SUMMARY
It is important to study commensal populations of Escherichia coli because they appear to be the reservoir of both extra-intestinal pathogenic E. coli and antibiotic resistant strains of E. coli. We studied 279 dominant faecal strains of E. coli from 243 adults living in the community in the Paris area in 2010. The phylogenetic group and sub-group [sequence type complex (STc)]of the isolates and the presence of 20 virulence genes were determined by PCR assays. The O-types and the resistance to 18 antibiotics were assessed phenotypically. The B2 group was the most frequently recovered (34.0%), followed by the A group (28.7%), and other groups were rarer. The most prevalent B2 subgroups were II (STc73), IV (STc141), IX (STc95) and I (STc131) with 22.1%, 21.1%, 16.8% and 13.7%, respectively, of the B2 group strains. Virulence factors (VFs) were more common in B2 group than other strains. One or more resistance was found in 125 strains (44.8% of the collection) but only six (2.2% of the collection) were multiresistant; no extended-spectrum beta-lactamase-producing strain was isolated. The C phylogroup and clonal group A strains were the most resistant. No trade-off between virulence and resistance was evidenced. We compared these strains to collections of strains gathered in the same conditions 30 and 10 years ago. There has been a parallel and linked increase in the frequency of B2 group strains (from 9.4% in 1980 to 22.7% in 2000 and 34.0% in 2010)and of VFs. Antibiotic resistance also increased, from 22.6% of strains resistant to at least one antibiotic in 1980 to 31.8% in 2000 and 44.8% in 2010; resistance to streptomycin, however, remained stable. Commensal human E. coli populations have clearly evolved substantially over time, presumably reflecting changes in human practices, and particularly increasing antibiotic use.
INTRODUCTION
Escherichia coli is a gut commensal of vertebrates, including humans(Berg, 1996). It can nevertheless cause a broad range of diseases from various diarrheal diseases to extra-intestinal diseases, and particularly urinary tract infections and bacteraemia(Russo & Johnson, 2003, Kaper et al., 2004). The gut is considered to be the reservoir of strains causing these extra-intestinal pathologies, and both epidemiological and experimental studies have led to the notion that virulence could be a by-product of commensalism(Nowrouzian et al., 2005, Le Gall et al., 2007, Diard et al., 2010).
Molecular typing, based onmultilocus sequence typing (MLST), various PCR based approaches and, more recently, whole genome sequencing, has provided a good understanding of the phylogenetic framework of the E. coli species(Clermont et al., 2015). The species E. colisensu strictois largely clonal(Desjardins et al., 1995), with seven main phylogenetic groups (A, B1, B2, C, D, E, and F) each composed of several clones/clonal complexes or sub-groups(Clermont et al., 2013). Escherichia clades have been described: they are phenotypically in distinguishable from E. coli but the nucleotide sequences are highly divergent, with clade I strains being the most closely related to E. coli sensu stricto(Walk et al., 2009). Membership of these phylogenetic entities is of particular interest, as there is a relationship between the genetic background of astrain and its virulence factors(VFs) (Escobar-Paramo et al., 2004). For example, some sequence types (STs) within the B2 phylogroup, as ST137, includes trains with numerous extra-intestinal genes and high virulence potential(Messika et al., 2012).
Recently, there have been some major shifts in the epidemiology of E. coli. Some clonal complexes, such as ST complex (STc)69, STc73, STc95 and STc131, have become more prevalent in extra-intestinal diseases(Bidet et al., 2007, Bert et al., 2010, Mahjoub-Messai et al., 2011, Bengtsson et al., 2012, Gibreel et al., 2012, Alhashash et al., 2013, Clermont et al., 2014). Some of these clonal complexes have members that spread resistance to various antibiotics such as the cotrimoxazole [clonal group A (CGA) or STc69](Manges et al., 2001)and third generation cephalosporins [ST131 producing extended-spectrum beta-lactamase (ESBL)](Nicolas-Chanoine et al., 2014). The gut has been found to be at the hub of this resistance (Carlet, 2012) and a major driver of the spread of antibiotic resistance in the community (Woerther et al., 2013).
In view of these major changes to E. coli epidemiology, it would be valuable to have recent and reliable data on human commensal strains. Unfortunately, such data are scarce and most relevant studies are based on specific populations, like hospitalised patients or travellers, and many are focused on resistant strains. We report a study of a collection of 279E. coli strains gathered from adult subjects living in the community in the Paris area(hereafter referred to as the “COLIVILLE” collection). We compared these strains to collections of strains gathered in the same conditions 30(Duriez et al., 2001) and 10(Escobar-Paramo et al., 2004)years ago by our group.
MATERIALS AND METHODS
Subjects of the COLIVILLE collection
Subjects were recruited by general practitioners of the Department of General Practice of the University Paris Diderot from the region of Ile-de-France (Paris, France, and its suburban area) from May 2009 to December 2011. All participants lived in the community and volunteered to self-collect a faecal swab sample. The inclusion criteria were: age of 18 years or more, no history of gastrointestinal disease, no symptoms of immunosuppression, no antibiotic therapy in the previous month and no hospitalisation in the 3 months preceding inclusion. Written informed consent was obtained from each participant, and the study was approved by the ethics evaluation committee of Institut National de la Santé et de la Recherche Médicale (INSERM) (CCTIRS no. 09.243, CNIL no. 909277, and CQI no. 01-014).
Isolation and characterisation of the COLIVILLE strains
Faecal samples were self-collected by the subjects. Immediately after stool emission, a swab was dipped in to the faeces, put in Amies transport medium (Medical Wire & Equipment Corsham, Wiltshire England) and sent by mail to the Avicenne hospital laboratory (Bobigny, France)(Smati et al., 2013). The swabs were plated onto Drigalski agar plates upon arrival (Bio-Rad, Life Science, Marnes-la-Coquette, France). After 24 hours of incubation at 37°C, the plates were inspected for the phenotypic aspect of the lactose-positive colonies. In most cases, only one type of colony was recovered. However, in some cases, two co-dominant types of colony were retrieved. One colony, or if appropriate two phenotypically distinct colonies, were randomly picked, allowing the recovery of the dominant E. coli clones of the commensal faecal microbiota from each subject(Smati et al., 2013). These isolates were identified as E. coli using API 20E (bioMérieux, Marcy l’Etoile, France), and stored in glycerol stock solution at −80°C.
E. coli genotyping
DNA was extracted from colonies of each strain using the Wizard genomic DNA purification kit (Promega, France) following the manufacturer instructions. The E. coli phylogroup (A, B1, B2, C, D, E, F) and Escherichia clade of each isolate were determined by the PCR quadruplex (Clermont et al., 2013)and aes and chuA allele-specific amplification (Clermont et al., 2011) methods, respectively. PCR-based methods were used to identify strains of CGA clonal complex (STc69) within the D phylogroup (Johnson et al., 2004)and to classify the B2 phylogroup strains into the 10 main subgroups (I to X)(Clermont et al., 2014, Clermont et al., 2015). Strains were screened for the presence of 20VFs representative of the main classes of E. coli extra intestinal virulence determinants known, including adhesins (iha, papC, hra, sfa/foc, papGII and papGIIIalleles of papG, and ibeA), iron capture systems (irp2, fyuA, iucC, iroNand ireA), protectins (ompT, traT and neuC, chromosomal) and toxins (usp, sat, clbQ, hlyC, and cnf1) (Johnson et al., 2008, Clermont et al., 2011). For each isolate, a virulence score, defined as the number of the 20 VFs tested that were present in that strain, was calculated [adapted from (Lefort et al., 2011)].
O-typing
Classical determination of O antigens was carried out according to Guinée & Jansen (1981), with all O (O1 to O181) antisera available. Subtyping of O2, O6, O25 and O45 types was performed by PCR as previously described (Clermont et al., 2011).
Antibiotic resistance phenotypes
The strains were tested for their antibiotic susceptibilities using adisk diffusion method according to the recommendations of the Antimicrobial Committee of the French Society for Microbiology(http://www.sfm-microbiologie.org). The following antimicrobial agents were tested: amoxicillin, amoxicillin-clavulanic acid, cefoxitin, cefotaxime, ertapenem, imipenem, streptomycin, gentamicin, tobramycin, netilmicin, amikacin, tetracycline, cotrimoxazole (sulfamethoxazole-trimethoprim), chloramphenicol, nalidixic acid, ofloxacin, fosfomycin, and nitrofurantoin. A strain was considered to be multiresistant if it was resistant to, at least, penicillins, cotrimoxazole and quinolones (Lefort et al., 2011). For each isolate, a resistance score was computed. The resistance score was defined as the number of antibiotic classes to which thestrain was resistant, among the ten tested (penicillins, cephalosporins, carbapenems, aminoglycosides, tetracyclines, sulfonamides, amphenicols, quinolones, phosphonic acids, furans).
Characteristics of other collections of commensal strains
Two previously published collections of human commensal strains isolated from the Paris area were studied for comparison. The criteria of selection of the subjects and protocols of E. coli isolation were similar to those of the COLIVILLE collection, except for the number of isolates studied for each individual. The VDG collection was from subjects sampled in 1980 and included 53 strains from 53 subjects (Duriez et al., 2001)(one strain per individual); the AEM collection was obtained in 2000 and included 44 strains from 27 subjects(Escobar-Paramo et al., 2004) (1 to 4 strains per individual). The phylogroup/subgroup membership, the presence of 10 VFs representative of the four main classes (sfa/foc, papC, iroN, iucC, fyuA, neuC, traT, hlyC, cnf1 and usp), and the susceptibility to seven antibiotics (amoxicillin, amoxicillin-clavulanic acid, streptomycin, chloramphenicol, cotrimoxazole, tetracycline and nalidixic acid)of these strains were determined as above. For all the strains of the three collections, we calculated a ‘reduced’ virulence score, defined as the number of the 10 tested VFs present in the strain.
Statistical analyses
Study of the COLIVILLE strain determinants
Several bacterial determinants were compared between phylogroups using Fisher exact tests and analyses of variance. Fisher exact tests were used to test associations between phylogroup and O-type and between phylogroup and VFs. Associations were tested for the most numerous O-types (10 or more strains), and all the studied VFs. Associations between the most frequent antibiotic resistances (at least five resistant strains) were similarly tested.
To evaluate the strength of association between each VF and B2 group strains, Fisher's exact test was used to compare the distribution of each VF between B2 group and all the other groups combined. As multiple tests were performed, the p values were adjusted using the Benjamini and Hochberg method(Benjamini & Hochberg, 1995). Virulence and resistance scores were compared between phylogroups by analyses of variance; if significant, Tuckey’s test was used for pairwise comparisons between phylogroups. Virulence scores were compared between B2 subgroups in a similar way. The Pearson correlation test was used to assess the relationship between virulence and resistance scores. The resistance scores of the clonal group A strains and the other phylogroup Dstrains were compared using a Wilcoxon-Mann-Whitney test.
Comparison with other commensal collections
The proportion of phylogenetic groups and of resistant strains were compared between the three Parisian collections (VDG, AEM and COLIVILLE) by Fisher exact tests. The ‘reduced’ virulence scores of the three collections were compared using a linear regression model with the date of sampling and the phylogenetic group as predictors.
All statistical analyses were performed using R software (R version 3.0.2). Data are shown as means with standard deviations (SD) for continuous variables and number and percentage for categorical variables. All tests were two-sided with a 5% type I error.
RESULTS
COLIVILLE subject population and strains
Two hundred and forty four subjects fulfilling our inclusion criteria were enrolled; there were 111 men and 133 women. The mean age was 57 years (11.4): 57 years for the men (12.5) and 56 years for the women(10.3). The dominant E. coli clone was isolated from the stools of these participants by plating on selective Drigalski medium. E. coli colonies were recovered from all subjects. For 208 subjects, colonies were phenotypically homogeneous and one was randomly isolated for study. For 36 subjects, there were two phenotypically distinct types of colonies, and one representative of each type was isolated and studied. Thus, 280 strains were isolated and phenotypically identified as E. coli using API 20E; 279 from 243 subjects were identified as E. coli sensu stricto by the quadruplex PCR assay (Clermont et al., 2013) and one strain from a subject exhibiting phenotypically homogeneous colonies was identified as Escherichia clade IV (Clermont et al., 2011)(Table S1). This last subject was not included in the subsequent analyses.
Phylogenetic group/subgroup, O-type and VF distribution
The PCR quadruplex method was used to classify all isolates among the seven main phylogroups. Group B2 was the most frequently recovered (34.0%), followed by group A (28.7%) and the group B1 (12.9%); members of groups F (9.7%), D (9.0%), C (3.2%) and E (2.5%) were less numerous(Table 1). Many of the group B2 strains were in four sub-groups: sub-group II (STc73) (22.1% of the B2 group), sub-group IV (STc141) (21.1%), sub-group IX (STc95) (16.8%) and sub-group I (STc131) (13.7%) (Table 1). Four sub-group Istrains belonged to ST131. Only 7.3% of the B2 strains were not assigned to a sub-group by our PCR-based assay (Clermont et al., 2014). Forty-eight % of the D strains belonged to CGA (STc69).
TABLE 1.
Phylogenic, virulence and resistance characteristics of the 279 E. coli strains of the COLIVILLE collection
| Phylogenetic group/subgroup | Strains N (%) | Mean of virulence score * (± SD) | Resistant strains † N (%) | Mean resistance score ‡ (± SD) |
|---|---|---|---|---|
| A | 80 (28.7) | 2.5 (± 2.5) | 28 (35.0) | 0.9 (± 1.5) |
| B1 | 36 (12.9) | 2.3 (± 2.1) | 17 (47.2) | 1.0 (± 1.4) |
| B2 | 95 (34.0) | 10.4 (± 3.0) | 43 (45.3) | 0.9 (± 1.3) |
| I § | ||||
| ST131 | 4 (4.2 ||||) | 7.8 (± 1.3) | 2 (50.0#) | 1.3 (± 1.5) |
| Non-ST131 | 9 (9.5) | 7.2 (± 1.9) | 8 (88.9) | 1.6 (± 1.7) |
| II | 21 (22.1) | 12.5 (± 2.3) | 8 (38.1) | 0.6 (± 1.0) |
| III | 2 (2.1) | 11.5 (± 0.7) | 1 (50.0) | 0.5 (± 0.7) |
| IV | 20 (21.1) | 10.7 (± 3.0) | 7 (35.0) | 0.6 (± 1.1) |
| V | 1 (1.1) | 13.0 | 1 (100.0) | 3.0 |
| VI | 3 (3.2) | 13.7 (± 0.6) | 2 (66.7) | 1.7 (± 2.1) |
| VII | 6 (6.3) | 10.0 (± 1.7) | 3 (50.0) | 0.5 (± 0.6) |
| VIII | 5 (5.2) | 5.8 (± 1.3) | 4 (80.0) | 1.0 (± 0.7) |
| IX | 16 (16.8) | 11.2 (± 1.3) | 4 (25.0) | 0.4 (± 0.9) |
| X | 1 (1.1) | 4.0 | 1 (100.0) | 2.0 |
| Unassigned | 7 (7.3) | 10.0 (± 3.7) | 4 (57.1) | 1.7 (± 2.0) |
| C | 09 (3.2) | 5.2 (± 2.0) | 08 (88.9) | 2.9 (± 1.8) |
| D | 25 (9.0) | 4.9 (± 3.2) | 14 (56.0) | 1.7 (± 2.0) |
| CGA** | 12 (48.0) | 5.7 (± 3.2) | 10 (83.3) | 2.7 (± 1.7) |
| Non-CGA | 13 (52.0) | 4.2 (± 3.1) | 4 (30.8) | 0.9 (± 1.8) |
| E | 07 (2.5) | 3.6 (± 1.9) | 02 (28.6) | 0.6 (± 1.1) |
| F | 27 (9.7) | 6.9 (± 3.8) | 13 (48.1) | 1.2 (± 1.6) |
| Total | 279 (100.0) | 5.9 (± 4.5) | 125 (44.8) | 1.1 (± 1.5) |
Virulence score, defined as the number of virulence genes present in the strain, among the 20 tested.
Resistant strain, defined as a strain resistant to at least one antimicrobial agent among the 18 tested.
Resistance score, defined as the number of antibiotic classes (penicillins, cephalosporins, carbapenems, aminoglycosides, amphenicols, sulfonamides, tetracyclines, quinolones, furans, phosphonic acids) to which the strain is resistant among the 10 tested.
The subgroups I, II, III, IV, V, VI, VII, VIII, IX and X correspond to the STc131, 73, 127, 141, 144, 12, 14, 452, 95 and 372, respectively (Clermont et al., 2015). ST131, sequence type 131.
Proportions of subgroups are reported as fractions of the respective phylogroups.
proportions of resistant strains within the subgroup.
CGA, clonal group A (STc69).
The standard O-typing assay failed to assign 22 strains to an O-type. Among the 257 typeable strains, 75 O-types were detected, 28 represented by single strains and 47 by at least two strains. There were at least 10 strains in each of five O-types: O6a, O1, O2b, O9 and O18. There was an association between O-type and phylogenetic group:most of the O6a, O1 and O2b strains belonged to the B2, F and B2 groups, respectively (p value= 2x10−9), and most non-typable strains belonged to the A group. Three of the ST131 strains belonged to the ST131-O25b clone and one belonged to the ST131-O16 clone. Most strains of the B2 subgroups II, IV, VI, VII and VIII were O6a-, O2b-, O4-, O75-, and O81-types, respectively, whereas the B2 subgroup I and IX strains were more heterogeneous (I: O25b, O9; IX: O1, O18, O2a). In the phylogroup D, CGA strains were O17 and O77 types, and these types were not detected among non-CGA strains (Table S1).
The 279 strains were screened for 20 VF genes. VF genes involved in iron acquisition and protectins were more frequent than those for adhesins and toxins (Table 2). Three VF genes were found in more than half of the strains: irp2 (60.9%) and fuyA (60.2%) both belonging to the high pathogenicity island (HPI) and involved in iron acquisition (Schubert et al., 2009), and the protectin gene ompT (58.4%) (Table 2). The carriage of each of the 20 tested virulence factors differed significantly between phylogroups (global adjusted p values ≤0.005, Table 2). Seventeen VF genes were significantly more prevalent among phylogroup B2 strains than among other strains (adjusted p values ≤0.002), whereas the three other (iha, traT and sat) were not(Table 2). Three VFs (papGIII, clbQ and cnf1) were found exclusively in the B2 strains.
TABLE 2.
Virulence traits of the 279 E. coli strains of the COLIVILLE collection, with respect to the phylogenetic group of the strains
| Virulence trait | Phylogenetic groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Function | Strains N (%) | A (n = 80) | B1 (n = 36) | B2 (n = 95) | C (n = 9) | D (n=25) | E (n = 7) | F (n = 27) | Global adjusted P values | Adjusted P values B2 versus other groups | |
| iha | Adhesin | 94 (33.7) | 18 | 7 | 35 | 1 | 12 | 5 | 16 | 0.0004 | 0.4 |
| papC | Adhesin | 65 (23.3) | 4 | 1 | 42 | 1 | 4 | 0 | 13 | 9×10−12 | 2 × 10−8 |
| hra | Adhesin | 61 (21.9) | 11 | 5 | 36 | 2 | 6 | 1 | 0 | 7 × 10−5 | 9 × 10−6 |
| sfa/foc | Adhesin | 51 (18.3) | 1 | 1 | 46 | 0 | 0 | 3 | 0 | 6 × 10−16 | 5 × 10−16 |
| papGII* | Adhesin | 32 (11.5) | 0 | 0 | 22 | 0 | 4 | 0 | 6 | 6 × 10−7 | 5 × 10−5 |
| ibeA | Adhesin | 30 (10.8) | 0 | 0 | 29 | 0 | 1 | 0 | 0 | 2 × 10−10 | 5 × 10−14 |
| papGIII* | Adhesin | 21 (7.5) | 0 | 0 | 21 | 0 | 0 | 0 | 0 | 4 × 10−7 | 5 × 10−11 |
| irp2 | Iron acquisition | 170 (60.9) | 28 | 10 | 88 | 8 | 15 | 0 | 21 | 6 × 10−16 | 5 × 10−16 |
| fyuA | Iron acquisition | 168 (60.2) | 27 | 9 | 88 | 8 | 15 | 0 | 21 | 6 × 10−16 | 5 × 10−16 |
| iucC | Iron acquisition | 128 (45.9) | 26 | 7 | 58 | 4 | 14 | 5 | 14 | 6 × 10−5 | 5 × 10−4 |
| iroN | Iron acquisition | 95 (34.1) | 5 | 7 | 71 | 4 | 3 | 3 | 2 | 6 × 10−16 | 5 × 10−16 |
| ireA | Iron acquisition | 57 (20.4) | 12 | 3 | 30 | 4 | 2 | 2 | 4 | 0.005 | 0.002 |
| ompT | Protectin | 163 (58.4) | 12 | 14 | 88 | 8 | 15 | 5 | 21 | 6 × 10−16 | 5 × 10−16 |
| traT | Protectin | 139 (49.8) | 27 | 19 | 51 | 6 | 16 | 1 | 19 | 0.002 | 0.4 |
| neuC | Protectin | 66 (23.7) | 9 | 0 | 41 | 0 | 1 | 0 | 15 | 2 × 10−12 | 10−7 |
| usp | Toxin | 106 (38.0) | 1 | 0 | 90 | 0 | 0 | 0 | 15 | 6 × 10−16 | 5 × 10−16 |
| sat | Toxin | 72 (25.8) | 15 | 1 | 25 | 1 | 12 | 0 | 18 | 5 × 10−8 | 0.9 |
| clbQ | Toxin | 55 (19.7) | 0 | 0 | 55 | 0 | 0 | 0 | 0 | 6 × 10−16 | 5 × 10−16 |
| hlyC | Toxin | 41 (14.7) | 0 | 0 | 39 | 0 | 2 | 0 | 0 | 2 × 10−15 | 5 × 10−16 |
| cnf1 | Toxin | 33 (11.8) | 0 | 0 | 33 | 0 | 0 | 0 | 0 | 5 × 10−13 | 5 × 10−16 |
Distinct alleles of the papG virulence gene.
The virulence score for the strains was from 0 to 17, with a mean of 5.9(4.5) (Table 1). Virulence score differed significantly between the phylogroups (p value=10−16). The virulence score of the B2 strains was significantly higher than those of the A, B1, C, D, E and F strains (p value= 10−13, 10−13, 10−6, 10−13,10−8 and 10−7, respectively). D strains had a significantly higher virulence score than A and B1 strains (p value= 0.004 and 0.01, respectively) and F strains had a significantly higher virulence score than A strains (p value= 10−10). The virulence score was significantly different between subgroups of the B2 group (p value=10−5): subgroups II, IV and IX had significantly higher virulence scores, with means of 12.5 (2.3), 10.7 (3.0) and 11.2 (1.3), respectively, than the sub-group I with mean 7.2 (1.9) (p value= 10−5, 0.004 and 0.001, respectively).
Antibiotic resistance phenotype
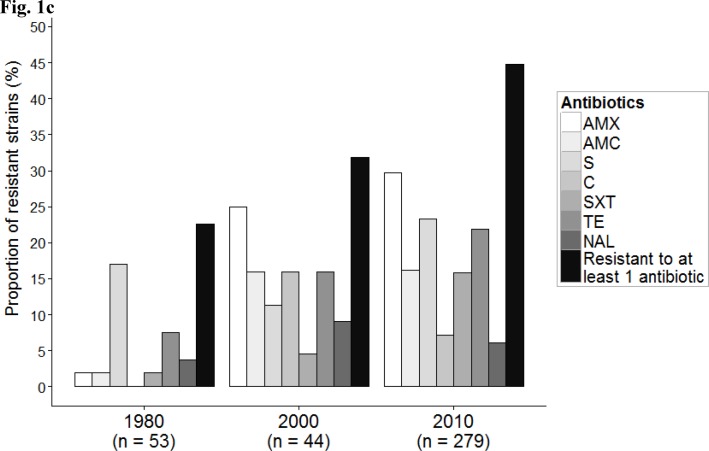
Resistance to one or more of the 18 tested antibiotics was found in 125 (44.8%) of the 279 strains (Table 1). Only 6 strains(2.2%) were multi resistant, i.e. resistant to, at least, amoxicillin, cotrimoxazole and nalidixic acid. Overall, 29.7% of the strains were resistant to amoxicillin and 16.1% to amoxicillin-clavulanic acid (Table 3). The only strain resistant tocefotaxime did not produce an ESBL but had a plasmid-encoded CMY-2 cephalosporinase (data not shown). No strain was resistant to carbapenems. Among the aminoglycosides, 23.3% of the strains were resistant to streptomycin and only 0.7%, 0.7% and 0.4% of the strains were resistant to gentamicin, tobramycin and netilmicin, respectively(Table 3). Resistance to tetracycline was found in 21.9% of the strains, to cotrimoxazole in 15.8% and to chloramphenicolin 7.2%;6.1% of strains were resistant to nalidixic acid and 3.2% to ofloxacin. Significant associations were found between penicillin resistance and resistance to the following antibiotics: chloramphenicol, cotrimoxazole, streptomycin, and tetracycline (adjusted p values<0.05). Associations between resistances to chloramphenicol, cotrimoxazole, streptomycin, and tetracycline were also significant (adjusted p values<10−3). Resistance to nalidixic acid was significantly associated with resistance to amoxicillin-clavulanic acid, streptomycin, cotrimoxazole and chloramphenicol (p value= 0.049, 0.048, 0.049 and 0.007, respectively).
TABLE 3.
Distribution of resistance to 18 antibiotics in the COLIVILLE collection (n = 279)
| Antibiotic class | Antibiotic | Strains N (%) |
|---|---|---|
| Penicillins | Amoxicillin | 83 (29.7) |
| Amoxicillin/clavulanic acid | 45 (16.1) | |
| Cephalosporins | Cefoxitin | 3 (1.1) |
| Cefotaxime | 1 (0.4) | |
| Carbapenems | Ertapenem | 0 (0.0) |
| Imipenem | 0 (0.0) | |
| Aminoglycosides | Streptomycin | 65 (23.3) |
| Gentamicin | 2 (0.7) | |
| Tobramycin | 2 (0.7) | |
| Netilmicin | 1 (0.4) | |
| Amikacin | 0 (0.0) | |
| Tetracyclines | Tetracycline | 61 (21.9) |
| Sulfonamides | Co-trimoxazole | 44 (15.8) |
| Amphenicols | Chloramphenicol | 20 (7.2) |
| Quinolones | Nalidixic acid | 17 (6.1) |
| Ofloxacin | 9 (3.2) | |
| Phosphonic acids | Fosfomycin | 1 (0.4) |
| Furans | Nitrofurantoin | 1 (0.4) |
Resistance scores were between 0 and 6, with a mean of 1.1(1.5)(Table 1). There was a significant difference of the resistance score between phylogroups (p value= 0.001). The resistance score of the C strains was significantly higher than those of the A, B1, B2, E and F strains (p value= 0.002, 0.02, 0.002, 0.03 and 0.03, respectively). The mean resistance score of the group D strains was 1.7(2.0); the mean resistance score of CGA strains was 2.7(1.7) and was significantly higher than the mean score of other group D strains, which was 0.9(1.8) (p value=0.009).
For the 279 strains as a whole, there was no significant correlation (Pearson coefficient= 0.06, p value=0.3; adjusted R2 of linear regression= 0.0006, p value= 0.3) between the virulence and the resistance scores.
Comparison with other collections of commensal E.coli
We compared the COLIVILLE collection to collections obtained in 1980 (VDG) and 2000 (AEM) in the same area. The most apparent observation was the increase in the proportion of group B2 strains from 9.4% in 1980 to 22.7% in 2000 and 34.0% in 2010, associated with a parallel decrease in the proportion of phylogroup A strains (52.8%, 27.3% and 28.7%, respectively, p value= 0.0002) (Fig. 1a). No CGA strain was evidenced in the VDG and AEM collections. The ‘reduced’ virulence score has increased significantly over time, from a mean of 1.5 (2.0) in 1980, to 2.3 (2.2) in 2000 and 3.2 (2.6) in 2010 (Fig. 1b). Our model indicated that this increase was due to the change in the phylogenetic distribution between 1980 and 2010 (effects of date of sampling were not significant), and in particular the increase in the proportion of the group B2 strains and the decrease in that of the A group strains. A more thorough analysis of the few B2 strains available from the VDG and AEM collections showed that classical extra-intestinal pathogenic E. coli(ExPEC) B2 subgroup strains (subgroup II/O6a and O22, subgroup III/O6a and subgroup IX/O1) with numerous VFs were already present (Table S2).
Figure 1.
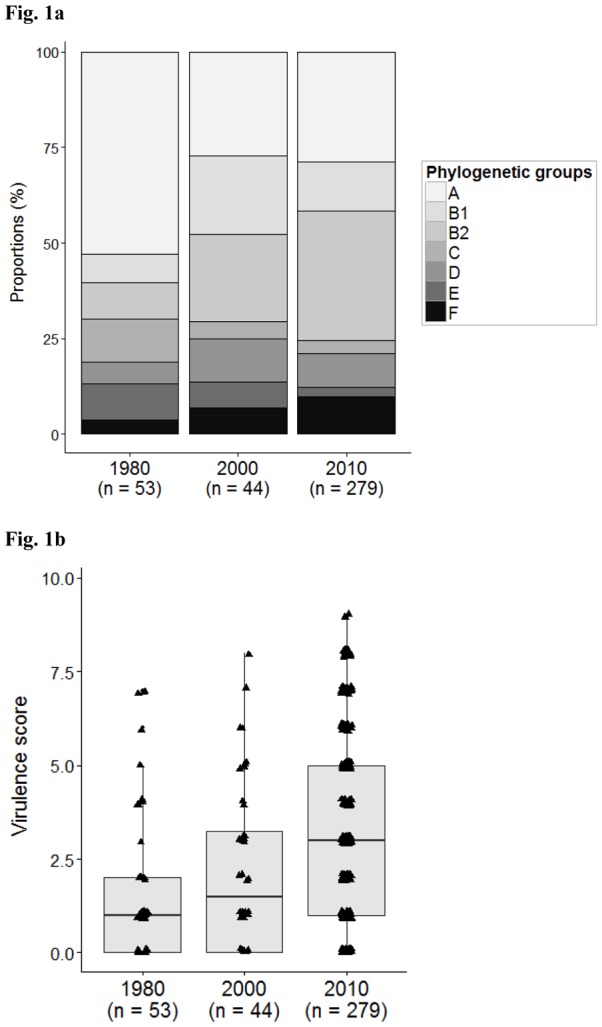
Phenotypic and genotypic characteristics of E. coli strains in each of the three collections. The COLIVILLE collection is the population isolated in (and labelled)“2010”. The VDG (“1980”) and AEM (“2000”) collections are from Duriez et al.(2001) and Escobar-Paramo et al. (2004), respectively.(a) Results are presented as a proportional stacked bar graph representing the phylogenetic distribution of E. coli. (b)Distribution of the ‘reduced’ virulence score. The black bars within each box plot show median values. The box covers the 25th percentile to the 75th percentile of the data. Bars above and below the box show 1.5 times the inter-quartile range. Dots located at some distance outside the bars correspond to outliers. (c)Histograms representing prevalence of resistance to amoxicillin (AMX), amoxicillin-clavulanic acid (AMC), streptomycin (S), chloramphenicol (C), cotrimoxazole (SXT), tetracycline(TE) and nalidixic acid (NAL), and antibiotic resistant strains.
We also studied the evolution of antibiotic resistance in these populations (Fig. 1c). The proportion of strains resistant to at least one antibiotic increased from 22.6% in 1980 to 31.8% in 2000 and 44.8% in 2010(p value=0.005). We observed the emergence of the resistance to amoxicillin and amoxicillin-clavulanic acid to attain 29.7% and 16.1%, respectively, by 2010. A similar trend was observed for cotrimoxazole and tetracycline whereas the resistance to streptomycin remained generally stable.
DISCUSSION
A major issue when isolating commensal strains of an opportunistic pathogenic species, such as E. coli, is the quality of the epidemiological data available for the human host. We therefore applied very strict inclusion criteria for the subjects, excluding individuals with any antibiotic therapy in the previous month and any hospitalisation in the 3 months preceding inclusion; the subjects were all epidemiologically unrelated and were recruited in the same geographic area and over a short period of time. To our knowledge, this is the largest series of “true” human commensal strains to be thoroughly studied, with data including population genetics, virulence and antibiotic resistance data. This collection will be useful to the scientific community for subsequent comparison purposes (Table S1).
The high frequency of the phylogroup B2 strains in 2010 (Table 1) is consistent with recent descriptions of human commensal strains in industrialised countries (Bailey et al., 2010, Bailey et al., 2010, Tenaillon et al., 2010). It contrasts with data obtained in developing countries where phylogroup A and B1 strains predominate (Unno et al., 2009, Bailey et al., 2010, Li et al., 2010, Tenaillon et al., 2010, Lescat et al., 2013, Tapader et al., 2014). Group A strains are the best adapted to a wide range of human hosts, whatever their lifestyle, diet and hygiene status. Our study is one of the first to use the quadruplex PCR assay (Clermont et al., 2013) allowing the identification of the minor phylogenetic groups C, E and F. Few group C and E strains were found (3.2% and 2.5%, respectively) whereas almost 10% of strains were group F, such that they were more numerous than group D strains (9.0%). Half of the group D strains were CGA strains, a clonal group including ExPEC(Manges et al., 2001). Only one Escherichia clade was found, confirming the rarity of other Escherichia clades in human (Clermont et al., 2011). We did not find any significant correlation between the phylogroup distribution and the age and or sex of the subjects (data not shown), contrary to observations in Australia(Gordon et al., 2005). We compared our 2010 collection to previous collections from the Paris area. The frequency of isolation of phylogroup B2 strains has increased with a parallel decrease of that of phylogroup A strains (Fig. 1a); this may be a consequence of modifications in the lifestyle of the Parisian population including changing food processing and hygiene procedures. Conversely, the prevalence of the minor groups (C, E and F) has remained stable over time and maybe less sensitive to these changes. The presence of VFs is linked to phylogenetic group membership, a well-known phenomenon (Escobar-Paramo et al., 2004), and we observed an increase of the number of VFs with time, paralleling the changes in phylogroup distribution (Fig. 1b).
Group B2 has various sub-groups or clonal complexes, three of them (sub-group I=STc131; sub-group II=STc73; sub-group IX=STc95) containing most ExPEC strains (Bengtsson et al., 2012, Gibreel et al., 2012, Alhashash et al., 2013, Clermont et al., 2014). We found numerous strains of sub-groups II and IX among the commensal B2 strains, with the classical ExPEC O-types (i.e. sub-group II/O6a and sub-group IX/O1-O18-O2a) whereas few sub-group I strains, also frequently recovered among commensal strains, were the classical ST131/O25b-O16 clones (Johnson et al., 2014). We also found many B2 sub-group IV (STc141) strains exhibiting the O2b type. It is therefore possible that two types of B2 strains in the human microbiota can be distinguished: the classical ExPEC clones that have both the property of being highly virulent and the property of being good gut colonizers, the extra-intestinal virulence being a by-product of commensalism (Nowrouzian et al., 2005, Le Gall et al., 2007, Diard et al., 2010);and the commensal-specific strains, as has been reported for the O81 B2 sub-group VIII (STc452) strains (Clermont et al., 2008).
Overall, the strains we isolated exhibited a low level of antibiotic resistance (Table 1) relative to French clinical strains isolated over the same period (http://www.onerba.org). For example, resistances to amoxicillin, cefotaxime, gentamicin, cotrimoxazole and fluoroquinolones were 51.8%, 7.6%, 5.6%, 23.9% and 14.4%, respectively, for clinical strains in 2010, but only 29.7%, 0.4%, 0.7%, 15.8%, 6.1%, respectively, in the COLIVILLE collection. This low prevalence of resistance in our collection argues indirectly for the “true” commensal character of the strains. Beside the well-known CGA(Manges et al., 2001), our work identifies, for the first time, phylogroup C as apotential reservoir of resistance among commensal strains(Table 1). A strain of this phylogroup producing an ESBL was responsible for an extensive outbreak of digestive colonisation in a neonatal ward with one case of meningitis (Moissenet et al., 2010).
Although antibiotic resistance in our 2010 collection was less prevalent than among clinical strains (see above), it was higher than in the earlier similar collections (Fig. 1c). This is consistent with a recent study in a Parisian check-up centre where a 10-fold increase of ESBL-producing E. coli strains was observed between 2006 and 2011(Nicolas-Chanoine et al., 2013). These effects are probably due to an increase in the exposure of the general population to antibiotic pressure (Woerther et al., 2013). Contrasting with this trend of increasing resistance, the prevalence of resistance to streptomycin appears to be stable through time in the Parisian population. A similar level of resistance to streptomycin (23%) was reported for E. coli strains isolated in England in 1991 (Chiew et al., 1998). Streptomycin therapy was frequent in the 1950s and 1960s but is not now widely used, and it largely restricted to tuberculosis. Thenon-disappearance of resistance to an (almost) unused antibiotic may be because the genes conferring this resistance are borne by genetic structures, including transposons and integrons, that possess other antibiotic resistance genes (Chiew et al., 1998). Indeed, we found an association in our collection between resistance to streptomycin and resistance to cotrimoxazole and chloramphenicol, all these resistances being mediated by co-localised integron-borne genes(Partridge et al., 2009).
In conclusion, our data indicates that commensal human E. coli population sevolve substantially over time and that precise and repeated characterisations are required to document the emergence of virulent and/or resistant strains in the community.
Acknowledgments
This work was partially supported by the grant CN2012/303(Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia and The European Regional Development Fund, ERDF).
ABBREVIATIONS
- CGA
Clonal Group A
- ExPEC
Extraintestinal Pathogenic Escherichia coli
- STc
Sequence Type complex
- ST
Sequence Type
- VF
Virulence Factor
- MLST
Multilocus Sequence Typing
- ESBL
Extended Spectrum Beta-Lactamase
- SD
Standard Deviation
- HPI
High Pathogenicity Island
References
- Alhashash F, Weston V, Diggle M, McNally A. Multidrug-resistant Escherichia coli bacteremia. Emerg Infect Dis. 2013;19:1699–1701. doi: 10.3201/eid1910.130309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JK, Pinyon JL, Anantham S, Hall RM. Commensal Escherichia coli of healthy humans: a reservoir for antibiotic-resistance determinants. J Med Microbiol. 2010;59:1331–1339. doi: 10.1099/jmm.0.022475-0. [DOI] [PubMed] [Google Scholar]
- Bailey JK, Pinyon JL, Anantham S, Hall RM. Distribution of human commensal Escherichia coli phylogenetic groups. J Clin Microbiol. 2010;48:3455–3456. doi: 10.1128/JCM.00760-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson S, Naseer U, Sundsfjord A, Kahlmeter G, Sundqvist M. Sequence types and plasmid carriage of uropathogenic Escherichia coli devoid of phenotypically detectable resistance. J Antimicrob Chemother. 2012;67:69–73. doi: 10.1093/jac/dkr421. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300. [Google Scholar]
- Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4:430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- Bert F, Johnson JR, Ouattara B, Leflon-Guibout V, Johnston B, Marcon E, Valla D, Moreau R, Nicolas-Chanoine MH. Genetic diversity and virulence profiles of Escherichia coli isolates causing spontaneous bacterial peritonitis and bacteremia in patients with cirrhosis. J Clin Microbiol. 2010;48:2709–2714. doi: 10.1128/JCM.00516-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidet P, Mahjoub-Messai F, Blanco J, Dehem M, Aujard Y, Bingen E, Bonacorsi S. Combined multilocus sequence typing and O serogrouping distinguishes Escherichia coli subtypes associated with infant urosepsis and/or meningitis. J Infect Dis. 2007;196:297–303. doi: 10.1086/518897. [DOI] [PubMed] [Google Scholar]
- Carlet J. The gut is the epicentre of antibiotic resistance. Antimicrob Resist Infect Control. 2012;1:39. doi: 10.1186/2047-2994-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew YF, Yeo SF, Hall LM, Livermore DM. Can susceptibility to an antimicrobial be restored by halting its use? The case of streptomycin versus Enterobacteriaceae. J Antimicrob Chemother. 1998;41:247–251. doi: 10.1093/jac/41.2.247. [DOI] [PubMed] [Google Scholar]
- Clermont O, Gordon D, Denamur E. Guide to the various phylogenetic classification schemes for Escherichia coli and the correspondence among schemes. Microbiology. 2015;161:980–988. doi: 10.1099/mic.0.000063. [DOI] [PubMed] [Google Scholar]
- Clermont O, Christenson JK, Denamur E, Gordon DM. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylogroups. Environ Microbiol Rep. 2013;8:58–65. doi: 10.1111/1758-2229.12019. [DOI] [PubMed] [Google Scholar]
- Clermont O, Gordon DM, Brisse S, Walk ST, Denamur E. Characterization of the cryptic Escherichia lineages: rapid identification and prevalence. Environ Microbiol. 2011;13:2468–2477. doi: 10.1111/j.1462-2920.2011.02519.x. [DOI] [PubMed] [Google Scholar]
- Clermont O, Christenson JK, Daubie AS, Gordon DM, Denamur E. Development of an allele-specific PCR for Escherichia coli B2 sub-typing, a rapid and easy to perform substitute of multilocus sequence typing. J Microbiol Methods. 2014;101:24–27. doi: 10.1016/j.mimet.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Clermont O, Lescat M, O’Brien CL, Gordon DM, Tenaillon O, Denamur E. Evidence for a human-specific Escherichia coli clone. Environ Microbiol. 2008;10:1000–1006. doi: 10.1111/j.1462-2920.2007.01520.x. [DOI] [PubMed] [Google Scholar]
- Clermont O, Olier M, Hoede C, Diancourt L, Brisse S, Keroudean M, Glodt J, Picard B, Oswald E, Denamur E. Animal and human pathogenic Escherichia coli strains share common genetic backgrounds. Infect Genet Evol. 2011;11:654–662. doi: 10.1016/j.meegid.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Desjardins P, Picard B, Kaltenbock B, Elion J, Denamur E. Sex in Escherichia coli does not disrupt the clonal structure of the population: evidence from random amplified polymorphic DNA and restriction-fragment-length polymorphism. J Mol Evol. 1995;41:440–448. doi: 10.1007/BF00160315. [DOI] [PubMed] [Google Scholar]
- Diard M, Garry L, Selva M, Mosser T, Denamur E, Matic I. Pathogenicity-associated islands in extraintestinal pathogenic Escherichia coli are fitness elements involved in intestinal colonization. J Bacteriol. 2010;192:4885–4893. doi: 10.1128/JB.00804-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duriez P, Clermont O, Bonacorsi S, Bingen E, Chaventre A, Elion J, Picard B, Denamur E. Commensal Escherichia coli isolates are phylogenetically distributed among geographically distinct human populations. Microbiology. 2001;147:1671–1676. doi: 10.1099/00221287-147-6-1671. [DOI] [PubMed] [Google Scholar]
- Escobar-Paramo P, Clermont O, Blanc-Potard AB, Bui H, Le Bouguenec C, Denamur E. A specific genetic background is required for acquisition and expression of virulence factors in Escherichia coli. Mol Biol Evol. 2004;21:1085–1094. doi: 10.1093/molbev/msh118. [DOI] [PubMed] [Google Scholar]
- Escobar-Paramo P, Grenet K, Le Menac’h A, et al. Large-scale population structure of human commensal Escherichia coli isolates. Appl Environ Microbiol. 2004;70:5698–5700. doi: 10.1128/AEM.70.9.5698-5700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibreel TM, Dodgson AR, Cheesbrough J, Fox AJ, Bolton FJ, Upton M. Population structure, virulence potential and antibiotic susceptibility of uropathogenic Escherichia coli from Northwest England. J Antimicrob Chemother. 2012;67:346–356. doi: 10.1093/jac/dkr451. [DOI] [PubMed] [Google Scholar]
- Gordon DM, Stern SE, Collignon PJ. Influence of the age and sex of human hosts on the distribution of Escherichia coli ECOR groups and virulence traits. Microbiology. 2005;151:15–23. doi: 10.1099/mic.0.27425-0. [DOI] [PubMed] [Google Scholar]
- Guinée PAM, Jansen WH. Escherichia coli associated with neonatal diarrhoea in piglets and calves. In: Leeww PM, Guinée PAM, editors. Laboratory diagnosis in neonatal calf and pig diarrhoea, current topics in veterinary and animal science. Martinus-Nijhoff; The Hague: 1981. pp. 126–162. [Google Scholar]
- Johnson JR, Owens K, Manges AR, Riley LW. Rapid and specific detection of Escherichia coli clonal group A by gene-specific PCR. J Clin Microbiol. 2004;42:2618–2622. doi: 10.1128/JCM.42.6.2618-2622.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JR, Johnston B, Kuskowski MA, Nougayrede JP, Oswald E. Molecular epidemiology and phylogenetic distribution of the Escherichia coli pks genomic island. J Clin Microbiol. 2008;46:3906–3911. doi: 10.1128/JCM.00949-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JR, Clermont O, Johnston B, Clabots C, Tchesnokova V, Sokurenko E, Junka AF, Maczynska B, Denamur E. Rapid and specific detection, molecular epidemiology, and experimental virulence of the O16 subgroup within Escherichia coli sequence type 131. J Clin Microbiol. 2014;52:1358–1365. doi: 10.1128/JCM.03502-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- Le Gall T, Clermont O, Gouriou S, Picard B, Nassif X, Denamur E, Tenaillon O. Extraintestinal virulence is a coincidental by-product of commensalism in B2 phylogenetic group Escherichia coli strains. Mol Biol Evol. 2007;24:2373–2384. doi: 10.1093/molbev/msm172. [DOI] [PubMed] [Google Scholar]
- Lefort A, Panhard X, Clermont O, Woerther PL, Branger C, Mentre F, Fantin B, Wolff M, Denamur E. Host factors and portal of entry outweigh bacterial determinants to predict the severity of Escherichia coli bacteremia. J Clin Microbiol. 2011;49:777–783. doi: 10.1128/JCM.01902-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescat M, Clermont O, Woerther PL, et al. Commensal Escherichia coli strains in Guiana reveal a high genetic diversity with host-dependant population structure. Environ Microbiol Rep. 2013;5:49–57. doi: 10.1111/j.1758-2229.2012.00374.x. [DOI] [PubMed] [Google Scholar]
- Li B, Sun JY, Han LZ, Huang XH, Fu Q, Ni YX. Phylogenetic groups and pathogenicity island markers in fecal Escherichia coli isolates from asymptomatic humans in China. Appl Environ Microbiol. 2010;76:6698–6700. doi: 10.1128/AEM.00707-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahjoub-Messai F, Bidet P, Caro V, Diancourt L, Biran V, Aujard Y, Bingen E, Bonacorsi S. Escherichia coli isolates causing bacteremia via gut translocation and urinary tract infection in young infants exhibit different virulence genotypes. J Infect Dis. 2011;203:1844–1849. doi: 10.1093/infdis/jir189. [DOI] [PubMed] [Google Scholar]
- Manges AR, Johnson JR, Foxman B, O’Bryan TT, Fullerton KE, Riley LW. Widespread distribution of urinary tract infections caused by a multidrug-resistant Escherichia coli clonal group. N Engl J Med. 2001;345:1007–1013. doi: 10.1056/NEJMoa011265. [DOI] [PubMed] [Google Scholar]
- Messika J, Magdoud F, Clermont O, Margetis D, Gaudry S, Roux D, Branger C, Dreyfuss D, Denamur E, Ricard JD. Pathophysiology of Escherichia coli ventilator-associated pneumonia: implication of highly virulent extraintestinal pathogenic Escherichia coli strains. Intensive Care Med. 2012;38:2007–2016. doi: 10.1007/s00134-012-2699-5. [DOI] [PubMed] [Google Scholar]
- Moissenet D, Salauze B, Clermont O, Bingen E, Arlet G, Denamur E, Merens A, Mitanchez D, Vu-Thien H. Meningitis caused by Escherichia coli producing TEM-52 extended-spectrum beta-lactamase within an extensive outbreak in a neonatal ward: epidemiological investigation and characterization of the strain. J Clin Microbiol. 2010;48:2459–2463. doi: 10.1128/JCM.00529-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas-Chanoine MH, Bertrand X, Madec JY. Escherichia coli ST131, an intriguing clonal group. Clin Microbiol Rev. 2014;27:543–574. doi: 10.1128/CMR.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas-Chanoine MH, Gruson C, Bialek-Davenet S, et al. 10-Fold increase (2006–11) in the rate of healthy subjects with extended-spectrum beta-lactamase-producing Escherichia coli faecal carriage in a Parisian check-up centre. J Antimicrob Chemother. 2013;68:562–568. doi: 10.1093/jac/dks429. [DOI] [PubMed] [Google Scholar]
- Nowrouzian FL, Wold AE, Adlerberth I. Escherichia coli strains belonging to phylogenetic group B2 have superior capacity to persist in the intestinal microflora of infants. J Infect Dis. 2005;191:1078–1083. doi: 10.1086/427996. [DOI] [PubMed] [Google Scholar]
- Partridge SR, Tsafnat G, Coiera E, Iredell JR. Gene cassettes and cassette arrays in mobile resistance integrons. FEMS Microbiol Rev. 2009;33:757–784. doi: 10.1111/j.1574-6976.2009.00175.x. [DOI] [PubMed] [Google Scholar]
- Russo TA, Johnson JR. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect. 2003;5:449–456. doi: 10.1016/s1286-4579(03)00049-2. [DOI] [PubMed] [Google Scholar]
- Schubert S, Darlu P, Clermont O, Wieser A, Magistro G, Hoffmann C, Weinert K, Tenaillon O, Matic I, Denamur E. Role of intraspecies recombination in the spread of pathogenicity islands within the Escherichia coli species. PLoS Pathog. 2009;5:e1000257. doi: 10.1371/journal.ppat.1000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smati M, Clermont O, Le Gal F, Schichmanoff O, Jaureguy F, Eddi A, Denamur E, Picard B. Real-time PCR for quantitative analysis of human commensal Escherichia coli populations reveals a high frequency of subdominant phylogroups. Appl Environ Microbiol. 2013;79:5005–5012. doi: 10.1128/AEM.01423-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapader R, Chatterjee S, Singh AK, Dayma P, Haldar S, Pal A, Basu S. The high prevalence of serine protease auto transporters of Enterobacteriaceae (SPATEs) in Escherichia coli causing neonatal septicemia. Eur J Clin Microbiol Infect Dis. 2014;33:2015–2024. doi: 10.1007/s10096-014-2161-4. [DOI] [PubMed] [Google Scholar]
- Tenaillon O, Skurnik D, Picard B, Denamur E. The population genetics of commensal Escherichia coli. Nat Rev Microbiol. 2010;8:207–217. doi: 10.1038/nrmicro2298. [DOI] [PubMed] [Google Scholar]
- Unno T, Han D, Jang J, Lee SN, Ko G, Choi HY, Kim JH, Sadowsky MJ, Hur HG. Absence of Escherichia coli phylogenetic group B2 strains in humans and domesticated animals from Jeonnam Province, Republic of Korea. Appl Environ Microbiol. 2009;75:5659–5666. doi: 10.1128/AEM.00443-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walk ST, Alm EW, Gordon DM, Ram JL, Toranzos GA, Tiedje JM, Whittam TS. Cryptic lineages of the genus Escherichia. Appl Environ Microbiol. 2009;75:6534–6544. doi: 10.1128/AEM.01262-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerther PL, Angebault C, Jacquier H, et al. Characterization of fecal extended-spectrum-beta-lactamase-producing Escherichia coli in a remote community during a long time period. Antimicrob Agents Chemother. 2013;57:5060–5066. doi: 10.1128/AAC.00848-13. [DOI] [PMC free article] [PubMed] [Google Scholar]