Abstract
Cheongguk-jang is a Korean traditional food produced by natural fermentation of boiled soybean. In cheongguk-jang, bacilli are dominant bacteria and produce highly viscous poly-γ-glutamic acid (γ-PGA), which improves human health functions. The purpose of this experiment was to find maximum production condition for the γ-PGA content during fermentation of cheongguk-jang with Bacillus subtilis 168. The most viscous cheongguk-jang was produced when soybean was cooked at 121 °C for 60 min in the presence of 50%(w/w) added water, followed by fermentation at 40 °C for 2 days. Additional conditions for maximum production of γ-PGA were the addition of 0.1%(w/w) FeCl3·6H2O, 3.0%(w/w) lactose and 3.0%(w/w) yeast extract as nutrients of inorganic salts, carbon source and nitrogen source, respectively. The three conditions did not show cumulative effect on the γ-PGA production and the addition of iron salt induced the most γ-PGA (0.97 ± 0.05%(w/w)), which corresponded to 2.7 times of the content in control cheongguk-jang.
Keywords: Bacillus subtilis 168, Cheongguk-jang, Fermentation, Poly-γ-glutamic acid
Introduction
Cheongguk-jang (CKJ) is a kind of Korean traditional fermented soybean food containing various nutrients. Korean people usually consume CKJ in the form of a hot soup after boiling in the presence of various seasonings. Traditional CKJ is made by fermenting cooked soybeans for 2–3 days at about 40 °C in contact with dried rice straw. The microorganisms involved in this natural fermentation are various bacteria, among which the predominant strains are Bacillus spp. The quality of CKJ varies considerably depending on the soybean variety, fermenting microorganisms, fermentation process, and the ratio of added ingredients (Lee et al., 2005). Several reports showed that CKJ is associated with antioxidant, antimicrobial, blood pressure lowering, antiallergenic and antiviral activities, which may come from isoflavones, peptides, phenols, and other flavonoids produced during fermentation (Baek et al., 2008; 2015; Wei et al., 2015).
Fermented soybean products including CKJ contain poly-γ-glutamic acid (γ-PGA) in the form of extracellular viscous substances produced by many Bacillus strains (Cheng et al., 1989; Goto and Kunioka, 1992; Hara and Ueda, 1982). γ-PGA is water soluble, biodegradable and edible, thus, it is non-toxic substance toward human and the environment (Luo et al., 2016). Several Bacillus strains producing γ-PGA have been isolated from various fermented soybean foods (Ashiuchi et al., 2001; Inatsu et al., 2002). Molecular weight (Mw) of bacterial γ-PGA usually ranges from 100 to over 2000 kDa (Bajaj and Singhal, 2009; Park et al., 2005; Richard and Margaritis, 2003; Shih and Van, 2001) and the biopolymer consists of repeating units of l-glutamic acid, and/or d-glutamic acid. The ratio of D/L content in γ-PGA changes readily depending on the types of microorganisms and environmental factors such as temperature and nutrient composition. Production yields of γ-PGA also depend on the microorganisms, temperature, and nutrients (Oh et al., 2007; Shih and Van, 2001). According to the strains used the nutrient requirements such as carbon sources, nitrogen sources, amino acids, and metal ions vary and mechanisms of γ-PGA synthesis are also different (Francis et al., 2003). Most commercial γ-PGAs are currently produced via microbial fermentation from biomass (Sirisansaneeyakul et al., 2017). γ-PGA can be used safely in a wide range of application including as thickeners, humectants, bitterness-relieving agents, cryoprotectants, sustained release materials, drug carriers, heavy metal absorbers, and animal feed additives (Luo et al., 2016). Previous studies about γ-PGA have identified potential anti-obesity, anti-cancer, anti-angiogenic, anti-tumorigenic, antibacterial, and anti-inflammatory activities (Davaatseren et al., 2013; Kim et al., 2007; Lee et al., 2013; Shin et al., 2015). Therefore, CKJ, which produces γ-PGA, is a very important food itself. The traditional fermented CKJ contained 2.6%(w/w) mucilage (Yang and Kim, 2013), and it was reported that CKJ fermented using a starter had an amount of about 5–6.3%(w/w) (Lee et al., 1991). Indeed, a substantial part of such mucus is known as γ-PGA. On the other hand, there is a report that γ-PGA is contained about 0.5%(w/w) in Japanese natto, which is a kind of CKJ (Tanimoto et al., 2001). Many studies have been conducted on the commercial production of γ-PGA using liquid culture (Sirisansaneeyakul et al., 2017; Wang et al., 2017). However, no study has been conducted to increase the amount of γ-PGA in the fermentation of CKJ which is solid fermentation and directly ingested as food. Therefore, in this study, fermentation conditions such as soybean cooking conditions, fermentation time and temperature, and added nutrients to produce γ-PGA maximally were investigated in the fermentation of CKJ using a starter, Bacillus subtilis 168 isolated from CKJ fermented by a traditional method.
Materials and methods
Microorganisms and cultivation
Bacillus subtilis 168 (B. subtilis KCCM 11609) isolated from CKJ was purchased from the Korean Culture Center of Microorganism (Seoul, Korea). The Bacillus strain was stored in liquid nitrogen during the experiment. The strain produced mucilage during the production process of CKJ. To prepare starter fermentation, the Bacillus was first grown on LB agar plates at 40 °C for 24 h. The microorganism was then transferred to a 500 mL flask containing 150 mL of edible LB broth, and the flask was cultured with shaking (180 rpm) at 40 °C for 24 h. The cooked soybeans were divided into 35 g portions in petri dishes and cooled to 40–50 °C. Then, CKJ fermentation was started by inoculating the soybean with 1.0%(v/w) B. subtilis 168.
Cooking of soybeans for CKJ
To investigate the optimum conditions for fermentation of CKJ, the effect of initial water content during soybean cooking on the fermentation was investigated according to the previous studies (Bajaj et al., 2008; Jian et al., 2005). The soybeans (Glycine max) were washed and soaked at room temperature for 14 h. Before the autoclave, distilled water was added to adjust the moisture content of soybeans. The combination of initial water content (50, 55, 60, 65, 70%(w/w) of soybean weight) and autoclave time (15, 30, 60 min) at 121 °C was applied in the experiment. The best cooking conditions for fermentation of CKJ were selected based on the viscosity measurement.
Changes in fermentation temperature and time
Three independent fermentations were performed at different temperatures (35, 40, 45 °C) and times (24, 48, 72 h) and then viscosities of the fermented soybeans were measured to determine the optimal temperature and time for the fermentation of CKJ.
Addition of mineral salts
Based on the previous study (Jian et al., 2005), aqueous mineral salt stock solution was prepared 41.52, 22.72, 47.92, 26.36%(w/w) for four kinds of inorganic salts MgSO4·7H2O, CaCl2·2H2O, FeCl3·6H2O, and NaCl, respectively and then subjected to moist heat sterilization. Salt stock solutions were added to the cooked soybeans to allow fermentation samples of different mineral concentrations. The final concentrations were 0.06, 0.1, and 0.2%(w/w) for MgSO4; 0.08, 0.15, and 0.2%(w/w) for CaCl2; 0.05, 0.1, and 0.2%(w/w) for FeCl3; and 0.07, 0.1, and 0.2%(w/w) for NaCl, respectively. The viscosities and γ-PGA contents of the fermented CKJs were measured to determine the effects of inorganic salts on CKJ.
Addition of carbon sources
Based on the previous report (Peng et al., 2015), five different solutions of carbon sources, i.e., glucose (23.08%(w/w)), sucrose (51.22%(w/w)), lactose (9.75%(w/w)), citric acid (42.49%(w/w)), and glycerol (99%(w/w)) were used. The stock solutions were added to the cooked soybeans to prepare fermentation samples of different carbon source concentrations. The final concentrations were 0.2, 1.0, and 2.0%(w/w) for glucose or citric acid, and 0.1, 0.5, and 1.0%(w/w) for sucrose, respectively. In case of lactose and glycerol, the final concentrations were 0.3, 1.0, and 3.0%(w/w). Both viscosities and γ-PGA contents of the fermented CKJs were measured to evaluate the effects of different carbon sources.
Addition of nitrogen sources
Peptone, yeast extract, and l-glutamic acid were dissolved in distilled water separately to give 13.04, 13.04, and 0.74%(w/w), respectively. Then these were sterilized by moist heat and added to the cooked soybeans. Both peptone and yeast extracts resulted in final concentrations of 0.3, 1.0, and 3.0%(w/w), and l-glutamic acid was 0.1, 0.2, and 0.3%(w/w), respectively. After fermentation, the viscosities and γ-PGA contents of the produced CKJs were measured and compared with one another.
Measurement of viscosity
To measure the viscosity, 30 g of CKJ was diluted with 30 g of distilled water and shaken (200 rpm) at 25 °C for 20 min. The mixture was filtered through a layer of muslin cloth and centrifuged at 2000 × g for 15 min. Viscosity of supernatant 30 mL was analyzed by Rapid Visco Analyzer (RVA, Tecmaster, Perten, Australia) at 25 °C, 160 rpm, for 5 min. The unit of viscosity was represented in centipoise (cp).
Measurement of mucilage and γ-PGA
The method of Jian et al. (2005) was partially modified for extraction. Fresh fermented substrate was diluted 10-fold with distilled water and mixed thoroughly in a shaker (200 rpm) for 1 h. The mixture was filtered through a double layer of muslin cloth and the filtrate was centrifuged at 15,000 × g for 15 min and filtered through a Whatman syringe filter (0.45 μm). A portion of this solution was dried to determine the weight of mucilage, and the remaining samples were used as HPLC samples to determine the amount of γ-PGA. Purification of γ-PGA was carried out by the previously reported methods (Goto and Kunioka, 1992; Nie et al., 2015). Ten milliliter of the above solution containing γ-PGA was diluted 5-fold with methanol and kept at 4 °C for 12 h. The solution was centrifuged (12,000 ×g) at 4 °C for 30 min to collect soluble crude γ-PGA, which was then dissolved in distilled water. The aqueous γ-PGA solution was dialyzed with Mw cutoff 3500 membrane against 1 L of distilled water for 12 h with 3 time water exchanges. The dialyzed solution was regarded as pure γ-PGA and the dry weight was measured by a 105 °C drying method. γ-PGA was analyzed using Shimadzu LC-20 HPLC equipped with HPLC SEC-250 column (300 × 7.8 mm) and UV detector (220 nm). As an eluting solution, 20 mM phosphate buffer (pH 7) containing 5 mM KCl was used. The flow rate of the mobile phase was 0.25 mL/min and the temperature of the column was 30 °C. The above purified γ-PGA was used as a standard for the determination of γ-PGA content in CKJ.
Statistical analysis
The experimental data were analyzed for statistical significance using two-way ANOVA analysis, variance and determination of confidence intervals, performed with a computer statistical program (IBM SPSS Statistical Analysis System, Version 23, SPSS Inc., Chicago, IL, USA). Duncan’s multiple range test was used to test the difference among means of data. A p value of < 0.05 was considered significant.
Results and discussion
Effects of soybean cooking conditions
The initial water content of fermentable substrate is one of the most important factors in solid-state fermentation (Jian et al., 2005). CKJ was prepared by fermenting soybeans cooked with different conditions, i.e., the added water amount (50, 55, 60, 65, and 70%(w/w) of the soybean weight) and the autoclave time (15, 30, 60 min). Since the viscosity is related to γ-PGA accumulation (Ashiuchi et al., 2001), viscosity was measured after collecting mucilages from CKJ as shown in Fig. 1. Statistical analysis showed that when soybeans were autoclaved at 121 °C for 15 or 30 min, there was no significant change in the viscosity even though there were large differences in cooking water contents. However, 60 min heat treatment of the same soybean significantly increased the viscosity of mucilage (p < 0.05), as the cooking water content decreased. The highest viscosity, 228.95 cp, was obtained when 50 mL of water was added to 100 g of soybeans and heated for 60 min at 121 °C. Two-way ANOVA using autoclave time and added water as variable showed a significant main effect of autoclave time for cooking soybean on the viscosity of CKJ (F(2, 15) = 23.4; p < 0.001) with a significant main effect of autoclave time (F(4,15) = 14.5; p < 0.001) as well as significant interaction between these two effects (F(8,15) = 12.7; p < 0.001), thus indicating that both autoclave time and the amount of added water for soybean cooking are important to the production of mucilage of CKJ. Viscosity of CKJ is important because it is one of criteria that consumers evaluate the CKJ quality. Since γ-PGA is known as highly viscous biopolymer (Luo et al., 2016), the viscosity of mucilage would be closely related to the γ-PGA content of CKJ. In summary, heating raw soybeans at 121 °C for 60 min was better than that for 15 min or 30 min to obtain high viscosity of CKJ and the amount of added water was the best at 50%(v/w) of soybean.
Fig. 1.
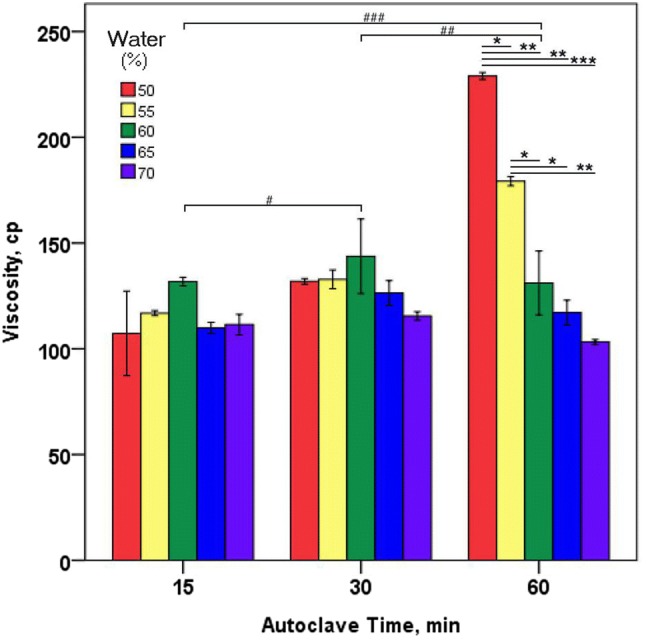
Viscosity of cheongguk-jang produced by different cooking conditions. Cooked soybeans for CKJ fermentation was prepared by changing two factors, the amount of added water and the heating time. The fermentation was carried out at 40 °C for 2 days. Values are mean ± SE. *p < 0.05; **p < 0.01; ***p < 0.001 viscosity versus amount of added water within each autoclave time; #p < 0.05; ##p < 0.01; ###p < 0.001 interaction of viscosity and autoclave time; two-way ANOVA with Scheffe post hoc test
Effects of fermentation time and temperature
CKJ was prepared by fermenting at 35, 40 and 45 °C for 3 days using B. subtilis 168. The viscosities of the produced mucilage were daily measured (Fig. 2). At the start of fermentation, mucilage was not observed. However, when fermenting at 35 or 45 °C, the viscosities increased to 100–150 cp on the first day of fermentation and were maintained until day three. However, when fermented at 40 °C, the viscosity significantly increased to more than 200 cp on the second day of fermentation compared to the first and third day fermentations. This value also showed significant difference (p < 0.05) with the viscosities of CKJ fermented at 35 or 45 °C for 2 days. In the case of liquid culture, however, γ-PGA in the fermentation using Bacillus sp. isolated from CKJ has been reported to increase and then decrease in terms of viscosity with increasing time (Ashiuchi et al., 2001). Therefore, from the results of this study proper fermentation time to obtain CKJ containing high amount of γ-PGA was 2 days. By the way, the best conditions for a high γ-PGA productivity using Bacillus subtilis C10 and Bacillus subtilis ZJU-7 were 32 h at 32 °C and 48 h at 37 °C, respectively (Chen et al., 2010; Zhang et al., 2012). Two-way ANOVA showed that there was a significant main effect of fermentation temperature on the viscosity of CKJ, F(2,9) = 4.86, p < 0.05 as well as fermentation time on the viscosity of CKJ (F(2,9) = 17.55, p < 0.01). Also there was a significant interaction between fermentation time and temperature on the viscosity of CKJ, (F(2,9) = 13.39, p < 0.05). Therefore, according to the previous reports and the results of this study, long-term fermentation is not necessary for the production of γ-PGA. Furthermore, the interaction of both fermentation temperature and time conditions is important for the viscosity of CKJ and conditions for the viscosity increase may be different for each Bacillus strain.
Fig. 2.
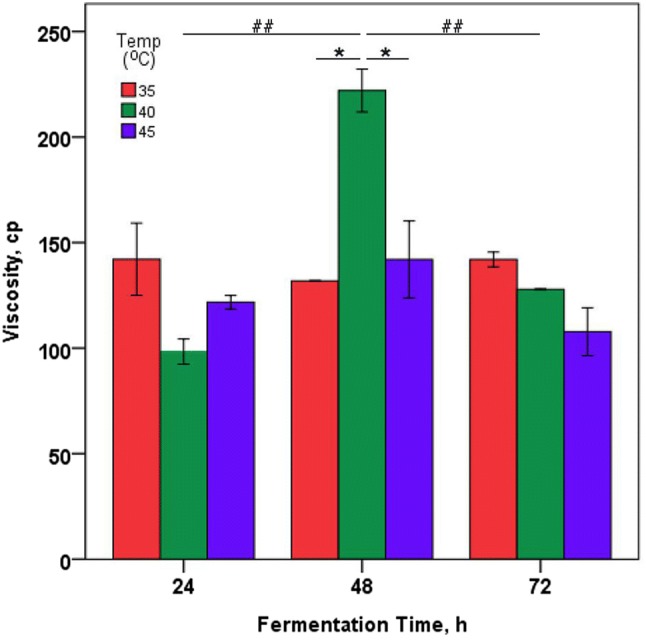
Effect of fermentation temperature and time on viscosity of cheongguk-jang. Fifty mL of water was added to 100 g soybeans and autoclaved at 121 °C for 60 min. Values are mean ± SE. *p < 0.05 viscosity versus fermentation temperature within each fermentation time; ##p < 0.01 interaction of viscosity and fermentation time; two-way ANOVA with Scheffe post hoc test
Effects of added mineral salts
In order to find the high γ-PGA producing conditions of CKJ fermentation, inorganic salts such as MgSO4, CaCl2, FeCl3 or NaCl solution were added to the cooked soybean. The viscosity and γ-PGA content of CKJ as affected by the mineral salts were shown in Table 1. The values obtained without added inorganic salts were 244 cp and 33%(w/w), respectively, as shown. Among the inorganic salts, MgSO4, FeCl3 or NaCl promoted the production of γ-PGA at low concentrations. However, CaCl2 in the range of 0.08–0.2%(w/w) was not associated with γ-PGA production. Magnesium sulfate significantly promoted the production of γ-PGA in the range of all concentrations treated. Ferric chloride and NaCl significantly stimulated the production of γ-PGA only at 0.1 and 0.07%(w/w), respectively. The highest content of γ-PGA (0.97 ± 0.05%(w/w)) was obtained with 0.1%(w/w) FeCl3·6H2O while 0.62 ± 0.01%(w/w) and 0.61 ± 0.01%(w/w) was shown with 0.1%(w/w) MgSO4 and 0.07%(w/w) NaCl, respectively. By the way, the viscosities of the mucilage of CKJ did not show any significant differences compared to the control (without mineral addition) group in all the treated concentrations. These inorganic salts such as FeCl3 may act as components of the coenzyme participating in the metabolism of bacteria, thus positively affecting the enzymatic reaction of γ-PGA synthesis (Jian et al., 2005).
Table 1.
Effect of added mineral salts on the viscosity and γ-PGA of cheongguk-jang
| Concentration, %(w/w) | γ-PGA, %(w/w) | Viscosity (cp) | |
|---|---|---|---|
| MgSO4·7H2O | 0 | 0.35 ± 0.03b | 244.16 ± 4.78ns |
| 0.06 | 0.58 ± 0.03a | 254.19 ± 6.10ns | |
| 0.1 | 0.62 ± 0.01a | 255.84 ± 46.63ns | |
| 0.2 | 0.58 ± 0.06a | 255.50 ± 29.40ns | |
| CaCl2·2H2O | 0 | 0.35 ± 0.03ns | 244.16 ± 4.78ns |
| 0.08 | 0.34 ± 0.01ns | 274.86 ± 38.18ns | |
| 0.15 | 0.48 ± 0.06ns | 274.20 ± 20.98ns | |
| 0.2 | 0.40 ± 0.10ns | 274.75 ± 13.05ns | |
| FeCl3·6H2O | 0 | 0.35 ± 0.03b | 244.16 ± 4.78ns |
| 0.05 | 0.41 ± 0.06b | 274.60 ± 41.87ns | |
| 0.1 | 0.97 ± 0.05a | 274.51 ± 2.77ns | |
| 0.2 | 0.44 ± 0.12b | 274.38 ± 17.73ns | |
| NaCl | 0 | 0.35 ± 0.03b | 244.16 ± 4.78ns |
| 0.07 | 0.61 ± 0.01a | 274.24 ± 30.86ns | |
| 0.1 | 0.46 ± 0.05b | 274.51 ± 5.33ns | |
| 0.2 | 0.45 ± 0.01b | 274.81 ± 50.66ns |
Data were obtained from duplicate experiments except control (n = 8) and expressed as the mean ± standard deviation
Different letters (a, b, c) within the same column indicate significant differences (p < 0.05)
ns not statistically significant
The results of this study, in which FeCl3 increased the yleld of γ-PGA, were consistent with the results of a recent report on Bacillus licheniformis (Feng et al., 2017). However, the maximum amount of γ-PGA was synthesized under the condition of 0.74 mM FeCl3 (i.e., 0.2%(w/w)), which was different from 0.1%(w/w) FeCl3 in this study. In addition, the yield of γ-PGA was 0.35%, which was significantly lower than 0.97%(w/w) of this study. The study (Feng et al., 2017) also showed that FeCl3 increased the expression of various enzymes involved in γ-PGA synthesis and degradation, especially pgsB expression, one of the synthetic enzymes.
Effect of added carbon sources
The aim of this study was to find carbon source that increases γ-PGA in CKJ. Lactose was the most potent carbon source for the purpose than other compounds as shown in Table 2. When the added lactose was 3.0%(w/w), the concentration of γ-PGA was the highest reaching 0.62 ± 0.06%(w/w). However, the increase in the concentration of added lactose did not change the viscosity of CKJ as shown in Table 2. Other carbon sources, such as glucose, sucrose, citric acid and glycerol did not increase the production of γ-PGA. Moreover, citric acid inhibited γ-PGA production. By the way in the presence of added citric acid, Bacillus subtilis CCTCC202048 produced the highest amount of γ-PGA (54.21 g/kg dry weight) in solid-state fermentation of mixture of soybean cake and wheat bran (Jian et al., 2005). Bacillus subtilis GS-2 produced the highest amount of γ-PGA (16.76 g/L) in media containing 3.0%(w/w) sucrose (Bang et al., 2012). Bacillus methylotrophicus SK19.001 showed a γ-PGA production rate of 14 g/L by 3%(w/w) glycerol (Peng et al., 2015). Thus, the effect of added carbon sources on γ-PGA is likely to depend on the strain of Bacillus sp.
Table 2.
Effect of the added carbon sources on the viscosity and γ-PGA of cheongguk-jang
| Concentration, %(w/w) | γ-PGA, %(w/w) | Viscosity (cp) | |
|---|---|---|---|
| Glucose | 0 | 0.35 ± 0.03ns | 213.35 ± 0.67b |
| 0.2 | 0.43 ± 0.02ns | 195.91 ± 1.68c | |
| 1 | 0.40 ± 0.05ns | 217.95 ± 2.56a | |
| 2 | 0.40 ± 0.01ns | 146.50 ± 0.28d | |
| Sucrose | 0 | 0.35 ± 0.03a | 213.35 ± 0.67ns |
| 0.1 | 0.37 ± 0.02a | 199.10 ± 18.59ns | |
| 0.5 | 0.31 ± 0.03ab | 202.74 ± 22.95ns | |
| 1 | 0.25 ± 0.04b | 193.28 ± 15.55ns | |
| Lactose | 0 | 0.35 ± 0.03c | 213.35 ± 0.67ns |
| 0.3 | 0.51 ± 0.01b | 219.32 ± 8.36ns | |
| 1 | 0.52 ± 0.01b | 205.00 ± 8.22ns | |
| 3 | 0.62 ± 0.06a | 196.02 ± 20.36ns | |
| Citric acid | 0 | 0.35 ± 0.03a | 213.35 ± 0.67a |
| 0.2 | 0.22 ± 0.01b | 220.97 ± 11.06a | |
| 1 | 0.23 ± 0.01b | 178.66 ± 41.54a | |
| 2 | 0.29 ± 0.03b | 51.65 ± 17.90b | |
| Glycerol | 0 | 0.35 ± 0.03a | 213.35 ± 0.67a |
| 0.3 | 0.24 ± 0.03b | 206.25 ± 0.41a | |
| 1 | 0.39 ± 0.04a | 216.64 ± 7.79a | |
| 3 | 0.26 ± 0.02b | 98.97 ± 12.95b |
Data were obtained from duplicate experiments except control (n = 10) and expressed as the mean ± standard deviation
Different letters (a, b, c) within the same column indicate significant differences (p < 0.05)
ns not statistically significant
Effect of added nitrogen sources
The added nitrogen sources were peptone, yeast extract, and l-glutamic acid. Peptone and yeast extract were added to the cooked soybeans at 0.3, 1.0, and 3.0%(w/w). However, l-glutamic acid was added to the range of 0.1, 0.2, and 0.3%(w/w), which did not affect taste of CKJ. Addition of peptone and yeast extract did not change the viscosity of CKJ as shown in Table 3. l-Glutamic acid decreased the viscosity as the amount of addition increased from 0.1 to 0.3%(w/w). Small amounts of peptone (0.3%(w/w)) increased the level of γ-PGA (0.42 ± 0.05%(w/w)), but the addition of large amounts inhibited its production. Yeast extract addition (3.0%(w/w)) increased γ-PGA production (0.75 ± 0.05%(w/w)), giving the most effective γ-PGA production, 2,2-fold, among the various nitrogen conditions. In the case of l-glutamic acid, the addition of only a small amount increased the production of γ-PGA. γ-PGA-producing bacteria are either l-glutamate-dependent or non-l-glutamate dependent (Luo et al., 2016). Therefore, Bacillus subtilis 168 may be classified as one of the glutamate dependent γ-PGA producers. Jian et al. reported that 4.9%(w/w) γ-PGA (w/dry weight) was obtained under 2.85%(w/w) yeast extract conditions by solid-state fermentation using Bacillus subtilis CCTCC202048 (Jian et al., 2005). Bacillus subtilis HA was reported to produce 11.1 g/L of γ-PGA by addition of 0.5%(w/w) ammonium sulfate in the medium (Seo et al., 2008). In this study, the addition of 3.0%(w/w) yeast extract to B. subtilis 168 could produce a large amount of γ-PGA in CKJ, which is used directly as food.
Table 3.
Changes in γ-PGA content and viscosity of cheongguk-jang by added nitrogen sources
| Nitrogen Source | ||||
|---|---|---|---|---|
| Final concentration, %(w/w) | Peptone | Yeast extract | l-glutamic acid | |
| Viscosity (cp) | 0 | 247.1 ± 0.18ns | 247.1 ± 0.18ns | 247.1 ± 0.18a |
| 0.1 | ND | ND | 256.08 ± 27.52a | |
| 0.2 | ND | ND | 237.94 ± 9.87ab | |
| 0.3 | 251.54 ± 21.66ns | 260.18 ± 0.37ns | 173.97 ± 43.62b | |
| 1 | 240.11 ± 44.9ns | 265.61 ± 29.43ns | ND | |
| 3 | 213.95 ± 23.74ns | 249.02 ± 0.40ns | ND | |
| γ-PGA, %(w/w) | 0 | 0.34 ± 0.01b | 0.34 ± 0.01b | 0.34 ± 0.01b |
| 0.1 | ND | ND | 0.46 ± 0.01a | |
| 0.2 | ND | ND | 0.43 ± 0.01a | |
| 0.3 | 0.42 ± 0.05a | 0.32 ± 0.03b | 0.51 ± 0.06a | |
| 1 | 0.25 ± 0.01c | 0.34 ± 0.08b | ND | |
| 3 | 0.27 ± 0.00c | 0.75 ± 0.05a | ND | |
Data were obtained from duplicate experiments and expressed as the mean ± standard deviation
Different superscripts (a, b, c) within the same column indicate significant differences (p < 0.05)
ns not statistically significant
γ-PGA production in CKJ under the combined nutritional condition
γ-PGA production was highest when 0.1%(w/w) FeCl3·6H2O, 3.0%(w/w) lactose or 3.0%(w/w) yeast extract was added. So, these conditions were applied all at once to ferment better CKJ and the produced γ-PGA content was measured (Table 4). Although the cumulative or synergistic effect of each ingredient was expected, the production of γ-PGA was only about 0.51 ± 0.02%(w/w). The level was higher compared to the control group (0.34 ± 0.01%(w/w)) but less than the amount of γ-PGA produced under each condition. By the way the viscosity of CKJ mucilage was not different from the control group under the same experimental conditions. The reason why the viscosity of mucilage of CKJ and its γ-PGA amount is not proportional is hard to explain. Probably the mucilage, which already has a fairly high viscosity, may not increase its viscosity even if the γ-PGA content is increased. In addition, Regestein Nee Meissner et al. reported that even though the amount of γ-PGA is important in the viscosity of mucilage, the influence of Mw of γ-PGA is greater (Regestein Nee Meissner et al., 2017). By the way, it is known that the Mw of γ-PGA can be greatly reduced as the fermentation progresses, thereby reducing viscosities (Feng et al., 2017). That is, in the case of liquid culture using Bacillus licheniformis ATCC 9945, the γ-PGA content was increased or maintained after 16 h cultivation, but the viscosity and Mw of γ-PGA were rapidly decreased (Jian et al., 2005).
Table 4.
Optimum condition for high γ-PGA production by fermentation cheongguk-jang
| Sample | Concentration, %(w/w) | Viscosity (cp) | γ-PGA, %(w/w) |
|---|---|---|---|
| Control | 0 | 214.14 ± 27.90a | 0.34 ± 0.01b |
| FeCl3·6H2O, lactose, yeast extract | 0.1, 3, 3 | 199.9 ± 21.26a | 0.51 ± 0.02a |
Data were obtained from duplicate experiments and expressed as the mean ± standard deviation
Different letters (a, b, c) within the same column indicate significant differences (p < 0.05)
ns not statistically significant
Taken together, we found that the most viscous CKJ with high γ-PGA content was produced when soybean was cooked at 121 °C for 60 min in the presence of 50%(w/w) added water, followed by fermentation with Bacillus subtilis 168 at 40 °C for 2 days in the presence of 0.1%(w/w) FeCl3·6H2O.
References
- Ashiuchi M, Kamei T, Baek DH, Shin SY, Sung MH, Soda K, Yagi T, Misono H. Isolation of Bacillus subtilis (chungkookjang), a poly-γ-glutamate producer with high genetic competence. Appl. Microbiol. Biotechnol. 2001;57:764–769. doi: 10.1007/s00253-001-0848-9. [DOI] [PubMed] [Google Scholar]
- Baek HI, Jung SY, Ha KC, Kim HM, Choi EK, Jung SJ, Park EO, Shin SW, Kim MG, Yun SK, Kwon DY, Yang HJ, Kim MJ, Kang HJ, Kim JH, Jeong DY, Jo SW, Cho BH, Chae SW. Effect of Chongkukjang on histamine-induced skin wheal response: a randomized, double-blind, placebo-controlled trial. J. Ethnic Foods. 2015;2:52–57. doi: 10.1016/j.jef.2015.04.003. [DOI] [Google Scholar]
- Baek LM, Park LY, Park KS, Lee SH. Effect of starter cultures on the fermentative characteristics of Cheonggukjang. Kor. J. Food Sci. Technol. 2008;40:400–405. [Google Scholar]
- Bajaj IB, Lele SS, Singhal RS. Enhanced production of poly (gamma-glutamic acid) from Bacillus licheniformis NCIM 2324 in solid state fermentation. J. Ind. Microbiol. Biotechnol. 2008;35:1581–1586. doi: 10.1007/s10295-008-0401-2. [DOI] [PubMed] [Google Scholar]
- Bajaj IB, Singhal RS. Enhanced production of poly (γ-glutamic acid) from Bacillus licheniformis NCIM 2324 by using metabolic precursors. Appl. Biochem. Biotechnol. 2009;159:133–141. doi: 10.1007/s12010-008-8427-5. [DOI] [PubMed] [Google Scholar]
- Bang BH, Rhee MS, Kim KP, Yi DH. Influences of culture medium components on the production poly (γ-glutamic acid) by Bacillus subtilis GS-2 isolated Chungkookjang. Korean J. Food Nutr. 2012;25:677–684. doi: 10.9799/ksfan.2012.25.3.677. [DOI] [Google Scholar]
- Chen J, Shi F, Zhang B, Zhu F, Cao W, Xu Z, Xu G, Cen P. Effects of cultivation conditions on the production of gamma-PGA with Bacillus subtilis ZJU-7. Appl. Biochem. Biotechnol. 2010;160:370–377. doi: 10.1007/s12010-008-8307-z. [DOI] [PubMed] [Google Scholar]
- Cheng C, Asada Y, Aida T. Production of γ-polyglutamic acid by Bacillus licheniformis A35 under denitrifying conditions. Agric. Biol. Chem. 1989;53:2369–2375. [Google Scholar]
- Davaatseren M, Hwang JT, Ho Park J, Kim MS, Wang S, Sung M. Poly-γ-glutamic acid attenuates angiogenesis and inflammation in experimental colitis. Mediators Inflamm. 2013;2013:982383. doi: 10.1155/2013/982383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Shi Q, Zhou G, Wang L, Chen A, Xie X, Huang X, Hu W. Improved production of poly-γ-glutamic acid with low molecular weight under high ferric ion concentration stress in Bacillus licheniformis ATCC 9945a. Process Biochem. 2017;56:30–36. doi: 10.1016/j.procbio.2017.02.017. [DOI] [Google Scholar]
- Francis F, Sabu A, Nampoothiri KM, Ramachandran S, Ghosh S, Szakacs G, Pandey A. Use of response surface methodology for optimizing process parameters for the production of α-amylase by Aspergillus oryzae. Biochem. Eng. J. 2003;15:107–115. doi: 10.1016/S1369-703X(02)00192-4. [DOI] [Google Scholar]
- Goto A, Kunioka M. Biosynthesis and hydrolysis of poly(gamma-glutamic acid) from Bacillus subtilis IF03335. Biosci. Biotechnol. Biochem. 1992;56:1031–1035. doi: 10.1271/bbb.56.1031. [DOI] [PubMed] [Google Scholar]
- Hara T, Ueda S. Regulation of poly glutamate production in Bacillus subtilis (natto): transformation of high pga productivity. Agric. Biol. Chem. 1982;46:2275–2281. [Google Scholar]
- Inatsu Y, Keitarou K, Yoshifumi I. Characterization of Bacillus subtilis strains isolated form fermented soybean foods in Southeast Asia: Comparison with B. subtilis (natto) starter strains. Jpn. Agric. Res. Q. 2002;36:169–175. doi: 10.6090/jarq.36.169. [DOI] [Google Scholar]
- Jian X, Shouwen C, Ziniu Y. Optimization of process parameters for poly γ-glutamate production under solid state fermentation from Bacillus subtilis CCTCC202048. Process Biochem. 2005;40:3075–3081. doi: 10.1016/j.procbio.2005.03.011. [DOI] [Google Scholar]
- Kim TW, Lee TY, Bae HC, Hahm JH, Kim YH, Park C, Kang TH, Kim CJ, Sung MH, Poo H. Oral Administration of high molecular mass poly-γ-glutamate induces NK Cell-Mediated Antitumor Immunity. J. Immunol. 2007;179:775–780. doi: 10.4049/jimmunol.179.2.775. [DOI] [PubMed] [Google Scholar]
- Lee BY, Kim DM, Kim KH. Physico-chemical properties of viscous substance extracted from Chungkook-jang. Kor. J. Food Sci. Technol. 1991;23:599–604. [Google Scholar]
- Lee EH, Son WC, Lee SE, Kim BH. Anti-Obesity Effects of poly-γ-glutamic acid with or without isoflavones on high-fat diet induced obese mice. Biosci. Biotechnol. Biochem. 2013;77:1694–1702. doi: 10.1271/bbb.130253. [DOI] [PubMed] [Google Scholar]
- Lee MY, Park SY, Jung KO, Park KY, Kim SD. Quality and functional characteristics of Chungkukjang prepared with various Bacillus sp. isolated from traditional Chungkukjang. J. Food Sci. 2005;70:M191–M196. doi: 10.1111/j.1365-2621.2005.tb07187.x. [DOI] [Google Scholar]
- Luo Z, Guo Y, Liu J, Qiu H, Zhao M, Zou W, Li S. Microbial synthesis of poly-γ-glutamic acid: current progress, challenges, and future perspectives. Biotechnol. Biofuels. 2016;9:134. doi: 10.1186/s13068-016-0537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie G, Zhu Z, Liu F, Nie Z, Ye Y, Yue W. Co-Production of nattokinase and poly (γ-glutamic acid) under solid-state fermentation using soybean and rice husk. Braz. Arch. Biol. Technol. 2015;58:718–724. doi: 10.1590/S1516-89132015050172. [DOI] [Google Scholar]
- Oh SM, Jang EK, Seo JH, Ryu MJ, Lee SP. Characterization of γ-polyglutamic acid produced from the solid-state fermentation of soybean milk cake using Bacillus sp. Food Sci. Biotechnol. 2007;16:509–514. [Google Scholar]
- Park C, Choi JC, Choi YH, Nakamura H, Shimanouchi K, Horiuchi T, Misono H, Sewaki T, Soda K, Ashiuchi M, Sung MH. Synthesis of super-high-molecular-weight poly-γ-glutamic acid by Bacillus subtilis subsp. chungkookjang. J. Mol. Catal. B: Enzym. 2005;35:128–133. doi: 10.1016/j.molcatb.2005.06.007. [DOI] [Google Scholar]
- Peng Y, Jiang B, Zhang T, Mu W, Miao M, Hua Y. High-level production of poly(γ-glutamic acid) by a newly isolated glutamate-independent strain. Bacillus methylotrophicus. Process Biochem. 2015;50:329–335. doi: 10.1016/j.procbio.2014.12.024. [DOI] [Google Scholar]
- Regestein Nee Meissner L, Arndt J, Palmen TG, Jestel T, Mitsunaga H, Fukusaki E, Buchs J. Investigation of poly(gamma-glutamic acid) production via online determination of viscosity and oxygen transfer rate in shake flasks. J. Biol. Eng. 2017;11:1–16. doi: 10.1186/s13036-017-0065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard A, Margaritis A. Rheology, oxygen transfer, and molecular weight characteristics of poly(glutamic acid) fermentation by Bacillus subtilis. Biotechnol. Bioeng. 2003;82:299–305. doi: 10.1002/bit.10568. [DOI] [PubMed] [Google Scholar]
- Seo JH, Kim CS, Lee SP. Physicochemical properties of poly-γ-glutamic acid produced by a novel Bacillus subtilis HA isolated from Cheonggukjang. J. Food Sci. Nutr. 2008;13:354–361. [Google Scholar]
- Shih IL, Van YT. The production of poly-(gamma-glutamic acid) from microorganisms and its various applications. Bioresour. Technol. 2001;79:207–225. doi: 10.1016/S0960-8524(01)00074-8. [DOI] [PubMed] [Google Scholar]
- Shin EJ, Sung MJ, Park JH, Yang HJ, Kim MS, Hur HJ, Hwang JT. Poly-γ-glutamic acid induces apoptosis via reduction of COX-2 expression in TPA-induced HT-29 human colorectal cancer cells. Int. J. Mol. Sci. 2015;16:7577–7586. doi: 10.3390/ijms16047577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirisansaneeyakul S, Cao MF, Kongklom N, Chuensangjun C, Shi ZP, Chisti Y. Microbial production of poly-gamma-glutamic acid. World J. Microbiol. Biotechnol. 2017;33:8. doi: 10.1007/s11274-017-2338-y. [DOI] [PubMed] [Google Scholar]
- Tanimoto H, Mori M, Motoki M, Torii K, Kadowaki M, Noguchi T. Natto mucilage containing poly-gamma-glutamic acid increases soluble calcium in the rat small intestine. Biosci. Biotechnol. Biochem. 2001;65:516–521. doi: 10.1271/bbb.65.516. [DOI] [PubMed] [Google Scholar]
- Wang FQ, Liang JZ, Xiao W, Wang W, Fu DW. Improved production of poly-gamma-glutamate by newly Bacillus subtilis 115. J. Biobased Mater. Bio. 2017;11:159–168. doi: 10.1166/jbmb.2017.1650. [DOI] [Google Scholar]
- Wei B, Cha SY, Kang M, Kim YJ, Cho CW, Rhee YK, Hong HD, Jang HK. Antiviral activity of Chongkukjang extracts against influenza A virus in vitro and in vivo. J. Ethnic Foods. 2015;2:47–51. doi: 10.1016/j.jef.2015.04.001. [DOI] [Google Scholar]
- Yang EI, Kim YS. Physiological properties of viscous substance from Cheonggukjang. J. Agric. Life Sci. 2013;44:10–14. [Google Scholar]
- Zhang H, Zhu J, Zhu X, Cai J, Zhang A, Hong Y, Huang J, Huang L, Xu Z. High-level exogenous glutamic acid-independent production of poly-(γ-glutamic acid) with organic acid addition in a new isolated Bacillus subtilis C10. Bioresour. Technol. 2012;116:241–246. doi: 10.1016/j.biortech.2011.11.085. [DOI] [PubMed] [Google Scholar]


