Abstract
Background and Purpose
Kazinol U is a prenylated flavan isolated from an extract of Broussonetia kazinoki Sieb (Moraceae). Kazinol U has shown cytoprotective effects against cytokine‐induced apoptotic cell death and induces AMP kinase (AMPK) activation through LKB1 activation. However, kazinol U has not been tested as a regulator of melanogenesis, although bark extract of B. kazinoki has been used as a cosmetic ingredient for skin conditioning.
Experimental Approach
We cultured mouse, human melanoma cells and normal human melanocytes to demonstrate anti‐melanogenic effects of kazinol U. A tyrosinase activity assay, Western blot, RT‐qPCR and a luciferase reporter gene assay were performed to determine the anti‐melanogenic mechanisms of kazinol U. We confirmed its effect on melanogenesis in vivo using zebrafish.
Key Results
Kazinol U inhibited the expression and activity of tyrosinase, the rate‐limiting enzyme in melanogenesis, and reduced tyrosinase expression and activity in response to cAMP‐inducing agents. Kazinol U reduced the expression of other melanogenic enzymes, such as tyrosinase‐related protein (Tyrp) 1 and Tyrp2, and down‐regulated microphthalmia‐associated transcription factor (MITF), the master regulator of the tyrosinase gene family. Moreover, kazinol U induced phosphorylation of AMPK and MAPK proteins, which are MITF inhibitors. It also exhibited anti‐melanogenic effects in zebrafish, a recently developed in vivo model.
Conclusions and Implications
Our findings suggest that kazinol U reduces melanogenesis via its inhibitory effect on MITF and its downstream target genes, tyrosinase, Tyrp1 and Tyrp2. This work may provide a basis for the application of kazinol U for the treatment of hyperpigmentation skin disorders.
Abbreviations
- α‐MSH
α‐melanocyte‐stimulating hormone
- AMPK
AMP kinase
- Dct/Trp2
dopachrome tautomerase
- MITF
microphthalmia‐associated transcription factor
- NHMs
normal human melanocytes
- Tyrp1
tyrosinase‐related protein 1
- UVR
UV radiation
Introduction
The visible pigmentation of mammals is determined by the synthesis and distribution of melanin in the eye, skin and hair bulbs (del Marmol and Beermann, 1996; Simon et al., 2009). Melanin is synthesized in the melanosomes of melanocytes through highly organized pathways and has various roles in the skin, such as determining its appearance and protecting it from UV radiation (UVR), toxic drugs and chemicals (Park et al., 2015; Slominski et al., 2015). Normally, exposure to solar UVR induces the synthesis of melanin, in particular black eumelanin, by melanocyte. UV irradiation increases DNA photoproducts and induces keratinocytes to secrete various autocrine and paracrine factors. α‐Melanocyte‐stimulating hormone (α‐MSH) is the most notable factor and is known as proopiomelanocortin, gene symbol POMC, which is post‐translationally cleaved into several peptides, such as β‐endorphin and α‐MSH. α‐MSH activates the melanocortin 1 receptor (MC1 receptor) on the plasma membrane of skin melanocytes and promotes cAMP‐dependent signalling, which finally induces melanin synthesis. Uncontrolled synthesis or the irregular distribution of melanin can cause hyperpigmentation skin disorders, such as age spots and melasma (Slominski et al., 2000; Slominski et al., 2004; Dessinioti et al., 2009; Abdel‐Malek et al., 2010; Cheli et al., 2010).
Melanin synthesis progresses through a series of reactions, including several intermediates and substrates. The non‐essential aromatic amino acid L‐tyrosine, a precursor of melanin pigment, is hydroxylated to L‐DOPA, which is oxidized to dopaquinone. L‐tyrosine can be directly oxidized to dopaquinone. After several enzymatic steps, melanin pigments are formed. However, many parts of melanin synthesis are still debatable. It has long been recognized that these steps are regulated by diverse factors, the most popular being the tyrosinase gene family, which includes tyrosinase, tyrosinase‐related protein 1 (Tyrp1) and tyrosinase‐related protein 2 (Tyrp2), in melanocytes. Tyrosinase is a membrane‐bound, copper‐containing, glycoprotein and the rate‐limiting enzyme in melanin biosynthesis; it principally participates in the conversion step of L‐tyrosine and L‐DOPA to melanin pigments (Yasumoto et al., 1994; Ando et al., 2007; Yamaguchi et al., 2010; Slominski et al., 2012). The microphthalmia‐associated transcription factor (MITF) is a master regulator of melanocyte survival and proliferation and plays a crucial role in melanogenesis as a key regulator of tyrosinase and Tyrp1 (Yasumoto et al., 1994; Widlund and Fisher, 2003; Abdel‐Malek et al., 2010; Liu and Fisher, 2010). Given that tyrosinase is a crucial factor for melanin production, various regulators of melanin synthesis have been studied for treating a variety of hyperpigmentation disorders and skin whitening (Ando et al., 2007; Gillbro and Olsson, 2011; Pillaiyar et al., 2015).
Kazinol U, a prenylated flavan, was isolated from an extract of Broussonetia kazinoki Sieb (Moraceae), which is distributed throughout China, Korea and Japan. B. kazinoki has been used as a diuretic, a tonic and a suppressant of oedema in Chinese folk medicine (Zhang et al., 2001; Lee et al., 2010). Several prenylated flavonols, flavanes and diphenyl propanes from B. kazinoki have been reported to have cytotoxic, anti‐oxidative, anti‐inflammatory, anti‐tumorigenic and tyrosinase inhibitory activities (Baek et al., 2009; Lee et al., 2010; Kim et al., 2012; Kim et al., 2015). Kazinol U has been reported to have phytoestrogenic, anti‐inflammatory and cytoprotective activity against cytokine‐induced apoptotic cell death and induce LKB1 activation (Lee et al., 2010; Bae et al., 2011; Kim et al., 2012). Although several anti‐melanogenic compounds from B. kazinoki have been evaluated as inhibitors of tyrosinase (Baek et al., 2009), kazinol U has not been tested as a regulator of melanogenesis. In this study, we report on the anti‐melanogenic activity of kazinol U in mouse and human melanoma cells and on the mechanisms related to its inhibitory activity.
Methods
Cell cultures
The B16F10 murine melanoma cell line was obtained from the American Type Culture Collection (ATCC Cat# CRL‐6475, RRID:CVCL_0159) and cultured in DMEM (Gibco/Invitrogen, Carlsbad, CA) containing 10% FBS (Gibco/Invitrogen) and 100 U·mL−1 of penicillin/100 μg·mL−1 of streptomycin (Gibco/Invitrogen) at 37°C in a humidified 95% air/5% CO2 atmosphere incubator. SK‐MEL‐2 (RRID:CVCL_0069), MNT‐1 (RRID:CVCL_5624), SK‐MEL‐5 (RRID:CVCL_0527), SK‐MEL‐28 (RRID:CVCL_0526) and M14 (RRID:CVCL_1395) human melanoma cell lines were kindly provided by Dr Min‐Geol Lee (Yonsei University College of Medicine, Seoul, Republic of Korea), Dr Jin Ho Chung (Seoul National University College of Medicine, Seoul, Republic of Korea) and Dr Sukjoon Yoon (Sookmyung Women's University, Seoul, Republic of Korea). MNT‐1 cells were cultured in the same conditioned media as B16F10 cells. SK‐MEL‐2, SK‐MEL‐5, SK‐MEL‐28 and M14 cells were cultured in RPMI1640 (Gibco/Invitrogen). Normal human melanocytes (NHMs) from the neonatal foreskin of moderately pigmented donors were purchased from Cascade Biologics/Invitrogen (SKU# C‐102‐5C) and cultured in Medium 254 (Cascade Biologics) supplemented with human melanocyte growth supplement (Cascade Biologics) at 37°C in a humidified 95% air/5% CO2 atmosphere incubator.
MTT assay
B16F10 cells were seeded in a 96‐well plate and incubated for 24 h for stabilization. Then, cells were treated with arbutin, kazinol U or DMSO for 48 h. After this incubation, cell viability was determined using 3‐(4,5‐dimethylthiazol‐2‐yl) 2,5‐diphenyltetrazolium bromide (MTT). The MTT solution (5 mg·mL−1) was added to each well, and then cells were incubated for 3 h at 37°C. The absorbance of the resulting formazan crystals dissolved in DMSO (200 μL) was measured using Victor3™ (PerkinElmer, Waltham, MA).
Tyrosinase activity assay
Cells were lysed with 1% Triton X‐100 in Dulbecco's PBS. The lysates were subjected to repeated freeze/thaw cycles for complete cell lysis, and the supernatant fractions were obtained by centrifugation at 10 000× g for 5 min at 4°C. The lysates were made up to the same concentration of protein using lysis buffer; 90 μL of lysate was reacted with 10 μL of L‐DOPA (10 mM) and incubated for 15–90 min at 37°C. Dopachrome formation was detected by measuring the absorbance at 475 nm using Victor3 and normalized to the total amount of protein. The activity was expressed in comparison with absorbance obtained from the reaction of L‐DOPA with various amounts of mushroom tyrosinase. The data are shown as a percentage of the value of the control sample.
RNA isolation and real‐time RT‐PCR
Total RNA was isolated using TRIzol (Invitrogen) according to the manufacturer's instructions. For the RT reaction, first‐strand cDNA was generated using M‐MLV reverse transcriptase (Promega, Madison, WI), oligo‐(dT) primers and dNTPs (Bioneer, Daejeon, Republic of Korea). Briefly, 5 μg of total RNA was reverse transcribed to cDNA. Quantitative real‐time PCR was performed using an ABI StepOnePlus™ real‐time PCR thermal cycler with Power SYBR Green PCR Master Mix according to the manufacturer's instructions (Applied Biosystems, CA). Primer sequences are listed in the Supporting Information Table S1 (Koo et al., 2010). Target mRNA levels were normalized to the expression of cyclophilin.
Western blot analysis
Cells were lysed on ice in protein extraction solution (iNtRON, Daegu, Republic of Korea) for 15 min. The supernatant fraction was reserved after centrifugation at 14 000× g for 15 min at 4°C. Total protein was separated by electrophoresis on an SDS‐PAGE gradient gel (8% to 15%) and transferred onto a PVDF membrane (Amersham Biosciences, Burkes, UK). The membranes were blocked with Tris‐buffered saline plus 0.05% Tween‐20 (TBST) containing 5% skimmed milk and incubated with primary antibodies overnight at 4°C. After this incubation, the membranes were washed with TBST and incubated with HRP‐conjugated secondary antibodies. The blots were visualized with an enhanced chemiluminescence system using an Ez‐Capture MG (ATTO Corporation, Tokyo, Japan).
Luciferase reporter assay
The pMITF‐Gluc reporter system harbouring the promoter region (GenBank seq. ID: D82874/502 bp/EcoRI/MITF‐sense 5′‐GGCGAATTCCTGCAGTCGGAAGTGGCAGTTA‐3′, BamHI/MITF‐anti‐sense 5′‐GGCGGATCCAGACTATCCCTCCCTCTACTTTC‐3′) of MITF and the pTyrosinase‐Gluc reporter system harbouring the promoter region (GenBank seq. ID: M27160/398 bp/EcoRI/Tyrosinase‐sense 5′‐GGCGAATTCCTCTCATTTGCAAGGTCAAATCA‐3′, BamHI/Tyrosinase‐anti‐sense 5′‐GGCGGATCCTTCCTCTAGTCCTCACAAGGT‐3′) were kindly provided by AmorePacific R&D Institute (Yongin, Gyeonggi‐do, Republic of Korea). For the luciferase reporter assay, semi‐confluent cells grown in 24‐well plates were co‐transfected with each luciferase plasmid and the pCMV‐β‐galactosidase reporter plasmid using PolyFect transfection reagent (Qiagen, Valencia, CA). Arbutin, kazinol U or DMSO treatments were applied immediately after transfection, and IBMX was added 1 h later. After incubation for 18 h, cells were harvested using passive lysis buffer, and the Gaussia luciferase activity was measured from the culture supernatants using a luminescence microplate reader set (Victor3) and the Gaussia Luciferase Assay Kit (New England Biolabs, Ipswich, MA) according to the manufacturer's instructions. β‐Galactosidase activity was measured using the substrate o‐nitrophenyl‐β‐D‐galactopyranoside and was used to adjust for any variability in transfection efficiency.
Knockdown of AMPK by siRNA
The control and AMP kinase (AMPK) siRNAs (Santa Cruz) were transfected into B16F10 cells using Lipofectamine™ RNAiMAX (Invitrogen).
Measurement of melanin concentration
NHMs were treated with kazinol U or arbutin for the indicated time points (0–4 days) and harvested. After centrifugation, the pellets obtained from cells or zebrafish embryos were dissolved in 1N NaOH/10% DMSO for 2 h at 80°C, and the concentration of solubilized melanin was determined by measuring the absorbance at 405 nm with Victor3. The melanin concentration was normalized to the cell number or the number of zebrafish.
Animals
All zebrafish husbandry and experimental protocols complied with institutional guidelines were approved by the local ethics boards (Sookmyung Women's University Animal Care and Use Committee, SMWU‐IACUC‐1712‐036), and they were maintained in the Sookmyung Zebrafish Facility (Sookmyung Women's University, Seoul, Korea). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010). Adult AB zebrafish (RRID:ZFIN_ZDB‐GENO‐960809‐7) were maintained in a 3 L polystyrene aquarium tank tanks (15 zebrafish per tank) under standard conditions at 28.5°C with a 14 h light/10 h dark cycle (Westerfield, 2007). Food with Artemia nauplii for adults and rotifers for larvae was fed twice per day. The eggs were obtained by natural mating with a ratio of two males to one female in a 1.0 L breeding tank and incubated in embryonic water at 28.5°C with a 14 h light/10 h dark cycle.
In vivo anti‐melanogenic assay in zebrafish
Embryos were obtained from natural crosses between the wild type AB strain fish and raised in embryonic water (sea salt, 0.06 g·L−1). Synchronized 24 hpf (hours post‐fertilization) embryos that were placed in 12‐well plates (10 embryos per well), in 5 mL embryonic water (maintained and changed daily) were treated with kazinol U, phenylthiourea (PTU), arbutin or DMSO for 48 h at 28.5°C with a 14 h light/10 h dark cycle. Embryos were dechorionated by forceps, anaesthetised in tricaine methanesulfonate solution (0.02%, Sigma‐Aldrich), mounted in 1% methyl cellulose and photographed under the stereomicroscope (Nikon SMZ1500, Tokyo, Japan). PTU (200 μM) was used as a positive control. To determine the tyrosinase activity and melanin contents, 50 zebrafish embryos (total 300 embryos) were treated with either kazinol U, PTU, arbutin or DMSO from 24 to 72 hpf, anaesthetised in tricaine methanesulfonate solution and lysed by 1% Triton X‐100 in DPBS. After the centrifugation, the supernatants were used for determining tyrosinase activity. The pellets were dissolved in 500 μL of 1N NaOH/10% DMSO for 2 h at 80°C and used for measuring the melanin concentration.
Data and statistical analysis
The results were analysed with GraphPad Prism (GraphPad Prism, Ver# 5, RRID:SCR_002798) statistic software (La Jolla, CA). All the values are presented as means ± SEM and analysed using ANOVA and the Newman–Keuls post hoc test when the F value was significant. P < 0.05 was considered statistically significant.
Materials
Preparation of kazinol U
The air‐dried root bark of B. kazinoki collected from the Gyeonggi Province, Republic of Korea (voucher specimen no. SPH 07002) (0.6 kg) was extracted for 24 h at room temperature with 2 L of ethanol. The extract (51 g) was suspended in water and successively partitioned with n‐hexane, EtOAc, CHCl3 and BuOH. The EtOAc fraction (31 g) was subjected to silica gel column chromatography eluted with an n‐hexane/acetone gradient system (20:1 → 1:10), and 11 fractions were collected. Fraction 8 was chromatographed on a RP‐C18 column with a gradient elution of MeOH (30% → 100%) to yield compound 1 (139 mg). Compound 1 was obtained as an oily substance, and the HREIMS showed the [M]+ ion at m/z 326.1516 (calcd 326.1518) corresponded to a molecular formula of C20H22O4. The structure of compound 1 was identified as kazinol U as previously reported (Lee et al., 2010).
Antibodies and chemicals
Antibodies against actin, α‐actinin (Cat# sc‐166 524, RRID:AB_2257995), tyrosinase (Cat# sc‐15 341, RRID:AB_2256771) and anti‐rabbit (Cat# sc‐2357, RRID:AB_628497) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against MITF (Cat# 12590, RRID:AB_2616024), phospho‐JNK (Cat# 9251, RRID:AB_331659) and phospho‐AMPK (Cat# 2531, RRID:AB_330330) were purchased from Cell Signaling Technology Inc. (Beverly, MA). Anti‐mouse (Cat# A4416, RRID:AB_258167), α‐MSH, IBMX, mushroom tyrosinase, L‐DOPA, arbutin, SP600125 and compound C were purchased from Sigma‐Aldrich (St. Louis, MO).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b).
Results
Kazinol U inhibits tyrosinase activity and melanin synthesis
Kazinol U is a prenylated flavan isolated from the EtOAc soluble fraction of B. kazinoki. It was identified as a new phytoestrogen with a novel structure (Lee et al., 2010) (Figure 1A). Initially, we determined the cytotoxicity of kazinol U against B16F10 mouse melanoma cells and MNT‐1 human melanoma cells by the MTT assay (Supporting Information Figure S1). Up to 20 μM, kazinol U did not affect cell viability and exhibited a minimal effect on the viability at 60 μM in B16F10 cells, whereas it did not affect the cell viability up to 60 μM in MNT‐1 cells. Its effect on the viability of other human melanoma cell lines was not significant in the similar concentration range (data not shown). Kazinol U reduced L‐DOPA oxidation of B16F10 cells in a dose‐dependent manner (Figure 1B). Consistently, tyrosinase mRNA expression was also dose‐dependently decreased (Figure 1C). Arbutin has been known to inhibit L‐DOPA oxidation by mushroom tyrosinase (Yagi et al., 1987) by reducing melanosomal tyrosinase activity rather than tyrosinase expression (Parvez et al., 2006; Tada et al., 2014). We observed that kazinol U more effectively decreases the tyrosinase activity compared with arbutin at the lower concentration of 20 μM (Figure 1D). α‐MSH is a regulator of melanocyte differentiation and melanogenesis by binding to the MC1R on melanocytes, leading to the induction of intracellular cAMP. The pharmacological agent IBMX is an inducer of intracellular cAMP (Park and Gilchrest, 1999; Kim et al., 2008; Jin et al., 2012). To determine whether kazinol U suppresses tyrosinase activity induced by IBMX or α‐MSH, tyrosinase activity was assessed after co‐treatment. Kazinol U inhibited tyrosinase activity induced by IBMX or α‐MSH with higher efficacy than arbutin (Figure 1E, F) and inhibited the pigmentation of the cell pellets (Figure 1G). Moreover, IBMX‐ or α‐MSH‐induced cell pigmentation was also substantially suppressed by kazinol U (Figure 1H, I). These findings indicate that kazinol U inhibits the endogenous tyrosinase activity, cAMP‐induced tyrosinase activity and cell pigmentation.
Figure 1.
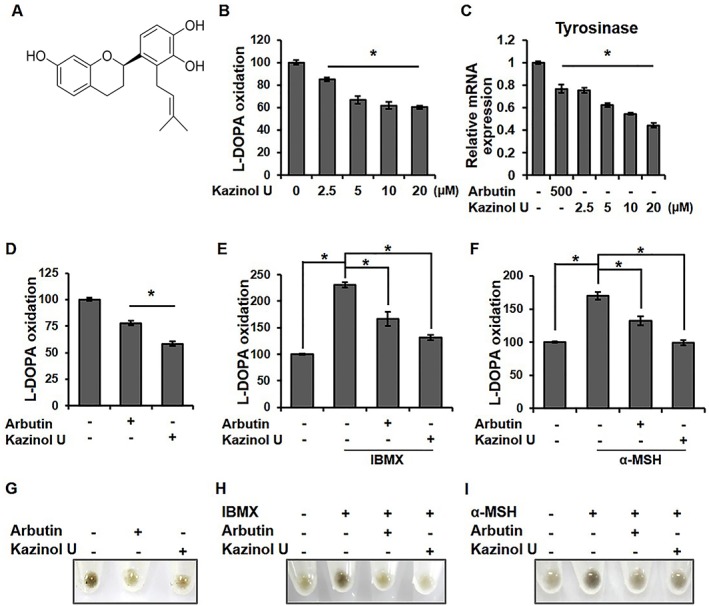
The effect of kazinol U on tyrosinase activity and pigmentation in B16F10 mouse melanoma cells. (A) Schematic of the chemical structure of kazinol U. (B) Cells were treated with the indicated concentrations of kazinol U or DMSO for 48 h. (C, D) Cells were treated with kazinol U, arbutin (500 μM) or DMSO for 24 h (C) or 48 h (D, G). (E, F and H, J) Cells were pretreated with arbutin, kazinol U or DMSO for 3 h before the addition of IBMX (100 μM) or α‐MSH (1 μM) for 48 h (B–F: n = 5). (G–I) Cell pellets were photographed using a Canon EOS 60D. One‐way ANOVA with a post hoc Newman–Keuls test was used for the statistical analysis; *P < 0.05.
Kazinol U represses the expression of melanogenic enzymes
Tyrosinase is stabilized by the expression of tyrosinase and Tyrp1, and dopachrome tautomerase (Dct) and Tyrp1 induce melanogenesis (Kobayashi et al., 1994; Kobayashi et al., 1998; Manga et al., 2000). To determine whether the inhibition of tyrosinase activity by kazinol U was caused by the modulation of the tyrosinase gene family, RT‐qPCR was performed. Kazinol U inhibited the mRNA levels of tyrosinase, Tyrp1 and Dct and suppressed IBMX‐induced expression of the tyrosinase gene family (Figure 2A–C). Kazinol U suppressed tyrosinase protein levels and IBMX‐ or α‐MSH‐induced tyrosinase expression (Supporting Information Figure S2A–C). Consistent with the qPCR results, kazinol U repressed the basal and IBMX‐induced tyrosinase promoter activity more effectively compared with arbutin (Figure 2D, E). Taken together, these results suggest that kazinol U efficiently reduces the tyrosinase expression and its promoter activity as well as the expression of tyrosinase‐related proteins in the presence of melanogenic inducers.
Figure 2.
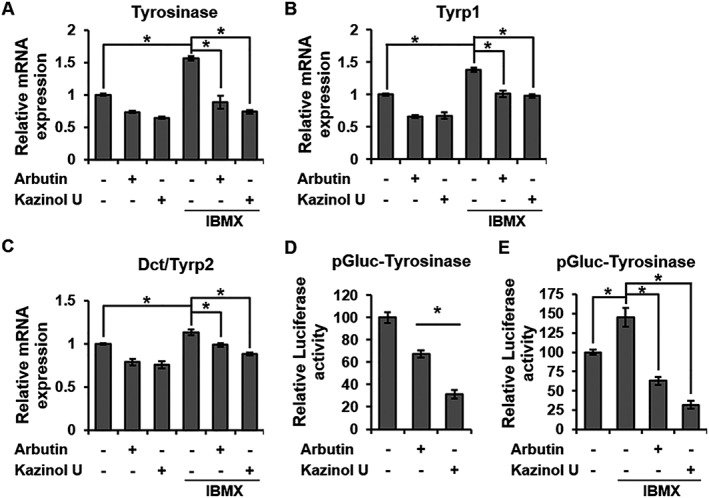
Inhibition of melanogenic enzyme by kazinol U. (A–C) B16F10 cells were pretreated with arbutin (500 μM), kazinol U (20 μM) or DMSO for 1 h before treatment with IBMX (100 μM). After treatment with IBMX for 24 h, tyrosinase (A), Tyrp1 (B) and Dct (C) mRNA expressions were measured by quantitative RT‐PCR using specific primers (n = 5). (D, E) Cells were co‐transfected with pTyrosinase‐Gluc and a β‐galactosidase plasmid and incubated for 18 h. Arbutin, kazinol U or DMSO was added to the cells after transfection. One hour later, the cells were treated with IBMX or nothing for 18 h before luciferase analysis. The luciferase activities were measured with a luminometer using a dual luciferase assay system (D: n = 6, E: n = 5). One‐way ANOVA with a post hoc Newman–Keuls test was used for the statistical analysis; *P < 0.05.
Kazinol U reduces the expression and transcriptional activation of MITF
MITF plays a crucial role in melanogenesis as a key transcriptional regulator of tyrosinase and tyrosinase‐related proteins. Thus, we further investigated whether kazinol U regulates MITF expression. Interestingly, MITF protein levels were inhibited by kazinol U. In addition, IBMX‐ and α‐MSH‐induced MITF expression was also inhibited by kazinol U (Figure 3A–C). Kazinol U also down‐regulated the basal and IBMX‐induced MITF mRNA levels in RT‐qPCR experiments in B16F10 mouse melanoma cells (Figure 3D). Furthermore, the effect of kazinol U on MITF expression was also observed in several human melanoma cell lines, including MNT1, M14, SK‐MEL‐2, SK‐MEL‐5 and SK‐MEL‐28 (Supporting Information Figure S3A–J). To verify whether kazinol U affects the transcriptional activation of MITF, reporter gene assays were performed. In accordance with the RT‐qPCR results, kazinol U suppressed basal and IBMX‐induced MITF transcriptional activation (Figure 3E, F). These results collectively indicate that kazinol U suppresses MITF expression and its transcriptional activation.
Figure 3.
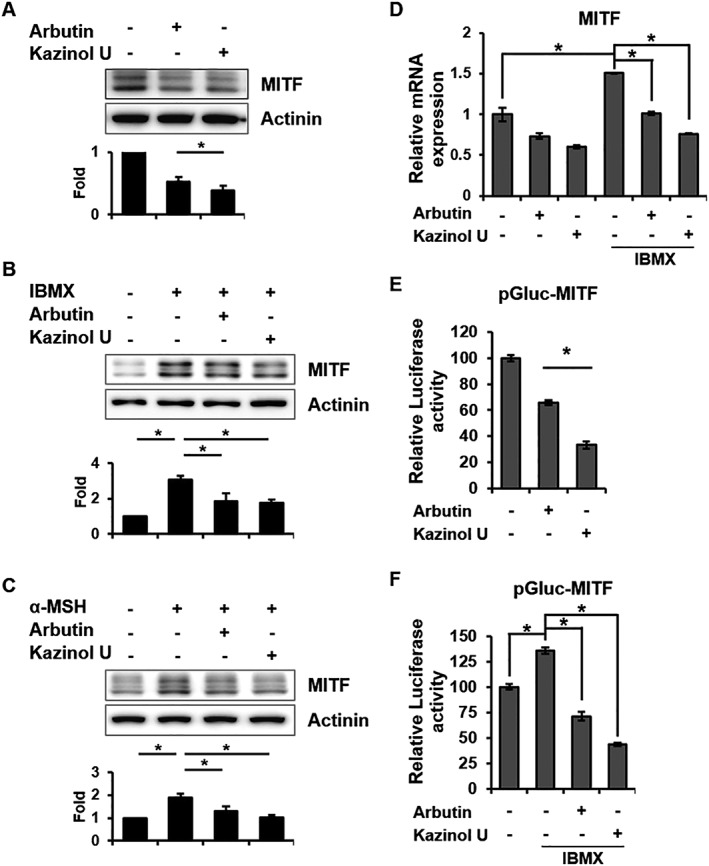
Inhibition of MITF mRNA, protein and transcriptional activity by kazinol U. (A) Cells were treated with arbutin (500 μM), kazinol U (20 μM) or DMSO for 6 h. (B–D) Cells were pretreated with arbutin, kazinol U or DMSO for 1 h and then cultured with IBMX (100 μM) or α‐MSH (1 μM) for 6 h (B, C) or 24 h (D). (A–C) MITF expression was detected by Western blot analysis (A: n = 5, B and C: n = 7). (D) MITF mRNA expression was measured by quantitative RT‐PCR using specific primers (n = 5). (E, F) Cells were co‐transfected with pMITF‐Gluc and a β‐galactosidase plasmid. The treatment condition was the same as in Figure 2. The changes in luciferase activity with respect to the DMSO‐control were calculated (E and F: n = 6). One‐way ANOVA with a post hoc Newman–Keuls test was used for the statistical analysis; *P < 0.05.
Kazinol U induces AMPK and JNK protein phosphorylation
MITF expression or stability is regulated by several pathways (Singh et al., 2005; Kono et al., 2006; Baek and Lee, 2015). In a previous study, we reported that melanogenesis is regulated by JNK phosphorylation (Lim et al., 2016). It has recently been reported that kazinol U induces LKB1, an upstream kinase of AMPK (Kim et al., 2012). Moreover, the correlation between AMPK activity and MITF expression has been reported in melanoma (Borgdorff et al., 2014; Lehraiki et al., 2014). However, the relationship between AMPK activity and melanogenesis has not been established to date. To identify the protein involved in melanogenesis and MITF inhibition by kazinol U, western blot analysis was performed. Interestingly, kazinol U significantly induced AMPK and JNK phosphorylation in B16F10 cells (Figure 4A). Consistent with these results, kazinol U increased the AMPK and JNK phosphorylation in human melanoma cell lines, whereas arbutin failed to increase the phosphorylation (Figure 4B–D and Supporting Information Figure S4), indicating that arbutin and kazinol U use different mechanisms for melanogenesis regulation. Furthermore, when we treated cells with compound C (AMPK inhibitor, dorsomorphin), the expression of MITF and tyrosinase markedly increased in parallel with the decrease in AMPK phosphorylation, which was partially reversed by co‐treatment with kazinol U in B16F10 cells (Figure 5A–C). We also confirmed these effects in human melanoma cell lines (Supporting Information Figure S5A–F). In fact, compound C significantly induced tyrosinase activity that was suppressed by kazinol U in B16F10 cells (Figure 5D). In addition, we transfected cells with AMPK siRNA to eliminate off‐target effects of compound C and found that kazinol U partially increased siAMPK‐inhibited phosphorylation of AMPK without changes in total AMPK levels. In accordance with AMPK phosphorylation, siAMPK‐induced MITF expression was reduced by kazinol U (Figure 5E, F).
Figure 4.
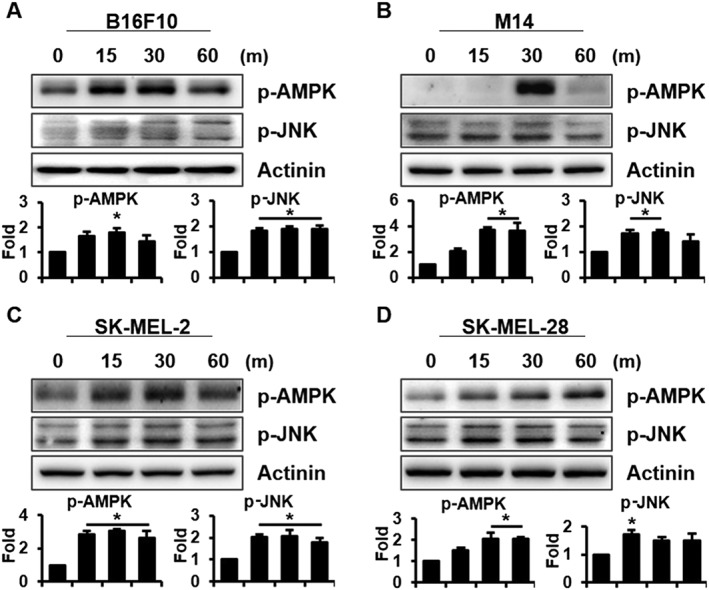
The effect of kazinol U on AMPK and JNK phosphorylation. (A–D) Cells were treated with kazinol U (20 μM) for the indicated time points. AMPK and JNK phosphorylations were analysed by Western blot in B16F10 (A), M14 (B), SK‐MEL‐2 (C) and SK‐MEL‐28 (D) melanoma cells (A–D: n = 5). One‐way ANOVA with a post hoc Newman–Keuls test was used for the statistical analysis; *P < 0.05.
Figure 5.
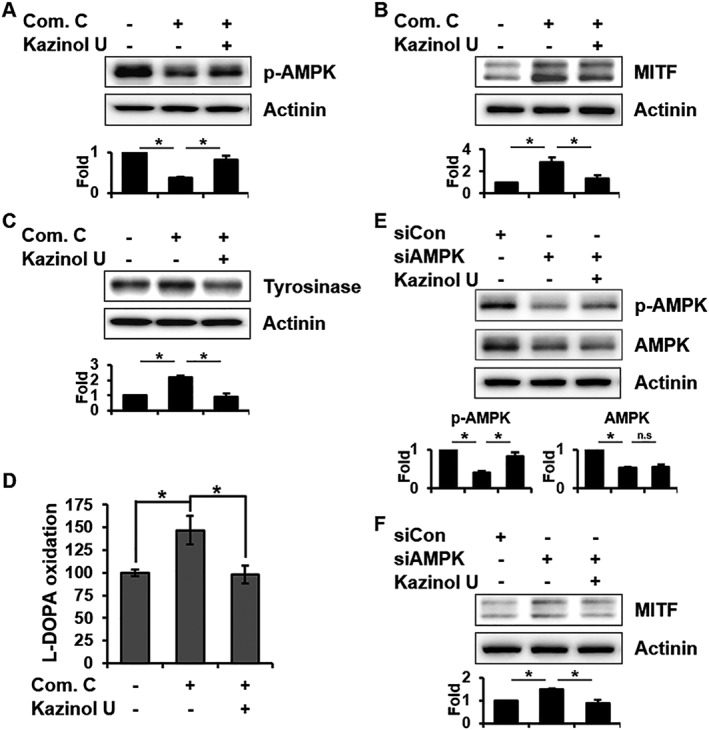
The effect of kazinol U on compound C‐ or siAMPK‐induced MITF and tyrosinase expression. (A) B16F10 cells were pretreated with compound C (20 μM) or DMSO for 1 h before treatment with kazinol U (20 μM) or DMSO for 1 h. (B) After pretreatment with kazinol U or DMSO for 1 h, the cells were treated with compound C for 7 h. (C) Cells pretreated with kazinol U or DMSO for 3 h were treated with compound C for 24 h. AMPK phosphorylation and MITF and tyrosinase expression were detected by Western blot analysis. (D) Cells were pretreated with kazinol U (20 μM) or DMSO for 3 h before the addition of compound C (20 μM) for 48 h. Tyrosinase activity was determined by measuring dopachrome formation. (E and F) Cells were transfected with AMPK siRNA for 24 h followed by treatment with kazinol U (20 μM) or DMSO for 1 h (E) or 6 h (F). MITF, AMPK and p‐AMPK expressions were detected by Western blot analysis (A–F: n = 5). One‐way ANOVA with a post hoc Newman–Keuls test was used for the statistical analysis; *P < 0.05.
To investigate whether JNK is also involved in anti‐melanogenic effects by kazinol U, the JNK inhibitor SP600125 was used. Kazinol U reversed the inhibition of JNK phosphorylation by SP600125 and suppressed MITF and tyrosinase up‐regulation by SP600125 (Figure 6A–C). Moreover, SP600125‐induced reduction in the phosphorylation of c‐Jun, a JNK downstream protein, was blocked by kazinol U (Supporting Information Figure S6A). In accordance with these results, SP600125‐induced tyrosinase activity, and the pigmentation of cell pellets was also reduced by kazinol U (Figure 6D, E). It has been reported that ERK and p38 participate in melanogenesis via MITF and tyrosinase regulation (Bellei et al., 2010; Baek and Lee, 2015; Zhou et al., 2017). We found that kazinol U significantly increased p38 and ERK phosphorylation in B16F10 and SK‐MEL‐28 cells (Supporting Information Figure S6B,C). Kazinol U restored p38 and ERK phosphorylation that was reduced by a treatment with SB203580 (p38 inhibitor) or PD98059 (ERK inhibitor) and suppressed MITF expression induced by SB203580 or PD98059 (Supporting Information Figure S6D–G). Taken together, our findings suggest that kazinol U exhibits anti‐melanogenic effects through the induction of AMPK and MAPK phosphorylation.
Figure 6.
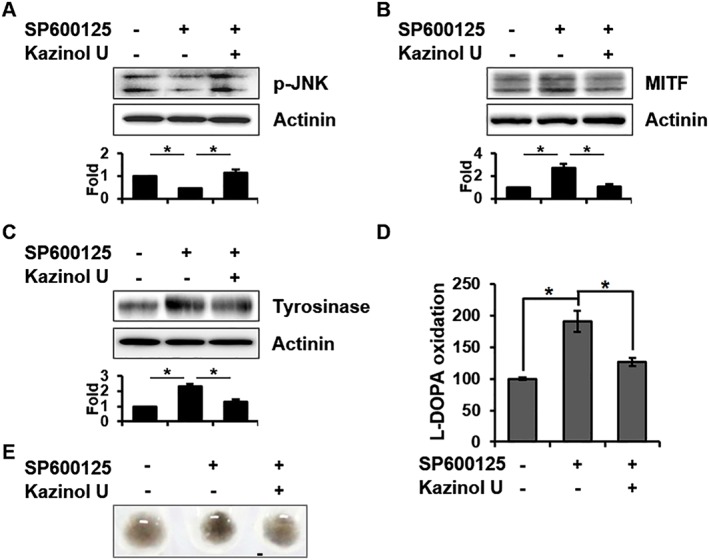
The effect of kazinol U on SP600125‐induced MITF and tyrosinase expression. (A) B16F10 cells were treated with kazinol U or DMSO for 30 min and cultured with SP600125 (20 μM) for 30 min. (B) After pretreatment with kazinol U or DMSO for 1 h, cells were treated with SP600125 for 7 h. (C) Cells pretreated with kazinol U or DMSO for 3 h were treated with SP600125 for 24 h. JNK phosphorylation and MITF and tyrosinase expression were detected by Western blot analysis (n ≥ 5). (D and E) Cells were pretreated with kazinol U (20 μM) or DMSO for 3 h before the addition of SP600125 (20 μM) for 48 h. Tyrosinase activity was determined by measuring dopachrome formation. Cell pigmentation was photographed using a Canon EOS 60D (A–D: n = 5). One‐way ANOVA with a post hoc Newman–Keuls test was used for the statistical analysis; *P < 0.05.
Kazinol U reduces melanogenesis in NHMs
To investigate whether kazinol U could reduce melanogenesis in NHMs, the cells were treated with kazinol U or arbutin. Kazinol U or arbutin did not affect the cell morphology of NHMs and showed no significant toxicity with treatment for 4 days (Supporting Information Figure S7A). Kazinol U inhibited both tyrosinase expression and activity in NHMs, whereas arbutin reduced tyrosinase activity alone (Figure 7A, B). As expected, kazinol U and arbutin inhibited MITF expression (Figure 7C) and kazinol U repressed IBMX‐ or α‐MSH‐induced MITF expression (Figure 7D, E). In accordance with the previous data using mouse and human melanoma cells, kazinol U induced phosphorylation of AMPK, JNK and c‐Jun in NHMs and increased p38 and ERK phosphorylation (Figure 7F and Supporting Information Figure S7B). Kazinol U also suppressed compound C‐ or SP600125‐induced MITF expression in NHMs (Supporting Information Figure S7C–F). Finally, the melanin content and tyrosinase activity were reduced by kazinol U in a time‐dependent manner (Figure 7G and Supporting Information Figure S7G), suggesting that kazinol U displays a remarkable anti‐melanogenic effect even in NHMs.
Figure 7.
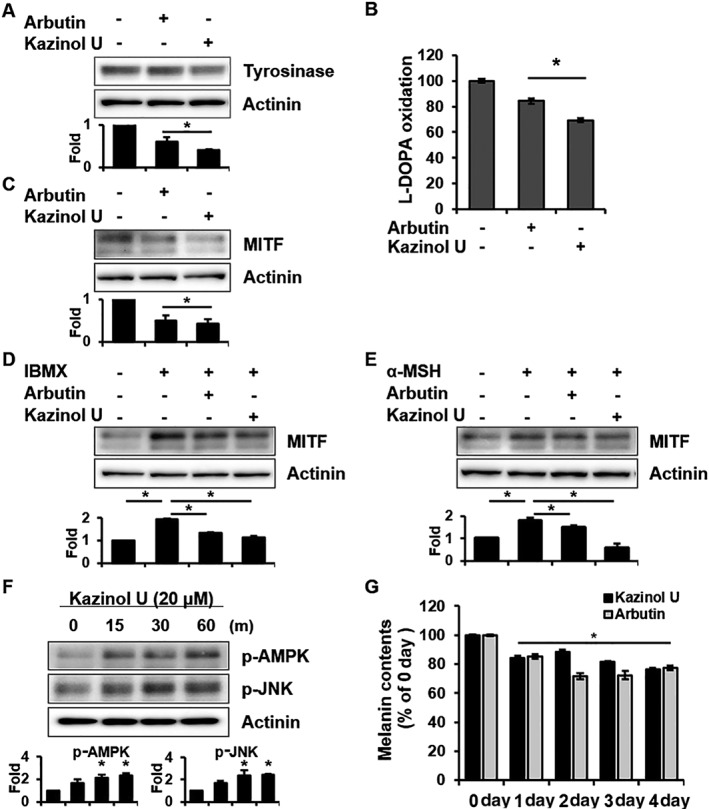
The effect of kazinol U on normal human melanocytes (NHMs). (A–C) NHMs were treated with arbutin (500 μM), kazinol U (20 μM) or DMSO for 18 h (A), 48 h (B) and 8 h (C). Tyrosinase and MITF expressions were detected by Western blot analysis (A and C), and tyrosinase activity was determined by measuring dopachrome formation (B). (D, E) The cells were pretreated with arbutin, kazinol U or DMSO for 1 h and then cultured with IBMX (100 μM) or α‐MSH (1 μM) for 8 h. MITF expression was detected by Western blot analysis. (F) The cells were treated with kazinol U (20 μM) for the indicated time points. AMPK and JNK phosphorylation was analysed by Western blot. (G) The cells were treated with arbutin or kazinol U for the indicated time points and harvested. Melanin content was determined by measuring the absorbance at 405 nm using Victor3 (A: n = 6, B–G: n = 5). One‐way ANOVA with a post hoc Newman–Keuls test was used for the statistical analysis; *P < 0.05.
Kazinol U suppresses melanogenesis in a zebrafish model
Zebrafish can be used as a whole animal model for phenotype‐based screening of melanogenic inhibitors or stimulators (Choi et al., 2007). Zebrafish have several advantages, including the cost‐effectiveness, small body size, short‐life spans, transparent embryos and physiological similarity to mammals (Malafoglia et al., 2013; Baek and Lee, 2015; Wu et al., 2015). Kazinol U was added to the embryo media for 48 h during embryonic development, and whole embryos were photographed under the stereomicroscope (Figure 8A). Kazinol U, but not arbutin, significantly suppressed melanogenesis in a dose‐dependent manner in zebrafish compared with the control group (Figure 8B). Phenylthiourea commonly used for inhibiting the melanization of zebrafish embryos showed the strongest effect at standard concentrations of 0.2 mM. Remarkably, kazinol U inhibited melanogenesis in the whole body compartments of zebrafish where melanin is generated. Of note, although the number of melanin‐containing granules in zebrafish was not significantly altered, melanin content was reduced by kazinol U (Figure 8C and Supporting Information Figure S8). Defects in the developmental processes were not observed at kazinol U concentrations less than 50 μM. Furthermore, tyrosinase activity in whole body lysates was suppressed by kazinol U in a dose‐dependent manner (Figure 8D). These results suggest that kazinol U clearly inhibits melanogenesis not only in vitro but also in vivo.
Figure 8.
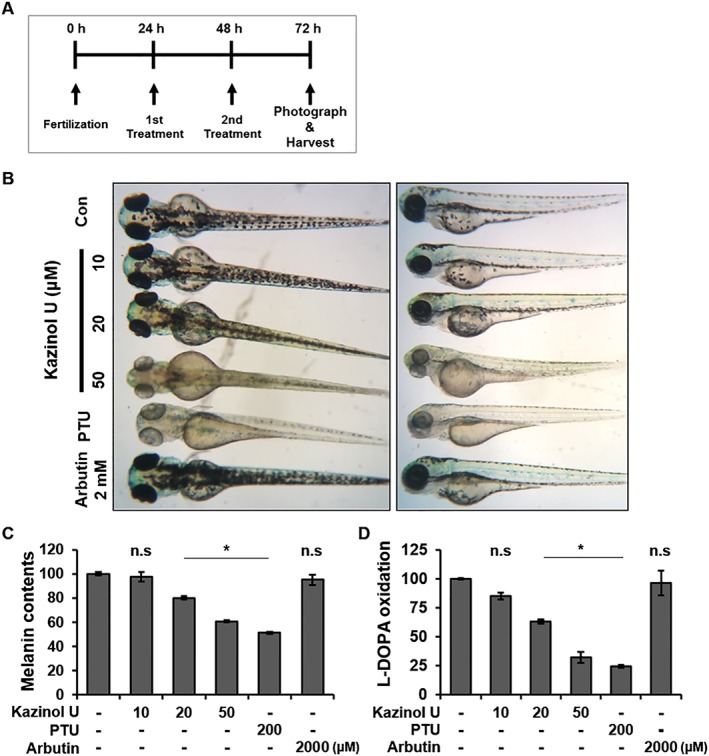
The effect of kazinol U on melanogenesis in zebrafish. (A) The experimental design for the zebrafish model. (B–D) Zebrafish embryos were treated with kazinol U (10–50 μM), phenylthiourea (PTU) (200 μM), arbutin (2 mM) or DMSO from 24 to 72 hpf. The pigmentation of the embryos was observed under a stereomicroscope. To determine the tyrosinase activity and melanin content, 50 zebrafish embryos were treated with each compound from 24 to 72 hpf and lysed with 1% Triton X‐100 in DPBS. After centrifugation, the supernatants were used to determine the tyrosinase activity. Pellets were dissolved in 500 μL of 1N NaOH/10% DMSO for 2 h at 80°C and used for measuring the absorbance at 405 nm using Victor3 (C: n = 6, D: n = 5). One‐way ANOVA with a post hoc Newman–Keuls test was used for the statistical analysis; *P < 0.05.
Discussion
Kazinol U from B. kazinoki Sieb (Moraceae) plants exhibits a variety of pharmacological activities (Baek et al., 2009; Lee et al., 2010; Kim et al., 2012; Kim et al., 2015). Baek et al. reported tyrosinase inhibitory effects of some compounds from this plant (Hwang and Lee, 2007; Baek et al., 2009), but detailed signalling mechanisms were not studied. Although remarkable tyrosinase inhibitors have been isolated from the wood and bark of the genus Broussonetia, each compound shows very weak whitening effects compared to the extracts. Kazinol U has been identified as a novel phytoestrogenic substance from B. kazinoki, and its effects on cytokine‐induced apoptotic cell death and LKB1 activation of kazinol U have been reported (Lee et al., 2010; Bae et al., 2011; Kim et al., 2012). In this study, we evaluated the anti‐melanogenic activity of kazinol U in B16F10 mouse melanoma cells and several human melanoma cells, including SK‐MEL‐2, SK‐MEL‐5, SK‐MEL‐28, M14 and MNT‐1. Kazinol U remarkably inhibited melanogenesis through the inhibition of tyrosinase and tyrosinase‐related proteins compared with arbutin. In accordance with these findings, it inhibited the expression of melanogenic enzymes and their enzymatic activity to reduce cell pigmentation. The higher activity of kazinol U compared to arbutin might partially result from the enhanced cell permeability assisted by the prenyl group in the structure (Kim et al., 2012). Although we did not assess the melanin synthesis in all five human melanoma cells, our findings clearly showed that key signalling pathways for melanin synthesis can be similarly interrupted by treatment with kazinol U.
AMPK is a highly conserved energy sensor related with growth and metabolism (Mihaylova and Shaw, 2011). AMPK signalling is directly or indirectly correlated with cAMP, CREB, AKT, p38 MAPK and ERK, which are associated with melanogenesis through MITF in melanocytes and melanoma and with metabolic pathways in various cells, including cancer cells (Horike et al., 2008; Mihaylova and Shaw, 2011; Pylayeva‐Gupta et al., 2011; Sanchez et al., 2012; Kim and He, 2013; Hardie and Ashford, 2014). Forskolin, an activator of adenylyl cyclase (AC), and IBMX, a cAMP inducer, phosphorylate the s485/491p site on AMPKα, leading to the inhibition of phosphorylation of the T172p site on AMPK, which is critical for AMPK kinase activity and is phosphorylated by LKB1 or CaMKK, an AMPK upstream kinase (Hurley et al., 2006). Nevertheless, the effect of AMPK activation on melanogenesis has rarely been reported. Previous reports demonstrated the anti‐melanogenic effect of metformin, which is an AMPK activator. Although metformin was shown to be an AMPK‐independent inhibitor of melanogenesis, the AMPK‐dominant negative form or siAMPK transfection caused MITF induction in NHMs and Mel501 human melanoma cells (Lehraiki et al., 2014). In contrast, suppression of AMPK inhibits MITF in primary human melanocytes that ectopically express BRAFV600E and HA‐MITF. siAMPK transfection reduced HA‐MITF expression, and compound C induced p‐HA‐MITF, which leads to MITF degradation. These results indicate that AMPK inhibition decreases MITF expression through ERK inactivation (Borgdorff et al., 2014). However, we observed that MITF expression and tyrosinase activity and expression were increased by compound C in numerous melanoma cells. Moreover, siAMPK treatment induced MITF expression in B16F10 mouse melanoma cells. Therefore, a further in‐depth study on how AMPK activation is involved in MITF regulation is needed.
As we reported previously, JNK phosphorylation is involved in anti‐melanogenesis (Lim et al., 2016). Kazinol U also induced JNK phosphorylation in several melanoma cells, whereas ERK and p38 MAPK phosphorylation showed differential expression patterns in each melanoma cell. Moreover, SP600125, a JNK inhibitor, induced MITF, tyrosinase expression and activity, and cell pigmentations, but kazinol U remarkably reversed these effects. A similar effect was found in experiments using p38 and ERK inhibitors, indicating that MAPKs are strongly involved in the anti‐melanogenesis effects of kazinol U. Finally, kazinol U also suppresses zebrafish melanogenesis in a dose‐dependent manner. Interestingly, it inhibited melanogenesis in whole body compartments of the zebrafish. Since zebrafish have visualizing pigmented cells on the skin and conserved features among zebrafish melanophores and human melanocytes, they are an excellent model for studying melanocyte development and diseases. While the migration of neural crest cells to the final destination is distinct from mice, chicks and zebrafish (Hou et al., 2006; Kelsh et al., 2009), most characteristics for skin structures and melanocyte biology, such as role of MITF in melanocyte development and transcriptional regulation of differentiation into melanocytes, are conserved in mammals and zebrafish (Le Guellec et al., 2004; Fuchs and Horsley, 2008; Kelsh et al., 2009; Mort et al., 2015; Kaufman et al., 2016). Thus, the pigmentation pattern of the zebrafish model facilitates studies on melanogenesis and melanocyte development, and this model can be used for pharmacological agent screening, biochemical stability testing and in vivo cytotoxicity assessments (Wu et al., 2015).
In summary, kazinol U exhibited anti‐melanogenic activity through the inhibition of MITF expression; inactivation of its downstream target genes, tyrosinase, Tyrp1 and Dct; and activation of AMPK and MAPK proteins (Supporting Information Figure S9). These findings indicate that kazinol U might be therapeutically and cosmetically applied for several hyperpigmentation skin disorders and skin whitening.
Author contributions
H.G.L., J.‐H.R. and J.‐S.L. coordinated the work. J.L., S.N., J.H.J. and M.J.K. performed the experiments. J.L., Y.Y., M.‐S.L. and J.‐S.L. performed data analysis and constructed the figures. The manuscript was drafted by J.L., J.‐H.R. and J.‐S.L. All authors were involved in the interpretation of the experimental data and critically revised and approved it for submission.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 Effect of kazinol U on B16F10 and MNT‐1 cells viability in different concentrations.
Figure S2 Inhibition of tyrosinase protein level by kazinol U.
Figure S3 Inhibition of MITF expression by kazinol U in five different human melanoma cell lines.
Figure S4 Effect of kazinol U and arbutin on AMPK and JNK phosphorylation in M14, SK‐MEL‐2 and SK‐MEL‐28 cells.
Figure S5 Effect of kazinol U on compound C‐induced MITF in melanoma cells.
Figure S6 Effect of kazinol U on MAPKs in melanoma cells.
Figure S7 Effect of kazinol U in NHMs.
Figure S8 Inhibition of melanogenesis, but not melanin‐containing granule formation, in zebrafish by kazinol U.
Figure S9 The mechanistic scheme of anti‐melanogenic effects of kazinol U.
Table S1 Quantitative PCR primer sequences.
Acknowledgements
This study was supported by grants from the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning, Republic of Korea, the Korean government (NRF‐2016R1A5A1011974 and 2011‐0030074).
Lim, J. , Nam, S. , Jeong, J. H. , Kim, M. J. , Yang, Y. , Lee, M.‐S. , Lee, H. G. , Ryu, J.‐H. , and Lim, J.‐S. (2019) Kazinol U inhibits melanogenesis through the inhibition of tyrosinase‐related proteins via AMP kinase activation. British Journal of Pharmacology, 176: 737–750. 10.1111/bph.14560.
Contributor Information
Jae‐Ha Ryu, Email: ryuha@sookmyung.ac.kr.
Jong‐Seok Lim, Email: jslim@sookmyung.ac.kr.
References
- Abdel‐Malek ZA, Kadekaro AL, Swope VB (2010). Stepping up melanocytes to the challenge of UV exposure. Pigment Cell Melanoma Res 23: 171–186. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando H, Kondoh H, Ichihashi M, Hearing VJ (2007). Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J Invest Dermatol 127: 751–761. [DOI] [PubMed] [Google Scholar]
- Bae UJ, Lee DY, Song MY, Lee SM, Park JW, Ryu JH et al (2011). A prenylated flavan from Broussonetia kazinoki prevents cytokine‐induced beta‐cell death through suppression of nuclear factor‐κB activity. Biol Pharm Bull 34: 1026–1031. [DOI] [PubMed] [Google Scholar]
- Baek SH, Lee SH (2015). Sesamol decreases melanin biosynthesis in melanocyte cells and zebrafish: possible involvement of MITF via the intracellular cAMP and p38/JNK signalling pathways. Exp Dermatol 24: 761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek YS, Ryu YB, Curtis‐Long MJ, Ha TJ, Rengasamy R, Yang MS et al (2009). Tyrosinase inhibitory effects of 1,3‐diphenylpropanes from Broussonetia kazinoki . Bioorg Med Chem 17: 35–41. [DOI] [PubMed] [Google Scholar]
- Bellei B, Maresca V, Flori E, Pitisci A, Larue L, Picardo M (2010). p38 regulates pigmentation via proteasomal degradation of tyrosinase. J Biol Chem 285: 7288–7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgdorff V, Rix U, Winter GE, Gridling M, Muller AC, Breitwieser FP et al (2014). A chemical biology approach identifies AMPK as a modulator of melanoma oncogene MITF. Oncogene 33: 2531–2539. [DOI] [PubMed] [Google Scholar]
- Cheli Y, Ohanna M, Ballotti R, Bertolotto C (2010). Fifteen‐year quest for microphthalmia‐associated transcription factor target genes. Pigment Cell Melanoma Res 23: 27–40. [DOI] [PubMed] [Google Scholar]
- Choi TY, Kim JH, Ko DH, Kim CH, Hwang JS, Ahn S et al (2007). Zebrafish as a new model for phenotype‐based screening of melanogenic regulatory compounds. Pigment Cell Res 20: 120–127. [DOI] [PubMed] [Google Scholar]
- del Marmol V, Beermann F (1996). Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett 381: 165–168. [DOI] [PubMed] [Google Scholar]
- Dessinioti C, Stratigos AJ, Rigopoulos D, Katsambas AD (2009). A review of genetic disorders of hypopigmentation: lessons learned from the biology of melanocytes. Exp Dermatol 18: 741–749. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Horsley V (2008). More than one way to skin. Genes Dev 22: 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillbro JM, Olsson MJ (2011). The melanogenesis and mechanisms of skin‐lightening agents—existing and new approaches. Int J Cosmet Sci 33: 210–221. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ashford ML (2014). AMPK: regulating energy balance at the cellular and whole body levels. Physiology (Bethesda) 29: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS guide to pharmacology in 2018: updates and expansion to encompass the new guide to immunopharmacology. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horike N, Sakoda H, Kushiyama A, Ono H, Fujishiro M, Kamata H et al (2008). AMP‐activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3β and thereby reduces cAMP‐responsive element transcriptional activity and phosphoenolpyruvate carboxykinase C gene expression in the liver. J Biol Chem 283: 33902–33910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Arnheiter H, Pavan WJ (2006). Interspecies difference in the regulation of melanocyte development by SOX10 and MITF. Proc Natl Acad Sci U S A 103: 9081–9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RL, Barre LK, Wood SD, Anderson KA, Kemp BE, Means AR et al (2006). Regulation of AMP‐activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J Biol Chem 281: 36662–36672. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Lee BM (2007). Inhibitory effects of plant extracts on tyrosinase, L‐DOPA oxidation, and melanin synthesis. J Toxicol Environ Health A 70: 393–407. [DOI] [PubMed] [Google Scholar]
- Jin ML, Park SY, Kim YH, Park G, Son HJ, Lee SJ (2012). Suppression of α‐MSH and IBMX‐induced melanogenesis by cordycepin via inhibition of CREB and MITF, and activation of PI3K/Akt and ERK‐dependent mechanisms. Int J Mol Med 29: 119–124. [DOI] [PubMed] [Google Scholar]
- Kaufman CK, Mosimann C, Fan ZP, Yang S, Thomas AJ, Ablain J et al (2016). A zebrafish melanoma model reveals emergence of neural crest identity during melanoma initiation. Science 351: aad2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsh RN, Harris ML, Colanesi S, Erickson CA (2009). Stripes and belly‐spots: a review of pigment cell morphogenesis in vertebrates. Semin Cell Dev Biol 20: 90–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Yang Y, Lee MS, Yoo YD, Lee HG, Lim JS (2008). NDRG2 gene expression in B16F10 melanoma cells restrains melanogenesis via inhibition of Mitf expression. Pigment Cell Melanoma Res 21: 653–664. [DOI] [PubMed] [Google Scholar]
- Kim AY, Lee CG, Lee DY, Li H, Jeon R, Ryu JH et al (2012). Enhanced antioxidant effect of prenylated polyphenols as Fyn inhibitor. Free Radic Biol Med 53: 1198–1208. [DOI] [PubMed] [Google Scholar]
- Kim HS, Lim J, Lee DY, Ryu JH, Lim JS (2015). Kazinol C from Broussonetia kazinoki activates AMP‐activated protein kinase to induce antitumorigenic effects in HT‐29 colon cancer cells. Oncol Rep 33: 223–229. [DOI] [PubMed] [Google Scholar]
- Kim I, He YY (2013). Targeting the AMP‐activated protein kinase for cancer prevention and therapy. Front Oncol 3: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Imokawa G, Bennett DC, Hearing VJ (1998). Tyrosinase stabilization by Tyrp1 (the brown locus protein). J Biol Chem 273: 31801–31805. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Urabe K, Winder A, Tsukamoto K, Brewington T, Imokawa G et al (1994). DHICA oxidase activity of TRP1 and interactions with other melanogenic enzymes. Pigment Cell Res 7: 227–234. [DOI] [PubMed] [Google Scholar]
- Kono M, Dunn IS, Durda PJ, Butera D, Rose LB, Haggerty TJ et al (2006). Role of the mitogen‐activated protein kinase signaling pathway in the regulation of human melanocytic antigen expression. Mol Cancer Res 4: 779–792. [DOI] [PubMed] [Google Scholar]
- Koo JH, Lee I, Yun SK, Kim HU, Park BH, Park JW (2010). Saponified sunflower and safflower oils inhibit melanogenesis in B16 melanoma cells. Mol Med Rep 3: 281–285. [DOI] [PubMed] [Google Scholar]
- Le Guellec D, Morvan‐Dubois G, Sire JY (2004). Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio). Int J Dev Biol 48: 217–231. [DOI] [PubMed] [Google Scholar]
- Lee DY, Kim DH, Lee HJ, Lee Y, Ryu KH, Jung BI et al (2010). New estrogenic compounds isolated from Broussonetia kazinoki . Bioorg Med Chem Lett 20: 3764–3767. [DOI] [PubMed] [Google Scholar]
- Lehraiki A, Abbe P, Cerezo M, Rouaud F, Regazzetti C, Chignon‐Sicard B et al (2014). Inhibition of melanogenesis by the antidiabetic metformin. J Invest Dermatol 134: 2589–2597. [DOI] [PubMed] [Google Scholar]
- Lim J, Nam S, Li H, Yang Y, Lee MS, Lee HG et al (2016). Antimelanogenic effect of 4‐hydroxylonchocarpin through the inhibition of tyrosinase‐related proteins and MAPK phosphatase. Exp Dermatol 25: 574–576. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Fisher DE (2010). Lighting a path to pigmentation: mechanisms of MITF induction by UV. Pigment Cell Melanoma Res 23: 741–745. [DOI] [PubMed] [Google Scholar]
- Malafoglia V, Bryant B, Raffaeli W, Giordano A, Bellipanni G (2013). The zebrafish as a model for nociception studies. J Cell Physiol 228: 1956–1966. [DOI] [PubMed] [Google Scholar]
- Manga P, Sato K, Ye L, Beermann F, Lamoreux ML, Orlow SJ (2000). Mutational analysis of the modulation of tyrosinase by tyrosinase‐related proteins 1 and 2 in vitro. Pigment Cell Res 13: 364–374. [DOI] [PubMed] [Google Scholar]
- Mihaylova MM, Shaw RJ (2011). The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13: 1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort RL, Jackson IJ, Patton EE (2015). The melanocyte lineage in development and disease. Development 142: 1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Gilchrest BA (1999). Signaling pathways mediating melanogenesis. Cell Mol Biol (Noisy‐le‐Grand) 45: 919–930. [PubMed] [Google Scholar]
- Park J, Chung H, Bang SH, Han AR, Seo EK, Chang SE et al (2015). (E)‐4‐(3,4‐Dimethoxyphenyl)but‐3‐en‐1‐ol enhances melanogenesis through increasing upstream stimulating factor‐1‐mediated tyrosinase expression. PLoS One 10: e0141988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez S, Kang M, Chung HS, Cho C, Hong MC, Shin MK et al (2006). Survey and mechanism of skin depigmenting and lightening agents. Phytother Res 20: 921–934. [DOI] [PubMed] [Google Scholar]
- Pillaiyar T, Manickam M, Jung SH (2015). Inhibitors of melanogenesis: a patent review (2009–2014). Expert Opin Ther Pat 25: 775–788. [DOI] [PubMed] [Google Scholar]
- Pylayeva‐Gupta Y, Grabocka E, Bar‐Sagi D (2011). RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer 11: 761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AM, Candau RB, Csibi A, Pagano AF, Raibon A, Bernardi H (2012). The role of AMP‐activated protein kinase in the coordination of skeletal muscle turnover and energy homeostasis. Am J Physiol Cell Physiol 303: C475–C485. [DOI] [PubMed] [Google Scholar]
- Simon JD, Peles D, Wakamatsu K, Ito S (2009). Current challenges in understanding melanogenesis: bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res 22: 563–579. [DOI] [PubMed] [Google Scholar]
- Singh SK, Sarkar C, Mallick S, Saha B, Bera R, Bhadra R (2005). Human placental lipid induces melanogenesis through p38 MAPK in B16F10 mouse melanoma. Pigment Cell Res 18: 113–121. [DOI] [PubMed] [Google Scholar]
- Slominski A, Tobin DJ, Shibahara S, Wortsman J (2004). Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 84: 1155–1228. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Luger T, Paus R, Solomon S (2000). Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev 80: 979–1020. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zmijewski MA, Pawelek J (2012). L‐tyrosine and L‐dihydroxyphenylalanine as hormone‐like regulators of melanocyte functions. Pigment Cell Melanoma Res 25: 14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski RM, Zmijewski MA, Slominski AT (2015). The role of melanin pigment in melanoma. Exp Dermatol 24: 258–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Kohno M, Niwano Y (2014). Alleviation effect of arbutin on oxidative stress generated through tyrosinase reaction with L‐tyrosine and L‐DOPA. BMC Biochem 15: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M (2007). The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th edn University of Oregon Press: Eugene, OR. [Google Scholar]
- Widlund HR, Fisher DE (2003). Microphthalamia‐associated transcription factor: a critical regulator of pigment cell development and survival. Oncogene 22: 3035–3041. [DOI] [PubMed] [Google Scholar]
- Wu SY, Wang HM, Wen YS, Liu W, Li PH, Chiu CC et al (2015). 4‐(Phenylsulfanyl)butan‐2‐one suppresses melanin synthesis and melanosome maturation in vitro and in vivo. Int J Mol Sci 16: 20240–20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi A, Kanbara T, Morinobu N (1987). Inhibition of mushroom‐tyrosinase by aloe extract. Planta Med 53: 515–517. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Hearing VJ, Maeda A, Morita A (2010). NADPH: quinone oxidoreductase‐1 as a new regulatory enzyme that increases melanin synthesis. J Invest Dermatol 130: 645–647. [DOI] [PubMed] [Google Scholar]
- Yasumoto K, Yokoyama K, Shibata K, Tomita Y, Shibahara S (1994). Microphthalmia‐associated transcription factor as a regulator for melanocyte‐specific transcription of the human tyrosinase gene. Mol Cell Biol 14: 8058–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PC, Wang S, Wu Y, Chen RY, Yu DQ (2001). Five new diprenylated flavonols from the leaves of Broussonetia kazinoki . J Nat Prod 64: 1206–1209. [DOI] [PubMed] [Google Scholar]
- Zhou J, Ren T, Li Y, Cheng A, Xie W, Xu L et al (2017). Oleoylethanolamide inhibits α‐melanocyte stimulating hormone‐stimulated melanogenesis via ERK, Akt and CREB signaling pathways in B16 melanoma cells. Oncotarget 8: 56868–56879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Effect of kazinol U on B16F10 and MNT‐1 cells viability in different concentrations.
Figure S2 Inhibition of tyrosinase protein level by kazinol U.
Figure S3 Inhibition of MITF expression by kazinol U in five different human melanoma cell lines.
Figure S4 Effect of kazinol U and arbutin on AMPK and JNK phosphorylation in M14, SK‐MEL‐2 and SK‐MEL‐28 cells.
Figure S5 Effect of kazinol U on compound C‐induced MITF in melanoma cells.
Figure S6 Effect of kazinol U on MAPKs in melanoma cells.
Figure S7 Effect of kazinol U in NHMs.
Figure S8 Inhibition of melanogenesis, but not melanin‐containing granule formation, in zebrafish by kazinol U.
Figure S9 The mechanistic scheme of anti‐melanogenic effects of kazinol U.
Table S1 Quantitative PCR primer sequences.


