Abstract
Our current knowledge of the structure, function, and diseases of the brain comes from direct examination of its substance. In the last centuries, only a few elite had managed to retrieve, gather, and preserve the elusive brain for their own research. The resulting brain collections, stored in formalin-filled jars or dried up in cabinets, served anatomical, neuropathological, anthropometric, ideological, and diagnostic purposes. In the 1960s, the first modern brain banks actively collecting and strategically preserving both diseased and healthy brains to be consequently distributed to the scientific community were instituted. In an era where state-of-the-art biochemical “Omic” studies and advanced metabolic and molecular neuroimaging exist, it is now, more than ever, that postmortem brain investigations must be performed. Only through the comparison and integration of postmortem neuropathological and biochemical findings and antemortem data from clinical, neuropsychological neuroimaging, and other biomarker examinations can we truly understand neurological disease mechanisms. Brain banks supplying brain specimens, antemortem information, and postmortem diagnosis are a major benefactor of brain research.
Keywords: Brain bank, Brain collection, Brain bank network, History of brain banking, History of neuroscience, Brain archiving, Encephalocentrism
1. Introduction
Among the various organs in our body, the brain is the most intricate such that our current understanding of its functions and disease mechanisms is still incomplete. One important factor obstructing brain research is the scarcity of tissue available to study. To overcome this dilemma, brain banks are born, actively collecting and distributing brains to researchers around the world. After an extensive review of the existing literature, we present a recount of the history of neuroscience and brain archiving, from the Greek philosophers and physicians who first considered the brain as the command center of the body and the earliest brain collectors and their contributions to the evolutionary changes that brought about the establishment of modern brain banks. The goal of this article is to raise awareness of the importance of brain banking, encourage human brain–based research, and invite researchers to “return” to neuropathology.
2. Encephalocentrism and brain dissections
In the early 5th century B.C., Alcmaeon of Croton (ca. 520-450 B.C.) introduced the concept of encephalocentrism wherein he considered the brain as the seat of the mind and soul [1], [2]. Alcmaeon showed deep interest in the special senses (hearing, seeing, tasting, and smelling) and was the first to perform anatomical dissections for learning purposes [1]. He concluded that all sensory organs were connected to the brain through channels or pores and that the brain was responsible for both sensory perception and its understanding [1], [2]. Alcmaeon promoted an empirical approach to knowledge, suggesting physicians and scholars should rely on practical observations and not on “divine revelations” [2].
The concept of encephalocentrism, with the brain as the command center of the body, was made popular by Hippocrates of Cos (460-370 B.C.) and his followers as documented in the collection of writings compiled in the Corpus Hippocraticum. In this Hippocratic collection, the brain was also described as the site where feelings, emotions, behavioral conduct, and good judgment originated. The correlations between brain damage and movement disorders, such as convulsions and paralyses, were also reported. Intriguingly, the modern concept of cerebral dominance was also implied [3].
Systematic human dissections began in Hellenistic Alexandria in the 3rd century B.C. Herophilus of Chalcedon (330-260 B.C.) and Erasistratus of Ceos (304-250 B.C.) performed dissections of cadavers, and even vivisections, of condemned criminals with permission from the Ptolemaic Royals. Although none of their original writings still exist, their works can be traced in the writings of Galen of Pergamon (129-216 A.D.). Herophilus distinguished nerves from tendons and blood vessels, motor from sensory nerves, cranial from spinal nerves, and cerebrum from cerebellum. Erasistratus suggested the correlation between intelligence and the number and complexity of brain convolutions [1], [3].
From the 3rd to 13th century A.D., much of the anatomical knowledge came from the works of Galen, who had spent most of his professional career in 2nd-century Rome, in a time and place were human dissections were prohibited. Dissections in Alexandria had stopped around 30 B.C. Galen shared Aristotle's belief that nothing except what could be experienced through the senses should be accepted; thus, he practiced dissections and vivisections. Given the religious and legal obstacles to human autopsies at the time, he resorted to studying animals. A few “chance encounters” with some wounded bodies and decomposed corpses provided a glimpse of the human body. He studied the anatomy of the brain, the cranial nerves, and the eyes. Among his many discoveries were the first descriptions of brain atrophy caused by aging, the association between stupor and brain pressure, and the alterations in sensation, perception, and cognition caused by head injuries [3].
The early 1300s witnessed the reinstitution of human dissections, but this time in Italy. The return to the dissecting table was influenced by medicolegal issues, epidemical problems, and necessity for medical training. Physician and anatomist Mondino de Luzzi (1270-1365) conducted dissections of executed criminals in Bologna, typically in front of a crowd of medical students and public bystanders [3]. In spite of the many human dissections, the artistic representations of human anatomy lagged behind, and drawings remained inexact with the exception of the works of Leonardo da Vinci (1452-1519). Da Vinci produced realistic drawings of neuroanatomical structures he had personally dissected including the eyes, meningeal arteries, cranial fossae, cranial nerves, skull, and ventricles. He also succeeded in reproducing a cast model of the ventricles by injecting molten wax inside the brain [4].
Considerable improvements in the anatomical drawings (with quality comparable with the illustrations of the modern day) were observed in the works of Andreas Vesalius (1514-1564). Vesalius taught anatomy in Padua and routinely performed dissections in the company of students, physicians, and scholars. Through collaborations with various artists, he was able to combine art and science in the anatomical text De Humani Corporis Fabrica and its companion volume, Epitome [5]. By means of direct observation of human remains during autopsies, Vesalius was also able to detect, contradict, and correct many inaccuracies in the widely accepted Galenical anatomy. Through Vesalius, the anatomical knowledge began to derive from what was directly observed and verified and no longer through what was written in the works of previous anatomists [3].
3. Brain collections
3.1. The 1600s-1700s: the perfection of preservation techniques and the rise of anatomy schools
The brain consists of soft tissue that is difficult to preserve. The prospect of archiving brains became practicable only after social restrictions were overcome and preservation techniques were made impeccable [6].
In the mid-1600s, Dutch anatomist Frederik Ruysch (1638-1731) perfected a method for preserving soft tissue by injecting veins and arteries with a mercuric sulfide–based mixture derived from cinnabar. Ruysch preserved and collected specimens showing congenital defects, malformations and tumors. Although he was able to observe brains, his collection included only very few brain samples. Ruysch's collection is now conserved at the Peter the Great Museum of Anthropology and Ethnography in Saint Petersburg [7].
Over the next hundred years, anatomical museums housing collections of different preserved body parts bloomed over Europe, including London, Paris, and Edinburgh. They were mainly associated with private anatomy schools and were used for educational purposes. However, only a few of said museums possessed archived brains due to the difficulty of their preservation [6].
In 18th century Britain, the scarcity of bodies forced anatomist to resort to illegal ways of obtaining them. Anatomist and obstetrician, William Hunter (1718-1783), ran an innovative private anatomy school where students were permitted to perform dissections. He accumulated some 2600 anatomical and pathological specimens throughout his life. The anatomical collection included 76 specimens of brain, spinal cord, and meninges obtained from postmortem autopsies. It is now housed at the Anatomy Museum of the University of Glasgow. The pathological neurological collection had 33 specimens showing arterial disease, tuberculosis, syphilis, and meningeal tumors, and is found at the Glasgow Royal Infirmary. Their sources included grave robbery (often through the help of resurrectionists, servants, and students), bribing of undertakers, body snatching to order, illegal trading, and seizing of unclaimed bodies and suicide victims. With the implementation of the 1752 Murder Act, access to cadavers became easier as dissection of bodies of hanged murderers became part of their sentences [8], [9].
3.2. The 1800s: the peak of the era of brain collections
Phrenology became popular in the early 1800s. Introduced by Franz Joseph Gall (1758-1828), Phrenology focused on the concept of brain function localization, affirming that intellectual faculties and personality traits corresponded to different brain areas and that cranial palpation was equivalent to examining the cortex. Gall dissected alcohol-fixed human brains and discovered the origins of chief cranial nerves, the significance of commissures, and the course and decussation of the pyramidal tract, among others. He also possessed a skull collection. Phrenology waned a few decades later due to heavy criticism [10].
In 1861, Paul Pierre Broca (1824-1880) vindicated the claim of phrenologists that language functions were located in the frontal lobes of the brain. Through direct observation of the brains of two deceased aphasic patients, he discovered a common lesion in the posterior half of the left third (or second) frontal convolution and suggested that the integrity of this area was indispensable to the exercise and faculty of articulate language [11]. Broca preserved the aphasic brains as a whole which were successively stored in formalin-filled jars at the Musée Dupuytren of the Pierre and Marie Curie University in Paris [12]. Recent re-examination of Broca's famous aphasic brains has demonstrated that the lesions originally found by Broca and the region we now call Broca's area, involving the pars opercularis and pars triangularis, present some dissimilarities and do not entirely overlap. Nonetheless, his discovery remains essential to the concept of brain function localization [13].
In the second half of the 1800s, anthropometric brain collections were established in France, USA, Japan, Russia, Germany, and Sweden to identify the substrate of genius. Their objective was to detect certain morphological features linkable to intelligence in the brains of people belonging to the superior “white” race and people of grand intellect capacity and high social rankings [14]. In 1889, Burt Green Wilder (1841-1925) began a collection of more than 600 brains, dividing them into “Brains of Educated and Orderly Persons”, belonging to scholars and eminent individuals, and “Brains of Unknown, Insane or Criminal Persons” including brains of women, murderers, racial minorities, and the mentally ill. Wilder searched for differences in size, shape, weight, and number of convolutions between the two groups and found no disparities visible with the naked eye. Of the original 600 brains collected, only 70 now remain at Uris Hall in Cornell University [15], [16].
Criminologist and physician Cesare Lombroso (1835-1909) believed that criminals, madmen, and geniuses all shared, in varying degrees of severity, a form of primitive psychobiological state caused by either a revolutionary throwback to an ancestral being (atavistic theory) or an arrest at a primitive early stage (born delinquent theory), leading to anthropological features and psychological reactions different from the “normal 19th-century man” (deviance theory). He considered the criminal as the most atavistic of all and linked certain craniofacial characteristics and variations to the primitive state [17]. He carried out craniometric measurements and started a collection in mid-1850s in Pavia. His collection included skulls, brains, plaster casts or wax models of faces, skeletons, photographs, and personal works of criminals and madmen, some of which originated from the local hospital and profanation of cemeteries, others donated by asylum directors, prison doctors, and pathologists from around Italy [18], and by international collaborators [17]. Although the collection was originally used for anthropometric studies and teaching sessions, it was subsequently transformed into a Museum of Criminal Anthropology now housed at the Palazzo degli Istituti Anatomici at the University of Turin [18].
Another prominent neuroscientist from late 19th century Italy was Carlo Giacomini (1840-1898), whose contribution to neuroanatomy included that of the discovery of a ribbon-shaped elevation surrounding the uncus (band or limbus of Giacomini) and the first description of the os odontoideum. Giacomini studied more than 1000 human brains using a method of dry preservation. It involved immerging the brain first in zinc chloride and later in glycerin to make it firm, and thus allow him to study many brains at a time. Giacomini observed that the brains of “healthy” individuals possessed broad morphological variability concerning the convolutions, and therefore strongly opposed Lombroso's atavistic theories and association of aberrant behavior and mental disposition to certain anatomical anomalies. Today, most of Giacomini's dried human brains are found at the Luigi Rolando Museum of Human Anatomy in Turin [19]. The dry brain specimens in display at the museum derive from both Giacomini's and Lombroso's collections.
In the mid-19th century Germany, Rudolph Virchow (1821-1902) began a pathological collection of human organs including brains at the Charité Hospital in Berlin. Driven by his interest in disease mechanisms, Virchow devoted most of his time to dissections and specimen preparation. By the opening of his Pathological Museum in 1901, his specimens had amounted to a total of 23,066 items. The specimens presented tumors, organic diseases and malformations. His museum was open to medical students and colleagues for teaching and research purposes and to the public to raise awareness of health and disease issues. After the two World Wars, most of the original content was destroyed. Only some 750 dry and wet pathological specimens can now be found at the Berlin Museum of Medical History at Charité [20].
From 1866 to 1876, under James Crichton-Browne (1840-1938), the West Riding Lunatic Asylum at Wakefield, Yorkshire operated as both a functional asylum and a research-oriented institution. Crichton-Browne believed that insanity was a physical degeneration of the brain warranting both a moral approach and a physical treatment. He associated the clinical presentation with postmortem pathological analysis of the brain to comprehend the nature and cause of mental and neurological diseases. He also compiled medical case notes and records of therapeutic interventions. He performed autopsies as a routine and instituted a laboratory for anatomy, neuropathology, and histology where about 1500 brains were extracted, weighed, dissected, observed under a microscope, and compared with each other. Postmortem records and drawings of pathological brains were also produced [21], [22], [23]. Wakefield's facilities and resources were made accessible not only to internal officers and clerks, but also to external young doctors aspiring to conduct scientific investigations and publish [21], [22], [23]. Under Crichton-Browne, the facilities and functions of the asylum were made to serve scientific research. With his systematic, scientific approach to studying and treating mental and neurological illnesses, Crichton-Browne is said to have constructed the foundations of modern neuroscience [23]. In many ways, the West Riding Lunatic Asylum acted as a predecessor to modern brain banks.
3.3. The 1900s: the systematization of brain collections
In the early 20th century, Harvey Cushing (1869-1939) started a collection based on brain tumors [24]. Cushing worked as a neurosurgeon at Johns Hopkins Hospital in Baltimore, Maryland. The Pathology Department had once misplaced a tissue sample of a pituitary cyst belonging to a previous case significant to Cushing. This event forced him to meticulously retain and preserve every specimen recovered during surgery or autopsy. From the late 1800s to 1936, he was able to gather about 2201 brain tumors and whole brain specimens. He also painstakingly made photographic copies of his collection [25]. The Cushing Collection, comprising over 2200 case studies including brain tumors and whole brain specimens, microscopic slides, about 50,000 pages of hospital records, notes and journal excerpts, and over 15,000 photographic negatives, can now be found at the Harvey Cushing Brain Tumor Registry at Yale University [26].
German physician and neuropathologist Julius Hallervorden (1882-1965) collected brain specimens from 1907 until 1960, but sometime between 1939 and 1945, during World War II (WWII), he received brains from victims of the “Euthanasia” killings of the mentally ill and physically disabled [27], [28], [29]. Initial investigations linking Hallervorden to the euthanasia killings came from a postwar report written by Major Leo Alexander in 1945 [30]. Three letters written by Hallervorden himself also attested his involvement [29]. He participated in brain extractions and provided glassware, fixatives, technicians and instructions about brain removal and processing to the killing centers. The opportunity for “wonderful brain material” blinded Hallervorden, outweighing any perceived moral obligations or personal hesitations [27]. The brains collected were dissected, preserved in glass slides, and stored making the infamous “H Series”. They were used for most of Hallervorden's post-WWII research on developmental disorders, malformations, and early childhood disease. Evidence suggests his collection was shared with other researchers and was exploited after his death. In 1990, owing to the dark nature of their origin, all specimens collected between 1933 and 1945 were buried, followed a few years later by a commemorated ceremony for euthanasia victims [29].
4. Modern brain banks
4.1. The 1950s to present: the era of active collection and purposeful distribution of brain tissues
Post-WWII England clinicians and researchers showed a heightened interest in exploring and expanding the field of neuropathology. It was viewed as a key to understanding many neurological and psychiatric diseases [31]. From 1947, John Arthur Nicholas Corsellis (1915-1994) worked as a neuropathologist at Runwell Psychiatric Hospital where he examined brains from Runwell patients along with brains from other psychiatric and general hospitals and coroner's office [32]. In 1951, he started a brain collection at Runwell of over 8000 brains affected by epilepsy, tumors, dementia, and psychiatric disorders. About 1000 brains belonged to a “control” group without any neurological or psychiatric disease [33]. The specimens (preserved in formalin, paraffin wax, and celloidin blocks) were properly archived accompanied by neuropathological reports, medical records, and neuropsychiatric notes [32]. With his vast number of affiliations and collaborators and his own expertise in the field of neuropathology, Corsellis was in the best position, not only to collect many brain specimens, but also to distribute brain samples to other researchers, and thus aiding various publications and transforming his collection into UK's earliest Brain Bank. In 1971, Corsellis provided David Bowen, a young biochemist, with fresh human brains obtained within 24 hours of death. Bowen demonstrated the presence of enzymatic activity in postmortem brain revealing the need to preserve brain tissue in ice to keep it fresh for biochemical studies [31], [34]. In 2016, the Corsellis Collection was distributed to different research organizations including the Scientific Initiative for Neuropsychiatric and Psychopharmacological Studies research center in Duffel which received most of its psychiatric components [33].
In 1961, Wallace Tourtellotte founded the National Neurological Research Bank at the Veterans Administration Wadsworth Medical Center in Los Angeles, California. Its purpose was to support research on the etiopathogenesis of developmental, neurological, and psychiatric disorders with no known cause, treatment, or animal model. This was carried out through proper acquisition, photographing, quick freezing, cryogenic storage, and distribution of donated human tissue and biological samples (cerebrum, brain stem, cerebellum, cerebrospinal fluid, and matching blood) to researchers throughout the world. Tourtellotte's Bank differed from brain collections because of the antemortem solicitation of target donors, their legal guardians and/or next of kin. Antemortem donation programs gave access to a complete medical history with information on acute and chronic diseases, their course and treatment, and agonal state leading up to the time of death. All brain tissue diagnoses were confirmed through comparison of clinical records with a detailed neuropathological report of major findings and diagnostic conclusions. Particular attention was given to quality control during brain tissue handling and archiving. In this way, Tourtellotte's Bank was able to distribute high quality, well-characterized brain specimens to researchers unable to obtain materials on their own [35], [36].
A decrease in autopsy rates was observed from the 1950s onward. This decline was attributed to emotional, cultural, and religious considerations; legal restraints; lack of belief of clinical value and benefit; and advances in antemortem diagnostic techniques [37]. Breakthrough in in vivo structural and functional brain imaging occurred in the 1970s gaining a central role in the study of the brain and the diagnosis of neurological diseases [38], [39].The lack of tissue available and the growing attention to neuroimaging became an important impediment to neuropathology and brain research. It was later on emphasized that although very informative and capable of detecting subtle or small central nervous system lesions (which could be inconspicuous on gross examination), neuroimaging was only a surrogate of gross neuropathology—it was not a substitute for tissue diagnosis [40]. Advanced biomarkers and modern molecular imaging derive from tissue observation and require tissue confirmation.
The 1970s also witnessed the expansion in the fields of immunohistochemistry, electron microscopy, and biochemistry. All these techniques demanded different methods of tissue preservation aside from the traditional formalin fixation. The biochemical “Omics” technologies especially required fresh, non-fixed brain tissue which a traditional brain collection typically failed to provide. Altogether, these factors contributed to the increasing necessity of good-quality brain tissue for research [34]. The “Omics” study the characteristics of the genome and its gene products, the interactions and anomalies in biological pathways, and the regulation of metabolic processes [41], [42].
Over the next decades, brain banks actively collecting and distributing brain tissue from both diseased patients and healthy controls thrived in the USA, Australia, and Europe. The establishment of brain banks provided enough tissue access to fuel brain tissue research. These brain banks follow precise ethical, social, and legal guidelines for the recruitment of potential donors in antemortem donation programs, postmortem examination, and data processing and sharing [43], [44], [45]. According to Gere [6], the new approach involving antemortem donation programs and prospective archiving signified that brains were no longer a passive subject of display like in brain collections but a means of active intervention. Brains from modern brain banks are well-characterized, accompanied by a personal, clinical, and drug history, and data on neurological function, brain imaging, genetic predisposition, clinical trials, and biomarker studies. The main forms of brain tissue produced are (1) formalin-fixed paraffin-embedded tissues used for neuropathological structural and immunohistochemical studies, (2) glutaraldehyde-fixed resin-embedded blocks for transmission electron microscopy and array tomography, and (3) frozen tissue sections reserved for biochemical techniques [44].
Brain banks began to form networks in the 21st century. BrainNet Europe (https://www.brainnet-europe.org) was an international consortium of 19 European Brain Banks united to promote brain banks, establish gold standards for tissue handling and quality control, and produce ethical guidelines. Other networks include the UK Brain Bank Network (https://mrc.ukri.org/research/facilities-and-resources-for-researchers/brain-banks), Australian Brain Bank Network (http://www.austbrainbank.org.au/index.html), and US National Institutes of Health-Neuro-BioBank (https://neurobiobank.nih.gov). All are defined by common standard operating procedures, combined protocols, and shared databases with mutual goals.
5. Discussion
Although both a form of brain archiving, brain collections and brain banks are actually very distinct. Brain collections were established for anatomical, neuropathological, anthropometric, ideological, educational, and diagnostic purposes by people interested in accumulating brains for their own personal use, or for that of immediate collaborators. Brain collections are housed in museums, preserved in formalin-filled jars or dried up in cabinets. Their sources included graveyards, hospitals, asylums, and black market.
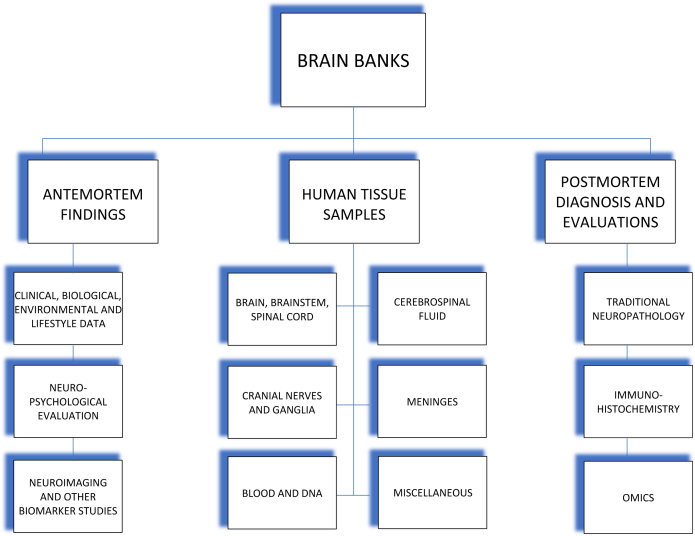
The decrease in autopsy rates from the 1950s onward and the upsurge in biochemical, molecular biology and immunohistochemical techniques called for the formation of brain banks which, to this day, actively collect and distribute high-quality, well-characterized brain tissue to researchers in the whole scientific community. Brain tissues from brain banks originate from donors enrolled in antemortem brain donation programs. Brains are properly extracted, dissected, and orderly stored either in formalin-fixed, paraffin-embedded blocks or cryopreserved in freezers. The brain specimens distributed are accompanied by antemortem findings, including clinical, biological, environmental, and lifestyle information, and results of neuropsychological evaluation, neuroimaging, and biomarker studies; and by postmortem findings including neuropathological diagnosis with immunohistochemical and biochemical study results (Fig. 1). Through brain banks, sufficient supply of nervous tissue is now available for research dedicated to understanding neurological disease mechanisms, to finding biomarkers, and to identifying risk genes, for an earlier diagnosis, better disease monitoring and innovative therapeutic interventions.
Fig. 1.
Shows the different types of tissue samples, antemortem findings, and postmortem analysis provided by brain banks.
The fact that the human brain can only be studied entirely after a person's demise makes elucidating the mechanisms underpinning certain neurological and neurodegenerative diseases arduous. Without brain banks mediating the process of acquiring human brain tissues, it is more challenging for many neuroscientists to do research. Animal models aiming to replicate human neurological diseases have been abundantly exploited in the last decades. Although very informative in some levels, animal models can be limiting and inefficient in perfectly replicating biochemical, cellular, and pathological changes commonly found in human neurological diseases, especially neurodegenerative ones. Aside from the structural and physiological differences between human and animal brains, the complexity of the human disorders such as variegated clinical picture and multiple trigger genes, the limited possibilities to monitor disease progression or therapeutic response in animals, and the heterogeneity of environmental and intrinsic factors all contribute to some poor results [46], [47].
Alzheimer's disease (AD) is the most prevalent cause of dementia worldwide. The number of persons affected by AD is expected to rise by 2050. The disease mechanism of action remains elusive to this day and efforts to find therapeutic and preventive measures are unsuccessful. Until now, most of AD research studies have been based on animal models, with less grants and funding given to human-based research. Because current and previous animal-based models have failed to yield significant game-changing results, focus should now be shifted to xeno-free human-based models. Other techniques such as induced pluripotent stem cells models, microfluids/organ-on-chip systems, and computational models can also be considered [47]. However, compared to these alternative models, neuropathology still remains the most valuable.
AD is a multifactorial syndrome caused by combined effects of genetic, epigenetic, nutritional, environmental, educational, and socioeconomic factors [42], [47]. To better understand the mechanisms of AD, consideration of the global and interconnected effects of these factors should be prioritized [47]. The National Institute on Aging funds about 30 Alzheimer's Disease Centers (https://www.nia.nih.gov/health/alzheimers-disease-research-centers) across the Unites States. The Alzheimer's Disease Centers perform basic, clinical, translational, and behavioral research to find a cure and, possibly, prevent AD, as well as to improve diagnosis and care for those affected. Through the National Alzheimer's Coordinating Center (https://www.alz.washington.edu), the Alzheimer's Disease Centers form a network which developed, adds, and shares a database of standardized clinical and neuropathological research data. The biological specimens including DNA, plasma, serum, RNA, cerebrospinal fluid, cell lines, and brain tissue together with clinical information are supplied by the National Centralized Repository for Alzheimer's Disease and Related Dementias (https://ncrad.iu.edu/history_and_mission.html).
Through the Alzheimer's Disease Genetics Consortium (http://www.adgenetics.org/content/program-overview), information about genetic risk variants associated with AD are being identified. Genome-wide association studies of neuropathological features of AD performed by Beecham et al [48] showed that direct analysis of neuropathological features can enhance or diminish the association between known genetic risk loci and specific neuropathological lesions of AD and also aid in the discovery of new genetic variants.
Another contribution of direct neuropathological studies of human brain is the discovery of distinct neurodegenerative disease variants and unprecedented pathological lesions [49], [50]. The definite diagnosis of neurodegenerative diseases, including AD, depends on postmortem neuropathological evaluation. In AD, both typical and atypical clinical phenotypes exist. Early-onset atypical AD differs from the typical amnesic form. It is associated with focal neuropsychological symptoms and with AD pathology in specific brain regions [49], [50]. Through a combined clinical, neuroimaging, and neuropathological study of brains with atypical AD, Kawakatsu et al [50] discovered how certain pathological lesions are responsible for the neuroimaging findings and how others are connected to the focal neuropsychological symptoms. Their study demonstrates the importance of relating clinicopathological and neuroimaging studies in the study of AD pathophysiology.
In vivo neuroimaging can be used as both a diagnostic marker, indicating the presence of disease pathology, and as a progression marker, describing the evolution of disease. The International Working Group 2 criteria [49] recognizes increased brain amyloid or tau retention on PET imaging as a biomarker of AD diagnosis. Conversely, structural MRI and FDG PET are considered biomarkers of disease staging and monitoring. The inclusion of biomarkers (CFS or PET tau and amyloid) in the criteria increases diagnostic accuracy. This highlights the value of biomarkers; the search for potential biomarkers becomes more imperative.
The Omics technologies include genomics, epigenomics, transcriptomics, proteomics, and metabolomics. Integration of data derived from different Omics techniques might help identify susceptibility genes, aberrant pathways, and new biomarkers for AD, Parkinson's disease, Huntington's disease, and other neurodegenerative diseases. Detecting dysregulated pathways not present in normal aging and pinpointing the molecular underpinnings of disease can lead to therapeutic solutions and might aid the field of personalized medicine [42]. With Omics, it is also possible to characterize biochemical, metabolic, and transcriptional differences among various cortical areas, and determine their susceptibility to distinct diseases.
Brain banks can opt to recruit and monitor donors involved in clinical trials or in longitudinal studies, giving the chance to study brains exposed to known therapeutic drugs and disease risk factors, respectively, and determine how these elements affect the brain's overall function. The recruitment of a control group with no known neurological disease is also fundamental. Healthy brains are used for comparison with diseased ones in order to recognize dissimilarities and abnormalities and distinguish physiological brain aging from disease. Even with sufficient and very detailed clinical, environmental, and socioeconomic data at hand, the examination of postmortem brain can hold a lot of surprises. In our brain bank, the Abbiategrasso Brain Bank, we have come across moderate to slightly severe neurodegenerative pathologies in the brains of subjects with normal cognitive functions, implying the need to further elucidate protective factors and coping mechanisms and determine how they alter the clinical picture.
6. Conclusions and future indications
The goal of this article is to raise awareness of the importance of brain banks, encourage human brain–based research and invite researchers to “return” to neuropathology. The long history of neuroscience and brain archiving, stretching from the initial introduction of encephalocentrism and the first brain dissections and collections to the formation of brain banks and modern research techniques, prove to us that it was through direct analysis of the human brain that scientific breakthroughs and major discoveries were achieved.
Even with the various in vivo neuroimaging and biomarker testing methods available nowadays, neuropathology still remains the only means to establish a definite diagnosis of neurodegenerative diseases. Only neuropathology can confirm or contest the correlations between the in vivo findings and neurodegenerative diseases. It is also through neuropathology that different neurodegenerative disease phenotypes and variants are discovered via detection of new pathological features and diverse pattern of distribution of known lesions.
Brain banks are nonprofit institutions which most often face financial challenges. Our brain bank is sustained by grants, sponsorships, and donations provided by the general public and private foundations. Directing economic and labor resources to human-centered research will yield profit in terms of general public health and disease prevention and treatment.
Our better understanding of neurological and neurodegenerative diseases depends on the possibility to integrate and correlate clinical, neuropsychological, and neuroimaging studies with neuropathology. With modern techniques such as biochemical and molecular biology studies and immunohistochemistry able to reinforce traditional neuropathology, it is now, more than ever, that direct examination of the brain must be exploited to understand neurological disease mechanisms including pathogenesis and progression; to find novel biomarkers necessary for in vivo disease identification and monitoring, and postmortem studies; and to identify genetic and environmental risk associations. The final goal is to boost early diagnoses, improve prognoses, implement innovative therapeutic interventions, and possibly discover preventive measures. Brain banks provide brain tissue and other biological materials accompanied by detailed antemortem clinical, neuropsychological, neuroimaging, and biomarker findings and postmortem biochemical, immunohistochemical, and neuropathological results to the research community. Brain banks are now considered to be the heart of neurological research.
Research in context.
-
1.
Systematic review: A comprehensive review of existing literature, both online and print, was conducted using all relevant keywords. Official websites of brain banks, brain bank networks and institutions involved in Alzheimer’s disease and other dementia research were also consulted. Knowledge and personal experience gained from operating our own Abbiategrasso Brain Bank also contributed to the preparation of the manuscript.
-
2.
Interpretation: The long history of neuroscience and brain archiving prove to us that it was through direct analysis of the human brain that scientific breakthroughs and major discoveries were achieved. Brain banks supplying human brain tissue along with antemortem findings and postmortem diagnosis are essential to brain research.
-
3.
Future directions: With modern biochemical studies and immunohistochemistry able to reinforce traditional neuropathology, it is now, more than ever, that direct examination of the brain must be performed to understand neurodegenerative disease mechanisms, to find biomarkers, and to identify risk genes. Human brain-based research must be encouraged, and researchers are invited to ‘return’ to neuropathology.
References
- 1.Crivellato E., Ribatti D. Soul, mind, brain: Greek philosophy and the birth of neuroscience. Brain Res Bull. 2007;71:327–336. doi: 10.1016/j.brainresbull.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Celesia G.G. Alcmaeon of Croton’s Observations on Health, Brain, Mind and Soul. J Hist Neurosci. 2012;21:409–426. doi: 10.1080/0964704X.2011.626265. [DOI] [PubMed] [Google Scholar]
- 3.Finger S. Oxford University Press; USA: 2000. Minds behind the brain: a history of the pioneers and their discoveries; pp. 27–60. [Google Scholar]
- 4.Pevsner J. Leonardo da Vinci’s contributions to neuroscience. Trends Neurosci. 2002 Apr;25:217–220. doi: 10.1016/s0166-2236(00)02121-4. [DOI] [PubMed] [Google Scholar]
- 5.Saunders J.B., O'Malley C.D. The World Publishing Company; Cleveland, Ohio: 1950. The illustrations from the works of Andreas Vesalius of Brussels. [Google Scholar]
- 6.Gere C. History of Neuroscience: A brief history of brain archiving. J Hist Neurosci. 2003;12:396–410. doi: 10.1076/jhin.12.4.396.27916. [DOI] [PubMed] [Google Scholar]
- 7.Boer L., Radziun A., Oostra R.J., Ruysch Frederik. 1638-1731): Historical perspective and contemporary analysis of his teratological legacy. Am J Med Genet A. 2017;173:16–41. doi: 10.1002/ajmg.a.37663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald S.W., Faithfull J.W. William Hunter’s sources of pathological and anatomical specimens, with particular reference to obstetric subjects. In: Hancock E., Pearce N., Campbell M., editors. William Hunter's World: the Art and Science of Eighteenth-Century Collecting. Ashgate Publishing; Farnham: 2015. pp. 45–58. [Google Scholar]
- 9.McDonald S.W. William Hunter’s anatomical and pathological specimens. In: Hancock E., Pearce N., Campbell M., editors. William Hunter’s World: the Art and Science of Eighteenth-Century Collecting. Ashgate Publishing; Farnham: 2015. pp. 97–111. [Google Scholar]
- 10.Simpson D. Phrenology and the neurosciences: contributions of FJ Gall and JG Spurzheim. ANZ J Surg. 2005;75:475–482. doi: 10.1111/j.1445-2197.2005.03426.x. [DOI] [PubMed] [Google Scholar]
- 11.Pearce J.M.S. Broca’s Aphasiacs. Eur Neurol. 2009;61:183–189. doi: 10.1159/000189272. [DOI] [PubMed] [Google Scholar]
- 12.LaPointe L.L. Paul Broca and French Brains: Portraits From the Life of an Eminent Neuroscientist. Commun Disord Q. 2014;36:29–34. [Google Scholar]
- 13.Dronkers N.F., Plaisant O., Iba-Zizen M.T., Cabanis E.A. Paul Broca's historic cases: high resolution MR imaging of the brains of Leborgne and Lelong. Brain. 2007;130:1432–1441. doi: 10.1093/brain/awm042. [DOI] [PubMed] [Google Scholar]
- 14.Spitzka E.A. A Study of the Brains of Six Eminent Scientists and Scholars Belonging to the American Anthropometric Society. Trans Am Philosophical Soc. 1907;21:175–308. [Google Scholar]
- 15.Lang S. A case for brains:Cornell’s cerebral display gets refurbishedhome. CornellChronicle. 2006. http://news.cornell.edu/stories/2006/05/two-students-upgrade-showcase-wilder-brain-collection 2006. Available at. Accessed May 2018.
- 16.Yaqub F. Brains: the mind as matter. Lancet Neurol. 2012 Aug;11:667. doi: 10.1016/s1474-4422(12)70174-6. [DOI] [PubMed] [Google Scholar]
- 17.Mazzarello P. Cesare Lombroso: an anthropologist between evolution and degeneration. Funct Neurol. 2011;26:97–101. [PMC free article] [PubMed] [Google Scholar]
- 18.Montaldo S. The Lombroso Museum from its origins to the present day. In: Knepper P., Yshtede P.J., editors. Cesare Lombroso Handbook. Routledge; 2013. pp. 98–112. [Google Scholar]
- 19.Perrini P., Montemurro N., Iannelli A. The contribution of Carlo Giacomini (1840-1898): the limbus Giacomini and beyond. Neurosurgery. 2013;72:475–481. doi: 10.1227/NEU.0b013e31827fcda3. [DOI] [PubMed] [Google Scholar]
- 20.Berliner Medizinhistorisches Museum der Charite. Berlin Museum of Medical History. http://www.bmm-charite.de/en/museum/history-of-the-museum.html Available at. Accessed May 2018.
- 21.Neve M., Turner T. What the Doctor thought and Did: Sir James Crichton-Browne (1840-1938) Med Hist. 1995;39:399–432. doi: 10.1017/s002572730006035x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jellinek E.H. Sir James Crichton-Browne (1840-1938): pioneer neurologist and scientific drop-out. J R Soc Med. 2005;98:428–430. doi: 10.1258/jrsm.98.9.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finn M.A. The West Riding Lunatic Asylum and the making of the modern brain sciences in the nineteenth century. PhD dissertation, University of Leeds, 2012.
- 24.Yale University Harvey Cushing/John Hay Whitney Medical Library Harvey Cushing and the Cushing Center. https://library.medicine.yale.edu/cushingcenter/history Available at. Accessed May 2018.
- 25.Fulton J.F. Harvey Cushing:a biography. C.C.Thomas, 1946.
- 26.Wahl C.J. Gone But Never Forgotten: Renaissance of the Harvey Cushing Brain Tumor Registry. 1996. http://www.neurosurgery.org/cybermuseum/tumorregistryhall/wahl.html Available at. Accessed May 2018.
- 27.Shevell M. Racial hygiene, active euthanasia, and Julius Hallervorden. Neurology. 1992;42:2214–2219. doi: 10.1212/wnl.42.11.2214. [DOI] [PubMed] [Google Scholar]
- 28.Schmuhl H.W. Brain Research and the Murder of the Sick: the Kaiser Wilhelm Institute for Brain Research,1937-1945. In: Heim S., Sachse C., Walker M., editors. The Kaiser Wilhelm Society under National Socialism. Cambridge University Press; 2009. pp. 99–119. [Google Scholar]
- 29.Wässle H. A Collection of brain sections of “Euthanasia” victims: Series H of Julius Hallervorden. Endevour. 2017;41:166–175. doi: 10.1016/j.endeavour.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Alexander L. Neuropathology and Neurophysiology, including Electro-encephalography, in Wartime Germany. 1945.
- 31.Schoefert A.K. Neither Physicians nor surgeons: whither neuropathological skill in post-war England? Med Hist. 2015 Jul;59:404–420. doi: 10.1017/mdh.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasper B., Taylor D., Janz D., Kasper E., Maier M., Williams M. Neuropathology of epilepsy and psychosis: the contributions of JAN Corsellis. Brain. 2010;133:3795–3805. doi: 10.1093/brain/awq235. [DOI] [PubMed] [Google Scholar]
- 33.SINAPS Scientific Initiative for Neuropsychiatric and Psychopharmacological Studies The Corsellis-SINAPS brain collection. https://www.sinapsduffel.com/brain-collection Available at. Accessed May 2018.
- 34.Overy C., Tansey E.M. vol 53. Queen Mary University of London; London: 2015. The development of brain banks in the UK c.1970-c.2010. (Wellcome Witnesses to Contemporary Medicine). [Google Scholar]
- 35.Tourtellotte W.W., Itabashi H.H., Rosario I., Berman K. The National Neurological Research Bank. A collection of cryopreserved human neurological specimens for neuroscientists. Ann N Y Acad Sci. 1984;436:513–516. doi: 10.1111/j.1749-6632.1984.tb14835.x. [DOI] [PubMed] [Google Scholar]
- 36.Tourtellotte W.W., Rosario I., Conrad A., Syndulko K. Human neuro-specimen banking 1961-1992. The National Neurological Research Specimen Bank (a donor program of pre- and post-mortem tissues and cerebrospinal fluid/blood; and a collection of cryopreserved human neurological specimens for neuroscientists) J Neural Transm Suppl. 1993;39:5–15. [PubMed] [Google Scholar]
- 37.Burton J.L., Underwood J. Clinical, educational, and epidemiological value of autopsy. Lancet. 2007;369:1471–1480. doi: 10.1016/S0140-6736(07)60376-6. [DOI] [PubMed] [Google Scholar]
- 38.Raichle M.E. Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci. 1998;95:765–772. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mishra S.K., Singh P. History of neuroimaging: the legacy of William Oldendorf. J Child Neurol. 2010;25:508–517. doi: 10.1177/0883073809359083. [DOI] [PubMed] [Google Scholar]
- 40.Vincentelli C., Hwang S.N., Holder C.A., Brat D.J. The Use of Neuroimaging to Guide the Histologic Diagnosis of Central Nervous System Lesions. Adv Anat Pathol. 2012;19:97–107. doi: 10.1097/PAP.0b013e318248b747. [DOI] [PubMed] [Google Scholar]
- 41.Geschwind D.H., Konopka G. Neuroscience in the era of functional genomics and systems biology. Nature. 2009;461:908–915. doi: 10.1038/nature08537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santiago J.A., Bottero V., Potashkin J.A. Dissecting the Molecular Mechanisms of Neurodegenerative Diseases through Network Biology. Front Aging Neurosci. 2017;9:166. doi: 10.3389/fnagi.2017.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bell J.E., Alafuzoff I., Al-Sarraj S., Arzberger T., Bogdanovic N., Budka H. Management of a twenty-first century brain bank: experience in the BrainNet Europe consortium. Acta Neuropathol. 2008;115:497–507. doi: 10.1007/s00401-008-0360-8. [DOI] [PubMed] [Google Scholar]
- 44.Samarasekera N., Al-Shahi S., Huitinga I., Klioveva N., McLean C.A., Kretzschmar H. Brain Banking for neurological disorders. Lancet Neurol. 2013;12:1096–1105. doi: 10.1016/S1474-4422(13)70202-3. [DOI] [PubMed] [Google Scholar]
- 45.Nussbeck S.Y., Wemheuer W.M., Beier K. Why brain banking should be regarded as a special type of biobanking:ethical, practical and data-management challenges. J biorepository Sci Appl Med. 2015;3:3–14. [Google Scholar]
- 46.JPND Research. Experimental models for neurodegenerative diseases. Report of the JPND Action Group, 2014.
- 47.Pistolatto F., Ohayon E.L., Lam A., Langley G.R., Novak T.J., Pamies D. Alzheimer disease research in the 21st century: past and current failures, new perspectives and funding priorities. Oncotarget. 2016;7:38999–39016. doi: 10.18632/oncotarget.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beecham G.W., Hamilton K., Naj A.C., Martin E.R., Huentelman M., Myers A.J. Genome-wide association meta-analysis of neuropathologic features of Alzheimer’s Disease and related dementias. Plos Genet. 2014;10:e1004606. doi: 10.1371/journal.pgen.1004606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dubois B., Feldman H.H., Jacova C., Hampet H., Molinuevo J.L., Blennow K. Advancing research diagnostic criteria for Alzheimer’s Disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 50.Kawakatsu S., Kobayashi R., Hayashi H. Typical and atypical appearance of early-onset Alzheimer’s disease: a clinical, neuroimaging and neuropathological study. Neuropathol. 2017;37:150–173. doi: 10.1111/neup.12364. [DOI] [PubMed] [Google Scholar]