Abstract
The mammalian penis is a complex hydraulic organ of cavernous (spongy) tissue supported by both smooth and skeletal muscle structures. In placental mammals, the paired Musculus ischiocavernosi anchor the corpora cavernosa to the pelvis (at the ischium), and the paired M. bulbospongiosi converge as they envelop the base of the corpus spongiosum. Male marsupials have a dramatically different anatomy, however, in which both sets of paired muscles remain separate, have a bulbous, globular shape and do not have any direct connection to the pelvis. Here we provide the first detailed anatomical investigation of the muscles of the penis in the western grey kangaroo (Macropus fuliginosus) incorporating dissection, histology, vascular casting and computed tomography. The M. ischiocavernosus and M. bulbospongiosus form massive, multipennate bodies of skeletal muscle surrounding the paired roots of the corpus cavernosum and corpus spongiosum, respectively. Bilateral vascular supply is via both the artery of the penis and the ventral perineal artery. Histological examination reveals cavernous tissues with substantial smooth muscle supported by fibroelastic trabeculae, surrounded by the thick collagenous tunica albuginea. The M. ischiocavernosus and M. bulbospongiosus are known to function during erection of the penis and ejaculation via muscular contraction increasing blood pressure within cavernous vascular tissues. The thick muscular anatomy of the kangaroo would be well suited to this function. The absence of any connection to the bony pelvis in marsupials suggests the possibility of different mechanisms of action of these muscles with regard to reduction of venous return, eversion from the cloaca, or movements such as penile flips, which have been described in some placental mammals. This highlights a greater diversity in form and function in the evolution of the mammalian penis than has been previously considered.
Keywords: erectile tissue, fibroelastic penis, M. bulbospongiosus, M. ischiocavernosus, Marsupialia, penile erection
Introduction
Many animals have hydraulic copulatory organs, derived from the tissues of the cloacal region, to facilitate internal fertilisation. The principal functional components of the mammalian penis are modelled around a consistent basic arrangement: essentially three columns of erectile tissue surrounded by multiple fascial layers (Awad et al. 2011). The erectile tissue – paired corpora cavernosa and single corpus spongiosum – is composed of open pore capillaries (sinusoids) with smooth muscle walls held together by fibrous connective tissue (Hsieh et al. 2012). The corpora cavernosa form the substantive part of the cavernous body of the penis. The corpus spongiosum encloses the spongy urethra to maintain patency and expands distally forming the glans penis, where present. Each of the erectile columns is surrounded by a thick collagen‐rich ‘fibrous skeleton’, the tunica albuginea (a single layer around the corpus spongiosum and two layers around the corpora cavernosa), which resists expansion and therefore increases pressure within the erectile tissue as it fills with blood, causing elongation of the penis during engorgement, as well as providing rigidity to resist bending during copulation (Kelly, 2002; Hsieh et al. 2012).
At the fundamental level, erection is produced by relaxation of the arterial walls within the penis, together with mechanisms that act to restrict venous return, such that vascular spaces become engorged with blood. Two skeletal muscles, the M. ischiocavernosus and M. bulbospongiosus, function to increase the hydrostatic pressure within the penis to produce suprasystolic pressures associated with increased rigidity at intromission and during copulation (Beckett et al. 1974; Purohit & Beckett, 1976; Lavoisier et al. 1986; Gerstenberg et al. 1990; Schmidt & Schmidt, 1993). At the base of the penis, the columns of corpora cavernosa diverge from one another as the two crura, which are enclosed in the M. ischiocavernosus (Chauveau, 1891; Hsieh et al. 2012), while the base of the corpus spongiosum is incompletely enclosed by the M. bulbospongiosi, which form a mass referred to as the urethral bulb (Awad et al. 2011). Blood pressure within the corpora cavernosa and corpus spongiosum, and thus rigidity of the penis, is tightly linked to the activity of these muscles (Schmidt & Schmidt, 1993).
The mammalian penis varies extensively in form and function between different taxa, commensurate with differences in reproductive behaviour and biology. However, differences in the form and function of the cavernous muscles of the penis have not previously been considered. No detailed structural or functional information on the muscles of the penis in marsupials is available, but general descriptions of the reproductive system of the western grey kangaroo (Macropus fuliginosus) (Martin et al. 2018) identified marked differences in the gross anatomy of the M. ischiocavernosus and M. bulbospongiosus in kangaroos compared with placental mammals. Similar arrangements of these muscles (often simply referred to as ‘bulbs’) have also been reported for other marsupials (Owen, 1839a; Young, 1879; Nogueira et al. 2004).
As form follows function, differences in the anatomy of the muscular cavernosi suggest potentially different mechanisms of action for these muscles during the processes of erection, intromission and copulation. These differences may represent the plesiomorphic anatomy in Mammalia or simply divergent evolution between the two clades. Therefore, a better understanding of the male reproductive anatomy in marsupials would shed light on the evolution of this functional system. We undertook a detailed study, incorporating dissection to describe the gross anatomy of the male reproductive tract and histological examination of the musculocavernous tissues of the penis, as well as vascular casting and CT scans to determine vascular supply of these muscular structures in one of the largest marsupials, the western grey kangaroo.
Materials and methods
The project was conducted under the approval of Murdoch University Animal Ethics Committee (Cadaver Notification), in compliance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (7th edition, 2004), and a Department of Parks and Wildlife Regulation 17 licence SF009843.
Gross anatomical descriptions
We documented the gross anatomy of the male kangaroo reproductive tract and the histological anatomy of the musculo‐cavernous tissues of the penis. Specimens of adult male western grey kangaroos (n = 54) from various locations around Pinjarra, southwest Western Australia, were purchased from professionals that culled at night under commercial licence for pet food products. Gross anatomical descriptions were made from dissections of fresh (frozen) adult material. Reproductive tracts were collected in the field from fresh carcasses by making a deep circular incision around the perineum externally to remove the entire perineal region and internal organs from the pelvic cavity intact. All material was collected with 12 h of death. The specimens were kept chilled during transport and frozen at −20 °C until dissection. We cleared the reproductive organs and accessory glands (Fig. 1) of connective tissue and then isolated the prostate gland, each of the bulbourethral (Cowper's) glands and the paired M. ischiocavernosi and M. bulbospongiosi. Connections between the various organs were described. As the anatomy of the perineal and pelvic regions is not well known in marsupials, we include a description of the muscle layers and associated organs encountered during dissection to serve as a useful guide to others.
Figure 1.
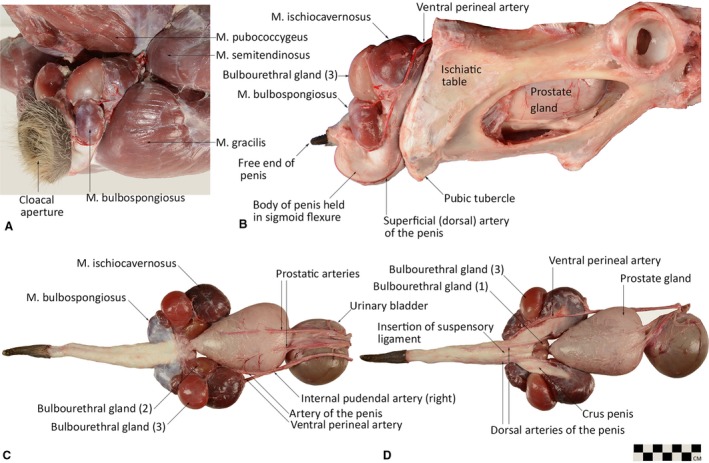
Male reproductive structures (late breeding season) of the western grey kangaroo (Macropus fuliginosus) in situ showing the relations of the muscles of the proximal tail and hindlimb (A) and bony pelvis (B), and dissected out showing the anatomical arrangement of penis, cavernosus muscles and accessory glands from dorsal (C) and ventral (D) views (modified from Martin et al. 2018). Scale bar in lower right corresponds to illustrations (c,d); total body mass 64 kg for this individual. Arteries were injected with coloured latex for greater resolution of vascular components.
Histology
Histological samples of the cavernous tissues of the penis and associated M. ischiocavernosus and M. bulbospongiosus were fixed in 10% neutral‐buffered formalin for 1 week prior to embedding in paraffin wax. Transverse and longitudinal sections were cut on a sliding microtome at 5 μm. Standard histological stains were used to give contrast to the tissues of interest: Martius Scarlet Blue (collagen), Smooth Muscle Actin immunohistochemistry stain (revealing smooth muscle) and Orcein (elastin).
Blood supply
Vascular casting and computed tomography (CT) scanning were used on two additional male cadavers to visualise the blood supply to the reproductive tract. Cadavers were placed in dorsal recumbency and the abdominal organs carefully removed within 3–5 h of death. A small incision was made in the ventral abdominal aorta, roughly 200 mm cranial to the bifurcation of the external iliac arteries, and two tubes (3.5 mm diameter) were inserted into the aorta and passed caudally into the right and left internal iliac arteries. The aorta was then ligated to hold the tubes in place.
The first individual (body mass m b 67.1 kg) was injected with a combination of Biodur Red E20, Biodur E2 and X‐OPAQUE‐HD barium sulphate powder (976.5 mg g−1 oral powder; MCI Forrest). A total of 160 g of E20, 72 g of E2 and 100 g of X‐OPAQUE‐HD were used as per the manufacturer's instructions and injected into the internal iliac arteries. The cadaver was left in the lab at room temperature for 8 h to allow the resin to cure before being placed in the chiller at 3 °C. The cadaver was then X‐rayed and CT‐scanned 24 h later.
The second individual (m b 63.4 kg) was injected with 300 mL mixture of latex rubber (Carolina) and Liquid Polibar barium sulphate at a ratio of 2 : 1. The kangaroo cadaver was then placed in the cool room at 3 °C for 24 h prior to dissection. Muscles from this individual were sectioned during dissection to examine the vascular arrangement.
Results
Gross anatomical descriptions
Perineal region
The perineal region forms a conspicuous feature of surface anatomy in kangaroos. Situated on the caudo‐ventral aspect of the trunk, at the base of the tail, this region encloses the distal rectum and, in males, the entirety of the retracted penis, paired M. ischiocavernosi (IC) and M. bulbospongiosi (BS) and three pairs of bulbourethral (Cowper's) glands (Fig. 1A). The dorsal anal and ventral preputial ostia open into a common space, the cloaca, which communicates with the external environment via the cloacal aperture (Fig. 1A). The skin of the perineum is very tough and covers layers of dense connective tissue and skeletal muscle (M. sphincter cloacae, M. sphincter ani ).
The large prostate gland and associated prostatic urethra are the only portions of the male reproductive system to reside within the pelvic cavity (Fig. 1B). The prostate is cushioned by the muscles of the pelvic diaphragm (M. levator ani and M. coccygeus) and a number of large fat pads that fill the contours of the bony pelvis.
The skeletal muscles of the perineum and pelvic floor are complex. M. sphincter cloacae are divisible into three portions. Pars cutanea is a circular cutaneous portion that passes around, and completely surrounds, the cloacal aperture. The transverse fibres of the sphincter cloacae pars superficialis cover the ventral and lateral aspects of the perineum and enclose the root of the penis, the M. bulbospongiosi, the bulbourethral glands, and distal third of the M. ischiocavernosi. The fibres insert into thick fascia along the lateral border of each crus. Sphincter cloacae pars profunda is a deep layer of thick transverse fibres that almost completely enclose the distal portion of the rectum, from the pelvic diaphragm to the anal ostium, and thus represents the M. sphincter ani externus. M. levator ani (M. ischiococcygeus), the most medial muscle of the pelvic diaphragm, from the medial table of the ischium, caudal to the obturator foramen, and passes ventrally to insert onto the proximal caudal vertebrae. M. coccygeus lies on the lateral aspect of the base of the tail, passing from the coccygeal fossa of the ischium to insert onto the distal transverse processes of the proximal eight caudal vertebrae (as described in Dawson et al. 2014).
Penis
In the western grey kangaroo, the penis has a mean total length of 166 ± 22 mm (Martin et al. 2018) and when retracted takes on a strong sigmoid flexure (Fig. 1b). It is held within dense fibrous connective tissue in the preputial sac at the base of the cloaca, ventral to the pelvic outlet (Fig. 1A,B). The free end of the retracted penis is caudo‐ventrally orientated. The free distal extremity of the penis is simple and tapered; there is no elaboration of a glans penis (Fig. 1C,D).
As observed histologically, the penile urethra was encircled by a very thin corpus spongiosum composed of collagen and smooth muscle (Fig. 2). The paired tracts of corpus cavernosus that form the body of the penis are C‐shaped in transverse section and, apart from a narrow fibrous ventral raphe, almost completely enclose the corpus spongiosum (Fig. 2). The corpora cavernosa are composed of deep elastic cavernous tissue surrounded by thick collagenous connective tissue (tunica albuginea).
Figure 2.
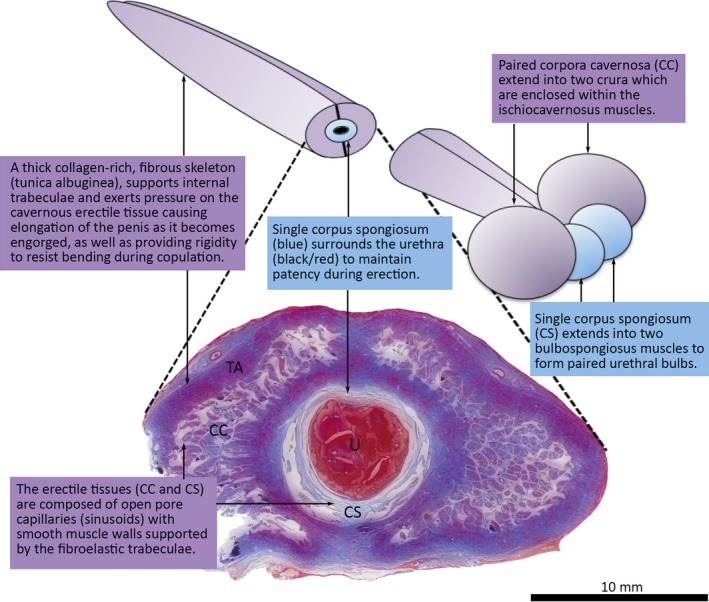
Transverse section of the western grey kangaroo (Macropus fuliginosus) penis reveals the arrangement of the three cavernous bodies: two lateral corpora cavernosa (CC) and a single corpus spongiosum (CS) which surrounds the urethra (U). The cavernous bodies extend into the paired M. ischiocavernosi and M. bulbospongiosi, respectively. Martius Scarlet Blue stain highlights the thick collagenous tunica albuginae (TA) surrounding the CC, and intervening medial septum. Urethra filled with red‐stained (fibrin) mucous exudate from bulbourethral glands postmortem.
The root of the penis is marked by the paired M. bulbospongiosi, which communicate on either side with the corpus spongiosum. From the root, two thick crura emerge and pass caudo‐laterally before becoming completely engulfed in the M. ischiocavernosi on either side.
Paired smooth muscles (M. retractor penis) originate on the ventral surface of the sacrum as long tendons; these become long bellies (circular in transverse section) as they pass ventrally on either side of the rectum and then along the urethral surface of the penis to insert approximately one‐third of the length from the terminal end. A short suspensory ligament from the bony tubercle on the caudal extremity of the ischiopubic symphysis inserts into the fibrous sheath of the root of the penis.
Ischiocavernosus muscle
The paired M. ischiocavernosi are large, bulbous structures, surrounding the terminal portion of the crura of the corpora cavernosa (Fig 1D). In situ, the M. ischiocavernosi lie dorsal‐most in the perineal region, filling a depression formed between M. pubococcygeus of the tail, and M. semitendinosus and M. gracilis of the thigh (Fig. 1A). They lie in close apposition to the largest of the bulbourethral glands, and from the ventral view completely obscure the remaining pairs of glands (Fig. 1C,D). Gross serial sections (Fig. 3) and histological sections (Fig. 4) reveal the massive, multipennate structure of the M. ischiocavernosi in relation to the crus penis. The blind‐ending crura have no attachment to the bony pelvis, nor do the M. ischiocavernosi. A small, indirect connection to the ischium is made via a blending of fleshy and dense aponeurotic fibres with those of the M. levator ani as it approaches its insertion into the fascia around the membranous urethra as it enters the base of the penis.
Figure 3.
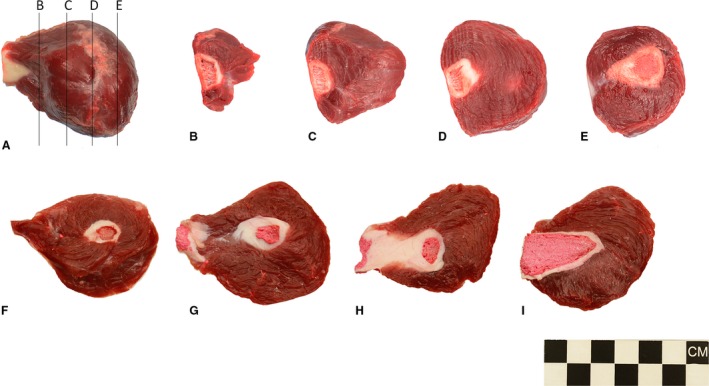
Gross appearance of the M. ischiocavernosus of the western grey kangaroo Macropus fuliginosus, dorsal view (A), serial transverse sections (B–E), and serial longitudinal from dorsal and ventral sections (F–I). Sections (F–I) have been cast with pigmented latex prior to dissection to highlight the vascular components.
Figure 4.
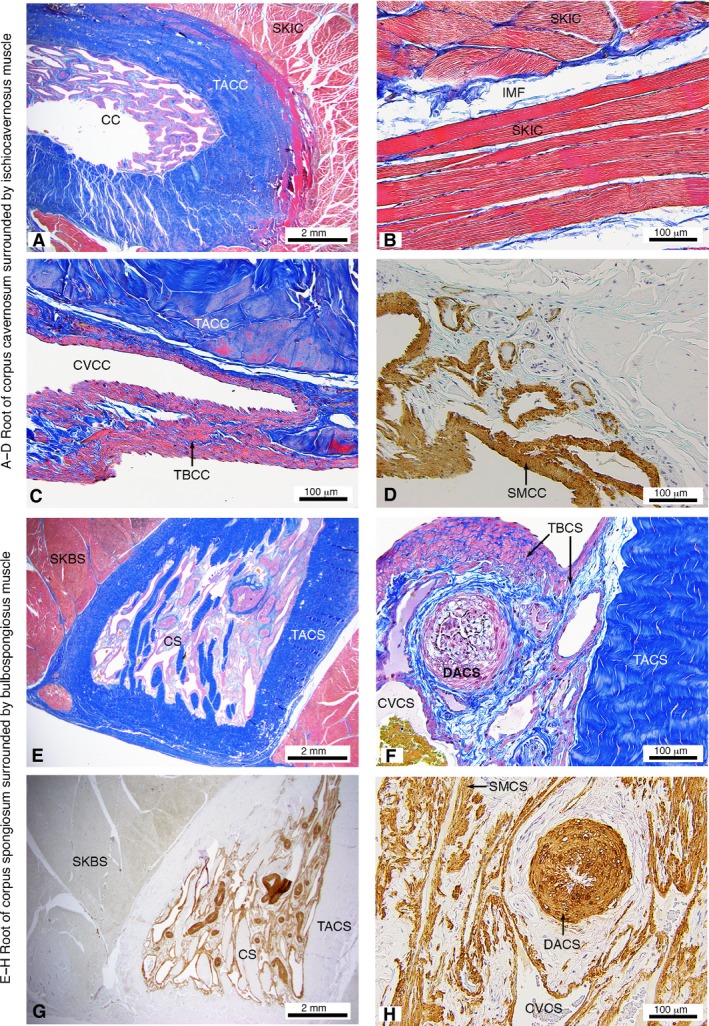
Histological appearance of the M. ischiocavernosus surrounding the root of the corpus cavernosum (A–D) and M. bulbospongiosus surrounding the root of the corpus spongiosum (E–H) of the western grey kangaroo Macropus fuliginosus (longitudinal sections). Martius Scarlet Blue highlighting collagen (blue) and muscle (red) (A–C,E–F) and Smooth Muscle Actin (D,G–H). CC, corpus cavernosum; CS, corpus spongiosum; CVCC, cavernous veins of corpus cavernosum; CVCS, cavernous veins of corpus spongiosum; DACS, deep artery of corpus spongiosum; IMF, intramuscular fascia; SKBS, skeletal muscle fibres of the M. bulbospongiosus; SKIC, skeletal muscle fibres of the M. ischiocavernosus; SMCC, smooth muscle in trabeculae of corpus cavernosum; SMCS, smooth muscle in trabeculae of corpus spongiosum; TACC, tunica albuginea of corpus cavernosum; TACS, tunica albuginea of corpus spongiosum; TBCC, trabecular of the corpus cavernosum; TBCS, trabecular of the corpus spongiosum.
Bulbospongiosus muscle
Although they are approximately a third the size of the M. ischiocavernosi, the M. bulbospongiosi have a similar multi‐pennate arrangement of skeletal muscle fibres, and a thick dorsal aponeurotic covering (Fig. 1C). In situ, the M. bulbospongiosi are located on either side of the base of the penis, lateral to the cloacal aperture and under cover of M. sphincter cloacae. The left and right M. bulbospongiosi are completely distinct from one another (unlike in placental mammals) and communicate with the cavernous components of the penis by distinct bilateral bulbs of corpus spongiosum.
Histology
Histological examinations of the M. ischiocavernosus and M. bulbospongiosus revealed thick bodies of skeletal muscle surrounding the roots of the corpora cavernosa and spongiosum, respectively (Fig. 4A,E). The fibre arrangement with the skeletal muscles is multi‐pennate, with fascicles arranged in various angles. For example, muscle fibres in Fig. 4b have been sectioned longitudinally, transversely and obliquely within the one section. Individual muscle fibres and fibre bundle fascicles (muscle stained red) are surrounded by fibroelastic connective tissue (collagen stained blue), the endomysium and perimysium, respectively (Fig. 4b). The muscles bundles are extensive and densely packed.
Enclosed within the M. ischiocavernosi and M. bulbospongiosi, respectively, the corpora cavernosa and spongiosa are covered by a thick layer of dense collagenous connective tissue, the tunica albuginea (Fig. 4A,C,E,F). The core of each corpus cavernosum is composed of numerous trabeculae surrounding the vascular spaces (cavernous veins). The trabeculae have a skeleton of collagen and elastin fibres (Fig. 4A,C,E,F), supporting layers of smooth muscle (Fig. 4D,G,H). The collagen fibres within the trabeculae and tunica albuginae are crimped (have a wavy appearance) along their long axis in the flaccid state. The regions of smooth muscle are relatively thicker in the corpus cavernosum than in the corpus spongiosum. Numerous deep arteries found throughout the cavernous tissues are highlighted with staining for smooth muscle actin (Fig. 4G,H).
Blood supply
The organs of the male reproductive tract in the kangaroo are supplied on either side by the long internal pudendal arteries, which (as in placental mammals) are the continuation of the internal iliac artery after the departure of the caudal gluteal artery (Fig. 5). The prostatic artery branches from the internal pudendal artery immediately after its separation from the caudal gluteal artery. The continuing internal pudendal artery passes into the pelvic cavity over the dorsal aspect of the bladder. Along the lateral aspect of the prostate, the vessel bifurcates into dorsal (artery of the penis) and ventral (ventral perineal artery) branches. The artery of the penis passes over the medial aspect of the M. ischiocavernosus, giving numerous small branches into the thick muscle, followed by a larger branch (deep artery of the penis), which divides to penetrate into the deep portions of the M. ischiocavernosus and also to supply the largest of the bulbourethral glands. There are small branches to the smaller bulbourethral glands before continuing as the dorsal artery of the penis. The ventral perineal artery also supplies the M. ischiocavernosus and the largest of the bulbourethral glands, and terminates within the M. bulbospongiosus as the ‘artery of the bulb’, thereby supplying the corpus spongiosum.
Figure 5.
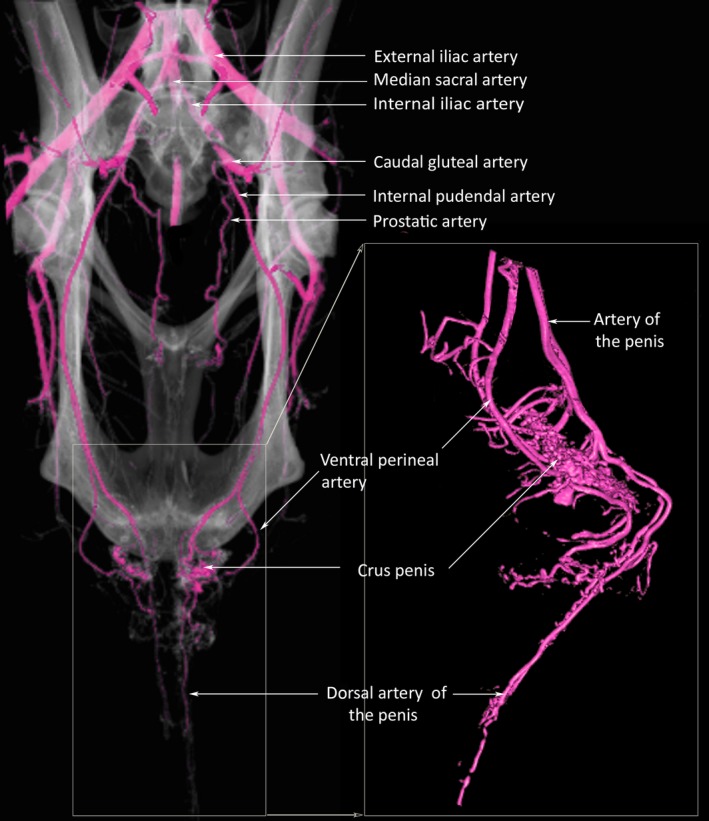
3D computed tomography reconstruction of the arterial blood supply of the reproductive tract of the male western grey kangaroo, Macropus fuliginosus. Contrast enhanced using casting agent of Biodur Red E20, Biodur E2 and X‐OPAQUE‐HD barium sulphate powder.
Discussion
Characterisation of the kangaroo penis and associated organs
This study describes the gross and histological anatomy of the western grey kangaroo penis and associated organs. The crura are continuous with the paired corpora cavernosa of the body of the penis and do not have any connection to the bony pelvis. Both corpora cavernosa contribute equally along the length of the penis. The corpus spongiosum surrounding the cavernous (spongy) urethra is relatively thin, and there is no expanded glans penis terminally. Histologically, the cavernous tissues of the penis are consistent with those found in placental mammals. Vascular spaces within the corpora cavernosa and spongiosa are surrounded by smooth muscle tissue supported on connective tissue trabeculae with collagen fibres and filaments of elastin. Presumably, the crimped collagen fibres of the trabeculae and tunica albuginae straighten during erection, allowing for some increase in trabecular length and expansion of the cavernous tissues, while at the same time maintaining the non‐circular cross‐sectional shape of the corpus cavernosum seen in other mammals (Kelly, 1999).
Among mammals, two main types of penis are recognised; the fibroelastic penis and the musculocavernous (vascular) penis. The anatomy of the western grey kangaroo penis appears to be consistent with a fibroelastic type of penis, with very thick connective tissue structures, especially tunica albuginea, composed of collagen and elastin, a strong sigmoid flexure, and long retractor penis muscles. In mammals with a fibroelastic penis (e.g. domestic cattle and pigs), the thick fibroelastic tissues exert pressure on the engorged vascular spaces and limit change in girth, resulting in rapid lengthening of the penis and straightening of the sigmoid flexure (Maia et al. 2006; Ribeiro et al. 2013). By contrast, in animals with a musculocavernous penis (e.g. rabbits, dogs or humans), the tunica albuginea is relatively thinner (Di Fiore, 1982) and engorgement results in greater changes in size, shape and girth of the penis (Maia et al. 2006). The characterisation of the kangaroo penis as a fibroelastic organ suggests that erection and copulation are likely relatively rapid events.
Structure and function of the cavernous muscles
In western grey kangaroos, the paired M. ischiocavernosi do not extend to attach to the pelvis (as they do in placental mammals). Rather, each blind‐ending corpus cavernosum is surrounded by a large, bulbous mass of muscle that has no connection to the bony pelvis. The paired M. bulbospongiosi, associated with the corpus spongiosum, remain completely separate from one another and have a similar globular appearance to the M. ischiocavernosus. The globular shape and anatomical arrangement of these muscles have been recorded for a number of marsupial species, including koalas, small insectivorous dasyurids and opossums, and appear to be consistent across South American and Australian clades of marsupials (Owen, 1839a; Young, 1879; Rodger & Hughes, 1973; Woolley & Webb, 1977; Nogueira et al. 2004).
This study highlights significant differences in anatomy between marsupial and placental male reproductive tracts. First, in placental mammals, the M. ischiocavernosus and M. bulbospongiosus function during the processes of engorgement of the penis, resulting in erection (Purohit & Beckett, 1976; Hart & Melese‐D'Hospital, 1983; Lavoisier et al. 1986) and ejaculation (Shafik, 1993a,b). The M. ischiocavernosus and M. bulbospongiosus contribute to the function of the penis via a number of mechanisms in placental mammals. Principally, contraction of the muscles exerts pressure upon a mass of incompressible fluid (blood) contained within a resisting tensile membrane (the tunica albuginea), leading to increased blood pressure within the columns of erectile tissues (corpora cavernosa and corpus spongiosum) (Purohit & Beckett, 1976; Holmes et al. 1991; Schmidt & Schmidt, 1993). During intromission and copulation, this is the primary role of the M. ischiocavernosus in increasing the internal fluid pressure within the corpus cavernosum. The M. bulbospongiosus contributes to erectile function in species that have an obvious glans penis, formed by the distal elaboration of the corpus spongiosum (Hart & Melese‐D'Hospital, 1983). During orgasm, rhythmic contractions of both the M. ischiocavernosus and M. bulbospongiosus assist the movement of semen along the urethra for ejaculation (Shafik, 1993a,b).
By contrast, in the kangaroo, these muscles are characterised by their globular shape and thick muscular walls composed of densely packed muscle bundles surrounding an internal cavity. The M. ischiocavernosus and M. bulbospongiosus in marsupials resemble the thick, muscular walls of cardiac ventricles (ventricular myocardium) or an avian gizzard (ventriculus; composed of smooth muscle), in which thick muscular walls provide the required mechanical compressive force on the internal chamber (Bennett & Cobb, 1969; Draeger et al. 1989; Torrent‐Guasp et al. 2005). The similarity in form of the thick and densely packed muscle bundles of the marsupial M. ischiocavernosus and M. bulbospongiosus suggests that they are suited to produce a large contractile force that could be used as a muscular pump. Their connection to the cavernous tissues of the corpus cavernosum and corpus spongiosum thus leads us to interpret that these muscles function as ‘pumps’ for generating suprasystolic pressures in the cavernous tissues of the penis during the process of engorgement/erection and ejaculation.
Secondly, in placental mammals, contraction of the M. ischiocavernosus also draws the root of the penis against the pelvis, compressing the deep dorsal vein of the penis and restricting venous drainage, thus increasing the blood pressure within the corpora cavernosa (Schmidt & Schmidt, 1993; Shafik et al. 2006). In species where the penis is retracted into the prepuce when flaccid, muscular actions of the M. ischiocavernosus in particular are involved in protrusion of the penis during erection (Nickel et al. 1973). Additionally, the connection of the M. ischiocavernosus to the pelvis has also been linked to penile movements, such as ‘penile flips’ in rats, which may play a role in the dislodgement of previously deposited seminal plugs (Hart & Melese‐D'Hospital, 1983).
The absence of extrinsic connections of the M. ischiocavernosus and M. bulbospongiosus in marsupials suggests that these muscles are unable to produce any leverage that might function in eversion of the penis from the cloaca or movements of the penis once erect. Further, there is no possibility of muscular contraction compressing the penis against the pubis to restrict venous blood flow. Indeed, this same observation was made by Cowper (1704) in his account of the male reproductive organs of the opossum over 300 years ago! It seems likely that this arrangement in marsupials may reflect the plesiomorphic anatomy of the Therian mammalian penis, and that the condition in placentals is more derived. It would be of great interest to examine the arrangement in monotremes to further inform this hypothesis. Owen (1839b) describes the proximal portion of the vascular tissue of the penis in monotremes as having two ‘lateral moieties’ separated by a median septum, both enclosed within a common dense fibrous sheath (Owen, 1839b, p. 392). There is no information, however, regarding how these structures relate to the arrangement of cavernous tissues within the penis, nor any muscular contributions to these structures. Further work on monotremes may help to clarify whether the condition in marsupials (as described here) does indeed reflect the plesiomorphic condition in Therian mammals or divergent evolution between the marsupial and placental clades.
Martin et al. (2018) reported that both M. ischiocavernosus and M. bulbospongiosus grow in proportion to body mass (m b) and are positively correlated with forelimb muscularity of the animal, a feature demonstrated to be under sexual selection in western grey kangaroos Warburton et al. (2018). Martin et al. (2018) found that M. ischiocavernosus (crural bulb) represented 0.121% of total m b in adult males (roughly 67 g for the average male mass of 54.3 kg) and were 40% heavier during the breeding (0.141% m b) than the non‐breeding (0.101% m b) season. The M. bulbospongiosus (urethral bulb) represents 0.037% of total m b in adult males, roughly 20 g for the average male, and were 48% heavier during the breeding (0.046% m b) than the non‐breeding (0.031% m b) season. As skeletal muscle responds to testosterone, it may be that increased circulating testosterone levels during the breeding season results in increased mass of the M. ischiocavernosus and M. bulbospongiosus. Skeletal muscles also respond to load; increased activity during the breeding season may therefore also contribute to observed increase in mass. Seasonal changes in the mass of the cavernous muscles have not (to our knowledge) been published for any other mammal. It would be interesting to know whether similar changes occur in placental mammals and the extent to which this varies between species with different penile structure and function.
In marsupials, as exemplified by the kangaroo, the left and right M. bulbospongiosi remain separate from one another, in contrast to placental mammals in which the two muscles fuse with one another ventrally along the medial raphe, forming a single urethral bulb. This double character of the bulb has been considered necessary to maintain the turgescence of the double or forked glans so characteristic of most marsupials (Young, 1879). However, our study suggests that the separate, paired nature of the M. bulbospongiosus is likely a plesiomorphic condition for Therian mammals. Elaboration of the glans is found in many didelphid (Didelphidae; Martin et al. 2018) and carnivorous marsupials (Dasyuridae, including genera with varied urethral appendages; Biggers, 1966), as well as bilbies (Thylacomyidae, which have bifurcation of both the glans and terminal urethra) (Woolley & Webb, 1977). As far as we are aware, however, the corpus spongiosum has not been shown to have any bilateral division within the body of the penis, and thus any separation of function between the two M. bulbospongiosi seems unlikely. Rather, the two separate M. bulbospongiosi are more likely to reflect the bilateral development of the reproductive organs. Partial division of the bulb of the corpus spongiosum is evident in a vascular cast of the dog penis (Murdoch University Veterinary Anatomy Museum) and the associated M. bulbospongiosus are paired, though closely situated. Future work to examine the structure and function of these muscles in a more diverse sample of mammals and reptiles would enable a much better understanding of the evolution of this functional system.
Conclusions
This investigation has clarified the gross and histological anatomy of the cavernous tissues of the kangaroo penis and associated muscular structures and confirms these as the M. ischiocavernosus and M. bulbospongiosus and therefore homologous with those of placental mammals. Further, it highlights an important structural difference, which is thus likely to be a functional difference, in the male reproductive system between marsupials and placentals, particularly in relation to the M. ischiocavernosus and M. bulbospongiosus. We suggest that these muscles in marsupials function purely as mechanical pumps for generating suprasystolic pressures in the cavernous tissues of the penis during the process of engorgement/erection and ejaculation, and, in contrast to the penis of placental mammals, they are not adapted for generating movement of the penis relative to the cloaca or pelvis. Ideally, in vivo testing of blood flow and pressure changes within these tissues during normal function would be required to confirm this hypothesis; however, such invasive experiments are difficult to perform. Further histological work to describe the composition and structure of the M. ischiocavernosus and M. bulbospongiosus in other vertebrate taxa (including reptiles, monotreme, marsupial and placental mammals) is needed to establish whether the muscular structures described here for kangaroos reflects an adaptation linked to their divergent patterns of reproduction and development or the plesiomorphic condition in Therian mammals. This work in marsupials demonstrates that the functional evolution of the mammalian penis is more diverse than has previously been considered. Broader phylogenetic comparisons will then enable an analysis of the evolutionary history of this intriguing functional system.
Conflict of interest
All authors declare that they have no conflict of interest.
Acknowledgements
D. Nottle, L. Boston, J. Hong and M. Gibberd for specimen acquisition and technical support. M. Martin, D. Supreme, K. Napier, V. Lenard and C. Flandrin for help with dissections. We also thank the editor and three anonymous reviewers for their positive and helpful suggestions for the improvement of our manuscript. Financial support was granted by Murdoch University to all authors.
References
- Awad A, Alsaid B, Bessede T, et al. (2011) Evolution in the concept of erection anatomy. Surg Radiol Anat 33, 301–312. [DOI] [PubMed] [Google Scholar]
- Beckett SD, Walker DF, Hudson RS, et al. (1974) Corpus cavernosum penis pressure and penile muscle activity in the bull during coitus. Am J Vet Res 35, 761–764. [PubMed] [Google Scholar]
- Bennett TH, Cobb JLS (1969) Studies on the avian gizzard: morphology and innervation of the smooth muscle. Cell Tissue Res 96, 173–185. [DOI] [PubMed] [Google Scholar]
- Biggers J (1966) Reproduction in male marsupials. Comparative biology of reproduction in mammals. Symposium of the Zoological Society. pp. 251–280, London: Academic Press. [Google Scholar]
- Chauveau A (1891) The Comparative Anatomy of the Domesticated Animals. 2nd English edn., rev. and enl. New York: D. Appleton. [Google Scholar]
- Cowper W (1704) An account of the anatomy of those parts of a male opossum that differ from the female: in a Letter to Dr Edward Tyson, from Mr William Cowper, Chirurgeon, and Fellow of the Royal Society, London. Philos Trans (1683‐1775) 24, 1576–1590. [Google Scholar]
- Dawson R, Milne N, Warburton NM (2014) Muscular anatomy of the tail of the western grey kangaroo, Macropus fuliginosus . Aust J Zool 62, 166–174. [Google Scholar]
- Di Fiore MSH (1982) Atlas of human histology; revised translation of Atlas de histologia normal, p. 267 Philadelphia: Lea & Febiger. [Google Scholar]
- Draeger A, Stelzer EH, Herzog M, et al. (1989) Unique geometry of actin‐membrane anchorage sites in avian gizzard smooth muscle cells. J Cell Sci 94, 703–711. [DOI] [PubMed] [Google Scholar]
- Gerstenberg TC, Levin RJ, Wagner G. (1990) Erection and ejaculation in man. Assessment of the electromyographic activity of the bulbocavernosus and ischiocavernosus muscles. Br J Urol 65, 395–402. [DOI] [PubMed] [Google Scholar]
- Hart BL, Melese‐D'Hospital PY (1983) Penile mechanisms and the role of the striated penile muscles in penile reflexes. Physiol Behav 31, 807–813. [DOI] [PubMed] [Google Scholar]
- Holmes GM, Chapple WD, Leipheimer RE, et al. (1991) Electromyographic analysis of male rat perineal muscles during copulation and reflexive erections. Physiol Behav 49, 1235–1246. [DOI] [PubMed] [Google Scholar]
- Hsieh C‐H, Liu S‐P, Hsu G‐L, et al. (2012) Advances in understanding of mammalian penile evolution, human penile anatomy and human erection physiology: clinical implications for physicians and surgeons. Med Sci Monit 18, RA118–RA125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DA (1999) Expansion of the tunica albuginea during penile inflation in the nine‐banded armadillo (Dasypus novemcinctus). J Exp Biol 202, 253–265. [DOI] [PubMed] [Google Scholar]
- Kelly DA (2002) The functional morphology of penile erection: tissue designs for increasing and maintaining stiffness. Integr Comp Biol 42, 216–221. [DOI] [PubMed] [Google Scholar]
- Lavoisier P, Courtois F, Barres D, et al. (1986) Correlation between intracavernous pressure and contraction of the ischiocavernosus muscle in man. J Urol 136, 936–939. [DOI] [PubMed] [Google Scholar]
- Maia RS, Babinski MA, Figueiredo MA, et al. (2006) Concentration of elastic system fibers in the corpus cavernosum, corpus spongiosum, and tunica albuginea in the rabbit penis. Int J Impot Res 18, 121–125. [DOI] [PubMed] [Google Scholar]
- Martin ML, Bateman PW, Auckland CH, et al. (2018) Is there evidence for a trade‐off between sperm competition traits and forelimb musculature in the western grey kangaroo? Biol J Lin Soc 123, 431–444. [Google Scholar]
- Nickel RS, Seiferle A, Nickel ER, et al. (1973) The Viscera of the Domestic Mammals. New York: Springer. [Google Scholar]
- Nogueira JC, Castro ACS, Câmara EVC, et al. (2004) Morphology of the male genital system of Chironectes minimus and comparison to other didelphid marsupials. J Mammal 85, 834–841. [Google Scholar]
- Owen R (1839a) Marsupialia In: Cyclopaedia of Anatomy and Physiology. (ed. Todd RB.), pp. 257–329, London: Sherwood, Gilbert and Piper. [Google Scholar]
- Owen R (1839b) Monotremata In: Cyclopaedia of Anatomy and Physiology. (ed. Todd RB.), pp. 366–406, London: Sherwood, Gilbert and Piper. [Google Scholar]
- Purohit RC, Beckett SD (1976) Penile pressures and muscle activity associated with erection and ejaculation in the dog. Am J Physiol 231, 1343–1348. [DOI] [PubMed] [Google Scholar]
- Ribeiro ICA, Abidu‐Figueiredo M, Costa FB, et al. (2013) Stereological study of the elastic fiber and smooth muscle cell system in the bovine and buffalo penis. Pesquisa Veterinária Brasileira 33, 107–112. [Google Scholar]
- Rodger JC, Hughes RL (1973) Studies of the accessory glands of male marsupials. Aust J Zool 21, 303–320. [Google Scholar]
- Schmidt MH, Schmidt HS (1993) The ischiocavernosus and bulbospongious muscles in mammalian penile rigidity. Sleep 16, 171–183. [DOI] [PubMed] [Google Scholar]
- Shafik A (1993a) The cervicocavernosus reflex: description of the reflex and its role in the sexual act. Int Urogynecol J 4, 70–73. [Google Scholar]
- Shafik A (1993b) Vaginocavernosus reflex. Clinical significance and role in sexual act. Gynecol Obstet Invest 35, 114–117. [DOI] [PubMed] [Google Scholar]
- Shafik A, Shafik I, El‐Sibai O, et al. (2006) Effect of external anal sphincter contraction on the ischiocavernosus muscle and its suggested role in the sexual act. J Androl 27, 40–44. [DOI] [PubMed] [Google Scholar]
- Torrent‐Guasp F, Kocica MJ, Corno AF, et al. (2005) Towards new understanding of the heart structure and function. Eur J Cardiothorac Surg 27, 191–201. [DOI] [PubMed] [Google Scholar]
- Warburton NM, Bateman PW, Fleming PA (2013) Sexual selection on forelimb muscles of western grey kangaroos (Skippy was clearly a female). Biol J Linn Soc 109, 923–931. [Google Scholar]
- Woolley PA, Webb SJ (1977) The penis of dasyurid marsupials In: The Biology of Marsupials. (eds Stonehouse B, Gilmore D.), pp. 307–323, London: Macmillan Press Ltd. [Google Scholar]
- Young AH (1879) The male generative organs of the koala (Phascolarctos cinereus). J Anat Physiol 13, 305–317. [PMC free article] [PubMed] [Google Scholar]


