Abstract
The objective of this study was to develop new drug delivery systems (DDS) and nutrient delivery systems (NDS), using starch as a carrier material for infusion technology. Corn, waxy rice, non-waxy rice, and potato starches were used as carrier materials. Sodium fluorescein was used as an infusion material for easy detection. Each starch suspension with sodium fluorescein was reacted in a water bath at 40, 50, and 60 °C for 30 min. After each reaction, the concentration of sodium fluorescein in the supernatant was measured using a fluorescence detector. Precipitated starch was observed using fluorescence microscopy. About 70% of sodium fluorescein infused in waxy rice and corn starches at 60 °C. Additionally, the granules of these two starches were luminous by green light when exposed to a fluorescence detector, suggesting that corn and waxy rice starches can be used as carrier materials in infusion technology for DDS and NDS.
Keywords: Drug delivery system, Nutrient delivery system, Infusion, Starch, Sodium fluorescein
Introduction
Drug delivery systems (DDS) and nutrient delivery systems (NDS) comprise one of the fastest growing areas in the healthcare sector, as well as an area of great promise for future technologies. The main focus of these systems is targeted drug/nutrient delivery. Nanotechnology is one of the most recent and successful drug delivery technologies; however, it is very complicated and expensive to produce. It can easily provide nutraceuticals and phytochemicals for humans and can create functional and value-added foods. Intracellular delivery of drugs may be possible for selective and prolonged pharmacological actions, and thereby may help to reduce side effects (Herrero-Vanrell and Refojo, 2001; Panyam and Labhasetwar, 2003).
Starch is the most significant carbon resource in plants and is a major component of the total crop yield worldwide. Starch is used in the food, cosmetics, paper, and textile industries due to its abundance, low cost, distinctive characteristics, and non-toxicity. Void spaces or cavities in the interior regions of various starch granules have been observed and these cavities were caused by the partial role of dehydration (Baldwin et al., 1994). Central cavities in the granules of many starch species were investigated by Huber and Bemiller (1997), and the microstructure of various types of starch granules have been studied extensively (Fannon et al., 2003; Huber and BeMiller, 2000; Kim and Huber, 2008). Additionally, infusion of catechin into native corn starch has been reported (Han et al., 2015). The use of starches in DDS and NDS has many advantages, such as reduced side effects, better patient acceptance and improved safety. In addition, various starches have different properties depending on the species, origin, and treatment of the starch. This means that specific controlled releases can be orchestrated using the varied properties of starches. Moreover, fluorescent nanoparticles have been used as signal magnifiers for a wide range of applications, including chemical analysis, biological research, and clinical diagnosis (Fehr et al., 2002; He et al., 2003; Stojanovic et al., 2000).
However, limited information is available on the infusion of certain materials into cavities, channels, and pores of various starch granules as applied to DDS and NDS. Therefore, the objective of this study was to investigate the effects of temperature and concentration on infusion efficiency of sodium fluorescein into various starches.
Materials and methods
Materials
Corn, potato, waxy rice, and non-waxy rice starches were used in this study. Corn and potato starches were obtained from Daesang Co. (Icheon, Korea) and Tureban Co., Ltd. (Seoul, Korea), respectively. Waxy and non-waxy rice starches were prepared using an alkaline method (Choi et al., 2006). Sodium fluorescein was purchased from Sigma-Aldrich Co. (Product # F6377, St. Louis, MO, USA).
Infusion tests
Starch–water suspensions (0.5 g, 40 mL) were prepared in conical tubes and 1 mL of sodium fluorescein (1 μM) was added. Samples were incubated in water baths at 25 °C, 40 °C, 50 °C, and 60 °C for 30 min each. After incubation, samples were centrifuged at 3000 rpm for 15 min and the supernatants were collected. Distilled water (40 mL) was added into the precipitates to wash the remaining sodium fluorescein that was not infused into the starches, and the mixtures were centrifuged. This washing process was performed three times and all of the washed solutions were collected into one beaker. All conical tubes were covered with aluminum foil to minimize photobleaching of fluorescein.
Qualitative and quantitative analysis of infusion
Before and after washing, the fluorescence of precipitated starches was observed using fluorescence microscopy (Inverted Fluorescence Microscope, Nikon TE2000U, Japan) for qualitative analysis.
For quantitative analysis, the concentrations of sodium fluorescein in the supernatant and washed solution were measured using a fluorescence detector (Tecan Infinite M200, San Jose, CA, USA). Fluorescent intensity of samples was measured with the absorption maximum at 494 nm and the emission maximum at 521 nm (in water). The amount of infused sodium fluorescein was determined by subtracting the amount of sodium fluorescein in both the supernatant and the washed solution from the initial amount of sodium fluorescein. On the other hand, the amount of easily removable sodium fluorescein was defined as the amount of sodium fluorescein in the washed solution.
Statistical analysis
All experiments were repeated three times. Experimental data were analyzed using Analysis of Variance (ANOVA), and were expressed as mean value ± standard deviation. Duncan’s multiple range test was conducted to assess significant differences among experimental mean values (p < 0.05). All statistical computations and analyses were conducted using SAS version 8.02 for Windows (SAS Institute, Inc., Cary, NC, USA).
Results and discussion
Qualitative analysis using fluorescence microscopy
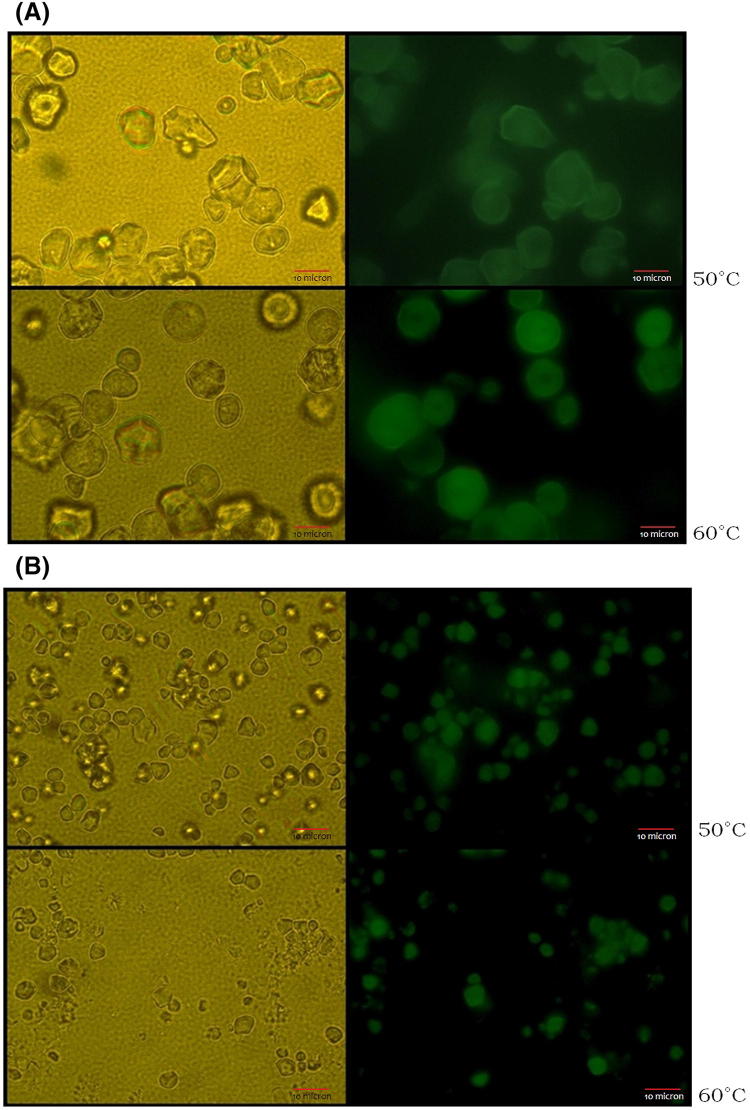
Light and fluorescent microscopic images of corn and waxy rice starches after infusion and washing are shown in Fig. 1. Among four kinds of starches, only two starch granules (corn and waxy rice) showed the highlighted regions by green color when exposed to a fluorescence microscope at 50 °C and 60 °C, respectively, after infusion and washing (Fig. 1). This result indicated that there was an infusion reaction in corn and waxy rice starch granules. It is well known that corn and sorghum starch granules have channels that connect to a central cavity from the external environment (Huber and Bemiller, 2000). On the other hand, channels within wheat starch granules are filled at least in part with protein, and removal of protein using protease enhanced access of chemicals to the channels and/or cavities (Kim and Huber, 2008). Potato and rice starch did not showed any penetration of sodium fluorescein into channels and/or cavities in this study. This suggested that these two starch granules may have no channels and pores or those filled with protein, resulting in no infusion of sodium fluorescein into the starch granules. However, corn and waxy rice starch granules may have open channels and/or cavities that are not always filled with protein, resulting in easy access of sodium fluorescein into internal starch granules without protease treatment.
Fig. 1.
Light and fluorescence microscopic images of corn (A) and waxy rice (B) starches at 50 °C and 60 °C after infusion and washing of sodium fluorescein
Infusion efficiency
Figure 2 shows the effects of temperature on infusion of sodium fluorescein into various starches. In order to differentiate the attached sodium fluorescein at the easily removable and the infused sodium fluorescein, the amounts of sodium fluorescein in both the supernatant and the washed solution were determined. No infusion phenomena occurred in any of the starches at 25 °C or 40 °C. However, above 50 °C, sodium fluorescein infused into corn and waxy rice starches. In the case of A-type starch granules, there are generally relatively large channels in the equatorial groove region, while finer channels are located in other regions of the granule (Kim and Huber, 2008). This may result in infusion of fluorescein into corn and waxy rice starches. However, this is not true for rice starch, which is the same A-type starch. This can be explained by the swelling capacity of rice starch, which has relatively lower swelling power than corn and waxy rice starches (Choi et al., 2006; Han et al., 2015). Swelling of rice starch occurs at relatively higher temperatures, resulting in no infusion of fluorescein until 60 °C. Therefore, swelling may be the most important factor influencing infusion of fluorescein. Generally, swelling is more likely to occur at higher temperatures as well. Thus, the highest infusion efficiency was observed at 60 °C in corn starch, which is just below the gelatinization temperature of corn starch. High diffusion takes place at high temperatures with swelling of starch granules, resulting in the highest infusion of fluorescein into any starch granule. It has been reported that fluorescein easily infuses into starch granules with swelling, as compared to non-swelled conditions (Kim and Huber, 2008). Therefore, infusion efficiency was higher at high temperatures than at low temperatures. The specific channels of each starch granule may also influence the infusion of fluorescein into the starch. Channels of starch granule appeared to facilitate the transfer of the chemical reagent into the granule matrix, though this effect was aided by granule swelling (hydration) and/or removal of channel-associated protein (Kim and Huber, 2008). This suggests that infusion of fluorescein is greatly influenced by starch varieties and infusion temperatures, which are strongly correlated with swelling, channels, pores, and cavities of starch granules. Certain starch granules, but not all starch granules, can be used as carrier materials for developing drug and nutrient delivery systems.
Fig. 2.
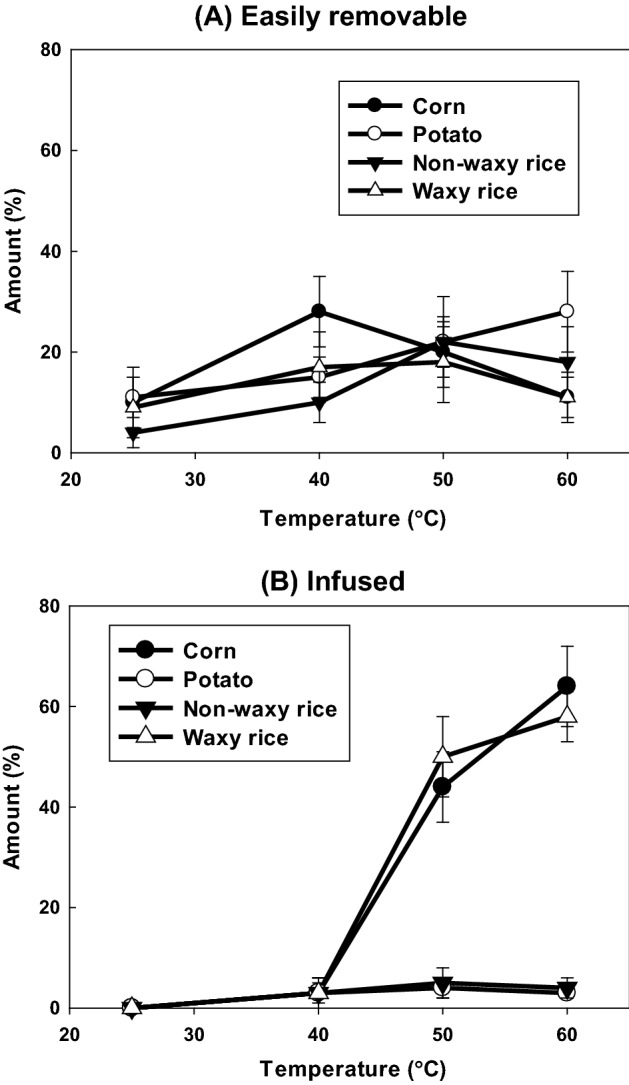
Effect of temperature on infusion of sodium fluorescein into various starches
Acknowledgements
This research was supported by the Main Research Program (E0164800-03) of the Korea Food Research Institute (KFRI) funded by the Ministry of Science, ICT & Future Planning.
References
- Baldwin PM, Adler J, Davies MC, Melia CD. Holes in starch granules: confocal, SEM and light microscopy studies of starch granule structure. Starch/Stärke. 1994;46:341–346. doi: 10.1002/star.19940460906. [DOI] [Google Scholar]
- Choi HW, Chung KM, Kim CH, Moon TH, Kim DS, Park CS, Baik MY. Physicochemical properties of cross-linked rice starches. J. Korean Soc. Appl. Biol. Chem. 2006;49:49–54. [Google Scholar]
- Fannon JE, Gray JA, Gunawan N, Huber KC, BeMiller JN. The channels of starch granules. Food Sci. Biotechnol. 2003;12:700–704. [Google Scholar]
- Fehr M, Frommer WB, Lalonde S. Visualization of maltose uptake in living yeast cells by fluorescent nanosensors. Proc. Natl. Acad. Sci. USA. 2002;99:9846–9851. doi: 10.1073/pnas.142089199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Choi SH, Kim BY, Baik MY. Infusion of catechin into native corn starch granule for drug and nutrient delivery systems. Food Sci. Biotechnol. 2015;24:2035–2040. doi: 10.1007/s10068-015-0270-1. [DOI] [Google Scholar]
- He H, Mortellaro MA, Leiner MJ, Fraatz RJ, Tusa JK. A fluorescent sensor with high selectivity and sensitivity for potassium in water. J Am Chem Soc. 2003;125:1468–1469. doi: 10.1021/ja0284761. [DOI] [PubMed] [Google Scholar]
- Herrero-Vanrell R, Refojo MF. Biodegradable microspheres for vitreoretinal drug delivery. Adv. Drug Deliv. Rev. 2001;52:5–16. doi: 10.1016/S0169-409X(01)00200-9. [DOI] [PubMed] [Google Scholar]
- Huber KC, BeMiller JN. Visualization of channels and cavities of corn and sorghum starch granules. Cereal Chem. 1997;74:537–541. doi: 10.1094/CCHEM.1997.74.5.537. [DOI] [Google Scholar]
- Huber KC, BeMiller JN. Channels of maize and sorghum starch granules. Carbohydr. Polym. 2000;41:269–276. doi: 10.1016/S0144-8617(99)00145-9. [DOI] [Google Scholar]
- Kim HS, Huber KC. Channels within soft wheat starch A- and B-type granules. J. Cereal Sci. 2008;48:159–172. doi: 10.1016/j.jcs.2007.09.002. [DOI] [Google Scholar]
- Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003;55:329–347. doi: 10.1016/S0169-409X(02)00228-4. [DOI] [PubMed] [Google Scholar]
- Stojanovic MN, Prada P, Landry DW. Flurescent sensors based on aptamer self-assembly. J. Am. Chem. Soc. 2000;122:11547–11548. doi: 10.1021/ja0022223. [DOI] [PubMed] [Google Scholar]