4). It is natural in correlation to the degradation of bioactive compound during roasting. Phenolic compounds especially chlorogenic acids (5-CQA) are thermally unstable (Hečimović et al., 2011; Vignoli et al., 2014), so there is a consistency in reducing these compounds with a more excessive roasting process. However, it is known that as the degree of roasting increases, large amounts of Maillard reaction products (MPRs) are formed, which have AA and tend to compensate losses of AA from phenolics (Ludwig et al., 2014; Vignoli et al., 2014).
Table 4.
DPPH and FRAP of coffee brew from beans roasted at different levels
| Level of roasting | DPPH IC50 (mg/mL) | FRAP (g TEAC/100 g) |
|---|---|---|
| Green bean | 3.07 ± 0.17ab | 25.13 ± 0.69a |
| Early yellow | 2.85 ± 0.13a | 26.03 ± 1.37ab |
| Brown | 2.97 ± 0.12a | 30.01 ± 0.16c |
| 1st crack done | 3.80 ± 0.26bc | 29.59 ± 0.28c |
| Very light | 4.14 ± 0.25c | 24.74 ± 0.91a |
| Light | 4.11 ± 0.15c | 29.14 ± 0.14c |
| Medium | 4.46 ± 0.36cd | 28.38 ± 2.21bc |
| Dark | 5.12 ± 0.76d | 27.21 ± 0.31abc |
| Ascorbic acid | 0.43 ± 0.00 | – |
Values with different superscript in the same column are significantly different (P < 0.05)
Average of four data ± standard deviation
The DPPH IC50 of coffee brew from the green beans was only 1.67 times stronger than the one in the coffee brew from the dark roasted bean. Meanwhile, the 5-CQA content in the green bean coffee brew was 18.2 times higher than in the dark roasted coffee brew. This result confirms that the newly-formed compound during roasting compensates losses of AA from native compounds.
In general, the AA of coffee brew measured by FRAP had slightly different behavior with those by DPPH. The AA by DPPH decreased significantly when the roasting proceeded after the first crack done level. FRAF also decreased after roasted at the very light level, but it rather increased in light level and showed no significant difference thereafter. This finding indicates that the ability of the reduction of ferric (Fe3+) to ferrous (Fe2+) in the Robusta coffee brew comes from the native and newly-formed bioactive compounds.
Determining critical roasting level from profiling of physicochemical change
Bioactive compounds changed differently as a function of Aw and MC (Fig. 1). Phenolic compounds (TPC and 5-CQA) dramatically declined when Aw decreased from 0.65 to 0.15, and the most significant decline was in Aw 0.22. Inversely, melanoidins dramatically rose at that Aw point. In addition, phenolic degradation or melanoidins formation continued when Aw reached a constant level. This profile supported a previous study of the incorporation of phenolic into melanoidins structures during roasting (Bartel et al., 2015). This phenomenon also confirms that low Aw is an ideal condition for the Maillard reaction, but it has an inverse effect on TPC. Caffeine was thermo stable because there were no significant changes in concentration during the Aw range (0.65–0.15).
Fig. 1.
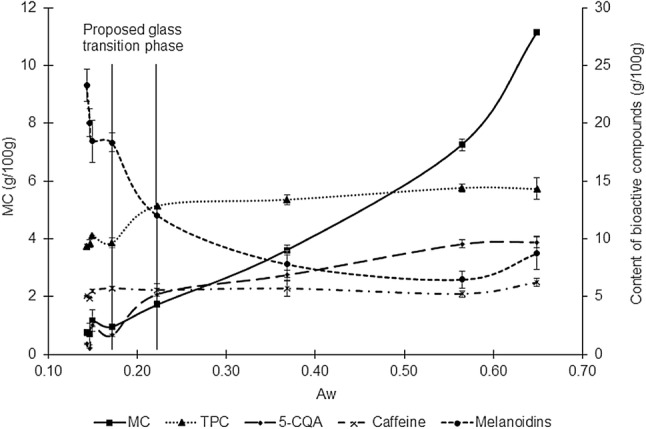
Profile of bioactive change during roasting as a function of Aw. MC Moisture content, TPC Total phenolic compounds, and 5-CQA = chlorogenic acid. Average of four data ± standard deviation
The remarkable decomposition of Arabica coffee beans has been detected at its glass transition point, as observed by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) (Rivera et al., 2011). At this point, the coffee matrix suffered the greatest transformation and greatest weight loss. Thus, the glass transition of Lampung Robusta coffee during roasting may occur when Aw decreases from 0.22 to 0.15 (Fig. 1). However, further analysis using DSC should be determined for confirmation.
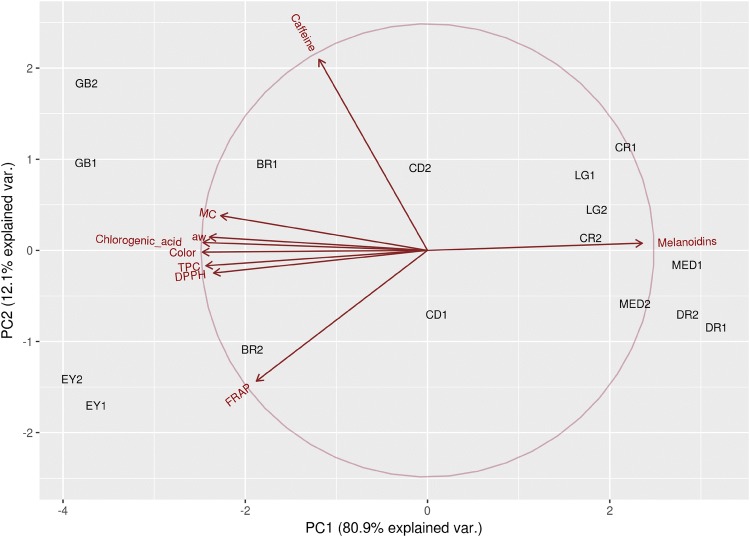
Biplot analysis discriminated coffee samples into different groups based on degree of roasting (PC1) and bioactive content (PC2) as presented in Fig. 2. Coffee samples are separated in tree locations according to the roasting levels (PC1). Light samples (green bean, early yellow roasted bean, and brown roasted bean) were located on the left side and were associated with higher MC, Aw, TPC, 5-CQA, and DPPH. Dark brown samples (very light, light, medium, and dark) were separated on the right side and were associated with melanoidins content. The 1st crack done sample had a unique position in the center of the figure between light and dark color sample groups. This discrimination strongly indicates that the critical roasting level determining bioactive content was the 1st crack done. Separation of sample groups based on PC2 was not clearly defined. Based on this result, it can be concluded that the critical roasting level for physicochemical change in Robusta coffee bean is in the first crack done level or when Aw decreased from 0.22 to 0.15.
Fig. 2.
Biplot of physicochemical properties of coffee bean from different roasting levels: green bean (GB), early yellow (EY), brown (BR), 1st crack done (CD), very light (CR), light (LG), medium (MED), and dark (DR)
Acknowledgements
The authors thank the Ministry of Research, Technology and Higher Education of the Republic of Indonesia for financial support [grant numbers 079/SP2H/LT/DRPM/II/2016, February 17th 2016]. The authors also acknowledge Aneka Coffee Industry-Indonesia for providing Robusta coffee beans and a roaster machine.
Compliance with ethical standards
Conflict of interest
The authors whose names are listed above state that they have no conflict in terms of either financial interest or non-financial interest.
References
- Amarasinghe UA, Hoanh CT, Dhaeze D, Hung TQ. Toward sustainable coffee production in Vietnam: more coffee with less water. Agric. Syst. 2015;136:96–105. doi: 10.1016/j.agsy.2015.02.008. [DOI] [Google Scholar]
- Andriot I, Le Quéré JC, Guichard E. Interactions between coffee melanoidins and flavour compounds: impact of freeze-drying (method and time) and roasting degree of coffee on melanoidins retention capacity. Food Chem. 2004;85:289–294. doi: 10.1016/j.foodchem.2003.07.007. [DOI] [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC Intl. 19th ed. Method 979.12. Association of Official Analytical Chemists, Maryland, USA (2012)
- Bae J-H, Park J-H, Im S-S, Song D-K. Coffee and health. Integr. Med. Res. 2014;3:189–191. doi: 10.1016/j.imr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel C, Mesias M, Morales FJ. Investigation on the extractability of melanoidins in portioned espresso coffee. Food Res. Int. 2015;67:356–365. doi: 10.1016/j.foodres.2014.11.053. [DOI] [Google Scholar]
- BeMiller JN, Huber KC. Carbihydrates. In: Damodaran S, Parkin KL, Fennema OR, editors. Fennema’s food chemistry. Boca Raton: CRC Press Inc; 2008. pp. 83–154. [Google Scholar]
- Ding M, Bhupathiraju SN, Chen M, van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematicreview and a dose-response meta-analysis. Diabetes Care. 2014;37:569–586. doi: 10.2337/dc13-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNunzio JE. Determination of caffeine in beverages by High Performance Liquid Chromatography. J. Chem. Educ. 1985;62:446. doi: 10.1021/ed062p446. [DOI] [Google Scholar]
- Fadai NT, Melrose J, Please CP, Schulman A, Van Gorder RA. A heat and mass transfer study of coffee bean roasting. Int. J. Heat Mass Tran. 2017;104:787–799. doi: 10.1016/j.ijheatmasstransfer.2016.08.083. [DOI] [Google Scholar]
- Gloess AN, Vietri A, Wieland F, Smrke S, Schönbächler B, López JAS, Petrozzi S, Bongers S, Koziorowski T, Yeretzian C. Evidence of different flavour formation dynamics by roasting coffee from different origins: on-line analysis with PTR-ToF-MS. Int. J. Mass Spectrom. 2014;365–366:324–337. doi: 10.1016/j.ijms.2014.02.010. [DOI] [Google Scholar]
- Hečimović I, Belščak-Cvitanović A, Horžić D, Komes D. Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem. 2011;129:991–1000. doi: 10.1016/j.foodchem.2011.05.059. [DOI] [PubMed] [Google Scholar]
- Iaccheri E, Laghi L, Cevoli C, Berardinelli A, Ragni L, Romani S, Rocculi P. Different analytical approaches for the study of water features in green and roasted coffee beans. J. Food Eng. 2015;146:28–35. doi: 10.1016/j.jfoodeng.2014.08.016. [DOI] [Google Scholar]
- International Coffee Organization. Total production by all exporting countries. http://www.ico.org/prices/po-production.pdf. Accessed Dec 6, 2017.
- Liang N, Xue W, Kennepohl P, Kitts DD. Interactions between major chlorogenic acid isomers and chemical changes in coffee brew that affect antioxidant activities. Food Chem. 2016;213:251–259. doi: 10.1016/j.foodchem.2016.06.041. [DOI] [PubMed] [Google Scholar]
- Li B, Zhang C, Peng L, Liang Z, Yan X, Zhu Y, Liu Y. Comparison of essential oil composition and phenolic acid content of selected Salvia species measured by GC-MS and HPLC methods. Ind. Crop. Prod. 2015;69:329–334. doi: 10.1016/j.indcrop.2015.02.047. [DOI] [Google Scholar]
- Li J, Seibold P, Chang-Claude J, Flesch-Janys D, Liu J, Czene K. Coffee consumption modifies risk of estrogen-receptor negative breast cancer. Breast Cancer Res. 2011;13:R49. doi: 10.1186/bcr2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kitts DD. Confirmation that the Maillard reaction is the principle contributor to the antioxidant capacity of coffee brews. Food Res. Int. 2011;44:2418–2424. doi: 10.1016/j.foodres.2010.12.037. [DOI] [Google Scholar]
- Ludwig IA, Sánchez L, Paz De Peña M, Cid C. Contribution of volatile compounds to the antioxidant capacity of coffee. Food Res. Int. 2014;61:67–74. doi: 10.1016/j.foodres.2014.03.045. [DOI] [Google Scholar]
- Massini R, Nicoli MC, Cassara A, Lerici CR. Study on physical and physico-chemical changes of coffee beans during roasting. Note 1. Ital. J. Food Sci. 1990;3:123–132. [Google Scholar]
- Rivera W, Velasco X, Gálvez C, Rincón C, Rosales A, Arango P. Effect of the roasting process on glass transition and phase transition of Colombian Arabic coffee beans. Procedia Food Sci. 2011;1:385–390. doi: 10.1016/j.profoo.2011.09.059. [DOI] [Google Scholar]
- Rodrigues NP, Bragagnolo N. Identification and quantification of bioactive compounds in coffee brews by HPLC-DAD-MSn. J. Food Compos. Anal. 2013;32:105–115. doi: 10.1016/j.jfca.2013.09.002. [DOI] [Google Scholar]
- Schenker S, Handschin S, Frey B, Perren R, Escher R. Pore structure of coffee beans. J. Food Sci. 2000;65:452–457. doi: 10.1111/j.1365-2621.2000.tb16026.x. [DOI] [Google Scholar]
- Shetty K, Curtis OF, Levin RE, Witkowsky R, Ang W. Prevention of vitrification aßociated with in vitro shoot culture of oregano. (Origanum Vulgare) by Pseudomonas spp. J. Plant Physiol. 1995;147:447–451. doi: 10.1016/S0176-1617(11)82181-4. [DOI] [Google Scholar]
- Van der Werf R, Marcic C, Khalil A, Sigrist S, Marchioni E. ABTS radical scavenging capacity in green and roasted coffee extracts. Lebensm.-Wiss. Technol. 2014;58:77–85. doi: 10.1016/j.lwt.2014.02.053. [DOI] [Google Scholar]
- Vignoli JA, Bassoli DG, Benassi MT. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: the influence of processing conditions and raw material. Food Chem. 2011;124:863–868. doi: 10.1016/j.foodchem.2010.07.008. [DOI] [Google Scholar]
- Vignoli JA, Viegas MC, Bassoli DG. Benassi MdT. Roasting process affects differently the bioactive compounds and the antioxidant activity of Arabica and Robusta coffees. Food Res. Int. 2014;61:279–285. doi: 10.1016/j.foodres.2013.06.006. [DOI] [Google Scholar]
- Wang X, Lim LT. Investigation of CO2 precursors in roasted coffee. Food Chem. 2017;219:185–192. doi: 10.1016/j.foodchem.2016.09.095. [DOI] [PubMed] [Google Scholar]
- Yu X, Bao Z, Zou J, Dong J. Coffee consumption and risk of cancers: a meta-analysis of cohort studies. BMC Cancer. 2011;11:96. doi: 10.1186/1471-2407-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin RC, Corso MP, Kitzberger CSG, Scholz MBdosS, Benassi MdeT. Good cup quality roasted coffees show wide variation in chlorogenic acids content. Lebensm.-Wiss. Technol. 2016;74:480–483. doi: 10.1016/j.lwt.2016.08.012. [DOI] [Google Scholar]
- Zhang Z, Hu G, Caballero B, Appel L, Chen L. Habitual coffee consumption and risk of hypertension: a systematic review and meta-analysis of prospective observational studies. Am. J. Clin. Nutr. 2011;93(6):1–8. doi: 10.3945/ajcn.110.004044. [DOI] [PubMed] [Google Scholar]