Abstract
Appropriate interventions might improve the prevention of essential hypertension. This requires a comprehensive view of modifiable lifestyle factors (MLFs) distribution and effect. To determine how six MLFs (general adiposity, abdominal adiposity, alcohol consumption, smoking, diet, physical inactivity) for risk of hypertension are distributed and how their combinations affect the risk, a prospective study cohort of 11,923 healthy participants from the population-based European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam Study was used. Of these, 1,635 developed hypertension during a mean follow-up of 10.3 years. Mutually exclusive combinations, clustering and interactions of MLFs were then investigated stratifying by sex, Hazard Ratios (HRs) and Population Attributable Risks (PARs%) were calculated. General adiposity alone was sufficient to increase the risk of hypertension (HR = 1.86, PAR% 3.36), and in this cohort it played a major role in enhancing the risk of hypertension, together with smoking and physical inactivity. MLFs had a different impact and a different modulation of risk in women and men, and they showed a remarkable tendency to occur in specific patterns with higher prevalence than expected. This indication can help to promote a holistic approach through multifactorial preventive strategies addressing more than a factor at a time. For prevention of hypertension addressing adiposity together with smoking, promoting at the same time physical activity should be the first choice.
Introduction
Despite massive improvements achieved in primary and secondary prevention, incidence and prevalence of essential hypertension (hereafter referred to as hypertension) are still rising worldwide, and further increases are foreseen if no effective counteractions are undertaken1,2. Population-based and/or individual interventions could be effective counteractions as they have shown to reduce blood pressure values and hypertension incidence3,4 and subsequently the risk of major cardiovascular diseases5–7. The implementation of interventions requires a good understanding of modifiable lifestyle factors (MLFs) within the targeted population8,9. This should also include insights on how the MLFs interact with each other and which MLFs profile could be a target of interventions with the highest potential impact.
So far the largest body of literature analysed MLFs separately, and information about how they are combined in the population and how such combinations impact risk are scarce, as well as information about clusters of association and mutual interactions. We identified six MLFs with sufficient evidence of a causal co-responsibility regarding the occurrence of hypertension: general and abdominal obesity10–12, excessive use of alcohol13,14, smoking15,16, lack of physical activity17 and low adherence to the DASH (Dietary Approaches to Stop Hypertension) diet18,19. Therefore, this study aimed to provide information about the prevalence and risk association of the MLFs combinations observed in a large adult German cohort, with insights about clustering and interactions of MLFs.
Methods
Study Design and population
The present study was performed in the prospective European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam cohort. The ethical committee of the state of Brandenburg, Germany, approved the methods of the EPIC Potsdam Study, following the principles of the Helsinki Declaration for human rights. Informed consent was obtained from all the participants before their recruitment20. Data collection at baseline examination included self-administered questionnaires, interviews and physical examinations21. Diet was assessed using a food frequency questionnaire (FFQ). Details about the modality, validity and reproducibility of the FFQ have been previously published22. Data on anthropometric variables and blood pressure were obtained during physical examinations21,23,24. All interviews and examinations were conducted by trained interviewers who were regularly supervised24.
Exclusion and inclusion criteria
The entire study population included 27,548 participants aged 40–65 (men) and 35–65 (women). Participants with prevalent primary or secondary hypertension (n = 11,057), with no follow-up time (n = 205), or with missing values in any of the variables used (n = 436) were excluded (Fig. 1). Participants with prevalent chronic diseases (T2DM and cancer), previous cardiovascular or cerebrovascular disorders (myocardial infarction and stroke) (n = 3,689), or morbid obesity (BMI > 40 kg m−2) (n = 27) were also excluded, to avoid bias due to comorbidities. After exclusion, 11,923 participants (8,107 women and 3,816 men) were eligible for analysis. Of these, 1,635 (1,046 women and 589 men) developed hypertension during a mean (SD) follow-up time of 10.3(2.6) years.
Figure 1.
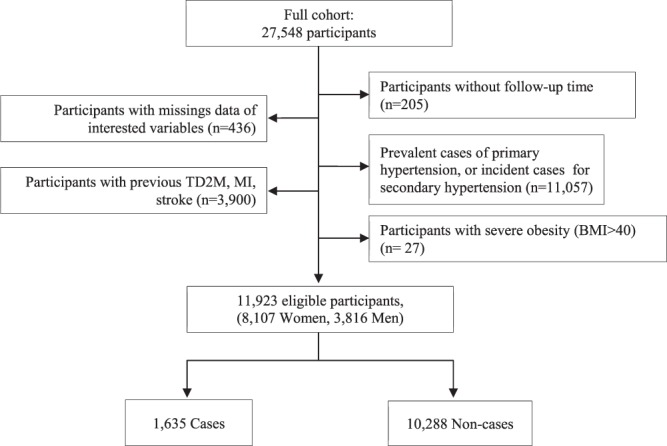
Flowchart of exclusions TD2M = Diabetes mellitus type 2; MI = myocardial infarction.
Definition and ascertainment of prevalent and incident hypertension
Prevalent cases are defined as participants with (i) systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg as determined by the mean of the second and third measurements, (ii) self-reported prevalent hypertension diagnosis, or (iii) use of antihypertensive medication. Self-administered questionnaires were used every 2 to 3 years to collect information about potential cases of incident hypertension and hypertension-specific medicines during the previous months. Incident cases were included in the analysis only after verification and the confirmation of the diagnosis by the treating physician (International Classification of Diseases Tenth Revision: I10)25.
Definition and categorisation of MLFs
Six acknowledged MLFs were selected: general adiposity, abdominal adiposity, physical activity, adherence to “Dietary Approach to Stop Hypertension” (DASH) diet, smoking, and alcohol consumption. Body Mass Index (BMI, kg/m²) and waist circumference accounted for general and abdominal adiposity, respectively. BMI was categorised as underweight/normal (<25), overweight (25–30) and obese (>30). Waist circumference was measured in cm and sex-specifically categorised as no risk (women < 80; men < 94), medium risk (women 80–88; men 94–102), and high risk (women > 88; men > 102). Smoking behaviour was categorised as never smoking, former and current smoking. The Improved Physical Activity Index (IPAI) adjusted for sex and age was used to account for physical activity; it was calculated as described in previous papers26 and divided into inactive, moderately active, and active/very active categories. Dietary intake data assessed by FFQ22 were used to calculate a DASH Diet Score27, that was modified due to lack of information about sodium intake. The modified DASH Score (mDASH) was divided into tertiles (low, medium and high) indicating adherence to the DASH diet. Alcohol intake from beverages was categorized as follows: no/light consumption (women 0–6 g/day; men 0–12 g/day), moderate (women > 6–12 g/day; men ≥ 12–24 g/day), high (women > 12–30 g/day; men > 24–60 g/day), and very high consumption (women > 30 g/day; men > 60 g/day). Based on previous knowledge, the following variables were used as adjusting variables in the risk models: educational attainment, age, and use of anti-inflammatory drugs. Educational attainment was categorised into high, medium, and low.
Statistical Analysis
Baseline characteristics were presented as means and standard deviations for continuous variables and as frequencies for categorical variables. First, the selected MLFs were analysed in the initial categories for their association with incidence of hypertension using the category with the assumed lowest risk as a reference. Hazard ratios (HRs) and 95% confidence intervals (CIs) were computed using Cox proportional hazards regression stratified by age. Entry time was defined as the participant’s age at recruitment (years) and exit time as age at either diagnosis of hypertension, death or return of the last follow-up questionnaire. In the subsequent analyses, to calculate the population attributable risk (PAR%), MLFs were dichotomised as risk/no risk, according to the findings from the initial analysis. The no risk category was used as a reference when calculating the HR. PAR% was calculated assuming a causal and independent relationship between the MLF and incident hypertension28. The following equation was used:
where Pe is the exposed proportion29. Further, mutually exclusive combinations of MLFs were investigated, using the binary system of presence (=1)/absence (=0) of the dichotomised MLFs. The prevalence of each combination was assessed, and the HRs calculated. The PAR% of each combination was also determined.
Finally, the ratio between observed and expected (O/E) prevalence of each combination was calculated according to a method previously published30,31. The expected prevalence for each combination assuming the factors aggregate by chance was computed by multiplying the individual probabilities of each factor based on their observed prevalence, as shown in the example below:
P1 to P5 represent the prevalence of the single factor observed in the whole study sample. 1 − P2 or 1 − P5 represents the complementary probability for the subject having the correspondent risk factor. We considered indicative of clustering an O/E prevalence ratio >1.
In addition, the risk performance of a combination was evaluated by comparing the observed HR with the expected HR assuming no interaction between factors. The expected HR was calculated by multiplying the HRs of each MLF:
Interaction of MLFs occurs when their observed HR differs from the one expected to result from their individual effect.
We considered indicative of synergistic interaction an O/E HR ratio >1.
All analyses were performed with SAS software, version 9.4, and SAS Enterprise Guide, version 6.1 (SAS Institute Inc., Cary, NC, USA).
Results
The baseline characteristics of the cohort are shown in Table 1. Participants who developed hypertension were older and more likely to be men and physically inactive, they had a higher BMI, waist circumference and slightly higher blood pressure at baseline. Men who developed hypertension but not women were more likely to be current or former smokers and to consume more than 24 g/day of alcohol. No appreciable difference was noticed in the DASH score between cases and non-cases in both sexes (Table 1).
Table 1.
Baseline General Characteristics of Participants.
| Women (n = 8107) | MEN (n = 3816) | |||
|---|---|---|---|---|
| Cases of incident hypertension (n = 1046) | Non-cases (n = 7061) | Cases of incident hypertension (n = 589) | Non-cases (n = 3227) | |
| Age, y (SD) | 50.1(9.1) | 45.6(8.6) | 52.2 (7.48) | 49.5 (7.6) |
| Follow-up time, y (SD) | 6.4 (2.9) | 10.8 (1.8) | 6.5(2.9) | 10.7(2) |
| BMI, Kg/m2 (SD) | 26.1 (3.9) | 24 (3.5) | 26.8 (2.9) | 25.4 (2.9) |
| Waist, cm (SD) | 81.4 (10.5) | 76.2 (9) | 94.4 (8.5) | 90.1 (8.6) |
| Systolic BP, mmHg (SD) | 123.5 (8.6) | 115.3 (9.8) | 127.5 (7.7) | 122.5 (9) |
| Diastolic BP, mmHg (SD) | 80.6 (5.8) | 76.1(6.8) | 82.6 (5.8) | 79.5 (6.2) |
| mDASH, score | 14.6 (4.4) | 14.6 (4.3) | 12.3 (4.1) | 12.3(4.3) |
| Smoking: | ||||
| Never (%) | 608 (7.50) | 3,861 (47.63) | 171 (4.48) | 1,147 (30.06) |
| Former (%) | 230 (2.84) | 1,781 (21.97) | 243 (6.37) | 1,196 (31.34) |
| Current (%) | 208 (2.57) | 1,419 (17.50) | 175 (4.59) | 884 (23.17) |
| Alcohol consumption: | ||||
| No/ light consumers (%) | 169 (2.08) | 1,056 (13.03) | 44 (1.15) | 303 (7.94) |
| Moderate consumers (%) | 676 (8.34) | 4,463 (55.05) | 329 (8.62) | 1,806 (47.33) |
| Heavy consumers (%) | 162 (2) | 1,263 (15.58) | 178 (4.66) | 956 (25.05) |
| Very heavy consumers (%) | 39 (0.48) | 279 (3.44) | 38 (1) | 162 (4.25) |
| Physical activity: | ||||
| Inactive (%) | 290 (3.58) | 1,447 (17.85) | 168 (4.40) | 694 (18.19) |
| Moderate (%) | 431 (5.32) | 2,887 (35.61) | 221 (5.79) | 1,300 (34.07) |
| Active/very act. (%) | 325 (4.01) | 2,727 (33.64) | 200 (5.24) | 1,233 (32.31) |
A multivariable-adjusted model was built to test the thresholds of the risk of hypertension in a preliminary analysis (data not shown). Alcohol was the only MLF that was not associated with the incidence of hypertension and was therefore excluded from the subsequent analyses.
According to the finding of the preliminary analysis, the MLFs were dichotomised and the categories characterised by higher risk of hypertension were defined as follows: BMI > 25 Kg/m2, waist circumference >88 cm for women and >102 cm for men, lowest tertile of mDASH score, current and former smoking, and “inactive” category of IPAI index.
In the dichotomised, multivariable-adjusted model (Table 2) the HRs of the category at risk compared to the one at no risk were in accordance with the results of the previous analysis. The HR (95% CI) for incident hypertension was 1.80 (1.56–2.07) in women and 1.78 (1.47–2.16) in men for a BMI > 25. The estimated HR (95% CI) for high-risk waist circumference was 1.42 (1.20–1.67) in women and 1.59 (1.28–1.97) in men. For low adherence to the DASH diet, the risk appeared to be higher (HR 1.47, 95% CI 1.19–1.82) in women than in men (HR 1.02, 95% CI 0.81–1.23) compared to higher adherence. Smoking had a HR (95% CI) of 1.07 (1.01–1.18) compared to non-smoking, and the risk was higher in men (HR 1.20, 95% CI 1.01–1.45) than in women (HR1.04, 95% CI 0.91–1.18). Inactivity showed a HR (95% CI) of 1.15 (1.02–1.31) in women and of 1.16 (1.01.1.36) in men compared to active/very active participants. The estimated PARs, representing the percentage of cases attributable to the presence of risk factors, were in women and men 39.3 and 50.3% for high BMI, 14.9 and 14.6% for high risk waist circumference, 10.5 and 16.6% for low adherence to DASH diet, 31.8 and 44.2% for smoking, 34.2 and 34.6% for low physical activity.
Table 2.
Hazard Ratios (CI 95%) and hypothesized Population Attributable Risks (PAR%) of hypertension in relation to single MLFs in men and women of the EPIC- POTSDAM cohort.
| MLF | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) | HR (95% CI) | aHR (95% CI) | PAR% | No. (%) | HR (95% CI) | aHR (95% CI) | PAR% | |
| BMI | ||||||||
| No risk (<25) | 1,646 (43.13) | 1.0 (reference) | 1.0 (reference) | 5,187 (63.98) | 1.0 (reference) | 1.0 (reference) | ||
| High risk (≥25) | 2,170 (56.87) | 2.01 (1.67–2.41) | 1.78 (1.47–2.16) | 50.37 | 2,920 (36.02) | 2.03 (1.79–2.31) | 1.80 (1.56–2.07) | 39.35 |
| Waist | ||||||||
| Below the limit | 3,405 (89.23) | 1.0 (reference) | 1.0 (reference) | 7,104 (87.63) | 1.0 (reference) | 1.0 (reference) | ||
| Above the limit | 411 (10.77) | 2.06 (1.68–2.54) | 1.59 (1.28–1.97) | 14.62 | 1,003 (12.37) | 2.01 (1.73–2.33) | 1.42 (1.20–1.67) | 14.97 |
| Dash Diet | ||||||||
| Medium/high adherence | 3,055 (80.06) | 1.0 (reference) | 1.0 (reference) | 7,464 (92.07) | 1.0 (reference) | 1.0 (reference) | ||
| Low adherence | 761 (19.94) | 1.01 (0.81–1.24) | 1.02 (0.81–1.23) | 16.65 | 643 (7.93) | 1.42 (1.15–1.76) | 1.47 (1.19–1.82) | 10.46 |
| Smoking | ||||||||
| Never | 1,318 (34.54) | 1.0 (reference) | 1.0 (reference) | 4,469 (55.13) | 1.0 (reference) | 1.0 (reference) | ||
| Former/current | 2,498 (65.46) | 1.29 (1.07–1.54) | 1.20 (1.01–1.45) | 44.17 | 3,638 (44.87) | 1.06 (0.94–1.21) | 1.04 (0.91–1.18) | 31.82 |
| Physical Activity | ||||||||
| Moderate/Active/Very active | 2,070 (54.25) | 1.0 (reference) | 1.0 (reference) | 4,468 (55.11) | 1.0 (reference) | 1.0 (reference) | ||
| Inactive | 1,746 (45.75) | 1.23 (1.05–1.45) | 1.16 (1.01.1.36) | 34.64 | 3,639 (44.89) | 1.22 (1.08–1.39) | 1.15 (1.02–1.31) | 34.17 |
HR: adjusted for educational attainment, age and use of anti-inflammatory drugs;
aHR: HR adjusted for all the other MLFs;
BMI: no risk: Body Mass Index <25 kg/m²; high risk: ≥25 kg/m²;
WAIST: below the limit: waist circumference <88 cm women; <102 cm men; above the limit: ≥ 88women; ≥ 102men
DASH DIET: “Dietary Approach to Stop Hypertension” diet; medium/high adherence: DASH score 9–28; low adherence: DASH score 0–8.
Combination of MLFs: prevalence, strength of association and PAR%
For simplicity of the combinations’ description we will use the following terms for the above-mentioned MLFs: BMI > 25: “BMI”; high-risk waist circumference: “waist”; low adherence to DASH diet: “diet”, former and current smoking: “smoking”; low physical activity: “inactivity”.
The analyses of specific combinations of MLFs revealed that a total of 1,580 women (19%) and 307 men (8%) were not exposed to any of the considered MLFs. (Tables 3–4). This group at no-risk was used as a reference. 2,885 women (35.6%) and 1,011 men (26.5%) were exposed to one single MLF only (Tables 3 and 4). The most common MLFs were smoking (15%women; 12%men), inactivity (12%women; 5%men), and BMI (7%women, 7%men). In this category, solely BMI showed an important association with the incidence of hypertension, with a HR of 1.78 (1.37–2.32) in women and 2.43 (1.43–4.13) in men (Tables 3 and 4).
Table 3.
HR (CI 95%) and PAR% for hypertension in relation to specific combinations of MLFs, Men.
| Combinations of mlfs | No. of cases among exposed (%) | Prevalence (%) | aHR (95% CI) | PAR % |
|---|---|---|---|---|
| No MLF | 20 (6, 5) | 307 (8.05) | ||
| 1 MLF | 111 (11) | 1, 011 (26.5) | ||
| BMI | 45 (15, 5 | 290 (7, 6) | 2.43 (1.43–4.13)* | 4.47 |
| Waist | 0 | 1 (0, 03) | ||
| Diet | 3 (5) | 61 (1, 6) | 0.83 (0.24–2.81) | |
| Smoking | 44 (9, 6) | 458 (12) | 1.62 (0.95–2.75) | |
| Inactivity | 19 (9, 5) | 201 (5, 3) | 1.55 (0.82–2.90) | |
| 2 MLFs | 193 (15) | 1, 288 (33) | ||
| BMI Inactivity | 45 (19, 6) | 230 (6) | 3.09 (1.82–5.25)* | 4.08 |
| BMI Smoking | 82 (16, 6) | 494 (12, 9) | 2.70 (1.66–4.42)* | 8.16 |
| BMI Waist | 8 (18, 6) | 43 (1, 1) | 2.93 (1.28–6.69)* | 0.74 |
| BMI Diet | 3 (7, 1) | 42 (1, 1) | 1.17 (0.35–3.97) | |
| Inactivity Diet | 5 (11, 4) | 44 (1, 1) | 2.03 (0.75–5.44) | |
| Inactivity Smoking | 40 (12, 5) | 320 (8, 4) | 1.95 (1.14–3.35)* | 4.10 |
| Diet Smoking | 10 (8, 7) | 115 (3) | 1.63 (0.76–3.50) | |
| 3 MLFs | 379 (43, 4) | 874 (22, 9) | ||
| BMI Smoking Inactivity | 86 (20, 3) | 424 (11, 1) | 3.13 (1.92–5.10)* | 7.56 |
| BMI Waist Smoking | 41 (37, 3) | 110 (2, 9) | 7.18 (4.19–12.32)* | 2.48 |
| BMI Diet Inactivity | 10 (23, 3) | 43 (1, 1) | 3.95 (1.84–8.48)* | 0.84 |
| BMI Diet Smoking | 20 (18) | 111 (2, 9) | 3.28 (1.76–6.12)* | 2.02 |
| BMI Waist Diet | 1 (20) | 5 (0, 1) | 3.57 (0–47–26.90) | |
| BMI Waist Inactivity | 10 (23, 8) | 42 (1, 1) | 3.85 (1.79–8.27)* | 0.81 |
| Diet Smoking Inactivity | 18 (12, 9) | 139 (3, 6) | 2.15 (1.13–4.08)* | 1.95 |
| 4 MLFs | 68 (22, 4) | 303 (7, 9) | ||
| BMI Diet Smoking Inactivity | 22 (17, 5) | 126 (3, 3) | 2.95 (1.61–5.43)* | 2.18 |
| BMI Waist Smoking Inactivity | 36 (26, 7) | 135 (3, 5) | 4.07 (2.35–7.05)* | 2.66 |
| BMI Waist Diet Inactivity | 2 (22, 2) | 9 (0, 2) | 4.22 (0.97–18.29) | |
| BMI Waist Diet Smoking | 8 (24, 2) | 33 (0, 9) | 3.96 (1.73–9.05)* | 0.64 |
| 5 MLFs | 11 (33, 3) | 33 (0, 9) | ||
| Waist Diet BMI Smoking Inactivity | 11 (33, 3) | 33 (0, 9) | 5.72 (2.71–12.08)* | 0.71 |
| Total | 589 (15, 4) | 3, 816 (100) |
aHR: HR adjusted for all the other combination of MLFs;
*p < 0,05.
Table 4.
HR (CI 95%) and PAR% for hypertension in relation to specific combinations of MLFs, Women.
| Combinations of mlfs | No. of cases among exposed (%) | Prevalence (%) | HRa (95% CI) | hPAR % |
|---|---|---|---|---|
| No MLF | 134 (8, 48) | 1, 580 (19, 5) | ||
| 1 MLF | 292 (10, 1) | 2, 885 (35, 6) | ||
| BMI | 96 (16, 6) | 579 (7, 1) | 1.78 (1.37–2.32)* | 3.14 |
| Waist | 2 (22, 2) | 9 (0, 1) | 2.44 (0.60–9.93) | |
| Diet | 10 (10, 8) | 93 (1, 1) | 1.45 (0.76–2.77) | |
| Smoke | 84 (6, 9) | 1221 (15, 1) | 0.95 (0.72–1.25) | |
| Inactivity | 100 (10, 2) | 983 (12, 1) | 1.08 (0.83–1.40) | |
| 2 MLFs | 616 (26, 6) | 2, 315 (28, 6) | ||
| BMI Inactivity | 106 (20) | 530 (6, 5) | 2.04 (1.57–2.64)* | 3.34 |
| BMI Smoke | 49 (12, 6) | 388 (4, 8) | 1.53 (1.10–2.13)* | 1.66 |
| BMI Waist | 49 (22, 9) | 214 (2, 6) | 2.41 (1.72–3.36)* | 1.54 |
| BMI Diet | 7 (25) | 28 (0, 3) | 2.52 (1.15–5.47)* | 0.21 |
| Waist Diet | 0 | /1 (0, 01) | ||
| Waist Inactivity | 2 (33, 3) | 6 (0, 1) | 4.29 (1.05–17.52)* | 0.06 |
| Waist Smoke | 0 | 4 (0, 05) | ||
| Inactivity Diet | 15 (12, 5) | 120 (/1, 5) | 1.51 (0.88–2.58) | |
| Inactivity Smoke | 87 (9, 5) | 916 (11, 3) | 1.19 (0.91–1.57) | |
| Diet Smoke | 9 (8, 3) | 108 (1, 3) | 1.20 (0.61–2.36) | |
| 3 MLFs | 213 (21, 3) | 998 (12, 3) | ||
| BMI Smoke Inactivity | 59 (18, 4) | 321 (4) | 2.17 (1.59–2.95)* | 2.14 |
| BMI Waist Smoke | 45 (22, 7) | 198 (2, 4) | 3.09 (2.19–4.33)* | 1.65 |
| BMI Diet Inactivity | 10 (25) | 40 (0, 5) | 2.97 (1.55–5.66)* | 0.33 |
| BMI Diet Smoke | 7 (31, 8) | 22 (0, 3) | 6.40 (2.97–13.79)* | 0.23 |
| BMI Waist Diet | 1 (8, 3) | 12 (0, 1) | 0.94 (0.13–6.76) | |
| BMI Waist Inactivity | 73 (28, 1) | 260 (3, 2) | 2.79 (2.08–3.74)* | 2.06 |
| Diet Smoke Inactivity | 17 (12, 2) | 139 (1, 7) | 1.91 (1.14–3.18)* | 0.82 |
| Waist Smoke Inactivity | 1 (20) | 5 (0, 1) | 2.21 (0.30–15.93) | |
| Waist Smoke Diet | 0 | 1 (0, 01) | ||
| 4 MLFs | 93 (29, 9) | 311 (3, 8) | ||
| BMI Diet Smoke Inactivity | 6 (16, 7) | 36 (0, 4 | 2.47 (1.08–5.61)* | 0.26 |
| BMI Waist Smoke Inactivity | 61 (24, 4) | 250 (3, 1) | 2.72 (2.00–3.70)* | 1.95 |
| BMI Waist Diet Inactivity | 3 (21, 4) | 14 (0, 2) | 2.15 (0.68–6.78) | |
| BMI Waist Diet Smoke | 3 (30) | 10 (0, 1) | 4.30 (1.36–13.64)* | 0.09 |
| Waist Diet Smoke Inactivity | 0 | 1 (0, 01) | ||
| 5 MLFs | 10 (55, 6) | 18 (/0, 2) | ||
| Waist Diet BMI Smoke Inactivity | 10 (55, 6) | 18 (0, 2) | 9.34 (4.85–17.99)* | 0.20 |
| Total | 1, 046 | 8, 601 |
aHR: HR adjusted for all the other combination of MLFs;
*p < 0,05.
Participants exposed to two MLFs were 2,315 women (28.6%) and 1,288 men (33%). The highest prevalence was observed for “smoking–inactivity” (11% women; 8% men), “BMI-smoking” (5% women; 13% men) and “BMI-inactivity”(6% women,men), with a HR (95% CI) of 1.19 (0.91–1.57), 1.53 (1.10–2.13), 2.04 (1.57–2.64) for women and 1.95 (1.14–3.35), 2.70 (1.66–4.42), 3.09 (1.82–5.25) for men, respectively. The highest impact was showed by “BMI-Inactivity” for women (PAR% 3.4), and by “BMI-Smoking” for men (PAR% 8.16).
Participants exposed to three MLFs were 998 women (12%) and 874 men (23%). The highest prevalence was observed for “BMI-smoking-inactivity” (4% women, 11% men), “waist-BMI-smoking” (2% women, 3% men), “waist-BMI-inactivity” (3% women,1% men) and “diet-smoking-inactivity”(1,7% women, 4% men), with a HR (95% CI) respectively of 2.17 (1.59–2.95), 3.09 (2.19–4.33), 2.79 (2.08–3.74), 1.91 (1.14–3.18) for women and of 3.13 (1.92–5.10), 7.18 (4.19–12.32), 3.85 (1.79–8.27), 2.15 (1.13–4.08) for men. The combination that showed the highest impact was “BMI-smoking-inactivity” (PAR% women 2.1; men 7.6). The combination of 3 MLFs at higher risk in women was “BMI-diet-smoking” with a HR (95% CI) of 6.40 (2.97–13.79).
Participants exposed to four MLFs were 311 women (4%) and 303 men (7.9%), with the highest prevalence showed by the combination of “BMI-waist-smoking-inactivity” (3.1% women, 3.5% men). This was also the combination at highest risk for men, with HR (95% CI) of 4.07 (2.35–7.05). For women, the combination at highest risk was “BMI-waist-diet-smoking” with a HR (95% CI) of 4.30 (1.36–13.64). The highest PAR% was shown by “BMI-waist-smoking-inactivity” (PAR% 1.9 women; 2.6 men). The simultaneous presence of all MLFs was detected only in 18 women (0.2%) and 33 men (0.9%), with a HR (95% CI) of 9.34 (4.85–17.99) for women and of 5.72 (2.71–12.08) for men.
Clustering and interactions
Clustering was defined for a combination when its observed prevalence was different from the one expected assuming the component MLFs aggregate by chance. Hereby “cluster” is intended as a combination of MLFs that shows clustering. A total of 14 clusters (C) of MLFs (Table 5) for both women and men were identified, of which only 8 showed a notable association with the incidence of hypertension, consistent between sexes.
Table 5.
Clustering and interactions of combined MLFs.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Prevalence | HR | Prevalence | HR | |||||
| Expected | O/E | Expected | O/E | Expected | O/E | Expected | O/E | |
| No MLF | 5.77 | 1.39 | ref. | 15.68 | 1.24 | ref. | ||
| 1 MLF | ||||||||
| BMI | 7.61 | 1 | — | — | 8.82 | 0.81 | — | — |
| Diet | 1.44 | 1.11 | — | — | 1.35 | 0.85 | — | — |
| Smoking | 10.94 | 1.1 | — | — | 12.76 | 1.18 | — | — |
| Inactivity | 4.87 | 1.08 | — | — | 12.77 | 0.95 | — | — |
| 2 MLFs | ||||||||
| BMI Inactivity | 6.42 | 0.94 | 3.77 | 1.5 | 7.19 | 0.91 | 1.92 | 0.99 |
| BMI Smoking | 14.43 | 0.9 | 3.94 | 1.26 | 7.18 | 0.67 | 1.69 | 0.82 |
| BMI Waist | 0.92 | 1.23 | 2.43 | 1.04 | 1.25 | 2.12 | 4.34 | 0.94 |
| BMI Diet | 1.90 | 0.58 | 2.02 | 0.64 | 0.76 | 0.46 | 2.58 | 0.1 |
| Inactivity Diet | 1.21 | 0.95 | 1.29 | 1.72 | 1.10 | 1.35 | 1.56 | 0.89 |
| Inactivity Smoking | 9.23 | 0.91 | 2.51 | 1.4 | 10.40 | 1.09 | 1.02 | 0.99 |
| Diet Smoking | 2.73 | 1.1 | 1.34 | 1.33 | 1.10 | 1.21 | 1.37 | 0.78 |
| 3 MLFs | ||||||||
| BMI Smoking Inactivity | 12.17 | 0.91 | 6.10 | 1.26 | 5.85 | 0.68 | 1.82 | 1.01 |
| BMI Waist Smoking | 1.74 | 1.65 | 3.94 | 2.11 | 1.01 | 2.41 | 4.12 | 1.16 |
| BMI Diet Inactivity | 1.60 | 0.71 | 3.13 | 1.88 | 0.61 | 0.79 | 2.78 | 0.98 |
| BMI Diet Smoking | 3.59 | 0.81 | 3.27 | 1.51 | 0.62 | 0.44 | 2.45 | 2.33 |
| BMI Waist Diet | 0.23 | 0.57 | 2.02 | 1.24 | 0.11 | 1.4 | 6.29 | 0.25 |
| BMI Waist Inactivity | 0.77 | 1.42 | 3.77 | 1.17 | 1.02 | 3.16 | 4.69 | 0.95 |
| Diet Smoking Inactivity | 2.30 | 1.58 | 2.08 | 1.51 | 0.16 | 1.1 | 1.48 | 1.1 |
| 4 MLFs | ||||||||
| BMI Diet Smoking Inactivity | 3.03 | 1.09 | 5.06 | 1.17 | 0.50 | 0.87 | 2.64 | 0.78 |
| BMI Waist Smoking Inactivity | 1.47 | 2.41 | 6.10 | 1.03 | 0.83 | 3.73 | 4.45 | 0.89 |
| BMI Waist Diet Inactivity | 0.19 | 1.24 | 3.13 | 1.26 | 0.09 | 1.94 | 6.80 | 0.5 |
| BMI Waist Diet Smoking | 0.43 | 1.98 | 3.27 | 1.14 | 0.08 | 1.37 | 5.98 | 1.1 |
| 5 MLFs | ||||||||
| Waist Diet BMI Smoking Inactivity | 0.37 | 2.35 | 5.06 | 1, 42 | 0.07 | 3.09 | 6.46 | 2.08 |
O/E = Observed/Expected ratio.
The most numerous clusters for women were “C-no risk” (19.5% of the female participants), “C-Smoking” (15%) and “C-Inactivity” (12%). For men, the most numerous clusters were “C-BMI-Smoking” (12.9% of the male participants), “C-Smoking” (12%) and “C-BMI-Smoking-Inactivity” (11%). In women, the clusters with the highest O/E prevalence ratios were “C-Waist-BMI-Smoking-Inactivity” (O/E prevalence = 3.73), “C-Waist-BMI-Diet-Smoking-Inactivity” (O/E prevalence = 3.09), and “C-Waist-BMI-Inactivity” (O/E prevalence = 3.16). In men the O/E prevalence ratios were generally lower, and the clusters with the highest ones were “C-Waist-BMI-Smoking-Inactivity” (O/E prevalence = 2.41), “C-Waist-BMI-Diet-Smoking-Inactivity” (O/E prevalence = 2.35) and “C-Waist-BMI-Diet-Smoking” (O/E prevalence = 1.98).
Among women, the most robust interaction was observed for “BMI-diet-smoking” (O/E HR: 2.33), followed by“C-Waist-BMI-Smoking” (O/E HR = 1.16). Interactions were slightly higher in men, and the highest O/E HR ratios were observed for “C-Waist-BMI-Smoking” (O/E HR = 2.11), “C-Waist-BMI-Diet-Smoking-Inactivity” “BMI-Diet-Inactivity”* (O/E HR = 1.88) and “Inactivity-Diet”* (O/E HR = 1.72) (Table 5). Notably, in women synergistic interactions were less frequent, and only 4 combinations of MFLs out of 25 showed an overlapping of clustering and interactions: “C-Waist-BMI-Smoking”, “C-Diet-Smoking-Inactivity”, “C-Waist-Diet-BMI-Smoking” and “C-Waist-BMI-Diet-Smoking-Inactivity”. In men, all the combinations of MLFs except for “BMI and diet”1* showed a O/E HR ratio >1, and an overlapping of clustering and interactions was observed in 10 of the 25 combinations (Table 5).
Discussion
In the prospective EPIC-Potsdam cohort study, combinations of five out of six well recognised high-risk MLFs for hypertension such as high general adiposity, abdominal adiposity, smoking, low adherence to DASH diet and physical inactivity, were associated with an increased risk of incident hypertension during follow-up.
General and abdominal adiposity, smoking and physical inactivity appear to play a major role in increasing the risk of incidence of hypertension. However, only general adiposity appeared to be a sufficient cause to enhance the risk of hypertension per se. Moreover, these MLFs showed a high tendency to cluster in specific patterns, as in some case they showed an observed prevalence over 3 times higher than how expected by chance.
When single MLFs were investigated one by one in the whole cohort, general adiposity showed the most notable association with incidence of hypertension. Similar risk estimates had been found in a recent meta-analysis for this MLF32, which further confirms that general adiposity plays a central role for risk, regardless of differences in genetical, sociocultural and environmental backgrounds. Abdominal obesity was found to increase the risk of developing hypertension in both sexes, consistently with findings from other cohort studies11,33.
Adherence to DASH diet was not uniformly distributed between sexes, with a remarkably higher percentage of men in a high-risk category compared to women. In the first analysis, low adherence to DASH diet increased the risk of hypertension only among women. This was somehow unexpected, as to date the benefits of a DASH diet regime are well recognised for both primary and secondary prevention19,34 in women and men34–37. However, a few studies have found no or weak evidence that greater concordance with DASH diet had a long-term impact on BP change or hypertension over time38,39. In our study, the lack of association might be the result of the low number of men in the reference group that increases the probability of a false negative relation.
In this cohort, smoking appeared to be associated with higher risk of hypertension in men but not in women. Smoking has been largely recognised as an important risk factor of hypertension. It is associated with phenomena involved in hypertension’s pathogenesis, such as endothelial dysfunction40, atherosclerotic plaque formation41 or arterial stiffness42 and it showed to have a role in the development of hypertension in epidemiological studies15,16,43,44. However, our findings are not isolated. In a large study involving 33,860 people, it was shown that any independent chronic effect of smoking on BP is small, with a significantly higher value of SBP only in men smokers45. In a study involving 28,236 women, Bowman et al. have found that risk for hypertension increases by consuming at least 15 cigarettes per day, whereas non-significant association have been observed for less amount46. In the present study, we did not consider the numbers of cigarettes consumed per day, and this might have underestimated the effect of heavy smoking on the incidence of hypertension.
Physical inactivity showed a similar increase in risk for hypertension in both sexes and comparable values in terms of PAR%, suggesting that this MLF is associated with hypertension regardless of behavioural and biological attributes to sex. This is consistent with the major findings so far47.
An unexpected finding was that alcohol consumption at baseline seemed not to increase the risk of hypertension despite evidence from other studies stating the opposite13,14. We repeated the analyses using a “lifetime alcohol” variable48, and obtained the same result as with baseline alcohol intake (data not shown). After performing sensitivity analyses, the results did not change (data not shown). We argue with a couple of possible explanations. First, this might be possibly the result of low statistical power due to a too small number of people in the reference group of no/light consumers, especially in men. Second, it might be related to the genetic backgrounds of the participants involved in the studies. The dose-response curve for alcohol is not homogenous among studies49,50, and there is evidence that the effect of alcohol is strictly related to the enzymatic pattern of individuals51–53. It is known that the genetic background of individuals plays a role in shaping their susceptibility to lifestyle factors’ effect on hypertension development54,55. Specifically, it has been found that apolipoprotein E polymorphism influences the effect of alcohol on hypertension, conferring more or less proneness to blood pressure increase depending on the allele carried56,57.
The analysis of combined MLFs examined separately subgroups of participants characterised by a defined set of MLFs. This allowed us to disentangle their effective contribution to risk modulation and helped to provide with detailed insights regarding how the MLFs are distributed.
The analysis of the subgroups with only one MLF showed that general adiposity is the only risk factor which in our cohort constitutes by itself a sufficient cause of hypertension. All other MLFs did not show an association with the incidence of hypertension if they occurred individually. Also, this analysis revealed that both smoking and physical inactivity were more frequent in women compared to men when they occur as isolated MLF. This is in contradiction with the finding of the first analysis, where the overall prevalence of physical inactivity was almost equal between sexes and the prevalence of smoking was higher in men compared to women (Table 2). We could notice only two combinations without BMI that increased the risk of hypertension: “smoking and inactivity” and “smoking, inactivity and diet”, for both sexes. These combinations without BMI had a notable prevalence and were important in explaining the risk.
Among men, PARs% were generally higher compared to women. For example, “BMI-smoking” has a PAR% of 8.2 in men, whereas 1.7 in women, and when those two factors go along with physical inactivity PAR% became 7.6 for men and 2.1 for women. This could be the consequence of an overall healthier behaviour for women so that the range of improvement is wider for men. The percentage of smoking among women with one or more risk factors is remarkably lower than the one observed in men. From this evidence, it might be speculated that in men this risk factor is more likely to be combined with overweight and inactivity, while in women smoking is more likely to occur while having an otherwise healthy lifestyle.
General adiposity combined with low adherence to DASH diet was less prevalent than expected in both sexes, and this might indicate that in the present cohort, overweight and obese people tend to have more attention for their diet. Moreover, this combination was associated with a higher risk of hypertension in women but not in men. This might be again the consequence of low statistical power due to the small number of men in the reference group. General and abdominal adiposity showed to be aligned as it could have been expected and show a tendency to aggregate and being combined with smoking and inactivity, consistently across sexes. Regarding synergistic interactions, higher risk than expected varied by sex but the combination of general adiposity, smoking and diet showed higher risk than expected in both sexes.
This study has a number of strengths. It is based on a cohort of the EPIC study, characterised by a large number of participants, with the availability of data on multiple risk factors for hypertension. Data collection and measurements were highly standardised and carried with a rigorous methodology. To the best of our knowledge, this is the first study that takes into account details about the specific combinations of MLFs on hypertension risk, allowing to study the interrelation and the relative contribution of each MLF to the risk of hypertension. Moreover, the analysis of clustering of hypertension risk factors is of particular importance because it provides insights into how MLFs tend to aggregate.
Nevertheless, some limitations of this study also need to be examined. It should be considered that hypertension has been recognised as a polygenic disease with complexities such as ‘gene-gene’ and ‘environment-gene’ interactions. The MLFs may modulate in different ways the effect of genes on BP54,55, and we could not quantify the contribution of this interaction. Despite it is well recognised the role of salt in the onset of hypertension58,59, we could not consider it because the information on salt consumption was lacking. Thus we had to build a modified DASH index without taking it into account, and this might have underestimated the impact of diet on the risk of hypertension.
Further, the crude dichotomous categorisation made for calculating the sets of combinations and the PARs might underestimate the actual effect of the various risk factors. However, it has been demonstrated elsewhere that dichotomised lifestyle factors have been useful for studying the association of lifestyle factors and risk of diseases in large populations60.
The results of this study support the need to investigate MLFs by detailing the specific combinations of co-occurrence, as we found evidence that the traditional analysis of single MLFs by mutually adjusted models might be misleading due to a loss of crucial information. In the study MLFs showed a remarkable tendency to occur in specific patterns, strengthening the rational to promote a more comprehensive approach through multifactorial interventions which target more than a factor at a time. For prevention of hypertension addressing adiposity together with smoking and promoting physical activity should be the first choice.
Acknowledgements
We thank the Human Study Centre (HSC) of the German Institute of Human Nutrition Potsdam-Rehbrücke, namely the trustee and the examination unit for the collection, the data hub for the processing, and the participants for the provision of the data, and the head of the HSC, Manuela Bergmann, for the contribution to the study design and leading the underlying processes of data generation. We thank the Leibniz Association’s Open Access Publishing Fund for financing this publication.
Author Contributions
V.A. wrote the main manuscript with input and support from H.B. V.A. performed all the statistical analyses with help of S.D., S.K. and W.B. All the authors revised the final version of the manuscript.
Data Availability
The datasets analysed during the current study are not publicly available due to data protection regulations. In accordance with German Federal and State data protection regulations, epidemiological data analyses of EPIC-Potsdam may be initiated upon an informal inquiry addressed to the secretariate of the Human Study Center (Office.HSZ@dife.de). Each request will then have to pass a formal process of application and review by the respective PI and a scientific board.
Competing Interests
The authors declare no competing interests.
Footnotes
*These combinations are not cluster, as their O/E prevalence ratio is <1.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kearney PM, et al. Global burden of hypertension: analysis of worldwide data. Lancet (London, England) 2005;365:217–223. doi: 10.1016/s0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Nelson, S., Whitsel, L., Khavjou, O., Phelps, D. & Leib, A. Projections of Cardiovascular Disease Prevalence and Costs (2016).
- 3.Puska P, Vartiainen E, Tuomilehto J, Salomaa V, Nissinen A. Changes in premature deaths in Finland: successful long-term prevention of cardiovascular diseases. Bulletin of the World Health Organization. 1998;76:419–425. [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell NR, et al. Lifestyle modifications to prevent and control hypertension. 1. Methods and an overview of the Canadian recommendations. Canadian Hypertension Society, Canadian Coalition for High Blood Pressure Prevention and Control, Laboratory Centre for Disease Control at Health Canada, Heart and Stroke Foundation of Canada. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 1999;160:S1–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Blood Pressure Lowering Treatment Trialists, C. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. The Lancet362, 1527–1535, 10.1016/S0140-6736(03)14739-3 (2003). [DOI] [PubMed]
- 6.Ettehad, D. et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. The Lancet387, 957–967, 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed]
- 7.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. Journal of hypertension. 2016;34:613–622. doi: 10.1097/hjh.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 8.Nations, U. Political Declaration of the High Level Meeting of the General Assembly on the Prevention and Control of Non-communicable diseases (2012).
- 9.WHO. Action Framework for Prevention and Control of Chronic Diseases (2006).
- 10.Gelber RP, Gaziano JM, Manson JE, Buring JE, Sesso HD. A prospective study of body mass index and the risk of developing hypertension in men. American journal of hypertension. 2007;20:370–377. doi: 10.1016/j.amjhyper.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shihab, H. M. et al. Body mass index and risk of incident hypertension over the life course: the Johns Hopkins Precursors Study. Circulation126, 2983–2989, 10.1161/circulationaha.112.117333 Epub 2012 Nov 14 (2012). [DOI] [PMC free article] [PubMed]
- 12.Must, A. & McKeown, N. M. In Endotext (eds De Groot, L. J. et al.) (MDText.com, Inc., 2000).
- 13.Cushman WC. Alcohol consumption and hypertension. Journal of clinical hypertension (Greenwich, Conn.) 2001;3:166–170. doi: 10.1111/j.1524-6175.2001.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs FD, Chambless LE, Whelton PK, Nieto FJ, Heiss G. Alcohol Consumption and the Incidence of Hypertension: The Atherosclerosis Risk in Communities Study. Hypertension. 2001;37:1242–1250. doi: 10.1161/01.hyp.37.5.1242. [DOI] [PubMed] [Google Scholar]
- 15.Halperin RO, Gaziano JM, Sesso HD. Smoking and the risk of incident hypertension in middle-aged and older men. American journal of hypertension. 2008;21:148–152. doi: 10.1038/ajh.2007.36. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko M, et al. Smoking was a Possible Negative Predictor of Incident Hypertension After a Five-Year Follow-up Among a General Japanese Population. Cardiol Res. 2012;3:87–93. doi: 10.4021/cr95w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beunza JJ, et al. Sedentary behaviors and the risk of incident hypertension: the SUN Cohort. American journal of hypertension. 2007;20:1156–1162. doi: 10.1016/j.amjhyper.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Craddick SR, et al. The DASH diet and blood pressure. Current atherosclerosis reports. 2003;5:484–491. doi: 10.1007/s11883-003-0039-5. [DOI] [PubMed] [Google Scholar]
- 19.Toledo E, et al. Hypothesis-oriented food patterns and incidence of hypertension: 6-year follow-up of the SUN (Seguimiento Universidad de Navarra) prospective cohort. Public Health Nutr. 2010;13:338–349. doi: 10.1017/s1368980009991066. [DOI] [PubMed] [Google Scholar]
- 20.Boeing, H., Korfmann, A. & Bergmann, M. M. Recruitment procedures of EPIC-Germany. European Investigation into Cancer and Nutrition. Annals of nutrition & metabolism 43, 205–215, doi:12787 (1999). [DOI] [PubMed]
- 21.Boeing, H., Wahrendorf, J. & Becker, N. EPIC-Germany–A source for studies into diet and risk of chronic diseases. European Investigation into Cancer and Nutrition. Annals of nutrition & metabolism 43, 195–204, doi:12786 (1999). [DOI] [PubMed]
- 22.Bohlscheid-Thomas S, Hoting I, Boeing H, Wahrendorf J. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the German part of the EPIC project. European Prospective Investigation into Cancer and Nutrition. International journal of epidemiology. 1997;26(Suppl 1):S59–70. doi: 10.1093/ije/26.suppl_1.S59. [DOI] [PubMed] [Google Scholar]
- 23.Kroke A, et al. Blood pressure measurement in epidemiological studies: a comparative analysis of two methods. Data from the EPIC-Potsdam Study. European Prospective Investigation into Cancer and Nutrition. J Hypertens. 1998;16:739–746. doi: 10.1097/00004872-199816060-00004. [DOI] [PubMed] [Google Scholar]
- 24.Kroke A, et al. Measures of quality control in the German component of the EPIC study. European Prospective Investigation into Cancer and Nutrition. Annals of nutrition & metabolism. 1999;43:216–224. doi: 10.1159/000012788. [DOI] [PubMed] [Google Scholar]
- 25.Dietrich S, et al. Identification of Serum Metabolites Associated With Incident Hypertension in the European Prospective Investigation into Cancer and Nutrition-Potsdam Study. Hypertension. 2016;68:471–477. doi: 10.1161/hypertensionaha.116.07292. [DOI] [PubMed] [Google Scholar]
- 26.Wientzek A, et al. The Improved Physical Activity Index for Measuring Physical Activity in EPIC Germany. PLoS ONE. 2014;9:e92005. doi: 10.1371/journal.pone.0092005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fung TT, et al. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Archives of internal medicine. 2008;168:713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 28.Jekel, J. F., Katz, D. L., Elmore, J. G. & Wild, D. Epidemiology, biostatistics and preventive medicine. (Elsevier Health Sciences, 2007).
- 29.Wacholder S, Benichou J, Heineman EF, Hartge P, Hoover RN. Attributable risk: advantages of a broad definition of exposure. American journal of epidemiology. 1994;140:303–309. doi: 10.1093/oxfordjournals.aje.a117252. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y, et al. Clustering of multiple healthy lifestyles among older Korean adults living in the community. Geriatrics & Gerontology International. 2012;12:515–523. doi: 10.1111/j.1447-0594.2011.00788.x. [DOI] [PubMed] [Google Scholar]
- 31.Dumith SC, Muniz LC, Tassitano RM, Hallal PC, Menezes AMB. Clustering of risk factors for chronic diseases among adolescents from Southern Brazil. Preventive medicine. 2012;54:393–396. doi: 10.1016/j.ypmed.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poorolajal, J., Hooshmand, E., Bahrami, M. & Ameri, P. How much excess weight loss can reduce the risk of hypertension? Journal of public health (Oxford, England), 10.1093/pubmed/fdw077 (2016). [DOI] [PubMed]
- 33.Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Archives of internal medicine. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 34.Harrington JM, et al. DASH diet score and distribution of blood pressure in middle-aged men and women. Am J Hypertens. 2013;26:1311–1320. doi: 10.1093/ajh/hpt106. [DOI] [PubMed] [Google Scholar]
- 35.Schwingshackl L, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. Journal of the Academy of Nutrition and Dietetics. 2015;115:780–800.e785. doi: 10.1016/j.jand.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Epstein DE, et al. Determinants and consequences of adherence to the dietary approaches to stop hypertension diet in African-American and white adults with high blood pressure: results from the ENCORE trial. Journal of the Academy of Nutrition and Dietetics. 2012;112:1763–1773. doi: 10.1016/j.jand.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lelong H, et al. Individual and Combined Effects of Dietary Factors on Risk of Incident Hypertension: Prospective Analysis From the NutriNet-Sante Cohort. Hypertension. 2017;70:712–720. doi: 10.1161/hypertensionaha.117.09622. [DOI] [PubMed] [Google Scholar]
- 38.Jiang J, et al. Concordance with DASH diet and blood pressure change: results from the Framingham Offspring Study (1991–2008) Journal of hypertension. 2015;33:2223–2230. doi: 10.1097/hjh.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 39.Folsom AR, Parker ED, Harnack LJ. Degree of Concordance With DASH Diet Guidelines and Incidence of Hypertension and Fatal Cardiovascular Disease*. American Journal of Hypertension. 2007;20:225–232. doi: 10.1016/j.amjhyper.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, et al. Vascular abnormalities in asymptomatic, healthy young adult smokers without other major cardiovascular risk factors: the Bogalusa Heart Study. American journal of hypertension. 2005;18:319–324. doi: 10.1016/j.amjhyper.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Sharrett AR, et al. Smoking, diabetes, and blood cholesterol differ in their associations with subclinical atherosclerosis: the Multiethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2006;186:441–447. doi: 10.1016/j.atherosclerosis.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Kim JW, et al. Acute and chronic effects of cigarette smoking on arterial stiffness. Blood pressure. 2005;14:80–85. doi: 10.1080/08037050510008896. [DOI] [PubMed] [Google Scholar]
- 43.Halimi JM, et al. The risk of hypertension in men: direct and indirect effects of chronic smoking. Journal of hypertension. 2002;20:187–193. doi: 10.1097/00004872-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Ain, Q. U. & Regmi, K. The effects of smoking in developing hypertension in Pakistan: A systematic review. 2015 5, 8, 10.3329/seajph.v5i1.24845 (2015).
- 45.Primatesta P, Falaschetti E, Gupta S, Marmot MG, Poulter NR. Association Between Smoking andBlood Pressure. Evidence From the Health Survey for England. 2001;37:187–193. doi: 10.1161/01.hyp.37.2.187. [DOI] [PubMed] [Google Scholar]
- 46.Bowman TS, Gaziano JM, Buring JE, Sesso HD. A prospective study of cigarette smoking and risk of incident hypertension in women. J Am Coll Cardiol. 2007;50:2085–2092. doi: 10.1016/j.jacc.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 47.Li W, et al. The effect of body mass index and physical activity on hypertension among Chinese middle-aged and older population. Scientific Reports. 2017;7:10256. doi: 10.1038/s41598-017-11037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bergmann MM, et al. The association of pattern of lifetime alcohol use and cause of death in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. International journal of epidemiology. 2013;42:1772–1790. doi: 10.1093/ije/dyt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thadhani R, et al. Prospective study of moderate alcohol consumption and risk of hypertension in young women. Archives of internal medicine. 2002;162:569–574. doi: 10.1001/archinte.162.5.569. [DOI] [PubMed] [Google Scholar]
- 50.Klatsky AL, Gunderson E. Alcohol and hypertension: a review. Journal of the American Society of Hypertension: JASH. 2008;2:307–317. doi: 10.1016/j.jash.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Ma C, et al. Associations between aldehyde dehydrogenase 2 (ALDH2) rs671 genetic polymorphisms, lifestyles and hypertension risk in Chinese Han people. Scientific reports. 2017;7:11136–11136. doi: 10.1038/s41598-017-11071-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Y, et al. Positive association between ALDH2 rs671 polymorphism and essential hypertension: A case-control study and meta-analysis. PloS one. 2017;12:e0177023–e0177023. doi: 10.1371/journal.pone.0177023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu N, Zhang Y, Nair S, Culver BW, Ren J. Contribution of ALDH2 polymorphism to alcoholism-associated hypertension. Recent patents on endocrine, metabolic & immune drug discovery. 2014;8:180–185. doi: 10.2174/1872214808666141020162000. [DOI] [PubMed] [Google Scholar]
- 54.Kunes J, Zicha J. The interaction of genetic and environmental factors in the etiology of hypertension. Physiol Res. 2009;58(Suppl 2):S33–41. doi: 10.33549/physiolres.931913. [DOI] [PubMed] [Google Scholar]
- 55.Hamet, P., Pausova, Z., Adarichev, V., Adaricheva, K. & Tremblay, J. Hypertension: genes and environment. Journal of hypertension16, 10.1097/00004872-199816040-00001 (1998). [DOI] [PubMed]
- 56.Leite MLC, Moriguchi EH, Lima-Costa MF. Interactive effects of ApoE polymorphism, alcohol and smoking on age-related trends of blood pressure levels in elderly men: the Bambuì Cohort Study of Ageing (1997–2008) Journal Of Human Hypertension. 2013;27:497. doi: 10.1038/jhh.2012.70. [DOI] [PubMed] [Google Scholar]
- 57.Kauma H, et al. Apolipoprotein E phenotype determines the effect of alcohol on blood pressure in middle-aged men. American journal of hypertension. 1998;11:1334–1343. doi: 10.1016/S0895-7061(98)00163-0. [DOI] [PubMed] [Google Scholar]
- 58.Ha SK. Dietary salt intake and hypertension. Electrolyte & blood pressure: E & BP. 2014;12:7–18. doi: 10.5049/EBP.2014.12.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He FJ, Campbell NR, MacGregor GA. Reducing salt intake to prevent hypertension and cardiovascular disease. Revista panamericana de salud publica = Pan American journal of public health. 2012;32:293–300. doi: 10.1590/S1020-49892012001000008. [DOI] [PubMed] [Google Scholar]
- 60.Fortin M, et al. Lifestyle factors and multimorbidity: a cross sectional study. BMC Public Health. 2014;14:686–686. doi: 10.1186/1471-2458-14-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are not publicly available due to data protection regulations. In accordance with German Federal and State data protection regulations, epidemiological data analyses of EPIC-Potsdam may be initiated upon an informal inquiry addressed to the secretariate of the Human Study Center (Office.HSZ@dife.de). Each request will then have to pass a formal process of application and review by the respective PI and a scientific board.


