Abstract
Introduction
Patiromer is a potassium (K+) binding polymer indicated for treating hyperkalemia. Among patients receiving chronic hemodialysis (HD), this study aimed to identify patient characteristics associated with patiromer initiation, describe patiromer utilization, and analyze serum K+ pre- and post-patiromer initiation.
Methods
In a retrospective cohort study, using electronic health record data from a large dialysis provider in the United States (study period: December 21, 2015, to December 20, 2016), HD patients were included who had a medication order for patiromer, sodium polystyrene sulfonate (SPS), or laboratory evidence of hyperkalemia (no K+ binder [NoKb] cohort). The index date was the first order for patiromer/SPS, or the first K+ ≥5.0 mEq/l (NoKb cohort), respectively. Using multivariable logistic regression, we identified patient characteristics associated with patiromer initiation. We evaluated patiromer utilization using Kaplan-Meier methodology and proportion of days covered. Serum K+ concentrations were assessed pre- versus post-patiromer initiation.
Results
Study cohorts included 527 (patiromer), 852 (SPS), and 8747 (NoKb) HD patients. Median follow-up was 141 days. Patiromer initiators were 2.6 times more likely to have had multiple prior episodes of hyperkalemia (odds ratio [OR]: 2.6; 95% confidence interval [CI]: 1.8–3.7). Most (61%) commenced patiromer on 8.4 g once daily; 60% of patients’ first patiromer order remained open after 180 days. Statistically significant reductions in K+, averaging approximately −0.5 mEq/l, were observed post-patiromer initiation (48% pre-patiromer vs. 22% post-patiromer had K+ ≥6.0 mEq/l [P < 0.001]).
Conclusion
Patiromer initiators receiving chronic hemodialysis had comparatively more severe, uncontrolled baseline hyperkalemia. Medication order data show long-term patiromer use was associated with significantly reduced K+.
Keywords: hemodialysis, hyperkalemia, patiromer
Hyperkalemia is a potentially life-threatening disorder due to alterations in cardiac conduction, which may result in arrhythmias and sudden death.1 This condition is a common and important complication among patients with end-stage kidney disease receiving chronic HD.2 Other conditions that place patients at increased risk for hyperkalemia include diabetes, heart failure, and use of renin-angiotensin-aldosterone system inhibitors.1, 3, 4
For patients receiving chronic HD, pharmacological therapeutic options for the outpatient treatment of hyperkalemia include SPS (Kayexalate; Sanofi-Aventis US LLC, Bridgewater, NJ), a nonspecific sodium-cation exchange resin5 and patiromer. However, there is limited evidence demonstrating the effectiveness of chronic SPS use. Further, SPS use is often limited by adverse events, including gastrointestinal symptoms and complications such as constipation, diarrhea, and nausea, in addition to other systemic toxicities, making it difficult for patients to tolerate it long-term.5 Patiromer (Veltassa; Relypsa, Inc., a Vifor Pharma Group Company, Redwood City, CA) was approved for the treatment of hyperkalemia by the US Food and Drug Administration in 2015 and by the European Medicines Agency in 2017.6, 7 Patiromer is a sodium-free nonabsorbed polymer that exchanges calcium for potassium (K+), thus removing K+ from the body and lowering serum potassium. Other small cations can also bind to patiromer but have a lower concentration in the colon than K+.8
In randomized clinical trials of patients with chronic kidney disease (not on dialysis) receiving concomitant therapy with renin-angiotensin-aldosterone system inhibitors who had hyperkalemia, patiromer was efficacious in reducing serum K+ up to 52 weeks.9, 10, 11 Despite patiromer being indicated for dialysis dependent patients with end-stage renal disease, its efficacy in this group is not well studied. In one prospective, inpatient metabolic study of 6 patients on chronic HD with moderate to severe hyperkalemia (serum K+ ≥5.5 mEq/l), patiromer 12.6 g daily was shown to decrease serum K+ levels and increase fecal K+ excretion.12
In addition to limited experimental evidence, few studies have evaluated the use and effectiveness of patiromer in the real-world dialysis practice. We addressed this evidence gap using electronic health record (EHR) data from a large dialysis organization in the United States to (i) examine the utilization of patiromer in patients requiring chronic HD, including demographic and clinical factors at initiation; (ii) identify independent patient characteristics associated with patiromer initiation; (iii) assess the initial dosing regimen and duration of use; and (iv) determine the effectiveness of patiromer in managing hyperkalemia.
Methods
Data Source and Study Design
We conducted a retrospective cohort study using EHR data spanning December 21, 2015, to December 20, 2016 (plus baseline data starting on January 1, 2015) from DaVita Kidney Care, a leading dialysis provider that operates 2400 dialysis clinics across the United States.13 The database was statistically de-identified pursuant to the Health Insurance Portability and Accountability Act Privacy Rule and included patient demographics, dialysis vintage (i.e., time from hemodialysis initiation), dialysis modality, comorbid conditions, data on hospital admissions, laboratory results, medication data, and detailed clinical data from each hemodialysis treatment session (e.g., missed HD treatments and dialysate K+ concentration). The medication data in this study comprised both medications ordered by a health care professional within DaVita clinics (e.g., a nephrologist) and other medications the patient was taking, as identified through periodic medication reconciliations that are mandated to occur quarterly.
Study Cohorts: Inclusion/Exclusion Criteria
The study population was identified over a 1-year study period from December 21, 2015 (when patiromer first became available for use following its October 21, 2015 approval by the Food and Drug Administration) to December 20, 2016, from patients aged ≥18 years receiving chronic in-center HD. Three study cohorts were assembled from among all patients. These consisted of those who (i) had a medication order for patiromer (patiromer cohort), (ii) had a medication order for SPS (SPS cohort), or (iii) had at least 2 K+ values ≥5.0 mEq/l separated by no more than 91 days but for whom neither SPS nor patiromer was recorded during the study period (NoKb cohort). Use of patiromer or SPS was identified from medication order-entry data using the search terms “Veltassa” or “patiromer” and “Kayexalate” or “sodium polystyrene sulfonate” or “SPS.” The index date was defined as the date of the first patiromer or SPS order, respectively, or the first of 2 K+ values ≥5.0 mEq/l for the NoKb cohort, within the defined study period. The study cohorts were mutually exclusive. Patients were first identified for inclusion in the patiromer cohort, followed by the SPS cohort, and last, the NoKb cohort. Patients whose first medication order for patiromer and SPS occurred on the same date were excluded. Patients were excluded who had a dialysis modality other than in-center HD (i.e., peritoneal dialysis, home HD, or nocturnal HD) and those who did not have at least 24 HD treatments in the 3 months (91 days) before the index date.
Baseline Patient Characteristics
Baseline variables were ascertained in the 12 months before and including the index date unless otherwise specified. Baseline demographic variables included age, sex, race (i.e., white, black or African American, Asian, other), region in the United States, and primary insurance carrier. Comorbid conditions were classified using International Classification of Diseases-9/10-Clinical Modification codes; the Charlson Comorbidity Score was calculated using comorbidity data.14 Medication orders and hospitalizations were examined. Dialysis-related factors including years since first HD treatment at DaVita, number of HD treatments 3 months before the index date, dialysate K+ concentration, and dialysis adequacy of the last baseline HD treatment were ascertained. The most recent body mass index and laboratory values within 91 days before the index date for K+, hemoglobin, calcium, albumin, blood urea nitrogen, and normalized protein catabolic rate were also recorded.
Patiromer Utilization
The dosage and frequency of administration in the first patiromer order were determined. Changes in the initial patiromer regimen were evaluated from the date of patiromer initiation until the first of the following censoring events: (i) patiromer discontinuation, (ii) loss to follow-up in the DaVita EHR (i.e., following kidney transplantation, dialysis discontinuation, transfer to a non-DaVita affiliated unit, or death), or (iii) the end of the study period (December 20, 2016). Patiromer use was considered terminated when a discontinuation order was identified in the EHR. However, if there were <30 days between a patiromer discontinuation and a subsequent patiromer order, patiromer exposure was considered to have continued.
Patiromer Duration of Use
Patiromer duration of use for each patient was assessed from patiromer initiation until the first censoring event (previously defined). Time to discontinuation of the first patiromer order was estimated using a Kaplan-Meier survival analysis. We calculated the proportion of days covered adherence metric,15 which was defined as the quotient of the number of days from the first patiromer order date to the patiromer discontinuation date (or another censoring event) divided by the number of days from the first patiromer order date to the first censoring event date (excluding patiromer discontinuation).
Serum Potassium Concentration Pre- and Post-Patiromer Initiation
For the patiromer cohort only, serum K+ concentration was assessed pre- and post-patiromer initiation. For these analyses only, patiromer exposure was classified using an intention-to-treat approach (i.e., patients who initiated patiromer were considered exposed to patiromer for the entire 3-month follow-up duration). Using last available K+ value (for each patient) in 3 sequential 30-day periods before and after the first patiromer order date, we described mean serum K+ concentration and 95% CI. To analyze the change in serum K+ pre- versus post-patiromer initiation, the last K+ value in each follow-up monthly interval (post-patiromer initiation) was compared with the last K+ value in the 3 months before patiromer initiation (i.e., baseline K+). Patients were included in the K+ change analyses who had both a baseline K+ value and a follow-up K+ value (in the 30-day interval being analyzed) and who remained uncensored to the end of the interval being analyzed. We also analyzed the proportion of patients with K+ ≥6.0 mEq/l (commonly considered to constitute severe hyperkalemia) pre- versus post-patiromer initiation. Additional sensitivity analyses were restricted to patiromer initiators with a baseline K+ ≥5.0 mEq/l and K+ ≥5.5 mEq/l.
Statistical Analysis
Baseline patient characteristics and patiromer utilization metrics were summarized as means with SDs and counts and percentages where appropriate. To compare baseline patient characteristics among the 3 study cohorts, we used the χ2 and Kruskal-Wallis tests for categorical and continuous variables, respectively. Time to discontinuation of the first patiromer order was depicted using a survival curve according to the method of Kaplan and Meier.
We used multivariable logistic regression to identify characteristics independently associated with patiromer use versus SPS use or versus NoKb status. Baseline characteristics that were statistically significant (P < 0.05) in univariate logistic regression models were included in the multivariable analysis, beginning with the most significant candidate predictor variable (i.e., with the highest Wald statistic). All significant variables identified from the univariate logistic regression were assessed for collinearity using Pearson’s correlation. When collinearity was identified (Pearson’s correlation coefficient >0.8), the variable more significantly associated with the outcome of initiating patiromer was included. Model validation was conducted using Monte Carlo (bootstrapping) simulation.16 The final model included variables that were included in ≥80% of the 1000 bootstrap-simulated models. Discriminative ability (area under the receiver operating characteristic) and calibration (using the Hosmer-Lemeshow goodness-of-fit statistic) of the final model were assessed.
For the patiromer cohort, the intraindividual changes in K+ concentration pre- versus post-patiromer initiation were analyzed using the paired t test. The McNemar χ2 test was used to analyze the proportion of patients with a K+ value ≥ 6.0 mEq/l pre- versus post-patiromer initiation. All statistical analyses were conducted using STATA Version 14 (StataCorp, LLC, College Station, TX).
Results
Characteristics of the Study Cohorts
A total of 10,126 chronic HD patients met the study inclusion criteria, with 527 in the patiromer cohort, 852 in the SPS cohort, and 8747 in the NoKb cohort; 3.8% of patiromer, 13.3% of SPS, and 6.8% of NoKb cohorts were excluded (details of the inclusion/exclusion criteria are provided in Supplementary Table S1). Patients in the patiromer, SPS, and NoKb cohorts originated from 311, 513, and 2103 dialysis facilities across the United States, respectively. The median follow-up time for patients pooled across the 3 cohorts was 141 days (interquartile range 156 days).
Baseline Patient Characteristics
Baseline demographic and clinical characteristics are presented in Table 1. Among the 3 study cohorts, the mean dialysis vintage (at DaVita) and mean age were approximately 4 years and 60 years, respectively. Fewer patients of black race were in the patiromer cohort compared with the SPS and NoKb cohorts. Multiple comorbid conditions were common among all study cohorts. Apart from SPS, insulin, cinacalcet, oral vitamin D, and nonsteroidal anti-inflammatory drug use, baseline medication utilization was similar. Hospitalizations were more common in the SPS cohort compared with the patiromer cohort; however, patients in the patiromer cohort had significantly more hyperkalemia-related hospitalizations than patients in the SPS or the NoKb cohort. In the 3 months before the index date, more patients in the patiromer and SPS cohorts had ≥40 HD treatments, longer HD run-time, and dialysate K+ concentration <2 mEq/l. Serum K+ concentration assessments were conducted more frequently for patiromer initiators who also had more severe and uncontrolled hyperkalemia compared with patients in the SPS and NoKb cohorts.
Table 1.
Baseline characteristics of chronic HD study patients
| Patient characteristicsa | Patiromer |
SPS |
NoKb |
P value |
|---|---|---|---|---|
| (n = 527) | (n = 852) | (n = 8747) | ||
| Demographic variables | ||||
| Age, y, mean (SD) | 59 (14) | 61 (14) | 62 (14) | <0.001 |
| Female, % | 43 | 47 | 45 | 0.35 |
| Race, % | <0.001 | |||
| White | 70 | 59 | 56 | |
| Black | 17 | 30 | 34 | |
| Primary insurance, % | 0.88 | |||
| Medicare | 80 | 80 | 81 | |
| Commercial | 10 | 9 | 10 | |
| Medicaid | 10 | 10 | 10 | |
| Clinical variables | ||||
| BMI ≥30 kg/m2, % | 33 | 34 | 37 | 0.28 |
| Charlson Comorbidity Index, mean (SD) | 5.4 (1.8) | 5.4 (1.9) | 5.5 (1.9) | 0.25 |
| Comorbid conditions, % | ||||
| Myocardial infarction | 8 | 8 | 8 | 0.99 |
| Congestive heart failure | 15 | 14 | 15 | 0.63 |
| Diabetes-uncomplicated | 69 | 69 | 69 | 1 |
| Diabetes-complicated | 58 | 56 | 54 | 0.13 |
| Arrhythmia | 2 | 1 | 1 | 0.18 |
| Peripheral vascular disease | 10 | 5 | 6 | <0.001 |
| Medication orders 12 months before index date, % | ||||
| SPS | 26 | 25 | 3 | <0.001 |
| SPS—3 months before index date | 11 | 5 | 1 | <0.001 |
| ACE inhibitor | 21 | 20 | 19 | 0.33 |
| ARB | 15 | 15 | 13 | 0.04 |
| Loop diuretic | 11 | 14 | 12 | 0.19 |
| Insulin | 24 | 30 | 22 | <0.001 |
| Cinacalcet | 39 | 30 | 24 | <0.001 |
| Vitamin D – oral | 28 | 24 | 20 | <0.001 |
| NSAID | 5 | 3 | 3 | 0.02 |
| Hospitalizations | ||||
| Hospitalized, % | 60 | 68 | 55 | <0.001 |
| Number of hospitalizations, mean (SD) | 2.3 (2.7) | 2.7 (2.8) | 2.1 (2.6) | <0.001 |
| Length of stay in hospital, d, mean (SD) | 6.7 (12.1) | 8.7 (13.8) | 7.2 (14.3) | 0.008 |
| Admitting hospitalization diagnosis, % | ||||
| Hyperkalemia-related | 14 | 9 | 4 | <0.001 |
| Cardiovascular-related | 14 | 18 | 12 | <0.001 |
| Dialysis-related variables | ||||
| HD vintage, yr, at DaVita, mean (SD) | 4.3 (2.3) | 4.1 (4.1) | 3.6 (3.6) | <0.001 |
| HD treatments (3 mo prior), mean (SD) | 37.8 (3.6) | 37.2 (4.2) | 36.9 (3.9) | <0.001 |
| ≥40 HD treatments (3 mo prior), % | 11 | 14 | 9 | <0.001 |
| HD run time ≥4 h (last HD in baseline), % | 29 | 26 | 22 | <0.001 |
| K+ dialysate <2 K mEq/l (last HD in baseline), % | 32 | 19 | 4 | <0.001 |
| Kt/V <1.2 (last HD in baseline), % | 4 | 5 | 6 | 0.02 |
| K+ last value 3 mo before index date | ||||
| K+ (mEq/l), mean (SD) | 5.8 (0.7) | 5.4 (1.0) | 5.4 (0.4) | <0.001 |
| K+ categories, % | <0.001 | |||
| K+ <5 mEq/l | 11 | 38 | 0 | |
| K+ ≥5–<5.5 mEq/l | 17 | 17 | 64 | |
| K+ ≥5.5–<6 mEq/l | 32 | 13 | 25 | |
| K+ ≥6–<6.5 mEq/l | 21 | 15 | 8 | |
| K+ ≥6.5 mEq/l | 18 | 17 | 3 | |
| All K+ assessments 3 mo before index date | ||||
| K+ tests, mean (SD) | 9.6 (4.2) | 7 (4.2) | 5.6 (3.2) | <0.001 |
| ≥ 3 K+ tests ≥6.0 mEq/l, % | 57 | 30 | 6 | |
| Other laboratory results last value before index date, % | ||||
| Hgb <9 g/dl | 5 | 6 | 6 | 0.31 |
| Calcium ≥10 mEq/l | 4 | 7 | 6 | 0.43 |
| Albumin <3.3 g/l | 7 | 11 | 11 | 0.003 |
| BUN ≥80 mg/dl | 30 | 21 | 17 | <0.001 |
| nPCR <0.8 g/kg/d | 12 | 21 | 20 | <0.001 |
ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor blockers; BMI, body mass index; BUN, blood urea nitrogen; HD, hemodialysis; Hgb, hemoglobin; K+, potassium; Kt/V, dialysis efficiency; nPCR, normalized protein catabolic rate; NoKb, no K+ binder; NSAID, nonsteroidal anti-inflammatory drug; SPS, sodium polystyrene sulfonate.
Baseline patient characteristics were classified in the 12-month baseline period before index date (inclusive of the index date), unless otherwise specified. If more than 1 value was available, the last value was used. The hyperkalemia, NoKb cohort included patients with at least 2 K+ values ≥5 mEq/l within 91 days.
Factors Associated With Patiromer Initiation
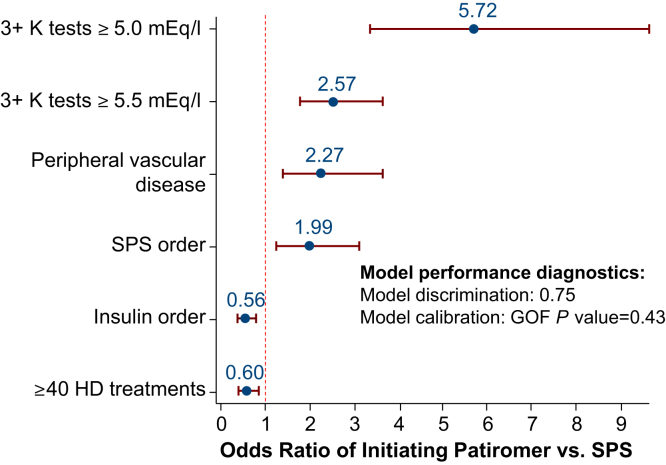
Multivariable analysis identified several clinical variables that were independently associated with patiromer initiation compared with patients in the SPS cohort (Figure 1). In the 3 months before the index date, patients initiating patiromer were more than twice as likely to have had multiple recent episodes of hyperkalemia (K+ value ≥5.5 mEq/l) compared with patients in the SPS cohort (adjusted OR: 2.57; 95% CI: 1.80–3.66) and twice as likely (OR: 1.99; 95% CI: 1.27–3.14) to have had a recent SPS order.
Figure 1.
Baseline characteristics independently associated with patiromer initiation versus patients in the sodium polystyrene sulfonate (SPS) cohort. GOF, goodness of fit; HD, hemodialysis; K, potassium.
By comparison with patients in the NoKb cohort, in the 3 months before the index date, patients in the patiromer cohort were more than 3 times as likely to have had multiple episodes of hyperkalemia (K+ ≥5.5 mEq/l; OR: 4.99; 95% CI: 3.54–6.98), to have had a prior SPS order (OR: 3.64; 95% CI: 2.75–4.83), and to have had an electrolyte-related hospitalization in the 12 months before the index date (OR: 3.32; 95% CI: 2.35–4.69). Patiromer users were more likely to have had a medication order for either oral vitamin D or cinacalcet, an HD run-time >4 hours, and a K+ dialysate concentration <2 mEq/l. Patiromer initiators were significantly less likely to be of black race, to have had a median HD vintage of <1 year, or a body mass index ≥35 kg/m2 (Supplementary Figure S1).
Patiromer Utilization
In 61% of patients initiating patiromer, their starting dose was 8.4 g once daily; this changed little over the study period (Supplementary Table S2). A minority of patients (<20%) received a dose of 8.4 g but less frequently than once daily. Only 4% of patients initiated patiromer at the 16.8 g dosage. Over the study period, most patiromer initiators (89%) had no changes to the initial dosing regimen. Overall, 7% of patiromer initiators had a dose or frequency of administration increase and 4% had a dose or frequency of administration decrease.
Duration of Patiromer Use
The Kaplan-Meier survival analysis showed 75% and 60% of patients’ first patiromer order remained open after 3 and 6 months, respectively (Supplementary Figure S2). The mean (SD) proportion of days covered was 83% (0.31), with 75% of patients’ first patiromer order covering ≥80% of all follow-up time (Supplementary Table S3).
Serum Potassium Concentrations Pre- and Post-Initiation of Patiromer
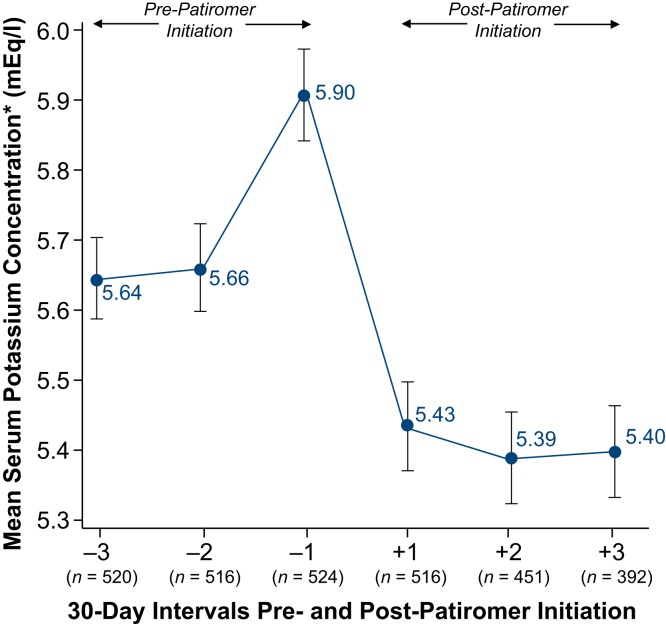
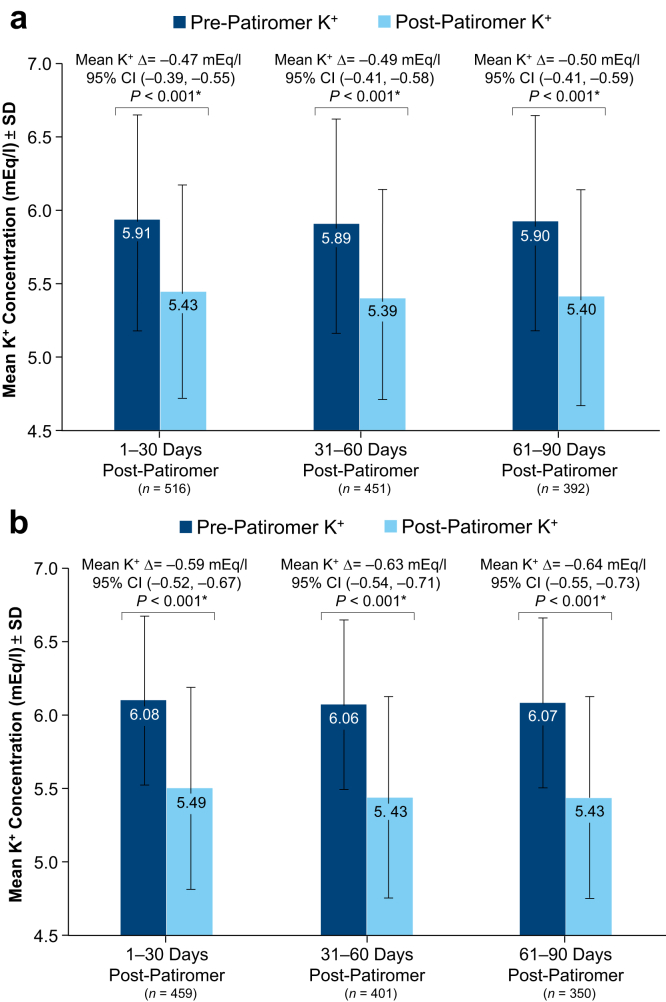
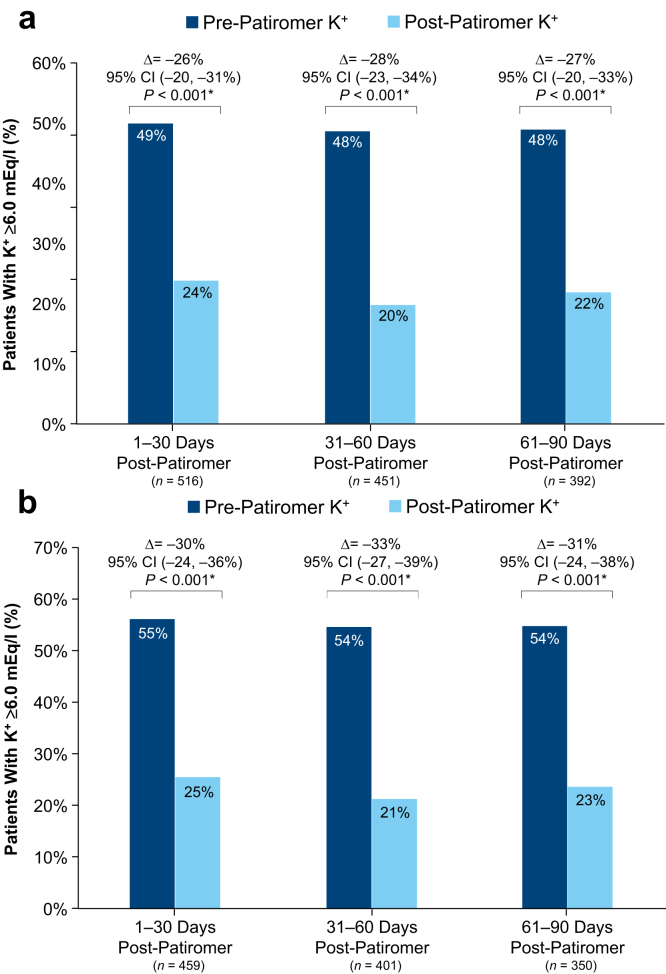
Mean K+ concentrations pre- and post-patiromer initiation are depicted in Figure 2. Compared with pre-patiromer K+ concentrations, we observed significant reductions in K+ concentrations in the three 30-day intervals following patiromer initiation (Figure 3a and b). For all patiromer initiators (Figure 3a) and patiromer initiators with a baseline K+ ≥5.0 mEq/l (Figure 3b), the mean K+ concentration reductions from the most recent pre-patiromer value were approximately −0.5 mEq/l and −0.6 mEq/l, respectively. The largest K+ concentration reduction (>1 mEq/l reduction) was observed among patients whose baseline K+ was ≥6.5 mEq/l (Supplementary Table S4). Figure 4a shows that the proportion of patiromer initiators with a K+ concentration ≥6.0 mEq/l was reduced from approximately 50% pre-patiromer to approximately 22% post-patiromer initiation. Among patiromer initiators with a baseline K+ ≥5.0 mEq/l, there was an approximately 30% reduction in the proportion of patients with a K+ concentration ≥6.0 mEq/l (Figure 4b). In sensitivity analyses restricted to patients with baseline K+ ≥5.5 mEq/l (approximately 75% of the overall patiromer cohort), the mean K+ reduction was approximately 0.7 mEq/l comparing baseline K+ to each monthly follow-up interval K+ (Supplementary Figure S3A). The restricted analyses further showed the proportion of patients with a K+ concentration ≥6.0 mEq/l was reduced from approximately 64% at baseline to 23% following patiromer initiation (Supplementary Figure S3B).
Figure 2.
Mean serum potassium and 95% confidence interval pre- versus post-patiromer initiation. *Using the last potassium value in each monthly interval for each included patient.
Figure 3.
Mean potassium change pre- versus post-patiromer initiation: all patiromer initiators (a); restricted to patiromer initiators with a baseline potassium ≥5.0 mEq/l (b). *P values and 95% confidence intervals (CIs) were estimated using the paired t test (H0: Δ = 0). The paired t test compared the last K+ value in the 3-month pre-patiromer to the last K+ value in each 30-day follow-up interval post-patiromer initiation. K+, potassium.
Figure 4.
Proportion of patients with potassium ≥6.0 mEq/l pre- versus post-patiromer initiation: all patiromer initiators (a); restricted to patiromer initiators with a baseline potassium ≥5.0 mEq/l (b). *P values were derived from the McNemar test, a within-subject z-test of equality of proportions for correlated data. This paired statistical test compares the proportion of patients with a K+ value ≥6.0 mEq/l pre-patiromer initiation versus post-patiromer initiation. The last K+ value in the 3-month pre-patiromer interval was compared with the last K+ value in each monthly follow-up interval. CI, confidence interval; K+, potassium.
Discussion
This study used real-world clinical data following the year after approval of patiromer, a novel potassium-lowering drug, to provide an early, but detailed description of its use among HD patients in a national HD cohort. This study showed that patients initiating patiromer were more likely to have had persistent hyperkalemia and prior use of SPS. Most HD patients (61%) commenced the recommended dosing regimen of 8.4 g patiromer once daily and 89% did not change this dosing regimen during follow-up. Approximately 60% of first patiromer orders remained open after 6 months. Serum K+ concentration reductions following patiromer initiation were, on average, −0.5 mEq/l among all patiromer initiators (Figure 3a) and −1.0 mEq/l for patients with severe hyperkalemia (≥6.5 mEq/l; Supplementary Table S4). Importantly, there was an approximately 50% relative percentage reduction in the proportion of patients with severe hyperkalemia (K+ ≥6.0 mEq/l) after patiromer initiation compared with before (Figure 4a and b).
Hyperkalemia is a common complication in patients with advanced kidney disease, including those on dialysis. It has been associated with a number of adverse outcomes including increased short-term and longer-term mortality, cardiovascular morbidity and mortality, hospitalization, and health care cost.17, 18 Thus, prevention of recurrent hyperkalemia may have implications in terms of improved health outcomes and reduced health care costs for patients receiving chronic HD. Before the introduction of patiromer, SPS was the only oral treatment option for hyperkalemia, but was neither specifically studied, nor commonly being used for chronic treatment and prevention of hyperkalemia. Recent randomized clinical trials evaluating the efficacy and safety of patiromer for the treatment of hyperkalemia demonstrated significant reductions in serum K+ concentration and good tolerance with low rates of discontinuation in patients with chronic kidney disease, including those with diabetic nephropathy and heart failure, and in patients taking renin-angiotensin-aldosterone inhibitors.10, 11, 19, 20 A small metabolic study of HD patients showed patiromer decreased serum K+ concentration and was associated with significant reductions in the proportion of patients with moderate to severe hyperkalemia (i.e., K+ ≥5.5, K+ ≥6.0, or K+ ≥6.5 mEq/l) following patiromer initiation.12 In this prospective clinical study of 6 patients treated with patiromer 12.6 g daily, no patients discontinued the study for adverse events and there were no serious adverse events. However, larger scale clinical studies of patiromer treatment in patients on HD were previously not available, an evidence gap that the present study attempts to fill. Comparing the findings of the small prospective clinical study of 6 patients with the present retrospective study of >500 early patiromer users, despite important methodologic differences (e.g., studying confined patients vs. patients treated in real-world clinical practice), both studies showed statistically significant reductions in the proportion of patients with moderate and severe hyperkalemia before compared with after patiromer initiation.
The selection criteria for the present study were broadly inclusive of patients in the United States undergoing chronic in-center HD with hyperkalemia treated in a typical clinical practice setting. For patients in the patiromer and SPS cohorts, we placed no restriction on baseline serum K+ concentration. However, consistent with other published studies of hyperkalemia, we conducted sensitivity analyses restricted to patients with baseline serum K+ concentrations ≥5.0 mEq/l (Figure 3, Figure 4b) and ≥5.5 mEq/l (Supplementary Figures S3A and B). Patient data originated from the EHR system of a large national dialysis provider operating more than 2000 dialysis clinics across the United States (patients in the patiromer cohort originated from 311 different dialysis clinics), thus highlighting the generalizability of the current patiromer utilization and effectiveness findings. Despite this, certain study limitations require careful consideration. Using medication orders from EHR data to define patiromer exposure (e.g., rather than pharmacy dispensing data) exposes the study to potential medication misclassification. This type of misclassification may occur if (i) the patiromer ordered was not “filled,” (ii) an open patiromer order was not closed or discontinued at the time the patient stopped taking the medication, or (iii) patient nonadherence occurred for a variety of reasons. Additional research, using alternative data sources, is warranted to confirm the study findings. An important limitation related to the effectiveness analysis (i.e., serum K+ concentration change from baseline) was the single-arm, within-patient “pre-versus-post” design to assess patiromer effectiveness. In the absence of a comparator or reference group, the ability to draw causal conclusions from the effectiveness results is limited. Further research, with a suitable comparator group to control for confounding, is planned in a subsequent study.
Conclusion
This study provides an early snapshot of patiromer use in real-world practice in patients receiving chronic HD. Patients initiating patiromer had more severe and recurrent hyperkalemia, failed attempts to control hyperkalemia with SPS, yet most of these patients had significant K+ reductions following patiromer initiation. Notably, the relative proportion of patients with severe hyperkalemia (i.e., ≥6.0 mEq/l) was reduced by approximately 50% following patiromer initiation. Together with the observed reductions in serum K+, the results regarding dose and duration of use suggest the providers’ intention for chronic patiromer use rather than for acute or short-term potassium control. Further research to investigate whether patiromer use and subsequent potassium control yields better patient outcomes is warranted.
Disclosure
CPK reports consulting fees from Amgen, AbbVie, Abbott, AstraZeneca, Bayer, Dr. Schar, Fresenius Medical Care, Keryx, Relypsa, Sanofi-Aventis, Takeda; lecture fees from Abbott, Keryx, Sanofi-Aventis; travel support from Amgen, AbbVie, Bayer, Fresenius Medical Care, Abbott, Keryx, Sanofi-Aventis; grant support from the National Institutes of Health; and royalties from UpToDate. CGR reports being a consultant to Relypsa, Keryx, Corvidia, and Halozyme. AC, DMS, and JF report employment by Relypsa, Inc., a Vifor Pharma Group Company. NO reports previous employment by Relypsa, Inc., a Vifor Pharma Group Company during the time of the study. JJC reports employment by DaVita, Inc.; is a consultant to GlaxoSmithKline plc, and is a scientific advisor for Relypsa, KBP Bioscience. WCW reports having served as a scientific advisor or consultant to Akebia, Amgen, AstraZeneca, Bayer, Daichii-Sankyo, Fibrogen, Relypsa, Vifor-Fresenius Medical Care Renal Pharma, and ZS Pharma.
Acknowledgments
This study was funded by Relypsa, Inc., a Vifor Pharma Group Company. The authors employed by Relypsa, Inc., were involved in research idea and study design, data acquisition, data analysis/interpretation, and contributed to intellectual content during manuscript drafting and revisions.
Editorial support services were provided by Impact Communication Partners, Inc., and funded by Relypsa, Inc., a Vifor Pharma Group Company.
Preliminary results of this study were presented as a poster at the American Society of Nephrology Kidney Week 2017 meeting (Rowan CG, et al; Patient characteristics and correlates of patiromer initiation for hyperkalemia in hemodialysis; TH-PO780).
Acknowledgments
Author Contributions
Research idea and study design: CPK, WCW, CGR, NO, JJC, DMS; data acquisition: CGR, NO; data analysis/interpretation: CPK, WW, JF, CGR, NO, AC, DMS, JJC; statistical analysis: CGR. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. CPK, CGR, and JF take responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Footnotes
Table S1. Patient inclusion/exclusion flow table.
Table S2. Patiromer ordered dosage and frequency of administration.
Table S3. Proportion of days covered for first patiromer order.
Table S4. Serum K+ concentration change (Δ) following patiromer initiation: stratified by baseline serum K+ concentration.
Table S5. Predictive model development data: patiromer versus SPS.
Table S6. Predictive model development data: patiromer versus NoKb.
Figure S1. Baseline characteristics independently associated with patiromer initiation: patiromer cohort versus no potassium binder cohort.
Figure S2. Kaplan-Meier survival analysis of time to discontinuation of first patiromer order.
Figure S3. Mean potassium change pre- versus post-patiromer initiation, restricted to patients with baseline K+ ≥5.5 mEq/l (a). Proportion of patients with K+ ≥6.0 mEq/l pre- versus post-patiromer initiation, restricted to patients with baseline K+ ≥5.5 mEq/l (b).
Supplementary material is linked to the online version of the paper at http://www.kireports.org/.
Supplementary Material
Patient inclusion/exclusion flow table.
Patiromer ordered dosage and frequency of administration.
Proportion of days covered for first patiromer order.
Serum K+ concentration change (Δ) following patiromer initiation: stratified by baseline serum K+ concentration.
Predictive model development data: patiromer versus SPS.
Predictive model development data: patiromer versus NoKb.
Baseline characteristics independently associated with patiromer initiation: patiromer cohort versus no potassium binder cohort.
Kaplan-Meier survival analysis of time to discontinuation of first patiromer order.
Mean potassium change pre- versus post-patiromer initiation, restricted to patients with baseline K+ ≥5.5 mEq/l (a). Proportion of patients with K+ ≥6.0 mEq/l pre- versus post-patiromer initiation, restricted to patients with baseline K+ ≥5.5 mEq/l (b).
References
- 1.Einhorn L.M., Zhan M., Hsu V.D. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156–1162. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovesdy C.P., Regidor D.L., Mehrotra R. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:999–1007. doi: 10.2215/CJN.04451206. [DOI] [PubMed] [Google Scholar]
- 3.Palmer B.F. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004;351:585–592. doi: 10.1056/NEJMra035279. [DOI] [PubMed] [Google Scholar]
- 4.Albert N.M., Yancy C.W., Liang L. Use of aldosterone antagonists in heart failure. JAMA. 2009;302:1658–1665. doi: 10.1001/jama.2009.1493. [DOI] [PubMed] [Google Scholar]
- 5.Chaitman M., Dixit D., Bridgeman M.B. Potassium-binding agents for the clinical management of hyperkalemia. P T. 2016;41:43–50. [PMC free article] [PubMed] [Google Scholar]
- 6.Veltassa (patiromer) for oral suspension [package insert] Relypsa, Inc.; Redwood City, CA: May, 2018. [Google Scholar]
- 7.European Medicines Agency. Veltassa. 2017. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004180/human_med_002141.jsp&mid=WC0b01ac058001d124. Accessed May 29, 2018.
- 8.Li L., Harrison S.D., Cope M.J. Mechanism of action and pharmacology of patiromer, a nonabsorbed cross-linked polymer that lowers serum potassium concentration in patients with hyperkalemia. J Cardiovasc Pharmacol Ther. 2016;21:456–465. doi: 10.1177/1074248416629549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitt B., Bakris G.L., Bushinsky D.A. Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors. Eur J Heart Fail. 2015;17:1057–1065. doi: 10.1002/ejhf.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weir M.R., Bakris G.L., Bushinsky D.A. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 11.Bakris G.L., Pitt B., Weir M.R. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314:151–161. doi: 10.1001/jama.2015.7446. [DOI] [PubMed] [Google Scholar]
- 12.Bushinsky D.A., Rossignol P., Spiegel D.M. Patiromer decreases serum potassium and phosphate levels in patients on hemodialysis. Am J Nephrol. 2016;44:404–410. doi: 10.1159/000451067. [DOI] [PubMed] [Google Scholar]
- 13.Kalantar-Zadeh K., Kuwae N., Regidor D.L. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 14.Romano P.S., Roos L.L., Jollis J.G. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 15.Steiner J.F., Prochazka A.V. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 16.Harrell F.E., Lee K.L., Mark D.B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Betts K.A., Woolley J.M., Mu F. The cost of hyperkalemia in the United States. Kidney Int Rep. 2018;3:385–393. doi: 10.1016/j.ekir.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain N., Kotla S., Little B.B. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;109:1510–1513. doi: 10.1016/j.amjcard.2012.01.367. [DOI] [PubMed] [Google Scholar]
- 19.Pitt B., Anker S.D., Bushinsky D.A. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32:820–828. doi: 10.1093/eurheartj/ehq502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakris G.L., Pitt B., Weir M.R. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the amethyst-dn randomized clinical trial. JAMA. 2015;314:151–161. doi: 10.1001/jama.2015.7446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient inclusion/exclusion flow table.
Patiromer ordered dosage and frequency of administration.
Proportion of days covered for first patiromer order.
Serum K+ concentration change (Δ) following patiromer initiation: stratified by baseline serum K+ concentration.
Predictive model development data: patiromer versus SPS.
Predictive model development data: patiromer versus NoKb.
Baseline characteristics independently associated with patiromer initiation: patiromer cohort versus no potassium binder cohort.
Kaplan-Meier survival analysis of time to discontinuation of first patiromer order.
Mean potassium change pre- versus post-patiromer initiation, restricted to patients with baseline K+ ≥5.5 mEq/l (a). Proportion of patients with K+ ≥6.0 mEq/l pre- versus post-patiromer initiation, restricted to patients with baseline K+ ≥5.5 mEq/l (b).