Abstract
Neutrophil extracellular traps (NETs) are extracellular DNA structures covered with antimicrobial peptides, danger molecules, and autoantigens that can be released by neutrophils. NETs are an important first-line defense mechanism against bacterial, viral, fungal, and parasitic infections, but they can also play a role in autoimmune diseases. NETs are immunogenic and toxic structures that are recognized by the autoantibodies of patients with antineutrophil cytoplasmic antibodies−associated vasculitis (AAV) (i.e., against myeloperoxidase or proteinase-3) and systemic lupus erythematosus (SLE) (i.e., against double-stranded DNA, histones, or nucleosomes). There is cumulating preclinical and clinical evidence that both excessive formation and impaired degradation of NETs are involved in the pathophysiology of AAV and SLE. These autoimmune diseases give rise to 2 clinically and pathologically distinct forms of glomerulonephritis (GN), respectively, crescentic pauci-immune GN and immune complex−mediated GN. Therefore, it is relevant to understand the different roles NET formation can play in the pathophysiology of these most prevalent renal autoimmune diseases. This review summarizes the current concepts on the role of NET formation in the pathophysiology of AAV and SLE, and provides a translational perspective on the clinical implications of NETs, such as potential therapeutic approaches that target NET formation in these renal autoimmune diseases.
Keywords: ANCA-associated vasculitis, autoimmune diseases, glomerulonephritis, neutrophil extracellular traps (NETs), systemic lupus erythematosus
Neutrophil Biology
Neutrophils are the most abundant (∼57%) subpopulation of circulating white blood cells and represent the most important effector cells of the innate immune system. They are typically recognized by the lobulated nucleus and have a relatively short lifespan of hours to days.1 Upon infection, neutrophils are the first responders of the immune system at the site of inflammation, and they recruit and activate other immune cells. To exert their primary defense function, neutrophils have the ability to attack pathogens by phagocytosis and by the release of different granules (called degranulation) that contain antimicrobial peptides and proteases, such as myeloperoxidase (MPO), neutrophil elastase, LL37, and matrix metalloproteinases.2 Recently, it has become clear that neutrophils also have the ability to directly attack and restrain pathogens by releasing neutrophil extracellular traps (NETs).3, 4
NETosis is a process that results in the release of extracellular DNA by neutrophils, which was originally believed to coincide with cell death5 and is phonetically classified among other regulated cell death pathways, such as apoptosis, pyroptosis, necroptosis, and ferroptosis.6 After the discovery of NETosis, similar processes have been described in other immune cells, including eosinophils,7 monocytes,8 and B cells,9 which are collectively referred to as “ETosis” and which are out of the scope of this review. These pathways may be classified by their caspase dependency and their immunogenicity. Classic apoptosis is typically seen as a caspase-dependent, non-immunogenic regulated cell death that is associated with the preservation of plasma membrane integrity throughout the process of cell death.10 In contrast, caspase-independent necroptosis and ferroptosis, as well as caspase-dependent pyroptosis, are all highly immunogenic forms of regulated cell deaths associated with the loss of plasma membrane integrity.6 Necroptosis and pyroptosis have been demonstrated to be relevant to fighting bacterial and viral infections,6 whereas ferroptosis has been implicated in cancer cell death and tissue injury.11 NETosis is a caspase-independent process, but studies are seemingly unclear on how to classify the process as immunogenic12, 13 or even anti-inflammatory.14 This is mainly due to the fact that the pathways leading to NETosis are still evolving,15 and many studies have demonstrated that distinct forms of the release of extracellular DNA by neutrophils exist.16, 17 Besides the classical suicidal NET formation that coincides with neutrophil death, it has also been demonstrated that NET formation can occur independently of cell death, which is referred to as vital NET formation.18, 19 NETs can also have anti-inflammatory effects, which has been demonstrated in mice models of gout14 and lupus-prone mice with defects in reduced NAD phosphate (NADPH) oxidase, which show a more severe phenotype.20
NETosis by neutrophils is an important mechanism in the innate immune system. However, it was recently described that neutrophils can play a role in the adaptive immune response through interaction with antigen-presenting cells21 and lymphocytes,22 both at sites of inflammation and in draining lymph nodes.22 In mice, it was shown that a subset of neutrophils have the ability to induce antibody production and class switching of marginal zone B cells by production of B-cell activating factor, a proliferation-inducing ligand, interleukin (IL)-21, CD40L expression, and NET formation.23 Moreover, fewer and hypomutated marginal zone B cells were observed in patients with congenital neutropenia, which supported this novel function of neutrophils as modulators of the adaptive immune response.23
Immunogenicity and Toxicity of NETs
The first and foremost assumption of NETs is that the extruded DNA is immunogenic and leads to overt inflammation that can then potentially lead to autoimmune diseases. However, it has long been known that DNA in itself is not immunogenic.24 Only DNA in combination with danger signals (i.e., danger-associated molecular patterns), such as LL37, a cathelicidin antimicrobial peptide,24, 25 or High Mobility Group Box Protein-1 (HMGB1),26 can activate antigen-presenting cells, in particular, plasmacytoid dendritic cells (DCs)24 and B cells.26, 27 This is mediated through Toll-like receptor-9 (TLR9) signaling that results in the production of interferon-α21 and (auto-)antibodies.27 Preclinical mouse models demonstrated that in vitro monocyte-derived DCs take up DNA particles from neutrophils undergoing NETosis, apoptosis, or necrosis.28 This internalization is mediated via the receptor for advanced glycation endproducts (RAGE)–TLR9 pathway.29, 30 Transfer of these DNA-loaded monocyte-derived DCs led to production of antibodies against dsDNA, MPO, and proteinase-3 (PR3) in mice.28 Autoantibody production was most significant when mice were injected with DNA-loaded monocyte-derived DCs that were exposed to NET-ting neutrophils. Other studies also demonstrated that nuclear material from NETs was more immunogenic than apoptotic material.12, 15
Besides the immunogenic effects of NETs, they are also believed to have a direct cytotoxic effect on human epithelial and endothelial cells through the externalization of histones31, 32, 33 and MPO.33, 34 NET-related histones were demonstrated to cause direct cytotoxicity of glomerular endothelial cells, podocytes, and parietal endothelial cells, which led to crescentic glomerulonephritis (GN) in preclinical models.35 Crescentic GN is typically seen in antineutrophil cytoplasmic antibodies (ANCA)−associated vasculitis (AAV) patients and is less frequent in lupus nephritis (LN). Moreover, extracellular MPO was demonstrated to induce oxidative damage,33 which was associated with glomerular and interstitial injury in AAV patients.34 In addition, endothelial cells have a limited capacity to internalize NETs.36 An overflow of NETs induce vascular leakage and endothelial-to-mesenchymal transition. In patients with systemic lupus erythematosus (SLE), glomerular presence of NETs was correlated with the severity of proteinuria and glomerular endothelial to mesenchymal transition,36 which emphasized the relevancy of this process.
Overall, break of self-tolerance toward autoantigens is a hallmark for a wide spectrum of systemic autoimmune diseases, including AAV37 and SLE.38 NETs are believed to be an important source of autoantigens in systemic autoimmune diseases.12, 21, 24, 25, 30, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 Indeed, 74% of these identified NET-associated proteins are recognized by autoantibodies in systemic autoimmune diseases.48 This NET autoimmunity was most prominent in SLE patients, and subsequently, in AAV patients. Proteomic studies of NETs derived from neutrophils of patients with AAV or SLE, or alternatively healthy neutrophils stimulated with AAV and SLE sera, are lacking. The currently identified range of peptides and enzymes localized to NETs are studied by proteomics of NETs induced by phorbol-12-myristate-13-acetate (PMA).49 Translating data from PMA-induced NETs to clinical disease should generally be done with caution, because the in vivo relevance of PMA as a chemical compound remains unclear.16 Nevertheless, several NET-related proteins found on PMA-induced NETs have also been identified with immunofluoresence microscopy studies on AAV- and SLE-induced NETs. The current data on NET-associated molecules, as identified by proteomics or immunofluorescence microscopy studies, that are known autoantigens in AAV and SLE are listed in Table 1.50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91
Table 1.
NET-associated molecules that are known autoantigens in AAV and/or SLE
| NET- molecules | Method of detection | Neutrophil localization | Auto-ag | Role in autoimmune disease | Ref. |
|---|---|---|---|---|---|
| Azurocidin | Proteomics of PMA-induced NETs49 | Azurophilic granules | AAV | Autoantibodies (atypical) present in AAV | 50 |
| Cathepsin G | Proteomics of PMA-induced NETs49 | Azurophilic granules | AAV SLE |
Autoantibodies (atypical) present in AAV Autoantibodies are present in SLE |
51, 52, 53, 54 |
| Neutrophil Elastase | Proteomics of PMA-induced NETs49 | Azurophilic granules | AAV SLE |
Anti-elastase antibodies present in AAV Anti-elastase antibodies are present in SLE. |
51, 52, 55 |
| Lactoferrin | Proteomics of PMA-induced NETs49 | Secondary granules | AAV SLE |
Atypical ANCA in AAV Autoantibodies are present in SLE |
52, 53, 54, 56, 57 |
| LAMP-2 | IF of PMA-induced NETs derived of AAV neutrophils | Lysosomal membrane of granules | AAV | Anti-LAMP autoantibodies are present in AAV patients Detected in AAV kidney biopsies |
58, 59, 60 |
| Lysozym C | Proteomics of PMA-induced NETs49 | Secondary granules | AAV | Atypical ANCA in AAV | 51, 52 |
| MPO | Proteomics of PMA-induced NETs49 IF of AAV-induced NETs and SLE-induced NETs 61 |
Azurophilic granules | AAV | Typical autoantigen for ANCAs in AAV Detected in AAV kidney biopies Anti-MPO ab are sometimes present in SLE |
34, 51, 58, 62 |
| PR3 | Proteomics of PMA-induced NETs49 Present on cell bodies of NET-ting neutrophils63 |
Azurophilic granules | AAV | Typical autoantigen for ANCAs autoantigen in AAV Detected in AAV kidney biopies |
58 |
| Alpha actinin 1/4 | Proteomics of PMA-induced NETs49 | Cytoskeleton | SLE | Autoag in LN, also bound by anti-dsDNA ab. Autoab associated with disease activity in SLE | 64, 65, 66, 67, 68, 69 |
| AENO | Proteomics of PMA-induced NETs49 | Glycolytic enzymes | SLE | Autoag eluted from LN biopsies. Autoab associated with disease activity in SLE |
70, 71, 72 |
| Annexin A1 | Proteomics of SLE-induced NETs/IF73 | Cytosol | SLE | Autoag eluted from LN biopsies. Autoantibodies present in SLE and associated with disease activity |
70, 72, 73 |
| C1q | IF of PMA-induced NETs incubated with SLE serum74 | − | SLE | Anti-C1q antibodies are present in SLE and associated with disease activity | 71, 74, 75, 76, 77 |
| Catalase | Proteomics of PMA-induced NETs49 | Peroxisomal | SLE | Autoantibodies present in SLE | 78 |
| Citrullinated histones | IF AAV-induced NETs79 and SLE-induced NETs80 | Cytoplasmic granules and nucleus | SLE | Anti-CCP antibodies are rarely detected in SLE | 81 |
| dsDNA | By definition present | Nucleus | SLE | Anti-dsDNA antibodies are hallmark of SLE and strongly correlate with disease activity | 81 |
| Histones (H2A, H2B, H3, H4) | Proteomics of PMA-induced NETs49 | Nucleus | SLE | Autoantigen in SLE Causing crescentic GN |
35 |
| HMGB1 | IF of RNP-ICx-induced NETs21 | Nucleus | SLE | Anti-HMGB1 AAbs levels correlate with disease activity, with anti-dsDNA Abs and with HMGB1 levels in SLE. HMGB1 binds (SLE)-ICx | 21, 26, 81, 82, 83 |
| HNP/α defensins | Proteomics of PMA-induced NETs.49 IF of PMA induced NETs and SLE-induced NETs 24 | Azurophilic Granules | SLE | HNP binds SLE-ICx Anti-HNP autoantibodies are present in SLE |
24, 84 |
| LL37 | IF of PMA induced and SLE-induced NETs24 | Nuclear | SLE | Anti-LL37 antibodies are present in SLE LL37 binds SLE-ICx |
21, 27, 85, 86 |
| mtDNA | IF of RNP-ICx-induced NETs42 | Mitochondria | SLE | Antimitochondrial antibodies are present in SLE patients | 42, 81 |
| Properdin | IF of AAV-induced NETs 87 IF of PMA induced-NETs88 |
Secondary granules | SLE | Properdin levels are decreased in SLE sera Case report of anti-properdin antibodies in SLE. Properdin is present in AAV kidney biopsies |
89, 90, 91 |
AAV, antineutrophil cytoplasmic antibodies (ANCA)−associated vasculitis; AENO, alpha enolase; IF, immunofluorescence; LAMP-2, lysosomal membrane protein-2; MPO, myeloperoxidase; NET, neutrophil extracellular trap; PMA, phorbol myristate acetate; PR3, proteinase-3; RNP, ribonucleoprotein; SLE, systemic lupus erythematosus.
The main antigens for ANCAs (i.e., MPO and PR3), which both originate from the azurophilic granules of neutrophils, were demonstrated on NETs.39, 63 In addition, co-localization of NETs (determined as extracellular histones) with MPO and PR3 was demonstrated in kidney biopsies of AAV patients.34, 39 Autoantigens for atypical ANCAs, including azurocidin,50, 92 cathepsin G,53 elastase,51, 53 lactoferrin,51, 53 lysosomal membrane protein-2,58 and lysozym C51 were demonstrated in NETs (Table 1). These atypical ANCAs are sometimes present in AAV patients,92 but are also commonly associated with other systemic inflammatory diseases.92
SLE patients, and especially those with immune complex (ICx)−mediated LN, can present with a wide range of circulating autoantibodies (>180 specificities) that recognize, among others, dsDNA, histones, nucleosomes, and extractable nuclear antigens.81 Many of these SLE-specific autoantigens can be found on NETs49 (Table 1), whereas some extractable nuclear antigens, including Ro, La, Smith, and ribonucleoprotein have not yet been identified on NETs.40, 49, 93 The combination of autoantibodies that recognize autoantigens on NETs convert these structures into highly immunogenic ICxs that can engage with TLRs and Fc-γ receptor (FcyR)IIa.48
Altogether, these cumulative data demonstrate that (i) NETs are immunogenic; (ii) NETs can directly mediate cytotoxicity to the glomerular tuft; and (iii) NETs contain relevant AAV and SLE autoantigens and contribute to the induction of autoimmunity.
Triggers and Pathways of NET Formation
Since the discovery of NETosis in 2004,3 the triggers and mechanisms of NET formation in vitro have been extensively studied, but unfortunately the exact in vivo processes remain to be fully elucidated.5, 94, 95 A profound understanding of the triggers and intracellular pathways leading to NETosis in autoimmune diseases is important to understand their role in disease pathophysiology and to identify potential, novel therapeutic strategies.
Over the years, a wide range of chemical and physiological triggers have been identified that can trigger NET formation in vitro. It is important to realize that although different stimuli can result in NET formation (i.e., the release of neutrophil-derived DNA to the extracellular space), it often involves signaling through distinct pathways.16, 18, 46, 96, 97 The main pathways that have been demonstrated to be involved in different forms of NET formation include activation of protein kinase C (PKC),98 NADPH oxidase,94, 95 reactive oxygen species (ROS),99 the Raf-mitogen-activated protein kinase (MEK)-extracellular signal–regulated kinase (ERK) pathway,100 the MPO/neutrophil elastase (NE) complex,101 autophagy,5, 58 microtubule polymerization,102 and protein arginine deiminase (PAD)-4/histone citrullination.103, 104, 105 In addition, during NET formation, the breakdown of the nuclear envelope will need to occur, which resembles the nuclear envelope disintegration during mitosis in dividing cells.106 In the following, we will focus on preclinical studies of known triggers and pathways of NET formation.
In vitro NET formation has been primarily studied after stimulation with PMA,3 a robust chemical compound that induces massive NET formation through PKC signaling, calcium influx, and ROS production.16 Subsequently, the azurosome is activated, which is a complex of MPO, NE, and cathepsin G, which leads to chromatin decondensation,101, 107 rupture of the plasma membrane, and release of chromosomal DNA.94, 97 PMA-induced NET formation is strictly dependent on NADPH-mediated ROS production.16, 94 This was primarily demonstrated in neutrophils derived from patients with chronic granulomatous disease that have mutations in their NADPH oxidase complex; therefore, their neutrophils are unable to produce ROS and are incapable of NET formation induced by PMA.16, 94 In line with this, PMA-induced NET formation can effectively be blocked by diphenyleneiodonium.108 In addition, PMA-induced NET formation does not usually involve PAD enzymes, because PMA activates PKCα, which inhibits PAD enzymes intracellularly.109 Consequently, citrullination of histones is generally low on PMA-induced NETs.16
The latter is in contrast to another widely used trigger of NET formation, calcium ionophores (CIs), which trigger DNA release through a calcium-dependent hyperactivation of PAD enzymes17 and results in hypercitrullination of histones.16, 17 This process is independent of PKC and ROS.104 Importantly, in some studies, PAD enzyme inhibition led only to a limited inhibition of CI-induced NET formation, which implied that citrullination itself might not be a prerequisite.110 CI-induced NET formation is intrinsically distinct from PMA-induced NET formation, but in the end, both pathways result in neutrophil-derived extracellular DNA release. Importantly, the citrullination of histones, as indicated by citrullinated histone H3 (CitH3) staining, is much more evident on CI-induced NET formation compared with PMA-induced NET formation.17
The involvement of PAD enzymes in NET formation originally came from the observation that PAD4 deficient mice could not make NETs (as measured by CitH3-positive NETs), when stimulated with CIs.103, 111 Because murine neutrophils are distinct from human neutrophils,112 results from mouse neutrophil experiments do not always directly translate to humans.113 For instance, there is a different balance of lymphocytes and neutrophils between humans and mice: human blood contains mainly neutrophils (50−70% neutrophils, 30−50% lymphocytes), whereas mouse blood contains mainly lymphocytes (75−90% lymphocytes vs. 10−25% neutrophils).114 Also in contrast to human neutrophils, murine neutrophils do not express defensins,115 FcαRI, FcγRIIA, and FcγRIIC,116 and various chemokines (e.g., IL-8).112 Moreover, histones present in NETs can, but will not always, undergo posttranslational modifications, such as citrullination.117 Thus, studies investigating for only the presence of CitH3-positive NETs as a quantitative measure for total NET formation potentially neglect CitH3-negative NETs, which are especially present when PAD enzymes are inhibited.16, 17, 118 Thus, citrullination of NET-related histones can occur during NET formation but is not required for NET formation.16, 17, 109, 119 As such, PAD inhibition can decrease NET formation dependently of the trigger used to induce NETs and will always result in decreased or absent citrullination of histones.17, 111 Therefore, the interpretation of CitH3 as a quantitative NET marker should be used with consideration.16, 17
All of the previously described triggers of NET formation involved lysis of the membrane, which is named lytic NET formation.18 Lytic NET formation is also referred to as suicidal NET formation, which typically takes a few hours, involves NADPH oxidase and ROS, and results in plasma membrane lysis, and consequently, DNA release, after which the neutrophil dies.46
In contrast, there are studies that demonstrated a nonlytic form of NET formation, which is also referred to as vital NET formation.18, 19, 120 During nonlytic NET formation, the neutrophils stay alive and retain their capability of phagocytosis.19 In contrast to the classic lytic forms, this does not involve plasma membrane rupture, and DNA is released through blebbing of vesicles.9, 120, 121
Nonlytic NET formation can be triggered by lipopolysaccharide (LPS),122 micro-organisms,19, 121, 123, 124 TLR4-activated platelets,122, 125 complement proteins together with TLR2 ligands,19 granulocyte-macrophage, colony-stimulating factor in combination with TLR4 or C5a,120 TLR9 triggering by CpG or non-CpG,9 or SLE-specific ICx,21, 42, 80, 126, 127 Nonlytic NET formation is triggered within minutes,121 and there is still controversy as to whether this is dependent on NADPH oxidase42, 120 or independent of NADPH oxidase.9, 121, 122, 126 However, chronic granulomatous disease patients who lack NADPH oxidase rely on mitochondrial ROS and form mitochondrial DNA enriched NETs. Moreover, nonlytic NETs were demonstrated to be enriched for interferogenic mitochondrial DNA.9, 120 The involvement of PAD enzymes and citrullination has not been studied in depth for vital NET formation,17 but it has been shown that Leishmania parasites induce vital NET formation within 10 minutes independently of both ROS and PAD enzymes.124 Taken together, these data demonstrate that NET formation is a highly specific regulated process that can be triggered by a wide range of different stimuli, all engaged on a different molecular pathway before finally leading to the extrusion of neutrophil-derived DNA in the extracellular environment. The involvement of the different pathways is intrinsically dependent on the specific trigger of NET formation. Therefore, the elucidation of the disease-specific triggers of NET formation and the pathways that are involved is essential to understand the role of NETs in autoimmune diseases such as AAV and SLE.
NETs in Renal Autoimmune Diseases
NET Formation in Autoimmune GN
In healthy humans, the formation of NETs has an antimicrobial function4 and is counterbalanced by the physiological degradation of NETs by DNAse.61 As expected, both excessive NET formation and impaired NET degradation has been demonstrated to play an important role in the pathogenesis of renal autoimmune diseases, including AAV and SLE.21, 24, 27, 61, 128
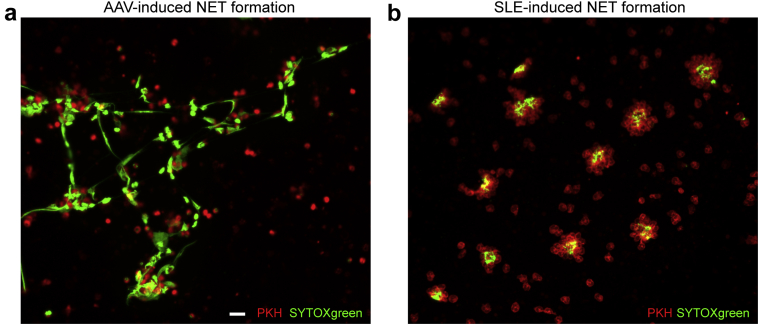
Recently, we demonstrated that an excess in ex vivo NET formation was characteristic for both patients with active AAV79 and patients with severe SLE.80 We also made a side-by-side comparison of AAV- and SLE-induced NET formation using confocal microscopy and immunohistochemistry, and showed lytic NET formation within hours in AAV versus nonlytic NET formation with clustering of NET-ting neutrophils within minutes in SLE (Figure 1) (unpublished data). Moreover, it was demonstrated that AAV-induced NET formation involved NADPH oxidase and PAD enzymes,79 whereas SLE-induced NET formation was independent of NADPH oxidase.126, 127 Recently, several studies linked necroptosis, a lytic form of cell death that is mediated by receptor interacting protein kinase (RIPK) and mixed lineage kinase domain (MLKL), to lytic NET formation,129 and specifically, to AAV-induced NET formation.130 In contrast, SLE-induced NETs have immunogenic properties with the presence of HMGB121 and oxidized mitochondrial DNA,42 which has not been seen on AAV-induced NETs (L.S. van Dam, unpublished data). NETs can also frequently contain posttranslational modifications (e.g., acetylation, methylation, citrullination).41, 47, 104, 117 In SLE, this leads to the development of autoantibodies against modified histones,47, 117 for instance, acetylated and methylated histones,117, 131, 132, 133 and modified ubiquinated MPO.134 Of note, ubiquinated-MPO−enriched NETs are highly capable of activating macrophages.134 In AAV, NETs were specifically enriched for citrullinated histones (L.S. van Dam, unpublished data), which were linked to causing crescentic GN in preclinical models.35 Another important distinction are the triggers of excessive NET formation, which is IgG-dependent in SLE80 but independent of IgG in AAV.79
Figure 1.
Ex vivo neutrophil extracellular trap (NET) formation in antineutrophil cytoplasmic antibodies (ANCA)−associated vasculitis (AAV) and systemic lupus erythematosus (SLE). Paul-Karl-Horan (PKH)26-labelled neutrophils (red) derived from a healthy donor were exposed to 10% serum of patients with AAV or SLE for 4 hours to induce NET formation. Extracellular DNA was stained with SYTOXgreen (green) and NET formation was imaged with immunofluorescence confocal microscopy. Images of AAV- (a) and SLE-induced (b) NET formation are shown at original magnification ×10; bar = 20 um.
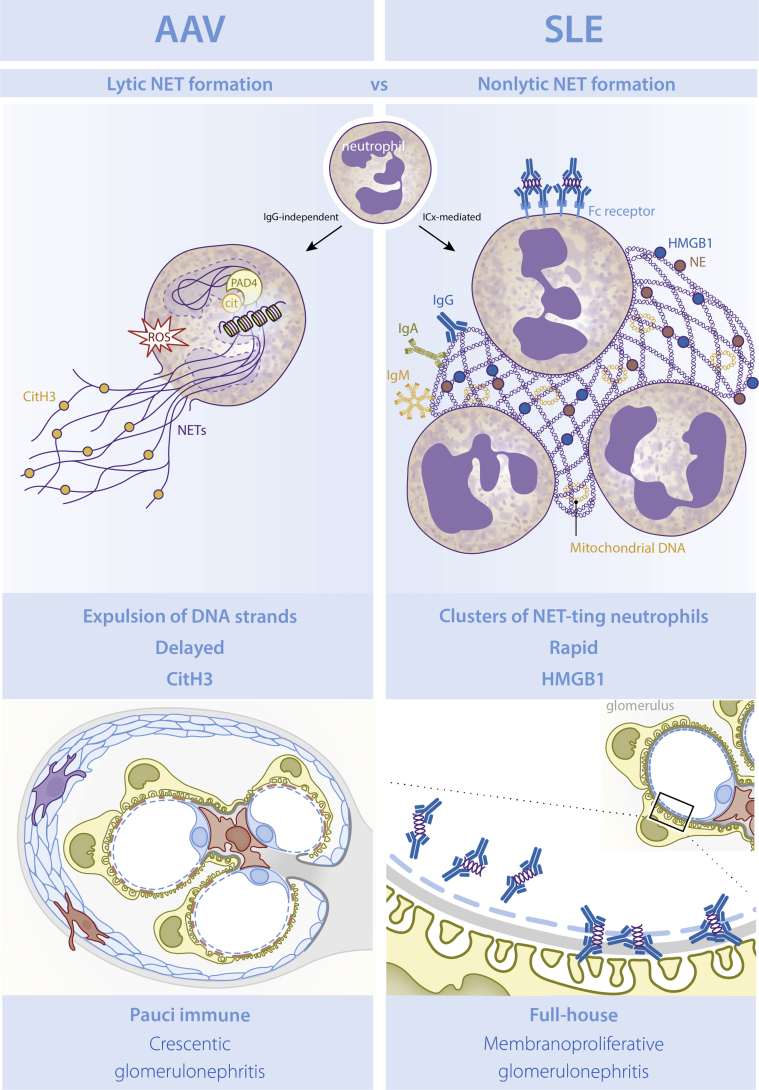
Taken together, cumulative evidence demonstrated that NET formation is not equal in SLE- and ANCA-associated renal autoimmune diseases and can be linked to the distinct forms of GN observed in these patients, which are features of AAV- and SLE-induced NET formation closely associated with the respective, typical features of pauci-immune, histone-induced crescentic GN in AAV and ICx-mediated, full-house LN in SLE (Figure 2).
Figure 2.
Overview of neutrophil extracellular trap (NET) formation in antineutrophil cytoplasmic antibodies (ANCA)−associated vasculitis (AAV) versus systemic lupus erythematosus (SLE). In AAV, lytic NET formation is induced involving reduced NAD phosphate oxidase and protein arginine deiminase (PAD) enzymes, which results in a lytic expulsion of NETs harboring citrullinated histones within hours. In SLE, nonlytic extrusion of NETs concomitant with clustering of neutrophils is induced within minutes. SLE-induced NETs have immunogenic properties, including enrichment for High Mobility Group Box Protein 1 (HMGB1), oxidized mitochondrial-derived DNA, and immune complex (ICx) formation, which was not the case for AAV-induced NETs. ROS, reactive oxygen species.
NET Degradation in Autoimmune GN
As mentioned previously, NETs also have a physiological role and become potentially pathogenic when they are not degraded efficiently.61, 128 For SLE patients, impaired degradation of NETs and other apoptotic material was associated with severity of lupus disease, and notably, LN.74, 128 The underlying reason for impaired NET degradation was demonstrated to be dependent upon at least 2 mechanisms in these SLE patients: (i) the presence of DNase1 inhibitors was shown to reduce the capacity of NET degradation; and (ii) the presence of anti-NET antibodies (i.e., a mix of antibodies against nuclear material) formed complexes that prevented the enzymatic degradation of NETs by the DNAse enzyme.24, 128 These phenomena were also shown to be present in MPO+ AAV patients who exhibited lower rates of NET degradation.61
Although impaired DNase1 is proposed as the main regulator NET degradation, it is unclear whether this enzyme can function in the tissues; impaired DNase activity was not associated with disease activity in AAV patients.61, 135 Macrophages have been reported as important effector cells that clear NETs. Defective phagocytosis by macrophages of mice deficient in milk fat globule epidermal growth factor-8 developed GN,136 and also lupus-prone mice with defective macrophages through deficiency of caspase-activated DNase resulted in higher anti-DNA antibody levels.136 Moreover, patients with acute respiratory distress syndrome demonstrated enhanced NET formation and diminished macrophage engulfment.137 Until now, no study has investigated the clearance of NETs by macrophages in AAV or SLE patients; however, this could be a potential contributing mechanism to the pathophysiology of these autoimmune diseases.
In summary, several studies demonstrated that both excessive NET formation and reduced NET degradation is a common autoimmune phenomenon found in AAV and SLE. However, the triggers and pathways leading to excessive NET formation in these renal autoimmune diseases are intrinsically different.
Excessive NET Formation: Focus on AAV
The role of NET formation in AAV was initially demonstrated by Kessenbrock et al. who observed that isolated ANCA was capable of inducing NET formation, and that NET structures were detected in renal biopsies of AAV patients.39 Although these findings were subsequently confirmed,61, 138, 139 others showed in vivo that circulating NET remnants were highly present in AAV patients with active disease but had an inverse correlation to serum ANCA levels.140 Recently, we demonstrated that NET formation was induced by IgG-depleted AAV sera,79 whereas IgA was not involved. These data suggested that not ANCAs, but other coinciding factors, affected neutrophils to form NETs in vivo, whereas the exact triggers controlling AAV-induced NET formation still remain unknown.
Importantly, AAV-induced NETs are proinflammatory,130 and were demonstrated to mediate vascular injury through inflicting endothelial injury in vitro.130, 140 As discussed previously, both processes are linked to glomerular injury and crescentic GN in AAV.34, 35 In addition, AAV-induced NETs are able to activate the alternative pathway of complement,87, 130 which is an important contributor to AAV pathogenesis, as demonstrated by the clinical success of C5aReceptor blockade in patients.141
In summary, NETs have a high clinical relevance in the pathophysiology of AAV because AAV patients display both an excessive formation and impaired degradation of NETs. NETs contain the main autoantigens for AAV and directly cause cytotoxicity, which leads to crescentic lesions in pauci-immune GN in AAV.
Excessive NET Formation: Focus on SLE
In the earliest publications that claimed NETs were related to SLE disease pathogenesis, investigators found that SLE neutrophils released significantly increased levels of DNA referred to as spontaneous NET formation.24 It has been known for a long time that neutrophils of SLE patients are different from those of healthy people; the existence of a subgroup of low-density granulocytes was demonstrated in 1986.142 Later, it was demonstrated that these neutrophils had an increased capability to form NETs.44, 143, 144
Morphologically, SLE sera can induce typical clustering of neutrophils.75, 145, 146 This phenomenon of neutrophil clustering preceded the discovery of NETs and has been known since 1990.75, 146 Clustering of neutrophils upon stimulation with SLE sera was correlated with lupus disease activity and was associated with the presence of anti-C1q autoantibodies.75 During SLE-induced NET formation, we observed a nonlytic form of NET formation that coincided with clustering of neutrophils (unpublished data). Importantly, SLE-induced NET formation was demonstrated to be NADPH/ROS-independent1, 127 and resulted in release of mitochondrial DNA,120 which are characteristics of nonlytic NET formation. SLE-induced NET formation can be triggered by ICx21, 80 or apoptotic microparticles.127 Ribonucleoprotein–ICxs, which are specifically present in SLE, are triggered in NET formation in a NADPH-dependent manner. These NETs also contained oxidized mitochondrial DNA.42
SLE-induced NETs are believed to be highly immunogenic because they contain oxidized mitochondrial DNA,42 HMGB1,21 and LL37.21 In addition, SLE-induced NETs can form ICx24, 74 and activate the complement system in vitro.74 HMGB1-nucleosome complexes were previously shown to induce an anti-dsDNA response in a TLR2 dependent manner in a non-autoimmune mice model, which supported an important role for the combination of NETs with danger-associated molecular patterns in SLE.147, 148 Recently, it was also demonstrated that LL37-DNA complexes originating from NETs are able to directly trigger autoantibody production by SLE memory B cells through endosomal uptake of LL37 and subsequent TLR9 receptor signaling.27 This study identified an important link between NETs and autoreactive B cells in SLE. Importantly, the excess of circulating NETs in SLE patients was associated with severe organ inflammation, and specifically, LN.128 Moreover, impaired degradation of NETs was also shown to be associated with SLE flares, disease activity, high autoantibody levels, and complement consumption.74
In summary, these data demonstrated the clinical relevance of NET formation in SLE, especially in ICx-mediated LN. NETs are involved in the pathophysiology of SLE: NETs induce autoantibodies that lead to ICx formation with NETs, which subsequently trigger more NET formation, causing a perpetuating, vicious cycle in SLE patients.
Unknowns About NET Formation in Renal Autoimmune Diseases
It has become apparent that lytic NET formation is associated with chromosomal DNA subject to post-translational modifications while nonlytic NET formation is enriched for mitochondrial DNA. Despite extensive preclinical studies on the mechanisms underpinning lytic and nonlytic NET formation, the clinical and translational studies in SLE and AAV are much more challenging and less unambiguous. As previously described, controversy remains as to whether NETs can be triggered in vivo by ANCA39 or not,79 whether in vivo NADPH oxidase/ROS is involved in SLE42 or not,126, 127 and the extent of mitochondrial DNA versus chromosomal DNA present in SLE-induced NETs.27, 42 Therefore, to better understand NET formation in autoimmune diseases, it is realistic to postulate that there is not only a sole mechanism of NET formation ongoing in vivo but rather that different forms of NET formation occur in parallel. Attention will need to be given to the chosen stimulus to induce NETs (e.g., whole serum vs. purified autoantibodies) and the use of healthy or AAV- or SLE-derived neutrophils for future studies.
Therapeutics Targeting NET Formation
There are several hypotheses on developing therapeutic targets that could interfere with NET formation. Because different forms of NET formation occur in AAV and SLE patients, it can be anticipated that the effects of potential therapeutic approaches will also be different. Obviously, depletion of neutrophils is not attractive because of the high risk of infection in patients with neutropenia. However, engagement of a signal inhibitory receptor on leucocyte-1, which is a specific protein expressed on phagocytes that negatively regulates neutrophil function,149 directly targets neutrophils without depleting them or diminishing their proinflammatory capabilities while reducing SLE-induced NET formation in vitro.149 A summary of reported, potential approaches that reduced NET formation in vitro are summarized in Table 2 and include targeting ROS with diphenyleneiodonium,98 targeting mitochondrial ROS with MitoTEMPO, a mitochondrially targeted antioxidant,42 or N-acetylcysteinine (NAC),154 inhibiting PAD enzymes by chlooramidine,150 or enhancing breakdown of NETs with DNase1.156 Thus far, none of these approaches have been successfully applied as a therapeutic approach. Recently, a novel antibody specifically targeting histones 2A and 4, named therapeutic anti-citrullinated protein antibodies (tACPA), demonstrated in vitro inhibition of CI-induced NET formation.158 Also, tofacitinib, a Janus kinases (JAK)/signal transducer and activator of transcription proteins (STAT) inhibitor reduced both spontaneous and LPS-induced NET formation in a mouse model of lupus,159 and is currently under clinical investigation in SLE patients (NCT02535689). Metformin was evaluated as a proof-of-concept treatment in a large cohort of SLE patients and demonstrated a reduction of in vitro PMA-induced NETs through an unknown mechanism and a decrease in flares of SLE patients.160 In addition, vitamin D decreased PMA-induced NET formation of SLE neutrophils in vitro.153 So far, there are no data on the effect of mycophenolate mofetil, azathioprine, rituximab, and cyclophosphamide on NET formation in AAV or SLE. However, corticosteroids, the cornerstone of induction treatment for both AAV and SLE patients, were demonstrated to impair ROS production by granulocytes and inhibit NET formation in vitro for both mouse and human neutrophils.170
Table 2.
Potential NET-targeted therapies in glomerular diseases
| Treatment | Target | Effect on NET formation | Clinical effect |
|---|---|---|---|
| ANCA-associated vasculitis | |||
| DPI | NADPH | Abrogation of PMA-induced NET formation98 | Not tested |
| Chlooramidin | PAD enzymes | Decreased NET formation in mouse model150 | Protection against renal, skin and vascular manifestations in mice models |
| Cortico-steroids | ROS, CLEC7A | Decreased in vitro (mouse and human) and in vivo (mouse) NET formation151 | Effective and widely used FDA approved therapy |
| C5a receptor antagonist | C5a receptor antagonist | Decreased NET formation and neutrophil activation.141, 152 Did not affect AAV serum−induced NET formation79 | Effective and safe in phase III study141 |
| NEC-1, NSA | Necroptosis pathways | Decreased AAV-induced NET formation130 | Not tested |
| Vitamin D | Unknown | Reduced PMA-induced NET formation in vitro153 | Improved endothelial function in SLE patients |
| Eculizumab | C5a mAb | Did not affect AAV serum induced NET formation79 | Case reports: effective and safe69 |
| Systemic lupus erythematosus | |||
| NAC | ROS scavenger | Decreased NET release154, 155 | Reduced disease activity in patients |
| MitoTEMPO | Mitochondrial ROS scavenger | Decreased NET formation and decreased oxidation of nucleic acids in NETs leading to decreased immunogenicity and IFN responses42 | Reduced disease activity in mice |
| DNase 1 | DNA | Enzymatic degradation of NETs156, 157 | Reduction of autoantibodies, proteinuria, delayed mortality in mouse model. Safe in phase I study, no change in disease activity |
| tACPA | Histones 2A, 4 | Inhibition of calcium ionophore induced NET formation158 | Not tested |
| SIRL-1 | SIRL-1 | Inhibition of SLE-induced NET formation149 | Not tested |
| Tofacitinib | Inhibition of JAK STAT | Reduced spontaneous and LPS-induced NETs in mouse model of lupus159 | Not tested |
| Metformin | Unknown mechanism | Reduced PMA-induced NET formation, decreased CPG-stimulated PDC IFN production160 | Decreased clinical flares, prednisone exposure |
| Corticosteroids | ROS, CLEC7A | Decreased in vitro (mouse and human) and in vivo (mouse) NET formation151 | Effective and widely used FDA-approved therapy |
| Vitamin D | Unknown | Reduced NET formation in vitro153 | Improved endothelial function in SLE patients |
| Eculizumab | C5a | Reduced NET formation and neutrophil activation161, 162 | Improved survival mouse model, safe and decreased haemolytic activity in SLE patients163, 172 |
| RTX+BLM | Plasma cells →ICx formation | Decreased NET formation80 | Reduction of anti-dsDNA, antihistones, antinucleosomes, anti-C1q, decreased disease activity |
| PIC1 | Complement protein 1 | Inhibition of ICx-induced NET formation inhibit NET formation by human neutrophils stimulated by PMA, MPO, or immune complex activated human sera164 | Not tested |
| HCQ | TLR9 | Decreased LPS-induced NET formation.165 Decreased IgG production of NET-stimulated SLE B cells27 | Effective and widely used FDA-approved therapy |
| Anifrolumab | IFN inhibitors | Anifrolumab decreased neutrophil NET complexes166, 167 | Reduced disease activity |
| Calcineurin inhibitors | T-cell activation | Modulation of calcium pools Reduced NET formation168 |
Improvement of renal disease169 Voclosporin: NCT03021499 |
AAV, antineutrophil cytoplasmic antibodies (ANCA)−associated vasculitis; BLM, belimumab; DPI, diphenyleneiodonium; FDA, Food and Drug Administration; ICx, immune-complex formation; HCQ, hydroxychloroquine; IFN, interferon; JAK, Janus kinases; NAC, N-acetyl cysteine; NEC-1, necrostatin-1; NET, neutrophil extracellular trap; NSA, necrosulfanomide; PIC1, peptide inhibitor of complement C1; PMA, phorbol myristate acetate; ROS, reactive oxygen species; RTX, rituximab; SLE, systemic lupus erythematosus; SIRL-1, signal inhibitory receptor on leukocytes-1; STAT, signal transducer and activator of transcription proteins; TLR9, Toll-like receptor-9.
Another potential successful approach can be to target the known triggers of NET formation (Table 2). In AAV, because the exact triggers are still unknown, C5a in combination with granulocyte-macrophage colony-stimulating factor was reported to induce NET formation,120 and C5a receptor inhibition with avacopan was demonstrated to be clinically effective in AAV patients.141 Although C5a, one of the components of the terminal complement system, has an important role in AAV,171, 172 in vitro C5a receptor blockade or the C5 antibody eculizumab were not able to inhibit AAV-induced NET formation.79 Another therapeutic approach in AAV was provided by recent studies that indicated that AAV-induced NET formation might involve the RIPK/MLKL-mediated necroptosis pathway. In vitro inhibition of the RIPK-complex by necrostatin-1 and inhibition of MLKL by necrosulfanomide both reduced AAV-induced NET formation.130 Therefore, future studies that investigate the potential of therapeutic RIPK/MLKL pathway inhibitors (ClinicalTrials.gov NCT02903966) are of high interest.
In SLE, ICxs are mainly responsible for excessive NET formation; therefore, the effective eradication of ICxs could decrease NET formation.80 Eradication of autoantibodies as defined by seroconversion (to negative) upon immunosuppressive treatment is not a major endpoint in clinical studies, although significant reductions in autoantibody levels can be observed. Recently, we showed that combined B-cell targeted treatment with rituximab combined with belimumab in patients with severe SLE resulted in a significant decrease of anti-dsDNA antibodies, which was also associated significantly with decreased excessive NET formation in vitro.80 Peptide inhibitor of complement factor C1 (compound name: PA-dPEG24) inhibited the activation of the classical complement pathway by ICx in vitro and also decreased NET formation when induced by PMA, MPO, or heat-aggregated ICx.164 Hydroxychloroquine, an effective and widely used therapy in SLE patients inhibits the DNA-sensing TLR9 pathway and was demonstrated to inhibit IgG secretion by B cells stimulated with NET-derived LL37-DNA complexes.27 In addition, LPS-induced NET formation was inhibited when human healthy and lupus neutrophils were pretreated with hydroxychloroquine.165 Anifrolumab, an interferon-α receptor antagonist, was investigated in a randomized clinical trial in SLE patients; it demonstrated an inhibitory effect on NET formation, was not observed in the placebo arm, and showed promising clinical efficacy.166 In addition, NET formation assessed by measuring 3 types of DNA complexed to MPO-, NE- or CitH3, as related to NETs, was significantly higher in SLE patients with a simultaneously high interferon signature status. The latter 2 studies indicated an association of NET formation with the interferon signaling pathway. Finally, there was some evidence that the calcineurin inhibitors, such as cyclosporine A, had an inhibitory effect on NET formation.168 Calcineurin inhibitors showed promising clinical efficacy for LN patients.173
In summary, there are several reports on therapeutics that are able to target NET formation in AAV and in SLE. These involve both newly developed but also currently used standard of care therapeutics. The distinct disease-specific forms of NET formation should be taken into account when evaluating targeted therapies at NET formation. Diminishing NET formation in AAV and SLE patients has been suggested to have a beneficial clinical effect based on reported preclinical and a few small clinical studies. Because NETs have a pivotal role in the pathophysiology of renal autoimmune diseases, targeting NETs might be clinically relevant for AAV and SLE patients.
NETs as a Biomarker of Disease Activity
As mentioned previously, NET formation has been demonstrated to be involved in the pathophysiology of renal autoimmune diseases. Therefore, NET formation could be a potential biomarker for disease activity. Both AAV and SLE are characterized by relapses and remissions of disease. We and others have demonstrated that excessive NET formation is predominantly seen in AAV patients with active disease and is low in AAV patients who were in remission or during an infection.79 In addition, 1 study demonstrated a longitudinal association of excessive NET formation with active disease within individual AAV patients.140 In contrast, NETs, as measured by cell-free DNA or MPO-DNA complexes, were not associated with disease activity in AAV patients by a third group.135 We recently also demonstrated that in SLE patients treated with Rituximab (RTX) + Belimumab (BLM), excessive NET formation correlated with disease activity.80 Moreover, it was demonstrated that NET formation of SLE neutrophils were significantly correlated with the titre of anti-LL37 autoantibodies in serum of SLE patients.27 Although the early identification and even prediction of disease flares would be advantageous to manage both AAV and SLE patients, the potential of NET formation as a possible biomarker has not been extensively studied yet. It is important to note that there is no gold standard to measure NET formation. The current methods used to evaluate NET formation in patients range from enzyme-linked immunosorbent assays, immunohistochemistry, immunofluorescence, and flow cytometric assays.174 All of these methods have their own specificity, objectivity, and ways to quantify NETs. Additional complexity is introduced by the different triggers used to induce and measure NETs.16, 17 Nevertheless, it is compelling to postulate that NET formation could be a measure of the autoantigenic load in patients and could plausibly be related to disease activity, remission, or predict relapses. As such, it would be of interest to investigate and quantify autoantigen formation in analogue to autoantibody formation throughout the course of follow-up of AAV and SLE patients.
Conclusions
The accumulating evidence on the pathogenic role of excessive NET formation in AAV and SLE and its relation to their respective forms of GN confirm the clinical relevance of NET formation in renal autoimmune diseases. NETs are a source of autoantigens in both AAV and SLE, are involved in shaping the humoral autoimmune response and cause direct glomerular inflammation and damage. Excessive NET formation and impaired degradation of NETs are jointly autoimmune phenomena that can lead to disease-relevant autoantibody production. Knowledge on the intrinsically distinct triggers and pathways of NET formation that are involved in AAV and SLE is growing and will undoubtedly foster further investigations into the potential of therapeutically targeting NET formation and the use of NETs as biomarkers.
Future studies on AAV- and SLE-induced NET formation should focus on the identification of specific NET-associated molecules, preferably assessed with proteomic-based approaches (Table 3). In addition, future studies should focus on evaluating if quantifying NET formation could serve as a biomarker for disease activity and/or prognosis in AAV and SLE patients. Finally, therapeutic targets should be identified that could potentially regress excessive NET formation or increase NET degradation in these renal autoimmune diseases. Together, addressing these research questions will increase our understanding of the in vivo NET formation processes in AAV and SLE.
Table 3.
Research questions for future translational research
| 1. Which NET-associated proteins are specifically present on AAV- and SLE-induced NETs as identified through proteomics? |
| 2. Could the quantification of NET formation serve as a biomarker for disease activity and prognosis in relation to conventional and novel therapies in AAV and SLE patients? |
| 3. Which targets can be identified that are capable of regressing excessive NET formation or increase NET degradation that could translate to a therapeutic approach in AAV and SLE? |
AAV, antineutrophil cytoplasmic antibodies (ANCA)− associated vasculitis; NET, neutrophil extracellular trap; SLE, systemic lupus erythematosus.
Disclosure
All the authors declared no competing interests.
Acknowledgements
We thank Manon Zuurmond (LUMC Leiden, the Netherlands) for the graphical design. This work is supported by the Dutch Kidney Foundation (KJPB12.028 & 17OKG04), Clinical Fellowship from the Netherlands Organization for Scientific Research (90713460) and FOREUM (SLE project).
References
- 1.Pillay J., den Braber I., Vrisekoop N. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 2.Faurschou M., Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann V., Reichard U., Goosmann C. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann V., Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 5.Remijsen Q., Kuijpers T.W., Wirawan E. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18:581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linkermann A., Stockwell B.R., Krautwald S., Anders H.J. Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat Rev Immunol. 2014;14:759–767. doi: 10.1038/nri3743. [DOI] [PubMed] [Google Scholar]
- 7.Yousefi S., Gold J.A., Andina N. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 8.Nakazawa D., Shida H., Kusunoki Y. The responses of macrophages in interaction with neutrophils that undergo NETosis. J Autoimmun. 2016;67:19–28. doi: 10.1016/j.jaut.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Ingelsson B., Soderberg D., Strid T. Lymphocytes eject interferogenic mitochondrial DNA webs in response to CpG and non-CpG oligodeoxynucleotides of class C. Proc Natl Acad Sci U S A. 2018;115:E478–E487. doi: 10.1073/pnas.1711950115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Chen X., Gueydan C., Han J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018;28:9–21. doi: 10.1038/cr.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Y., Hou W., Song X. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S., Kaplan M.J. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat Rev Nephrol. 2016;12:402–413. doi: 10.1038/nrneph.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee K.H., Kronbichler A., Park D.D. Neutrophil extracellular traps (NETs) in autoimmune diseases: a comprehensive review. Autoimmun Rev. 2017;16:1160–1173. doi: 10.1016/j.autrev.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Schauer C., Janko C., Munoz L.E. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20:511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 15.Lightfoot Y.L., Kaplan M.J. Disentangling the role of neutrophil extracellular traps in rheumatic diseases. Curr Opin Rheumatol. 2017;29:65–70. doi: 10.1097/BOR.0000000000000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenny E.F., Herzig A., Kruger R. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife. 2017;6 doi: 10.7554/eLife.24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konig M.F., Andrade F. A critical reappraisal of neutrophil extracellular traps and NETosis mimics based on differential requirements for protein citrullination. Front Immunol. 2016;7:461. doi: 10.3389/fimmu.2016.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yipp B.G., Kubes P. NETosis: how vital is it? Blood. 2013;122:2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 19.Yipp B.G., Petri B., Salina D. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell A.M., Kashgarian M., Shlomchik M.J. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci Transl Med. 2012;4:157ra141. doi: 10.1126/scitranslmed.3004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Romo G.S., Caielli S., Vega B. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leliefeld P.H., Koenderman L., Pillay J. How neutrophils shape adaptive immune responses. Front Immunol. 2015;6:471. doi: 10.3389/fimmu.2015.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puga I., Cols M., Barra C.M. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2011;13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lande R., Ganguly D., Facchinetti V. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lande R., Gregorio J., Facchinetti V. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 26.Tian J., Avalos A.M., Mao S.Y. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 27.Gestermann N., Di Domizio J., Lande R. Netting neutrophils activate autoreactive B cells in lupus. J Immunol. 2018;200:3364–3371. doi: 10.4049/jimmunol.1700778. [DOI] [PubMed] [Google Scholar]
- 28.Sangaletti S., Tripodo C., Chiodoni C. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood. 2012;120:3007–3018. doi: 10.1182/blood-2012-03-416156. [DOI] [PubMed] [Google Scholar]
- 29.Bertheloot D., Naumovski A.L., Langhoff P. RAGE enhances TLR responses through binding and internalization of RNA. J Immunol. 2016;197:4118–4126. doi: 10.4049/jimmunol.1502169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carmona-Rivera C., Carlucci P.M., Moore E. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci Immunol. 2017;2(10) doi: 10.1126/sciimmunol.aag3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brinkmann V., Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol. 2012;198:773–783. doi: 10.1083/jcb.201203170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen R., Kang R., Fan X.G., Tang D. Release and activity of histone in diseases. Cell Death Dis. 2014;5:e1370. doi: 10.1038/cddis.2014.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saffarzadeh M., Juenemann C., Queisser M.A. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Sullivan K.M., Lo C.Y., Summers S.A. Renal participation of myeloperoxidase in antineutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis. Kidney Int. 2015;88:1030–1046. doi: 10.1038/ki.2015.202. [DOI] [PubMed] [Google Scholar]
- 35.Kumar S.V., Kulkarni O.P., Mulay S.R. Neutrophil extracellular trap-related extracellular histones cause vascular necrosis in severe GN. J Am Soc Nephrol. 2015;26:2399–2413. doi: 10.1681/ASN.2014070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pieterse E., Rother N., Garsen M. Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arterioscler Thromb Vasc Biol. 2017;37:1371–1379. doi: 10.1161/ATVBAHA.117.309002. [DOI] [PubMed] [Google Scholar]
- 37.Lamprecht P., Kerstein A., Klapa S. Pathogenetic and clinical aspects of anti-neutrophil cytoplasmic autoantibody-associated vasculitides. Front Immunol. 2018;9:680. doi: 10.3389/fimmu.2018.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zharkova O., Celhar T., Cravens P.D. Pathways leading to an immunological disease: systemic lupus erythematosus. Rheumatology (Oxford) 2017;56(suppl_1):i55–i66. doi: 10.1093/rheumatology/kew427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kessenbrock K., Krumbholz M., Schonermarck U. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villanueva E., Yalavarthi S., Berthier C.C. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khandpur R., Carmona-Rivera C., Vivekanandan-Giri A. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5:178ra140. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lood C., Blanco L.P., Purmalek M.M. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22:146–153. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carmona-Rivera C., Purmalek M.M., Moore E. A role for muscarinic receptors in neutrophil extracellular trap formation and levamisole-induced autoimmunity. JCI Insight. 2017;2:e89780. doi: 10.1172/jci.insight.89780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grayson P.C., Carmona-Rivera C., Xu L. Neutrophil-related gene expression and low-density granulocytes associated with disease activity and response to treatment in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2015;67:1922–1932. doi: 10.1002/art.39153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grayson P.C., Kaplan M.J. At the bench: neutrophil extracellular traps (NETs) highlight novel aspects of innate immune system involvement in autoimmune diseases. J Leukoc Biol. 2016;99:253–264. doi: 10.1189/jlb.5BT0615-247R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jorch S.K., Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017;23:279–287. doi: 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- 47.Knight J.S., Carmona-Rivera C., Kaplan M.J. Proteins derived from neutrophil extracellular traps may serve as self-antigens and mediate organ damage in autoimmune diseases. Front Immunol. 2012;3:380. doi: 10.3389/fimmu.2012.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darrah E., Andrade F. NETs: the missing link between cell death and systemic autoimmune diseases? Front Immunol. 2012;3:428. doi: 10.3389/fimmu.2012.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urban C.F., Ermert D., Schmid M. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao M.H., Lockwood C.M. Azurocidin is a novel antigen for anti-neutrophil cytoplasmic autoantibodies (ANCA) in systemic vasculitis. Clin Exp Immunol. 1996;103:397–402. doi: 10.1111/j.1365-2249.1996.tb08293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schultz D.R., Tozman E.C. Antineutrophil cytoplasmic antibodies: major autoantigens, pathophysiology, and disease associations. Semin Arthritis Rheum. 1995;25:143–159. doi: 10.1016/s0049-0172(95)80027-1. [DOI] [PubMed] [Google Scholar]
- 52.Silva de Souza A.W. Autoantibodies in systemic vasculitis. Front Immunol. 2015;6:184. doi: 10.3389/fimmu.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao M.H., Liu N., Zhang Y.K., Wang H.Y. Antineutrophil cytoplasmic autoantibodies (ANCA) and their target antigens in Chinese patients with lupus nephritis. Nephrol Dial Transplant. 1998;13:2821–2824. doi: 10.1093/ndt/13.11.2821. [DOI] [PubMed] [Google Scholar]
- 54.Manolova I., Dancheva M., Halacheva K. Antineutrophil cytoplasmic antibodies in patients with systemic lupus erythematosus: prevalence, antigen specificity, and clinical associations. Rheumatol Int. 2001;20:197–204. doi: 10.1007/s002960100108. [DOI] [PubMed] [Google Scholar]
- 55.Nassberger L., Jonsson H., Sjoholm A.G., Sturfelt G., Heubner A. Circulating anti-elastase in systemic lupus erythematosus. Lancet. 1989;1:509. doi: 10.1016/s0140-6736(89)91420-7. [DOI] [PubMed] [Google Scholar]
- 56.Schulte-Pelkum J., Radice A., Norman G.L. Novel clinical and diagnostic aspects of antineutrophil cytoplasmic antibodies. J Immunol Res. 2014;2014:185416. doi: 10.1155/2014/185416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carmona-Rivera C., Kaplan M.J. Detection of SLE antigens in neutrophil extracellular traps (NETs) Methods Mol Biol. 2014;1134:151–161. doi: 10.1007/978-1-4939-0326-9_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang S., Zhang Y., Yin S.W. Neutrophil extracellular trap formation is associated with autophagy-related signalling in ANCA-associated vasculitis. Clin Exp Immunol. 2015;180:408–418. doi: 10.1111/cei.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kain R. L29. Relevance of anti-LAMP-2 in vasculitis: why the controversy. Presse Med. 2013;42:584–588. doi: 10.1016/j.lpm.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 60.Kain R., Rees A.J. What is the evidence for antibodies to LAMP-2 in the pathogenesis of ANCA associated small vessel vasculitis? Curr Opin Rheumatol. 2013;25:26–34. doi: 10.1097/BOR.0b013e32835b4f8f. [DOI] [PubMed] [Google Scholar]
- 61.Nakazawa D., Shida H., Tomaru U. Enhanced formation and disordered regulation of NETs in myeloperoxidase-ANCA-associated microscopic polyangiitis. J Am Soc Nephrol. 2014;25:990–997. doi: 10.1681/ASN.2013060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pradhan V.D., Badakere S.S., Bichile L.S., Almeida A.F. Anti-neutrophil cytoplasmic antibodies (ANCA) in systemic lupus erythematosus: prevalence, clinical associations and correlation with other autoantibodies. J Assoc Physic India. 2004;52:533–537. [PubMed] [Google Scholar]
- 63.Panda R., Krieger T., Hopf L. Neutrophil extracellular traps contain selected antigens of anti-neutrophil cytoplasmic antibodies. Front Immunol. 2017;8:439. doi: 10.3389/fimmu.2017.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Renaudineau Y., Deocharan B., Jousse S. Anti-alpha-actinin antibodies: a new marker of lupus nephritis. Autoimmun Rev. 2007;6:464–468. doi: 10.1016/j.autrev.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 65.Seret G., Canas F., Pougnet-Di Costanzo L. Anti-alpha-actinin antibodies are part of the anti-cell membrane antibody spectrum that characterize patients with lupus nephritis. J Autoimmun. 2015;61:54–61. doi: 10.1016/j.jaut.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 66.Renaudineau Y., Croquefer S., Jousse S. Association of alpha-actinin-binding anti-double-stranded DNA antibodies with lupus nephritis. Arthritis Rheum. 2006;54:2523–2532. doi: 10.1002/art.22015. [DOI] [PubMed] [Google Scholar]
- 67.Mason L.J., Ravirajan C.T., Rahman A. Is alpha-actinin a target for pathogenic anti-DNA antibodies in lupus nephritis? Arthritis Rheum. 2004;50:866–870. doi: 10.1002/art.20103. [DOI] [PubMed] [Google Scholar]
- 68.Zhao Z., Weinstein E., Tuzova M. Cross-reactivity of human lupus anti-DNA antibodies with alpha-actinin and nephritogenic potential. Arthritis Rheum. 2005;52:522–530. doi: 10.1002/art.20862. [DOI] [PubMed] [Google Scholar]
- 69.Manenti L., Urban M.L., Maritati F. Complement blockade in ANCA-associated vasculitis: an index case, current concepts and future perspectives. Intern Emerg Med. 2017;12:727–731. doi: 10.1007/s11739-017-1636-6. [DOI] [PubMed] [Google Scholar]
- 70.Bruschi M., Galetti M., Sinico R.A. Glomerular autoimmune multicomponents of human lupus nephritis in vivo (2): planted antigens. J Am Soc Nephrol. 2015;26:1905–1924. doi: 10.1681/ASN.2014050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mosca M., Chimenti D., Pratesi F. Prevalence and clinico-serological correlations of anti-alpha-enolase, anti-C1q, and anti-dsDNA antibodies in patients with systemic lupus erythematosus. J Rheumatol. 2006;33:695–697. [PubMed] [Google Scholar]
- 72.Bruschi M., Sinico R.A., Moroni G. Glomerular autoimmune multicomponents of human lupus nephritis in vivo: alpha-enolase and annexin AI. J Am Soc Nephrol. 2014;25:2483–2498. doi: 10.1681/ASN.2013090987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bruschi M., Petretto A., Vaglio A. Annexin A1 and autoimmunity: from basic science to clinical applications. Int J Mol Sci. 2018;19(5) doi: 10.3390/ijms19051348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leffler J., Martin M., Gullstrand B. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol. 2012;188:3522–3531. doi: 10.4049/jimmunol.1102404. [DOI] [PubMed] [Google Scholar]
- 75.Sturfelt G., Jonsson H., Hellmer G., Sjoholm A.G. Clustering of neutrophil leucocytes in serum: possible role of C1q-containing immune complexes. Clin Exp Immunol. 1993;93:237–241. doi: 10.1111/j.1365-2249.1993.tb07972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orbai A.M., Truedsson L., Sturfelt G. Anti-C1q antibodies in systemic lupus erythematosus. Lupus. 2015;24:42–49. doi: 10.1177/0961203314547791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Horvath L., Czirjak L., Fekete B. Levels of antibodies against C1q and 60 kDa family of heat shock proteins in the sera of patients with various autoimmune diseases. Immunol Lett. 2001;75:103–109. doi: 10.1016/s0165-2478(00)00287-x. [DOI] [PubMed] [Google Scholar]
- 78.Mansour R.B., Lassoued S., Gargouri B. Increased levels of autoantibodies against catalase and superoxide dismutase associated with oxidative stress in patients with rheumatoid arthritis and systemic lupus erythematosus. Scand J Rheumatol. 2008;37:103–108. doi: 10.1080/03009740701772465. [DOI] [PubMed] [Google Scholar]
- 79.Kraaij T., Kamerling S.W.A., van Dam L.S. Excessive neutrophil extracellular trap formation in ANCA-associated vasculitis is independent of ANCA. Kidney Int. 2018;94:139–149. doi: 10.1016/j.kint.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 80.Kraaij T., Kamerling S.W.A., de Rooij E.N.M. The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus. J Autoimmun. 2018;91:45–54. doi: 10.1016/j.jaut.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 81.Yaniv G., Twig G., Shor D.B. A volcanic explosion of autoantibodies in systemic lupus erythematosus: a diversity of 180 different antibodies found in SLE patients. Autoimmun Rev. 2015;14:75–79. doi: 10.1016/j.autrev.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 82.Abdulahad D.A., Westra J., Bijzet J. High mobility group box 1 (HMGB1) and anti-HMGB1 antibodies and their relation to disease characteristics in systemic lupus erythematosus. Arthritis Res Ther. 2011;13:R71. doi: 10.1186/ar3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Avalos A.M., Kiefer K., Tian J. RAGE-independent autoreactive B cell activation in response to chromatin and HMGB1/DNA immune complexes. Autoimmunity. 2010;43:103–110. doi: 10.3109/08916930903384591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tamiya H., Tani K., Miyata J. Defensins- and cathepsin G-ANCA in systemic lupus erythematosus. Rheumatol Int. 2006;27:147–152. doi: 10.1007/s00296-006-0173-9. [DOI] [PubMed] [Google Scholar]
- 85.Neumann A., Berends E.T., Nerlich A. The antimicrobial peptide LL-37 facilitates the formation of neutrophil extracellular traps. Biochem J. 2014;464:3–11. doi: 10.1042/BJ20140778. [DOI] [PubMed] [Google Scholar]
- 86.Neumann A., Vollger L., Berends E.T. Novel role of the antimicrobial peptide LL-37 in the protection of neutrophil extracellular traps against degradation by bacterial nucleases. J Innate Immun. 2014;6:860–868. doi: 10.1159/000363699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang H., Wang C., Zhao M.H., Chen M. Neutrophil extracellular traps can activate alternative complement pathways. Clin Exp Immunol. 2015;181:518–527. doi: 10.1111/cei.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yuen J., Pluthero F.G., Douda D.N. NETosing neutrophils activate complement both on their own NETs and bacteria via alternative and non-alternative pathways. Front Immunol. 2016;7:137. doi: 10.3389/fimmu.2016.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pauly D., Nagel B.M., Reinders J. A novel antibody against human properdin inhibits the alternative complement system and specifically detects properdin from blood samples. PLoS One. 2014;9:e96371. doi: 10.1371/journal.pone.0096371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nozal P., Garrido S., Martinez-Ara J. Case report: lupus nephritis with autoantibodies to complement alternative pathway proteins and C3 gene mutation. BMC Nephrol. 2015;16:40. doi: 10.1186/s12882-015-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hilhorst M., van Paassen P., van Rie H. Complement in ANCA-associated glomerulonephritis. Nephrol Dial Transplant. 2017;32:1302–1313. doi: 10.1093/ndt/gfv288. [DOI] [PubMed] [Google Scholar]
- 92.Schultz H., Csernok E., Herlyn K. ANCA against bactericidal/permeability-increasing protein, azurocidin, calprotectin and defensins in rheumatic and infectious diseases: prevalence and clinical associations. Clin Exp Rheumatol. 2003;21(6 Suppl 32):S117–S120. [PubMed] [Google Scholar]
- 93.Chauhan S.K., Rai R., Singh V.V. Differential clearance mechanisms, neutrophil extracellular trap degradation and phagocytosis, are operative in systemic lupus erythematosus patients with distinct autoantibody specificities. Immunol Lett. 2015;168:254–259. doi: 10.1016/j.imlet.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 94.Fuchs T.A., Abed U., Goosmann C. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Remijsen Q., Vanden Berghe T., Wirawan E. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011;21:290–304. doi: 10.1038/cr.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Phillipson M., Kubes P. The neutrophil in vascular inflammation. Nat Med. 2011;17:1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Delgado-Rizo V., Martinez-Guzman M.A., Iniguez-Gutierrez L. Neutrophil extracellular traps and its implications in inflammation: an overview. Front Immunol. 2017;8:81. doi: 10.3389/fimmu.2017.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gray R.D., Lucas C.D., MacKellar A. Activation of conventional protein kinase C (PKC) is critical in the generation of human neutrophil extracellular traps. J Inflamm (Lond) 2013;10:12. doi: 10.1186/1476-9255-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nishinaka Y., Arai T., Adachi S. Singlet oxygen is essential for neutrophil extracellular trap formation. Biochem Biophys Res Commun. 2011;413:75–79. doi: 10.1016/j.bbrc.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 100.Hakkim A., Fuchs T.A., Martinez N.E. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011;7:75–77. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- 101.Papayannopoulos V., Metzler K.D., Hakkim A., Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neeli I., Dwivedi N., Khan S., Radic M. Regulation of extracellular chromatin release from neutrophils. J Innate Immun. 2009;1:194–201. doi: 10.1159/000206974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martinod K., Demers M., Fuchs T.A. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A. 2013;110:8674–8679. doi: 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Y., Li M., Stadler S. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fuhrmann J., Clancy K.W., Thompson P.R. Chemical biology of protein arginine modifications in epigenetic regulation. Chem Rev. 2015;115:5413–5461. doi: 10.1021/acs.chemrev.5b00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Amulic B., Knackstedt S.L., Abu Abed U. Cell-cycle proteins control production of neutrophil extracellular traps. Dev Cell. 2017;43:449–462 e445. doi: 10.1016/j.devcel.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 107.Metzler K.D., Fuchs T.A., Nauseef W.M. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117:953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ostafin M., Pruchniak M.P., Ciepiela O. Different procedures of diphenyleneiodonium chloride addition affect neutrophil extracellular trap formation. Anal Biochem. 2016;509:60–66. doi: 10.1016/j.ab.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 109.Neeli I., Radic M. Opposition between PKC isoforms regulates histone deimination and neutrophil extracellular chromatin release. Front Immunol. 2013;4:38. doi: 10.3389/fimmu.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lewis H.D., Liddle J., Coote J.E. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol. 2015;11:189–191. doi: 10.1038/nchembio.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kusunoki Y., Nakazawa D., Shida H. Peptidylarginine deiminase inhibitor suppresses neutrophil extracellular trap formation and MPO-ANCA production. Front Immunol. 2016;7:227. doi: 10.3389/fimmu.2016.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mestas J., Hughes C.C. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 113.Bardoel B.W., Kenny E.F., Sollberger G., Zychlinsky A. The balancing act of neutrophils. Cell Host Microbe. 2014;15:526–536. doi: 10.1016/j.chom.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 114.Doeing D.C., Borowicz J.L., Crockett E.T. Gender dimorphism in differential peripheral blood leukocyte counts in mice using cardiac, tail, foot, and saphenous vein puncture methods. BMC Clin Pathol. 2003;3:3. doi: 10.1186/1472-6890-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Risso A. Leukocyte antimicrobial peptides: multifunctional effector molecules of innate immunity. J Leukoc Biol. 2000;68:785–792. [PubMed] [Google Scholar]
- 116.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 117.Liu C.L., Tangsombatvisit S., Rosenberg J.M. Specific post-translational histone modifications of neutrophil extracellular traps as immunogens and potential targets of lupus autoantibodies. Arthristic Res Ther. 2012;14:R25. doi: 10.1186/ar3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Claushuis T.A.M., van der Donk L.E.H., Luitse A.L. Role of peptidylarginine deiminase 4 in neutrophil extracellular trap formation and host defense during Klebsiella pneumoniae-induced pneumonia-derived sepsis. J Immunol. 2018;201:1241–1252. doi: 10.4049/jimmunol.1800314. [DOI] [PubMed] [Google Scholar]
- 119.Neeli I., Radic M. Knotting the NETs: analyzing histone modifications in neutrophil extracellular traps. Arthritis Res Ther. 2012;14:115. doi: 10.1186/ar3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yousefi S., Mihalache C., Kozlowski E. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 121.Pilsczek F.H., Salina D., Poon K.K. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 122.Pieterse E., Rother N., Yanginlar C. Neutrophils discriminate between lipopolysaccharides of different bacterial sources and selectively release neutrophil extracellular traps. Front Immunol. 2016;7:484. doi: 10.3389/fimmu.2016.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.DeSouza-Vieira T., Guimaraes-Costa A., Rochael N.C. Neutrophil extracellular traps release induced by Leishmania: role of PI3Kgamma, ERK, PI3Ksigma, PKC, and [Ca2+] J Leukoc Biol. 2016;100:801–810. doi: 10.1189/jlb.4A0615-261RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rochael N.C., Guimaraes-Costa A.B., Nascimento M.T. Classical ROS-dependent and early/rapid ROS-independent release of neutrophil extracellular traps triggered by Leishmania parasites. Sci Rep. 2015;5:18302. doi: 10.1038/srep18302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Carestia A., Kaufman T., Schattner M. Platelets: new bricks in the building of neutrophil extracellular traps. Front Immunol. 2016;7:271. doi: 10.3389/fimmu.2016.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pieterse E., Rother N., Yanginlar C. Cleaved N-terminal histone tails distinguish between NADPH oxidase (NOX)-dependent and NOX-independent pathways of neutrophil extracellular trap formation. Annals Rheum Dis. 2018;77:1790–1798. doi: 10.1136/annrheumdis-2018-213223. [DOI] [PubMed] [Google Scholar]
- 127.Rother N., Pieterse E., Lubbers J. Acetylated histones in apoptotic microparticles drive the formation of neutrophil extracellular traps in active lupus nephritis. Front Immunol. 2017;8:1136. doi: 10.3389/fimmu.2017.01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hakkim A., Furnrohr B.G., Amann K. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Desai J., Kumar S.V., Mulay S.R. PMA and crystal-induced neutrophil extracellular trap formation involves RIPK1-RIPK3-MLKL signaling. Eur J Immunol. 2016;46:223–229. doi: 10.1002/eji.201545605. [DOI] [PubMed] [Google Scholar]
- 130.Schreiber A., Rousselle A., Becker J.U. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc Natl Acad Sci U S A. 2017;114:E9618–E9625. doi: 10.1073/pnas.1708247114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dieker J., Berden J.H., Bakker M. Autoantibodies against modified histone peptides in SLE patients are associated with disease activity and lupus nephritis. PLoS One. 2016;11:e0165373. doi: 10.1371/journal.pone.0165373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van Bavel C.C., Dieker J., Muller S. Apoptosis-associated acetylation on histone H2B is an epitope for lupus autoantibodies. Mol Immunol. 2009;47:511–516. doi: 10.1016/j.molimm.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 133.van Bavel C.C., Dieker J.W., Kroeze Y. Apoptosis-induced histone H3 methylation is targeted by autoantibodies in systemic lupus erythematosus. Ann Rheum Dis. 2011;70:201–207. doi: 10.1136/ard.2010.129320. [DOI] [PubMed] [Google Scholar]
- 134.Barrera-Vargas A., Gomez-Martin D., Carmona-Rivera C. Differential ubiquitination in NETs regulates macrophage responses in systemic lupus erythematosus. Ann Rheum Diseases. 2018;77:944–950. doi: 10.1136/annrheumdis-2017-212617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang H., Sha L.L., Ma T.T. Circulating level of neutrophil extracellular traps is not a useful biomarker for assessing disease activity in antineutrophil cytoplasmic antibody-associated vasculitis. PLoS One. 2016;11:e0148197. doi: 10.1371/journal.pone.0148197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hanayama R., Tanaka M., Miwa K. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 137.Gregoire M., Uhel F., Lesouhaitier M. Impaired efferocytosis and neutrophil extracellular trap clearance by macrophages in ARDS. Eur Respir J. 2018;52(2) doi: 10.1183/13993003.02590-2017. [DOI] [PubMed] [Google Scholar]
- 138.Ohlsson S.M., Ohlsson S., Soderberg D. Neutrophils from vasculitis patients exhibit an increased propensity for activation by anti-neutrophil cytoplasmic antibodies. Clin Exp Immunol. 2014;176:363–372. doi: 10.1111/cei.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yoshida M., Sasaki M., Sugisaki K. Neutrophil extracellular trap components in fibrinoid necrosis of the kidney with myeloperoxidase-ANCA-associated vasculitis. Clin Kidney J. 2013;6:308–312. doi: 10.1093/ckj/sft048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Soderberg D., Kurz T., Motamedi A. Increased levels of neutrophil extracellular trap remnants in the circulation of patients with small vessel vasculitis, but an inverse correlation to anti-neutrophil cytoplasmic antibodies during remission. Rheumatology (Oxford) 2015;54:2085–2094. doi: 10.1093/rheumatology/kev217. [DOI] [PubMed] [Google Scholar]
- 141.Jayne D.R.W., Bruchfeld A.N., Harper L. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol. 2017;28:2756–2767. doi: 10.1681/ASN.2016111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hacbarth E., Kajdacsy-Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthr Rheum. 1986;29:1334–1342. doi: 10.1002/art.1780291105. [DOI] [PubMed] [Google Scholar]
- 143.Carmona-Rivera C., Kaplan M.J. Low-density granulocytes: a distinct class of neutrophils in systemic autoimmunity. Semin Immunopathol. 2013;35:455–463. doi: 10.1007/s00281-013-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kaplan M.J. Neutrophils in the pathogenesis and manifestations of SLE. Nat Rev Rheumatol. 2011;7:691–699. doi: 10.1038/nrrheum.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van der Linden M., van den Hoogen L.L., Westerlaken G.H.A. Neutrophil extracellular trap release is associated with antinuclear antibodies in systemic lupus erythematosus and anti-phospholipid syndrome. Rheumatology (Oxford) 2018;57:1228–1234. doi: 10.1093/rheumatology/key067. [DOI] [PubMed] [Google Scholar]
- 146.Jonsson H., Sturfelt G. A novel assay for neutrophil clustering activity of human sera: relation to disease activity and neutropenia in systemic lupus erythematosus. Ann Rheum Dis. 1990;49:46–50. doi: 10.1136/ard.49.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Urbonaviciute V., Furnrohr B.G., Meister S. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med. 2008;205:3007–3018. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Urbonaviciute V., Starke C., Pirschel W. Toll-like receptor 2 is required for autoantibody production and development of renal disease in pristane-induced lupus. Arthriti Rheum. 2013;65:1612–1623. doi: 10.1002/art.37914. [DOI] [PubMed] [Google Scholar]
- 149.Van Avondt K., Fritsch-Stork R., Derksen R.H., Meyaard L. Ligation of signal inhibitory receptor on leukocytes-1 suppresses the release of neutrophil extracellular traps in systemic lupus erythematosus. PLoS One. 2013;8:e78459. doi: 10.1371/journal.pone.0078459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Knight J.S., Subramanian V., O'Dell A.A. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann Rheum Dis. 2015;74:2199–2206. doi: 10.1136/annrheumdis-2014-205365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kalleda N., Amich J., Arslan B. Dynamic immune cell recruitment after murine pulmonary Aspergillus fumigatus infection under different immunosuppressive regimens. Front Microbiol. 2016;7:1107. doi: 10.3389/fmicb.2016.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huang Y.M., Wang H., Wang C. Promotion of hypercoagulability in antineutrophil cytoplasmic antibody-associated vasculitis by C5a-induced tissue factor-expressing microparticles and neutrophil extracellular traps. Arthritis Rheumatol. 2015;67:2780–2790. doi: 10.1002/art.39239. [DOI] [PubMed] [Google Scholar]
- 153.Handono K., Sidarta Y.O., Pradana B.A. Vitamin D prevents endothelial damage induced by increased neutrophil extracellular traps formation in patients with systemic lupus erythematosus. Acta Med Indones. 2014;46:189–198. [PubMed] [Google Scholar]
- 154.Garcia R.J., Francis L., Dawood M. Attention deficit and hyperactivity disorder scores are elevated and respond to N-acetylcysteine treatment in patients with systemic lupus erythematosus. Arthritis Rheum. 2013;65:1313–1318. doi: 10.1002/art.37893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lai Z.W., Hanczko R., Bonilla E. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012;64:2937–2946. doi: 10.1002/art.34502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Davis J.C., Jr., Manzi S., Yarboro C. Recombinant human Dnase I (rhDNase) in patients with lupus nephritis. Lupus. 1999;8:68–76. doi: 10.1191/096120399678847380. [DOI] [PubMed] [Google Scholar]
- 157.Macanovic M., Sinicropi D., Shak S. The treatment of systemic lupus erythematosus (SLE) in NZB/W F1 hybrid mice; studies with recombinant murine DNase and with dexamethasone. Clin Exp Immunol. 1996;106:243–252. doi: 10.1046/j.1365-2249.1996.d01-839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chirivi R.G., van Rosmalen J.W., Kambas K. Netosis-inhibiting t-acpa therapy for use in different net-driven human autoimmune diseases. Ann Rheum Dis. 2018;77(suppl 2):205. [Google Scholar]
- 159.Furumoto Y., Smith C.K., Blanco L. Tofacitinib ameliorates murine lupus and its associated vascular dysfunction. Arthritis Rheumatol. 2017;69:148–160. doi: 10.1002/art.39818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang H., Li T., Chen S., Gu Y., Ye S. Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-concept trial of metformin. Arthritis Rheumatol. 2015;67:3190–3200. doi: 10.1002/art.39296. [DOI] [PubMed] [Google Scholar]
- 161.Wang Y., Hu Q., Madri J.A. Amelioration of lupus-like autoimmune disease in NZB/WF1 mice after treatment with a blocking monoclonal antibody specific for complement component C5. Proc Natl Acad Sci U S A. 1996;93:8563–8568. doi: 10.1073/pnas.93.16.8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.van Bijnen S.T.A., Wouters D., van Mierlo G.J. Neutrophil activation and nucleosomes as markers of systemic inflammation in paroxysmal nocturnal hemoglobinuria: effects of eculizumab. J Thromb Haemost. 2015;13:2004–2011. doi: 10.1111/jth.13125. [DOI] [PubMed] [Google Scholar]
- 163.Barilla-Labarca M.L., Toder K., Furie R. Targeting the complement system in systemic lupus erythematosus and other diseases. Clin Immunol. 2013;148:313–321. doi: 10.1016/j.clim.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 164.Hair P.S., Enos A.I., Krishna N.K., Cunnion K.M. Inhibition of immune complex complement activation and neutrophil extracellular trap formation by peptide inhibitor of complement C1. Front Immunol. 2018;9:558. doi: 10.3389/fimmu.2018.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Smith C.K., Vivekanandan-Giri A., Tang C. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 2014;66:2532–2544. doi: 10.1002/art.38703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.White W., Seto N., Playford M. Alteration of mediators of vascular inflammation by anifrolumab in the phase iib muse study in sle. Ann Rheum Dis. 2018;77:136–137. [Google Scholar]
- 167.Furie R., Khamashta M., Merrill J.T. Anifrolumab, an anti-interferon-alpha receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. 2017;69:376–386. doi: 10.1002/art.39962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gupta A.K., Giaglis S., Hasler P., Hahn S. Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS One. 2014;9:e97088. doi: 10.1371/journal.pone.0097088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee Y.H., Lee H.S., Choi S.J., Dai Ji J., Song G.G. Efficacy and safety of tacrolimus therapy for lupus nephritis: a systematic review of clinical trials. Lupus. 2011;20:636–640. doi: 10.1177/0961203310389486. [DOI] [PubMed] [Google Scholar]
- 170.Kalleda N., Amich Elias J., Poreddy S. Corticosteroids impair granulocyte transfusion therapy by targeting NET formation and neutrophil antifungal functions via ROS/Dectin1 pathways. Blood. 2016;128:2506. [Google Scholar]
- 171.Huugen D., van Esch A., Xiao H. Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int. 2007;71:646–654. doi: 10.1038/sj.ki.5002103. [DOI] [PubMed] [Google Scholar]
- 172.Xiao H., Schreiber A., Heeringa P. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170:52–64. doi: 10.2353/ajpath.2007.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kraaij T., Bredewold O.W., Trompet S. TAC-TIC use of tacrolimus-based regimens in lupus nephritis. Lupus Sci Med. 2016;3:e000169. doi: 10.1136/lupus-2016-000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Masuda S., Nakazawa D., Shida H. NETosis markers: quest for specific, objective, and quantitative markers. Clin Chim Acta. 2016;459:89–93. doi: 10.1016/j.cca.2016.05.029. [DOI] [PubMed] [Google Scholar]