Abstract
Diabetic kidney disease (DKD) is a complex and multifactorial disorder associated with deregulations in a large number of different biological pathways on the molecular level. Using the 2 established biomarkers, estimated glomerular filtration rate (eGFR) and albuminuria will not allow allocating patients to tailored therapy. Molecular multimarker panels as sensors for the deregulation of the various disease mechanisms combined with a better understanding of how investigational as well as approved drugs interfere with these disease processes forms the basis for platform trials in DKD. In these platform trials, patients with DKD are assigned to the most suitable treatment arm based on their molecular marker profile. Close monitoring of biomarkers after treatment initiation together with assessment of renal function and “off-target” effects will allow identification of therapy responders, with nonresponders shifted to the next-best treatment arm based on their molecular profile. In this viewpoint article, we summarize evidence on the variation of DKD disease progression as well as the response to therapy and outline procedures to model disease pathophysiology supporting biomarker panel construction. Finally, the use of biomarkers in clinical trial setup is discussed.
Keywords: biomarker panel, clinical trial design, diabetic kidney disease, pathophysiology, predictive marker
Worldwide the number of patients with diabetes mellitus (DM) increased from 108 million in 1980 to 422 million in 2014. In 2012, 1.5 million deaths were directly caused by DM and another 2.2 million were attributable to high blood glucose. DM (and especially type 2 DM) will be the seventh leading cause of mortality by 2030.1 This epidemic increases the incidence and prevalence of DM-associated complications including DKD. DKD is especially common in specific populations, including the elderly and obese, those with onset of DM at a younger age, and certain ethnic and disadvantaged groups. Unfortunately, the increase in the prevalence of DM is most prominent in these high-risk individuals, and therefore, DKD will affect approximately 50% of patients with type 2 DM.2
The presence, progression, and severity of DKD markedly influence the prognosis. In the Third National Health and Nutrition Examination Survey, the 10-year adjusted cardiovascular mortality in subjects with DKD was 6.1% compared with 3.0% in subjects with DM and normal renal function.3 Paradoxically, the recent improvements in cardiovascular survival will allow more patients to develop renal impairment.4, 5
The aim of this viewpoint article was to summarize evidence on the variation of DKD disease progression and the variation of therapy response. We furthermore outline ways of modeling DKD disease pathophysiology supporting biomarker identification and biomarker panel construction. We finally discuss the use of biomarkers in clinical trial setup.
DKD: Variation in Disease Progression
In 1996 Nelson et al.6 serially assessed glomerular function over a period of 4 years in 194 Pima Indians, a population with an exceptionally high incidence of type 2 DM and DKD. The glomerular filtration rate (GFR) was elevated as long as normo- or micro-albuminuria was present, but declined once higher urinary albumin loss developed. This sequence of events resembled the one described earlier in patients with type 1 DM.7 “Diabetic nephropathy” was therefore considered to be driven by a uniform pathophysiology (mainly metabolic and hemodynamic disturbances) following a predictable clinical course that could be reliably captured by the 2 biomarkers albuminuria and GFR.
This paradigm was maintained for several years, although some studies supported a different view. Renal biopsies obtained from 30 patients with type 2 DM, microalbuminuria, and preserved GFR were analyzed by Fioretto et al.8 Despite this seemingly “homogeneous” (laboratory) phenotype, only one-third of the study population showed histological lesions typical for “diabetic nephropathy,” whereas two-thirds either had no or atypical findings under light microscopy.8 Some years later it became evident that the sequence of laboratory changes during the progression of renal disease varies, again questioning the concept of a uniform pathophysiology. During a 15-year follow-up period, 38% of the participants of the UK Prospective Diabetes Study developed micro- or macroalbuminuria and 29% chronic kidney disease (defined by a creatinine clearance below 60 ml/min). However 51% of the latter never had increased albuminuria.9 Further support suggesting that the course of DKD is variable comes from studies showing that the slope of eGFR decline is extremely diverse. In the placebo group of the Irbesartan Diabetic Nephropathy Trial, the creatinine clearance decreased on average by 6.5 ml/min per 1.73 m2 per year with an SD of 8.8 ml/min per 1.73 m2 and a coefficient of variation of 135%.10 This variability in the renal disease progression occurred despite strict baseline inclusion and exclusion criteria (especially regarding the accepted range of albuminuria and GFR), which aimed to form a “homogeneous” study population.
In summary, during the past 2 decades, our view on renal disease in patients with DM has changed. As renal biopsies are still rarely performed, it is mostly clinical observations supporting the concept that the pathophysiology driving the incidence and progression of DKD is not uniform in all patients and at all stages.
DKD: Variation in Drug Response
Another line of evidence for heterogeneity of DKD comes from interventional trials. In general intensified metabolic and/or blood pressure control, the latter, especially if achieved by the use of agents that block the activity of the renin angiotensin aldosterone system (RAAS), have been proven effective in interventional studies decreasing the incidence and slowing the progression of DKD.10, 11, 12, 13, 14 Novel therapeutic strategies like sodium-glucose cotransporter 2 inhibition or glucagon-like peptide 1 receptor agonist therapy may even have beneficial effects beyond blood glucose control and/or blood pressure lowering.15, 16, 17, 18 All of these large interventional outcome trials nevertheless focus on mean differences between the studied patient groups. A considerable interindividual variability in the response to all investigated interventions is, however, observable. In the Irbesartan Diabetic Nephropathy Trial, for example, 1715 hypertensive patients with type 2 DM with proteinuria levels >900 mg per day and serum creatinine concentrations between 1 and 3 mg/dl were randomly assigned to treatment with either 300 mg irbesartan, 10 mg amlodipine, or placebo.10 During a mean follow-up of 2.6 years, the primary endpoint, a composite of doubling of serum creatinine, end-stage renal disease, or death from any cause, occurred in 32.6% and 41.1% of irbesartan- and amlodipine-treated patients, respectively, as compared with 39% in the placebo group. Obviously angiotensin receptor blocker (ARB) therapy was superior to calcium antagonist or placebo treatment, but it was by far not effective in all participants, as reflected by the high residual risk despite irbesartan treatment. Subsequent analyses from the Irbesartan Diabetic Nephropathy Trial study demonstrated that individuals with the largest reduction in albuminuria during the first month of therapy showed the greatest renal risk reduction during subsequent follow-up.19 Conversely, renal risk did not decrease among 26% of patients who did not experience a reduction in albuminuria during irbesartan treatment.
Next to interindividual variability in treatment response at a specific point, the same has been reported intraindividually over time. Kröpelin et al.20 analyzed the albuminuria-lowering effect of ARBs in the Reduction of Endpoints in NIDDM [non–insulin-dependent DM] with the Angiotensin II Antagonist Losartan trial and the Irbesartan Diabetic Nephropathy Trial. Following therapy initiation, 36.3% of the patients showed a reduction in albuminuria >30% during the first 3 months. Among these, albuminuria further decreased in 44.8%, remained stable in 31.7%, but increased in 23.5%. Similar albuminuria fluctuations were observed in subjects with an initial albuminuria reduction of <30%. These studies suggest indirectly that the pathophysiology underlying a DKD phenotype is different between patients and changes within an individual over time.
In addition to the variation in response in the intended or on-target risk marker, it has been shown that many drugs affect multiple other renal or cardiovascular risk markers than the one intended (i.e., an ARB may affect hemoglobin, serum potassium, or uric acid) and individual patients again show a large variation in responses in these off-target/unintended risk markers.21 The interindividual variability in drug responses in off-target risk markers may also have played a role in recent clinical trials that were stopped early due to unexpected severe side effects. The BEACON (Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes) trial aimed to characterize the long-term renal efficacy and safety of the anti-inflammatory antioxidant agent bardoxolone methyl. The trial was stopped early due to excess edema, heart failure, and mortality in the bardoxolone methyl treatment arm, allegedly due to the sodium-retaining effects of the drug. A post hoc analysis identified a subgroup of patients with brain natriuretic peptide >200 pg/ml or previous heart failure that did not tolerate the drug and experienced heart failure. Thus, the variation in response is not only present in the on-target but also in the off-target risk markers.
To complicate things even more, a patient with a response in the on-target risk marker may not necessarily exhibit a response in the off-target marker. Thus, one individual may experience a reduction in blood pressure and albuminuria during ARB treatment while another experiences a blood pressure reduction but no albuminuria reduction or vice versa. These variations in response in on-target, off-target risk markers that are even time-dependent underscore the marked heterogeneity of DKD. From a practical clinical perspective, these findings highlight the need to strictly monitor the individual’s response to multiple risk markers over time to determine which patients will benefit most from treatment.
Determinants of Variation in Response
Variability in drug response in DKD can be attributed to different factors. First, therapy adherence is an obvious and important determinant. Patients with DKD can be prescribed more than 20 pills.22 Such a high pill burden is associated with a lower therapy adherence and subsequently poorer individual drug response. Individual variation in drug absorption, distribution, metabolism, or excretion is another factor that may explain why patients respond differently. As an example, a study with the endothelin receptor antagonist atrasentan found that Asian patients showed, in general, a larger reduction of albuminuria than North American patients. This difference between the populations was explained by the higher exposure to atrasentan in Asian patients due to genetic differences in drug metabolism. Environmental factors can also play a role. Dietary habits, such as sodium and phosphate intake, have been shown to influence the response to RAAS inhibition.23, 24, 25 Finally, the underlying disease pathophysiology of DKD is a key determinant of the individual response. For example, increased RAAS activity is observed in some but not all patients with DKD and associated with a higher rate of renal function decline. The insertion/deletion polymorphism of the angiotensin-converting enzyme gene influences the systemic and renal activity of the RAAS and has been implicated in the progression of renal and cardiovascular disease. A prespecified analysis of the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan trial demonstrated that the DD angiotensin-converting enzyme polymorphism is associated with a higher risk of end-stage renal disease and, interestingly, a better response to losartan compared with the insertion/deletion or II genotype.26 These findings were replicated in an independent study of the Ramipril Efficacy in Nephropathy trial in nondiabetic kidney disease and illustrate that underlying disease pathophysiology, reflected here by the RAAS activity, determines disease progression and response to treatment.27
Biomarkers in Clinical Trial Designs
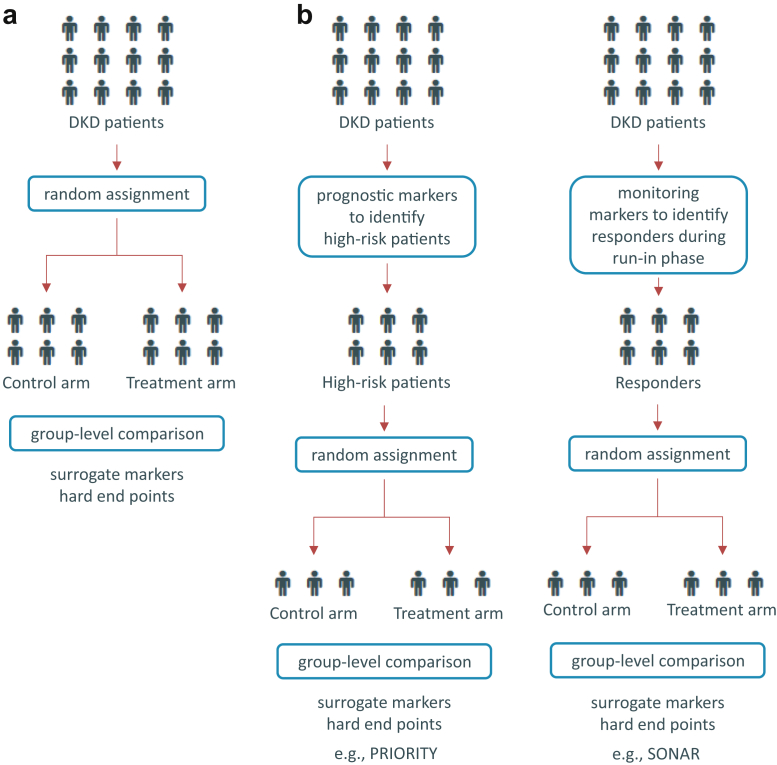
In the classical design of clinical trials, patients are randomly assigned to either a treatment or placebo group (Figure 1a). In situations of a variable and prolonged clinical course (like DKD), an a priori selection of patients based on risk (enrichment) is useful to reduce the necessary sample size and/or follow-up period (Figure 1b). The incorporation of molecular biomarkers to improve the clinical trial design by enriching the population for patients with higher risk or more likely to respond to the new intervention has been applied for quite some time now in oncology research and is gaining momentum also in chronic disease areas, including the cardiovascular and renal field.
Figure 1.
Clinical trial designs in diabetic kidney disease (DKD). (a) The classical trial setup is depicted where DKD patients are randomly assigned to either the treatment or the placebo control arm. (b) Two different marker enrichment trial setups are displayed in which high-risk patients with DKD are either identified by prognostic markers or responders are identified in a run-in phase with a later assignment to either the treatment or placebo control arm. PRIORITY, proteomic prediction and renin angiotensin aldosterone system inhibition prevention of early diabetic nephropathy in type 2 diabetic patients with normoalbuminuria; SONAR, study of diabetic nephropathy with the endothelin receptor antagonist atrasentan.
The PRIORITY (Proteomic prediction and renin angiotensin aldosterone system inhibition prevention of early diabetic nephropathy in type 2 diabetic patients with normoalbuminuria) study applies a risk enrichment strategy making use of a proteomics marker panel to identify subjects with DM at risk of progression from normo- to microalbuminuria.28 This capillary electrophoresis-mass spectrometry–based urinary multimarker classifier was developed in a cross-sectional study in patient groups with varying etiologies of chronic kidney disease.29 In PRIORITY, those patients identified by the proteomics panel being at high risk are randomly assigned to either receive spironolactone or placebo (Figure 1b). The trial is currently still ongoing and results of this marker-based enrichment strategy trial are expected in 2019.
A disadvantage of a risk enrichment strategy that solely focuses on the prognostic aspect is the fact that it is unknown if the biomarker-enriched population will actually respond to the investigational treatment. Clinical trial enrichment strategies enrolling populations that are more likely to respond to the new intervention is another approach. This type of enrichment approach was taken in the study of diabetic nephropathy with the endothelin receptor antagonist atrasentan (SONAR). After a first run-in phase to optimize RAAS blockade, the key element of SONAR was a subsequent enrichment period to separate atrasentan responders from nonresponders based on the level of reduction in urinary albumin-to-creatinine ratio (>30% decrease) (Figure 1b). In this case, albuminuria was not primarily used as a prognostic marker but rather to describe treatment efficacy, as the post hoc analyses from multiple trials indicate that the drug-induced change in albuminuria is an indicator of the patient’s responsiveness to the drug. Importantly, not only does the SONAR trial focus on the on-target risk marker, but it also integrates the individual’s response in off-target risk markers: patients with a rise in body weight or B-type natriuretic peptide level, as proxies for sodium retention, at the end of the enrichment period are excluded to ensure the safety of the intervention.30 The SONAR trial with its enrichment design focusing on the individual response to atrasentan may therefore be a precedent for future clinical trials in DKD. It should, however, be mentioned that the SONAR trial was in the meantime stopped early due to a lower event rate than expected. Unintended consequences of this new trial design may occur and the final results of the SONAR trial will establish the utility of this type of enrichment design.
Applications of Biomarkers in Other Areas of Medicine
The previously described enrichment strategies have already been applied for quite some time in other areas of medicine. In modern oncology, biomarkers are not only used to predict prognosis and monitor disease activity, but to assess individual pathophysiology of a tumor. For example, the antineoplastic antibody trastuzumab was found to be effective in patients with advanced gastric or gastroesophageal junction cancer with an upregulation of the HER2 receptor (human epidermal growth factor receptor 2).31 Notably, the protein marker HER2 is also the drug target and serves for identifying patients eligible for the respective therapy. Ideally, baseline predictive biomarkers also change during treatment (dynamic predictive biomarker), thus also allowing monitoring of treatment efficacy. In the BATTLE (Biomarker-integrated Approaches of Targeted Therapy for Lung Cancer Elimination) study, biomarker data on the genetic markers epidermal growth factor receptor and KRAS/BRAF, as well as expression status of vascular endothelial growth factor/vascular endothelial growth factor-2 and retinoid X receptors/Cyclin D1 in cancer tissue, were used to assign patients to 1 of 4 treatment arms (erlotinib, vandetanib, erlotinib-bexarotene, or sorafenib) to increase efficacy.32 The BATTLE study is the first completed prospective, adaptively randomized study that mandated tumor profiling with "real-time" biopsies, taking a substantial step toward realizing personalized lung cancer therapy by integrating real-time molecular laboratory findings in delineating specific patient populations for individualized treatment.
There are a couple of reasons why the number of marker-guided clinical trials in the area of oncology is much higher than in the area of chronic diseases. A set of fairly well-characterized mechanisms being critical for cancer development and growth have been described,33 and a number of therapies directly affecting targets in core biological processes are available. Nonetheless, other disease areas are also moving toward better personalizing therapies. Procalcitonin-guided antibiotic therapy improves the diagnostic and therapeutic management of patients presenting with respiratory illness,34 and in resistant hypertension, a metabolic marker panel linked to citric acid metabolism was reported to predict the response to spironolactone.35
Personalizing Medicine: Matching DKD Pathophysiology With Drug Mechanism of Action
The pathophysiology of DKD is multifactorial and the pathways involved in initiation and progression, confounded by the ones involved in comorbidities and drug response, constitute a wide and complex as well as redundant molecular network. Not all pathways that drive a specific phenotype must, however, be active in all patients at all stages of the disease as also discussed by McCarthy36 in the context of type 2 DM, which again would require frequent monitoring of the patients (ideally by noninvasive biomarkers) and adjustment of tailored therapy. Phases of tubulointerstitial inflammation, for example, might be followed by progressive fibrosis, both contributing to a drop of GFR but probably mandating different therapy. A panel of 2 biomarkers, one for inflammation and the other for fibrosis, could allow quantitative and/or qualitative upfront stratification of patients, thereby supporting therapeutic decision making. Even though this approach seems straightforward, one major obstacle is that it depends on the availability of a “complete” inventory of the pathophysiologically relevant molecular processes of DKD and identification of associated markers. Our capacity to analyze deregulations associated with a disease on a molecular level has increased dramatically with the widespread introduction of various “omics” techniques, and changes of the molecular phenotype have been described in DKD at the level of the genome, transcriptome, proteome, and metabolome.37
There are, however, challenges in implementing the various “omics” techniques in DKD, one being transforming the massive amount of data into meaningful context. Biological vocabularies, such as the gene ontology (GO) can be of help in this endeavor, with the Renal Gene Ontology Annotation Initiative specifically dedicated to renal development and disease.38 Proteins involved in renal development, function, and disease are annotated with information on (i) their respective molecular function(s) (i.e., the activities that they can directly perform), (ii) contribution to biological process(es), and (iii) subcellular location(s). Using this categorization, the proteins are allocated to specific GO terms, which are organized into a graph, each term being linked to 1 or more general “parent” terms and 1 or more specific “child” terms if applicable.
Using published data on deregulated proteins in DKD and the GO vocabulary, Heinzel et al.39 defined involved GO biological processes via enrichment analysis, thereby constructing a GO process model of DKD. Another approach uses molecular pathways rather than GO terms. Proteins are assigned to pathways, and the respective information can be retrieved from databases, like the Kyoto Encyclopedia of Genes and Genomes or Reactome, as well as consolidated efforts, like PathwayCommons, National Center for Biotechnology Information Biosystems, or GeneCards.39 Alternatively, one can use experimentally derived or computationally inferred information on protein-protein interactions to construct molecular network models informing on deregulated molecular processes in DKD.39, 40
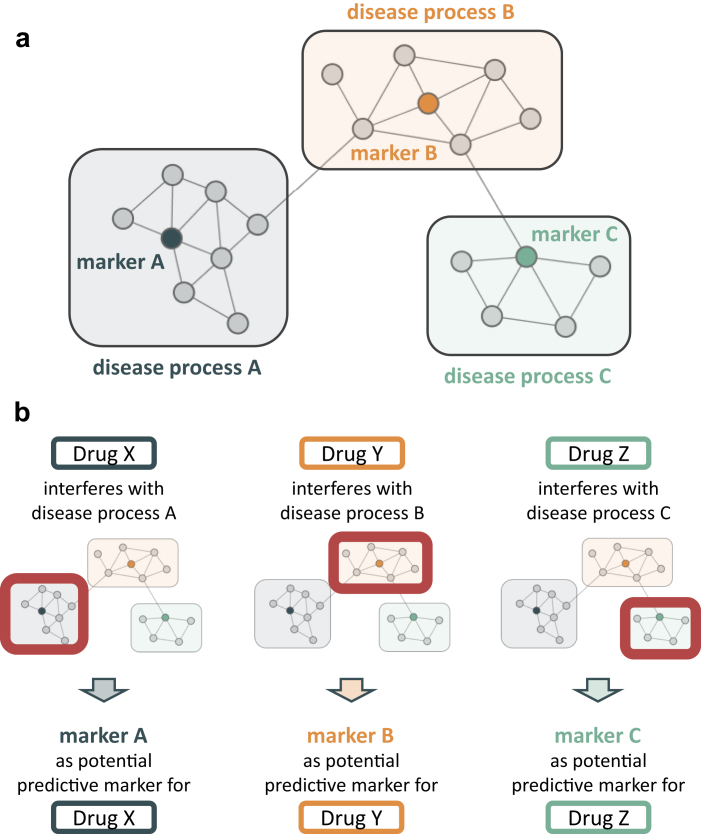
Irrespective of the modeling method used, genes/proteins are grouped to make the massive amount of information obtained from “omics” experiments accessible for interpretation. However, many input data used come from association studies and thus the relevance of groups (or processes) identified with regard to a specific phenotype (e.g., progression of disease) yet needs to be established. Therefore, in a next step, biomarkers can be chosen to represent each group/process and their potential to predict the event of interest can be tested in baseline samples of a prospective cohort or sometimes an interventional study. The various models of DKD currently available hold 20 to 40 processes. After complete characterization, some of them (and their associated biomarkers) will form a panel that summarizes “all” pathophysiologically relevant processes contributing to the phenotype of interest in the population. In case some processes are active only in some patients or at some specific stage of the disease, the biomarker panel will give positive results in only a fraction of the tested markers. Repetitive measurements will allow monitoring changes in the activity of relevant pathways/processes and ultimately guide combination/sequential therapy if the molecular drug mode of action can be adequately linked to the pathophysiologically relevant process(es). Figure 2 schematically depicts the delineation of predictive biomarkers via network interference analysis on the molecular level.
Figure 2.
Scheme for delineating predictive markers. (a) Schematic diabetic kidney disease (DKD) pathophysiology model. Multiple processes contribute to DKD development and progression. Representative markers serve as proxies for monitoring deregulation in certain processes. (b) Drugs affect the individual DKD disease processes in different ways and magnitudes. A representative biomarker of a disease process may qualify as predictive marker for selection of a targeted drug showing interference on a molecular level with the respective disease process.
A recent article41 used the molecular process model representation of DKD as described by Mayer et al.40 as well as Heinzel et al.39 In brief, large-scale -omics profiling was combined with scientific literature mining for deriving a set of 2466 protein coding genes (PCGs) deregulated in DKD. This feature set was mapped onto a hybrid protein interaction network holding more than 16,000 PCGs as nodes and more than 600,000 connecting edges reflecting their interactions. The resulting subgraph was further segmented into 34 internally highly connected clusters of PCGs using the Molecular Complex Detection method.42, 43 These processes covered 688 PCGs, the size of each ranging from 3 to 128 nodes. Sixteen markers that best represented the 4 largest processes holding in total 398 PCGs were selected and measured in a discovery study for their ability to predict a decline in eGFR.44 In a subsequent study using baseline serum samples of 1765 patients from 2 large clinical trials, an ultimate panel of 9 of these makers (CHI3L1, GH1, HGF, MMP2, MMP7, MMP8, MMP13, TIE2, TNFR1) was measured for validation. The explained variability of annual eGFR loss by the biomarkers indicated by the adjusted R2 was 15% and 34% for patients with ≥60 and <60 ml/min per 1.73 m2, respectively, and by clinical predictors 20% and 29%, respectively. A combination of molecular and clinical predictors increased the adjusted R2 to 35% and 64%, respectively.41
Identifying specific molecular processes associated with a specific phenotype of DKD and biomarkers associated with these processes, based on a molecular model of DKD, can be used to characterize the progression of patients based on individual pathophysiology. Matching the molecular mode of action of drug(s) to these specific molecular processes might allow selecting a specific drug or drug combinations that prevent or reverse deregulations in identified molecular pathways and thus guide therapy. This situation mirrors the one applied in infectious diseases, in which repetitively pathogens are identified and antimicrobial therapy is adjusted according to the results obtained. Matching a DKD disease progression model to a drug mechanism of action model was used in a study by Pena et al.45 A panel of serum metabolites being linked to molecular processes of inflammation and stress response, as well as downstream consequences of fibrosis and extracellular matrix rearrangement, was able to predict albuminuria response to ARBs in both type 1 and type 2 DM. This observation supports the concept that improved molecular characterization of drug effect and disease pathophysiology can predict treatment response.
Using Biomarker Panels to Monitor the Individual Drug Response
Using biomarker panels to match individual patients in whom specific molecular processes are deregulated and drive disease progression, with the molecular mode of action to reverse these pathological processes may lead to more targeted drug development and prescription. However, even when these novel drug-patient matches are identified, it remains important to monitor the individual response. Panels that capture the individual short-term response to an intervention on multiple biomarkers and integrate and translate this short-term individual response in a predicted effect on clinical outcomes are necessary. A recently developed response panel, the so-called multiple parameter response efficacy (PRE) score integrates multiple short-term drug effects on clinical parameters to predict the long-term drug effect on renal and cardiovascular outcomes. The PRE score is composed of clinical parameters that are measured in routine clinical care. Parameters that are used in the PRE score include, among others, urinary albumin-to-creatinine ratio, systolic blood pressure, hemoglobin, uric acid, potassium, and cholesterol levels. The clinical markers included in the PRE score have been associated with renal disease in type 2 DM and, importantly, changes in these markers in the short term (up to a few weeks) have been associated with renal and cardiovascular risk changes. For example, in patients with type 2 DM and nephropathy, short-term reductions in albuminuria during treatment with ARBs have been associated with reductions in the risk of end-stage renal disease in the long term.46, 47 Similarly, reductions in uric acid during treatment with losartan have been shown to be associated with reductions in risk for renal and cardiovascular endpoints.47, 48 Conversely, some drugs, such as renin-angiotensin-aldosterone-blockade and increases in potassium in the short term are associated with increased renal and cardiovascular risk.49 Because many drugs used for the management of DKD affect multiple cardiovascular risk markers, changes in these risk markers should be measured and integrated in a response panel (PRE score) to predict the effect of a drug on long-term clinical outcomes.
This response panel was originally developed in patients with DKD within the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan trial,14 and subsequently validated in several other clinical trials.21, 50, 51, 52 In all analyses so far, the score predicted at a population level the treatment effect on clinical endpoints during 3- to 4-year follow-up based on the short-term, 3 to 6 months, treatment effect on multiple biomarkers. Importantly, the score also improved predictions for individual patients who would benefit from ARB therapy.21
The previously mentioned studies that test the ability of the PRE score to predict cardio and renal events have shown that applying this multiple response score instead of using the response in 1 single marker improves the prediction of long-term drug effects on a population level as well as in individual patients. In clinical practice, such a multiple response score can be used as a tool for clinicians to predict long-term treatment effects more accurately, and accordingly adjust treatment strategies and/or monitoring frequency if necessary. It is important to emphasize that the current clinical parameters used in the PRE score are usually measured in routine clinical care and consist of standard physical and biochemical laboratory parameters. The advantage is that this approach may be implemented with relatively low extra costs and efforts. The disadvantage is that the effects of novel interventions that target deregulated molecular pathways in DKD, such as inflammation, oxidative stress, or fibrosis, are likely not predicted by the PRE score, as to date it does include only clinical biochemical parameters that probably do not predict effect of novel anti-inflammatory or antifibrotic agents. It is therefore likely that additional biomarkers (e.g., peptides or metabolites) are needed to fully capture the ultimate effect of these novel therapies on cardiovascular and renal outcome.
Conclusions
DKD disease initiation and progression is heterogeneous, and because of the different mechanisms involved, there is also variation in drug response. This variation can be between patients in the on-target parameters, but also in the off-target parameters. Furthermore, there can be variation in response within an individual within a panel of markers. This variation in response can be due to a number of factors, such as adherence; heterogeneity in drug absorption, metabolism, or excretion; environmental factors, such as dietary habits; and underlying pathophysiology of DKD. The large variation in drug response indicates that part of the treated population does not benefit or is even harmed by the prescribed drug.
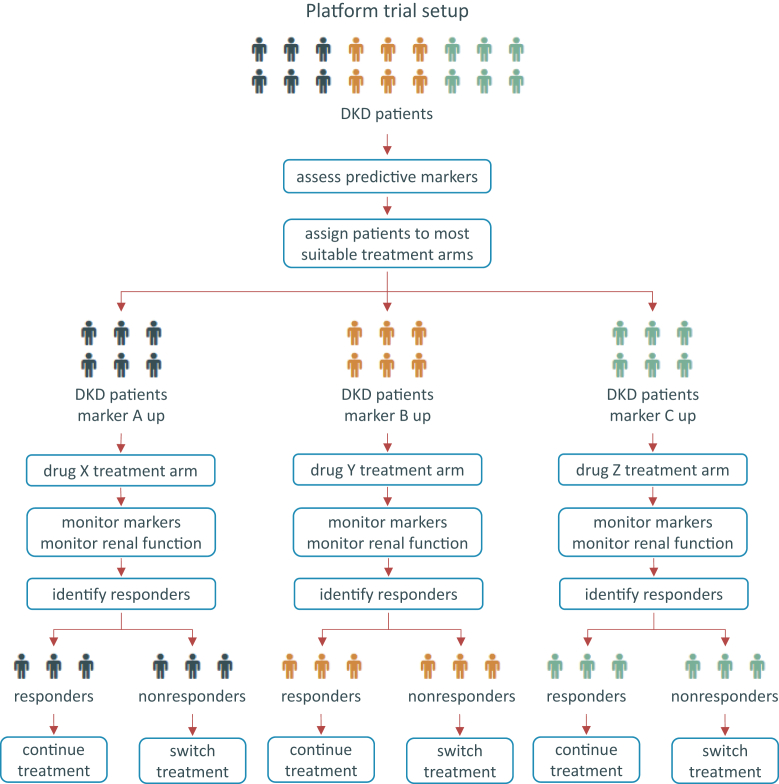
Biomarker panels can be used to minimize the response variability and the concomitant therapy resistance. Biomarker panels can help to identify patients at high risk in whom more intensified risk factor management is required, they can be used to identify populations more likely to respond to the prescribed drug, and, finally, they can be used to monitor the individual therapy response (Figure 3).
Figure 3.
Proposed platform trial design for diabetic kidney disease (DKD). Schematic representation of a platform trial design for DKD in which patients are assigned to a treatment arm based on concentration levels of a set of predictive markers for the available treatment options. Markers and renal function parameters are used for patient monitoring and identification of responders who remain in the assigned treatment arm, whereas nonresponders are shifted to the next-best suitable treatment based on marker profiles.
Despite the ever-increasing number of biomarker papers in DKD, the set of markers used in daily clinical practice is mainly limited to serum creatinine, eGFR, and albuminuria, mostly to predict prognosis and judge treatment efficacy. Novel omics methodologies are developed and are being used in other fields of medicine, such as oncology. The challenge for the future will be to broadly implement these techniques in the management of chronic diseases like DKD. Omics technologies enable the concerted cataloging of molecular processes being associated with DKD development and progression. Biomarker panels reflecting these molecular processes and disease pathophysiology will guide treatment selection. Biomarker panels that capture the short-term drug response after the drug is selected will then contribute in clinical decision making. The complementary use of biomarker panels integrated into systems medicine models makes implementation of personalized medicine into clinical practice more and more realistic.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We thank all consortium partners of the BEAt-DKD (Biomarker Enterprise to Attack DKD; www.beat-dkd.eu/) IMI2 project for fruitful discussions during project meetings.
This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No. 115974 (BEAt-DKD). This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation program and the EFPIA European Federation of Pharmaceutical Industries and Associations with the Juvenile Diabetes Research Foundation.
References
- 1.World Health Organization http://www.who.int/mediacentre/factsheets/fs312/en/ WHO diabetes key facts. Available at:
- 2.Thomas M.C., Cooper M.E., Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016;12:73–81. doi: 10.1038/nrneph.2015.173. [DOI] [PubMed] [Google Scholar]
- 3.Afkarian M., Sachs M.C., Kestenbaum B. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–308. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawshani A., Rawshani A., Franzén S. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376:1407–1418. doi: 10.1056/NEJMoa1608664. [DOI] [PubMed] [Google Scholar]
- 5.Gæde P., Oellgaard J., Carstensen B. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia. 2016;59:2298–2307. doi: 10.1007/s00125-016-4065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson R.G., Bennett P.H., Beck G.J. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Engl J Med. 1996;335:1636–1642. doi: 10.1056/NEJM199611283352203. [DOI] [PubMed] [Google Scholar]
- 7.Wajchenberg B.L., Sabbaga E., Fonseca J.A. The natural history of diabetic nephropathy in type I diabetes and the role of metabolic control in its prevention, reversibility and clinical course. Acta Diabetol Lat. 1983;20:1–18. doi: 10.1007/BF02629124. [DOI] [PubMed] [Google Scholar]
- 8.Fioretto P., Mauer M., Brocco E. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia. 1996;39:1569–1576. doi: 10.1007/s001250050616. [DOI] [PubMed] [Google Scholar]
- 9.Retnakaran R., Cull C.A., Thorne K.I. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55:1832–1839. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 10.Lewis E.J., Hunsicker L.G., Clarke W.R. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 11.Perkovic V., Heerspink H.L., Chalmers J. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83:517–523. doi: 10.1038/ki.2012.401. [DOI] [PubMed] [Google Scholar]
- 12.Wong M.G., Perkovic V., Chalmers J. Long-term benefits of intensive glucose control for preventing end-stage kidney disease: ADVANCE-ON. Diabetes Care. 2016;39:694–700. doi: 10.2337/dc15-2322. [DOI] [PubMed] [Google Scholar]
- 13.DCCT/EDIC Research Group. de Boer I.H., Sun W. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365:2366–2376. doi: 10.1056/NEJMoa1111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenner B.M., Cooper M.E., de Zeeuw D. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 15.Marso S.P., Daniels G.H., Brown-Frandsen K. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marso S.P., Bain S.C., Consoli A. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 17.Wanner C., Inzucchi S.E., Lachin J.M. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 18.Neal B., Perkovic V., Mahaffey K.W. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 19.Atkins R.C., Briganti E.M., Lewis J.B. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis. 2005;45:281–287. doi: 10.1053/j.ajkd.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Felix Kröpelin T., de Zeeuw D., Holtkamp F.A. Individual long-term albuminuria exposure during angiotensin receptor blocker therapy is the optimal predictor for renal outcome. Nephrol Dial Transplant. 2016;31:1471–1477. doi: 10.1093/ndt/gfv429. [DOI] [PubMed] [Google Scholar]
- 21.Schievink B., de Zeeuw D., Parving H.-H. The renal protective effect of angiotensin receptor blockers depends on intra-individual response variation in multiple risk markers. Br J Clin Pharmacol. 2015;80:678–686. doi: 10.1111/bcp.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnier M., Pruijm M., Wuerzner G. Drug adherence in chronic kidney diseases and dialysis. Nephrol Dial Transplant. 2015;30:39–44. doi: 10.1093/ndt/gfu015. [DOI] [PubMed] [Google Scholar]
- 23.Lambers Heerspink H.J., Holtkamp F.A., Parving H.-H. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 2012;82:330–337. doi: 10.1038/ki.2012.74. [DOI] [PubMed] [Google Scholar]
- 24.Vegter S., Perna A., Postma M.J. Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol. 2012;23:165–173. doi: 10.1681/ASN.2011040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zoccali C., Ruggenenti P., Perna A. Phosphate may promote CKD progression and attenuate renoprotective effect of ACE inhibition. J Am Soc Nephrol. 2011;22:1923–1930. doi: 10.1681/ASN.2011020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parving H.-H., de Zeeuw D., Cooper M.E. ACE gene polymorphism and losartan treatment in type 2 diabetic patients with nephropathy. J Am Soc Nephrol. 2008;19:771–779. doi: 10.1681/ASN.2007050582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruggenenti P., Perna A., Remuzzi G. ACE inhibitors to prevent end-stage renal disease: when to start and why possibly never to stop: a post hoc analysis of the REIN trial results. Ramipril Efficacy in Nephropathy. J Am Soc Nephrol. 2001;12:2832–2837. doi: 10.1681/ASN.V12122832. [DOI] [PubMed] [Google Scholar]
- 28.Proteomic Prediction and Renin Angiotensin Aldosterone System Inhibition Prevention Of Early Diabetic nephRopathy In TYpe 2 Diabetic Patients With Normoalbuminuria (PRIORITY). Available at: https://clinicaltrials.gov/ct2/show/NCT02040441. Accessed May 10, 2018. [DOI] [PMC free article] [PubMed]
- 29.Good D.M., Zürbig P., Argilés A. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics. 2010;9:2424–2437. doi: 10.1074/mcp.M110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Study of diabetic nephropathy with atrasentan (SONAR). Available at: https://clinicaltrials.gov/ct2/show/NCT01858532. Accessed May 10, 2018.
- 31.Bang Y.-J., Van Cutsem E., Feyereislova A. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 32.Kim E.S., Herbst R.S., Wistuba The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov. 2011;1:44–53. doi: 10.1158/2159-8274.CD-10-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Wirz Y., Branche A., Wolff M. Management of Respiratory infections with use of procalcitonin: moving toward more personalized antibiotic treatment decisions. ACS Infect Dis. 2017;3:875–879. doi: 10.1021/acsinfecdis.7b00199. [DOI] [PubMed] [Google Scholar]
- 35.Martin-Lorenzo M., Martinez P.J., Baldan-Martin M. Citric acid metabolism in resistant hypertension: underlying mechanisms and metabolic prediction of treatment response. Hypertension. 2017;70:1049–1056. doi: 10.1161/HYPERTENSIONAHA.117.09819. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy M.I. Painting a new picture of personalised medicine for diabetes. Diabetologia. 2017;60:793–799. doi: 10.1007/s00125-017-4210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harder J.L., Hodgin J.B., Kretzler M. Integrative biology of diabetic kidney disease. Kidney Dis (Basel) 2015;1:194–203. doi: 10.1159/000439196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alam-Faruque Y., Dimmer E.C., Huntley R.P. The renal gene ontology annotation initiative. Organogenesis. 2010;6:71–75. doi: 10.4161/org.6.2.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heinzel A., Mühlberger I., Stelzer G. Molecular disease presentation in diabetic nephropathy. Nephrol Dial Transplant. 2015;30(Suppl 4):iv17–iv25. doi: 10.1093/ndt/gfv267. [DOI] [PubMed] [Google Scholar]
- 40.Mayer P., Mayer B., Mayer G. Systems biology: building a useful model from multiple markers and profiles. Nephrol Dial Transplant. 2012;27:3995–4002. doi: 10.1093/ndt/gfs489. [DOI] [PubMed] [Google Scholar]
- 41.Mayer G., Heerspink H.J.L., Aschauer C. Systems biology-derived biomarkers to predict progression of renal function decline in type 2 diabetes. Diabetes Care. 2017;40:391–397. doi: 10.2337/dc16-2202. [DOI] [PubMed] [Google Scholar]
- 42.Bader G.D., Hogue C.W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics. 2003;4:2. doi: 10.1186/1471-2105-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinzel A., Mühlberger I., Fechete R. Functional molecular units for guiding biomarker panel design. Methods Mol Biol. 2014;1159:109–133. doi: 10.1007/978-1-4939-0709-0_7. [DOI] [PubMed] [Google Scholar]
- 44.Pena M.J., Heinzel A., Heinze G. A panel of novel biomarkers representing different disease pathways improves prediction of renal function decline in type 2 diabetes. PLoS One. 2015;10:e0120995. doi: 10.1371/journal.pone.0120995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pena M.J., Heinzel A., Rossing P. Serum metabolites predict response to angiotensin II receptor blockers in patients with diabetes mellitus. J Transl Med. 2016;14:203. doi: 10.1186/s12967-016-0960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hellemons M.E., Persson F., Bakker S.J.L. Initial angiotensin receptor blockade-induced decrease in albuminuria is associated with long-term renal outcome in type 2 diabetic patients with microalbuminuria: a post hoc analysis of the IRMA-2 trial. Diabetes Care. 2011;34:2078–2083. doi: 10.2337/dc11-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heerspink H.J.L., Ninomiya T., Persson F. Is a reduction in albuminuria associated with renal and cardiovascular protection? A post hoc analysis of the ALTITUDE trial. Diabetes Obes Metab. 2016;18:169–177. doi: 10.1111/dom.12600. [DOI] [PubMed] [Google Scholar]
- 48.Miao Y., Ottenbros S.A., Laverman G.D. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension. 2011;58:2–7. doi: 10.1161/HYPERTENSIONAHA.111.171488. [DOI] [PubMed] [Google Scholar]
- 49.Miao Y., Dobre D., Heerspink H.J.L. Increased serum potassium affects renal outcomes: a post hoc analysis of the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. Diabetologia. 2011;54:44–50. doi: 10.1007/s00125-010-1922-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smink P.A., Miao Y., Eijkemans M.J.C. The importance of short-term off-target effects in estimating the long-term renal and cardiovascular protection of angiotensin receptor blockers. Clin Pharmacol Ther. 2014;95:208–215. doi: 10.1038/clpt.2013.191. [DOI] [PubMed] [Google Scholar]
- 51.Smink P.A., Hoekman J., Grobbee D.E. A prediction of the renal and cardiovascular efficacy of aliskiren in ALTITUDE using short-term changes in multiple risk markers. Eur J Prev Cardiol. 2014;21:434–441. doi: 10.1177/2047487313481754. [DOI] [PubMed] [Google Scholar]
- 52.Schievink B., de Zeeuw D., Smink P.A. Prediction of the effect of atrasentan on renal and heart failure outcomes based on short-term changes in multiple risk markers. Eur J Prev Cardiol. 2016;23:758–768. doi: 10.1177/2047487315598709. [DOI] [PMC free article] [PubMed] [Google Scholar]