Abstract
Propranolol is the first-line treatment for infants suffering from infantile hemangioma. Recently, some authors raised the question of potential neurologic side effects of propranolol due to its lipophilic nature and thus its ability to passively cross the blood-brain barrier (BBB) and accumulate into the brain. Hydrophilic beta-blockers, such as atenolol and nadolol, where therefore introduced in clinical practice. In addition to their classical mode of action in the brain, circulating factors may modulate the release of reactive oxygen/nitrogen species (ROS/RNS) from endothelial cells that compose the BBB without entering the brain. Due to their high capacity to diffuse across membranes, ROS/RNS can reach neurons and modify their activity. The aim of this study was to investigate other mechanisms of actions in which these molecules may display a central effect without actually crossing the BBB. We first performed an oral treatment in mice to measure the accumulation of propranolol, atenolol and nadolol in different brain regions in vivo. We then evaluated the ability of these molecules to induce the release of nitric oxide (NO) and hydrogen peroxide (H2O2) ex vivo in the hypothalamus. As expected, propranolol is able to cross the BBB and is found in brain tissue in higher amounts than atenolol and nadolol. However, all of these beta-blockers are able to induce the secretion of signaling molecules (i.e., NO and/or H2O2) in the hypothalamus, independently of their ability to cross the BBB, deciphering a new potential deleterious impact of hydrophilic beta-blockers in the brain.
Keywords: beta-blockers, propranolol, blood-brain barrier, infantile hemangioma, reactive oxygen species
Introduction
Since its discovery in 1960 by J. W. Black, the non-selective beta-blocker propranolol has been widely used in the treatment of hypertension, tachycardia and other cardiac disorders. More recently, propranolol has been introduced as first-line therapy for infantile hemangiomas, the most common soft-tissue tumors of childhood (Leaute-Labreze et al., 2015).
As propranolol is a lipophilic molecule, its use in infants raised the question of its ability to passively cross the blood-brain barrier (BBB) and directly activate adrenoreceptors on neuronal cells, and to subsequently impact the neurologic development of the child. Even if many reports have documented that propranolol treatment during infancy does not alter further brain development when compared to child and adolescents from the general population examined several years after cessation of treatment (Moyakine et al., 2016, 2017; Gonzalez-Llorente et al., 2017; Mahon et al., 2018), other beta-blockers with hydrophilic properties have been here or there introduced in the treatment of infantile hemangiomas. The most commonly used molecules are nadolol and atenolol, which display hydrophilic properties and are therefore suggested to be unable to cross the BBB (Neil-Dwyer et al., 1981; Dahlof and Dimenas, 1990) and thus potentially decrease the risk to induce deleterious central effects (de Graaf et al., 2013; Pope et al., 2013; Tasani et al., 2017; Alexopoulos et al., 2018). However, these assumptions are based on biophysical characteristics of these molecules, and data regarding long-term safety are clearly lacking.
Alternative mechanisms in which some molecules are able to induce signaling pathways in the brain without crossing the blood-brain barrier have been described. One mechanism, that requires diffusible factors between two partners, implies central neurons and endothelial cells located in vascular wall. Indeed, brain endothelial cells have the capacity to produce second messengers such as nitric oxide (NO) to control the release of neurotransmitters (Knauf et al., 2001). This is especially the case in the mediobasal hypothalamus that includes the median eminence, a circumventricular organ where the BBB is physiologically absent (Rodriguez et al., 2005, 2010; Horsburgh and Massoud, 2013). For example, circulating hormones like estrogen (Prevot et al., 1999) or apelin (Duparc et al., 2011) induce endothelial NO release, with consequences on physiological functions, such as ovulation and glucose homeostasis, respectively. Hydrogen peroxide (H2O2) is another way to induce a signaling pathway in this brain region. As previously described for NO signaling, high dose of apelin can modify the release of hypothalamic H2O2 that could participate to an over-activation of the sympathetic system leading to the development of a type 2 diabetic state (Drougard et al., 2014). Thus, some molecules can modulate the activity of neuronal cells despite the absence of passage through the BBB and of direct activation of specific receptors on these cells.
The aim of the present study was to investigate whether propranolol, atenolol and nadolol induce the secretion of signaling molecules (i.e., NO and H2O2) in the hypothalamus, independently of their ability to directly stimulate adrenoreceptors on neuronal cells.
Methods
Animals and Mass Spectrometry Analyses
All procedures are performed in accordance with the Directive 2010/63/UE recommendations and with French Veterinary Authorities agreement. Ex vivo design and procedures were approved by Ethical Committee (under protocol CEEA-122 2014-53). Eight weeks-old C57BL/6J male mice (n = 10/group, mean body weight = 25 g) were orally treated with either propranolol (3 mg/kg/day), atenolol (2 mg/kg/day) or nadolol (1 mg/kg/day) during 7 days, once a day for vehicle, nadolol and atenolol groups (at 8.00 am) and twice a day for propranolol group (at 8.00 am and 6.00 pm). Dose and administration scheme were selected to mimic therapeutic use of these molecules in infants suffering from infantile hemangioma. Mice were euthanized 1 h after the last gavage. Cortex, hypothalamus, cerebellum and brainstem were collected and immediately frozen in liquid nitrogen.
The concentrations of propranolol, atenolol and nadolol were determined after solid phase extraction followed by LC/ESI-MS/MS detection. Tissues were diluted in 9 ml of ultrapure water for 1 g of brain and homogenized by sonication over crushed ice. Atenolol-D7 and propranolol-D7 were used as internal standards. Dynamic concentration range was comprised between 1 and 8000 ng/ml for each compound. The chromatographic peaks for tested compound and internal standards were identified according to their retention times and MRM ion transitions and integrated by analytical software (MassLynx version 4.1, Waters).
Nitric Oxide and Hydrogen Peroxyde ex vivo Amperometric Measurements
Calibration of the electrochemical sensor was performed as previously published (Duparc et al., 2011; Drougard et al., 2014; Fournel et al., 2017; Abot et al., 2018). After dissection, hypothalamus was washed in Krebs-Ringer bicarbonate/glucose buffer (pH 7.4) in an atmosphere of 95%O2–5%CO2 and then immersed in Eppendorf tubes containing the same medium. Spontaneous NO or H2O2 release was measured at 37°C for 10 min by using either a NO-specific (ISO-NOPF, World Precision Instruments) or a H2O2-specific (ISO-HPO, World Precision Instruments) amperometric probe implanted in the hypothalamus. Ten micro liter saline solution (vehicle) was injected directly in the survival medium. After 10 min of record, 10 μl beta-blocker solution at increasing concentration was injected (final concentrations: 50 and 250 ng/ml). These concentrations were chosen to mimic plasma concentration of these molecules after an oral therapeutic dose in humans (i.e., approximately 50 ng/ml for propranolol and nadolol, and 250 ng/ml for atenolol). The concentration of NO or H2O2 gas in solution was measured in real-time (TBR1025, World Precision Instruments). DataTrax2 software (World Precision Instruments) performed data acquisition. Data are expressed as delta variation of NO or H2O2 release from basal.
Statistical Analyses
All statistical analyses were performed using GraphPad Prism 5.0 for Windows (GraphPad Software Inc., San Diego, CA, United States). Two-way ANOVA and Bonferroni’s post hoc tests when used when appropriate. All values are presented as mean ± SEM. Statistical significance was set at p < 0.05.
Results
Propranolol, Atenolol, and Nadolol Concentration in the Brain
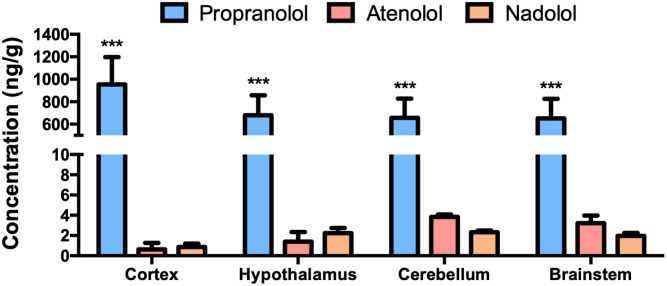
We have first evaluated the capacity of the three beta-blockers to reach brain tissues (Figure 1). As previously described, propranolol is found at a high concentration in the brain after an oral treatment. In the same experimental conditions, the concentrations of nadolol and atenolol are lower in all brain regions, including a neurohemal structure such as the hypothalamus. This last result suggests that hydrophilic molecules cannot massively penetrate into the brain whether or not the BBB is leaky.
FIGURE 1.
Accumulation of propranolol, atenolol, and nadolol in different brain regions after a chronic oral treatment in mice. Concentration of propranolol, atenolol, and nadolol measured in the cortex, hypothalamus, brainstem, and cerebellum after 1 week of daily oral treatment in mice; n = 10/group; ∗∗∗p < 0,001 vs. atenolol and nadolol.
Hypothalamic NO Release in Response to Beta-Blockers
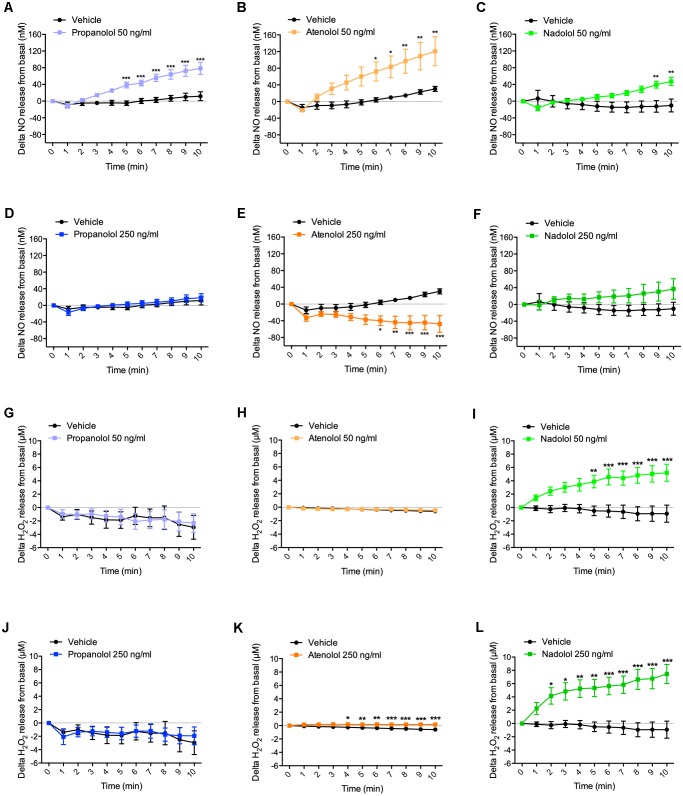
At the dose of 50 ng/ml, propranolol rapidly increased NO release from hypothalamus from 5 to 10 min (Figure 2A). Interestingly, atenolol was, at the same dose, also able to increase NO secretion from 6 to 10 min (Figure 2B), while delta variation of NO release in response to nadolol treatment was significantly induced after 9 min (Figure 2C). Taken together, these results show that, at a low dose, all three tested beta-blockers are able to induce NO release from hypothalamus.
FIGURE 2.
Hypothalamic NO and H2O2 release in response to propranolol, atenolol, and nadolol treatment. Ex vivo NO (A–F) and H2O2 (G–L) release from hypothalamus in response to propranolol (A,D,G,J), atenolol (B,E,H,K), or nadolol (C,F,I,L) at either low dose 50 ng/mL (A–C,G–I) or high dose 250 ng/mL (D–F,J–L); n = 10/group; ∗p < 0,05, ∗∗p < 0,01, and ∗∗∗p < 0,001 vs. vehicle.
When hypothalamus was treated with propranolol at a higher dose (i.e., 250 ng/ml), no significant variation in NO release was observed (Figure 2D). Surprisingly, atenolol displayed an opposite effect at the high dose compared to low dose treatment by significantly decreasing NO release after 6 min of treatment (Figure 2E). As observed for propranolol, nadolol treatment at this same dose did not induce any significant variation of NO release by the hypothalamus (Figure 2F).
These data indicate that lipophilic propranolol and hydrophilic atenolol and nadolol are all able to induce NO release from the hypothalamus at a low dose. Contrasting results observed at a higher dose suggest that alternative mechanisms may be activated to counterbalance the effects of massive NO release in the brain.
Hypothalamic H2O2 Release in Response to Beta-Blockers
Treatment with a low dose of propranolol (i.e., 50 ng/ml) did not induce any significant variation of H2O2 release from the hypothalamus (Figure 2G). Similar results have been observed upon atenolol treatment (Figure 2H). However, in response to nadolol at a low dose, delta variation of H2O2 release from basal significantly increased from 5 min (Figure 2I).
At a higher dose of 250 ng/ml, no significant changes of H2O2 production were observed in response to propranolol treatment (Figure 2J), while both atenolol (Figure 2K) and nadolol (Figure 2L) treatment rapidly increased H2O2 release.
Altogether, these results clearly show that, while propranolol treatment does not induce H2O2 release from the hypothalamic explants at any dose, a high dose of atenolol or nadolol rapidly induce H2O2 release from the hypothalamus. The amplitude of H2O2 production is particularly high in response to nadolol treatment, either at a low or high dose.
Discussion
We have shown that, despite the inability of hydrophilic beta-blockers to cross the BBB and thus to accumulate into the brain, all three beta-blockers tested in this study, either lipophilic or hydrophilic, were able to modulate the release of NO and/or H2O2 in the hypothalamus. As these small molecules can passively diffuse in brain tissue, they could have an impact on neuronal activity of central neurons. This could explain some of the potential deleterious effects of these molecules in the brain such as sleep disorders (de Graaf et al., 2013; Pope et al., 2013; Labreze et al., 2015; Leaute-Labreze et al., 2015; Randhawa et al., 2015; Ji et al., 2016) and in periphery such as hypoglycemia (Holland et al., 2010; Poterucha et al., 2015).
Despite its ability to accumulate into the brain, propranolol failed to increase the release of hypothalamic H2O2 that is usually associated with oxidative stress (Fisher-Wellman and Neufer, 2012; Angelova and Abramov, 2018). Little is known in the literature to explain the mode of action of propranolol to avoid H2O2 release. It has been shown that, in the skin of frog, propranolol is able to decrease water permeability induced by arginine vasotocin (AVT) (Ogushi et al., 2010; Saitoh et al., 2014). In this model, aquaporins are translocated at the membrane in response to AVT. We can speculate that propranolol, by acting on aquaporins translocation, could decrease the negative impact of H2O2, which is known to use aquaporins to cross cell membrane (Tamma et al., 2018).
The effect of atenolol on hypothalamic NO/H2O2 release is also unexpected. At low dose, atenolol induces NO release from hypothalamic explants, but at higher dose of 250 ng/ml, corresponding to plasma concentration measured after an oral administration at the therapeutic dose, it decreases NO release and increases H2O2 secretion. Even if this dual effect seems surprising, our group has previously demonstrated the existence of such physiological phenomenon in another physiological context. Indeed, apelin, an adipokine released by the adipose tissue, stimulates the release of hypothalamic NO at a low dose (Duparc et al., 2011), but inhibits NO release and stimulates H2O2 release at a high dose (Duparc et al., 2011; Drougard et al., 2014). In our experimental model, this dual effect can be explained by pharmacological and physiological hypotheses. For instance, the decrease of NO release could be due to its interaction with H2O2 to generate hydroxyl radical (Nappi and Vass, 1998).
In addition, we observed that nadolol induces both NO and H2O2 secretion either at a low or high dose. The low dose reproduces plasma concentration measured after an oral therapeutic dose of nadolol. This result demonstrates that the ability to cross the BBB is not mandatory to induce signaling in the brain, and thus further activation/inactivation of specific neuronal populations.
Finally, the magnitude of NO/H2O2 release in response to the different treatments also needs to be considered. Indeed, while a release in the range of pM to nM induces physiological effects such as signal transduction and neurotransmission (either beneficial or detrimental depending on targeted neurons), a release in the range of nM to μM induces deleterious effects such as oxidative stress and DNA damage leading to cellular dysfunction (Bredt, 1999; Mustafa et al., 2009). In this study we show that, at a concentration mimicking plasma concentration of the three beta-blockers after a therapeutic dose, propranolol induced a release of 80 nM of NO and no change in H2O2 production. In contrast, atenolol decreased NO release but increased H2O2 production in the range of μM. Nadolol was responsible of the release of 50 nM of NO and, more importantly, induced a 5 μM release of H2O2, which is probably leading to oxidative stress and cellular toxicity. Altogether, these data indicate that, even if they do not cross the BBB and do not accumulate in brain tissues, both atenolol and nadolol are able to induce central toxicity.
Of course, our study is limited by the use of murine ex vivo models which do not reflect the exact physiological conditions observed in vivo. A similar approach (i.e., NO/H2O2 real-time measurements) could be performed in vivo in different brain regions in mice in response to an oral load of beta-blockers (Fournel et al., 2017).
To conclude, our study brings new elements to decipher the mode of action (and the potential related side effects) of beta-blockers in the brain. The mode of communication through endothelial cells and production of NO/H2O2 that does not require the entry of a beta-blocker molecule into the brain has to be considered when using such molecule.
Author Contributions
All authors have designed the experiments and wrote the manuscript. AA had performed the experiments.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was funded by Pierre Fabre Dermatologie. AD is employed by Pierre Fabre. CK is co-founder of Enterosys SAS (Labège, France).
References
- Abot A., Lucas A., Bautzova T., Bessac A., Fournel A., Le-Gonidec S., et al. (2018). Galanin enhances systemic glucose metabolism through enteric nitric oxide synthase-expressed neurons. Mol. Metab. 10 100–108. 10.1016/j.molmet.2018.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos A., Thanopoulou I., Dakoutrou M., Georgiadou E., Chrousos G. P., Kakourou T. (2018). Atenolol treatment for severe infantile hemangiomas: a single-centre prospective study. J. Eur. Acad. Dermatol. Venereol. 32e117–e119. 10.1111/jdv.14590 [DOI] [PubMed] [Google Scholar]
- Angelova P. R., Abramov A. Y. (2018). Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett. 592 692–702. 10.1002/1873-3468.12964 [DOI] [PubMed] [Google Scholar]
- Bredt D. S. (1999). Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radic Res. 31 577–596. 10.1080/10715769900301161 [DOI] [PubMed] [Google Scholar]
- Dahlof C., Dimenas E. (1990). Side effects of beta-blocker treatments as related to the central nervous system. Am. J. Med. Sci. 299 236–244. 10.1097/00000441-199004000-00004 [DOI] [PubMed] [Google Scholar]
- de Graaf M., Raphael M. F., Breugem C. C., Knol M. J., Bruijnzeel-Koomen C. A., Kon M., et al. (2013). Treatment of infantile haemangiomas with atenolol: comparison with a historical propranolol group. J. Plast Reconstr. Aesthet. Surg. 66 1732–1740. 10.1016/j.bjps.2013.07.035 [DOI] [PubMed] [Google Scholar]
- Drougard A., Duparc T., Brenachot X., Carneiro L., Gouaze A., Fournel A., et al. (2014). Hypothalamic apelin/reactive oxygen species signaling controls hepatic glucose metabolism in the onset of diabetes. Antioxid. Redox Signal. 20 557–573. 10.1089/ars.2013.5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duparc T., Colom A., Cani P. D., Massaly N., Rastrelli S., Drougard A., et al. (2011). Central apelin controls glucose homeostasis via a nitric oxide-dependent pathway in mice. Antioxid. Redox Signal. 15 1477–1496. 10.1089/ars.2010.3454 [DOI] [PubMed] [Google Scholar]
- Fisher-Wellman K. H., Neufer P. D. (2012). Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol. Metab. 23 142–153. 10.1016/j.tem.2011.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournel A., Drougard A., Duparc T., Marlin A., Brierley S. M., Castro J., et al. (2017). Apelin targets gut contraction to control glucose metabolism via the brain. Gut 66 258–269. 10.1136/gutjnl-2015-310230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Llorente N., Del Olmo-Benito I., Munoz-Ollero N., Descalzo M. A., Garcia-Doval I., Torrelo A. (2017). Study of cognitive function in children treated with propranolol for infantile hemangioma. Pediatr. Dermatol. 34 554–558. 10.1111/pde.13229 [DOI] [PubMed] [Google Scholar]
- Holland K. E., Frieden I. J., Frommelt P. C., Mancini A. J., Wyatt D., Drolet B. A. (2010). Hypoglycemia in children taking propranolol for the treatment of infantile hemangioma. Arch. Dermatol. 146 775–778. 10.1001/archdermatol.2010.158 [DOI] [PubMed] [Google Scholar]
- Horsburgh A., Massoud T. F. (2013). The circumventricular organs of the brain: conspicuity on clinical 3T MRI and a review of functional anatomy. Surg. Radiol. Anat. 35 343–349. 10.1007/s00276-012-1048-2 [DOI] [PubMed] [Google Scholar]
- Ji Y., Wang Q., Chen S., Xiang B., Xu Z., Li Y., et al. (2016). Oral atenolol therapy for proliferating infantile hemangioma: a prospective study. Medicine 95:e3908. 10.1097/MD.0000000000003908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf C., Ferreira S., Hamdane M., Mailliot C., Prevot V., Beauvillain J. C., et al. (2001). Variation of endothelial nitric oxide synthase synthesis in the median eminence during the rat estrous cycle: an additional argument for the implication of vascular blood vessel in the control of GnRH release. Endocrinology. 142 4288–4294. 10.1210/endo.142.10.8443 [DOI] [PubMed] [Google Scholar]
- Labreze C., Voisard J. J., Delarue A., Moore N. (2015). Risk of neurodevelopmental abnormalities in children treated with propranolol. Br. J. Dermatol. 173 1562–1564. 10.1111/bjd.14000 [DOI] [PubMed] [Google Scholar]
- Leaute-Labreze C., Hoeger P., Mazereeuw-Hautier J., Guibaud L., Baselga E., Posiunas G., et al. (2015). A randomized, controlled trial of oral propranolol in infantile hemangioma. N. Engl. J. Med. 372 735–746. 10.1056/NEJMoa1404710 [DOI] [PubMed] [Google Scholar]
- Mahon C., Heron G., Perkins D., Drage A., Wargon O. (2018). Oral propranolol for infantile haemangioma may be associated with transient gross motor delay. Br. J. Dermatol. 178 1443–1444. 10.1111/bjd.16334 [DOI] [PubMed] [Google Scholar]
- Moyakine A. V., Kerstjens J. M., Spillekom-van Koulil S., van der Vleuten C. J. (2016). Propranolol treatment of infantile hemangioma (IH) is not associated with developmental risk or growth impairment at age 4 years. J. Am. Acad. Dermatol. 75 59.e1–63.e1. 10.1016/j.jaad.2016.02.1218 [DOI] [PubMed] [Google Scholar]
- Moyakine A. V., Spillekom-van Koulil S., van der Vleuten C. J. M. (2017). Propranolol treatment of infantile hemangioma is not associated with psychological problems at 7 years of age. J. Am. Acad. Dermatol. 77 105–108. 10.1016/j.jaad.2017.01.025 [DOI] [PubMed] [Google Scholar]
- Mustafa A. K., Gadalla M. M., Snyder S. H. (2009). Signaling by gasotransmitters. Sci. Signal. 2 re2 10.1126/scisignal.268re2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi A. J., Vass E. (1998). Hydroxyl radical formation resulting from the interaction of nitric oxide and hydrogen peroxide. Biochim. Biophys. Acta. 1380 55–63. 10.1016/S0304-4165(97)00125-6 [DOI] [PubMed] [Google Scholar]
- Neil-Dwyer G., Bartlett J., McAinsh J., Cruickshank J. M. (1981). Beta-adrenoceptor blockers and the blood-brian barrier. Br. J. Clin. Pharmacol. 11 549–553. 10.1111/j.1365-2125.1981.tb01169.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogushi Y., Kitagawa D., Hasegawa T., Suzuki M., Tanaka S. (2010). Correlation between aquaporin and water permeability in response to vasotocin, hydrin and {beta}-adrenergic effectors in the ventral pelvic skin of the tree frog Hyla japonica. J. Exp. Biol. 213 288–294. 10.1242/jeb.036871 [DOI] [PubMed] [Google Scholar]
- Pope E., Chakkittakandiyil A., Lara-Corrales I., Maki E., Weinstein M. (2013). Expanding the therapeutic repertoire of infantile haemangiomas: cohort-blinded study of oral nadolol compared with propranolol. Br. J. Dermatol. 168 222–224. 10.1111/j.1365-2133.2012.11131.x [DOI] [PubMed] [Google Scholar]
- Poterucha J. T., Bos J. M., Cannon B. C., Ackerman M. J. (2015). Frequency and severity of hypoglycemia in children with beta-blocker-treated long QT syndrome. Heart Rhythm. 12 1815–1819. 10.1016/j.hrthm.2015.04.034 [DOI] [PubMed] [Google Scholar]
- Prevot V., Croix D., Rialas C. M., Poulain P., Fricchione G. L., Stefano G. B., et al. (1999). Estradiol coupling to endothelial nitric oxide stimulates gonadotropin-releasing hormone release from rat median eminence via a membrane receptor. Endocrinology. 140 652–659. 10.1210/endo.140.2.6484 [DOI] [PubMed] [Google Scholar]
- Randhawa H. K., Sibbald C., Garcia Romero M. T., Pope E. (2015). Oral Nadolol for the Treatment of Infantile Hemangiomas: a Single-Institution Retrospective Cohort Study. Pediatr. Dermatol. 32 690–695. 10.1111/pde.12655 [DOI] [PubMed] [Google Scholar]
- Rodriguez E. M., Blazquez J. L., Guerra M. (2010). The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides. 31 757–776. 10.1016/j.peptides.2010.01.003 [DOI] [PubMed] [Google Scholar]
- Rodriguez E. M., Blazquez J. L., Pastor F. E., Pelaez B., Pena P., Peruzzo B., et al. (2005). Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int. Rev. Cytol. 247 89–164. 10.1016/S0074-7696(05)47003-5 [DOI] [PubMed] [Google Scholar]
- Saitoh Y., Ogushi Y., Shibata Y., Okada R., Tanaka S., Suzuki M. (2014). Novel vasotocin-regulated aquaporins expressed in the ventral skin of semiaquatic anuran amphibians: evolution of cutaneous water-absorbing mechanisms. Endocrinology. 155 2166–2177. 10.1210/en.2013-1928 [DOI] [PubMed] [Google Scholar]
- Tamma G., Valenti G., Grossini E., Donnini S., Marino A., Marinelli R. A., et al. (2018). Aquaporin membrane channels in oxidative stress, cell signaling, and aging: recent advances and research trends. Oxid. Med. Cell Longev. 2018:1501847. 10.1155/2018/1501847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasani M., Glover M., Martinez A. E., Shaw L. (2017). Atenolol treatment for infantile haemangioma. Br. J. Dermatol. 176 1400–1402. 10.1111/bjd.15317 [DOI] [PubMed] [Google Scholar]