Abstract
Tissue plasminogen activator is the only U.S. FDA-approved therapy for ischemic stroke, while there is no specific medication for hemorrhagic stroke. Therefore, the treatment of acute stroke continues to be a major unmet clinical need. We explored the effects of miR-195 on neurovascular protection and its potential in treating acute stroke. Using both cellular and animal studies, we showed that miR-195’s beneficial effects are mediated by four mechanisms: (1) anti-apoptosis for injured neural cells by directly suppressing Sema3A/Cdc42/JNK signaling, (2) neural regeneration by promoting neural stem cell proliferation and migration, (3) anti-inflammation by directly blocking the NF-kB pathway, and (4) improvement of endothelial functions. We intravenously injected miR-195 carried by nanoparticles into rats with either ischemic or hemorrhagic stroke in the acute stage. The results showed that miR-195 reduced the size of brain damage and improved functional recovery in both types of stroke rats. The reduction of injured brain volume could be up to 45% in ischemic stroke and approximately 30% in hemorrhagic stroke. The therapeutic window between stroke onset and miR-195 treatment could be up to 6 h. Our data demonstrated that miR-195 possesses the potential to become a new drug to treat acute ischemic and hemorrhagic stroke.
Keywords: microRNA, stroke, neuroprotection, neural stem cell, Sema3A
Introduction
Stroke is a leading cause of death and disability worldwide. Stroke can be characterized as ischemic or hemorrhagic, with ischemic stroke accounting for 85% of instances.1 The intravenous administration of tissue plasminogen activator (tPA) within 3 h of stroke onset, and within 4.5 h of stroke onset in select patients,2 is currently the only U.S. Food and Drug Administration (FDA)-approved therapy for ischemic stroke. Because of the time-dependent nature of tPA therapy and a fear of hemorrhagic complications, only 1%–8% of potentially eligible patients have been treated with tPA.3 In contrast to treatment for ischemic stroke, the recommendation for managing hemorrhagic stroke is supportive treatment because no specific medication has been developed.4, 5 Therefore, treatments for acute ischemic and hemorrhagic stroke continue to be a major unmet clinical need.
We previously showed that miR-195 exerts vasculoprotective effects.6 In addition, aberrant expression of miR-195 has been reported to play a role in neurological and cardiovascular diseases.7, 8, 9, 10 Stroke is a vascular disorder that leads to neural death. Because miR-195 plays roles in both the cerebrovascular and neural systems, dys-regulation of miR-195 levels may be associated with stroke event and prognosis. The primary aim of the present study is to test whether and how miR-195 is involved in stroke. To test our aim, we first investigated the mechanisms behind the of beneficial effects exhibited by miR-195 using endothelial cells, vascular smooth muscle cells, neural cells, and neural stem cells (NSCs). On the basis of the results of these cellular studies, we reasoned that miR-195 may be useful when applied to cerebrovascular events, regardless of ischemic or hemorrhagic stroke. Subsequently, we used miR-195 to treat animals with acute ischemic and hemorrhagic stroke. The results of these animal studies confirmed the potential role of miR-195 in stroke therapy.
Results
miR-195 Exerts Neuroprotection
miR-195 Increases Viability of Damaged Cells
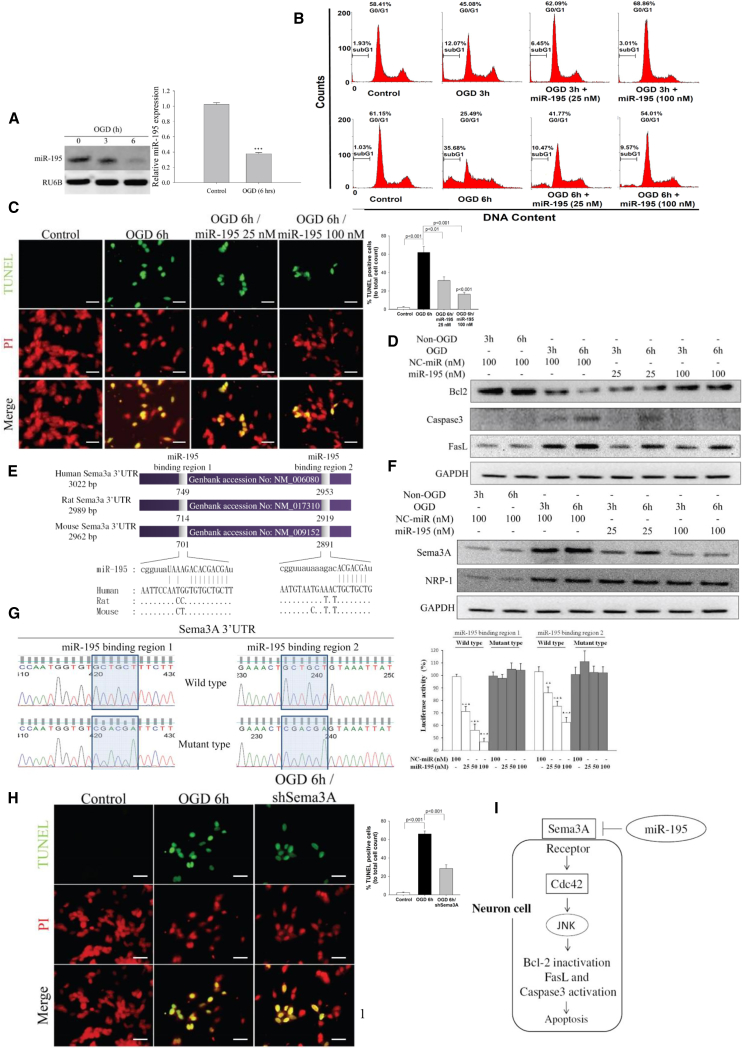
SH-SY5Y neural cells were subjected to oxygen-glucose deprivation (OGD) for 3 or 6 h to mimic ischemic conditions in the brain. At 24 h, and miR-195 levels were significantly reduced (Figure 1A). In addition, OGD reduced cell viability and increased lactate dehydrogenase (LDH) release in a duration-dependent manner (Figures S1A and S1B). Transfection of miR-195 dose-dependently increased cell viability and decreased LDH release (Figures S1C and S1D). Flow cytometry analysis revealed that OGD increased SH-SY5Y cell entry into the sub-G1 phase, while miR-195 reduced the number of cells in the sub-G1 phase and restored the normal cell distribution in the G0/G1 phase (Figure 1B). TUNEL assay further demonstrated that miR-195 substantially decreased OGD-induced cellular apoptosis (Figure 1C). Besides, an increase in intracellular miR-195 levels led to upregulation of anti-apoptotic factor (Bcl-2) and downregulation of two apoptotic molecules (FasL and caspase-3) (Figure 1D). These results suggest that miR-195 possesses a neuroprotective effect by anti-apoptosis.
Figure 1.
miR-195 Reversed OGD-Induced Apoptosis by Knockdown of Sema3A
All data presented in this figure were measured 24 h after the initiation of OGD. (A) Under 6 h OGD treatment, miR-195 levels were significantly decreased as measured by northern blot and qPCR. See also Figure S1. (B) Typical DNA histogram plots from flow cytometry of OGD-treated SH-SY5Y cells. miR-195 was transfected to the cells after 3 or 6 h of OGD. OGD increased the proportion of cells in sub-G1, and miR-195 reversed the cell distribution in the cell cycle. (C) TUNEL assay shows an increase of OGD-induced SH-S5Y5 apoptosis and miR-195 anti-apoptosis in a dose-dependent manner. Images were obtained using a Leica DMI6000B microscope with 400× magnification. Scale bar, 20 μm. (D) Transfection of miR-195 could reverse the detrimental effects of OGD by increasing an anti-apoptotic factor (Bcl-2) and suppressing apoptotic factors (FasL and caspase-3). (E) Schematic representation of miR-195 binding sites in the Sema3A 3′ UTR of human, rat, and mouse genome. (F) OGD treatment increased protein levels of Sema3A and its receptor Nrp-1, but miR-195 reversed the OGD effect on Sema3A. See RNA data in Figure S2. (G) The two miR-195 binding sites in the Sema3A 3′ UTR were individually tested using the luciferase reporter assay. miR-195 dose-dependently inhibited luciferase activity for the constructs carrying the wild-type sequence, but miR-195 had no effects on the constructs carrying the mutant sequence. (H) TUNEL assay demonstrates that Sema3-shRNA (short hairpin RNA) reduced apoptosis in the 6 h OGD-treated cells. See also Figures S3A–S3C. Images were obtained using a Leica DMI6000B microscope with 400× magnification. Scale bar, 20 μm. (I) Schematic diagram shows how miR-195 exerts its effect to prevent cell apoptosis. NC-miR, normal control microRNA; PI, propidium iodide stain. Lipofectamine 2000 was used for miR-195 transfection. **p < 0.01 and ***p < 0.001. All values were expressed as mean ± SE.
miR-195 Suppresses Sema3A Expression
Semaphorin 3A (Sema3A) is a potent inducer for neuronal death.11, 12 The Sema3A gene was predicted as a direct target of miR-195 by bioinformatic databases. There are two potential miR-195 binding sites at the Sema3A mRNA 3′ UTR in human, rat, and mouse (Figure 1E). We also experimentally confirmed that Sema3A can be knocked down by miR-195 (protein data in Figure 1F; RNA data in Figure S2). The luciferase reporter assay confirmed that miR-195 can bind to both binding sites in the Sema3A 3′ UTR (Figure 1G). The expression levels of Sema3A and its receptor, neuropilin (Nrp-1), were significantly increased in OGD-treated SH-SY5Y cells (protein data in Figure 1F; RNA data in Figure S2).
Knockdown of Sema3A reduced cell apoptosis (Figure 1H), increased cell count, and reduced LDH release (Figures S3A–S3C), confirming that miR-195’s neuroprotective effect can be mediated through Sema3A repression. Cdc42 can affect cell cycle progression, regulate FasL expression,13 activate caspase-3, and induce apoptosis.14, 15 We previously demonstrated that Cdc42 is also a direct target of miR-1956; furthermore, Cdc42 is a downstream molecule in the Sema3A signaling pathway.16, 17 Therefore, suppression of Sema3A also resulted in Cdc42 downregulation (Figure S3D). These data suggest that miR-195 can regulate Sema3A and Cdc42 to achieve a neuroprotective effect. The miR195/Sema3A signaling pathway is schematically presented in Figure 1I.
miR-195 Improves Endothelial Functions
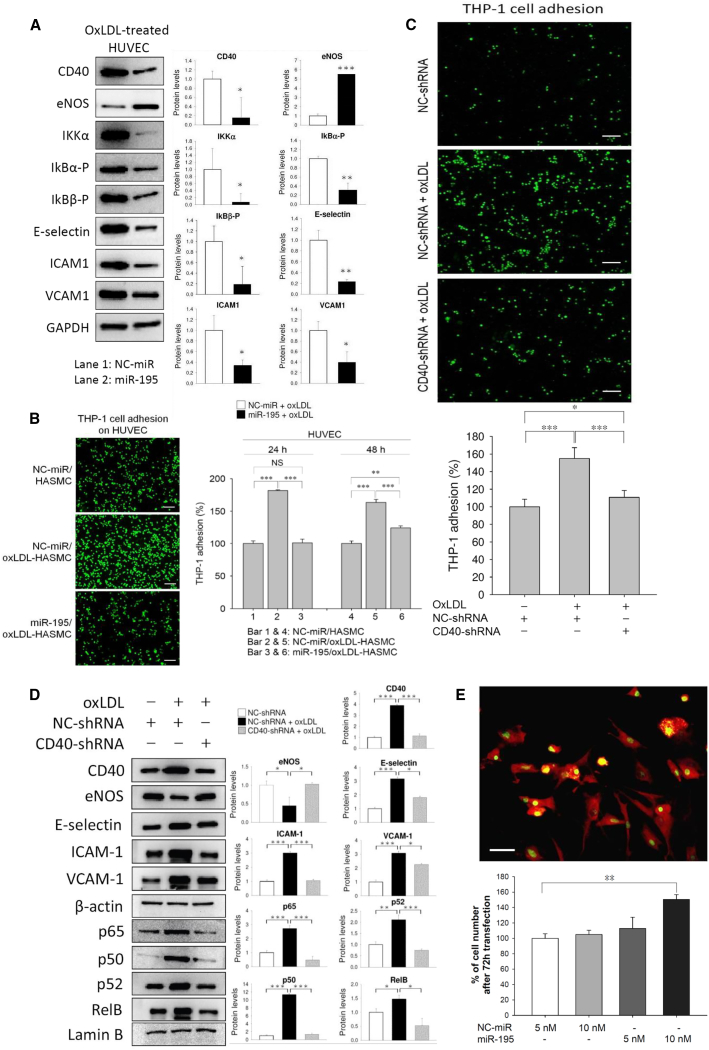
Given that endothelial cells (ECs) play multiple pivotal roles in stroke recovery, we tested the effects of miR-195 on endothelial functions. Transfection of miR-195 to human umbilical vein ECs (HUVECs) downregulated the expression of adhesion molecules and upregulated endothelial nitric oxide synthase (eNOS) expression (Figure 2A). To further mimic in vivo conditions, we assessed the effects of miR-195 on HUVECs in a HUVEC/human aortic smooth muscle cell (HASMC) co-culture. In the adhesion assay, oxidized low-density lipoprotein (oxLDL) treatment to HASMCs increased THP-1 adhesion to HUVECs in the HUVEC/HASMC co-culture. However, if oxLDL-treated HASMCs were also transfected with miR-195, THP-1 adhesion to HUVECs was reduced (Figure 2B).
Figure 2.
miR-195 Improved Endothelial Functions
(A) Protein markers in miR-195-transfected and oxLDL-treated HUVECs. We used oxLDL to decrease endothelial functions, and therefore the beneficial effects of miR-195 could be detected. Quantitative data from the western blot are shown at right. See also Figures S4 and S5. (B) HUVECs were co-cultured with microRNA-transfected (50 nmol/L) and oxLDL-treated HASMCs for 5 h, and the adhesion test was performed using THP-1 cells. Left: results obtained using a fluorescence microscope with 100× magnification; right: quantitative data. Scale bar, 100 μm. Lipofectamine 2000 was used as a transfection reagent. (C) HUVECs were transfected with CD40-shRNA (1 μg/mL), and THP-1 cells were added to the HUVEC culture for the adhesion assay at 5 h post-transfection. Images were obtained using a Leica DMI6000B microscope with 100× magnification. Scale bar, 100 μm. (D) Western blot for adhesion molecules, eNOS, and the NF-κB-related molecules was measured at 48 h post-transfection of shRNA. (E) Rat neural stem cells (NSCs) isolated from the subventricular zone (SVZ) were first confirmed by the positive staining of Nestin (red in cytoplasm) and Sox2 (green in nucleus and cytoplasm). Transfection of miR-195 by HiPerFect transfection reagent increased the proliferation of NSC at 72 h. Images were obtained using a Leica DMI6000B microscope with 400× magnification. Scale bar, 20 μm. Values are presented as mean ± SEM from three independent experiments performed in triplicate. *p < 0.05, **p < 0.01, and ***p < 0.001. NC-miR, normal control microRNA. All values were expressed as mean ± SE.
miR-195 Inhibits NF-κB Signaling in ECs
NF-κB signaling affects the expression of adhesion and inflammatory molecules in ECs.18, 19 We conducted a series of experiments to show that miR-195 inhibits NF-κB signaling, including IKKα and phosphorylated IκBs (p-IκBα and p-IκBβ) (Figure 2A). These inhibitory effects subsequently reduced ubiquitin-dependent IκB degradation and led to suppression of p65/p50 (p65 is also known as RelA) and RelB/p52 nuclear transposition. (Figure S4).
CD40 is a protein expressed in the brain’s neural and vascular cells that can stimulate the NF-κB pathway and was predicted to be a miR-195 direct target (Figure S5A).20 CD40 expression is markedly upregulated in the stroke hemisphere, and its levels are related to post-ischemia brain inflammation.20 Our experiments confirmed that CD40 was directly suppressed by miR-195 (Figures 2A and 5B). Knockdown of CD40 in HUVECs reduced cell adhesion (Figures 2C and 2D), decreased NF-κB signaling, and increased eNOS levels (Figure 2D).
miR-195 Stimulates Neural Stem Cells
Proliferation and mobilization of NSCs are important factors for stroke recovery.21
We used Nestin and SOX2 as NSC markers. Transfection of miR-195 to rat Nestin+/SOX2+ NSCs increased NSC proliferation (Figure 2E). SDF-1 can increase stem cell proliferation and promote NSC function. SDF-1 secretion was increased by transfection of miR-195 to HASMCs (Figure S6A), but miR-195 did not have the same effect when transfected to HUVEC, mouse brain ECs, or astrocytes (Figures S6A–S6C).
Exogenous miR-195 Treats Acute Ischemic Stroke
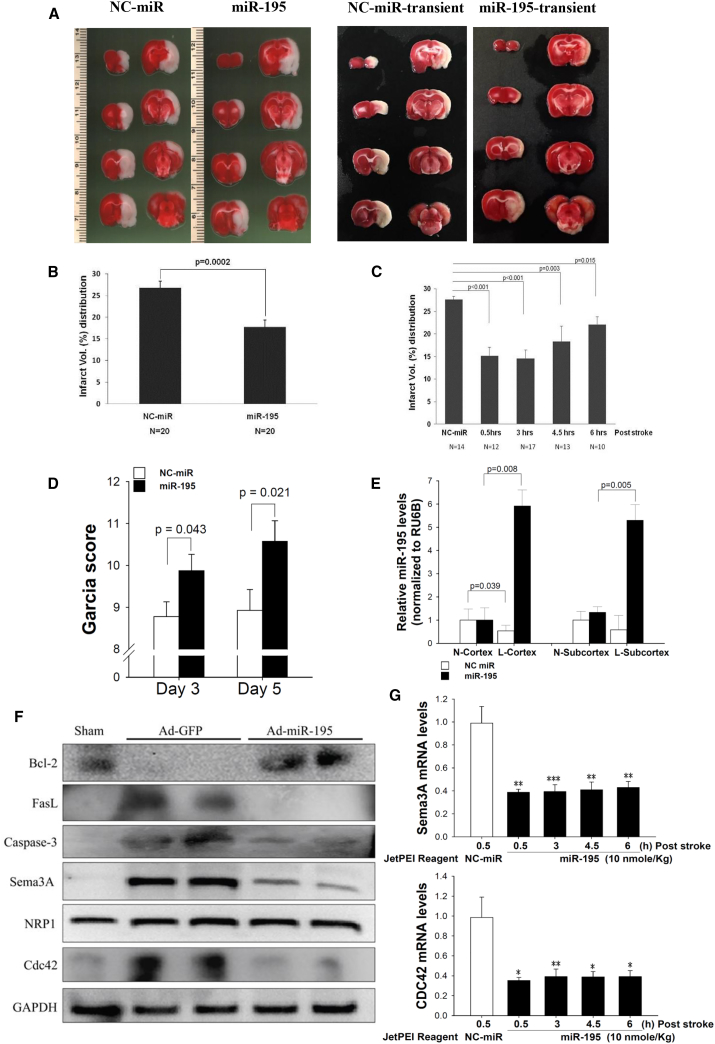
Given that miR-195 has multiple beneficial effects on the neurovascular system, we tested whether miR-195 could be used to treat acute stroke. First, we induced ischemic stroke in rats by middle cerebral artery occlusion (MCAO).22 We used two different types of MCAO: (1) permanent MCAO, in which the artery was occluded for 24 h and the rats were sacrificed directly thereafter, and (2) transient MCAO, in which the artery was occluded for 2 h and the rats were sacrificed on day 5. miR-195 carried by the commercial PEI-based nanoparticles (called in vivo-jetPEI) was IV injected via the tail vein. Our preliminary study found that the optimal concentration was 10 nmol/kg (Figure S7). For the rats subjected to permanent MCAO, miR-195 was injected at different time windows (0.5, 3, 4.5, and 6 h) after stroke. For the rats subjected to transient MCAO, miR-195 was given only at 6 h after stroke. NC-miR carried by in vivo-jetPEI was used as a placebo. To make the data comparable among rats subjected to transient MCAO, we calculated Garcia scores at 6 h post-stroke and selected only the rats with the scores between 6 and 8 for subsequent studies. As a result, all rats had similar levels of neurological deficiency upon enrollment for the study of transient MCAO.
The average infarct area of the treated group was significantly smaller than that of the placebo group for both permanent (Figures 3A and 3B) and transient (Figure 3C) MCAO models. More important, miR-195 retained its therapeutic effect at the intervention time window of 6 h. Significant improvements in Garcia score were noted on days 3 and 5 in the transient MCAO rats (Figure 3D). In the miR-195-treated rats, miR-195 levels were higher in the lesion cortex and subcortex than in the contralateral normal cortex and subcortex (Figure 3E). Conversely, in the placebo group, miR-195 levels were significantly lower in the lesion cortex and subcortex than in their counterparts. Furthermore, there was a negative correlation between miR-195 levels in the infarct hemisphere and infarct size (Pearson correlation = −0.78, p = 0.0075; Table S1).
Figure 3.
miR-195 Improved Outcomes of Ischemic Stroke in Rats
Stroke rats were IV injected with miR-195 or NC-miR carried by jetPEI nanoparticles. The rodent brains subjected to permanent MCAO were collected at 24 h post-stroke, while brains of transient MCAO rodents were collected on day 5. (A) Representative brain slices from the permanent MCAO model (left panel) and the transient MCAO model (right panel). Rats subjected to permanent MCAO received NC-miR (left) or miR-195 (right) treatment at 3 h post-stroke, and they were sacrificed at 24 h. Rats subjected to transient MCAO received NC-miR or miR-195 treatment at 6 h post-stroke, and they were sacrificed on day 5. TTC staining (white) shows the infarct regions. (B) miR-195 significantly reduced the infarct volume in the permanent MCAO rats even when the treatment window was 6 h post-stroke. The infarct volumes are presented as a percentage of total brain volume. (C) miR-195 also significantly reduced the infarct volume in the transient MCAO rats treated at 6 h post-stroke. (D) miR-195 treatment improved the Garcia scores of the transient MCAO rats. N = 20 for each group. (E) miR-195 treatment of the permanent MCAO rats led to a high concentration of miR-195 in the stroke hemisphere. NC-miR treatment did not change miR-195 concentration in the lesion hemisphere. These rats received IV injection at 3 h post-stroke. L, stroke lesion hemisphere; N, normal hemisphere. N = 6 for NC-miR, n = 10 for miR-195. (F) Western blot for Sema3A, Nrp-1, Cdc42, Bcl-2, FasL, and caspase-3 in the brains of permanent MCAO rats treated with Ad-GFP or Ad-miR-195 at 3 h. GAPDH was used as the internal control. Ad-miR-195, adenovirus expressing miR-195; Ad-GFP, control adenovirus expressing GFP. (G) Brain sema3A and Cdc42 mRNA levels in the permanent MCAO rats treated with miR-195 or NC-miR at different time windows. N = 6, 10, 10, 10, and 8 for NC-miR and miR-195 at 0.5, 3, 4.5, and 6 h, respectively. *p < 0.05, **p < 0.01, and ***p < 0.001. All values were expressed as mean ± SE.
Pharmacokinetics and Biodistribution
Seven normal Sprague-Dawley (SD) rats were independently used to test miR-195 concentrations in the circulation (i.e., pharmacokinetic study). Five minutes after injection of nanoparticle-carried miR-195 (10 nmol/kg), circulating miR-195 levels had increased by more than 390-fold (ranging from 390-fold to 1,025-fold). The half-life (T1/2) varied among animals from a minimum of 893 min to a maximum of 6,650 min (Table S2). jetPEI can interfere with miR-195 detection. The wide range of T1/2 may be because the dissociation of miR-195 from jetPEI varied among animals. Another 18 normal SD rats received the same treatment and were used to measure the distribution of miR-195 in the rats’ organs. At each of the six time points (pre-dose and 1, 6, 24, 48, and 72 h), three rats were sacrificed. As expected, the highest concentration was in the serum, followed by the heart and then the lungs (Figure S8). Because these animals did not have stroke, the exogenous miR-195 barely affected miR-195 levels in the brain.
Confirmation of miR-195 Effects In Vivo
To replicate the effects of miR-195 demonstrated in our cellular studies, we measured anti-apoptotic markers, inflammatory substances, endothelial functions, and NSCs in the rodent brains subjected to permanent MCAO. The expression patterns of Sema3A, Nrp1, caspase-3, FasL, CdC42, and Bcl-2 in the brains were consistent with the results we obtained in the cellular studies (Figures 3F and 3G). Similarly, miR-195 possessed anti-inflammatory effects by decreasing TNFα, IL-1β, IL-6, and MCP-1 levels in the brains (Figure S9). Again, improvement of endothelial function by miR-195 was demonstrated through changes of the mRNA levels of inducible nitric oxide synthase (iNOS), eNOS, vascular cell adhesion molecule (VCAM), and intercellular adhesion molecule (ICAM) in the brains (Figure S10).
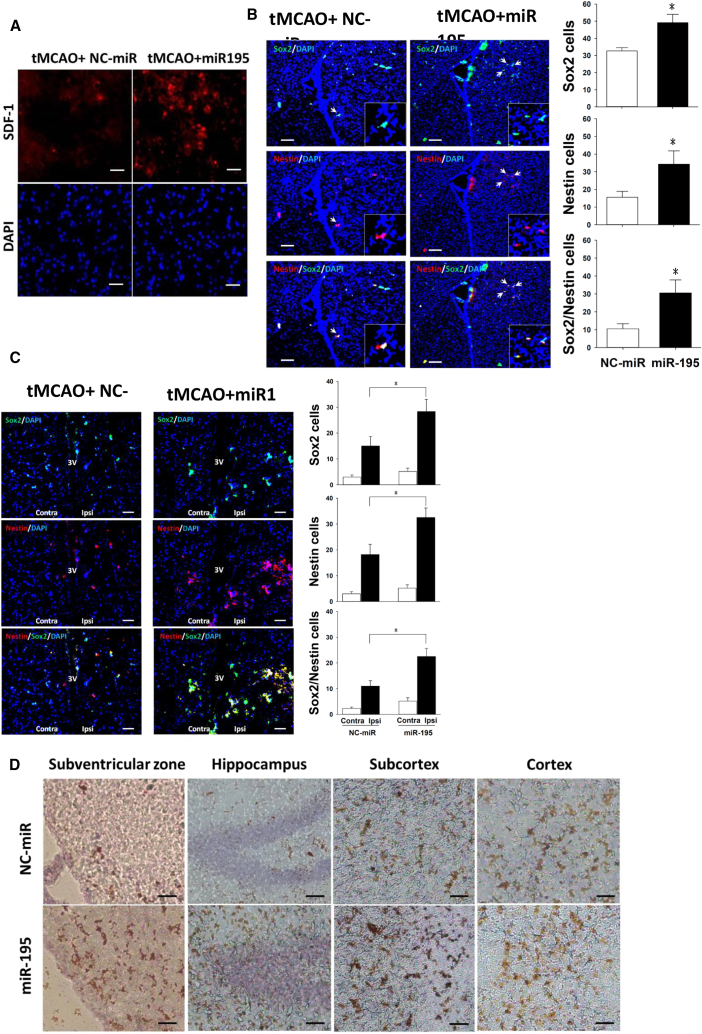
To demonstrate the effect of miR-195 on NSCs in vivo, we examined the brains from rats subjected to transient MCAO. On day 5 post-stroke, SDF-1 levels were higher in the miR-195-treated rats than in the NC-miR placebo rats (Figure 4A). Most NSCs co-expressed both SOX2 and Nestin markers. Both SOX2+/Nestin+ cells in the subventricular zone (SVZ) (Figure 4B) and peri-third ventricle (Figure 4C) were significantly increased in the miR-195-treated group compared with the placebo group. Notably, because stroke can also increases NSC proliferation, as a result there were more NSCs on the ischemic side (ipsilateral) than the uninjured contralateral side of the placebo group (Figure 4C). GAP43 is highly expressed in neuronal growth cones during development and axonal regeneration; therefore, the GAP43+ cells indicated that miR-195 promoted neuronal regeneration in multiple areas of the infarct brain (Figure 4D).
Figure 4.
miR-195 Promoted NSCs by Increasing SDF-1
(A) Stroke was induced by transient MCAO (tMCAO). miR-195 increased SDF-1 proteins in the cortex of stroke hemisphere, as indicated by immunofluorescent staining. Magnification, 200×. Scale bar, 20 μm. (B and C) miR-195 increased the number of SOX2+ and Nestin+ NSCs in the SVZ (B) and in the peri-third ventricle (3v) (C). NSCs were indicated by immunofluorescent staining. Insets show higher magnification for double-immunoreactive cells. *p < 0.05. Ipsi, the side ipsilateral to stroke lesion; Contra, the side contralateral to stroke lesion. Magnification, 100× for images in (B) and (C) and 400× for insets. Scale bar, 50 μm. The sample sizes for the bar charts in (B) and (C) were n = 6 for NC-miR and n = 6 for miR-195. (D) miR-195 increased the number of GAP43+ cells (brown) indicated by immunohistochemistry staining in multiple areas of the stroke hemisphere. Magnification, 100×. Scale bar, 200 μm. tMCAO, transient middle carotid artery occlusion. Images were obtained using a Leica DMI6000B microscope. All values were expressed as mean ± SE.
Exogenous miR-195 Treats Acute Hemorrhagic Stroke
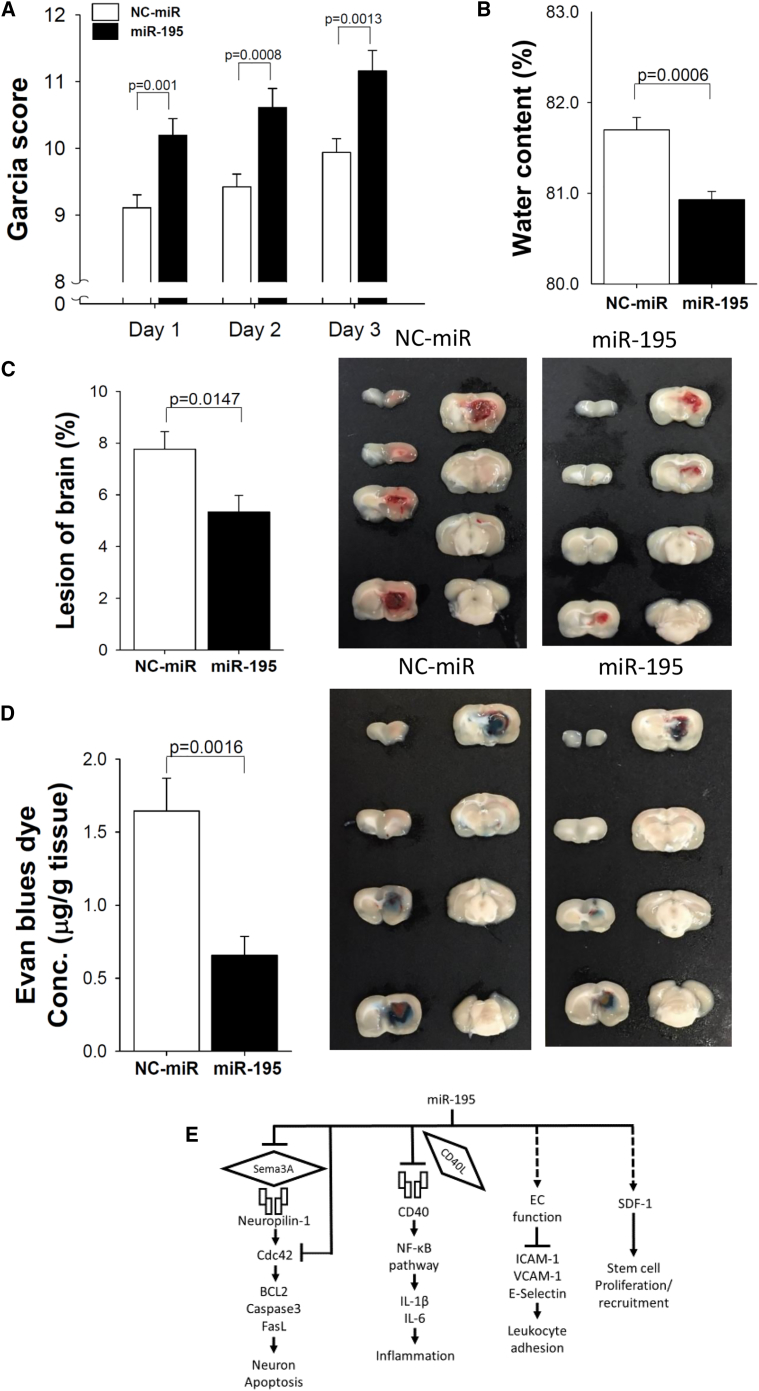
We further examined the effect of miR-195 on acute hemorrhagic stroke in rats by IV injecting identically formulated miR-195 at 4 h post-stroke. The miR-195-treated group contained 31 rats, and the NC-miR placebo group contained 32 rats. All rats were evaluated for Garcia score, and the rats were sacrificed on day 3. Because the preparation of brain tissue for the measurement of edema, stroke lesion volume, and permeability differs, the rats were divided into groups of 16, 29, and 18 to be used for these three measurements, respectively. The results showed that miR-195 treatment significantly improved the functional outcome, as indicated by a higher Garcia score (all p values were 0.001 on days 1, 2, and 3; Figure 5A). miR-195 also significantly reduced brain edema (p = 0.0006; Figure 5B), lesion volume (p = 0.01; Figure 5C), and permeability (p = 0.002; Figure 5D) on day 3.
Figure 5.
miR-195 Improved the Outcome of Hemorrhagic Stroke in Rats
(A) miR-195 treatment improved the Garcia score in the rats of hemorrhagic stroke; n = 32 for NC-miR, n = 31 for miR-195. (B) Reduced brain edema; n = 9 for NC-miR, n = 7 for miR-195. (C) Reduced percentage of lesion volume; n = 14 for NC-miR, n = 15 for miR-195. (D) Reduced blood-brain barrier (BBB) permeability; n = 9 for NC-miR, n = 9 for miR-195. (E) Schematic showing the effects of miR-195 on neurovascular protection. Values are presented as mean ± SEM from three independent experiments performed in triplicate. *p < 0.05, **p < 0.01, and ***p < 0.001. All values were expressed as mean ± SE.
Discussion
We showed that miR-195 can inhibit apoptosis in damaged neural cells, increase SDF-1 to promote NSCs for neurogenesis, block the NF-kB pathway for anti-inflammation, and improve endothelial functions. Figure 5E schematically shows the effects of miR-195 discovered in this study. These beneficial effects were also revealed by reduced brain damage and improved functional recovery in animals that had suffered from ischemic and hemorrhagic stroke. Accordingly, miR-195 possesses the potential to serve as a new drug to treat patients with acute stroke and other types of brain injury.
Current treatment and many ongoing clinical trials for ischemic stroke focus on thrombolytic therapy. However, it is important to distinguish whether the stroke is caused by ischemia or hemorrhage before using thrombolytic therapy, because this treatment may cause brain hemorrhaging that can further complicate the already injured brain. miR-195, on the other hand, has the potential to be used for both ischemic and hemorrhagic stroke; this advantage may make it more suitable for emerging treatment regardless of the stroke type. A recent breakthrough in treating acute ischemic stroke, intra-arterial clot retrieval, was validated in five clinical trials published in 2015.23, 24, 25, 26, 27 Thus far, two strategies have been tested to treat ischemic stroke: restoring blood flow (tPA, clot retrieval) and reducing neural injury. These two strategies can have a synergistic effect. If miR-195 can be further proved effective in reducing brain injury, it may be applied to ischemic stroke patients before the procedure of clot retrieval is performed.
The change of miR-195 levels in damaged cells and tissues can be time, location, and cell type dependent. For example, the miR-195 level rapidly decreased in infarcted and border zones but dramatically increased in remote zones 1 h after myocardial ischemia.28 On the contrary, prolonged myocardial ischemia caused the elevation of miR-195 levels in infarcted and border zones but suppression in remote zones.28 Previous studies have reported that miR-195 can suppress anti-apoptosis gene BCL2 in cancer cell lines29 and cardiomyoctes,30 which opposes our finding in OGD-treated SY5Y cells. We also tested for the effect of miR-195 on mouse neural cell line (N2A cells) and found that miR-195 did not suppress BCL2 expression (Figure S11). Similar to our findings, Ai et al.7 showed that miR-195 decreased in hypoxia-damaged neurons and reported that miR-195 prevented neuron death by suppressing the caspase pathway.31 It should be noted that microRNA’s suppressive effect is influenced by its target gene expression level, and a single microRNA can exert opposite effects under different conditions.32, 33 Therefore, the effects of miR-195 in different cells and organs may not be directly comparable.
Sema3A is upregulated when the brain is injured.34 The increase in Sema3A signaling elevates cerebrovascular permeability,35 and secreted Sema3A can additionally interact with its receptor Nrp-1 to inhibit neuronal regeneration and enhance neuronal death.11, 34, 36 Sema3A can upregulate Cdc42 expression, and a decrease in Cdc42 expression has been reported to attenuate neuronal apoptosis in the rodent stroke model.37 By inhibiting the cascade of Sema3A/Nrp-1/Cdc42 and increasing Bcl-2 levels, miR-195 can achieve an anti-apoptotic effect in the injured brain and reduce neuronal death. Consistent with our results, a recent study further demonstrated a significant decrease of infarct volume in Sema3A-knockout mice subjected to ischemic stroke.35
NSCs play a critical role in neurogenesis after stroke. NSCs need to migrate from the stem cell niches to the peri-infarct area to perform neurogenesis. We examined NSCs in the classic SVZ niche and a novel niche38 (peri-third ventricle), and our data showed that miR-195 increased NSCs in both niches in the animals that had suffered strokes. These results were consistent with the previous report indicating that neurogenesis can be attributed to NSCs from multiple niches.39 Numerous studies using the MCAO model have uniformly shown that proliferating NSCs are capable of migrating toward the injured sites in order to differentiate to new neurons.38 However, NSCs can deplete over time after ischemic injury.38 Our data revealed that miR-195 increases the number of NSCs and that these stem cells are likely to differentiate to functional neurons and glia because increased GAP43 expression was considered a marker of neuronal development and axonal regeneration.40 Notably, GAP43 plays a role to regulate nerve sprout associated with adult plasticity.41 Collectively, miR-195 may wake up stem cells from the niches and increase stem cell functions, leading to a better post-stroke outcome.
Stroke is more prevalent in the elderly, who often have chronic disease comorbidities. In this study, we induced artificial stroke in young animals. Therefore, the beneficial effects attributed to miR-195 may be attenuated in elderly subjects who suffer stroke. In the present study we did not measure physiological parameters for the stroke rats, and also we did not conduct longer term follow-up, which must be addressed in follow-up studies. Brain stroke triggers a complex and highly interconnected cascade of cellular and molecular events. Targeting multiple components of the neurovascular unit, rather than just neurons, should be a priority in stroke research.42
In conclusion, the present study not only demonstrated the detailed mechanisms of neurovascular protection controlled by miR-195 but also showed that miR-195 can be used to treat both acute ischemic and acute hemorrhagic stroke in experimental animals. Furthermore, the long time window for miR-195 treatment can be important for future clinical practice. Therefore, the potential of miR-195 to treat cerebral vascular accidents warrants further investigation.
Materials and Methods
Detailed information can be found in the Supplemental Information.
Cell Studies
OGD was used to mimics ischemia in vivo. Cells were cultured in the OGD condition for 3 or 6 h, and then cells were maintained in glucose-containing DMEM with normoxia for 21 h (if OGD duration was 3 h) or 18 h (if OGD duration was 6 h). THP-1 cells were labeled with calcein AM and were added to the culture wells containing HUVECs for the monocyte adhesion assay. The luciferase reporter assay was used to test the existence of miR-195 binding sites in the targeted genes.
Target Site Prediction
Three algorithms were used to predict miR-195 target genes: miRanda (http://microrna.sanger.ac.uk/targets/v5/), TargetScan (http://targetscan.org), and PicTar (http://pictar.mdc-berlin.de).
Viral miR Preparation
Adenoviruses were amplified by infection into HEK293A cells. Viral MOI was estimated on the basis of in vitro HEK293A transduction efficiency: 0.5 mL of undiluted viral stocks were added to 106 HEK293A cells, and number of GFP-positive cells was counted 48 h after transduction.
Animal Study
The procedure for balloon injury6 and for ischemic stroke by MCAO is described elsewhere.22, 43 In the present study’s permanent MCAO model, male SD rats were subjected to occlusion for 24 h and sacrificed directly thereafter, because of the high mortality rate after 24 h MCAO. In the transient MCAO model, rats were subjected to occlusion for 2 h. In this model, rats were included for further evaluation only if their Garcia scores were between 6 and 8 at 6 h post-stroke. The eligible transient MCAO rats were then sacrificed on day 5. For hemorrhagic stroke, male SD rats were subjected to stereotaxic infusion of bacterial collagenase to induce intracranial hemorrhage (ICH). Similarly, we only included hemorrhagic stroke rats with moderate to severe neurological impairment, and these animals were sacrificed on day 3.
miR-195 mimic or normal control microRNA (NC-miR) was formulated with a commercial nanoparticle called jetPEI and then intravenously (IV) administrated via the tail vein. The operators were blinded to treatment assignment of miR-195 or NC-miR. The ischemic brain slices were stained with 0.1% 2,3,5-TTC. Digital photographs of the brain slices were taken, and lesion volume was computed using ImageJ version 1.40 (NIH). The total lesion volume was calculated as the sum of lesion area in each section.
The Garcia score was used to evaluate each rat’s neurological function. The researchers who conducted the Garcia assessment were not informed of whether the rats were in the treated or placebo group. The assessment was performed on days 3 and 5 for transient MCAO rats and on days 1, 2, and 3 for hemorrhagic stroke rats. Because brain edema peaked on day 3 in hemorrhagic stroke, the brain was removed on day 3 and dried in an oven to obtain the dry weight. The cerebellum was used as an internal control. Water content was expressed as a percentage of wet weight: [(wet weight) − (dry weight)] (wet weight)−1 × 100%. Blood-brain barrier permeability was assessed using a modified Evans blue extravasation method on day 3.
Immunostaining
Immunofluorescent staining was used to detect cellular expression of SDF-1, SOX2, Nestin, and GAP43. Immunohistochemistry (IHC) staining was used to stain the markers in brain slices.
Study Approval
The Animal Care and Use Committee of Kaohsiung Medical University approved the animal experimental protocols (approval number IACUC-101068), which strictly conforms to the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Statistical Analysis
All values in the text and figures are expressed as mean ± SE. Statistical differences were evaluated using Student’s t test or analysis of covariance (ANCOVA). A p value less than 0.05 was considered to indicate statistical significance in all experiments. Analysis of the data and plotting of the figures were performed using SigmaPlot 10 software (Systat Software, San Jose, CA, USA).
Author Contributions
H.-Y.C. designed studies, conducted the experiments, interpreted the data, and wrote the draft. Y.-S.W. designed studies, conducted the experiments, interpreted the data, and wrote the draft. P.-Y.H. conducted the experiments and wrote the draft. C.-Y.C. conducted the experiments. Y.-C.L. designed studies, interpreted the data, and wrote the draft. S.-H.H.J. designed and supervised studies, interpreted the data, and wrote and proved the draft.
Conflicts of Interest
H.-Y.C., Y.-S.W., and S.-H.H.J. have filed patents on the basis of the data from this study. The other authors have no competing interests. The authors have no additional financial interests.
Acknowledgments
This work was supported by grants from the following organizations: the Department of Health (100TM014), the Ministry of Science and Technology (Taiwan, R.O.C., MOST103-2314-B-037-026-MY3, MOST 103-2314-B-037-027-MY2, MOST 105-2314-B-039-050, and MOST 106-2314-B-039-021), the National Health Research Institutes (Taiwan, R.O.C., NHRI-EX106-10605PI), Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10601010036), and “Drug Development Center, China Medical University” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Footnotes
Supplemental Information includes eleven figures, two tables, and Supplemental Materials and Methods and can be found with this article online at https://doi.org/10.1016/j.omtm.2018.11.011.
Supplemental Information
References
- 1.Feigin V.L., Lawes C.M., Bennett D.A., Barker-Collo S.L., Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 2.Cheng N.T., Kim A.S. Intravenous thrombolysis for acute ischemic stroke within 3 hours versus between 3 and 4.5 hours of symptom onset. Neurohospitalist. 2015;5:101–109. doi: 10.1177/1941874415583116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reeves M.J., Arora S., Broderick J.P., Frankel M., Heinrich J.P., Hickenbottom S., Karp H., LaBresh K.A., Malarcher A., Mensah G., Paul Coverdell Prototype Registries Writing Group Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry. Stroke. 2005;36:1232–1240. doi: 10.1161/01.STR.0000165902.18021.5b. [DOI] [PubMed] [Google Scholar]
- 4.Morgenstern L.B., Hemphill J.C., 3rd, Anderson C., Becker K., Broderick J.P., Connolly E.S., Jr., Greenberg S.M., Huang J.N., MacDonald R.L., Messé S.R., American Heart Association Stroke Council and Council on Cardiovascular Nursing Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–2129. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steiner T., Al-Shahi Salman R., Beer R., Christensen H., Cordonnier C., Csiba L., Forsting M., Harnof S., Klijn C.J., Krieger D., European Stroke Organisation European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int. J. Stroke. 2014;9:840–855. doi: 10.1111/ijs.12309. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y.S., Wang H.Y., Liao Y.C., Tsai P.C., Chen K.C., Cheng H.Y., Lin R.T., Juo S.H. MicroRNA-195 regulates vascular smooth muscle cell phenotype and prevents neointimal formation. Cardiovasc. Res. 2012;95:517–526. doi: 10.1093/cvr/cvs223. [DOI] [PubMed] [Google Scholar]
- 7.Ai J., Sun L.H., Che H., Zhang R., Zhang T.Z., Wu W.C., Su X.L., Chen X., Yang G., Li K. MicroRNA-195 protects against dementia induced by chronic brain hypoperfusion via its anti-amyloidogenic effect in rats. J. Neurosci. 2013;33:3989–4001. doi: 10.1523/JNEUROSCI.1997-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu H.C., Wang L.M., Wang M., Song B., Tan S., Teng J.F., Duan D.X. MicroRNA-195 downregulates Alzheimer’s disease amyloid-β production by targeting BACE1. Brain Res. Bull. 2012;88:596–601. doi: 10.1016/j.brainresbull.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Sun X.Y., Lu J., Zhang L., Song H.T., Zhao L., Fan H.M., Zhong A.F., Niu W., Guo Z.M., Dai Y.H. Aberrant microRNA expression in peripheral plasma and mononuclear cells as specific blood-based biomarkers in schizophrenia patients. J. Clin. Neurosci. 2015;22:570–574. doi: 10.1016/j.jocn.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Long G., Wang F., Duan Q., Yang S., Chen F., Gong W., Yang X., Wang Y., Chen C., Wang D.W. Circulating miR-30a, miR-195 and let-7b associated with acute myocardial infarction. PLoS ONE. 2012;7:e50926. doi: 10.1371/journal.pone.0050926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Winter F., Holtmaat A.J., Verhaagen J. Neuropilin and class 3 semaphorins in nervous system regeneration. Adv. Exp. Med. Biol. 2002;515:115–139. doi: 10.1007/978-1-4615-0119-0_10. [DOI] [PubMed] [Google Scholar]
- 12.Gagliardini V., Fankhauser C. Semaphorin III can induce death in sensory neurons. Mol. Cell. Neurosci. 1999;14:301–316. doi: 10.1006/mcne.1999.0787. [DOI] [PubMed] [Google Scholar]
- 13.Su J.L., Lin M.T., Hong C.C., Chang C.C., Shiah S.G., Wu C.W., Chen S.T., Chau Y.P., Kuo M.L. Resveratrol induces FasL-related apoptosis through Cdc42 activation of ASK1/JNK-dependent signaling pathway in human leukemia HL-60 cells. Carcinogenesis. 2005;26:1–10. doi: 10.1093/carcin/bgh220. [DOI] [PubMed] [Google Scholar]
- 14.Manna K., Khan A., Kr Das D., Bandhu Kesh S., Das U., Ghosh S., Sharma Dey R., Das Saha K., Chakraborty A., Chattopadhyay S. Protective effect of coconut water concentrate and its active component shikimic acid against hydroperoxide mediated oxidative stress through suppression of NF-κB and activation of Nrf2 pathway. J. Ethnopharmacol. 2014;155:132–146. doi: 10.1016/j.jep.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 15.Thomas A., Giesler T., White E. p53 mediates bcl-2 phosphorylation and apoptosis via activation of the Cdc42/JNK1 pathway. Oncogene. 2000;19:5259–5269. doi: 10.1038/sj.onc.1203895. [DOI] [PubMed] [Google Scholar]
- 16.Hou S.T., Jiang S.X., Desbois A., Huang D., Kelly J., Tessier L., Karchewski L., Kappler J. Calpain-cleaved collapsin response mediator protein-3 induces neuronal death after glutamate toxicity and cerebral ischemia. J. Neurosci. 2006;26:2241–2249. doi: 10.1523/JNEUROSCI.4485-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Hare M.J., Hou S.T., Morris E.J., Cregan S.P., Xu Q., Slack R.S., Park D.S. Induction and modulation of cerebellar granule neuron death by E2F-1. J. Biol. Chem. 2000;275:25358–25364. doi: 10.1074/jbc.M001725200. [DOI] [PubMed] [Google Scholar]
- 18.Kouroedov A., Eto M., Joch H., Volpe M., Lüscher T.F., Cosentino F. Selective inhibition of protein kinase Cbeta2 prevents acute effects of high glucose on vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 2004;110:91–96. doi: 10.1161/01.CIR.0000133384.38551.A8. [DOI] [PubMed] [Google Scholar]
- 19.Tzima E., Del Pozo M.A., Kiosses W.B., Mohamed S.A., Li S., Chien S., Schwartz M.A. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 2002;21:6791–6800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klohs J., Gräfe M., Graf K., Steinbrink J., Dietrich T., Stibenz D., Bahmani P., Kronenberg G., Harms C., Endres M. In vivo imaging of the inflammatory receptor CD40 after cerebral ischemia using a fluorescent antibody. Stroke. 2008;39:2845–2852. doi: 10.1161/STROKEAHA.107.509844. [DOI] [PubMed] [Google Scholar]
- 21.Arvidsson A., Collin T., Kirik D., Kokaia Z., Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 22.Candelario-Jalil E., Mhadu N.H., González-Falcón A., García-Cabrera M., Muñoz E., León O.S., Fiebich B.L. Effects of the cyclooxygenase-2 inhibitor nimesulide on cerebral infarction and neurological deficits induced by permanent middle cerebral artery occlusion in the rat. J. Neuroinflammation. 2005;2:3. doi: 10.1186/1742-2094-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berkhemer O.A., Fransen P.S., Beumer D., van den Berg L.A., Lingsma H.F., Yoo A.J., Schonewille W.J., Vos J.A., Nederkoorn P.J., Wermer M.J., MR CLEAN Investigators A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 24.Goyal M., Demchuk A.M., Menon B.K., Eesa M., Rempel J.L., Thornton J., Roy D., Jovin T.G., Willinsky R.A., Sapkota B.L., ESCAPE Trial Investigators Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 25.Campbell B.C., Mitchell P.J., Kleinig T.J., Dewey H.M., Churilov L., Yassi N., Yan B., Dowling R.J., Parsons M.W., Oxley T.J., EXTEND-IA Investigators Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 26.Saver J.L., Goyal M., Bonafe A., Diener H.C., Levy E.I., Pereira V.M., Albers G.W., Cognard C., Cohen D.J., Hacke W., SWIFT PRIME Investigators Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 27.Jovin T.G., Chamorro A., Cobo E., de Miquel M.A., Molina C.A., Rovira A., San Román L., Serena J., Abilleira S., Ribó M., REVASCAT Trial Investigators Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 28.Hang P., Sun C., Guo J., Zhao J., Du Z. BDNF-mediates down-regulation of microRNA-195 inhibits ischemic cardiac apoptosis in rats. Int. J. Biol. Sci. 2016;12:979–989. doi: 10.7150/ijbs.15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh R., Saini N. Downregulation of BCL2 by miRNAs augments drug-induced apoptosis—a combined computational and experimental approach. J. Cell Sci. 2012;125:1568–1578. doi: 10.1242/jcs.095976. [DOI] [PubMed] [Google Scholar]
- 30.Zhu H., Yang Y., Wang Y., Li J., Schiller P.W., Peng T. MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovasc. Res. 2011;92:75–84. doi: 10.1093/cvr/cvr145. [DOI] [PubMed] [Google Scholar]
- 31.Chen X., Jiang X.M., Zhao L.J., Sun L.L., Yan M.L., Tian Y., Zhang S., Duan M.J., Zhao H.M., Li W.R. MicroRNA-195 prevents dendritic degeneration and neuron death in rats following chronic brain hypoperfusion. Cell Death Dis. 2017;8:e2850. doi: 10.1038/cddis.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukherji S., Ebert M.S., Zheng G.X., Tsang J.S., Sharp P.A., van Oudenaarden A. MicroRNAs can generate thresholds in target gene expression. Nat. Genet. 2011;43:854–859. doi: 10.1038/ng.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shu J., Xia Z., Li L., Liang E.T., Slipek N., Shen D., Foo J., Subramanian S., Steer C.J. Dose-dependent differential mRNA target selection and regulation by let-7a-7f and miR-17-92 cluster microRNAs. RNA Biol. 2012;9:1275–1287. doi: 10.4161/rna.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang S.X., Whitehead S., Aylsworth A., Slinn J., Zurakowski B., Chan K., Li J., Hou S.T. Neuropilin 1 directly interacts with Fer kinase to mediate semaphorin 3A-induced death of cortical neurons. J. Biol. Chem. 2010;285:9908–9918. doi: 10.1074/jbc.M109.080689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou S.T., Nilchi L., Li X., Gangaraju S., Jiang S.X., Aylsworth A., Monette R., Slinn J. Semaphorin3A elevates vascular permeability and contributes to cerebral ischemia-induced brain damage. Sci. Rep. 2015;5:7890. doi: 10.1038/srep07890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasterkamp R.J., De Winter F., Holtmaat A.J., Verhaagen J. Evidence for a role of the chemorepellent semaphorin III and its receptor neuropilin-1 in the regeneration of primary olfactory axons. J. Neurosci. 1998;18:9962–9976. doi: 10.1523/JNEUROSCI.18-23-09962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J., Pei D.S., Zhang Q.G., Zhang G.Y. Down-regulation Cdc42 attenuates neuronal apoptosis through inhibiting MLK3/JNK3 cascade during ischemic reperfusion in rat hippocampus. Cell. Signal. 2007;19:831–843. doi: 10.1016/j.cellsig.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Lin R., Iacovitti L. Classic and novel stem cell niches in brain homeostasis and repair. Brain Res. 2015;1628:327–342. doi: 10.1016/j.brainres.2015.04.029. Pt B. [DOI] [PubMed] [Google Scholar]
- 39.Lin R., Cai J., Nathan C., Wei X., Schleidt S., Rosenwasser R., Iacovitti L. Neurogenesis is enhanced by stroke in multiple new stem cell niches along the ventricular system at sites of high BBB permeability. Neurobiol. Dis. 2015;74:229–239. doi: 10.1016/j.nbd.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 40.Shen Y., Mani S., Donovan S.L., Schwob J.E., Meiri K.F. Growth-associated protein-43 is required for commissural axon guidance in the developing vertebrate nervous system. J. Neurosci. 2002;22:239–247. doi: 10.1523/JNEUROSCI.22-01-00239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frey D., Laux T., Xu L., Schneider C., Caroni P. Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outgrowth, and anatomical plasticity. J. Cell Biol. 2000;149:1443–1454. doi: 10.1083/jcb.149.7.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Candelario-Jalil E. Injury and repair mechanisms in ischemic stroke: considerations for the development of novel neurotherapeutics. Curr. Opin. Investig. Drugs. 2009;10:644–654. [PubMed] [Google Scholar]
- 43.Wang H.Y., Liu C.B., Wu H.W., Kuo J.S. Direct profiling of phospholipids and lysophospholipids in rat brain sections after ischemic stroke. Rapid Commun. Mass Spectrom. 2010;24:2057–2064. doi: 10.1002/rcm.4620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.