Abstract
Introduction
Hepatitis C virus (HCV) infection is common in patients with end-stage renal disease. We investigated the safety and efficacy of ombitasvir (OBV)/paritaprevir (PTV)/ritonavir (r) ± dasabuvir (DSV) ± ribavirin (RBV) in 2 phase 3, open-label, multicenter studies in patients with stage 4 or 5 chronic kidney disease (CKD).
Methods
RUBY-I, Cohort 2 enrolled treatment-naïve or -experienced patients with HCV genotype (GT) 1a or 1b infection, with or without cirrhosis. Patients received 12 weeks (24 weeks for GT1a patients with cirrhosis) of OBV/PTV/r + DSV; all GT1a patients received RBV. RUBY-II enrolled treatment-naïve patients with GT1a or GT4 infection without cirrhosis. All patients received 12 weeks of RBV-free treatment: OBV/PTV/r + DSV for GT1a-infected patients; OBV/PTV/r for GT4-infected patients. The primary endpoint was sustained virologic response at posttreatment week 12 (SVR12).
Results
RUBY-I, Cohort 2 and RUBY-II enrolled 66 patients, including 50 (76%) on dialysis; 15 (23%) had compensated cirrhosis. Overall, the SVR12 rate was 95% (63/66); 1 patient had virologic failure. There were 3 discontinuations due to adverse events. Seventy-three percent (27/37) of patients receiving RBV had adverse events leading to RBV dose modification. The RBV-free RUBY-II study had no hemoglobin-associated adverse events.
Conclusion
Treatment with OBV/PTV/r ± DSV ± RBV was well tolerated and patients with HCV GT1 or 4 infection and stage 4 or 5 CKD had high SVR12 rates, including patients with compensated cirrhosis and/or prior treatment experience.
Keywords: chronic kidney disease, NS5A inhibitor, NS3/4A protease inhibitor, renal disease, RUBY
See Commentary on Page 191
HCV is responsible for an estimated >70 million infections worldwide,1 which can lead to the development of cirrhosis and hepatocellular carcinoma.2 HCV is also a risk factor for progression to end-stage renal disease (ESRD),3, 4 and patients with ESRD have a much higher incidence of HCV infection than the general population.5 Few direct-acting antiviral (DAA) regimens can be administered to patients with severe renal insufficiency,6, 7 and before the approval of regimens that are safe and effective in this population, most of these patients were left untreated.8
Regimens containing the nucleoside polymerase inhibitor sofosbuvir, including coformulated combinations with NS5A inhibitors ledipasvir and velpatasvir, and the NS3 protease inhibitor voxilaprevir are not recommended for patients with advanced renal impairment (estimated glomerular filtration rate [eGFR] <30 ml/min per 1.73 m2) because the principal sofosbuvir metabolite, GS-331007, is cleared primarily by the kidney, with up to 20-fold higher exposures in patients with ESRD.9, 10, 11, 12 Before the approval of elbasvir/grazoprevir in 201613 and glecaprevir/pibrentasvir in 2017,14 regimens recommended in current HCV treatment guidelines for patients with stage 4 or 5 CKD, the combination of OBV (NS5A inhibitor), PTV (NS3/4A protease inhibitor; discovered by AbbVie and Enanta and administered with low-dose ritonavir [r] as a pharmacokinetic enhancer), and DSV (non-nucleoside NS5B polymerase inhibitor) was the only commercially available DAA combination regimen recommended for use in patients with advanced CKD.15
The DAA combination of OBV/PTV/r ± DSV contains 4 components that are eliminated via biliary excretion or metabolism with minimal renal excretion.15 This regimen thus requires no dose adjustment in patients with any degree of renal impairment.15 RUBY-I was a phase 3 study that evaluated the safety, pharmacokinetics, and efficacy of this combination in patients with stage 4 or 5 CKD and was coadministered with an adjusted RBV dosage of 200 mg daily. Cohort 1 of this study, conducted in treatment-naïve patients without cirrhosis, had drug exposures comparable to those seen in healthy patients and SVR in 18 (95%) of 19 patients who completed treatment and follow-up.16 Here, we report the results of RUBY-I, Cohort 2 (n = 48), which includes patients with compensated cirrhosis, prior interferon (IFN)-based treatment experience, and ESRD on dialysis, including peritoneal dialysis.
Despite the use of a low initial dose of RBV in RUBY-I, Cohort 1, RBV therapy was interrupted or discontinued in a high percentage of patients due to anemia. However, it is unclear whether RBV is essential in this population. Evidence suggests that hepatic fibrosis and inflammation are less extensive,17 and plasma HCV RNA levels are lower in HCV-infected patients on hemodialysis than HCV-infected patients with normal renal function. The latter observation has been attributed to adhesion of circulating virions to dialysis membranes.18 To investigate whether these favorable prognostic factors allow a subset of GT1a-infected patients to be successfully treated with OBV/PTV/r ± DSV without RBV, we also present results from the exploratory RUBY-II study (n = 18), which evaluated the RBV-free OBV/PTV/r ± DSV regimen in patients who are noncirrhotic with GT1a or GT4 infection and CKD stage 4 or 5. Safety and efficacy results from both studies are reported.
Methods
Study Overview and Regimens
All patients signed informed consent, and the studies were conducted in accordance with their respective protocols, International Conference on Harmonization guidelines, and the Declaration of Helsinki. All authors had access to all relevant data and reviewed and approved the final manuscript before submission.
RUBY-I, Cohort 2 (NCT002207088) and RUBY-II (NCT02487199) were phase 3b, open-label, multicenter studies that evaluated the safety and efficacy of OBV/PTV/r and DSV with or without RBV in patients with HCV and CKD stage 4 (eGFR 15–30 ml/min per 1.73 m2) or stage 5 (eGFR <15 ml/min per 1.73 m2 or on dialysis). OBV/PTV/r 25/150/100 mg was administered once daily and DSV 250 mg was administered twice daily. For GT1a-infected patients enrolled in RUBY-I, Cohort 2, RBV 200 mg was also administered once daily. All patients who received at least 1 dose of study drug were followed for 24 weeks after completion or discontinuation of study drug to monitor safety, HCV RNA levels, and, in the case of virologic failures, emergence of resistance-associated polymorphisms (RAPs). Pharmacokinetic data on DAA regimen components were collected in both studies. The disposition of patients is shown in Supplementary Figure S1.
RUBY-I, Cohort 1 Study Overview
Findings from the first cohort of RUBY-I were previously published.16 Briefly, the study enrolled 20 noncirrhotic, GT1-infected adults with stage 4 or 5 CKD, with no history of prior HCV treatment. Cirrhosis and CKD stage criteria were the same as defined later in this article. GT1a-infected patients received OBV/PTV/r + DSV + RBV and GT1b-infected patients received the regimen without RBV, both for 12 weeks.
Patient Selection Criteria and Study Design of RUBY-I, Cohort 2 and RUBY-II
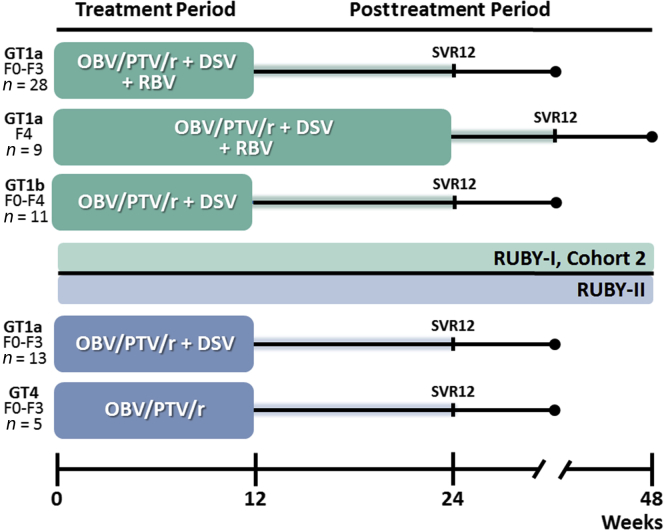
The study designs for both studies are shown in Figure 1 and the main inclusion and exclusion criteria are shown in Table 1.
Figure 1.
RUBY-I, Cohort 2 and RUBY-II study design. Cohort 2 of RUBY-I included 2 arms, A and B, that enrolled patients with genotype (GT)1a infection and were treated with ombitasvir (OBV)/paritaprevir (PTV)/ritonavir (r) + dasabuvir (DSV) ± ribavirin (RBV). Patients in arm A were noncirrhotic and were treated for 12 weeks. Patients with cirrhosis were enrolled into arm B and were treated for 24 weeks. Patients in arm C of RUBY-I, Cohort 2 had GT1b infection and were treated with OBV/PTV/r + DSV without RBV for 12 weeks. In RUBY-II, all patients were noncirrhotic; those with GT1a were treated with OBV/PTV/r + DSV without RBV for 12 weeks. Patients with GT4 infection were treated with OBV/PTV/r without RBV for 12 weeks. SVR12, sustained virologic response at posttreatment week 12.
Table 1.
Comparison of main inclusion and exclusion criteria
| RUBY-I, Cohort 2 | RUBY-II |
|---|---|
| Main inclusion | |
| Male or female aged ≥18 yr | Male or female aged ≥18 yr |
| Positive anti-HCV antibody test | Positive anti-HCV antibody test |
| HCV RNA >1000 IU/ml | HCV RNA >1000 IU/ml |
| GT1 | GT1a or 4 |
| Untreated or PegIFN ± RBV experienced | Untreated or PegIFN ± RBV experienced |
| With or without compensated cirrhosis (Metavir F0–4)a | Without cirrhosis (Metavir F0–3)a |
| eGFR <30 ml/min per 1.73 m2 (CKD stage 4 or 5 including HD and PD) | eGFR <30 ml/min per 1.73 m2 (CKD stage 4 or 5 including HD and PD) |
| Main exclusion | |
| Coinfection with HBV, HIV | Coinfection with HBV, HIV |
| Current or past clinical evidence of Child-Pugh class B or C disease or a clinical history of hepatic decompensation | Current or past clinical evidence of cirrhosis |
| Screening laboratory test abnormalities | Screening laboratory test abnormalities |
| Albumin <2.8 g/dl | Albumin <3.5 g/dl |
| Hemoglobin <10 g/dl | Hemoglobin <8 g/dl |
| Platelets <25,000/mm3 | Platelets <120,000/mm3 |
| Total bilirubin ≥3.0 mg/dl | Total bilirubin ≥3.0 mg/dl |
| INR >2.3 | INR >2.3 |
| Use of known strong inducers and inhibitors of CYP2C8, strong or moderate inducers of CYP3A, medications contraindicated with ritonavir, within 2 weeks or 10 half-lives, whichever is longer, before the first dose of study drug | Use of known strong inducers and inhibitors of CYP2C8, strong or moderate inducers of CYP3A, medications contraindicated with ritonavir, within 2 weeks or 10 half-lives, whichever is longer, before the first dose of study drug |
CKD, chronic kidney disease; CYP, cytochrome P450; eGFR, estimated glomerular filtration rate by Modified Diet in Renal Disease equation; GT, genotype; HBV, hepatitis B virus; HCV, hepatitis C virus; HD, hemodialysis; INR, international normalized ratio; PD, peritoneal dialysis; PegIFN, pegylated interferon; RBV, ribavirin.
Based on the results of FibroTest, FibroScan, or liver biopsy at screening.
RUBY-I, Cohort 2 enrolled adults at least 18 years of age with stage 4 or 5 CKD, including those on hemodialysis or peritoneal dialysis, who had chronic HCV GT1 infection with or without compensated cirrhosis and were treatment naïve or pegylated IFN ± RBV experienced. Plasma samples for HCV genotype were collected at screening and assessed with the Versant HCV Genotype Inno LiPA Assay, version 2.0 or higher. Patients were classified as having stage 4 or 5 CKD based on an eGFR of 15 to 30 ml/min per 1.73 m2 or <15 ml/min per 1.73 m2 (or requiring dialysis), respectively; this was calculated using the Modification of Diet in Renal Disease equation.19
Exclusion criteria included coinfection with hepatitis B or human immunodeficiency virus, current or past evidence of liver decompensation or Child-Pugh B or C disease, and laboratory values shown in Table 1.
Absence of cirrhosis (METAVIR <3, Ishak <4) was determined by liver biopsy within 24 months before (or during) screening, transient elastography result of <12.5 kPa within 6 months before (or during) screening, or a screening FibroTest score of ≤0.72 and an aspartate aminotransferase–to-platelet ratio index ≤2. Baseline fibrosis stage determinants are described in Supplementary Table S1.
RUBY-I, Cohort 2 patients were treated according to US prescribing information for Viekira Pak15: GT1a-infected patients without cirrhosis received OBV/PTV/r + DSV + RBV for 12 weeks, and those with cirrhosis received the regimen with RBV for 24 weeks. GT1b-infected patients with or without cirrhosis received OBV/PTV/r + DSV for 12 weeks. Patients were followed for 24 weeks posttreatment.
RUBY-II enrolled adults at least 18 years of age with stage 4 or 5 CKD or ESRD, including those on dialysis, who had chronic HCV GT1a or 4 infection and were treatment naïve or pegylated IFN experienced. Exclusion criteria included evidence of HCV genotypes other than 1a or 4, coinfection with hepatitis B or human immunodeficiency virus, current or past evidence of liver cirrhosis, and laboratory values shown in Table 1.
Criteria for determination of eGFR, cirrhosis, fibrosis, and HCV RNA quantification were consistent with those for RUBY-I, Cohort 2, described previously. In contrast to RUBY-I, Cohort 2, RUBY-II was RBV free; GT1a-infected patients received OBV/PTV/r + DSV and patients with GT4 received OBV/PTV/r, both for 12 weeks. Patients were followed for 24 weeks posttreatment.
Assessment of Efficacy, Safety, Resistance, and Pharmacokinetics
Plasma samples were collected at screening and again at each study visit (weeks 1, 2, 4, 8, 12, 16, 20, and 24). These samples were used for routine laboratory surveillance, quantifying levels of HCV RNA, analyzing plasma concentrations of study drugs and liver biomarkers, and population sequencing for identifying RAPs in patients with virologic failure.
Efficacy
Plasma HCV RNA levels were determined by a central laboratory using the Roche (Basel, Switzerland) COBAS TaqMan real-time reverse-transcriptase polymerase chain reaction assay v2.0, with a lower limit of detection of 15 IU/ml and a lower limit of quantification (LLOQ) of 25 IU/ml. A patient was considered to achieve SVR12 if he or she had HCV RNA <LLOQ for the duration between end of treatment and posttreatment week 12. Any patient who had confirmed HCV RNA ≥LLOQ during that time was considered to have relapsed. The primary endpoint of both studies was the percentage of patients who achieved SVR12. A patient whose HCV RNA was assessed at ≥LLOQ after it had been measured at <LLOQ during treatment, or had a confirmed increase >1 log10 IU/ml above nadir in 2 consecutive measurements, was considered to have virologic breakthrough.
Safety
Serious adverse events (AEs) were collected between signing of informed consent until 30 days after study drug discontinuation; AEs were collected from the first administration of study drug until 30 days after study drug discontinuation. Assessment of the relatedness of each AE was made with respect to both DAAs and RBV, and AEs were classified using the MedDRA system organ class and preferred term.
Resistance
Baseline RAPs were determined by performing population sequencing (detection threshold of 15%) on HCV RNA from day 1 plasma samples for each patient. Known polymorphisms within the NS3, NS5A, or NS5B viral proteins targeted by the OBV/PTV/r + DSV regimen were analyzed at amino acid positions where they had been previously identified for GT1 (RUBY-I, Cohort 2) (Supplementary Table S2). For patients who had virologic failure or treatment discontinuation, the sample nearest in time to failure/discontinuation with an HCV RNA level ≥1000 IU/ml was retested for persistence or emergence of RAPs.
Ribavirin Management for Decreases in Hemoglobin in RUBY-I, Cohort 2
Any patient whose hemoglobin decreased more than 2 g/dl during any 4-week period, or fell below 10 g/dl at any time, had RBV dosing interrupted after the decrease was confirmed by retesting. If confirmed, RBV was not resumed until hemoglobin levels returned to an acceptable level, and resumption was at the discretion of the investigator. Use of hematologic growth factors (eg, erythropoietin) or blood transfusion for hemoglobin decreases were permitted at the discretion of the investigator.
Statistical Analyses
The primary endpoint was the percentage of patients with SVR12 (HCV RNA < LLOQ 12 weeks after the last actual dose of study drugs) according to the intention-to-treat principle. All patients who received at least 1 dose of study drug were included in the intention-to-treat analysis. Patients with missing values were considered not to have achieved SVR12. The number and percentage of patients achieving SVR12 were calculated and 2-sided 95% confidence intervals were computed using the Wilson score method for binomial proportions. Analyses were performed using SAS Version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Baseline Patient Demographics
RUBY-I, Cohort 2
Fifty-seven patients were screened between September 21 and December 4, 2015; 9 patients failed screening and 48 were enrolled and received study drug in the RUBY-I, Cohort 2. Most patients were male, were of black race, and had stage 5 CKD at baseline; 67% (32/48) were on dialysis at baseline. The median baseline hemoglobin was 11.8 g/dl. One patient started dialysis while on treatment, which was planned before study initiation. Twenty-eight patients had HCV GT1a without cirrhosis and received OBV/PTV/r + DSV + RBV for 12 weeks, 9 patients had GT1a with compensated cirrhosis and received OBV/PTV/r + DSV + RBV for 24 weeks, and 11 patients had GT1b and received OBV/PTV/r + DSV without RBV for 12 weeks, regardless of cirrhosis. Detailed patient demographics for RUBY-I, Cohort 2 are shown in Table 2.
Table 2.
RUBY-I, Cohort 2 patient demographics and clinical characteristics
| Characteristic | GT1a F0-F3 with RBV 12 wk n = 28 | GT1a F4-only with RBV 24 wk n = 9 | GT1b F0-F4 without RBV 12 wk n = 11 |
|---|---|---|---|
| Age, median, yr (range) | 59 (32–76) | 56 (44–64) | 58 (50–77) |
| Male, n (%) | 23 (82) | 9 (100) | 8 (73) |
| Race, n (%) | |||
| White | 12 (43) | 3 (33) | 4 (36) |
| Black | 16 (57) | 4 (44) | 6 (55) |
| Other | 0 | 2 (22) | 1 (9) |
| Hispanic or Latino ethnicity, n (%) | 9 (32) | 2 (22) | 3 (27) |
| BMI, median kg/m2 (range) | 28 (17–42) | 27 (21–35) | 25 (19–31) |
| IL28B non-CC genotype, n (%) | 24 (86) | 5 (55) | 8 (73) |
| Former IDU, n (%) | 10 (36) | 1 (11) | 3 (27) |
| Treatment experienced, n (%) | 4 (14) | 3 (33) | 3 (27) |
| Baseline fibrosis stage | |||
| F0-F1 | 14 (50) | 0 | 3 (27) |
| F2 | 9 (32) | 0 | 1 (9) |
| F3 | 5 (18) | 0 | 1 (9) |
| F4 | 0 | 9 (100) | 6 (55) |
| CKD stage, n (%) | |||
| 4 (eGFR 15–30 ml/min per 1.73 m2) | 4 (14) | 2 (22) | 2 (18) |
| 5 (eGFR <15 ml/min per 1.73 m2 or dialysis) | 24 (86) | 7 (78) | 9 (82) |
| Baseline dialysis, n (%) | |||
| Peritoneal | 1 (4) | 1 (11) | 0 |
| Hemodialysis | 18 (64) | 7 (78) | 8 (73) |
| Erythropoietin use at baseline | 7 (25) | 2 (22) | 1 (9) |
| Baseline HCV RNA, median log10 IU/ml (range) | 6.2 (5.0–7.7) | 6.0 (5.3–7.4) | 5.8 (3.3–7.3) |
| Hemoglobin, median g/dl (range) | 11.8 (9.9–16.4) | 11.6 (10.1–12.9) | 12.3 (10.7–13.7) |
| Platelet count, median × 109/l (range) | 199 (104–346) | 127 (73–186) | 171 (58–292) |
BMI, body mass index; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate by Modification of Diet in Renal Disease study; GT, genotype; HCV, hepatitis C virus; IDU, injection drug user; IL28B, interleukin 28B; RBV, ribavirin.
A combined analysis of data from RUBY-I (Cohort I, n = 20, and Cohort 2, n = 48) showed that most patients with available data (83%; 50/60) had no detectable baseline RAPs (Supplementary Table S3).
RUBY-II
Twenty-three patients were screened between January 21 and April 5, 2016; 5 patients failed screening, and 18 were enrolled and received study drug. Most patients were male, were of white race, and had stage 5 CKD at baseline; 94% (17/18) of patients were on dialysis at baseline. The median baseline hemoglobin was 11.9 g/dl. All enrolled patients had stage F0-F3 fibrosis; 5 patients had prior treatment experience with pegylated IFN ± RBV. Of those enrolled, 13 patients had HCV GT1a infection and received OBV/PTV/r + DSV for 12 weeks, and 5 patients had GT4 and received OBV/PTV/r for 12 weeks. Detailed patient demographics for RUBY-II are shown in Table 3.
Table 3.
RUBY-II patient demographics and clinical characteristics
| Characteristic | GT1a F0-F3 12 wk n = 13 | GT4 F0-F3 12 wk n = 5 |
|---|---|---|
| Age, median yr (range) | 57 (34–76) | 58 (31–67) |
| Male, n (%) | 9 (70) | 3 (60) |
| Race, n (%) | ||
| White | 8 (62) | 3 (60) |
| Black | 1 (8) | 2 (40) |
| Other | 4 (31) | 0 |
| Hispanic or Latino ethnicity, n (%) | 1 (8) | 0 |
| BMI, median kg/m2 (range) | 26 (20–32) | 28 (19–41) |
| IL28B non-CC genotype, n (%) | 7 (54) | 5 (100) |
| Former IDU, n (%) | 5 (38) | 0 |
| Baseline fibrosis stage | ||
| F0-F1 | 8 (62) | 3 (60) |
| F2 | 1 (8) | 1 (20) |
| F3 | 4 (31) | 1 (20) |
| CKD stage, n (%) | ||
| 4, eGFR 15–30 ml/min per 1.73 m2 | 0 | 1 (20) |
| 5, eGFR <15 ml/min per 1.73 m2 or dialysis | 13 (100) | 4 (80) |
| Baseline dialysis | ||
| Peritoneal | 5 (38) | 0 |
| Hemodialysis | 8 (62) | 4 (80) |
| Erythropoietin use at baseline | 7 (54) | 2 (2) |
| Baseline HCV RNA, median log10 IU/ml (range) | 5.8 (4.6–7.3) | 5.7 (4.6–6.2) |
| Baseline mean alanine aminotransferase, IU/l | 43.6 | 25.0 |
| Baseline mean aspartate aminotransferase, U/l | 45.2 | 26.0 |
| Prior treatment experience, n (%) | 4 (31) | 1 (20) |
| Hemoglobin, median g/dl (range) | 12.0 (10.4–13.9) | 11.8 (10.0–13.0) |
| Platelet count, median × 109/l (range) | 222 (163–365) | 204 (136–243) |
BMI, body mass index; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate by Modification of Diet in Renal Disease study; GT, genotype; HCV, hepatitis C virus; IDU, injection drug use; IL28B, interleukin 28B.
SVR and Efficacy
RUBY-I, Cohort 2
Ninety-six percent (46/48, 95% confidence interval 86–99) of patients achieved SVR12 in the intention-to-treat population (Figure 1). Of the 2 patients who failed to achieve SVR, 1 patient had confirmed virologic failure. This patient was on hemodialysis, was HCV treatment naïve, and had GT1a infection. Although plasma HCV RNA was suppressed <LLOQ by treatment week 2, the patient discontinued the study on their own initiative on day 66 and had virologic relapse at week 12. At baseline, this patient had a polymorphism in NS5B (556G), and at the time of virologic failure, substitutions were present in NS3, NS5A, and NS5B: D168V, Q30R, and Y448H (Supplementary Table S4). A second patient was lost to follow-up at day 4 after withdrawing from the study due to a volvulus unrelated to study drug.
RUBY-II
The overall SVR12 rate was 94% (17/18, 95% confidence interval 74–99) in the intention-to-treat population (Figure 2). No patients in this study had virologic failure. The single patient who did not achieve SVR12 had ESRD and elected to undergo a renal transplant at treatment week 2 and withdrew from the study. Thirteen patients with HCV GT1a were treated without RBV, and the SVR12 rate was 100% (13/13) for these patients.
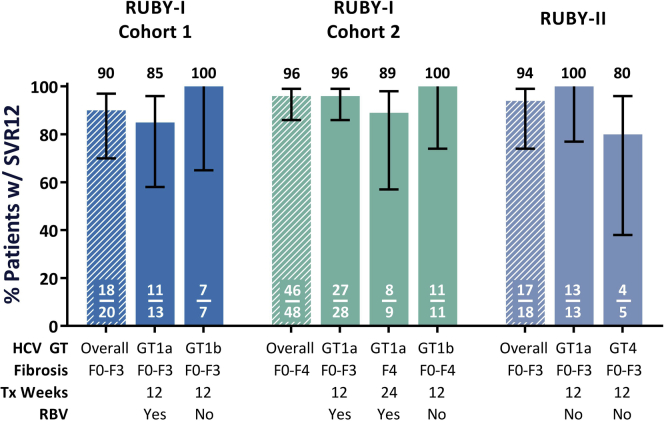
Figure 2.
Sustained virologic response at posttreatment week 12 (SVR12) rates across the RUBY study series. This graph shows SVR12 rates across all of the RUBY studies, including RUBY-I, Cohort 1.21 Both RUBY-I, Cohort 2 and RUBY-II included patients with prior hepatitis C virus (HCV) treatment experience; however, in RUBY-II, patients were noncirrhotic and ribavirin (RBV) was not coadministered for patients with any HCV genotype. Overall, in patients with stage 4 or 5 chronic kidney disease, treatment with ombitasvir (OBV)/paritaprevir (PTV)/ritonavir (r) ± dasabuvir (DSV) regimens resulted in high SVR rates for patients with HCV genotype (GT)1 or 4 infection, including those with concomitant cirrhosis and prior treatment experience. Tx, treatment; w/, with.
Thus, if the results of these studies are considered in combination and the first cohort of RUBY-I is included, the overall SVR12 rate for this regimen in patients with stage 4 or 5 CKD or ESRD was 94% (81/86, 95% confidence interval 87–97), with only 2 patients experiencing virologic failure.16
Safety and AEs
Across both studies, most patients experienced at least 1 AE, and 2 (3%) patients withdrew due to an AE. One patient in RUBY-I withdrew on day 4 due to a serious AE (volvulus) deemed unrelated to study drugs, and 1 patient in RUBY-II discontinued treatment at week 2 to undergo an elective renal transplant. Another patient in RUBY-II discontinued study drug due to an asymptomatic alanine aminotransferase elevation on treatment day 66 (peak of 255 U/l from a baseline of 18 U/l), but still achieved SVR12. Two other patients experienced asymptomatic alanine aminotransferase elevations >5 times the upper limit of normal during treatment, both of which returned to normal range within 10 days without interruption of study drug dosing. The most frequent AEs across both studies were fatigue, diarrhea, and nausea. Anemia and hemoglobin decreases were frequent AEs only in the RBV-containing treatment arms of RUBY-I; RBV management is discussed in more detail below. Treatment-emergent AEs and laboratory abnormalities are summarized in Table 4.
Table 4.
Treatment-emergent AEs and laboratory abnormalities
| Characteristic | RUBY-I, Cohort 2 |
RUBY-II |
|||
|---|---|---|---|---|---|
| GT1a F0-F3 with RBV 12 wk n = 28 | GT1a F4 only with RBV 24 wk n = 9 | GT1b F0-F4 without RBV 12 wk n = 11 | GT1a F0-F3 without RBV 12 wk n = 13 | GT4 F0-F3 without RBV 12 wk n = 5 | |
| Any AE, n (%) | 27 (96) | 8 (89) | 6 (55) | 13 (100) | 5 (100) |
| Serious AE, n (%) | 8 (29) | 4 (44) | 1 (9) | 3 (23) | 1 (20) |
| AEs leading to study drug discontinuation, n (%) | 0 | 1 (11)a | 0 | 1 (8)b | 1 (20)c |
| AEs in ≥15% of patients, n (%) | |||||
| Anemia | 16 (57) | 3 (33) | 0 | 0 | 0 |
| Fatigue | 9 (32) | 3 (33) | 1 (9) | 3 (23) | 1 (20) |
| Diarrhea | 7 (25) | 2 (22) | 0 | 4 (31) | 0 |
| Hemoglobin decrease | 7 (25) | 2 (22) | 0 | 0 | 0 |
| Nausea | 5 (17) | 3 (33) | 0 | 4 (31) | 0 |
| Headache | 3 (11) | 1 (11) | 1 (9) | 3 (23) | 0 |
| Vomiting | 7 (25) | 1 (11) | 0 | 2 (8) | 0 |
| Pruritus | 4 (14) | 0 | 2 (18) | 2 (15) | 1 (20) |
| Abdominal pain | 2 (7) | 0 | 0 | 4 (31) | 0 |
| Hypertension | 2 (7) | 1 (11) | 0 | 3 (23) | 1 (20) |
| AEs leading to RBV dose modification, n (%) | 25 (89) | 5 (56) | N/A | N/A | N/A |
| Hemoglobin, n (%) | |||||
| Grade 2, <10–8 g/dl | 19 (68) | 4 (50) | 3 (27) | 4 (31) | 2 (40) |
| Grade 3, <8–6.5 g/dl | 1 (4) | 2 (25) | 0 | 0 | 0 |
| Total bilirubin, n (%) | |||||
| Grade 2, >1.5–3 × ULN | 1 (4) | 2 (25) | 0 | 0 | 0 |
| Grade 3, >3–20 × ULN | 0 | 1 (13) | 0 | 0 | 0 |
| ALT, n (%) | |||||
| Grade 3, >5–20 × ULN | 1 (4) | 0 | 0 | 1 (8) | 1 (20) |
Hemoglobin and total bilirubin were assessed post baseline; ALT was assessed post nadir.
AE, adverse event; ALT, alanine aminotransferase; GT, genotype; N/A, not applicable; RBV, ribavirin; ULN, upper limit of normal.
Volvulus assessed as not related to study drug.
Discontinued study drug, but still achieved SVR12.
Discontinued study drug due to renal failure and transplant.
Ribavirin Management for Decreases in Hemoglobin in RUBY-I, Cohort 2
The baseline RBV dose for GT1a-infected patients was 200 mg/d, and the median baseline hemoglobin level was 11.8 g/dl. Anemia (19/37) and hemoglobin decreases (9/37) were common AEs in RUBY-I, Cohort 2. A total of 10 patients required RBV dose modification due to hemoglobin decreases (Supplementary Table S5). The median time for the first RBV dose modification was at treatment day 28.5 (range: day 15–43). Two of the 10 patients were on erythropoietin at baseline. Another 2 patients interrupted RBV dosage due to AEs of rash and intermittent worsening of nausea and vomiting. Eleven of the 12 patients who underwent RBV dose modification achieved SVR12. The patient who did not achieve SVR12 was the one who discontinued study drug on treatment day 66 on his own initiative (Supplementary Table S4).
Discussion
The objective of the RUBY-I, Cohort 2 and RUBY-II clinical trials, presented in this report, was to evaluate the efficacy and safety of OBV/PTV/r ± DSV treatment with or without RBV in patients with stage 4 or 5 CKD, including patients on peritoneal dialysis or hemodialysis. The collective results show that this combination produces high SVR12 rates in this population regardless of the presence of cirrhosis or prior treatment experience. Despite the use of RBV in many patients, there was a low rate of serious AEs or treatment discontinuations.
In both RUBY-I Cohorts 1 and 2, GT1a-infected patients received a low initial dose of RBV (200 mg/d). Despite frequent hemoglobin reductions, the overall SVR12 rate was 92% (46/50).16 Only patients who received RBV had hemoglobin levels <8 g/dl (4/50; 8%). Twelve patients in Cohort 2 had RBV dose modifications: 10 due to hemoglobin decreases and 2 due to other AEs. RBV discontinuation or dose modification occurred most frequently between treatment weeks 4 and 5 (day 28.5), which is consistent with a previous analysis that showed that considerable decreases in hemoglobin occur within the first 2 to 4 weeks of initiating treatment with RBV.20 No patient discontinued the study due to an anemia-related AE.
Although low initial doses of RBV are recommended in patients with renal insufficiency, and dose modifications are recommended to manage RBV-associated hematologic toxicity in such patients,21 adherence to these recommendations does not eliminate hematologic toxicity; thus, RBV-free treatment regimens are highly desirable. Both the US15 and EU22, 23 labels for OBV/PTV/r + DSV recommend coadministration of RBV for patients with GT1a and GT4 infection. In RUBY-II, an RBV-free regimen (OBV/PTV/r ± DSV) was studied in 18 patients with GT1a or 4. A high SVR12 rate was observed (17/18; 94%) with no episodes of anemia or AEs associated with decreased hemoglobin. The results of the comparative phase 3 PEARL-IV study are instructive. Among GT1a-infected patients who received OBV/PTV/r + DSV, SVR rates were 90% in those who received a RBV-free regimen and 97% in those who received RBV.24 Due to the small number of patients in RUBY-II, it is not possible to say whether the RBV-free regimen was more efficacious than an RBV-containing regimen in GT1a-infected patients; however, it is reassuring that no virologic failures occurred. As noted in the introduction, HCV-infected dialysis patients may have low plasma HCV RNA levels and be “easier to treat” than patients with higher HCV RNA levels.25 However, in spite of data from RUBY-II that suggest that RBV coadministration may not be necessary in some GT1a- or GT4-infected patients on hemodialysis, larger studies would be needed to confirm these results. Given that OBV/PTV/r ± DSV is no longer recommended as first-line treatment in patients with HCV infection and CKD,6, 7 it is unlikely that such studies will be performed.
The results of the RUBY studies are consistent with the results of others that have evaluated this combination in patients with CKD, including patients on hemodialysis.26, 27, 28, 29, 30 SVR12 rates in these trials were consistently high (range 96% to 100%). Use of RBV was associated with hematologic AEs in these studies. For example, grade 3 or 4 anemia occurred in 9% of patients in the largest of these studies.29
There was 1 death in RUBY-I, Cohort 1: a 60-year-old man on hemodialysis who died of left ventricular systolic dysfunction 11 days after treatment ended. The death was not attributed to DAA or RBV and appears to have resulted from the patient’s underlying cardiovascular morbidity. There was a low rate of study drug discontinuation in these studies (6% overall), including 1 patient who discontinued treatment to receive an elective renal transplant.
The major route of elimination for OBV, PTV, ritonavir, and DSV is biliary excretion or metabolism with minimal renal excretion, and the regimen requires no dose adjustment in patients with any degree of renal impairment.31 The pharmacokinetic profiles of OBV, PTV, and DSV in patients with renal impairment and ESRD from RUBY-I, Cohort 2, presented elsewhere, showed plasma exposures consistent with levels seen in other phase 3 studies containing patients with normal or mildly impaired renal function.32 In addition, hemodialysis did not impact DAA concentrations in venous versus arterial samples, suggesting the regimen can be administered without regard to dialysis. These findings are consistent with those from RUBY-I, Cohort 1.16
These studies have several limitations. First, the Modification of Diet in Renal Disease study equation was used to estimate GFR and classify patients. Use of an alternative method such as the Chronic Kidney Disease Epidemiology Collaboration equation to estimate GFR may have altered the number of patients with CKD stage 4 or 5 disease.33 However, this would not have altered the outcome or conclusions of the trial, because of the high overall SVR12 rates regardless of CKD class. Second, very few patients consented to intensive pharmacokinetic analysis (10/68; 14%), so the intensive pharmacokinetic data comes from a small sample of patients. Therefore, it was difficult to draw firm conclusions about drug exposure comparisons between subgroups (eg, CKD stage 4 vs. 5, hemodialysis vs. peritoneal dialysis, or patients with or without cirrhosis). Third, only 18 patients were enrolled into the RBV-free RUBY-II study; only 5 of these patients had HCV GT4 and the 4 patients who completed treatment achieved SVR12. Fourth, patients with CKD are at increased risk of major adverse cardiovascular events,34 and RBV has the potential to cause hemolytic anemia and provoke myocardial ischemia in patients who experience substantial decreases in hemoglobin during treatment.35 Overall, patients in these studies had a median hemoglobin level of 12.0 g/dl. The safety results of this study population may not be generalizable to patients with lower baseline hemoglobin levels, who might be at greater risk for adverse outcomes with further hemoglobin decrease. In this context, it is noteworthy that no anemia-related AEs were observed in patients treated without RBV. An RBV-free treatment option therefore seems most appropriate for such patients. Finally, clinical trials by their nature enroll select patients who are defined by the eligibility criteria and patients who consent to participate in clinical trials may be more health conscious than nonparticipants. For these reasons, the results may not be generalizable to the general population of patients with HCV infection and CKD.
Current guidelines recommend 2 RBV-free regimens for patients with HCV infection and stage 4 or 5 CKD, elbasvir/grazoprevir, which is approved for the treatment of patients with GT1 or 4 infection, and glecaprevir/pibrentasvir, which has pangenotypic activity. Elbasvir/grazoprevir produced an SVR12 rate of 94% after 12 weeks of treatment in patients with CKD stage 4 or 5 and HCV GT1 infection enrolled in a phase 3 study.36 Similarly, glecaprevir/pibrentasvir produced a high overall SVR12 rate (98%) in patients with GT1 to 6 infection and stage 4 or 5 CKD in a phase 3 study.37 For this reason, the regimens examined in the studies presented here would be considered to be alternatives to the 2 recommended regimens.
In conclusion, the RUBY-I, Cohort 2 and RUBY-II studies demonstrated high efficacy for OBV/PTV/r ± DSV ± RBV treatment in patients with HCV GT1 or GT4 and stage 4 and 5 CKD, including patients with compensated cirrhosis and prior HCV treatment experience, and in those receiving peritoneal dialysis or hemodialysis. Similar to the findings of RUBY-I, Cohort 1,16 treatment was well tolerated with a very low discontinuation rate. There were no cases of hepatic decompensation among patients with compensated cirrhosis (n = 15). As expected, hemoglobin decreases were frequently reported in patients who received RBV, although these patients still achieved a high SVR12 rate. Patients with HCV GT1a infection treated with OBV/PTV/r + DSV without RBV had a high SVR rate in RUBY-II. These findings confirm the suitability of OBV/PTV/r ± DSV ± RBV as an IFN-free DAA therapy for HCV-infected patients with ESRD, including those with compensated cirrhosis.
Disclosures
AbbVie sponsored the studies (NCT02207088, NCT02487199), contributed to their design, collection, analysis, and interpretation of the data, and participated in the writing, review, and approval of the content of the manuscript. All authors had access to all relevant data. This article contains information on the investigational use of ombitasvir/paritaprevir/r and dasabuvir.
EC, JZ, DW, NS, and DEC are employees of AbbVie and may hold AbbVie stock or options.
EL: Speaker: Gilead, GSK, Kadmon, Merck, Vertex; Research/Grant Support: AbbVie, Achillion, Boehringer Ingelheim, BMS, Gilead Sciences, GSK, Idenix, Intercept Pharmaceuticals, Janssen, Medtronic, Merck, Novartis, Presidio, Roche, Santaris Pharmaceuticals, Vertex; Advisor: AbbVie, Achillion, BioCryst, Biotica, Enanta, Idenix, Janssen, Merck, Novartis, Santaris, Theravance, Vertex.
EG: Advisor: AbbVie, Gilead, Achillion, Novartis, Roche, Merck, Janssen.
JV: Research support: AbbVie, Biotest, Bristol-Myers Squibb, Gilead, Janssen, Vertex, Merck, Genentech, Genfit, Hyperion, Immuron, Intercept, Ocera, Sundise, Exalenz, Taiwan J, Shire, Conatus, Galectin, Globeimmune, Pfizer, Novartis, Mallinckrodt, Ikaria; Advisory board: AbbVie, BioIncept, Boehringer Ingelheim, Bristol-Myers Squibb, Exalenz, Gilead, Janssen, Merck, Novartis, HepQuant, Hyperion, Intercept, Salix, Sundise, Taiwan J, Umecrine S.
KA: Speaker bureau/consultancy: Achillion, Arbutus, BMS, Gilead, GSK, Intercept, Janssen, Merck, Novartis, Roche; Research support: AbbVie, BMS, Gilead, Roche.
TH: Grants/Research support: AbbVie (Advisory Board), Boehringer Ingelheim, Bristol-Myers Squibb (Advisory Board), Eisai, Gilead Sciences, Janssen, Idenix, Ikaria, Mochida, Roche, Ocera, Taigen, Takeda, Salix, Sundise, Vertex; Speaker: Baxter, Bristol-Myers Squibb, Gilead, Salix.
PSM: Grant/Research Funding: AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Mass Biologics, Merck, Bayer-Onyx, Santaris, Salix, Vital Therapies, Vertex; Consultant/Advisor: AbbVie, Bristol-Myers Squibb, Gilead, Johnson and Johnson, Merck, Salix, Genentech, Vertex; Fees for Non-CME/CE services: Bayer-Onyx, Gilead, Janssen, Merck, Salix, Vertex.
PJP: Speaker/Consultant/Advisor: Gilead, AbbVie, Merck, Intercept; Research Support paid to Scripps Health: Gilead, AbbVie, Intercept, Bristol-Myers Squibb, Merck, Conatus, Beckman, Genfit.
MB owns stock in AbbVie.
GM: Grant/Research Funding: AbbVie.
KRR: Advisor: Gilead, Bristol-Myers Squibb, AbbVie, Merck, Janssen; Research Support (paid to the University of Pennsylvania): Gilead, Bristol-Myers Squibb, AbbVie, Merck, Janssen. The other author declared no competing interests.
Acknowledgments
AbbVie and the authors express their gratitude to the patients who participated in this study and their families. They also thank all of the participating study investigators and coordinators. Medical writing was provided by Ryan J. Bourgo, PhD, of AbbVie, and by Blair Jarvis MSc, ELS, of Medical Expressions, funded by AbbVie.
AbbVie sponsored the studies (NCT02207088, NCT02487199), contributed to their design, collection, analysis, and interpretation of the data, and participated in the writing, review, and approval of the content of the manuscript.
Author contributions: Study concept: EC, NSS, DEC; acquisition of data: EL, EG, JV, KA, TH, PSM, PJP, MB, NK, GM, KRR; analysis and interpretation of data: EL, EG, EC, JV, KA, TK, PSM, PJP, MB, NK, GM, JZ, DW, NSS, DEC, KRR; critical review and revision of the manuscript: EL, EG, EC, JV, KA, TK, PSM, PJP, MB, NK, GM, JZ, DW, NSS, DEC, KRR; statistical analysis: DW.
Footnotes
Figure S1. PRISMA diagram showing the number of patients screened, through completion of treatment.
Table S1. Baseline fibrosis staging.
Table S2. Polymorphisms within the HCV NS3, NS5A, or NS5B viral proteins that alter susceptibility to the OBV/PTV/r + DSV regimen.
Table S3. RUBY-I, cohort 2: baseline resistance-associated polymorphisms.
Table S4. RUBY-I, cohort 2: characteristics of patient with virologic breakthrough.
Table S5. RUBY-I, cohort 2: ribavirin and hemoglobin management.
Supplementary material is linked to the online version of the paper at http://www.kireports.org/.
Supplementary Material
PRISMA diagram showing the number of patients screened, through completion of treatment.
Baseline fibrosis staging.
Polymorphisms within the HCV NS3, NS5A, or NS5B viral proteins that alter susceptibility to the OBV/PTV/r + DSV regimen.
RUBY-I, cohort 2: baseline resistance-associated polymorphisms.
RUBY-I, cohort 2: characteristics of patient with virologic breakthrough.
RUBY-I, cohort 2: ribavirin and hemoglobin management.
References
- 1.Blach S., Zeuzem S., Manns M. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 2.Perz J.F., Armstrong G.L., Farrington L.A., Hutin Y.J., Bell B.P. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Okoh E.J., Bucci J.R., Simon J.F., Harrison S.A. HCV in patients with end-stage renal disease. Am J Gastroenterol. 2008;103:2123–2134. doi: 10.1111/j.1572-0241.2008.01981.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee J.J., Lin M.Y., Chang J.S. Hepatitis C virus infection increases risk of developing end-stage renal disease using competing risk analysis. PLoS One. 2014;9:e100790. doi: 10.1371/journal.pone.0100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C.H., Kao J.H. Treatment of hepatitis C virus infection in patients with end-stage renal disease. J Gastroenterol Hepatol. 2011;26:228–239. doi: 10.1111/j.1440-1746.2010.06488.x. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 7.AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. Updated May 24, 2018. Available at: https://www.hcvguidelines.org. Accessed July 23, 2018.
- 8.Goodkin D.A., Bieber B., Jadoul M. Mortality, hospitalization, and quality of life among patients with hepatitis C infection on hemodialysis. Clin J Am Soc Nephrol. 2017;12:287–297. doi: 10.2215/CJN.07940716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sovaldi (sofosbuvir) tablets for oral use [prescribing information]. Foster City, CA: Gilead Sciences Inc; revised 11/2017.
- 10.Harvoni (ledipasvir and sofosbuvir) tablets for oral use [prescribing information]. Foster City, CA: Gilead Sciences Inc; revised 11/2017.
- 11.Epclusa (sofosbuvir and velpatasvir) tablets for oral use [prescribing information]. Foster City, CA: Gilead Sciences Inc; revised 11/2017.
- 12.Vosevi (sofosbuvir, velpatasvir and voxilaprevir) tablets for oral use [prescribing information]. Foster City, CA: Gilead Sciences Inc; revised 11/2017.
- 13.Zepatier (elbasvir and grazoprevir) tablets for oral use [prescribing information]. Whitehouse Station, NJ: Merck and Co.; revised 06/2018.
- 14.Mavyret (glecaprevir and pibrentasvir) tablets for oral use [prescribing information]. North Chicago, IL: AbbVie Inc.; revised 12/2017.
- 15.Viekira pak (ombitasvir, paritaprevir, and ritonavir tablets, dasabuvir tablets), co-packaged for oral use. [prescribing information]. North Chicago, IL: AbbVie Inc.; revised 11/2017.
- 16.Pockros P.J., Reddy K.R., Mantry P.S. Efficacy of direct-acting antiviral combination for patients with HCV genotype 1 infection and severe renal impairment or end-stage renal disease. Gastroenterology. 2016;150:1590–1598. doi: 10.1053/j.gastro.2016.02.078. [DOI] [PubMed] [Google Scholar]
- 17.Trevizoli J.E., de Paula Menezes R., Ribeiro Velasco L.F. Hepatitis C is less aggressive in hemodialysis patients than in nonuremic patients. Clin J Am Soc Nephrol. 2008;3:1385–1390. doi: 10.2215/CJN.01330308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizuno M., Higuchi T., Yanai M., Kanmatsuse K., Esumi M. Dialysis-membrane-dependent reduction and adsorption of circulating hepatitis C virus during hemodialysis. Nephron. 2002;91:235–242. doi: 10.1159/000058398. [DOI] [PubMed] [Google Scholar]
- 19.Levey A.S., Bosch J.P., Lewis J.B. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 20.Reau N., Hadziyannis S.J., Messinger D. Early predictors of anemia in patients with hepatitis C genotype 1 treated with peginterferon alfa-2a (40KD) plus ribavirin. Am J Gastroenterol. 2008;103:1981–1988. doi: 10.1111/j.1572-0241.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Food and Drug Administration. REBETOL (ribavirin USP) [prescribing information]. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020903s052,021546s008lbl.pdf. Updated 1998. Accessed March 2, 2015.
- 22.Viekirax (ombitasvir 12.5 mg/ paritaprevir 75 mg/ ritonavir 50 mg) film-coated tablets [summary of product characteristics]. Ludwigshafen, Germany: AbbVie Deutschland GmbH; revised 7/2018.
- 23.Exviera (dasabuvir 250 mg) film-coated tablets [summary of product characteristics]. Ludwigshafen, Germany: AbbVie Deutschland GmbH; revised 5/2018.
- 24.Ferenci P., Bernstein D., Lalezari J. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–1992. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 25.Fabrizi F., Bunnapradist S., Lunghi G., Martin P. Kinetics of hepatitis C virus load during hemodialysis: novel perspectives. J Nephrol. 2003;16:467–475. [PubMed] [Google Scholar]
- 26.Abad S., Vega A., Hernandez E. Universal sustained viral response to the combination of ombitasvir/paritaprevir/ritonavir and dasabuvir with/without ribavirin in patients on hemodialysis infected with hepatitis C virus genotypes 1 and 4. Am J Nephrol. 2017;45:267–272. doi: 10.1159/000454819. [DOI] [PubMed] [Google Scholar]
- 27.Munoz-Gomez R., Rincon D., Ahumada A. Therapy with ombitasvir/paritaprevir/ritonavir plus dasabuvir is effective and safe for the treatment of genotypes 1 and 4 hepatitis C virus (HCV) infection in patients with severe renal impairment: a multicentre experience. J Viral Hepat. 2017;24:464–471. doi: 10.1111/jvh.12664. [DOI] [PubMed] [Google Scholar]
- 28.Ponziani F.R., Siciliano M., Lionetti R. Effectiveness of paritaprevir/ritonavir/ombitasvir/dasabuvir in hemodialysis patients with hepatitis C virus infection and advanced liver fibrosis: Case reports. Am J Kidney Dis. 2017;70:297–300. doi: 10.1053/j.ajkd.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 29.Sanai F.M., Alghamdi A.S., Afghani A.A. High efficacy of ombitasvir/paritaprevir/ritonavir plus dasabuvir in hepatitis C genotypes 4 and 1-infected patients with severe chronic kidney disease. Liver Int. 2018;38:1395–1401. doi: 10.1111/liv.13674. [DOI] [PubMed] [Google Scholar]
- 30.Sperl J., Kreidlova M., Merta D. Paritaprevir/ritonavir/ombitasvir plus dasabuvir regimen in the treatment of genotype 1 chronic hepatitis C infection in patients with severe renal impairment and end-stage renal disease: a real-life cohort. Kidney Blood Press Res. 2018;43:594–605. doi: 10.1159/000488965. [DOI] [PubMed] [Google Scholar]
- 31.Khatri A., Dutta S., Marbury T.C. Pharmacokinetics and tolerability of anti-hepatitis C virus treatment with ombitasvir, paritaprevir, ritonavir, with or without dasabuvir, in subjects with renal impairment. Clin Pharmacokinet. 2017;56:153–163. doi: 10.1007/s40262-016-0429-9. [DOI] [PubMed] [Google Scholar]
- 32.Shuster D.L., Menon R.M., Ding B. Ombitasvir, paritaprevir, ritonavir, dasabuvir and ribavirin pharmacokinetics in HCV-infected subjects with chronic kidney disease stage 4 (severe renal impairment) or stage 5 (end-stage renal disease) Hepatology. 2016;64:975A. [Google Scholar]
- 33.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mok Y., Ballew S.H., Bash L.D. International validation of the thrombolysis in myocardial infarction (TIMI) risk score for secondary prevention in post-MI patients: A collaborative analysis of the chronic kidney disease prognosis consortium and the risk validation scientific committee. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.008426. pii: e008426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lenci I., Piccolo P., Francioso S. Recurrent myocardial ischaemia during combination antiviral therapy in a patient with chronic hepatitis C and normal aminotransferase levels. Dig Liver Dis. 2008;40:785–790. doi: 10.1016/j.dld.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Roth D., Nelson D.R., Bruchfeld A. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537–1545. doi: 10.1016/S0140-6736(15)00349-9. [DOI] [PubMed] [Google Scholar]
- 37.Gane E., Lawitz E., Pugatch D. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med. 2017;377:1448–1455. doi: 10.1056/NEJMoa1704053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA diagram showing the number of patients screened, through completion of treatment.
Baseline fibrosis staging.
Polymorphisms within the HCV NS3, NS5A, or NS5B viral proteins that alter susceptibility to the OBV/PTV/r + DSV regimen.
RUBY-I, cohort 2: baseline resistance-associated polymorphisms.
RUBY-I, cohort 2: characteristics of patient with virologic breakthrough.
RUBY-I, cohort 2: ribavirin and hemoglobin management.