Abstract
Several beneficial effects on oral health are ascribed to melatonin. Due to its lipophilic nature, non‐protein‐bound circulating melatonin is usually thought to enter the saliva by passive diffusion through salivary acinar gland cells. Recently, however, using transmission electron microscopy (TEM), melatonin was found in acinar secretory granules of human salivary glands. To test the hypothesis that granular located melatonin is actively discharged into the saliva by exocytosis, i.e. contrary to the general belief, the β‐adrenergic receptor agonist isoprenaline, which causes the degranulation of acinar parotid serous cells, was administered to anaesthetised rats. Sixty minutes after an intravenous bolus injection of isoprenaline (5 mg kg−1), the right parotid gland was removed; pre‐administration, the left control gland had been removed. Samples were processed to demonstrate melatonin reactivity using the immunogold staining method. Morphometric assessment was made using TEM. Gold particles labelling melatonin appeared to be preferentially associated with secretory granules, occurring in their matrix and at membrane level but, notably, it was also associated with vesicles, mitochondria and nuclei. Twenty‐six per cent of the total granular population (per 100 μm2 per cell area) displayed melatonin labelling in the matrix; three‐quarters of this fraction disappeared (P < 0.01) in response to isoprenaline, and melatonin reactivity appeared in dilated lumina. Thus, evidence is provided of an alternative route for melatonin to reach the gland lumen and the oral cavity by active release through exocytosis, a process which is under the influence of parasympathetic and sympathetic nervous activity and is the final event along the so‐called regulated secretory pathway. During its stay in granules, anti‐oxidant melatonin may protect their protein/peptide constituents from damage.
Keywords: exocytosis, immunogold method, melatonin, parotid gland, secretion, transmission electron microscopy
Introduction
Important roles are ascribed to melatonin (N‐acetyl‐5‐methoxytryptamine) in the protection of oral health. Despite usually being associated with the pineal gland and the circadian rhythm, the hormone also has a number of extrapineal sources and functions (Acuña‐Castroviejo et al. 2014). Once in the systemic circulation, the lipophilic nature of melatonin is thought to allow the non‐protein‐bound fraction of the hormone passively to diffuse across cell membranes, including those of salivary glands, and to enter the fluid secreted into the oral cavity. Moreover, an endogenous production of melatonin has been suggested for striated ducts in major salivary glands (Shimozuma et al. 2011).
Salivary melatonin has potential beneficial implications in caries, periodontitis, mucositis, candidiasis, herpes infection, xerostomia, the osseo‐integration of dental implants and even oral cancer (Gómez‐Moreno et al. 2010; Reiter et al. 2015; Permuy et al. 2017). These effects may be related to anti‐inflammatory, anti‐oxidant, free‐radical scavenging, antimicrobial, immunomodulatory and bone growth‐promoting activities of the hormone (Czesnikiewicz‐Guzik et al. 2007; Cengiz et al. 2012; Permuy et al. 2017).
Recent ultrastructural studies suggest that, in addition to its passive entry into the saliva, melatonin may be actively taken up by the secretory cells and secreted. In the acinar serous cells of the human parotid gland, melatonin occurs in close contact with the secretory granules or in fact in the granular matrix (Isola & Lilliu, 2016). Moreover, in the parotid glands of humans and rats, melatonin receptors of the MT1 and MT2 types, belonging to the G‐protein family, are found not only in the plasma cell membranes but also, and predominantly, in association with the secretory granules (Isola et al. 2013, 2016, 2017). In addition, using the rat parotid gland as an experimental model (Isola et al. 2016), a high load of circulating melatonin increases not only the number of secretory granules marked for MT1 receptors but also the density of this type of receptor per granule, suggesting an intracellular adaptive melatonin receptor‐dependent transport and granular storage system in the gland. If located in granules, the gland would lose melatonin due to exocytosis. In the current study, using transmission electron microscopy (TEM) and immunogold‐labelled melatonin, this hypothesis is put to the test by exposing anaesthetized rats to a large intravenous dose of the sympathetic β‐adrenergic receptor agonist isoprenaline (Amsterdam et al. 1969; Peter et al. 1995). The consequent marked degranulation of parotid, a gland that has the advantage of consisting of just one type of acinar cell, allowed us to assess this kind of melatonin release.
Materials and methods
Seven adult female Sprague‐Dawley rats (Harlan Inc., UK), weighing 245 ± 6 g (mean ± SEM) were used. The experiments were approved by the Ethics Committee for Animal Experiments (CESA), University of Cagliari, Italy, and conformed to the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Council of Europe No. 123, Strasbourg, 1985). The rats were housed in standard plastic cages with wood chip bedding in a room with light–dark cycles of 12 h (lights on at 08:00 h). The room temperature was between 22 and 23 °C. A standard rat pelleted diet (Mucedola s.r.l., Italy) was always available, except for about 15 h preceding the experiment. A fasting period was necessary to prevent the animals from reflexively stimulating their salivary glands in response to food intake, thereby evoking exocytosis. The experiments started at about 10:00 h, i.e. at a time when the blood level of melatonin is low (Jaworek et al. 2004). Tap water was always available. The experiments were as humane as possible. The animals were anaesthetised with an intraperitoneal dose (0.5 mL 100 g–1) of Equithesin (a fresh mixture of 4.25% chloral hydrate, 2.1% magnesium sulphate and 0.97% pentobarbital sodium). Body temperature was monitored using a rectal probe and was maintained at 38 °C by means of a thermostatically controlled blanket. The animals were fitted with a femoral venous polyethylene catheter to serve as a conduit for the administration of isoprenaline, and a tracheal cannula. Prior to the drug administration, the left parotid gland was carefully dissected, removed and weighed for use as control tissue. Bleeding, if any, was stopped with Spongostan™ and the wound was closed. Isoprenaline (5 mg kg−1 bodyweight; Sigma, St. Louis, MO, USA) was infused slowly over 4–5 min. In one rat, the right gland had already been removed 10 min after the start of the administration of isoprenaline for exploratory purposes. In the remaining six rats, the right parotid gland was removed after 60 min and weighed. The animals were killed by an overdose of Equithesin.
Transmission electron microscopy
Glandular fragments of 1 mm3 were treated for TEM observation. The specimens were fixed in a mixture of 1.25% glutaraldehyde and 1% paraformaldehyde in 0.15 m cacodylate buffer (pH 7.2) for 2 h at room temperature, post‐fixed with 2% osmium, dehydrated and embedded in epoxy resin (Embed‐812, Electron Microscopy Sciences, Hatfield, UK). Semithin sections (2 μm), stained with toluidine blue, were examined by light microscopy, using a Leica DMR HC, to check the histological appearance. Light microscopy images were obtained with a Leica DC300 camera and elaborated using Leica IM50 v: 1.2 software (Leica Microsystem Ag, Germany). Ultrathin sections were observed by a Jeol 100S TEM operating at 80 kV.
Transmission electron microscopy and immunogold technique
Samples from non‐treated and treated parotid glands were cut into small pieces (1 mm3) and fixed with a mixture of 3% paraformaldehyde and 1% glutaraldehyde in 0.1 m cacodylate buffer (pH 7.2) for 2 h at room temperature. To preserve the antigenicity of the tissue, most pieces were rinsed in cacodylate buffer to which 3.5% sucrose was added, dehydrated and embedded in epoxy resin; treatment with osmium tetroxide was omitted. Randomly selected tissue blocks were used to prepare ultrathin sections (80 nm) for electron microscopic study. Ultrathin sections were collected on nickel grids and processed for the immunohistochemical analysis (Isola et al. 2017). Though the acrylic resin/UV light method is usually regarded as more sensitive (Gocht, 1992) than the currently used, well established immunohistochemical method, the outcome of the comparisons between isoprenaline‐treated and control glands is likely to be the same regardless of method.
Melatonin immunohistochemical analysis
For melatonin immunolocalisation, the grids were treated with 1% bovine serum albumin (BSA) and 5% normal goat serum (NGS) in phosphate‐buffered saline (PBS) solution to block non‐specific binding. They were then incubated overnight at 4 °C with a rabbit polyclonal antibody specific to melatonin (Thermo Fisher Scientific Inc., Waltham, MA, USA; PA:1‐85053) diluted 1 : 20 in PBS + 1% BSA and 5% NGS. The grids were then incubated for 1 h at room temperature with the secondary antiserum, a goat anti‐rabbit IgG conjugated to 15‐nm gold particles (GE Healthcare, UK), diluted 1 : 30 in PBS + 1% BSA.
Negative control samples were obtained either by incubating salivary tissue with the diluted primary antibody pre‐absorbed (antibody/melatonin ratio: 1 : 5 and 1 : 10) for 24 h at 4 °C, by only incubating with non‐immune serum or by omitting the primary antibody.
Quantitative evaluation of labelling and statistical analysis
A total of 54 randomly selected electron micrographs were used to quantify immunoreactivity using the image‐pro plus program (v: 4.5; Media Cybernetics, Inc.); 30 images came from non‐treated glands and 24 from treated glands. All the granules in the micrographs randomly selected were considered. A total of 1524 granules were considered for the statistical analysis; 1190 granules came from non‐treated parotid glands and 334 granules from isoprenaline‐treated parotid glands. We considered those gold particles directly placed onto the granular membranes and those gold particles within a range of about ± 25 nm related to the membranes to be associated with the granular membrane. The labelling density of the granules was calculated for unstimulated and stimulated parotids and expressed as the number of gold particles per 1 μm2. The percentage of melatonin‐positive granules was calculated by counting the number of labelled granules with respect to the total granule number per surface unit (100 μm2). The values obtained from non‐treated samples were compared with those obtained from treated ones. With respect to each parameter, a mean value was calculated for each gland (when appropriate). An unpaired Student's t‐test with Welch's correction was used to calculate statistical significances based on log values (with respect to the morphometric assessment). All data were expressed as means ± SEM. The significance level was set at P < 0.05.
Results
The weights of the parotid glands exposed to isoprenaline for 60 min (158.4 ± 15.1 mg, n = 6) did not differ statistically from those of the control glands removed before the administration of the drug (168.3 ± 13.7 mg, n = 7); the gland exposed to isoprenaline for just 10 min weighed 134.3 mg.
Parotid morphology
In unstimulated glands, observed by light microscopy and TEM, the acinar cells were full of secretory granules and the lumina of the acini were restricted and devoid of profiles signalling exocytosis.
Melatonin localisation
The unstimulated parotid glands expressed immunoreactivity for melatonin. The reactivity was preferentially localized to the secretory granules of the acinar cells (Fig. 1a); the gold particles occurred both in the granular membrane and in the granular matrix. The nuclei, rough endoplasmic reticulum and the membranes of mitochondria also showed immunoreactivity for melatonin (Fig. 1b). Moreover, melatonin labelling occurred in small vesicles located among the granules.
Figure 1.
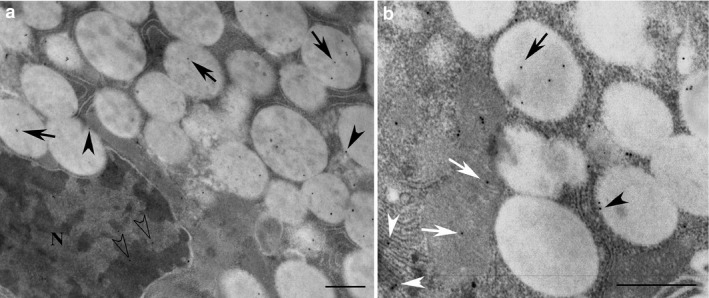
Acinar cells of non‐stimulated parotid glands used for immunogold localisation of melatonin. The reactivity for melatonin (black arrows) was observed in secretory granules. The gold particles (black dots) were also localised in the membrane (black arrowheads) of the granules (a). In addition, melatonin reactivity (b) was found associated with rough endoplasmic reticulum (white arrowheads) and mitochondria (white arrows), and often in the nucleus (transparent arrowheads). N, nucleus. Scale bar: 1 μm.
In contrast to acinar granules, the samples examined showed the secretory granules of intercalated and striated duct cells to lack melatonin labelling (Fig. 2a–c). However, in both types of ducts, small vesicles expressed reactivity for melatonin (Fig. 2a–c). In the striated duct cells, the labelled vesicles were principally found to be located in the apical portion of the cells (Fig. 2b); reactivity was also found in the folds of basal membranes of the cells (Fig. 2b), in the cristae and also here, in the membranes of mitochondria. In addition, nuclei of the duct cells displayed reactivity for melatonin (Fig. 2a,c).
Figure 2.
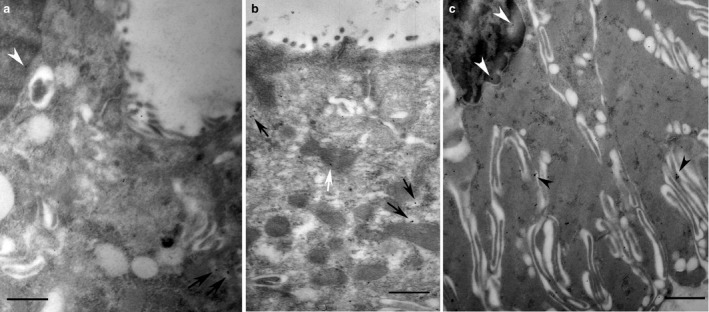
Intercalated (a) and striated (b,c) duct cells of non‐stimulated parotid glands used for the immunogold localisation of melatonin. The secretory granules of both intercalated and striated ducts displayed no immunoreactivity for melatonin. The small vesicles diffused in the cytoplasm appear to be, however, labelled (black arrows). In striated duct cells (b) melatonin labelling seemed to be also in the folds (arrowheads) of basal membranes and in mitochondria (white arrow). Often, in both ducts, melatonin reactivity occurred in the nuclei (a,c). Scale bar: 1 μm.
No reactivity for melatonin was demonstrated in those sections in which the primary antibody had been omitted; alternatively, only non‐immune serum had been incubated or pre‐adsorbed primary antibody combined with melatonin had been used.
Effects of isoprenaline
The acini of glands exposed to isoprenaline for 60 min displayed dilated lumina and enlarged intercellular canaliculi and, further, the cells exhibited omega profiles of exocytosis and, most strikingly, the loss of secretory granules, as shown by both light microscopy and TEM (Fig. 3 and 4). After just 10 min, morphological changes indicating exocytosis were evident, as shown in the single observation at that time (Fig. 5).
Figure 3.
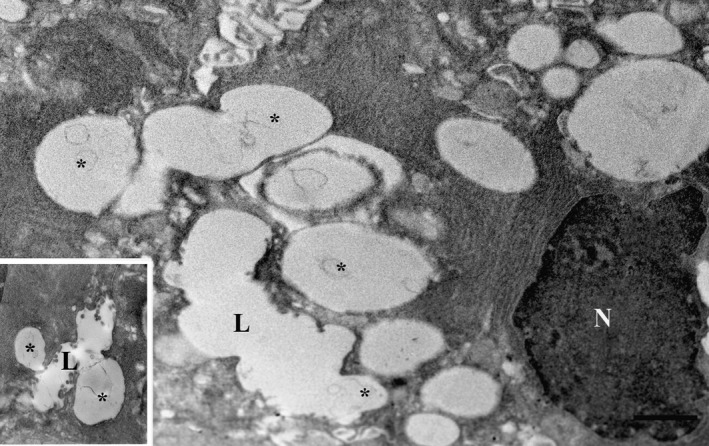
Cells of parotid gland by transmission electron microscopy (TEM) exposed to isoprenaline for 60 min post‐fixed with osmium to enhance morphology. The lumina (L) were dilated and profiles of exocytosis (asterisks) were visible. N, nucleus. Insert: Omega exocytotic profiles clearly connected to the lumen. Scale bar: 1 μm.
Figure 4.
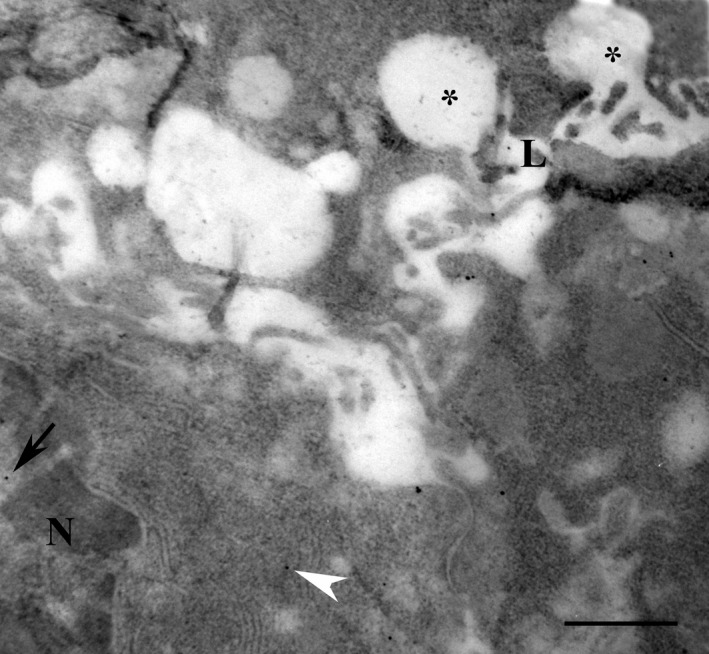
Serous cells of parotid gland exposed to isoprenaline for 60 min and used for the immunogold localisation of melatonin. Melatonin reactivity was observed in the lumen (close to the letter L) and in the membranes (white arrowhead) as well as in the nucleus (arrow). N: nucleus. *Omega exocytosis profiles. Scale bar: 1 μm.
Figure 5.
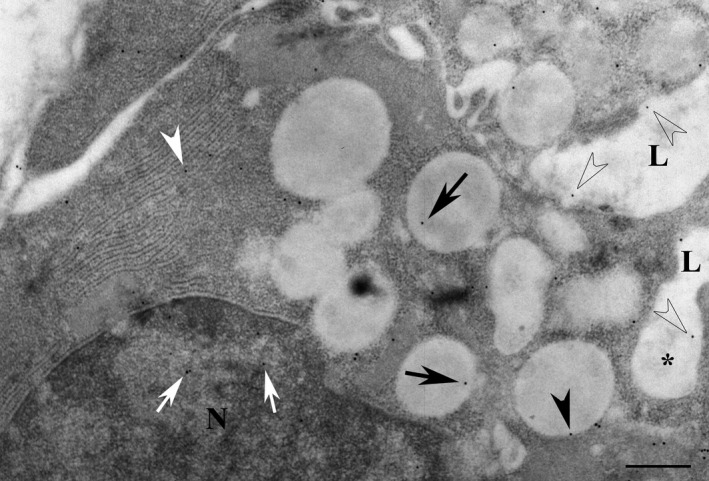
Serous cells of parotid gland exposed to isoprenaline for 10 min and used for the immunogold localisation of melatonin. Melatonin reactivity (transparent arrows) was observed in the lumen (L) and in omega exocytosis profiles (*). Reactivity was also observed in membranes (arrowhead) and in the matrix of persisting granules (black arrows), as well as in the rough endoplasmic reticulum (white arrowhead) and in the nucleus (white arrows). Compared with the unstimulated contralateral gland, the isoprenaline‐stimulated gland had lost 25% of its content of acinar secretory granules. N, nucleus. Scale bar: 1 μm.
With respect to melatonin‐labelled granule size, the mean area per granule was 0.96 ± 0.02 μm2 and 0.83 ± 0.08 μm2 (P < 0.05) in control (n = 413) and isoprenaline‐treated samples (n = 52, 60 min treatment), respectively. This difference in mean area between treated and non‐treated samples may reflect a shift in the composition of the total labelled granular population so that the proportion of small‐sized granules is increased and the proportions of granules of larger sizes are decreased after the isoprenaline treatment. Considering a hypothetical spheroidal granule with 1‐μm2 area, we established three ranges: granules with a size < 0.33 μm2, which are probably those cut at their polar level; granules with a size > 0.66 μm2, which are probably those cut at their equatorial level; and granules with a size between 0.33 and 0.66 μm2 in between those two cases. Our results are shown in Fig. 6.
Figure 6.
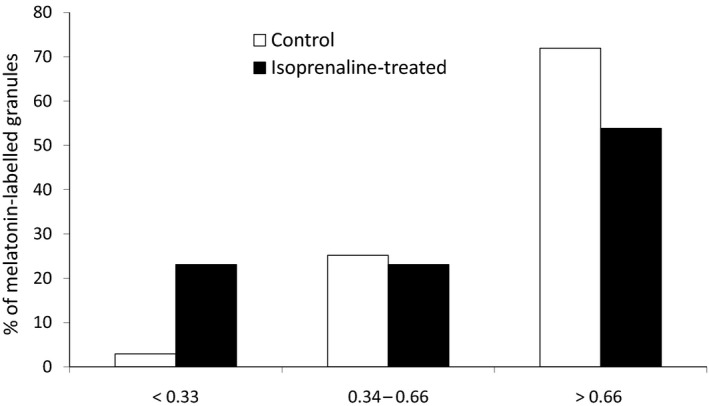
Percentage of the total number of melatonin‐labelled granules in the chosen range sizes (< 0.33 μm2, from 0.33 to 0.66 μm2 and > 0.66 μm2) in isoprenaline‐treated samples compared to control ones.
With respect to cell membranes, mitochondria, vesicles and nuclei, reactivity for melatonin was still demonstrable after isoprenaline. However, no quantitative analyses were performed on these structures.
As is evident from Table 1, the total number of granules per 100 μm2 of acinar cell area was significantly lower in the stimulated glands than in the control glands, differing by 74% (P < 0.001), and the granular populations labelled with melatonin in any way were similarly affected (by 75–76%, P < 0.01).
Table 1.
Melatonin‐labelled acinar secretory granules of the rat parotid gland and the exocytotic response to isoprenaline (5 mg kg−1, i.v.). Number of granule profiles of control and stimulated glands expressed per 100 μm2 of acinar cell area. Values are means ± SEM
| Total number of granule profiles | Total number of labelled granule profiles | Matrix with or without membrane‐ labelled granule profiles | Only matrix‐labelled granule profiles | |
|---|---|---|---|---|
| Control glands (n = 7) | 40.8 ± 6.2 | 14.3 ± 3.2 (35%)† | 11.0 ± 2.4 (27%)† | 9.1 ± 1.8 (22%)† |
| Stimulated glands (n = 6) | 10.6 ± 2.2 (26%)*** |
3.5 ± 1.5 (33%)†
(24%)** |
2.8 ± 1.4 (26%)†
(25%)** |
2.2 ± 1.0 (21%)†
(24%)** |
†When compared with the total number of granules within each group of glands.
** P < 0.01 and ***P < 0.001 when compared with corresponding figures for control glands.
The percentage distribution of melatonin in the granule population (Table 1), as related to the total number of granules per 100 μm2 of acinar cell area, was in the same range in stimulated and control glands with respect to the total number of granules labelled (33% vs. 35%), the number of granules displaying melatonin in the matrix with or without labelled membranes (26% vs. 27%) and, also, when singled out, those granules only displaying melatonin in the matrix (21% vs. 22%).
The figures for mean labelling density (i.e. gold particles per 1 μm2 of labelled granula) were, however, higher (P < 0.05) in the six stimulated glands (2.9 ± 0.4) than in the seven control glands (1.8 ± 0.2) with respect to those granules labelled in the matrix or at the membranes, or at both sites. When only considering those granules displaying melatonin in the matrix of the granula, the relationship between stimulated and non‐stimulated glands persisted but no statistically significant difference (P > 0.05) was attained, the figures being 2.5 ± 0.5 and 1.6 ± 0.2, respectively (data not shown in the table).
Discussion
The present study shows that melatonin is located in the secretory granules of the acinar serous cells of the rat parotid gland and therefore is likely to be released by exocytosis into the lumen, as a consequence of the mobilization of the major regulated secretory pathway (Castle & Castle, 1996). Exocytosis by the β‐adrenergic receptor agonist, isoprenaline, is a well known phenomenon in salivary glands, having been demonstrated not only in rodents (Amsterdam et al. 1969; Peter et al. 1995) but also in humans (Testa Riva et al. 2006; Del Fiacco et al. 2015). The present findings thus suggest a role for sympathetic activity, by β‐adrenergic receptors, in the secretion of melatonin. There are, in fact, a number of stimulants that are known to mobilise the regulated secretory pathway of the acinar cells and are therefore potential contributors to the secretion of melatonin. Muscarinic agonists and, in particular, the co‐transmitter of acetylcholine, vasoactive intestinal peptide, evoke exocytosis, demonstrating a regulatory role in this process for parasympathetic transmitters in both animals (Ekström et al. 1994; Ekström & Ekström, 2001; Ekström, 2002) and humans (Del Fiacco et al. 2015). In agreement with the exocytotic responses to autonomimetics, animal experiments show varying degrees of acinar granule depletion in response to the electrical stimulation of the sympathetic and parasympathetic innervations (Garrett & Thulin, 1975; Ekström et al. 1994, 1996). Moreover, hormones, including melatonin, of particular interest in the present context, have been shown to cause the secretion of protein/amylase (Çevik Aras & Ekström, 2008) and to evoke exocytosis in salivary glands (Loy et al. 2012, 2015); luzindole, an MT1/MT2 antagonist with greater affinity for MT2 receptors (Radio et al. 2006), abolished these secretory events evoked by melatonin. As a result, melatonin itself may not only stimulate its own uptake from the circulation and intracellular traffic, bound to its receptors, but also influence its own release. In addition, melatonin has been shown to stimulate the synthesis of salivary secretory proteins (Çevik‐Aras et al. 2011) and, further, to exert anti‐inflammatory actions in the glands (Çevik‐Aras & Ekström, 2010).
Interestingly, the previous demonstration of melatonin in human salivary glands showed that the number of secretory granules labelled with melatonin, contained in the serous cells, varied between the two types of examined glands (Isola & Lilliu, 2016). About 90% of the granules were labelled in the parotid glands, whereas the corresponding figure was 40% in the submandibular glands. The submandibular gland, being a mixed gland in terms of acinar cell type, seemed to lack melatonin labelling of mucous granules. The rat parotid glands showed a percentage figure (i.e. 35%) similar to that of the human submandibular glands.
In the current study, the total pool of labelled granules of isoprenaline‐treated glands and non‐treated glands, respectively, was found to differ. After the treatment, the percentage of small‐sized granules was higher and the percentage of large‐sized granules lower, probably reflecting de novo granules developing in the cells exposed to the exocytotic drug.
No preferential release of labelled or non‐labelled granule content in response to isoprenaline was found. There were, however, higher figures for the labelling density of melatonin (per granule) in the residual population of those granules being labelled after the exposure to isoprenaline (for 60 min). Whether this tendency represents melatonin that has newly entered from the circulation and reflects specific melatonin‐preferring features of certain granules is open to speculation. The fact that the labelling density may vary has been shown in the recently studied human glands (Isola & Lilliu, 2016), where the labelling density per granule was two times higher in the parotid glands than in the submandibular glands; the cause of this difference is unknown. Whether the isoprenaline treatment affected, in any direction, the quantity of the immunogold particles associated with various cell compartments, other than granules, was not assessed in the current study.
The present findings of labelled secretory granules in the acinar cells but not in the intercalated and striated duct cells may be combined with our previous demonstration of melatonin receptors associated with the acinar granules but not with the ductal cells in the rat parotid gland (Isola et al. 2016). Moreover, this difference in labelling of melatonin between granules of acinar cells and those of ductal cells may serve to underline that melatonin does not indiscriminately bind to the secretory granules of the gland.
On the assumption that granule melatonin is associated with the MT1 and MT2 receptors, hormone and receptor have not yet been demonstrated in the same image, it may be wondered whether the hormone‐receptor complex is cleaved once in the saliva. This would be in analogy to the receptor‐mediated passage of immunoglobulin A and transferrin following their transcytotic passage over the acinar cells (Nashida et al. 2009; Xu et al. 2013). Oral mucosal MT1 and MT2 receptors have been described in the rat (Shimozuma et al. 2011), opening the door to the possibility that the complex, as such, may anchor to the mucosa.
The level of melatonin in the saliva is about one‐third of that in the plasma, corresponding to the non‐protein‐bound fraction of melatonin in the plasma and thought to reflect the passive diffusion of the hormone (Kennaway & Voultsios, 1998). Recently, enzymes involved in the synthesis of melatonin were demonstrated in the striated ducts, but not the acinar cells, of rat salivary glands and human submandibular glands (Shimozuma et al. 2011), suggesting a possible ductal source of salivary melatonin. Although the present study provides the first evidence of the salivary gland secretion of melatonin by exocytosis, it may be worth considering a role for exocytotic melatonin not only in the saliva but also in the granules. In the granules, melatonin may, through its anti‐oxidant properties, protect the stored content of the granule, including the enzymes to be secreted, from damage, as pointed out earlier (Isola et al. 2016).
As in human salivary glands (Isola & Lilliu, 2016), melatonin also occurred in vesicles of the rat parotid gland, both in those vesicles among the acinar granules and in those in the striated ducts. Previously, the vesicles have also been shown to contain the receptors for melatonin (Isola et al. 2013, 2016). In the acinar cells, melatonin‐containing vesicles may indicate vesicles fusing with the granules, or budding from the granules to be secreted continuously by constitutive‐like pathways and not by exocytosis (Castle & Castle, 1996; Huang et al. 2001). In the striated ducts, the vesicle‐containing melatonin may reflect the ductal synthesis of melatonin (Shimozuma et al. 2011) or the endocytotic capture of melatonin from the ductal lumen.
Despite being characterised as lipophilic and thought to diffuse passively through membranes, melatonin (Yu et al. 2016), like some other lipophilic compounds such as thyroid hormones and glucocorticoids (Suzuki & Abe, 2008; Mason et al. 2010), may, in addition, be subjected to transport mechanisms. The glucose transporter GLUT1 has been suggested to carry melatonin over membranes in prostate cancer cells (Hevia et al. 2015). Furthermore, the anti‐oxidant activities of melatonin in mitochondria have recently attracted interest (Hardeland, 2017; Reiter et al. 2017). Both melatonin synthesis and the presence of an uptake system to concentrate melatonin in the mitochondria are the subject of discussion. It has been suggested that the human oligopeptide transporters (PEPT 1/2) located in the mitochondrial membranes transfer melatonin to the mitochondria (Huo et al. 2017). In this connection, our current finding of melatonin labelling in the mitochondrial membranes, combined with our previous demonstration of melatonin receptors of both the MT1 and MT2 types attached to the mitochondrial membranes in the rat parotid gland, are of interest (Isola et al. 2016). Similarly, the currently demonstrated presence of melatonin in the nuclei, combined with our previous findings of melatonin receptors at this location (Isola et al. 2016), may tentatively suggest that melatonin exerts protective anti‐oxidant actions of DNA (Venegas et al. 2012). Whether the two types of melatonin receptors are responsible for actually carrying melatonin over various membranes in salivary glands is, however, at present unknown.
With respect to the secretion of melatonin from the pineal gland, melatonin is thought to bypass the storage‐stage and, as it is synthesised, to leak from the pinealocytes due to its high membrane permeability (Yu et al. 2016).
Though exocrine functions and associated ultrastructural changes of salivary glands have been the focus of the current study, it is of interest to point out that melatonin may influence the morphology of salivary glands during their embryogenesis, as shown in vitro in mice submandibular glands (Obana‐Koshino et al. 2015).
To conclude, although melatonin is usually assumed to reach the saliva by passive diffusion through the epithelial cells, the present study demonstrates the occurrence of melatonin in acinar secretory granules and the release of the hormone by granule exocytosis in response to the activity of the regulated secretory pathway, a pathway known to be mobilised by both divisions of the autonomic nervous system, as well as by hormones, including melatonin itself. Apart from a number of beneficial effects in the oral cavity, melatonin in the granules may exert anti‐oxidant activities during its stay and thus protect the granular constituents.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
J. Ekström, M. Isola, R. Isola and F. Loy performed sampling collection, data analysis and interpretation, drafting of the manuscript and critical revision.
Acknowledgements
J. Ekström acknowledges support from the University of Cagliari for periods as visiting professor.
References
- Acuña‐Castroviejo D, Escames G, Venegas C, et al. (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci 71, 2997–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A, Ohad I, Schramm M (1969) Dynamic changes in the ultrastructure of the acinar cell of the rat parotid gland during the secretory cycle. J Cell Biol 41, 753–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle JD, Castle AM (1996) Two regulated secretory pathways for newly synthesized parotid salivary proteins are distinguished by doses of secretagogues. J Cell Sci 109, 2591–2599. [DOI] [PubMed] [Google Scholar]
- Cengiz Mİ, Cengiz S, Wang HL (2012) Melatonin and oral cavity. Int J Dent, 2012, 491872 10.1155/2012/491872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çevik Aras H, Ekström J (2008) Melatonin‐evoked in vivo secretion of protein and amylase from the parotid gland of the anaesthetized rat. J Pineal Res 45, 413–421. [DOI] [PubMed] [Google Scholar]
- Çevik‐Aras H, Ekström J (2010) Anti‐inflammatory action of cholecystokinin and melatonin in the rat parotid gland. Oral Dis 16, 661–667. [DOI] [PubMed] [Google Scholar]
- Çevik‐Aras H, Godoy T, Ekström J (2011) Melatonin‐induced protein synthesis in the rat parotid gland. J Physiol Pharmacol 62, 95–99. [PubMed] [Google Scholar]
- Czesnikiewicz‐Guzik M, Konturek SJ, Loster B, et al. (2007) Melatonin and its role in oxidative stress related diseases of oral cavity. J Physiol Pharmacol 58, 5–19. [PubMed] [Google Scholar]
- Del Fiacco M, Quartu M, Ekström J, et al. (2015) Effect of the neuropeptides vasoactive intestinal peptide, peptide histidine methionine and substance P on human major salivary gland secretion. Oral Dis 21, 216–223. [DOI] [PubMed] [Google Scholar]
- Ekström J (2002) Muscarinic agonist‐induced non‐granular and granular secretion of amylase in the parotid gland of the anaesthetized rat. Exp Physiol 87, 147–152. [DOI] [PubMed] [Google Scholar]
- Ekström J, Ekström PF (2001) Acinar degranulation in the rat parotid gland induced by neuropeptides. Exp Physiol 86, 733–738. [DOI] [PubMed] [Google Scholar]
- Ekström J, Asztély A, Helander HF, et al. (1994) Depletion of secretory granules from the feline parotid gland: action of NANC transmitters per se . Acta Physiol 150, 83–88. [DOI] [PubMed] [Google Scholar]
- Ekström J, Asztély A, Tobin G (1996) Non‐adrenergic, non‐cholinergic influences on parotid acinar degranulation in response to stimulation of the parasympathetic innervation in the anaesthetized rat. Exp Physiol 81, 935–942. [DOI] [PubMed] [Google Scholar]
- Garrett JR, Thulin A (1975) Changes in parotid acinar cells accompanying salivary secretion in rats on sympathetic or parasympathetic nerve stimulation. Cell Tissue Res 159, 179–193. [DOI] [PubMed] [Google Scholar]
- Gocht A (1992) Use of LR white resin for post‐embedding immunolabelling of brain tissue. Acta Anat (Basel) 145, 327–339. [DOI] [PubMed] [Google Scholar]
- Gómez‐Moreno G, Guardia J, Ferrera MJ, et al. (2010) Melatonin in diseases of the oral cavity. Oral Dis 16, 242–247. [DOI] [PubMed] [Google Scholar]
- Hardeland R (2017) Melatonin and the electron transport chain. Cell Mol Life Sci 74, 3883–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevia D, González‐Menéndez P, Quiros‐González I, et al. (2015) Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J Pineal Res 58, 234–250. [DOI] [PubMed] [Google Scholar]
- Huang AY, Castle AM, Hinton BT, et al. (2001) Resting (basal) secretion of proteins is provided by the minor regulated and constitutive‐like pathways and not granule exocytosis in parotid acinar cells. J Biol Chem 276, 22296–22306. [DOI] [PubMed] [Google Scholar]
- Huo X, Wang C, Yu Z, et al. (2017) Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: an implication of the therapeutic potential. J Pineal Res 62, e12390. 10.1111/jpi.12390. [DOI] [PubMed] [Google Scholar]
- Isola M, Lilliu MA (2016) Melatonin localization in human salivary glands. J Oral Pathol Med 45, 510–515. [DOI] [PubMed] [Google Scholar]
- Isola M, Ekström J, Diana M, et al. (2013) Subcellular distribution of melatonin receptors in human parotid glands. J Anat 223, 519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isola M, Ekström J, Lilliu MA, et al. (2016) Dynamics of the melatonin MT1 receptor in the rat parotid gland upon melatonin administration. J Physiol Pharmacol 67, 111–119. [PubMed] [Google Scholar]
- Isola M, Lilliu MA, Loy F, et al. (2017) Diabetic status influences the storage of melatonin in human salivary glands. Anat Rec (Hoboken) 301, 711–716. [DOI] [PubMed] [Google Scholar]
- Jaworek J, Konturek SJ, Tomaszewska R, et al. (2004) The circadian rhythm of melatonin modulates the severity of caerulein‐induced pancreatitis in the rat. J Pineal Res 37, 161–170. [DOI] [PubMed] [Google Scholar]
- Kennaway DJ, Voultsios A (1998) Circadian rhythm of free melatonin in human plasma. J Clin Endocrinol Metab 83, 1013–1015. [DOI] [PubMed] [Google Scholar]
- Loy F, Diana M, Isola R, et al. (2012) Morphological evidence that pentagastrin regulates secretion in the human parotid gland. J Anat 220, 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy F, Isola M, Isola R, et al. (2015) Ultrastructural evidence of a secretory role for melatonin in the human parotid gland. J Physiol Pharmacol 66, 847–853. [PubMed] [Google Scholar]
- Mason BL, Pariante CM, Jamel S, et al. (2010) Central nervous system (CNS) delivery of glucocorticoids is fine‐tuned by saturable transporters at the blood‐CNS barriers and nonbarrier regions. Endocrinology 151, 5294–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashida T, Yoshie S, Imai A, et al. (2009) Transferrin secretory pathways in rat parotid acinar cells. Arch Biochem Biophys 487, 131–138. [DOI] [PubMed] [Google Scholar]
- Obana‐Koshino A, Ono H, Miura J, et al. (2015) Melatonin inhibits embryonic salivary gland branching morphogenesis by regulating both epithelial cell adhesion and morphology. PLoS ONE 10, e0119960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permuy M, López‐Peña M, González‐Cantalapiedra A, et al. (2017) Melatonin. A review of its potential functions and effects on dental diseases. Int J Mol Sci 19, 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter B, Van Waarde MA, Vissink A, et al. (1995) Degranulation of rat salivary glands following treatment with receptor‐selective agonists. Clin Exp Pharmacol Physiol 22, 330–336. [DOI] [PubMed] [Google Scholar]
- Radio NM, Doctor JS, Witt‐Enderby PA (2006) Melatonin enhances alkaline phosphatase activity in differentiating human adult mesenchymal stem cells grown in osteogenic medium via MT2 melatonin receptors and the MEK/ERK (1/2) signaling cascade. J Pineal Res 40, 332–342. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Rosales‐Corral SA, Liu XY, et al. (2015) Melatonin in the oral cavity: physiological and pathological implications. J Periodontal Res 50, 9–17. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Rosales‐Corral S, Tan DX, et al. (2017) Melatonin as a mitochondria‐targeted antioxidant: one of evolution's best ideas. Cell Mol Life Sci 74, 3863–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimozuma M, Tokuyama R, Tatehara S, et al. (2011) Expression and cellular localization of melatonin‐synthesizing enzymes in rat and human salivary glands. Histochem Cell Biol 135, 389–396. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Abe T (2008) Thyroid hormone transporters in the brain. Cerebellum 7, 75–83. [DOI] [PubMed] [Google Scholar]
- Testa Riva F, Puxeddu R, Loy F, et al. (2006) Cytomorphological study on human submandibular gland following treatment with secretagogue drugs. Cell Tissue Res 324, 347–352. [DOI] [PubMed] [Google Scholar]
- Venegas C, García JA, Escames G, et al. (2012) Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res 52, 217–227. [DOI] [PubMed] [Google Scholar]
- Xu S, Ma L, Evans E, et al. (2013) Polymeric immunoglobulin receptor traffics through two distinct apically targeted pathways in primary lacrimal gland acinar cells. J Cell Sci 126, 2704–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Dickson EJ, Jung SR, et al. (2016) High membrane permeability for melatonin. J Gen Physiol 147, 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]


