Abstract
Orexins/hypocretins exert cardiovascular effects which are centrally mediated. In the present study, we tested whether orexins and their receptors may also act in an autocrine/paracrine manner in the heart exerting direct effects. Quantitative reverse transcription-PCR (RT-PCR), immunohistochemical and Western blot analyses revealed that the rat heart expresses orexins and orexin receptors (OXR). In isolated rat cardiomyocytes, only orexin-B (OR-B) caused an increase in contractile shortening, independent of diastolic or systolic calcium levels. A specific orexin receptor-2 (OX2R) agonist ([Ala11, d-Leu15]-Orexin B) exerted similar effects as OR-B, whereas a specific orexin receptor-1 (OX1R) antagonist (SB-408124) did not alter the responsiveness of OR-B. Treatment of the same model with OR-B resulted in a dose-dependent increase in myosin light chain and troponin-I (TnI) phosphorylation. Following ischaemia/reperfusion in the isolated Langendorff perfused rat heart model, OR-B, but not OR-A, exerts a cardioprotective effect; mirrored in an in vivo model as well. Unlike OR-A, OR-B was also able to induce extracellular signal-regulated kinase (ERK) 1/2 (ERK1/2) and Akt phosphorylation in rat myocardial tissue and ERK1/2 phosphorylation in human heart samples. These findings were further corroborated in an in vivo rat model. In human subjects with heart failure, there is a significant negative correlation between the expression of OX2R and the severity of the disease clinical symptoms, as assessed by the New York Heart Association (NYHA) functional classification. Collectively, we provide evidence of a distinct orexin system in the heart that exerts a cardioprotective role via an OR-B/OX2R pathway.
Keywords: myocardial infarction, orexins, orexin receptors
Introduction
Cardiovascular disease (CVD), including myocardial infarction, is the leading cause of morbidity and mortality in the Western world [1,2]. Orexins, also referred to as hypocretins, exist in two functional forms, i.e. orexin A (OR-A) and orexin B (OR-B), which are derived from a common 130 amino acid precursor peptide [3,4] and exert their actions by activating the orexin-1 (OX1R) and orexin-2 (OX2R) receptors. OXRs belong to the G protein-coupled receptor (GPCR) superfamily [3] and are distributed throughout both central and peripheral sites where they regulate endocrine, metabolic and cardiovascular functions [5–7]. Of note, several established cardiovascular drugs exert their cardiac actions via GPCRs, such as the muscarinic, angiotensin, adrenergic, and endothelin receptors [8].
Growing evidence indicates that central orexin neurons are implicated in cardiovascular regulation [5]. For example, in the central nervous system (CNS), both orexin and OX2R-containing nerve fibres have been identified in the paraventricular nucleus (PVN) [9], an area which constitutes a vital central site for integration of sympathetic outflow and cardiovascular function [10–17]. Thus, although orexins can influence the autonomic control of the cardiovascular system, little is known about any direct effects of orexins in the heart.
We and others have provided evidence for a wide distribution of orexins and their receptors peripherally [18–20]. We have also demonstrated the presence of OXRs in human adipose tissue, where they modulate adipogenesis and adipose tissue metabolism [7]. Herein, we hypothesised that orexins may also exert direct cardiac effects and we sought to investigate the expression and function of cardiac OXRs using in vitro, ex vivo and in vivo models, corroborating this with clinical data.
Materials and methods
Drugs and experimental solutions
Study treatment agents were: OR-A, OR-B (Phoenix Peptides), OX2R agonist ([Ala11, d-Leu15]-OR-B; Tocris), OX1R antagonist (SB-408124; Tocris), wortmannin (PI3Kinase (PI3K) inhibitor; Sigma–-Aldrich) and mitogen-activated protein kinase (MAPK) inhibitor U0126 (Tocris). According to the manufacturer’s specifications on biological activity, the used OX2R agonist ([Ala11, d-Leu15]-OR-B; Tocris) is a ‘highly potent and selective OX2 receptor agonist; displays 400-fold selectivity over OX1 receptors. EC50 values are 0.13 and 52 nM for human OX2 and OX1 receptors respectively’. Similarly, the used OX1R antagonist (SB-408124) is a ‘selective non-peptide orexin OX1 receptor antagonist (Kb values are 21.7 and 1405 nM for human OX1 and OX2 receptors respectively)’. Triphenyl-tetrazolium chloride (TTC, Sigma–Aldrich) was used to determine the infarct size following ischaemia and reperfusion. All other reagents used were of the highest purity commercially available.
Animal experiments
All animal experiments were performed in accordance with U.K. legal requirements and the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. This investigation also conforms to the guidelines for the welfare of animals in experimental neoplasia as they have been developed by the United Kingdom Coordinating Committee of Cancer Research (UKCCCR). Adult male Wistar rats (250–300 g) were housed in environmentally controlled conditions (22 ± 2°C, humidity 40–60%) under a 12:12-h light–dark schedule (lights on 06:00). After a week of habituation to these conditions, rats were killed using cervical dislocation. Hearts were immediately removed for the following studies: (i) dissection and immediate snap-freezing for later tissue analysis; (ii) isolation of ventricular cardiomyocytes by enzymatic digestion; (iii) immersion in ice-cold oxygenated Tyrode’s solution and then perfusion via the aortic cannula in the modified Langendorff model with Tyrode’s solution at 37°C, as previously described [21]. In brief, contractile parameters were measured by insertion of a fluid-filled latex balloon through the left atrium (LA) into the left ventricle (LV), this was connected to a pressure transducer (MTL0380, ADInstruments Ltd, U.K.) and the balloon volume was adjusted to give end-diastolic pressure of <10 mmHg. Aortic perfusion pressure was monitored with a second pressure transducer in series with the aortic cannula. Data were continuously recorded using a Power Lab 8 preamplifier/digitiser (ADInstruments Ltd, U.K.). Hypothalamic dissection took place as previously described [22]. All procedures were approved by the ethics committee and complied with the University’s care and welfare guidelines, in strict accordance with the Home Office Guidance (PPL 70/7175) on Research and Testing using animals and the Animal (Scientific Procedures) Act, 1986 and the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Human cardiovascular multiple tissue cDNA panel
Commercially available human cardiovascular multiple tissue cDNA (MTC) panel (Clontech) were used for the present study. All tissues were normal (i.e. non-diseased) and the amount of pooled patients varied from 11 up to 32. The sets of primers used for reverse transcription-PCR (RT-PCR) amplification were: (i) OX1R sense 5′-ccttcctggctgaagtgaag-3′ and antisense 5′-agtgggagaaggtgaagcag-3′ and (ii) OX2R sense 5′-gtcgcaactggtcatctgct-3′ and antisense 5′-cgtcctcatgtggtggttct-3′.
Harvesting of single-cell mRNA and cDNA synthesis
Under the microscope, single rat cardiomyocytes (n=6) were harvested via the patch clamp micro-electrode pipette, without losing the gigaseal to prevent contamination with extracellular fluid [23]. Subsequently, the pipette contents were expelled into a sterile 0.5 ml tube.
PCR
Total RNA from different rat heart chambers (n=3) was extracted and cDNA synthesis was performed. PCRs were carried out as previously described [7].
Immunofluorescence for orexins and OXRs in cardiomyocytes
Adult rat cardiomyocytes, seeded on glass cover-slips were fixed with 4% paraformaldehyde in PBS. The primary rabbit antiserum OR-A and OR-B (Phoenix Europe GmbH, Karlsruhe, Germany) and the primary goat polyclonal OX1R (Santa Cruz Biotechnology Inc., U.S.A.) and a monoclonal OX2R antibody (Abcam, U.K.) were used at a 1:500 dilution. All dilutions were made in 3% BSA in PBS-0.01% Triton X-100. Specimens were incubated with primary antibody for 60 min, then washed three times with PBS (5 min each time) before incubation with anti-rabbit IgG-FITC and Texas Red anti-goat secondary antibody (Vector Laboratories, Peterborough, U.K.) for 45 min. The slides were then thoroughly rinsed with PBS, and the cell nuclei were visualised by applying the DNA-specific dye DAPI at a final concentration of 1.5 µg/ml.
Immunohistochemistry for orexin and OXRs in the rat heart
Whole heart tissue sections from adult rats (n=3) were cut at 3 μm. Endogenous peroxidase was blocked by incubating sections in a 1.5% H2O2 solution for 20 min. The detection system used was an immunoperoxidase-based system (Vector Universal Elite ABC kit) with a diaminobenzidine tetrahydrachloride visualisation agent. Sections were incubated with primary antibody (OX1R, OX2R, OR-A and OR-B) for 60 min at room temperature. Sections were then washed for 2 × 5 min in buffer. Secondary biotinylated antibody was applied for 30 min at room temperature. The avidin–biotin complex solution was then applied to the sections and incubated for 30 min at room temperature (Vector Elite ABC reagent). DAB solution was prepared according to the manufacturer’s instructions and applied to the sections for 5 min. Sections were then dehydrated, cleared and mounted.
For controls, rat spleen, testes (positive controls) and thymus (negative control) were stained using paraffin-embedded tissues from US Biomax (#RA162, MD, U.S.A.). Furthermore, we have repeated the OR-A staining on rat heart array (#TR031; US Biomax MD, U.S.A.).
Western blotting for prepro-orexin and orexins
Protein lysates from rat brain, rat heart and human myometrium were separated using SDS/PAGE (15% polyacrylamide) under reducing conditions and then transferred on to immunoblot nitrocellulose membrane. Blocking of non-specific binding was achieved by placing the membrane in a blocking buffer (1% BSA in 1× TBST) at 4°C overnight. The following day, membrane was rinsed with TBST for 5–10 min. Membranes were then incubated with primary anti-rabbit OR-A and OR-B antibodies (Phoenix Peptides), diluted 1:500 and further incubated at 4°C overnight under gentle agitation. The next day, after rinsing the membrane three times in TBST for 10 min to remove unbound primary antibodies, the membranes were exposed to anti-rabbit IgG–HRP conjugate (Sigma–Aldrich) (diluted 1:2000 in blocking solution) for 1 h at room temperature. Detection of immunocomplexes took place using the ECL method, exposing X-ray film (Fuji) at different time points (30 s to 1 min) following by developing. Molecular weight approximations are taken by comparing the stained bands to that of the marker proteins.
Orexin-mediated signalling in cardiomyocytes
Following the isolation of calcium tolerant cardiomyocytes (n=6), the cells were treated in a concentration (0.01–100 nM) and time (0–60 min) dependent manner with both OR-A and OR-B. Following these optimisation experiments, orexin-induced phosphorylation of various signalling cascades, i.e. Akt, extracellular signal-regulated kinase (ERK) 1/2 (ERK1/2), Troponin-I (TnI), and Myosin Light Chain were determined by Western blot analyses, as described previously [24].
Measurement of cell contraction and intracellular calcium
Contraction and intracellular calcium [Ca2+]i were determined in isolated ventricular cardiomyocytes (n=6), as previously described [21]. Cells were classified as either contractile or non-contractile in response to electrical stimulation. To measure [Ca2+]i, myocytes were loaded with fura-2 and excited alternately at 340 and 380 nm with a monochromator and emitted light collected at >520 nm and measured using a photomultiplier tube (Photon Technology International). Contraction strength is shown as percentage cell shortening and [Ca2+]i as Fura2 ratio.
Determination of infarction following ischaemia/reperfusion in the isolated Langendorff perfused rat heart
All experiments lasted for 195 min. Hearts were allowed to stabilise for 30 min with the standard Tyrode’s solution perfusion. Contractile parameters from the data files were calculated by averaging a minimum of six cycles per data point to obtain the mean value for left ventricular systolic pressure (LVSP mmHg), and left ventricular end diastolic pressure (LVEDP mmHg) (measured at t0, t30, t40, t75, t85, t100, t130, t160 and t195 min). The difference between these two pressure values represented the left ventricular developed pressure (LVDP, mmHg). Heart rate (HR, beats.min−1) was determined at the same time points together with the rate pressure product (RPP, mmHg), which was calculated by multiplying the LVDP and HR (average value of LVDP is obtained from averaging both systolic and diastolic values, then the mean is multiplied by HR to obtain RPP).
Global ischaemia was produced for 30 min by stopping flow of physiological solution to the heart. At the end of reperfusion, the heart was frozen at −20°C before being sliced into 2 mm thick transverse sections and stained with 2% TTC, which reacts with intracellular dehydrogenases present in viable cells producing a red pigment and staining viable tissue red (whereas infarcted tissue remains pale) and then fixed in 10% formalin overnight to enhance the contrast. Each section was then photographed with a digital camera and then traced on to a clear acetate sheet to determine infarct size (% infarct area = infarct area on each slice/total area of the heart slice × 100) [25]; the area was calculated using computer-assisted planimetry [NIH image 1.57] [26–28].
For the inhibitor studies, the rats were randomly assigned to one of the following groups: (i) control group, where they were subjected to 30 min of global ischaemia followed by 120 min of reperfusion, and the treated heart groups, which received inhibitor (wortmannin; 1 μmol/l or U0126; 10 μmol/l) together with OR-B perfusion which was added to Tyrode’s buffer after stabilisation and perfused for 10 min prior to 30-min global ischaemia and switched back to normal Tyrode’s during 120 min reperfusion. In addition, some hearts only received the inhibitors (wortmannin or U0126) to rule out any influence they may have directly upon infarct size.
Inducing ischaemia in rats in vivo
Rats were anaesthetised with 3% isoflurane inhalation and then intubated via a tracheostomy and ventilated with a mixture of O2 and 1.5–3% isoflurane, using a rodent ventilator. A left thoracotomy was performed through the fourth intercostal space, pericardium opened and the left coronary artery (LCA) located and ligated with a 4-0 silk suture. The ligation was confirmed successfully when the anterior wall of the LV turned pale around the area that was tied off using a snare. Following 30 min of regional ischaemia, the snare was released and blood allowed to flow freely during 120 min of reperfusion. At the end of the experimental protocol, the hearts were removed and perfused on to the Langendorff system for staining. The ligation was tied off and hearts perfused with 1% Evans Blue to demarcate the area at risk (AAR), followed by TTC staining to identify the viable tissue staining deep red. The infarct area within the risk area was then calculated (I/R %). The same surgical procedure was followed for sham group without occlusion of the LCA (data not shown).
Determination of infarction following ischaemia/reperfusion in the in vivo rat heart
At the end of reperfusion, the rat was overdosed with pentobarbital and the heart was rapidly removed and mounted on to the Langendorff apparatus as above. The left anterior descending artery was ligated, 1 ml of 0.25% Evans Blue (in PBS) was then injected into the heart and allowed to perfuse followed by normal Tyrode’s for a further 2 min to delineate the area not at risk (ANAR) staining it blue [29]. The heart was stored at −20°C before being sectioned into 2-mm sections and stained with TTC to determine the infarct size as stated above. The percentage infarct tissue (I) within the AAR was calculated as I/AAR %. Furthermore, the ischaemic area (AAR) was distinguished from ANAR.
Orexin in the human heart
The clinical study was approved by the Research Ethics Committee South-Central Oxford C (11/SC/0140). All participants provided written informed consent, in accordance with the Declaration of Helsinki.
Orexin gene expression in human myocardial tissue
To explore whether our findings from the cell cultures and animal model could be of relevance to CVD in humans, we investigated OX2R gene expression levels in human myocardium samples. A cohort of 54 patients undergoing coronary artery bypass grafting with available myocardial cDNA library was used for this purpose (Table 1); relative expression was calculated using the Pfaffl method, with phosphoglycerate kinase 1 (PGK1) as housekeeping gene [30].
Table 1. Selected key patient characteristics prior to cardiac surgery and tissue collection for the heart failure study patients, including age, gender, hypertension, hyperlipidaemia, type 2 diabetes mellitus, smoking status and body mass index.
| Participants (n) | 54 |
| Age (years) | 65.2 ± 1.3 |
| Male gender (%) | 50 (92.6) |
| Hypertension (%) | 30 (55.6) |
| Hyperlipidaemia (%) | 31 (57.4) |
| Type 2 diabetes mellitus (%) | 16 (29.6) |
| Smoking - active (%) | 26 (48.1) |
| - ex (%) | 13 (24.1) |
| BMI (kg/m2) | 27.29 ± 0.50 |
Data are presented as mean ± S.E.M. or as frequencies. Abbreviation: BMI, body mass index.
Orexin signalling in the human myocardium
To examine the direct effects of OR-A and OR-B on intracellular signalling in the human heart, human myocardial tissue samples were collected from the right atrial appendage of eight patients undergoing cardiac surgery. These myocardial tissue samples were washed in ice-cold Krebs HEPES buffer and then cut into thin strips containing all myocardial layers. The tissue was first equilibrated for 10 min in Krebs HEPES buffer at 37°C and then incubated for 20 min in the presence or absence of human recombinant OR-A (100 nM) or OR-B (100 nM) (Phoenix Pharmaceuticals Inc., Burlingame, CA, U.S.A.). At the end of the experiment, incubated tissue samples were collected and stored at −80°C until assayed. Western immunoblotting was used to examine the direct effects of OR-A and OR-B on p-Akt (Ser473), total-Akt, (Cell Signaling, Danvers, MA), and phospho/total ERK1/2 (Abcam, Cambridge, England, United Kingdom) in human myocardial tissue. Briefly, myocardial tissue samples were homogenised for 30 s using a pre-cooled electric Polytron homogenizer in 220 μl of lysis buffer (Invitrogen, U.K.) containing a protease plus phosphatase inhibitor cocktail (Roche Applied Science). Homogenates were spun at 13000 rpm for 10 min at 4°C. The protein concentration of the supernatants was then measured using the BCATM Protein Assay kit (Pierce, U.K.). Protein lysates were separated on 12% gradient SDS-NuPAGE gel (Invitrogen, U.K.), and proteins transferred to PVDF membranes (Amersham UK Ltd.), followed by blocking with 5% powdered skimmed milk. The membranes were incubated with the respective primary antibodies overnight and immunodetection of the primary antibodies was performed with horseradish-peroxidase–conjugated secondary antibodies (Promega), and enhanced chemifluorescence (Amersham Bioscience UK Ltd.), and quantified in relation to the house-keeping protein GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical analysis
All results are expressed as mean ± S.E.M., unless indicated otherwise. For Western blotting experiments, the densities of the immunoreactive bands were measured using a scanning densitometer coupled to scanning software (ImageQuant; Molecular Dynamics, Amersham Pharmacia, U.K.). Data were evaluated as in the study by Maulik et al. [31]. Statistical analyses among the Langendorff model perfused groups were performed using one-way ANOVA followed by Bonferroni’s post hoc test with significance determined at the level of P<0.05.
Results
Detection of cardiac prepro-orexin and OXRs
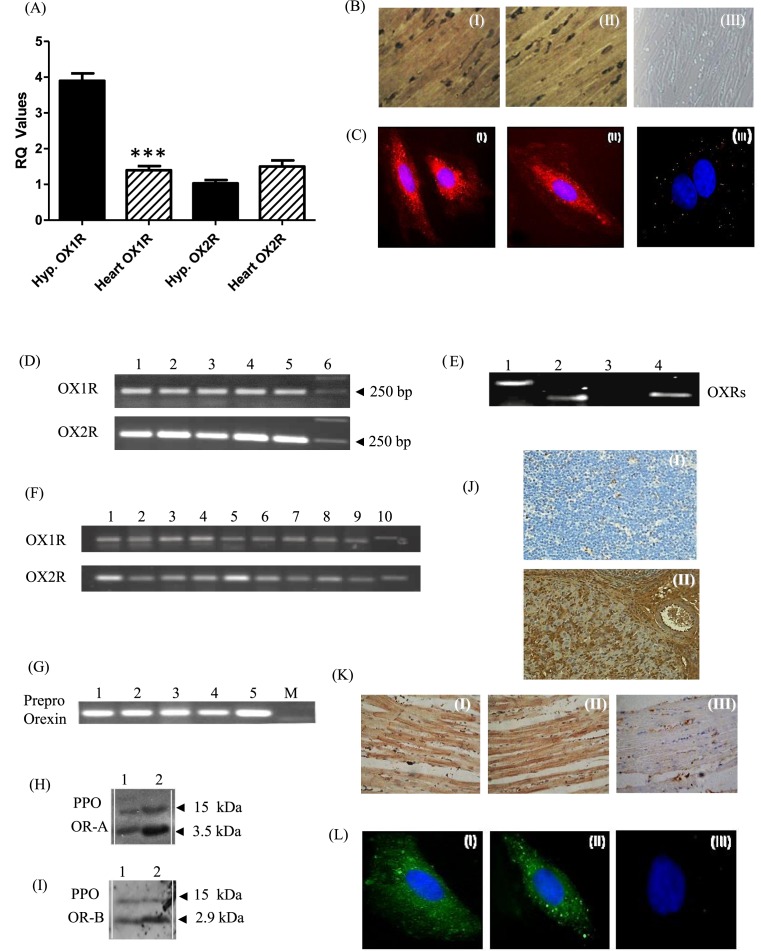
Using qRT-PCR, we demonstrate that both OXRs are expressed in the rat heart. Using rat hypothalamus as a positive control, the expression of OX1R was significantly less in the heart (P<0.001), whereas OX2R expression levels were similar between the heart and the hypothalamus (Figure 1A). Both OXRs were uniformly expressed throughout the rat heart including the LV, left auricle (also known as left atrial appendage), interventricular septum, right ventricle (RV) and right auricle (Figure 1D). Single-cell PCR from cardiomyocytes revealed expression of both OXRs in single cells investigated (Figure 1E).
Figure 1. Expression of orexin-1 (OX1R) and orexin-2 (OX2R) receptors in the rat and human heart.
(A) Quantificative RT-PCR analysis of OX1R and OX2R in the rat hypothalamus (hypo) and rat heart (***P<0.001 for heart compared with hypothalamus). (B) Immunohistochemical analysis of rat heart ventricular sections (n=3) for OX1R (I) and OX2R (II). Magnification ×400. Negative control (III) confirmed the specificity of the immunostaining. (C) Immunohistochemical analysis of OX1R (I) and OXR2 (II) in rat cardiomyocytes and negative control (III). Identical results obtained from four independent experiments; measuring at least 50 cells. (D) Expression of OX1R and OX2R in rat heart compartments (n=3). Lane 1 cDNA from LV; Lane 2 cDNA from left auricle; Lane 3 cDNA from septum; Lane 4 cDNA from RV; Lane 5 cDNA from right auricle; and Lane 6 is DNA marker. (E) Single-cell RT-PCR in cardiomyocytes (n=6): Lane-1: β-Actin; Lane-2: OX1R; Lane-3: negative control; Lane-4: OX2R. (F) Expression of OX1R and OX2R in different human cardiac compartments: Lane-1: aorta; Lane-2: apex of the heart; Lane-3: LA; Lane-4: right atrium (RA); Lane-5: right auricle; Lane-6: left auricle; Lane-7: LV; Lane-8: RV; Lane-9: interventricular septum; and Lane-10: atrioventricular (AV) node. (G) Expression of prepro-orexin in rat heart chambers (n=3). Prepro-orexin is expressed as a 302-bp PCR product in the LV (lane 1), LA (lane 2), interventricular septum (lane 3), RV (lane 4), and RA (lane 5). M = Marker; DNA ladder. (H,I) Prepro-orexin (PPO) and the cleaved peptides OR-A and OR-B were detected using Western blotting, with molecular weights of 15 kDa for PPO, 3.5 and 2.9 kDa for OR-A (H) and OR-B (I), respectively; Lane 1: rat heart lysates; and Lane 2: rat hypothalamus lysates (n=3). (J) Immunohistochemical analysis for OR-A on rat thymus section (I) and rat spleen (II). (K) Immunohistochemical analysis of rat heart ventricular sections (n=3) for OR-A (I) and OR-B (II) and negative control (III). Magnification ×400. (L) Rat cardiomyocyte immunofluorescent analysis of OR-A (I), OR-B (II) and negative control (III). Identical results obtained from four independent experiments, measuring at least 50 cells.
Using Western blotting, we also confirmed the expression of OXRs at the protein level in total rat heart and cardiomyocyte cell lysates (data not shown). Immunohistochemical analysis of ventricular sections using specific antibodies for OX1R (Figure 1B(I)) and OX2R (Figure 1B(II)) revealed a widespread cytoplasmic and membrane distribution of both OXRs across the rat ventricle. Cellular distribution of OXRs was further investigated in rat cardiomyocytes in vitro. Immunofluorescence revealed a similar distribution of OX1R (Figure 1C(I)) and OX2R (Figure 1C(II)). This is consistent with the expression of GPCRs in the cytoplasm (due to trafficking or internalisation), as well as on the cell membrane [32]. We then proceeded to study the expression of OXRs in the human heart using a cardiovascular MTC panel demonstrating expression of both OXRs in multiple human cardiac compartments (Figure 1F).
We also demonstrated that prepro-orexin is expressed as a 302-bp PCR product in the LV, LA, interventricular septum, RV and right atrium (RA) of the rat heart (Figure 1G). We have used Western blotting to demonstrate that the prepropeptide can be cleaved into full-length OR-A (Figure 1H) and OR-B (Figure 1I), with molecular weights of approximately 3.5 and 2.9 kDa, respectively. We then assessed the protein expression using immunohistochemistry. Antibodies were validated using rat thymus as a negative control and rat spleen and rat testes as positive control (Figure 1J and Supplementary Figure S2). Subsequent immunohistochemical analysis of rat ventricular sections revealed protein expression of both cleaved peptides across the ventricle (Figure 1K and Supplementary Figure S2). Similar expression was evident in individual cardiomyocytes (Figure 1L).
Cardiomyocyte contractility and calcium measurements
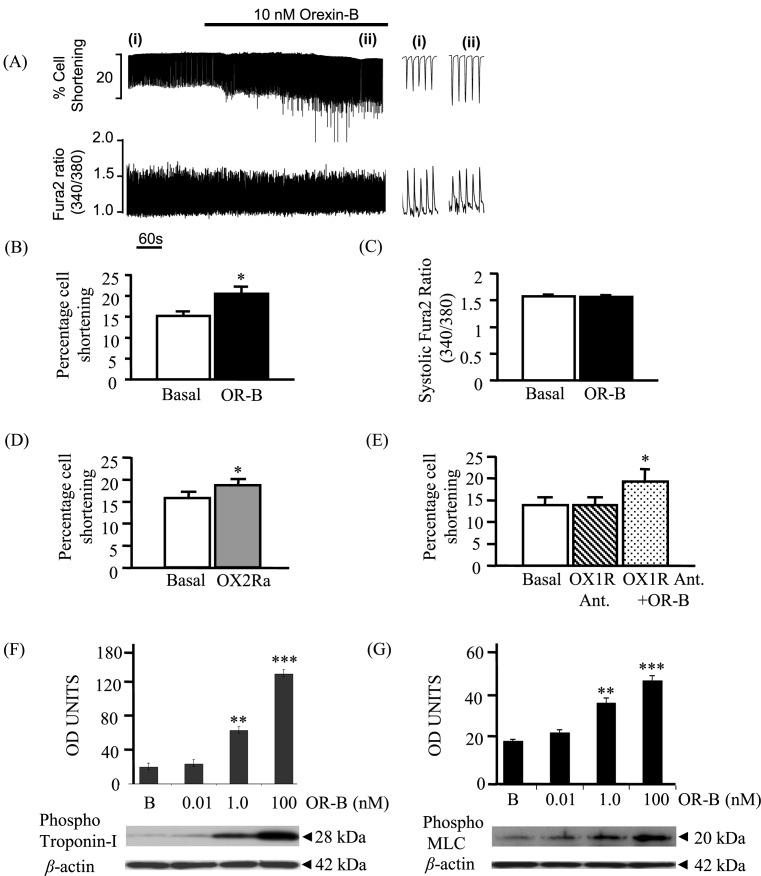
Superfusion of cardiomyocytes with Tyrode’s solution containing 10 nM OR-B increased the shortening of twitch contraction (Figure 2A), from 15.2 ± 1.2 to 20.6 ± 1.7% (n=6, P<0.01; Figure 2B). This was not associated with any change on either the amplitude (Figure 2A) or the rate of the Ca2+ transient decline (n=6, P=NS (no supplement); Figure 2C). OR-A had no effect on the rat cardiomyocyte cell length even with concentrations as high as 100 µM (data not shown; P=0.07). The OR-B effect was replicated using the specific OX2R agonist ([Ala11, d-Leu15]-OR-B) (Figure 2D), while it was not blocked by an OX1R-specific antagonist (SB-408124) (Figure 2E), suggesting that these effects are predominantly mediated via an OR-B/OX2R pathway.
Figure 2. Effects of OR-B on contractility of rat cardiomyocytes and on the phosphorylation of TnI and myosin light chain.
Using video-edge detection system, changes in cell length of individual cardiomyocytes (n=6) were recorded upon challenge with orexins. (A,B) Superfusion of the myocyte with OR-B (10 nM) increased the strength of twitch contraction (**P<0.01). (A,C) No effect on the amplitude of the Ca2+ transient was observed. (D) Similar effects on the strength of twitch contraction was observed following treatment with a specific OX2R agonist (OX2Ra: [Ala11, d-Leu15]-Orexin B) (*P<0.05). (E) Addition of an OX1R-specific antagonist (OX1R Ant.: SB-408124) did not alter the OR-B cardiomyocyte contractile response (*P<0.05). (F) OR-B was able to induce phosphorylation of TnI in a concentration-dependent manner (**P<0.01, ***P<0.001). (G) OR-B was able to induce phosphorylation of MLC in a concentration-dependent manner (**P<0.01, ***P<0.001). For the above experiments, equal protein loading was confirmed, using β-actin as an internal control. B = basal (no OR-B). Abbreviation: MLC, myosin light chain.
Furthermore, OR-B, but not OR-A (data not shown), significantly induced phosphorylation of TnI at Ser23/Ser24 in cardiomyocytes. This effect was dose-dependent, with a maximum activation at 100 nM of OR-B (P<0.001; Figure 2F). Similarly, OR-B, but not OR-A (data not shown), induced significant phosphorylation of the myosin light chain (MLC) at Ser19 with maximal effects at 100 nM and 15 min (P<0.001; Figure 2G).
OR-B exerts a cardioprotective effect
In both the isolated rat heart and the in vivo rat model, we measured LVDP, LV pressure decay and HR. The RPP was then calculated; this was used as a clear and direct indication of the energy demand of the heart and, thus, a good measure of the energy consumption of the heart pre- and post-ischaemia.
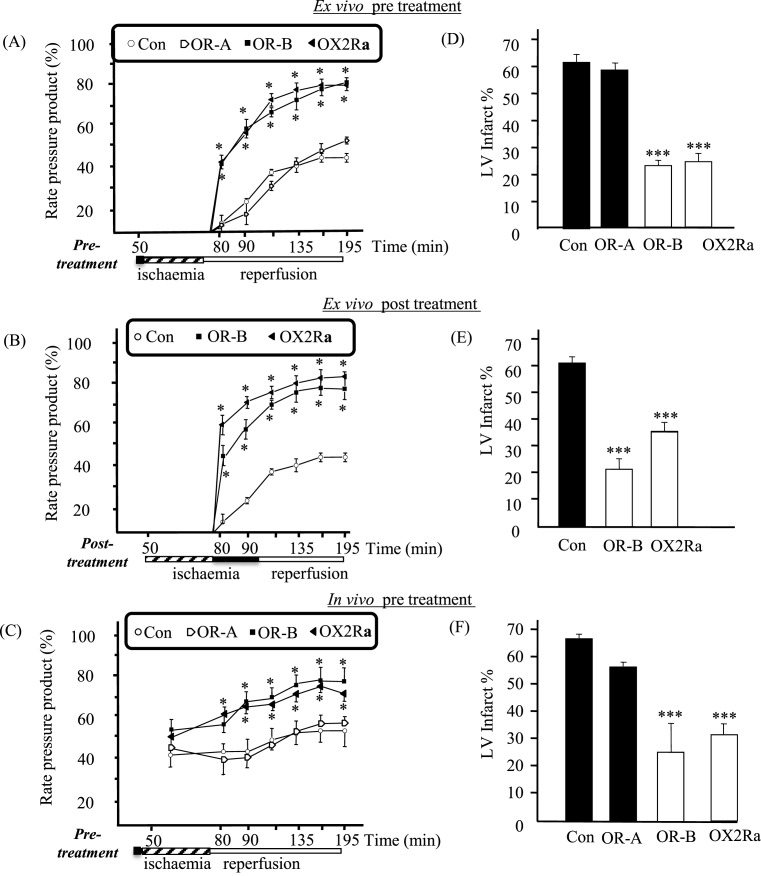
Initially, pre-treatment with either OR-A, OR-B or OX2R agonist, prior to the ischaemic insult, did not alter the haemodynamic parameters or HR between any of the groups (data not shown). Contractile parameters were recorded at various time points throughout the experimental protocol (Supplementary Figure S1). Following 30 min of global ischaemia in the isolated Langendorff perfused rat heart model, the expected decline in cardiac function during reperfusion, as observed in the control hearts, was not affected by pre-treatment with OR-A (Figure 3A). In contrast, the pre- and post-treatment groups for both OR-B and the OX2R agonist resulted in significantly improved recovery compared with control during reperfusion (P<0.05; Figure 3A,B). This was further replicated in the in vivo rat model where the control group (saline treated) demonstrated poor cardiac functional recovery and cardiac function following 30 min of regional ischaemia and 120 min reperfusion, whereas the OR-B pre-treated group [bolus injection (i.v.) of OR-B (100 nM) 10 min prior to regional ischaemia] showed significant improvement in functional recovery during reperfusion (P<0.05; Figure 3C). Concentration-dependent effects were noted with OR-B (20–100 nM), with a maximal response at 100 nM (data not shown).
Figure 3. Cardiac functional parameters and infarct size determination following ischaemia (n=8).
(A–C) Panels depict the effects on left RPP = HR × LVDP, following 30 min of ischaemia (45–75 min) for: (A) Control (Con); pre-treatment: OR-A, OR-B or OX2R agonist (OX2Ra) in the in situ rat model (ex vivo; isolated Langendorff model) (*P<0.05, compared with control). (B) Control, post-treatment OR-B or OX2Ra in the in situ rat model (*P<0.05, compared with control). (C) Control, bolus pre-treatment OR-A, OR-B, OX2Ra in the in vivo rat model (*P<0.05, compared with control). (D–F) Depict the effects on the LV infarct size (% LV; transverse sections of the rat heart were obtained at the end of the experimental protocol and the sections were stained using TTC so that the infarcted tissue appears pale, while viable tissue stains red). More specifically (D) Control; pre-treatment with OR-A, OR-B and OX2Ra in the ex vivo model (***P<0.001, compared with control). (E) Control; post-treatment with OR-B or OX2Ra in the ex vivo model (***P<0.001, compared with control). (F) In vivo model with bolus pre-treatment with OR-A, OR-B and OX2Ra prior to regional ischaemia and reperfusion (***P<0.001, compared with control).
Effects of OR-B reducing the LV infarct size
In isolated rat heart model pre-treated with OR-B for 10 min prior to global ischaemia and reperfusion, the LV infarct size was reduced from 61.5 ± 3.0% in the control to 22.6 ± 1.9% in the treated group (P<0.001; Figure 3D). In addition, the OR-B post-ischaemic treatment was just as effective at providing myocardial protection as pre-treatment, since it also significantly reduced the LV infarct size to 21.8 ± 3.9% (P<0.001; Figure 3E). The OX2R-specific agonist exhibited a similar protective effect to OR-B treatment, inducing a reduction in LV infarct size both during pre-treatment (25.7 ± 3.6%) and at the start of reperfusion (34.7 ± 3.1%, both P-values <0.001 compared with control; Figure 3D,E). However, pre-treatment with OR-A alone showed no significant reduction compared with control (58.3 ± 3.0 compared with 61.5 ± 3.0%, respectively; P=NS) in the LV infarct size (Figure 3D). Moreover, in the in vivo model pre-treatment with a bolus injection (i.v.) of OR-B (and of ORX2Ra, but not of OR-A) 10 min prior to regional ischaemia and reperfusion significantly reduced the %LV infarct size compared with the control (saline) group (66.7 ± 3.0 compared with 26.8 ± 14.9% in the control and OR-B treated group, respectively; P<0.001; Figure 3F).
Involvement of distinct signalling pathways in myocardial protection
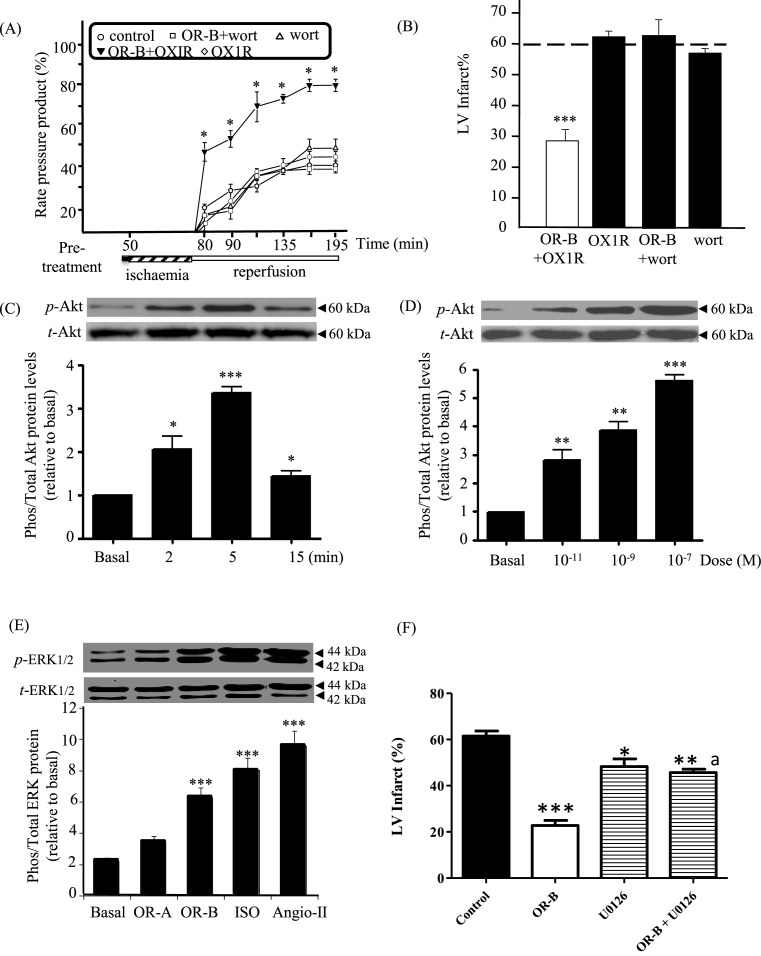
The isolated rat heart model was pre-treated either with an OX1R-specific antagonist or a PI3 kinase (PI3K) inhibitor (wortmannin) in the presence or absence of OR-B prior to ischaemia/reperfusion. Pre-treatment with either the OX1R-specific antagonist or wortmannin alone showed no change in functional recovery compared with the control group (Figure 4A) and no effect on the %LV infarct size (61.5 ± 3.0% in the control group compared with 61.8 ± 1.9% with OX1R antagonist alone, and 56.5 ± 1.5% with wortmannin alone; P=NS, Figure 4B). Pre-treatment with OR-B together with wortmannin showed no myocardial protection on cardiac functional recovery (P=NS; Figure 4A); and, abolished the OR-B protective effect on LV infarct size (P=NS, Figure 4B), suggesting the importance of the PI3K/Akt pathway in OR-B induced cardioprotection. Improved functional recovery with OR-B pre-treatment was not inhibited by the simultaneous administration of an OX1R-specific antagonist (P<0.05; Figure 4A). Similarly, the OR-B protective effect on %LV infarct size was not blocked by the OX1R-specific antagonist (P<0.001; Figure 4B). Both these findings suggest that the noted effect is OX2R specific.
Figure 4. OR-B/ OX2R effects on key signalling pathways involved in myocardial protection using the isolated Langendorff perfused rat heart model (n=8).
(A) Cardiac functional performance measured and represented as left RPP in rat hearts pre-treated with control (saline treated), OR-B plus OX1R antagonist, OX1R antagonist alone, OR-B plus wortmannin (wort; a PI3K inhibitor) and wortmannin alone (*P<0.05 compared with control). (B) LV infarct size determination (%LV) in pre-treated hearts with OR-B plus OX1R antagonist, OX1R antagonist alone, OR-B plus wortmannin and wortmannin alone (***P<0.001 compared with control). Dotted line across denotes control levels. (C) OR-B time-dependent treatment and Akt phosphorylation: exposure of rat cardiomyocytes to OR-B (100 nM) induced maximal phosphorylation at 5 min. Change in the phosphorylation was evident as early as 2 min post-treatment (*P<0.05, ***P<0.001 compared with basal). (D) OR-B induced a dose-dependent induction in the phosphorylation status of Akt, becoming significant at 1 nM and reaching a plateau at 100 nM (**P<0.01, ***P<0.001 compared with basal). (E) OR-B treatment increased the phosphorylation of ERK1/2 compared with basal levels (***P<0.001). Isoproterenol (ISO) and Angiotensin-II (Angio-II) were employed as positive controls. (F) Inhibition of the MAPK pathway using a selective MAPK inhibitor (U0126) abrogates the protective effect of OR-B on LV infarct size (*P<0.05, **P<0.01, ***P<0.001 compared with control; aP<0.05 compared with OR-B).
Given that ischaemic preconditioning protects the heart by phosphorylating the pro-survival kinases Akt and ERK1/2 at reperfusion, we investigated further whether orexins can affect their phosphorylation [33]. Treatment with OR-B increased the phosphorylation of both Akt and ERK1/2 compared with basal levels (P-values <0.001; Figure 4C–E); in contrast with OR-A which had no significant effect on ERK1/2 (Figure 4E) and Akt (data not shown). The OR-B effect was concentration dependent (Figure 4D). The time course for the ERK1/2 activation was for 3, 5, 10 and 15 min. The involvement of an MAPK pathway was further corroborated using the selective MAPK inhibitor U0126, demonstrating that inhibition of the MAPK pathway using U0126 abrogates the protective effect of OR-B on LV infarct size (Figure 4F).
OX2R expression in patients with heart failure, and involvement of the ERK1/2 pathway
To further evaluate the potential implication of orexins and their receptors in cardiac disease in humans, we studied OXR downstream signalling and expression in cardiac tissue samples from adult patients undergoing cardiac surgery and coronary artery bypass grafting. In the context of these studies, all human myocardial tissue samples were collected from the right atrial appendage during cardiac surgery.
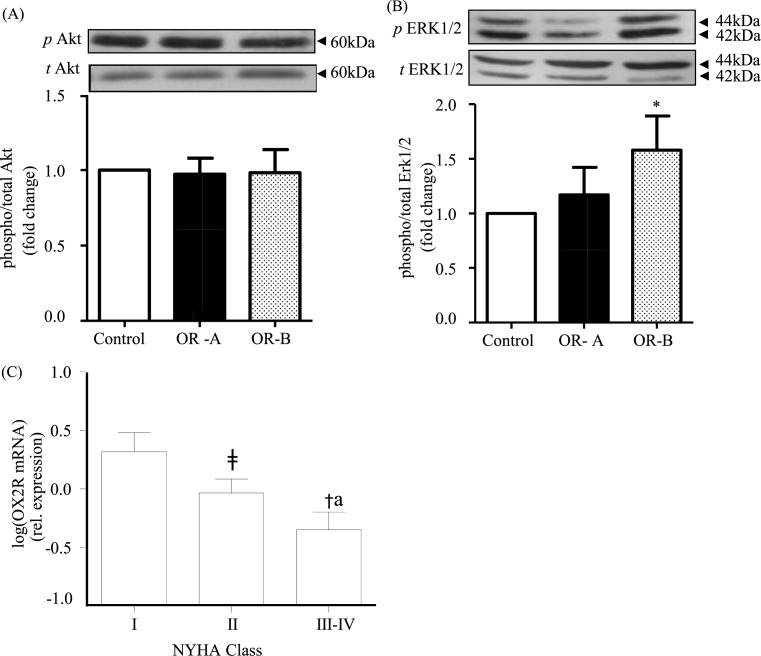
Using Western blotting, we examined the direct effects of OR-A and OR-B on the phosphorylation of Akt and ERK1/2 in heart tissue samples from eight patients. Treatment with OR-A or OR-B did not change Akt phosphorylation compared with basal levels (Figure 5A), while only OR-B significantly increased ERK1/2 phosphorylation compared with control (non-treated heart tissue; P<0.05; Figure 5B).
Figure 5. Orexin-induced signalling and orexin receptor expression in heart samples from cardiac surgery patients.
(A,B) In human heart tissue obtained from patients undergoing coronary artery bypass (n=8), both OR-A and OR-B showed no change in Akt phosphorylation compared with the non-treated heart tissue (100 nM). Change in the phosphorylation state was evident for ERK1/2 signalilng in the OR-B treatment group (*P<0.05 compared with control). (C) Expression of OX2R in heart samples from heart failure patients (n=54) demonstrated a significant decrease in OX2R in NYHA-II compared with NYHA-I patients (‡P<0.05) and in NYHA-III/IV compared with NYHA-II (*P<0.05) and to NYHA-I patients (†P<0.05) and NYHA-III/IV compared with NYHA-I (aP<0.05). NYHA: New York Heart Association Functional Classification for heart failure stages.
In addition, we measured OXR expression levels in heart tissue samples from heart failure patients undergoing coronary artery bypass grafting (n=54; Table 1). Our results demonstrated a negative correlation between the severity of heart failure symptoms and the expression of OX2R. As such, when patients were categorised using the New York Heart Association (NYHA) functional classification of heart failure (classes I–IV based on increasing severity of clinical symptoms) there was a significant decrease in OX2R in NYHA-II compared with NYHA-I and in NYHA-III/IV compared with NYHA-II and NYHA-I (P-values <0.05; Figure 5C).
Discussion
In the present study, we have shown that the heart constitutes a source of orexins and expresses functional OXRs. Moreover, our experiments revealed that OR-B can exert direct cardioprotective effects in both ex vivo and in vivo rat heart models.
Indeed, using the Langendorff perfused rat heart model, pre- and post-myocardial ischaemia treatments with both OR-B and a selective OX2R agonist resulted in significantly increased RPP which is a direct indication of the energy demand of the heart and, thus, a good measure of the energy consumption of the heart pre- and post-ischaemia.
In the case of the ex vivo studies that replicate the in vivo results, additional interactions between sympathetic activity, which is increased in ischaemia [34], and OXR signalling through cAMP [35] cannot be ruled out. Additionally, treatment of ventricular cardiomyocytes with OR-B, but not OR-A, lead to significantly increased Tn-I and MLC phosphorylation, followed by a concomitant increase in the strength of their twitch contraction. Of note, these findings agree with previous studies demonstrating that changes in the phosphorylation of key cardiac regulatory proteins can affect cardiac function [36,37].
This is the first observation of a differential specificity of OR-A and OR-B in the heart. OR-A activates both OX1R and OX2R receptors with similar potencies (∼36 nM), whereas OR-B selectively activates OX2R compared with OX1R (∼420 nM) [38]. Both receptors belong to the GPCR superfamily and are highly promiscuous in terms of their G-protein coupling characteristics [39]. These tend to be cell- and tissue-specific and influence subsequent activation of downstream signalling pathways. For example, we have previously shown that expression of human OX2R is regulated by a complex involving a proximal PKA/PKC-regulated promoter and a distal promoter regulating tissue-specific expression of alternative transcripts which in turn post-transcriptionally regulate receptor levels [40]. Therefore, future studies are required to provide further insight into the regulation of the promoter of OX2R in the myocardium.
Moreover, a dichotomous role of OXRs is evident in the rat brain, where OX1Rs mediate the neurobiological effects that drive drug seeking, whereas OX2Rs play a crucial role in sleep/wake cycle regulation and arousal [41]. Interestingly, OR-A, but not OR-B, can activate electrophysiologically the subfornical organ of rat neurons [42]. In a more recent study, OR-A (100 nM) and OR-B (100 nM) increased leptin gene expression in differentiated preadipocytes by 49.9 and 71.3% respectively [43], indicative of differential modulation of transcriptional responses by these two peptides.
This phenomenon has been previously described for other GPCRs. In the case of smooth muscle cell CRH-receptors, urocortin, but not CRH, activated the MAPK signal transduction pathway despite similar binding affinities [44]. Thus, it is possible that differences on OR-A and OR-B responses could also be influenced by the cardiac microenvironment, leading to alternative coupling of G proteins and activation of distinct signalling pathways. In previous studies, we have shown that OX2Rs can differentially couple to Gq, Gi/o and Gs proteins in different tissues [19,22,39]. Emerging studies have also shown that OXRs can form functional dimers. It is possible that dimerisation can also take place at the cardiac level and therefore influence pharmacological properties, such as the affinity of the receptors for orexin ligands, as well as cellular distribution and trafficking [45]. Finally, polymorphisms of the OX2R gene have also been described previously [46]. To this date, dimerisation or polymorphisms have not been explored in the myocardium. Future studies should determine whether dimerisation or polymorphisms of cardiac OXRs may affect ligand binding, or indirectly affect ligand binding by altering receptor structure.
In the present study, using an OX1R antagonist and an OX2R agonist, we conclude that the primary signalling at the rat heart is mediated via an OR-B/OX2R pathway. OXRs are able to activate certain key plasticity-regulating cascades, such as PI3K and ERK, and p38 MAPK. Here, pre-treatment with wortmannin abolished the OR-B protective effect on LV infarct size; suggesting the involvement of the PI3K/Akt pathway in OR-B induced cardioprotection. This signalling pathway has been implicated in myocardial protection; and is referred to as the reperfusion injury signalling kinase (RISK) pathway [33]. Mediators that target the activation of the RISK (PI3K/Akt) pathway have been also shown to ameliorate recovery following ischaemic stress [25,47]. In our study, OR-B, but not OR-A, was able to induce the phosphorylation status of both Akt and ERK1/2. Our data contradict previous studies showing that both orexin peptides can increase Akt phosphorylation. For example, in a study of neuronal cell cultures OR-B was more potent than OR-A, but the maximal effect of both peptides on the Akt activation was very similar [48]. This apparent contradiction could be attributed to the fundamental differences between cardiomyocytes and neuronal cells including morphological polarity, shape and function of action potentials, synaptic and homoeostatic plasticity; not to mention the spatial expression of OXRs in the brain [49]. As such, it is plausible that orexins exert their effect in an organ- and cell-specific manner.
The OR-B selectivity in post-translational modification of key mediators is corroborated by previous studies in other tissues. Indeed, in the human testis, when IP3 turnover was measured, OR-B exerted a more potent effect compared with OR-A [18]. Similarly, in human H295R adrenocortical cells’ diverse roles have been noted for OR-A and OR-B in terms of activation of MAPKs and cortisol release; i.e. only OR-A (at 1 nM) activated ERK1/2 and p38 [50].
Based on our findings, herein we propose the presence of a signalling pathway involving OX2R-ERK1/2-MLC in the heart with a net effect of increased Ca2+ sensitivity and cell length/contractility following OR-B superfusion in cardiomyocytes [21,51–54]. Activation of ERK1/2 is involved in the activation of contractile responses through mechanisms involving direct phosphorylation of the Ca2+/calmodulin-dependent MLC kinase (MLCK) [33,55–57].
In a seminal review by Spinazzi et al. [58], a case is made for mediation of Ca2+ by OXRs. However, this effect of OX1R and OX2R on Ca2+-release is through IP3 receptor activation, which, although evident in the hypothalamus, does not impact on the contraction of rat ventricular cardiac muscle under basal conditions, where the ryanodine receptors (RyR) dominate excitation–contraction (E–C) coupling [59]. As such, this may have more of a role in pathophysiology of heart failure and arrhythmias [60]. Interestingly, the dominant expression of IP3 receptors is on the nuclear membrane where they are thought to be involved in cardiac hypertrophy [61].
Conversely, inhibition of ERK attenuates force development by lowering MLC phosphorylation in cardiac tissue [62]. Because of its central role in the regulation of contraction and relaxation of the heart, cardiac TnI (cTnI) is also phosphorylated by OR-B to ensure appropriate function. Changes in the phosphorylation status of cTnI are well documented during acute cardiac events and in patients with heart failure [63].
We have expanded on these observations using human heart tissue samples from heart failure patients. So far, a number of studies indicated that there is a dysregulation of the orexin system in the CNS in models of heart failure [64–66]. Here we further report that in adults with heart failure (NYHA classes I–IV) there was a significant negative correlation between the severity of clinical symptoms (NYHA class) and OX2R expression. The fact that OX2R expression was significantly lower in patients with NYHA classes III/IV compared with less symptomatic patients with NYHA class I/II, indicates that OX2R signalling is compromised in more severe heart failure. These observations are further supported by the findings of Perez et al. [67], where in a heart failure model, hypocretin receptor 2 (HCRTR2)/OX2R-deficient mice exhibited poorer cardiac function, and greater myocardial scarring. Moreover, in the same study, differential OX2R expression data from microarray experiments were recorded in patients with dilated cardiomyopathy and ischaemic cardiomyopathy [67].
Based on the established role that orexins have in maintaining the awake state, orexin antagonism has also been identified as a novel therapeutic approach for the treatment of insomnia [68]. As such, suvorexant, a non-selective dual orexin (OX1R/OX2R) receptor antagonist (DORA), has been approved by the FDA, in 2014, for treating insomnia patients with difficulties in sleep onset and/or sleep maintenance (http://www.acc.org/latest-in-cardiology/articles/2015/06/16/08/40/new-insomnia-drugs-in-the-context-of-cardiovascular-disease - BELSOMRA® (suvorexant) package insert: http://www.merck.com/product/usa/pi_circulars/b/belsomra/belsomra_pi.pdf). To date, suvorexant use has not been associated with any reported adverse cardiovascular safety outcomes; however, it must be highlighted that it is unknown whether suvorexant has adverse effects in CVD patients, since patients with significant and/or recent CVD (e.g. with acute coronary syndrome or congestive heart failure) were excluded from the relevant trials (http://www.acc.org/latest-in-cardiology/articles/2015/06/16/08/40/new-insomnia-drugs-in-the-context-of-cardiovascular-disease). Therefore, and based on the findings from our study and from others [67], the use of orexin antagonists may warrant further assessment and careful monitoring for CVD adverse effects, particularly in heart failure patients [69].
Finally, our human ex vivo study results were also consistent with the in vitro rat data, since orexin treatment of human myocardial tissue demonstrated that OR-B, but not OR-A, was able to induce ERK1/2 phosphorylation. However, there was no change in Akt phosphorylation upon treatment with OR-A or OR-B. The latter could be due to the fact that chronic heart failure reduces Akt phosphorylation, so signalling is already compromised at basal levels. Indeed, compared with subjects with normal cardiac function, human skeletal muscle of heart failure patients demonstrates reduced Akt phosphorylation [70]. It should be noted that the cardiovascular and musculoskeletal system are interrelated, whereby a functional deterioration in one is reflected similarly in the other [71]. Differences in the phosphorylation of Akt can also be due to species differences, since certain proteins, like ETS-related gene (ERG), are differentially expressed in the rat and human heart [72].
We also acknowledge certain limitations with our experimental system using healthy young adult rats compared with patients used in the present study that have certain comorbidities. To date, it has proven difficult to find the ‘perfect’ translational in vivo model for CVD. This is primarily due to the high level of complexity, as well as heterogeneity of these diseases, in addition to potential impact/influence from environmental or genetic factors. Future studies can make use of different animal models for examples such as; heart failure, aged rats or rats fed on a high-fat diet to generate further data on the involvement of OR-B/OX2R at the cardiac level. However, to the best of our knowledge, there is no perfect in vivo model to represent the entire repertoire of defects/comorbidities at the cardiac level.
In summary, our present findings document that OR-B exhibits a protective role on cardiac function pre- and post-ischaemia, leading to reduction in the infarct size. We also provide novel conclusive evidence that the rat heart expresses functional OXRs and is a source of orexins. Cardiac OXRs can also influence contractile tone via mechanisms involving phosphorylation of TnI and MLC. Thus, in addition to the known CNS effects of orexins/OX2R [5,73], our data illustrate the importance of the OX2R as a potential therapeutic target directly at the heart level, suggesting that targeted use of OX2R-specific agonists may have a role in the prevention and treatment of CVD.
Clinical perspectives
Several cardiovascular drugs exert their cardiac actions via GPCRs, and orexins acting via their GPCRs can affect the heart centrally. In the present study, we tested whether orexins and their receptors can exert a direct effect at cardiac level.
The human and rat heart constitute a source of orexins and express functional OXRs. OR-B can exert direct cardioprotective effects in both ex vivo and in vivo rat heart models. In adults with heart failure there was a significant negative correlation between the severity of clinical symptoms (NYHA class) and OX2R expression in the human heart.
Based on the findings from our study, the use of orexin antagonists warrants further assessment and careful monitoring for CVD adverse effects, particularly in heart failure patients.
Supporting information
Supplementary Figure 1. Experimental protocol for the isolated Langendorff perfused rat heart and the in vivo model of ischaemia reperfusion (n=8).
Supplementary Figure 2. A: Rat testes (positive control), B and C: Different sections of rat heart stained positive for OR-A, D: Rat Heart; a region that is not positive for OR-A; demonstrating antibody specificity. Magnification x40.
Abbreviations
- AAR
area at risk
- ANAR
area not at risk
- [Ca2+]i
intracellular calcium
- CNS
central nervous system
- cTnI
cardiac troponin I
- CVD
cardiovascular disease
- ERK
extracellular signal-regulated kinase
- GPCR
G protein-coupled receptor
- HR
heart rate
- LA
left atrium
- LCA
left coronary artery
- LV
left ventricle
- LVDP
left ventricular developed pressure
- MAPK
mitogen-activated protein kinase
- MTC
multiple tissue cDNA
- MLC
myosin light chain
- NS
no supplement
- NYHA
New York Heart Association
- OR-A
orexin A
- OR-B
orexin B
- OX1R
orexin receptor-1
- OX2R
orexin receptor-2
- OXR
orexin receptor
- PI3K
PI3 kinase
- RISK
reperfusion injury signalling kinase
- RPP
rate pressure product
- RT-PCR
reverse transcription-PCR
- RV
right ventricle
- Tn
troponin
- TTC
triphenyl-tetrazolium chloride
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the British Heart Foundation [grant number 03/131/16192]; and the Heart Research U.K. [grant number RG2564/08/09].
Author contribution
H.S.R. conceived and planned the experiments. V.H.P., J.C., E.K., C.A., A.A. and B.K.T. carried out the experiments. V.H.P., E.K., I.K., H.S.M., G.K.D., G.R., C.A., E.W.H., A.N. and H.S.R. contributed to the interpretation of the results. H.S.R. and E.K. took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript.
References
- 1.Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M.. et al. (2015) Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation 131, e29–e322 [DOI] [PubMed] [Google Scholar]
- 2.Townsend N., Nichols M., Scarborough P. and Rayner M. (2015) Cardiovascular disease in Europe 2015: epidemiological update. Eur. Heart J. 36, 2673–2674 10.1093/eurheartj/ehv428 [DOI] [PubMed] [Google Scholar]
- 3.Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R.M., Tanaka H.. et al. (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 10.1016/S0092-8674(00)80949-6 [DOI] [PubMed] [Google Scholar]
- 4.de Lecea L., Kilduff T.S., Peyron C., Gao X., Foye P.E., Danielson P.E.. et al. (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U.S.A. 95, 322–327 10.1073/pnas.95.1.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrive P. and Kuwaki T. (2017) Orexin and central modulation of cardiovascular and respiratory function. Curr. Top. Behav. Neurosci. 33, 157–196 10.1007/7854_2016_46 [DOI] [PubMed] [Google Scholar]
- 6.Peyron C., Tighe D.K., van den Pol A.N., de Lecea L., Heller H.C., Sutcliffe J.G.. et al. (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 18, 9996–10015 10.1523/JNEUROSCI.18-23-09996.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Digby J.E., Chen J., Tang J.Y., Lehnert H., Matthews R.N. and Randeva H.S. (2006) Orexin receptor expression in human adipose tissue: effects of orexin-A and orexin-B. J. Endocrinol. 191, 129–136 10.1677/joe.1.06886 [DOI] [PubMed] [Google Scholar]
- 8.Salazar N.C., Chen J. and Rockman H.A. (2007) Cardiac GPCRs: GPCR signaling in healthy and failing hearts. Biochim. Biophys. Acta 1768, 1006–1018 10.1016/j.bbamem.2007.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S., Blache D., Vercoe P.E., Adam C.L., Blackberry M.A., Findlay P.A.. et al. (2005) Expression of orexin receptors in the brain and peripheral tissues of the male sheep. Regul. Pept. 124, 81–87 10.1016/j.regpep.2004.07.010 [DOI] [PubMed] [Google Scholar]
- 10.Shirasaka T., Miyahara S., Kunitake T., Jin Q.H., Kato K., Takasaki M.. et al. (2001) Orexin depolarizes rat hypothalamic paraventricular nucleus neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R1114–R1118 10.1152/ajpregu.2001.281.4.R1114 [DOI] [PubMed] [Google Scholar]
- 11.Kannan H., Hayashida Y. and Yamashita H. (1989) Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am. J. Physiol. 256, R1325–R1330 [DOI] [PubMed] [Google Scholar]
- 12.Kuru M., Ueta Y., Serino R., Nakazato M., Yamamoto Y., Shibuya I.. et al. (2000) Centrally administered orexin/hypocretin activates HPA axis in rats. Neuroreport 11, 1977–1980 10.1097/00001756-200006260-00034 [DOI] [PubMed] [Google Scholar]
- 13.Samson W.K., Gosnell B., Chang J.K., Resch Z.T. and Murphy T.C. (1999) Cardiovascular regulatory actions of the hypocretins in brain. Brain Res. 831, 248–253 10.1016/S0006-8993(99)01457-2 [DOI] [PubMed] [Google Scholar]
- 14.Shirasaka T., Nakazato M., Matsukura S., Takasaki M. and Kannan H. (1999) Sympathetic and cardiovascular actions of orexins in conscious rats. Am. J. Physiol. 277, R1780–R1785 [DOI] [PubMed] [Google Scholar]
- 15.Chen C.T., Hwang L.L., Chang J.K. and Dun N.J. (2000) Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R692–R697 10.1152/ajpregu.2000.278.3.R692 [DOI] [PubMed] [Google Scholar]
- 16.Smith P.M., Samson W.K. and Ferguson A.V. (2007) Cardiovascular actions of orexin-A in the rat subfornical organ. J. Neuroendocrinol. 19, 7–13 10.1111/j.1365-2826.2006.01497.x [DOI] [PubMed] [Google Scholar]
- 17.Jochem J., Zwirska-Korczala K., Zabielski R., Kato I. and Kuwahara A. (2006) Cardiovascular effects of centrally acting orexin A in haemorrhage-shocked rats. J. Physiol. Pharmacol. 57, 115–124 [PubMed] [Google Scholar]
- 18.Karteris E., Chen J. and Randeva H.S. (2004) Expression of human prepro-orexin and signaling characteristics of orexin receptors in the male reproductive system. J. Clin. Endocrinol. Metab. 89, 1957–1962 10.1210/jc.2003-031778 [DOI] [PubMed] [Google Scholar]
- 19.Randeva H.S., Karteris E., Grammatopoulos D. and Hillhouse E.W. (2001) Expression of orexin-A and functional orexin type 2 receptors in the human adult adrenals: implications for adrenal function and energy homeostasis. J. Clin. Endocrinol. Metab. 86, 4808–4813 10.1210/jcem.86.10.7921 [DOI] [PubMed] [Google Scholar]
- 20.Johren O., Bruggemann N. and Dominiak P. (2004) Orexins (hypocretins) and adrenal function. Horm. Metab. Res. 36, 370–375 10.1055/s-2004-814569 [DOI] [PubMed] [Google Scholar]
- 21.Rodrigo G.C., Lawrence C.L. and Standen N.B. (2002) Dinitrophenol pretreatment of rat ventricular myocytes protects against damage by metabolic inhibition and reperfusion. J. Mol. Cell Cardiol. 34, 555–569 10.1006/jmcc.2002.1536 [DOI] [PubMed] [Google Scholar]
- 22.Karteris E., Machado R.J., Chen J., Zervou S., Hillhouse E.W. and Randeva H.S. (2005) Food deprivation differentially modulates orexin receptor expression and signaling in rat hypothalamus and adrenal cortex. Am. J. Physiol. Endocrinol. Metab. 288, E1089–E1100 10.1152/ajpendo.00351.2004 [DOI] [PubMed] [Google Scholar]
- 23.Fauconnier J., Bedut S., Le Guennec J.-Y., Babuty D. and Richard S. (2003) Ca2+ current-mediated regulation of action potential by pacing rate in rat ventricular myocytes. Cardiovasc. Res. 57, 670–680 10.1016/S0008-6363(02)00731-9 [DOI] [PubMed] [Google Scholar]
- 24.Karteris E., Hillhouse E.W. and Grammatopoulos D. (2004) Urocortin II is expressed in human pregnant myometrial cells and regulates myosin light chain phosphorylation: potential role of the type-2 corticotropin-releasing hormone receptor in the control of myometrial contractility. Endocrinology 145, 890–900 10.1210/en.2003-1210 [DOI] [PubMed] [Google Scholar]
- 25.Bose A.K., Mocanu M.M., Carr R.D. and Yellon D.M. (2007) Myocardial ischaemia-reperfusion injury is attenuated by intact glucagon like peptide-1 (GLP-1) in the in vitro rat heart and may involve the p70s6K pathway. Cardiovasc. Drugs Ther. 21, 253–256 10.1007/s10557-007-6030-6 [DOI] [PubMed] [Google Scholar]
- 26.Sharma A. and Singh M. (2000) Effect of ethylisopropyl amiloride, a Na+ - H+ exchange inhibitor, on cardioprotective effect of ischaemic and angiotensin preconditioning. Mol. Cell. Biochem. 214, 31–38 10.1023/A:1007167519596 [DOI] [PubMed] [Google Scholar]
- 27.Baxter G.F., Marber M.S., Patel V.C. and Yellon D.M. (1994) Adenosine receptor involvement in a delayed phase of myocardial protection 24 hours after ischemic preconditioning. Circulation 90, 2993–3000 10.1161/01.CIR.90.6.2993 [DOI] [PubMed] [Google Scholar]
- 28.Woolfson R.G., Patel V.C. and Yellon D.M. (1996) Pre-conditioning with adenosine leads to concentration-dependent infarct size reduction in the isolated rabbit heart. Cardiovasc. Res. 31, 148–151 10.1016/S0008-6363(95)00185-9 [DOI] [PubMed] [Google Scholar]
- 29.Wu Y., Yin X., Wijaya C., Huang M-H and McConnell B.K. (2011) Acute myocardial infarction in rats. J. Vis. Exp. 48, 2464 10.3791/2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaffl M.W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maulik A., Davidson S.M., Piotrowska I., Walker M. and Yellon D.M. (2018) Ischaemic preconditioning protects cardiomyocytes from anthracycline-induced toxicity via the PI3K pathway. Cardiovasc. Drugs Ther. 32 (3), 245–253 10.1007/s10557-018-6793-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calebiro D., Godbole A., Lyga S. and Lohse M.J. (2015) Trafficking and function of GPCRs in the endosomal compartment. Methods Mol. Biol. 1234, 197–211 10.1007/978-1-4939-1755-6_16 [DOI] [PubMed] [Google Scholar]
- 33.Hausenloy D.J., Tsang A., Mocanu M.M. and Yellon D.M. (2005) Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am. J. Physiol. Heart Circ. Physiol. 288, H971–H976 10.1152/ajpheart.00374.2004 [DOI] [PubMed] [Google Scholar]
- 34.Jardine D.L., Charles C.J., Ashton R.K., Bennett S.I., Whitehead M., Frampton C.M.. et al. (2005) Increased cardiac sympathetic nerve activity following acute myocardial infarction in a sheep model. J. Physiol. 565, 325–333 10.1113/jphysiol.2004.082198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmqvist T., Johansson L., Ostman M., Ammoun S., Akerman K.E.O. and Kukkonen J.P. (2005) OX1 orexin receptors couple to adenylyl cyclase regulation via multiple mechanisms. J. Biol. Chem. 280, 6570–6579 10.1074/jbc.M407397200 [DOI] [PubMed] [Google Scholar]
- 36.Layland J., Solaro R.J. and Shah A.M. (2005) Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc. Res. 66, 12–21 10.1016/j.cardiores.2004.12.022 [DOI] [PubMed] [Google Scholar]
- 37.Morano I. (1999) Tuning the human heart molecular motors by myosin light chains. J. Mol. Med. (Berl.) 77, 544–555 10.1007/s001099900031 [DOI] [PubMed] [Google Scholar]
- 38.Boss C. and Roch C. (2015) Recent trends in orexin research-2010 to 2015. Bioorg. Med. Chem. Lett. 25, 2875–2887 10.1016/j.bmcl.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 39.Karteris E. and Randeva H.S. (2003) Orexin receptors and G-protein coupling: evidence for another “promiscuous” seven transmembrane domain receptor. J. Pharmacol. Sci. 93, 126–128 10.1254/jphs.93.126 [DOI] [PubMed] [Google Scholar]
- 40.Chen J. and Randeva H.S. (2010) Genomic organization and regulation of the human orexin (hypocretin) receptor 2 gene: identification of alternative promoters. Biochem. J. 427, 377–390 10.1042/BJ20091755 [DOI] [PubMed] [Google Scholar]
- 41.Baimel C., Bartlett S.E., Chiou L.-C., Lawrence A.J., Muschamp J.W., Patkar O.. et al. (2015) Orexin/hypocretin role in reward: implications for opioid and other addictions. Br. J. Pharmacol. 172, 334–348 10.1111/bph.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ono K., Kai A., Honda E. and Inenaga K. (2008) Hypocretin-1/orexin-A activates subfornical organ neurons of rats. Neuroreport 19, 69–73 10.1097/WNR.0b013e3282f32d64 [DOI] [PubMed] [Google Scholar]
- 43.Wojciechowicz T., Skrzypski M., Szczepankiewicz D., Hertig I., Kolodziejski P.A., Billert M.. et al. (2016) Original Research: Orexins A and B stimulate proliferation and differentiation of porcine preadipocytes. Exp. Biol. Med. (Maywood) 241, 1786–1795 10.1177/1535370216649261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grammatopoulos D.K., Randeva H.S., Levine M.A., Katsanou E.S. and Hillhouse E.W. (2000) Urocortin, but not corticotropin-releasing hormone (CRH), activates the mitogen-activated protein kinase signal transduction pathway in human pregnant myometrium: an effect mediated via R1alpha and R2beta CRH receptor subtypes and stimulation of Gq-protei. Mol. Endocrinol. 14, 2076–2091 [DOI] [PubMed] [Google Scholar]
- 45.Thompson M.D., Sakurai T., Rainero I., Maj M.C. and Kukkonen J.P. (2017) Orexin receptor multimerization versus functional interactions: neuropharmacological implications for opioid and cannabinoid signalling and pharmacogenetics. Pharmaceuticals (Basel) 10, 10.3390/ph10040079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson M.D., Comings D.E., Abu-Ghazalah R., Jereseh Y., Lin L., Wade J.. et al. (2004) Variants of the orexin2/hcrt2 receptor gene identified in patients with excessive daytime sleepiness and patients with Tourette’s syndrome comorbidity. Am. J. Med. Genet. B Neuropsychiatr. Genet. 129B, 69–75 10.1002/ajmg.b.30047 [DOI] [PubMed] [Google Scholar]
- 47.Bopassa J.C., Eghbali M., Toro L. and Stefani E. (2010) A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 298, H16–H23 10.1152/ajpheart.00588.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sokolowska P., Urbanska A., Bieganska K., Wagner W., Ciszewski W., Namiecinska M.. et al. (2014) Orexins protect neuronal cell cultures against hypoxic stress: an involvement of Akt signaling. J. Mol. Neurosci. 52, 48–55 10.1007/s12031-013-0165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J., Karteris E., Collins D. and Randeva H.S. (2006) Differential expression of mouse orexin receptor type-2 (OX2R) variants in the mouse brain. Brain Res. 1103, 20–24 10.1016/j.brainres.2006.05.054 [DOI] [PubMed] [Google Scholar]
- 50.Ramanjaneya M., Conner A.C., Chen J., Kumar P., Brown J.E.P., Johren O.. et al. (2009) Orexin-stimulated MAP kinase cascades are activated through multiple G-protein signalling pathways in human H295R adrenocortical cells: diverse roles for orexins A and B. J. Endocrinol. 202, 249–261 10.1677/JOE-08-0536 [DOI] [PubMed] [Google Scholar]
- 51.Rodrigo G.C., Davies N.W. and Standen N.B. (2004) Diazoxide causes early activation of cardiac sarcolemmal KATP channels during metabolic inhibition by an indirect mechanism. Cardiovasc. Res. 61, 570–579 10.1016/j.cardiores.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 52.Morano I., Ritter O., Bonz A., Timek T., Vahl C.F. and Michel G. (1995) Myosin light chain-actin interaction regulates cardiac contractility. Circ. Res. 76, 720–725 10.1161/01.RES.76.5.720 [DOI] [PubMed] [Google Scholar]
- 53.Morano I., Hofmann F., Zimmer M. and Ruegg J.C. (1985) The influence of P-light chain phosphorylation by myosin light chain kinase on the calcium sensitivity of chemically skinned heart fibres. FEBS Lett. 189, 221–224 10.1016/0014-5793(85)81027-9 [DOI] [PubMed] [Google Scholar]
- 54.Franks K., Cooke R. and Stull J.T. (1984) Myosin phosphorylation decreases the ATPase activity of cardiac myofibrils. J. Mol. Cell Cardiol. 16, 597–604 10.1016/S0022-2828(84)80624-0 [DOI] [PubMed] [Google Scholar]
- 55.Spinazzi R., Ziolkowska A., Neri G., Nowak M., Rebuffat P., Nussdorfer G.G.. et al. (2005) Orexins modulate the growth of cultured rat adrenocortical cells, acting through type 1 and type 2 receptors coupled to the MAPK p42/p44- and p38-dependent cascades. Int. J. Mol. Med. 15, 847–852 [PubMed] [Google Scholar]
- 56.Rapundalo S.T. (1998) Cardiac protein phosphorylation: functional and pathophysiological correlates. Cardiovasc. Res. 38, 559–588 10.1016/S0008-6363(98)00063-7 [DOI] [PubMed] [Google Scholar]
- 57.Filatov V.L., Katrukha A.G., Bulargina T V. and Gusev N.B. (1999) Troponin: structure, properties, and mechanism of functioning. Biochemistry (Mosc.) 64, 969–985 [PubMed] [Google Scholar]
- 58.Spinazzi R., Andreis P.G., Rossi G.P. and Nussdorfer G.G. (2006) Orexins in the regulation of the hypothalamic-pituitary-adrenal axis. Pharmacol. Rev. 58, 46–57 10.1124/pr.58.1.4 [DOI] [PubMed] [Google Scholar]
- 59.Bers D.M. (2008) Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 70, 23–49 10.1146/annurev.physiol.70.113006.100455 [DOI] [PubMed] [Google Scholar]
- 60.Kockskamper J., Zima A.V., Roderick H.L., Pieske B., Blatter L.A. and Bootman M.D. (2008) Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J. Mol. Cell Cardiol. 45, 128–147 10.1016/j.yjmcc.2008.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu X., Zhang T., Bossuyt J., Li X., McKinsey T.A., Dedman J.R.. et al. (2006) Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J. Clin. Invest. 116, 675–682 10.1172/JCI27374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D’Angelo G. and Adam L.P. (2002) Inhibition of ERK attenuates force development by lowering myosin light chain phosphorylation. Am. J. Physiol. Heart Circ. Physiol. 282, H602–H610 10.1152/ajpheart.00221.2001 [DOI] [PubMed] [Google Scholar]
- 63.Wijnker P.J.M., Murphy A.M., Stienen G.J.M. and van der Velden J. (2014) Troponin I phosphorylation in human myocardium in health and disease. Neth. Heart J. 22, 463–469 10.1007/s12471-014-0590-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szczepanska-Sadowska E., Cudnoch-Jedrzejewska A., Ufnal M. and Zera T. (2010) Brain and cardiovascular diseases: common neurogenic background of cardiovascular, metabolic and inflammatory diseases. J. Physiol. Pharmacol. 61, 509–521 [PubMed] [Google Scholar]
- 65.Yoshida T., Tabony A.M., Galvez S., Mitch W.E., Higashi Y., Sukhanov S.. et al. (2013) Molecular mechanisms and signaling pathways of angiotensin II-induced muscle wasting: potential therapeutic targets for cardiac cachexia. Int. J. Biochem. Cell Biol. 45, 2322–2332 10.1016/j.biocel.2013.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hayward L.F., Hampton E.E., Ferreira L.F., Christou D.D., Yoo J.-K., Hernandez M.E.. et al. (2015) Chronic heart failure alters orexin and melanin concentrating hormone but not corticotrophin releasing hormone-related gene expression in the brain of male Lewis rats. Neuropeptides 52, 67–72 10.1016/j.npep.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 67.Perez M V., Pavlovic A., Shang C., Wheeler M.T., Miller C.L., Liu J.. et al. (2015) Systems genomics identifies a key role for hypocretin/orexin Receptor-2 in human heart failure. J. Am. Coll. Cardiol. 66, 2522–2533 10.1016/j.jacc.2015.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Christopher J.A. (2014) Small-molecule antagonists of the orexin receptors. Pharm. Pat. Anal. 3, 625–638 10.4155/ppa.14.46 [DOI] [PubMed] [Google Scholar]
- 69.Pan S., Cabral C.S., Ashley E.A. and Perez M.V. (2017) Orexin: a missing link between sleep disorders and heart failure? Curr. Heart Fail. Rep. 14, 100–105 10.1007/s11897-017-0322-3 [DOI] [PubMed] [Google Scholar]
- 70.Toth M.J., Ward K., van der Velden J., Miller M.S., Vanburen P., Lewinter M.M.. et al. (2011) Chronic heart failure reduces Akt phosphorylation in human skeletal muscle: relationship to muscle size and function. J. Appl. Physiol. 110, 892–900 10.1152/japplphysiol.00545.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chaanine A.H. and Hajjar R.J. (2011) AKT signalling in the failing heart. Eur. J. Heart Fail. 13, 825–829 10.1093/eurjhf/hfr080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pond A.L., Scheve B.K., Benedict A.T., Petrecca K., Van Wagoner D.R., Shrier A.. et al. (2000) Expression of distinct ERG proteins in rat, mouse, and human heart. Relation to functional I(Kr) channels. J. Biol. Chem. 275, 5997–6006 10.1074/jbc.275.8.5997 [DOI] [PubMed] [Google Scholar]
- 73.Huang S.-C., Dai Y.-W.E., Lee Y.-H., Chiou L.-C. and Hwang L.-L. (2010) Orexins depolarize rostral ventrolateral medulla neurons and increase arterial pressure and heart rate in rats mainly via orexin 2 receptors. J. Pharmacol. Exp. Ther. 334, 522–529 10.1124/jpet.110.167791 [DOI] [PubMed] [Google Scholar]