To the Editor:
It is well-established that racial/ethnic minorities have a higher prevalence of diabetes and chronic kidney disease than white individuals.1 Racial/ethnic disparities in end-stage kidney disease are profound in the United States, with Hispanic individuals having 2-fold, and non-Hispanic black individuals (NHBs) having 3- to 4-fold greater risk as compared with non-Hispanic white individuals (NHWs).2, 3, 4 Multiple factors likely contribute to these disparities, including differential access to high-quality health care services, health behaviors (e.g., dietary patterns) and genetic factors, among others.5 Approximately 40% of persons with diabetes mellitus have diabetic kidney disease (DKD),6 rendering diabetes the leading cause of chronic kidney disease and end-stage kidney disease in the United States.4
Previous studies have investigated temporal trends in DKD prevalence in the US population overall.6, 7 These studies suggest a trend toward declining prevalence of albuminuria, more so among younger persons and NHWs with diabetes, and an overall increase in the prevalence of reduced estimated glomerular filtration rate.6, 7 The effect of these temporal trends on racial/ethnic disparities in DKD has not been fully explored, and could inform ongoing and future efforts to reduce disparities in kidney disease. Therefore, in the present study, we aimed to determine separately the prevalence of reduced estimated glomerular filtration rate, albuminuria, or both across 3 racial/ethnic groups (NHW, NHB, and Hispanic) over the past decade and examine changes in racial/ethnic disparities.
Results
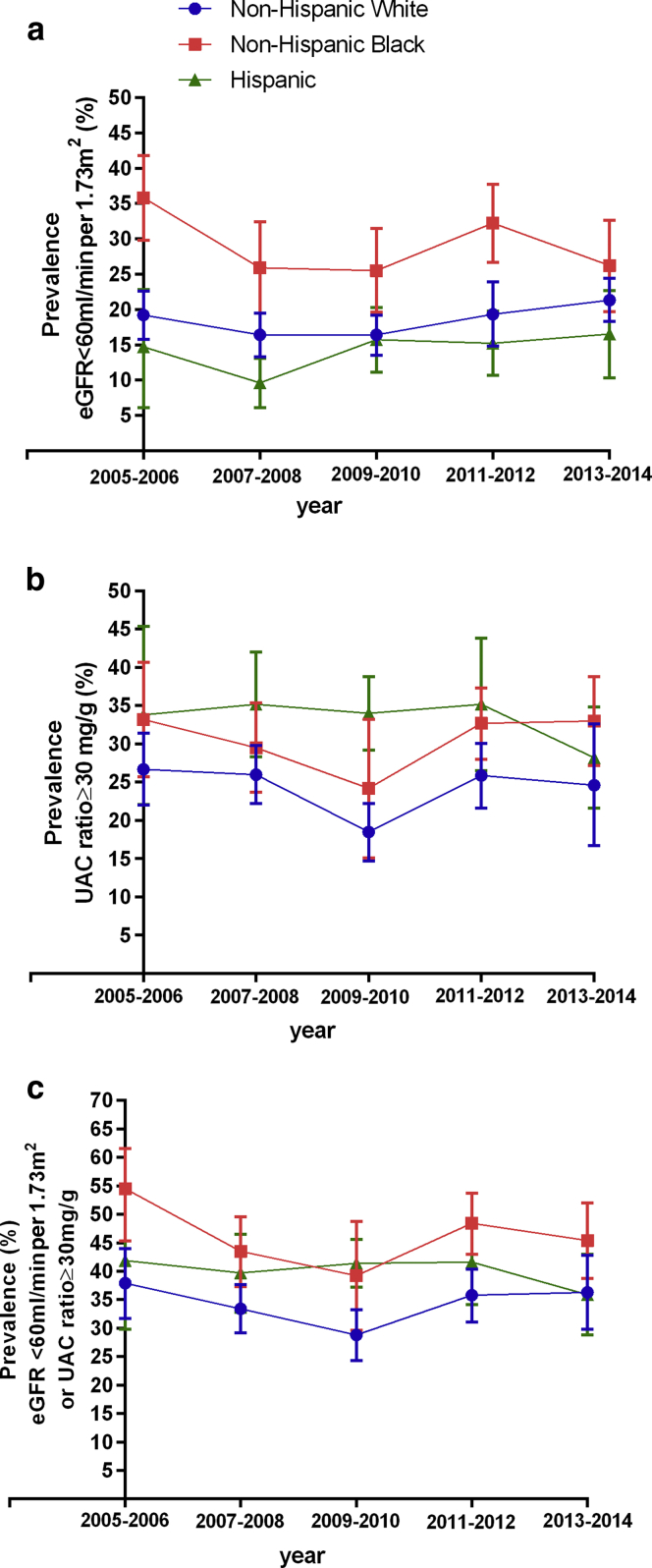
Our sample included 3874 persons with diabetes, representing 114,710,140 US adults. There were several sociodemographic and clinical differences between the racial/ethnic groups examined (Table 1). In 2005–2006, the prevalence of reduced kidney function was 19.2% (95% confidence interval [CI], 15.8–22.6) among NHWs, time trend P = 0.06; 35.8% (95% CI, 29.8–41.8) for NHBs, time trend P = 0.06; and 14.7% (95% CI, 6.6–22.7) for Hispanic individuals, time trend P = 0.21 (Figure 1a).
Table 1.
Sociodemographic and clinical characteristics of adults aged ≥18 years with diabetes, by race/ethnicity, NHANES 2005–2014
| NHANES survey |
|||||
|---|---|---|---|---|---|
| 2005–2006 |
2007–2008 |
2009–2010 |
2011–2012 |
2013–2014 |
|
| Total population survey | n = 608 | n = 986 | n = 807 | n = 748 | n = 725 |
| Non-Hispanic white (obs) | 240 | 416 | 329 | 215 | 275 |
| Age, yr | 61 ± 10 | 60 ± 12 | 62 ± 10 | 62 ± 11 | 61 ± 10 |
| Sex-female, % | 50.8 (44.0–57.8) | 45.4 (39.0–52.0) | 44.7 (37.7–51.8) | 46.3 (36.0–56.9) | 42.10 (35.8–48.6) |
| Education status | |||||
| < High school | 19.0 (14.6–24.3) | 21.8 (15.9–29.1) | 20.2 (15.3–26.2) | 19.0 (12.9–27.1) | 14.2 (10.3–19.2) |
| High school | 32.4 (23.4–43.0) | 32.0 (24.0–41.1) | 21.7 (15.1–30.3) | 28.7 (19.0–41.0) | 23.8 (19.8–28.4) |
| College graduate & above | 19.5 (13.0–28.1) | 19.2 (13.8–25.9) | 25.6 (19.1–33.4) | 23.4 (15.4–33.8) | 18.7 (13.4–25.3) |
| Physiological parameters | |||||
| BMI, kg/m2 | 33.0 ± 5.6 | 32.4 ± 5.8 | 32.9 ± 5.9 | 33.1 ± 5.2 | 33.7 ± 6.0 |
| Systolic BP, mm Hg | 134.9 ± 14.4 | 130.1 ± 15.5 | 126.8 ± 14.9 | 130.5 ± 12.9 | 129.4 ± 12.1 |
| Diastolic BP, mm Hg | 69.7 ± 8.8 | 69.1 ± 10.4 | 65.5 ± 10.6 | 69.4 ± 10.0 | 67.6 ± 11.1 |
| Fasting glucose, mg/dl | 146.7 ± 35.0 | 150.3 ± 51.6 | 140.9 ± 32.1 | 155.8 ± 34.9 | 159.7 ± 39.5 |
| Hemoglobin A1C, % | 6.8 ± 1.1 | 6.9 ± 1.2 | 7.0 ± 1.1 | 7.3 ± 1.1 | 7.4 ± 1.1 |
| Serum creatinine, mg/dl | 0.98 ± 0.20 | 0.96 ± 0.25 | 0.95 ± 0.24 | 0.96 ± 0.26 | 0.97 ± 0.25 |
| Urine ACR, mg/g | 98.5 ± 319.8 | 88.7 ± 414.2 | 58.9 ± 183.4 | 91.9 ± 270.6 | 130.4 ± 457.4 |
| eGFR, ml/min per 1.73 m2 | 76.9 ± 15.7 | 80.8 ± 18.6 | 79.5 ± 17.9 | 79.8 ± 17.2 | 79.9 ± 17.3 |
| Non-Hispanic black (obs) | 183 | 251 | 148 | 239 | 160 |
| Age, yr | 57 ± 17 | 55 ± 19 | 57 ± 17 | 56 ± 20 | 57 ± 16 |
| Sex-female, % | 52.5 (44.1–60.8) | 57.2 (50.5–63.6) | 54.8 (46.2–63.1) | 57.2 (50.1–64.0) | 50.5 (43.1–57.8) |
| Education status | |||||
| < High school | 36.9 (27.5–47.3) | 36.4 (31.7–41.2) | 30.2 (21.0–41.5) | 29.1 (21.6–37.9) | 22.9 (15.2–32.9) |
| High school | 31.3 (23.8–39.8) | 25.7 (21.7–30.2) | 26.1 (16.7–38.4) | 21.9 (17.4–27.1) | 31.7 (26.3–37.7) |
| College graduate & above | 9.6 (5.3–16.7) | 11.2 (7.7–15.9) | 6.3 (3.1–12.6) | 18.8 (12.9–26.3) | 18.4 (12.5–26.3) |
| Physiological parameters | |||||
| BMI, kg/m2 | 33.8 ± 11.0 | 34.6 ± 12.1 | 34.4 ± 10.7 | 34.7 ± 12.4 | 34.8 ± 11.0 |
| Systolic BP, mm Hg | 137.0 ± 27.0 | 129.7 ± 26.6 | 133.3 ± 24.1 | 134.1 ± 29.5 | 134.1 ± 22.3 |
| Diastolic BP, mm Hg | 70.7 ± 24.1 | 72.7 ± 19.6 | 71.5 ± 21.0 | 71.4 ± 21.9 | 71.4 ± 22.1 |
| Fasting glucose, mg/dl | 145.1 ± 70.5 | 175.4 ± 124.2 | 152.5 ± 66.8 | 163.6 ± 94.9 | 155.0 ± 95.1 |
| Hemoglobin A1C, % | 7.6 ± 2.3 | 7.5 ± 2.7 | 7.9 ± 2.8 | 8.0 ± 3.2 | 7.8 ± 2.6 |
| Serum creatinine, mg/dl | 1.08 ± 0.47 | 1.01 ± 0.51 | 1.00 ± 0.45 | 1.04 ± 0.63 | 1.05 ± 0.47 |
| Urine ACR, mg/g | 181.6 ± 1193.6 | 242.2 ± 1542.6 | 70.7 ± 343.5 | 124.5 ± 573.6 | 138.1 ± 540.3 |
| eGFR, ml/min per 1.73 m2 | 73.8 ± 29.2 | 80.4 ± 31.6 | 78.9 ± 29.8 | 77.4 ± 36.2 | 75.6 ± 28.5 |
| Hispanic (obs) | 185 | 319 | 330 | 294 | 290 |
| Age, yr | 53 ± 16 | 56 ± 18 | 55 ± 20 | 54 ± 16 | 55 ± 17 |
| Sex-female, % | 56.6 (48.1–64.9) | 50.9 (44.2–57.6) | 48.5 (40.8–56.2) | 47.8 (41.0–54.8) | 46.1 (42.2–50.0) |
| Education status | |||||
| < High school | 46.6 (32.8–61.0) | 47.5 (40.8–54.2) | 52.2 (44.9–59.5) | 45.1 (34.5–55.9) | 39.2 (33.1–45.6) |
| High school | 21.3 (16.5–27.0) | 20.7 (13.4–30.5) | 13.8 (9.7–19.1) | 20.1 (16.4–24.5) | 18.8 (13.1–26.2) |
| College graduate & above | 12.1 (4.9–26.8) | 10.8 (7.4–15.4) | 10.3 (7.1–14.9) | 13.3 (9.2–19.1) | 17.9 (13.0–24.3) |
| Physiological parameters | |||||
| BMI, kg/m2 | 30.1 ± 8.3 | 30.8 ± 9.1 | 31.5 ± 9.3 | 32.3 ± 8.0 | 30.8 ± 8.0 |
| Systolic BP, mm Hg | 127.2 ± 26.2 | 128.6 ± 27.3 | 129.7 ± 20.8 | 127.3 ± 20.8 | 127.3 ± 21.0 |
| Diastolic BP, mm Hg | 71.5 ± 13.0 | 70.1 ± 19.4 | 68.5 ± 20.1 | 70.8 ± 14.4 | 70.7 ± 15.5 |
| Fasting glucose, mg/dl | 178.1 ± 97.8 | 160.1 ± 94.7 | 162.7 ± 85.3 | 159.3 ± 69.3 | 163.4 ± 78.1 |
| Hemoglobin A1C, % | 7.6 ± 2.5 | 7.3 ± 2.4 | 7.8 ± 2.5 | 7.9 ± 2.1 | 8.0 ± 2.5 |
| Serum creatinine, mg/dl | 0.87 ± 0.35 | 0.81 ± 0.38 | 0.84 ± 0.44 | 0.83 ± 0.30 | 0.88 ± 0.45 |
| Urine ACR, mg/g | 156.6 ± 698.3 | 196.2 ± 918.4 | 177.3 ± 1352.3 | 89.3 ± 380.3 | 204.5 ± 1083.4 |
| eGFR, ml/min per 1.73 m2 | 90.0 ± 25.3 | 93.0 ± 29.7 | 92.3 ± 33.6 | 93.4 ± 26.0 | 90.4 ± 28.8 |
Data are expressed in weighted means ± SD and proportions ± SD.
BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; NHANES, National Health and Nutrition Examination Survey; obs, observations; Urine ACR, urine albumin to creatinine ratio.
Figure 1.
Race/ethnicity trends in chronic kidney disease among US adults with diabetes. The prevalence of reduced eGFR adjusted for age and sex (a), the prevalence of albuminuria adjusted for age and sex (b), and the prevalence of reduced eGFR or albuminuria adjusted for age and sex (c). eGFR, estimated glomerular filtration rate; UAC ratio, urine albumin-to-creatinine ratio.
Despite there being no statistically significant changes in the prevalence of reduced kidney function for any racial/ethnic group over the period examined, we observed opposite, although statistically insignificant, trends in the prevalence of reduced kidney function among NHWs (with increasing prevalence) and NHBs (with decreasing prevalence) (P = 0.06 for both). Thus, disparities in prevalence of reduced kidney function in NHBs compared with NHWs decreased between 2005 and 2006 (16.6%; 95% CI, 8.8–24.3; P < 0.001) and 2013 and 2014 (4.8%; 95% CI, −3.0 to 12.83; P = 0.23). The difference in prevalence of reduced kidney function between Hispanic and NHW individuals varied little from 2005 to 2006 (−4.5%; 95% CI, −14.1 to 5.11; P = 0.35) and 2013 to 2014 (−4.8%; 95% CI, −11.54 to 1.91; P = 0.16). Therefore, the disparities in prevalence of reduced kidney function overtime from 2005 to 2014 between NHB or Hispanic individuals compared with NHWs varied little, respectively, P = 0.35 and P = 0.48 (Figure 1a).
In 2005–2006, the prevalence of albuminuria was 26.7% (95% CI, 22.0–31.4) among NHWs, time trend P = 0.16; 33.1% (95% CI, 25.7–40.67) among NHBs, time trend P = 0.52 and 33.8% (95% CI, 22.1–45.8) among Hispanic individuals, time trend P = 0.62. (Figure 1b). There were no statistically significant differences in the prevalence of albuminuria in NHBs compared with NHWs in 2005–2006 (6.4%; 95% CI, −2.3 to 15.2; P = 0.14); however, in 2013–2014, NHBs had significantly greater prevalence of albuminuria (8.4%; 95% CI, 1.2–15.5; P = 0.02). The prevalence of albuminuria in Hispanic compared with NHW individuals was similar in 2005–2006 (P = 0.23) and 2013–2014 (P = 0.49). Disparities in prevalence of albuminuria over time (from 2005–2014) between NHB or Hispanic individuals compared with NHWs were stable, P = 0.91 and 0.36, respectively.
In 2005–2006, the prevalence of either reduced kidney function or albuminuria was 37.9% (95% CI, 31.7–44.0) among NHWs, time trend P = 0.14, 53.5% (95% CI, 45.3–61.6) among NHBs, time trend P = 0.17; and 41.9% (95% CI, 29.8–54.0) among Hispanic individuals, time trend P = 0.75 (Figure 1c). Disparities in prevalence of either reduced kidney function or albuminuria in NHB compared with NHW individuals were 15.6% (95% CI, 6.1–25.1); P = 0.001 in 2005–2006 and 9.1% (95% CI, 15.7–16.6); P = 0.02 in 2013–2014. The prevalence of reduced kidney function or albuminuria among Hispanic compared with NHW individuals was similar in 2005–2006 (P = 0.56) and 2013–2014 (P = 0.93). Disparities in prevalence of reduced kidney function or albuminuria over time (from 2005–2014) between NHB or Hispanic individuals compared with NHWs were stable, P = 0.85 and P = 0.26, respectively.
Discussion
We found that the overall prevalence of DKD (defined by reduced kidney function or albuminuria) among each of the 3 racial/ethnic groups examined remained similar during most of the past decade, which is consistent with previous studies reporting the prevalence of DKD in the US population overall.6, 7 Our study extends these studies by noting that disparities in DKD prevalence comparing NHB and Hispanic individuals with NHWs have persisted over the past decade. Notably, however, at different time points through the decade examined, there were some declining trends in the NHB versus NHW disparity in reduced kidney function. This finding should be considered in the context of previous reports of increasing prevalence of reduced kidney function in the general US population of persons with diabetes,6, 7 which may have been due to increasing prevalence among NHWs.
A key limitation of our study was the lack of sufficient data in the National Health and Nutrition Examination Survey to allow for analyses of other racial/ethnic groups, such as American Indian and Asian individuals. Similarly, we were not able to examine subpopulations of Hispanic individuals, which may share genetic and social risks similar to NHBs (e.g., black Hispanic individuals).
The underlying reasons for the persistence of racial/ethnic disparities in DKD are likely multifactorial, to include constitutional factors (e.g., genetic background), health behaviors (e.g., dietary patterns) and socially determined factors (e.g., access to high-quality health care). Further exploration of these findings could identify opportunities to narrow disparities in DKD prevalence. Strategies that have proven successful in decreasing rates of end-stage kidney disease among minority populations with diabetes include the program of the Indian Health Service and the Centers for Disease Control and Prevention, which used a population health approach to treating kidney disease, in the context of routine diabetes care.8 Whether such approaches could improve DKD outcomes among other racial/ethnic minority groups, such as NHB and Hispanic individuals, should be explored.
Disclosure
All the authors declared no competing interests.
Acknowledgments
DCC was supported by grant K23DK097184 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland. RSA was supported by the Bloomberg Professorship in Diabetes at Johns Hopkins University.
Author contributions: Study concept and design: JPD, DCC, RSA; Acquisition, analysis, or interpretation of data: JPD, MS, DCC; Drafting of the manuscript: JPD; Critical revision of the manuscript for important intellectual content: all authors; Statistical Analysis: JPD, MS; Administrative, technical, or material support: SHG, RSA; Study supervision: RSA, DCC.
Footnotes
Supplementary Methods.
Supplementary material is linked to the online version of the paper at www.kireports.org/.
Supplementary Material
References
- 1.Spanakis E.K., Golden S.H. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13:814–823. doi: 10.1007/s11892-013-0421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClellan W., Warnock D.G., McClure L. Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol. 2006;17:1710–1715. doi: 10.1681/ASN.2005111200. [DOI] [PubMed] [Google Scholar]
- 3.Ricardo A.C., Flessner M.F., Eckfeldt J.H. Prevalence and correlates of CKD in Hispanics/Latinos in the United States. Clin J Am Soc Nephrol. 2015;10:1757–1766. doi: 10.2215/CJN.02020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saran R., Robinson B., Abbott K.C. US Renal Data System 2017 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71:A7. doi: 10.1053/j.ajkd.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholas S.B., Kalantar-Zadeh K., Norris K.C. Racial disparities in kidney disease outcomes. Semin Nephrol. 2013;33:409–415. doi: 10.1016/j.semnephrol.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afkarian M., Zelnick L.R., Hall Y.N. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA. 2016;316:602–610. doi: 10.1001/jama.2016.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer I.H., Rue T.C., Hall Y.N. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narva A. Population health for CKD and diabetes: lessons from the Indian Health Service. Am J Kidney Dis. 2018;71:407–411. doi: 10.1053/j.ajkd.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.