Abstract
High temperature reduces influenza viral replication; however, the treatment of fevers is thought to be necessary to improve patients' conditions. We examined the effects of high temperature on viral replication and infection-induced damage to human tracheal epithelial cells. Cell viability and dome formation were reduced, the number of detached cells was increased and lactate dehydrogenase (LDH) levels tended to be increased from 72 h to 120 h in uninfected cells cultured at 40 °C. Long-term (72 h and/or 120 h) exposure to high temperatures (39 °C and/or 40 °C) decreased RNA levels and/or viral titers of eight influenza virus strains. Cell viability and dome formation were reduced, and the number of detached cells and LDH levels were increased to a similar extent after infection with the A/H1N1 pdm 2009 virus at 37 °C and 40 °C. High temperature increased the endosomal pH, where the viral RNA enters the cytoplasm, in uninfected cells. High temperature reduced the production of IL-6, which mediate viral replication processes, and IL-1β and IL-8 in uninfected and infected cells. Based on these findings, high temperature may cause similar levels of airway cell damage after infection to cells exposed normal temperatures, although high temperature reduces viral replication by affecting the function of acidic endosomes and inhibiting IL-6-mediated processes.
Keywords: Cell biology, Microbiology, Physiology, Virology
1. Introduction
High temperature enhances defense mechanisms against infection by many viruses [1] and decreases influenza virus replication [2]. The pyrexial substances that are produced during influenza virus infection, such as interferon (IFN), exert antiviral effects [3]. Thus, a high temperature aids in inhibiting influenza virus replication.
In contrast, fever is the major symptom of influenza virus infection, and the use of antipyretic drugs to treat fever is thought necessary in children suffering from adverse effects of high temperature, such as febrile seizures [1, 4], as well as in patients with dehydration and severe outcomes caused by high temperature-induced excessive sweating and anorexia [5, 6]. However, the toxic effects of high temperature on human airway epithelial cells during influenza virus infection require further study.
The effects of high temperature on influenza virus replication vary between viral strains and the methods used to measure viral replication. For example, the release of seasonal influenza viruses (H3N2) from allantois-on-shell cultures is decreased at 41 °C or 40 °C [2]. Similarly, significantly more viruses were shed in nasal washes of ferrets in which fever was suppressed with sodium salicylate [7]. In contrast, the growth capacity of an influenza virus [A/WSN/1933 (A/H1N1)] in Madin-Darby Canine Kidney (MDCK) cells is similar at 33 °C and at 39.5 °C [8].
Several effects of high temperature on influenza viral replication processes have been reported, including enhanced viral RNA polymerase mRNA production [9] and inhibition of nuclear export of the influenza virus ribonucleoprotein complex by heat shock protein 70 [10]. The influenza virus is internalized via receptor-mediated endocytosis, and the low pH of the endosome triggers viral and endosomal membrane fusion [11], resulting in another round of viral replication. Vacuolar H+-ATPase and ion transport across Na+/H+ exchangers regulate endosomal pH [12, 13]; however, the effects of high temperature on endosomal pH and influenza viral replication in human airway epithelial cells require further study.
The present study examined the effects of clinically high temperatures on influenza viral replication, cell damage and cell function related to viral replication using primary cultures of human tracheal epithelial (HTE) cells.
2. Results
2.1. Effects of high temperature on cell damage in the absence or presence of viral infection
Based on the results of preliminary experiments, an A/H1N1 pdm 2009 viral infection induced similar levels of epithelial cell damage in cells cultured at 37 °C and 40 °C for 120 h post-infection, although lower viral titers were observed in cells cultured at 40 °C than in cells cultured at 37 °C. Therefore, we investigated the effects of long-term exposure to high temperatures on the damage to uninfected and infected cells.
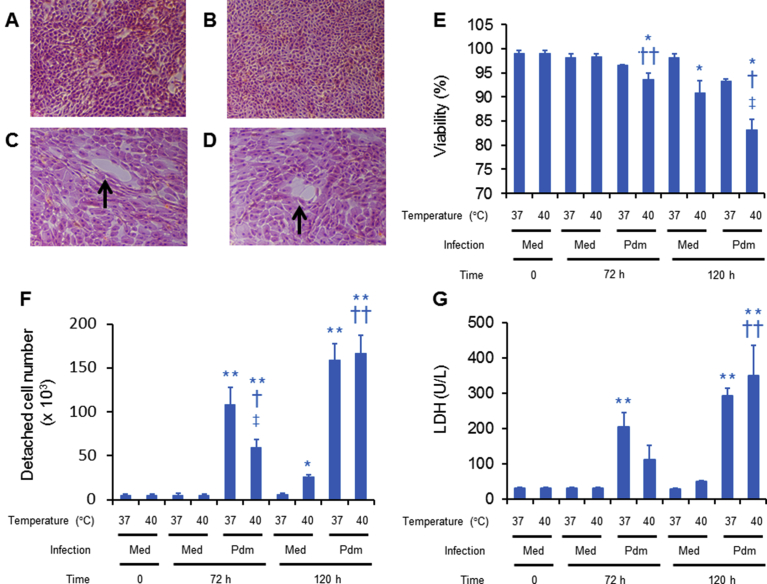
Hematoxylin eosin staining of the uninfected cells showed confluent cell sheets, and the shape and magnitude of staining of the cells cultured at 40 °C for 120 h did not differ from those at 37 °C (Fig. 1A, B). In contrast, a significant proportion of culture vessels were not covered with cells at 120 h post-infection after an incubation at 37 °C and 40 °C (Fig. 1C, D), which might be caused by cell detachment.
Fig. 1.
(A–D) Hematoxylin-eosin staining of human tracheal epithelial (HTE) cells cultured in slide glasses for 120 h at 37 °C (A, C) or 40 °C (B, D) following infection without (A, B) or with (C, D) the A/H1N1 pdm 2009 virus. Arrows show slide glasses that were not covered by cells (magnification: x 100). (E–G) Viability of attached cells (E), numbers of detached cells (F), and LDH levels in the supernatants (G) of uninfected (med) and infected (pdm) cells before (time 0) or after culture at 37 °C or 40 °C for 72 h or 120 h. (E–G) The results are expressed as the means ± SEM of five tracheae. Significant differences from uninfected cells cultured at 37°C are indicated by ∗p < 0.05 and ∗∗p < 0.01. Significant differences from uninfected cells cultured at 40 °C are indicated by †p < 0.05 and ††p < 0.01. Significant differences from infected cells cultured at 37 °C are indicated by ‡p < 0.05.
The viability of the uninfected cells cultured at 37 °C did not decrease at 72 h or 120 h (Fig. 1E). In contrast, the viability of uninfected cells cultured at 40 °C for 120 h decreased compared with the viability of cells cultured at 37 °C, although the viability of uninfected cells cultured at 40 °C for 72 h did not decrease (Fig. 1E).
Furthermore, the viability of infected cells cultured at 40 °C for 72 h and 120 h decreased compared with the viability of uninfected cells cultured at 37 °C and 40 °C. Lower viability was observed for infected cells cultured at 40 °C than infected cells cultured at 37 °C for 120 h post-infection (Fig. 1E). Viability tended to decrease in infected cells cultured at 37 °C for 72 h and 120 h compared with the viability of uninfected cells cultured at 37 °C, but the different was not statistically significant.
The number of detached cells in supernatants of uninfected cells cultured at 37 °C did not increase at 72 h and 120 h (Fig. 1F). The number of detached cells in uninfected cells cultured at 40 °C for 72 h also did not differ from the number of detached cells cultured at 37 °C. In contrast, a greater number of uninfected cells that had been cultured at 40 °C for 120 h were detached than uninfected cells cultured at 37 °C (Fig. 1F).
Viral infection increased the number of detached cells cultured at 37 °C and 40 °C for 72 h and 120 h post-infection compared with the number of uninfected detached cells cultured at 37 °C (Fig. 1F). Viral infection also increased the number of detached cells cultured at 40 °C for 72 h and 120 h post-infection compared with uninfected cells cultured at 40 °C. Fewer detached cells were observed in the infected cultures that were incubated at 40 °C for 72 h than in the infected cells cultured at 37 °C (Fig. 1F). In contrast, the number of detached cells in the infected cultures at 40 °C for 120 h did not differ from the infected cells cultured at 37 °C (Fig. 1F).
Lactate dehydrogenase (LDH) levels in supernatants of uninfected cells cultured at 37 °C did not change at 72 h and 120 h (Fig. 1G). LDH levels in uninfected cells cultured at 40 °C for 72 h also did not differ from the levels observed in cells cultured at 37 °C. LDH levels in uninfected cells cultured at 40 °C for 120 h tended to be higher than the levels observed in uninfected cells at 37 °C, but a statistically significant difference was not observed (Fig. 1G).
Viral infection increased the LDH levels in cells cultured at 37 °C for 72 h and 120 h post-infection compared with the levels in uninfected cells at 37 °C (Fig. 1G). Viral infection also increased the LDH levels in the cells cultured at 40 °C for 120 h post-infection compared with the LDH levels of uninfected cells cultured at 37 °C and 40 °C (Fig. 1G). Viral infection tended to increase the LDH levels in the cells cultured at 40 °C for 72 h compared with the LDH levels in uninfected cells cultured at 37 °C and 40 °C, but the difference was not significant (Fig. 1G). LDH levels in infected cells cultured at 40 °C for 72 h and 120 h did not differ from the levels in infected cells at 37 °C, although the levels in the infected cells cultured at 40 °C for 72 h tended to be lower than cells the infected cultures at 37 °C.
2.2. Effects of high temperature on dome formation in the absence or presence of viral infection
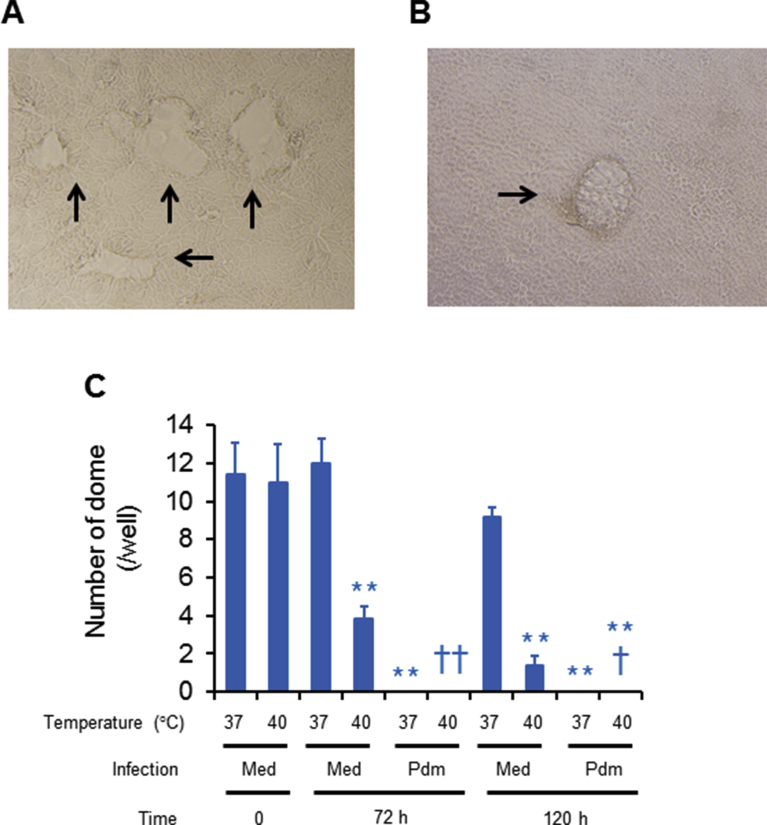
Uninfected cells cultured in the wells exhibited dome formation (Fig. 2A), and fewer domes were observed on uninfected cell monolayers cultured at 40 °C for 72 h and 120 h than on cells cultured at 37 °C (Fig. 2B, C). Dome formation disappeared in infected cell monolayers cultured at 37 °C and 40 °C for 72 h and 120 h post-infection (Fig. 2C).
Fig. 2.
(A and B) Phase contrast photographs of uninfected cell monolayers cultured at 37 °C (A) or 40 °C (B) for 120 h. Arrows show dome formation (magnification: x 100). (C) The number of domes on the cell monolayers of uninfected (med) and infected (pdm) cells before (time 0) or after culture at 37 °C or 40 °C for 72 h or 120 h. The results are expressed as the means ± SEM of five tracheae. Significant differences from uninfected cells cultured at 37 °C are indicated by **p < 0.01. Significant differences from uninfected cells cultured at 40 °C are indicated by †p < 0.05 and ††p < 0.01.
2.3. Effects of high temperature on A/H1N1 pdm 2009 and A/H3N2 Aichi viral release and RNA replication
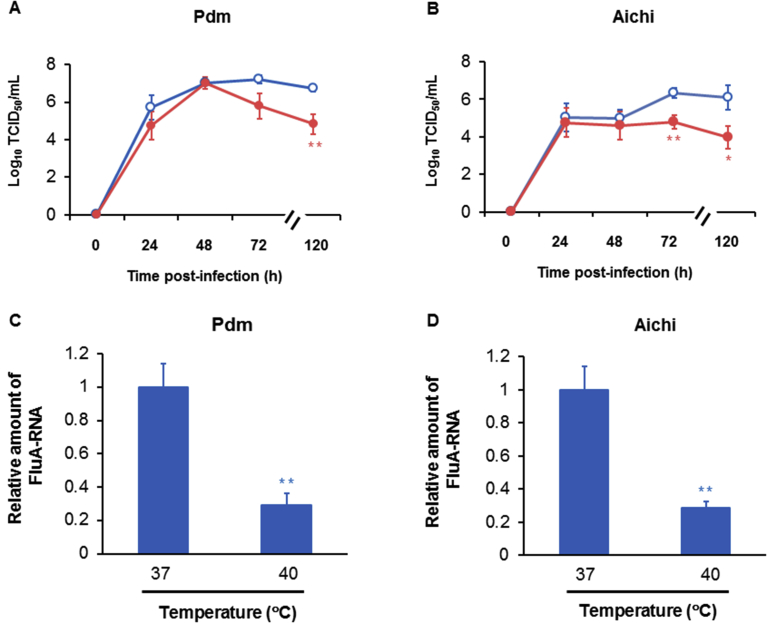
The titers of the A/H1N1 pdm 2009 virus in cells cultured at 40 °C for 24 h and 48 h post-infection did not differ from cells cultured at 37 °C (Fig. 3A, B). The titers of the A/H1N1 pdm 2009 virus in cells cultured at 40 °C at 72 h post-infection were tended to be lower than those of the cells cultured at 37 °C, but the difference was not significant (Fig. 3A). In contrast, the titers of the A/H1N1 pdm 2009 virus in cells cultured at 40 °C for 120 h post-infection were lower than cells cultured at 37 °C (Fig. 3A).
Fig. 3.
(A and B) Time course analysis of viral titers in the supernatants of HTE cells at different times after infection with A/H1N1 pdm 2009 (pdm) (A) and A/H3N2 Aichi (Aichi) (B) viruses and culture at 37 °C (open circles) or 40 °C (closed circles). Viral titers are expressed as Log10 TCID50/mL. The results are expressed as the means ± SEM of nine (day 3) or five (other than day 3) different tracheae. Significant differences from cells cultured at 37 °C are indicated by ∗p < 0.05 and ∗∗p < 0.01. (C and D) Viral RNA replication in HTE cells at 120 h post-infection with the A/H1N1 pdm 2009 (C) and A/H3N2 Aichi (D) viruses detected using real-time quantitative RT-PCR. The results are expressed as the relative amount of RNA expression compared with the expression of the influenza viral RNA (FluA-RNA) in cells cultured at 37 °C and are reported as the means ± SEM of four samples. Significant differences from cells cultured at 37 °C are indicated by ∗∗p < 0.01.
Similarly, the titers of the A/H3N2 Aichi virus in cells cultured at 40 °C collected at for 24 h and 48 h post-infection did not differ from cells cultured at 37 °C (Fig. 3B). In contrast, lower titers of the A/H3N2 Aichi virus were observed in the cells cultured at 40 °C for 72 h and 120 h post-infection than in cells cultured at 37 °C (Fig. 3B).
Lower levels of the A/H1N1 pdm 2009 and A/H3N2 Aichi viral RNAs were detected in cells cultured at 40 °C than in cells cultured at 37 °C for 120 h post-infection (Fig. 3C, D).
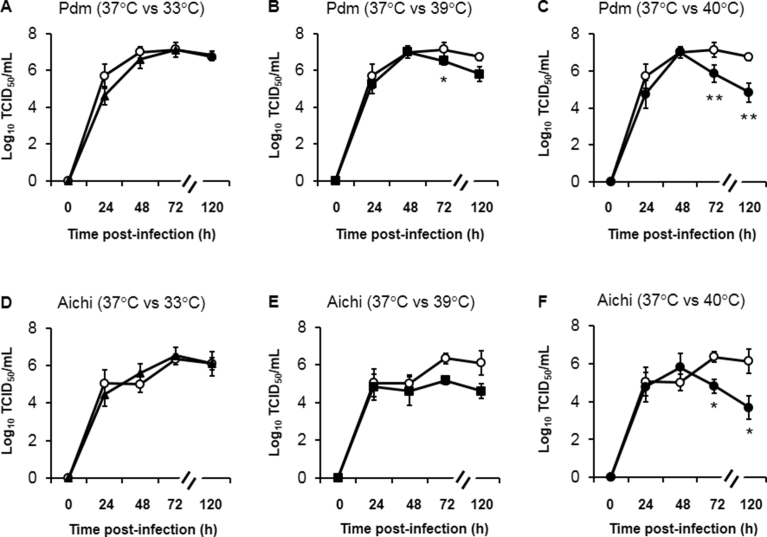
When viral titers were compared in the tracheal cells cultured at four temperatures (33 °C, 37 °C, 39 °C and 40 °C) using samples collected at four time points (24 h, 48 h, 72 h and 120 h post-infection), A/H1N1 pdm 2009 viral titers in the cells cultured at 33 °C did not differ from the titers in cells cultured at 37 °C (Fig. 4A). In contrast, lower A/H1N1 pdm 2009 viral titers were recorded at 72 h post-infection in cells cultured at 39 °C than in cells cultured at 37 °C (Fig. 4B). A/H1N1 pdm 2009 viral titers in cells cultured at 39 °C for 120 h also tended to be lower than in cells cultured at 37 °C, but a statistically significant difference was not observed (Fig. 4B). In contrast, lower A/H1N1 pdm 2009 viral titers were observed at 72 h and 120 h post-infection in cells cultured at 40 °C than in cells cultured at 37 °C (Fig. 4C).
Fig. 4.
Time course analysis of viral titers in the supernatants of HTE cells at different times after infection with A/H1N1 pdm 2009 (pdm) (A–C) and A/H3N2 Aichi (Aichi) (D–F) viruses and culture at 33 °C (closed triangles), 37 °C (open circles), 39 °C (closed squares) or 40 °C (closed circles). Viral titers are expressed as Log10 TCID50/mL. The results are expressed as the means ± SEM of five different tracheae. Significant differences from cells cultured at 37 °C at each time point are indicated by ∗p < 0.05 and ∗∗p < 0.01.
Similarly, A/H3N2 Aichi viral titers in cells cultured at 33 °C did not differ from the titers in cells cultured at 37 °C (Fig. 4D). A/H3N2 Aichi viral titers in cells cultured at 39 °C for 72 h and 120 h tended to be lower than in cells cultured at 37 °C, but a statistically significant difference was not observed (Fig. 4E). In contrast, lower A/H3N2 Aichi viral titers were observed at 72 h and 120 h post-infection in cells cultured at 40 °C than in cells cultured at 37 °C (Fig. 4F).
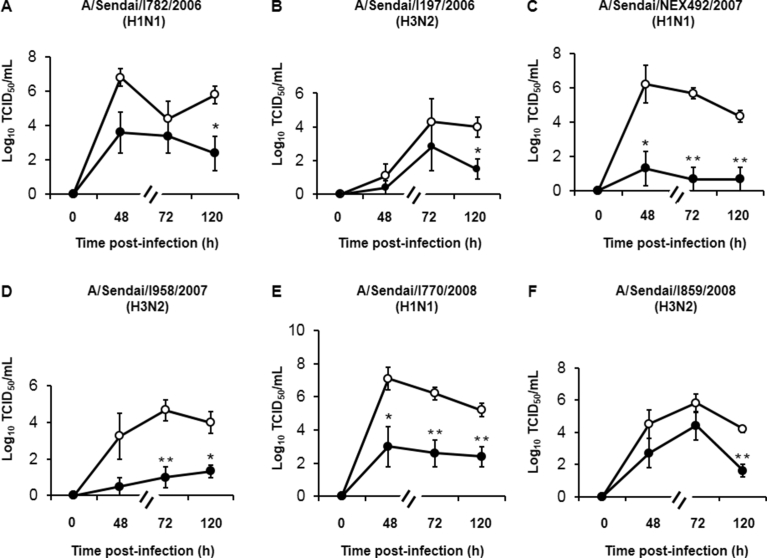
2.4. Effects of high temperature on the release of six other influenza virus strains
We examined viral titers in supernatants collected at 48, 72, and 120 h after cells were infected with six strains of influenza viruses other than A/H1N1 pdm 2009 and A/H3N2 Aichi to confirm the inhibitory effects of high temperature (40 °C) on viral replication.
At 48 h post-infection, lower titers of two strains of viruses [A/Sendai/NEX492/2007 (H1N1) and A/Sendai/I770/2008 (H1N1)] were observed in cells cultured at 40 °C than in cells cultured at 37 °C (Fig. 5C, E). In contrast, the titers of four other strains of viruses measured at 48 h post-infection in cells cultured at 40 °C did not differ from the titers in cells cultured at 37 °C, although the titers of these strains in cells cultured at 40 °C tended to be lower than the titers in cells cultured at 37 °C (Fig. 5A, B, D, and F).
Fig. 5.
Viral titers in supernatants collected at 48 h, 72 h or 120 h after infection with six strains of influenza viruses other than A/H1N1 pdm 2009 and A/H3N2 Aichi in cells cultured at 37 °C and 40 °C. Viral titers are expressed as Log10 TCID50/mL. The results are expressed as the means ± SEM of three (C, D), four (B) or five (A, E, and F) different tracheae. Significant differences from cells cultured at 37 °C are indicated by *p < 0.05 and **p < 0.01.
At 72 h post-infection, lower titers of three viral strains [A/Sendai/NEX492/2007 (H1N1), A/Sendai/I958/2007 (H3N2), A/Sendai/I770/2008 (H1N1)] were observed in cells cultured at 40 °C than in cells cultured at 37 °C (Fig. 5C–E). In contrast, the titers of the three other strains in cells cultured at 40 °C did not differ from the titers in cells cultured at 37 °C, although the titers of these strains in cells cultured at 40 °C tended to be lower than the titers in cells cultured at 37 °C (Fig. 5A, B, and F).
In contrast, at 120 h post-infection, lower titers were observed for all influenza virus strains in cells cultured at 40 °C than in cells cultured at 37 °C (Fig. 5).
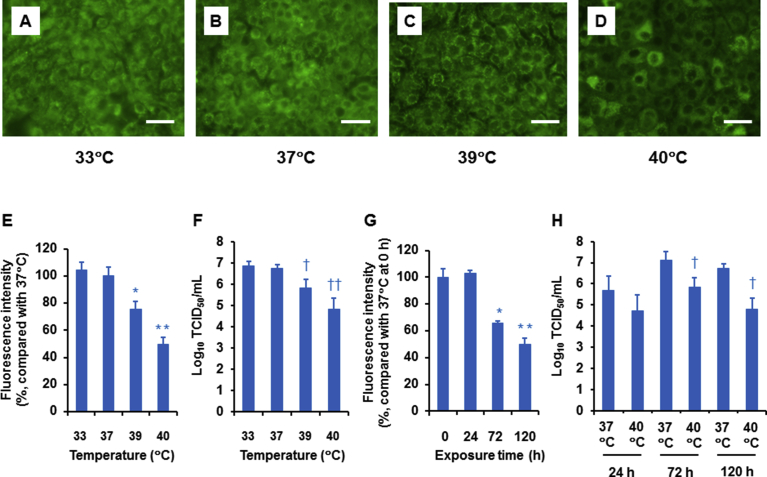
2.5. Effects of high temperature on acidic endosomes
Acidic endosomes in uninfected HTE cells were stained green with LysoSensor DND-189 (Fig. 6A–D). The number and fluorescence intensity of labeled cells cultured at 39 °C and 40 °C for 120 h were lower than in cells cultured at 37 °C (Fig. 6A–E). The fluorescence intensity in cells was reduced in a temperature-dependent manner, and the most potent inhibitory effects were observed at 40 °C (Fig. 6E). Similar to the fluorescence intensity, the A/H1N1 pdm 2009 viral titers were reduced in temperature-dependent manner, and the most potent inhibitory effects on viral titers were observed at 40 °C (Fig. 6F), suggesting a relationship between the temperature to which the cells were exposed and the reductions in the fluorescence intensity of acidic endosome and viral titers.
Fig. 6.
(A–D) Changes in the distribution of acidic endosomes exhibiting green fluorescence in uninfected HTE cells cultured at 33 °C (A), 37 °C (B), 39 °C (C), or 40 °C (D) for 120 h (bar = 100 μm). (E and F) The effects of the four temperatures on the fluorescence intensity of acidic endosomes in uninfected cells cultured for 120 h (E) and on the A/H1N1 pdm 2009 viral titers in cells cultured for 120 h (F). The results are expressed as the means ± SEM of eight (E) and five (F) tracheae. Viral titers are expressed as Log10 TCID50/mL. (G and H) The effects of the time of exposure to high temperature (40 °C) on fluorescence intensity (G) and the A/H1N1 pdm 2009 viral titers (H). The results are expressed as the means ± SEM of eight (G) and five (H) tracheae. (E–H) Significant differences from uninfected cells cultured at 37 °C are indicated by ∗p < 0.05 and ∗∗p < 0.01 (E and G). Significant differences from infected cells cultured at 37 °C are indicated by †p < 0.05 and ††p < 0.01 (F and H).
Furthermore, the fluorescence intensity in cells cultured at 40 °C was reduced in a time-dependent manner, and the most potent inhibitory effects were observed on cells exposed to the high temperature for 120 h (Fig. 6G). The A/H1N1 pdm 2009 viral titers were also reduced in a time-dependent manner, and inhibitory effects on viral titers were observed on cells cultured at 40 °C for 72 h and 120 h, but not 24 h, post-infection (Fig. 6H), suggesting a relationship between the time of exposure to high temperatures and the reductions in the fluorescence intensity of acidic endosome and viral titers.
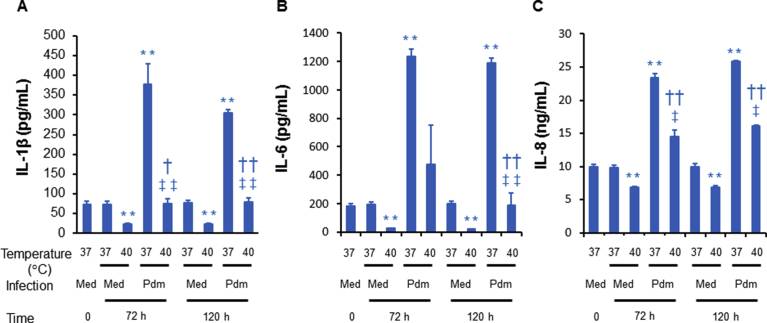
2.6. Effects of high temperature on the production of inflammatory cytokines in cells infected with and without the virus
Levels of IL-1β, IL-6 and IL-8 in the supernatants of uninfected cells cultured at 37 °C for 72 h and 120 h did not differ from the levels in uninfected cells before exposure to high temperature (time 0) (Fig. 7). In contrast, lower levels of IL-1β, IL-6 and IL-8 were observed in the supernatants of uninfected cells cultured at 40 °C for 72 h and 120 h than in uninfected cells cultured at 37 °C (Fig. 7).
Fig. 7.
The levels of IL-1β, IL-6 or IL-8 in the cell supernatants in cells cultured before (time 0) or at 72 h or 120 h after infection with sham (med) or the A/H1N1 pdm 2009 virus (pdm) and culture at 37 °C or 40 °C. The results are expressed as the means ± SEM of three tracheae. Significant differences from uninfected cells cultured at 37 °C are indicated by **p < 0.01. Significant differences from uninfected cells cultured at 40 °C are indicated by †p < 0.05 and ††p < 0.01. Significant differences from infected cells cultured at 37 °C are indicated by ‡p < 0.05 and ‡‡p < 0.01.
Infection with the A/H1N1 pdm 2009 virus increased the levels of IL-1β, IL-6 and IL-8 in cells cultured at 37 °C for 72 h and 120 h than in uninfected cells cultured at 37 °C (Fig. 7). Infection with the A/H1N1 pdm 2009 virus also increased the levels of IL-1β and IL-8 in cells cultured at 40 °C for 72 h and 120 h and the levels of IL-6 in cells cultured at 40 °C for 120 h than in uninfected cells cultured at 40 °C (Fig. 7).
Furthermore, lower levels of these cytokines were observed in the infected cells cultured at 40 °C than in infected cells cultured at 37 °C, although IL-6 production was not significantly decreased at 72 h post-infection in infected cells cultured at 40 °C (Fig. 7).
3. Discussion
As shown in the present study, lower viral titers were observed in the supernatants of HTE cells exposed to high temperature (39 °C and/or 40 °C) for 72 h and/or 120 h after infection with the A/H1N1 pdm 2009 and A/H3N2 Aichi viruses than in cells cultured at 37 °C. Long-term exposure to high temperatures also decreased RNA replication in these two viral strains. Lower viral titers of six other influenza virus strains were observed in cells cultured at 40 °C for 48 h, 72 h and/or 120 h post-infection than in cells cultured at 37 °C. The number and fluorescence intensity of acidic endosomes, by which viral ribonucleoproteins containing the influenza virus RNA enter the cytoplasm [11], were decreased in cells cultured at 40 °C for 72 h or longer. These findings suggest that long-term exposure to high temperatures decreases influenza virus replication by affecting the function of acidic endosomes. Furthermore, cell viability decreased, the number of detached cells increased and LDH levels tended to increase from 72 h to 120 h in uninfected cells cultured at 40 °C, which might explain the decreased viral titers observed at the 120 h time point.
According to Tang et al. [4], a high temperature is indicated by a 39.4 °C for the mean peak body temperature, and 3–4 days of febrile episodes in children with seizures induced by influenza virus infection. In the present study, the titers of the A/H1N1 pdm 2009 virus decreased in cells cultured at 39 °C for 72 h post-infection (Fig. 4), and the titers of the A/H3N2 Aichi virus decreased in cells cultured at 40 °C for 72 h post-infection (Fig. 3). Furthermore, decreased viability and increased numbers of detached cells and LDH levels in supernatants were observed in infected cells cultured at 37 °C and/or 40 °C for 72 h compared with uninfected cells cultured at 37 °C. Fewer domes were observed on uninfected cell monolayers cultured at 40 °C for 72 h than on uninfected cell monolayers cultured at 37 °C, and dome formation disappeared in infected cell monolayers cultured at 37 °C and 40 °C for 72 h post-infection. Thus, exposure to high temperatures observed in humans for 3–4 days after influenza virus infection [4] may induce human airway epithelial cell damage and may reduce the replication of pandemic and seasonal human influenza viruses, although exposure to high temperature of 40 °C for more than 96 h may not be occur in humans.
In contrast, the A/H1N1 pdm 2009 and A/H3N2 Aichi viral titers in cells exposed to high temperature of 40 °C for 24 h did not differ from cells cultured at 37 °C. Short-term exposure to a high temperature of 40 °C for 24 h did not decrease the number or fluorescence intensity of acidic endosomes; thus, short-term exposure to a high temperature may be insufficient to decrease viral replication.
The influenza virus is internalized by receptor-mediated endocytosis, and the low pH of the endosome triggers viral and endosomal membrane fusion [11], resulting in subsequent viral replication. Vacuolar H+-ATPase and ion transport across Na+/H+ exchangers regulate the endosomal pH [12, 13]. No studies have examined the effects of high temperature on the H+-ATPase and Na+/H+ exchangers in airway epithelial cells, but a high temperature of 39 °C affects H+/K+-ATPase mRNA levels in gastric epithelial cells [14]. Therefore, a high temperature may affect the functions of the ion channels that regulate endosomal pH.
As shown in our previous study, an anti-IL-6 receptor antibody and an NF- κB inhibitor, caffeic acid phenethyl ester, reduce the titers of the A/H1N1 pdm 2009 virus [15]. Furthermore, IL-6 activates caspases [16] that enhance the release of viral ribonucleoprotein complexes from the nucleus [17]. In our previous study, a caspase-3 inhibitor, Z-DEVD-fmk, reduced the A/H1N1 pdm 2009 viral titers in the supernatants [15]. The finding that high temperature reduced IL-6 levels in the present study is consistent with results from a previous reports showing that high temperature inhibits IL-6 production induced by lipopolysaccharides in mice [18] and inhibits NF-κB signaling [19], which is associated with IL-6 production [20]. Therefore, the high temperature-induced decrease in IL-6 production might also be associated with the decreased influenza virus replication in the present study. Furthermore, reduced production of IL-1β and IL-8 following exposure to high temperature might also be mediated by the inhibition of NF-κB [19] under these conditions, which is also associated with the production of IL-1β and IL-8 [21].
We demonstrated that long-term exposure to high temperatures decreased viral replication. However, influenza virus infection induced epithelial cell damage at 40 °C to a similar extent as observed at 37 °C. Therefore, we examined whether long-term exposure to high temperatures induces damage in cells that were not infected with the influenza virus. Long-term exposure to high temperature (40 °C) decreased the viability of attached cells, increased the numbers of detached cells, tended to increase LDH levels in the supernatants and reduced the number of domes on cell monolayers. The LDH levels and cell viability parameters are markers of apoptosis and necrosis [22, 23]. Dome formation is associated with sodium movement and may indicate membrane integrity [24, 25]. Furthermore, high temperature has been shown to induce cancer cell apoptosis by producing reactive oxygen species [26] and inhibiting the mitochondrial membrane potential. In the present study, virus infection decreased cell viability and increased the number of detached cells and the LDH levels in the supernatant, consistent with our previous study [15]. Therefore, the cytotoxic effects of a high temperature might have increased cell damage induced by infection with the A/H1N1 pdm 2009 virus at 40 °C in the present study.
Dome formation indicates fluid absorption and epithelial cell differentiation [24, 27]. Epithelial sodium channels play key roles in sodium absorption in airway epithelial cells [28], and inhibition of this channel decreases the dome formation frequency [29]. Sodium absorption from the apical membrane of cells is regulated by various ion channels, including a basolateral potassium channel, a Na+/K+/2Cl co-transporter and Na+/K+-ATPase [24, 30]. Intracellular free sodium levels induce sodium transport outside of cells across the basolateral membrane [24] and regulate membrane integrity [25], whereas high temperature decreases intracellular free sodium levels [31]. Therefore, these effects of high temperature may be associated with the decreased in the number of domes formed on cell monolayers.
Furthermore, increased paracellular permeability reduces domes in the epithelial cells [32] and a relationship between epithelial cell damage and increased permeability has been reported [33]. Therefore, the decreased number of domes observed in the present study may suggest cell damage induced by high temperatures and influenza virus infection.
High temperature decreases influenza virus release from allantois-on-shell cultures at 24 h post-infection and viral titers in ferret nasal washes at 18–66 h post-infection [2, 7]. In contrast, the growth capacity of an influenza [A/WSN/1933 (A/H1N1)] virus in MDCK cultured at 33 °C and 39.5 °C was similar at 48 h post-infection [8]. In the present study, A/H1N1 pdm 2009 and A/H3N2 Aichi viral titers were decreased in cells exposed to high temperatures for 72 h or longer. The potency of the inhibitory effects of high temperature on viral replication also differed between influenza virus species in the present study. The reasons for the different effects of high temperature on viral replication between influenza virus species and between different studies are uncertain, but differences in measurement methods, observation times after viral infection and temperature-sensitivity of influenza virus strains [34] may underlie these differences.
We examined the effects of high temperatures on the replication of eight strains of seasonal influenza viruses. We also measured viral RNA replication in A/H1N1 pdm 2009 and A/H3N2 Aichi viruses to confirm the viral replication of seasonal human A/H1N1 and A/H3N2 influenza viruses, as we previously reported [15]. Furthermore, we studied the effects of high temperatures on cell damage and inflammatory cytokine production in the absence or presence of the A/H1N1 pdm 2009 virus, because we previously observed that this type of influenza virus induced the greatest amount of cell damage among the human seasonal influenza viruses we examined [15].
Human bronchial epithelial cells have been used in previous studies to reveal pathophysiological effects of influenza virus infection [35]. Similarly, human tracheal epithelial (HTE) cells express the receptor for influenza virus [36] and have also been used in studies in influenza virus infection [35, 37]. We established HTE cell culture methods [38], confirmed the expression of the receptor for influenza [39], and used these cells in influenza virus infection studies [15, 39]. Based on these findings, we used HTE cells in the present study.
Cells isolated from the human trachea comprise basal and intermediate cells, with occasional ciliated and goblet cells [40]. As shown in our previous study, cells cultured with air-interface methods on filter membranes coated with collagen gel form multilayered structures, and the luminal surface contains cilia and secretory granules [38]. Although we cultured cells in wells in the present study and did not determine the specific cell types, we could detect cilia beating under the microscope and a good cell condition before influenza virus infection.
Supernatants of HTE cells contain a significant amount of influenza virus, and the virus consistently replicated in HTE cells after infection with virus stocks generated using HTE cells [15]. This infection system may recapitulate influenza virus replication in airway cells infected with the virus that is released from adjacent cells. Therefore, we used virus stocks that were grown in HTE cells, as we previously reported [15], rather than MDCK cells, which are cultured in MEM supplemented with trypsin.
The effects of anti-influenza drugs on improving symptoms, including fever, and on reducing viral replication have previously been reported [41, 42]. Fever is the major symptom of influenza virus infection, and a high temperature of 39.4 °C for mean peak body temperature has been reported in children with seizures induced by influenza virus infection [4, 5], indicating that high temperature correlates with influenza symptoms, including febrile seizures.
Therefore, the use of antipyretic drugs to treat fever is thought to be necessary in children suffering from the adverse effects of high temperature, such as febrile seizures [4, 5], as well as in patients with dehydration and severe outcomes caused by high temperature-induced excessive sweating and anorexia [5, 6]. Scientific evidence of the antipyretic efficacy and safety of acetaminophen in children has also been reported [43]. Because a similar extent of airway epithelial cell damage was also observed after influenza virus infection at both high and normal temperatures, prompt reduction of fevers using antipyretic and anti-influenza drugs could help improve patients' airway conditions.
This study had some limitations. The culture system contained surface epithelial cells alone and may not have contained other cells [38] involved in defense mechanisms against viral infection [1]. Second, significant amounts of the antiviral substrate, IFN, were undetected in the HTE cell supernatants, as we previously reported [15]. Finally, we did not examine the effects of high temperature on the viral RNA polymerase activity or on inhibition of nuclear export of the influenza virus ribonucleoprotein complex by heat shock protein [9, 10]. However, according to Da Costa et al., the RNA polymerase activity of influenza A virus is affected by high temperatures and may alter virus replication [8]. Furthermore, influenza A viruses from different species have different temperature optima [8].
4. Conclusions
Long-term exposure to a clinically high temperature may reduce viral replication by affecting the function of acidic endosomes and inhibiting the IL-6-mediated processes. However, in contrast to the beneficial effects observed on other cells, high temperature may cause similar levels of airway cell damage after infection to cells exposed normal temperatures. Prompt reductions in fevers using antipyretic and anti-influenza drugs may help improve patients' airway conditions.
5. Materials and methods
5.1. Human tracheal epithelial cell cultures
HTE cells were cultured at 37 °C prior to influenza virus infection, as previously described [15]. Cells were plated in plastic 24-well plates (Becton Dickinson, Franklin Lakes, NJ, USA) or 96-well plates, on slide glasses, or on coverslips in petri dishes and cultured in 0.2 mL or 1 mL of DMEM-Ham's F-12 (DF12) containing 2% Ultroser G. Cells were cultured at 33 °C, 37 °C, 39 °C or 40 °C after infection with A/H1N1 pdm 2009 and A/H3N2 Aichi viruses.
Preliminary studies showed that long-term exposure to high temperatures reduced the titers of these two viral strains; therefore, cells were cultured at 37 °C and 40 °C after infection with six different viral strains to confirm the inhibitory effects of long-term exposure to high temperature on viral replication. Tracheas used for cell cultures were obtained from 30 patients after death (age, 71 ± 13 yrs; 14 females and 16 males). This study was approved by the Tohoku University Ethics Committee (IRB number: 2016-1-803).
5.2. Measurement of cell damage and dome formation
Cells were plated in 24-well dishes, and the viability of adhered cells, number of floating cells that had detached from the adherent cell monolayers on the culture vessels, and lactate dehydrogenase (LDH) concentrations in the supernatants were measured [15]. Adhered cells were isolated by exposure to trypsin-EDTA solution, and the viability of the collected cells was measured by trypan blue exclusion. Floating cells in the supernatants were collected by centrifugation, and the cell numbers were measured. LDH was measured using the Japanese Society of Clinical Chemistry (JSCC) recommended method (lactate to pyruvate direction) [44, 45]. Because dome formation indicates fluid absorption and epithelial cell differentiation [24, 27], the number of domes per unit area (0.36 cm2) was measured in 96-well plates.
5.3. Virus stocks
Influenza virus stocks were generated by collecting the supernatants after infecting HTE cells with one of the eight following human influenza virus strains using the methods reported in our previous study [15]: the pandemic A/H1 2009 virus [A/H1N1 pdm 2009, A/Sendai-H/N0633/2009 (H1N1) pdm09], A/H3N2 Aichi influenza virus [A/H3N2 Aichi, A/Aichi/2/68 (H3N2)] and 6 human influenza virus strains [A/Sendai/I782/2006 (H1N1), A/Sendai/I197/2006 (H3N2), A/Sendai/NEX492/2007 (H1N1), A/Sendai/I958/2007 (H3N2), A/Sendai/I770/2008 (H1N1), and A/Sendai/I859/2008 (H3N2)]. The 6 viruses, other than the A/H1N1 pdm 2009 and A/H3N2 Aichi influenza viruses, were also isolated from Japanese patients with symptoms of influenza virus infection, including fever.
5.4. Viral detection and titration
Influenza viruses in the culture supernatants were detected and titrated using the endpoint method by infecting replicate MDCK cells in plastic 96-well plates with 10-fold dilutions of virus-containing supernatants, as previously described [15, 46, 47]. Then, the presence of the characteristic cytopathic effects of influenza virus was determined. The TCID50 (TCID, tissue culture infective dose) was calculated using previously described methods [46]; the viral titers in the supernatants are reported as TCID50/mL [15].
5.5. Viral infection of cells
HTE cells were infected with influenza viruses using previously described methods [15]. An influenza virus stock solution was added to the cells in 24-well plates [400 μL per well, 1.0 × 103 TCID50/mL, multiplicity of infection (MOI) of 0.0008]. After a 1 h incubation, the viral solution was removed and the cells were cultured in 1 ml of fresh medium at 37 °C in a 5% CO2-95% air atmosphere.
5.6. Supernatant collection
Supernatants (400 μL) were collected at 24 h, 48 h or 72 h post-infection, and then the same volume (400 μL) of fresh medium was added after the collection of supernatants, as previously reported [15, 48]. The entire supernatant volume (1 mL) was also collected 120 h after infection. Using these methods, we observed consistent viral release [15, 48].
5.7. Quantification of influenza virus RNA
To confirm the effects of hyperthermia on A/H1N1 pdm 2009 and A/H3N2 Aichi viral replication, a two-step real-time quantitative reverse transcription (RT)-PCR assay was performed using TaqMan® Gene Expression Master Mix (Applied Biosystems, Bedford, CA, USA), as described previously [15].
5.8. Measurement of changes in acidic endosomes
We examined the effects of high temperature on acidic endosomes through which viral RNA enters the cytoplasm [11] to determine the mechanisms by which high temperature alters viral replication. The distribution and fluorescence intensity of the acidic endosomes in cells were measured with LysoSensor DND-189 dye (Molecular Probes, Eugene, OR, USA) and live-cell imaging using previously described methods [39]. Cells cultured on coverslips in petri dishes were observed with a fluorescence microscope (OLYMPUS IX70; OLYMPUS Co. Ltd., Tokyo, Japan). The excitation wavelength was 443 nm, and the light emitted from the cells was detected through a 505-nm filter. The fluorescence intensity was calculated using a fluorescence image analyzer system (Lumina Vision®; Mitani Co. Ltd., Fukui, Japan) equipped with a fluorescence microscope. Uninfected cells were cultured on coverslips in petri dishes at 33 °C, 37 °C, 39 °C or 40 °C for 24, 72 or 120 h. The fluorescence intensity of the acidic endosomes was measured in 100 cells, and the mean value of the fluorescence intensity was expressed as a percentage of the control value compared with the fluorescence intensity of the cells cultured at 37 °C.
5.9. Measurement of inflammatory cytokine levels
We also measured IL-6 levels in the supernatants because we previously showed that the level of IL-6 production is associated with the extent of influenza virus replication [15]. Furthermore, we measured the levels of IL-1β and IL-8 to study the effects of high temperature on the production of inflammatory cytokines following influenza viral infection [15, 39]. IL-6 levels were measured using a specific enzyme-linked immunosorbent assay (ELISA; a solid phase chemiluminescence ELISA kit, QuantinGlo® ELISA, R&D Systems, MN, USA). IL-1β and IL-8 levels were also measured using a Human IL-1 beta/IL-1F2 Quantikine HS ELISA kit (R&D Systems) and an enzyme immunoassay kit (IL-8 EASIA kit, Invitrogen, CA, USA), respectively.
5.10. Statistical analysis
The results are expressed as means ± SEM. Statistical analyses were performed using two-way repeated measures analysis of variance (ANOVA). Subsequent post-hoc analyses were performed using Bonferroni's method. Student's t-tests were performed for comparisons between two groups. Values of p < 0.05 were considered significant for all analyses. In all experiments, n refers to the number of donors (tracheae) from whom the cultured epithelial cells were obtained. All analyses were performed using SPSS version 20 (IBM Japan, Tokyo, Japan).
Declarations
Author contribution statement
Mutsuo Yamaya: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Hidekazu Nishimura: Conceived and designed the experiments; Performed the experiments.
Nadine Lusamba Kalonji, Xue Deng, Yoshitaka Shimotai: Performed the experiments.
Haruki Momma: Analyzed and interpreted the data.
Ryoichi Nagatomi: Conceived and designed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Suguru Omiya, the staff at Sendai National Hospital, and the staff at the Biomedical Research Unit and the Department of Pathology at Tohoku University Hospital for providing technical support.
References
- 1.Roberts N.J., Jr. Temperature and host defense. Microbiol. Rev. 1979;43:241–259. doi: 10.1128/mr.43.2.241-259.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Overton H.A. Molecular studies of the differential replication at pyrexial temperatures of two influenza viruses differing in virulence for ferrets. Virus Res. 1986;5:235–251. doi: 10.1016/0168-1702(86)90021-3. [DOI] [PubMed] [Google Scholar]
- 3.Weber F. Inverse interference: how viruses fight the interferon system. Viral Immunol. 2004;17:498–515. doi: 10.1089/vim.2004.17.498. [DOI] [PubMed] [Google Scholar]
- 4.Tang J. Relationship between common viral upper respiratory tract infections and seizures in children from Suzhou, China. J. Child Neurol. 2014;29:1327–1332. doi: 10.1177/0883073813515074. [DOI] [PubMed] [Google Scholar]
- 5.Dalziel S.R., Pediatric Emergency Research Networks H1N1 Working Group Predictors of severe H1N1 infection in children presenting within pediatric emergency research Networks (PERN): retrospective case-control study. Br. Med. J. 2013;347:f4836. doi: 10.1136/bmj.f4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujikura Y. Mortality and severity evaluation by routine pneumonia prediction models among Japanese patients with 2009 pandemic influenza A (H1N1) pneumonia. Respir. Investig. 2014;52:280–287. doi: 10.1016/j.resinv.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Husseini R.H. Elevation of nasal viral levels by suppression of fever in ferrets infected with influenza viruses of differing virulence. J. Infect. Dis. 1982;145:520–524. doi: 10.1093/infdis/145.4.520. [DOI] [PubMed] [Google Scholar]
- 8.Da Costa B. Temperature-sensitive mutants in the influenza A virus RNA polymerase: alterations in the PA linker reduce nuclear targeting of the PB1-PA dimer and result in viral attenuation. J. Virol. 2015;89:6376–6390. doi: 10.1128/JVI.00589-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashiwagi T. Artificial hybrids of influenza A virus RNA polymerase reveal PA subunit modulates its thermal sensitivity. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirayama E. Heat shock protein 70 is related to thermal inhibition of nuclear export of the influenza virus ribonucleoprotein complex. J. Virol. 2004;78:1263–1270. doi: 10.1128/JVI.78.3.1263-1270.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sieczkarski S.B. Role of protein kinase C-II in influenza virus entry via late endosomes. J. Virol. 2003;77:460–469. doi: 10.1128/JVI.77.1.460-469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshansky V. Proton gradient formation in early endosomes from proximal tubules. Biochim. Biophys. Acta. 1996;1284:171–180. doi: 10.1016/s0005-2736(96)00123-x. [DOI] [PubMed] [Google Scholar]
- 13.Mellman I. Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 14.Tabuchi Y. New gastric epithelial cell lines from mice transgenic for temperature-sensitive Simian virus 40 large T antigen show distinct types of cell differentiation. Digestion. 2003;67:71–81. doi: 10.1159/000070396. [DOI] [PubMed] [Google Scholar]
- 15.Yamaya M. Magnitude of influenza virus replication and cell damage is associated with interleukin-6 production in primary cultures of human tracheal epithelium. Respir. Physiol. Neurobiol. 2014;202:16–23. doi: 10.1016/j.resp.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Cai Q. Recombinant adenovirus Ad-RUNrf2 reduces paraquat-induced A549 injury. Hum. Exp. Toxicol. 2012;31:1102–1112. doi: 10.1177/0960327112450902. [DOI] [PubMed] [Google Scholar]
- 17.Wurzer W.J. Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J. 2003;22:2717–2728. doi: 10.1093/emboj/cdg279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welc S.S. The impact of hyperthermia on receptor-mediated interleukin-6 regulation in mouse skeletal muscle. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janus P. Cross talk between cytokine and hyperthermia-induced pathways: identification of different subsets of NF-κB-dependent genes regulated by TNFα and heat shock. Mol. Genet. Genom. 2015;290:1979–1990. doi: 10.1007/s00438-015-1055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Z. Rhinovirus stimulation of interleukin-6 in vivo and in vitro. Evidence for nuclear factor κB-dependent transcriptional activation. J. Clin. Invest. 1996;97:421–430. doi: 10.1172/JCI118431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah S.A. Oxytetracycline inhibits mucus secretion and inflammation in human airway epithelial cells. Chemotherapy. 2017;62:301–306. doi: 10.1159/000475983. [DOI] [PubMed] [Google Scholar]
- 22.Catalani S. Metabolism modifications and apoptosis induction after CellfoodTM administration to leukemia cell lines. J. Exp. Clin. Canc. Res. 2013;32:63. doi: 10.1186/1756-9966-32-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cechetti F. Effect of treadmill exercise on cell damage in rat hippocampal slices submitted to oxygen and glucose deprivation. Brain Res. 2007;1157:121–125. doi: 10.1016/j.brainres.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 24.Widdicombe J.H. Primary cultures of the dog’s tracheal epithelium: fine structure, fluid and electrolyte transport. Cell Tissue Res. 1987;247:95–103. doi: 10.1007/BF00216551. [DOI] [PubMed] [Google Scholar]
- 25.Lakos Z. The effect of transmembrane potential on the dynamic behavior of cell membranes. Biochim. Biophys. Acta. 1990;1023:41–46. doi: 10.1016/0005-2736(90)90007-b. [DOI] [PubMed] [Google Scholar]
- 26.Fu Q. Association of elevated reactive oxygen species and hyperthermia induced radiosensitivity in cancer stem-like cells. Oncotarget. 2017;8:101560–101571. doi: 10.18632/oncotarget.21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondo M. Simple technique for culture of highly differentiated cells from dog tracheal epithelium. Am. J. Physiol. 1991;261:L106–L117. doi: 10.1152/ajplung.1991.261.2.L106. [DOI] [PubMed] [Google Scholar]
- 28.Schild L. Structure function relationship of ENaC and its role in sodium handling. Adv. Exp. Med. Biol. 2001;502:305–314. doi: 10.1007/978-1-4757-3401-0_20. [DOI] [PubMed] [Google Scholar]
- 29.Brouard M. Epithelial sodium in human epidermal keratinocytes: expression of its subunits and relation to sodium transport and differentiation. J. Cell Sci. 1999;112:3343–3352. doi: 10.1242/jcs.112.19.3343. [DOI] [PubMed] [Google Scholar]
- 30.Mason R.J. Transepithelial transport by pulmonary alveolar type II cells in primary culture. Proc. Natl. Acad. Sci. U.S.A. 1982;79:6033–6037. doi: 10.1073/pnas.79.19.6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amorino G.P. Effects of hyperthermia on intracellular chloride. J. Membr. Biol. 1996;152:217–222. doi: 10.1007/s002329900099. [DOI] [PubMed] [Google Scholar]
- 32.Cattaneo I. Shear stress reverses dome formation in confluent renal tubular cells. Cell. Physiol. Biochem. 2011;28:673–682. doi: 10.1159/000335813. [DOI] [PubMed] [Google Scholar]
- 33.Van Spaendonk H. Regulation of intestinal permeability: the role of proteases. World J. Gastroenterol. 2017;23:2106–2123. doi: 10.3748/wjg.v23.i12.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiseleva I. Phenotypic characteristics of novel swine-origin influenza A/California/07/2009 (H1N1) virus. Influenza Other Respir. Viruses. 2010;4:1–5. doi: 10.1111/j.1750-2659.2009.00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan R.W. Use of ex vivo and in vitro cultures of the human respiratory tract to study the tropism and host responses of highly pathogenic avian influenza A (H5N1) and other influenza viruses. Virus Res. 2013;178:133–145. doi: 10.1016/j.virusres.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baum L.G. Sialyloligosaccharides of the respiratory epithelium in the selection of human influenza virus receptor specificity. Acta Histochem. Suppl. 1990;40:35–38. [PubMed] [Google Scholar]
- 37.Davis A.S. Validation of normal human bronchial epithelial cells as a model for influenza A infections in human distal trachea. J. Histochem. Cytochem. 2015;63:312–328. doi: 10.1369/0022155415570968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaya M. Differenciated structure and function of cultures from human tracheal epithelium. Am. J. Physiol. 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- 39.Yamaya M. Clarithromycin inhibits type A seasonal influenza virus infection in human airway epithelial cells. J. Pharmacol. Exp. Ther. 2010;333:81–90. doi: 10.1124/jpet.109.162149. [DOI] [PubMed] [Google Scholar]
- 40.Widdicombe J.H. Electrical properties of monolayers cultured from cells of human tracheal mucosa. J. Appl. Physiol. 1985;58:1729–1735. doi: 10.1152/jappl.1985.58.5.1729. [DOI] [PubMed] [Google Scholar]
- 41.Saito R. Reduced effectiveness of oseltamivir in children infected with oseltamivir-resistant influenza A (H1N1) viruses with His275Tyr mutation. Pediatr. Infect. Dis. J. 2010;29:898–904. doi: 10.1097/INF.0b013e3181de9d24. [DOI] [PubMed] [Google Scholar]
- 42.Treanor J.J., For the US Oral Neuraminidase Study Group Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized control trial. J. Am. Med. Assoc. 2000;283:1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 43.Temple A.R. Dosing and antipyretic efficacy of oral acetaminophen in children. Clin. Ther. 2013;35:1361–1375. doi: 10.1016/j.clinthera.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 44.Marui Y. Multi-enzyme reference material from established human cell lines and human sources. Clin. Chim. Acta. 1995;233:19–38. doi: 10.1016/0009-8981(94)05963-s. [DOI] [PubMed] [Google Scholar]
- 45.Maekawa M. Lactate dehydrogenase (LD) (English Abstract) Rinsho Byori Suppl. 2001;116:81–89. [PubMed] [Google Scholar]
- 46.Numazaki Y. A microplate method for isolation of viruses from infants and children with acute respiratory infections. Microbiol. Immunol. 1987;31:1085–1095. doi: 10.1111/j.1348-0421.1987.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 47.Condit R.C. Principles of virology. In: Knipe D.M., Howley P.M., editors. sixth ed. vol. 1. Lippincott Williams & Wilkins Inc; Philadelphia: 2013. pp. 21–51. (Fields Virology). [Google Scholar]
- 48.Yamaya M. Effects of neuraminidase inhibitors on the release of oseltamivir-sensitive and oseltamivir-resistant influenza viruses from primary cultures of human tracheal epithelium. J. Med. Virol. 2015;87:25–34. doi: 10.1002/jmv.23974. [DOI] [PubMed] [Google Scholar]