Short abstract
Migraine is the seventh most disabling disorder globally, with prevalence of 11.7% worldwide. One of the prevailing mechanisms is the activation of the trigeminovascular system, and calcitonin gene-related peptide (CGRP) is an important therapeutic target for migraine in this system. Recent studies suggested an emerging role of pituitary adenylate cyclase-activating peptide (PACAP) in migraine. However, the relation between CGRP and PACAP and the role of PACAP in migraine remain undefined. In this study, we established a novel repetitive (one, three, and seven days) electrical stimulation model by stimulating dura mater in conscious rats. Then, we determined expression patterns in the trigeminal ganglion and the trigeminal nucleus caudalis of the trigeminovascular system. Electrical stimulation decreased facial mechanical thresholds, and the order of sensitivity was as follows: vibrissal pad >inner canthus >outer canthus (P < 0.001). The electrical stimulation group exhibited head-turning and head-flicks (P < 0.05) nociceptive behaviors. Importantly, electrical stimulation increased the expressions of CGRP, PACAP, and the PACAP-preferring type 1 (PAC1) receptor in both trigeminal ganglion and trigeminal nucleus caudalis (P < 0.05). The expressions of two vasoactive intestinal peptide (VIP)-shared type 2 (VPAC1 and VPAC2) receptors were increased in the trigeminal ganglion, whereas in the trigeminal nucleus caudalis, their increases were peaked on Day 3 and then decreased by Day 7. PACAP was colocalized with NEUronal Nuclei (NeuN), PAC1, and CGRP in both trigeminal ganglion and the trigeminal nucleus caudalis. Our results demonstrate that the repetitive electrical stimulation model can simulate the allodynia during the migraine chronification, and PACAP plays a role in the pathogenesis of migraine potentially via PAC1 receptor.
Keywords: Migraine, electrical stimulation, calcitonin gene-related peptide, pituitary adenylate cyclase-activating peptide, PAC1 receptor
Introduction
Migraine is a severe brain disorder, listed as the seventh most disabling disorder globally by the World Health Organization,1 with prevalence of 11.7% worldwide2 and 9.3% in China.3 One of the prevailing mechanisms of migraine is the activation of the trigeminovascular system, which results in the pain feeling associated with multiple head regions. Nociceptive nerve fibers from the peripheral terminals of the trigeminal ganglion (TG) innervate intra- and extracranial vasculature and meninges. Applying mechanical stimulation, chemical stimulation, or electrical stimulation (ES) to these structures, particularly to the dura mater, can result in migraine-like headache, which provides a reasonable and feasible way to simulate migraine in animal models. Central terminals of the TG project to second-order neurons in the spinal trigeminal nucleus caudalis (Sp5C) and the upper cervical spinal cord (C1–C2), which together are named the trigeminocervical complex (TCC). Afferents from the TCC then synapse on third-order thalamocortical neurons.4 Vasoactive neuropeptides such as calcitonin gene-related peptide (CGRP) and pituitary adenylate cyclase-activating peptide (PACAP) are thought to be released from the nociceptive fibers innervating dura mater upon stimulation, causing vasodilation of dura vessels.5,6
The role of PACAP in migraine has gained increasing attention recently.7–10 Evidence from three aspects supports the involvement of PACAP in the pathogenesis of migraine: (1) PACAP infusion induced migraine-like attacks in migraine patients without aura,11,12 (2) plasma PACAP levels were increased in the ictal period of migraine attacks and were decreased in the interictal period,13,14 and (3) PACAP infusion caused headache and dilation of middle meningeal artery in healthy volunteers, which could be reversed by migraine abortive treatments.15 Some animal studies applied exogenous PACAP16 or used isolated tissue17 to study the role of PACAP in migraine. We propose that at least two important questions are left unanswered: (1) what is the role of endogenous PACAP in migraine? and (2) Is there any relationship between PACAP and migraine-like behaviors in conscious animals?
The effects of PACAP are mediated through G-protein-coupled receptors (GPCRs): two vasoactive intestinal peptide (VIP)-shared type 2 (VPAC1 and VPAC2) receptors, and PACAP-preferring type 1 (PAC1) receptor. VPAC1 and VPAC2 have the same affinity for PACAP and VIP, whereas PAC1 has a 1000-fold higher affinity for PACAP.18 Based on the structural and functional similarities between PACAP and VIP, Amin et al. compared their effects in female migraine patients without aura. Interestingly, infusion of PACAP caused migraine-like attacks in 73% patients (16 of the 22), whereas VIP caused attacks in 18% patients (4 of the 22),19 suggesting that the role of PACAP in migraine might be mediated by PAC1 receptor. However, previous studies arrived at inconsistent conclusions on the question that which is the specific receptor of PACAP in the pathogenesis of migraine.17,20,21 Our recent study established a chronic migraine model by repetitively stimulating dura mater using inflammatory soup. We found a decrease in PACAP level and a selective increase in PAC1 level, suggesting that PACAP is involved in the development of migraine potentially through PAC1 receptor.22
The role of CGRP in migraine has been intensively studied since 1990s.23–25 Recently, CGRP receptor antagonists and CGRP antibodies have been approved effective in the acute treatment and the prevention of migraine, respectively.26–28 PACAP shares many similarities with CGRP, such as abilities of inducing light aversion in mice and causing vasodilation during neurogenic inflammation.29 However, the relationship between CGRP and PACAP in migraine remains undefined. Therefore, this study established a novel migraine model by repetitively stimulating the dura mater surrounding the superior sagittal sinus electrically in conscious adult rat and then investigated the expressions of PACAP, PAC1, VPAC1, VPAC2, and CGRP in the TG and the trigeminal nucleus caudalis (TNC) of the trigeminovascular system, hoping to reveal the role of PACAP in the pathogenesis of migraine.
Materials and methods
Animals
Since migraine is three-fold more prevalent in females than in males,30 and that a part of (<10%) female patients suffer from menstrual migraine,31 only male animals were utilized in this study to minimize the influence of menstrual cycles. Male Sprague–Dawley rats (n = 24, weight: 180–200 g) were housed individually in a temperature-controlled (22 ± 2°C) and light-controlled (12 h dark/light cycle with the light turned on at 07:00 a.m.) environment with free access to food and water. This study was approved by the Committee on Animal Use for Research and Education of the Laboratory Animals Centre at Chinese PLA General Hospital (Beijing, China), following the ethical guidelines for experimental pain in conscious animals to minimize the suffering.32
Experimental design
Rats were randomly assigned into four groups (n = 6 in each group): Sham stimulation for seven-day group (Sham), ES for one-day group (ES-1D), ES for three-day group (ES-3D), ES for seven-day group (ES-7D). Rats were allowed to habituate in the home cage for three days before the surgery, during which the basal mechanical thresholds (MTs) of facial areas (outer canthus, inner canthus, and vibrissal pad) were determined using von Frey monofilaments (North Coast Medical Co., Ltd., USA). Stimulation electrodes were implanted as previously described to stimulate the dura mater surrounding the superior sagittal sinus.33,34 After the surgery, rats were allowed to recover for three days to ensure that MTs were back to baseline. The experimental apparatus and detailed stimulation procedures were applied as previously described.33 Rats in the ES-1D, ES-3D, and ES-7D groups received an ES (10 min, 20 Hz, 250 μs pulse duration, and 3–5 mA intensity) once daily for one day, consecutive three days, and seven days, respectively. Rats in the Sham group (Sham) were only connected to the stimulator without stimulation 10 min daily for seven days to control the possible influences of surgical and experimental procedures. Behaviors during the stimulating period (10 min) were recorded and analyzed by two researchers who were blind to the experimental design. Before daily stimulus, baselines of facial MTs on each day were determined using von Frey monofilaments. Afterward, facial MTs were determined at 0 min, 30 min, 60 min, and 90 min after stimulus.
Facial MT
Six patches of facial skin were tested clockwise (left vibrissal pad, left inner canthus, left outer canthus, right outer canthus, right inner canthus, and then right vibrissal pad) with von Frey filaments of force values determined in preexperiments (26, 15, 10, 8, 6, 4, 2, and 1 g, rats that did not respond to 26 g were assigned as 26 g) using an “up-down” paradigm.35 Von Frey filaments were applied perpendicularly to the tested area for 3 to 6 s until a positive response were observed: asymmetrical face grooming, withdraw, escape, or attack response. The force value at which two positive responses were noted in three trials was recorded as the MT. Average integrated value of bilateral areas was accepted as the final result for each animal.
Tissue preparation
Rats in different groups were humanely sacrificed at 90 min after the final stimulus. The TNC and bilateral TGs were carefully isolated and embedded in Tissue-Tek OCT Compound (Sakura Finetek, Torrance, CA, USA). Then, the base mold containing embedded tissue was submerged into liquid nitrogen for 10 to 20 s till the entire tissue block being frozen completely. Frozen tissue blocks were kept in −80°C and were placed in −20°C for 24 h prior to sectioning. Frozen tissue blocks were cut into 20-µm-thick serial sections using a freezing microtome (CM1850; Leica, Wetzlar, Germany), followed by fixation in precooled acetone (−20°C) for 20 min. Hematoxylin and eosin (H&E) staining and Nissl staining were carried out following standard protocols.36
To control for the uneven distribution of TG neurons, we prepared 12 sets of sequential slides per animal (6 × 20-µm-thick sections/slide) using an “S-shape” strategy, which means that Slide 1 had the 1st, 13th, 25th, 37th, 49th, and 61st sequential sections of the TG. In addition, slides of the same number (e.g. Slide 1) from different animals were used for the staining of one specified marker (e.g. immunohistochemistry (IHC) for PACAP). This strategy would hopefully lessen the disturbance of uneven distribution of the TG neurons and provide an average estimation of the whole TG neurons.
Immunohistochemistry
Frozen sections underwent heat-induced epitope retrieval (submerged in 90°C water bath for 2 min. Retrieval solution: 0.3% sodium citrate, 0.04% citric acid, and pH 6.0), endogenous enzyme interference (incubated in 3% H2O2 solution), and endogenous biotin interference (incubated in IHC Biotin Block Kit, BLK-0002; Maixin Biological Technology, Ltd., Fujian, China). Afterward, sections were blocked with 10% goat serum (ZLI-9005, ZSGB-BIO, China) at 37°C for 1 h and were incubated at 4°C overnight with respective diluted primary antibodies (Table 1). On the second day, sections were incubated with secondary antibody for 1 h at room temperature (HRP-Polymer anti-Mouse/Rabbit IHC Kit, KIT-5030; Maixin Biological Technology, Ltd.), followed by incubation of 3,3′-diaminobenzidine (ZLI-9018, ZSGB-BIO, China) for 1 to 5 min at room temperature.
Table 1.
Primary Antibodies Used for Immunohistochemistry.
| Name | Host | Dilutions | Catalog | Source | Citations |
|---|---|---|---|---|---|
| PACAP | Mouse | 1:80 | sc-25439 | Santa Cruz, USA | Castorina et al.37 |
| CGRP | Mouse | 1:50 | ab81887 | Abcam, UK | Eftekhari et al.38 |
| PAC1 | Mouse | 1:400 | ab54980 | Abcam, UK | Kanasaki et al.39 |
| VPAC1 | Rabbit | 1:50 | sc-30019 | Santa Cruz, USA | Csati et al.40 |
| VPAC2 | Rabbit | 1:50 | sc-30020 | Santa Cruz, USA | Csati et al.40 |
CGRP: calcitonin gene-related peptide; PACAP: pituitary adenylate cyclase-activating peptide; PAC1: ■; VPAC1: ■; VPAC2: ■.
Immunofluorescence
Frozen sections underwent endogenous enzyme interference and endogenous biotin interference and blocking as described earlier. Thereafter, sections were incubated at 4°C overnight with combinations of diluted primary antibodies: anti-PACAP and anti-NEUronal Nuclei (NeuN), anti-PACAP and anti-PAC1, and anti-PACAP and anti-CGRP (Table 2). On the second day, sections were incubated with the combination of rabbit immunoglobulin (IgG) secondary antibody (1:500, A-11034; Thermo Fisher) and mouse IgG secondary antibody (1:1000, A-21424, Thermo Fisher). For the analysis of IHC and immunofluorescence (IF) results, six images (one image/section, six sections/animal) at 20× magnification of the TG and six images (bilateral images/section, three sections/animal) at 50× magnification of the TNC were randomly selected using a microscope (DP73; Olympus, Tokyo, Japan). Average integrated density of six images was accepted as the final result for each animal.
Table 2.
Primary Antibodies Used for Immunofluorescence.
| Name | Host | Dilutions | Catalog | Source | Citations |
|---|---|---|---|---|---|
| PACAP | Rabbit | 1:50 | ab174982 | Abcam, UK | Kortesi et al.41 |
| CGRP | Mouse | 1:50 | ab81887 | Abcam, UK | Eftekhari et al.38 |
| PAC1 | Mouse | 1:100 | ab54980 | Abcam, UK | Kanasaki et al.39 |
| NeuN | Mouse | 1:3000 | MAB337 | Merck Millipore, Germany | Borsani et al.42 |
CGRP: calcitonin gene-related peptide; PACAP: pituitary adenylate cyclase-activating peptide; PAC1: ■; NeuN: ■.
Statistical analysis
SPSS 19.0 software was applied for the statistical analysis, and Graphpad Prism 6 software was used to generate the graphs. Shapiro–Wilk test was first conducted to test the normality followed by Levene’s test for the homogeneity. Normally distributed data were analyzed using analysis of variance (ANOVA) and Fisher’s least significant difference test (with regular variance) or Dunnett’s T3 test (with irregular variance) for comparisons between the groups. For abnormally distributed data, Kruskal–Wallis test was used to determine differences among the groups. Repeated measures ANOVA was used to compare differences in MTs between groups. The results were accepted from “sphericity assumed” option if the assumption of Mauchly’s test was met or from “lower-bound” option if the assumption was violated. A value of P < 0.05 was considered significant.
Results
Repetitive ES of the dura mater decreased facial MTs via chronic effect
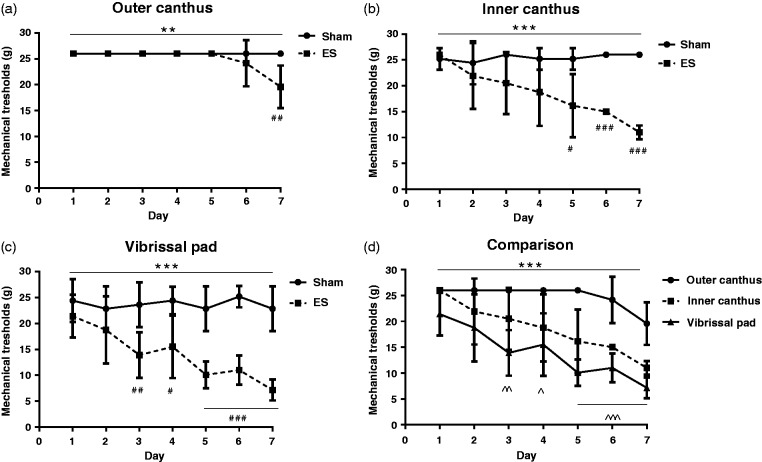
To investigate whether repetitive ES of the dura mater surrounding the superior sagittal sinus can simulate the allodynia of migraine, we first determined the MTs of outer canthus, inner canthus, and vibrissal pad before and after stimulation (within 90 min), which represented for the chronic and acute effects of ES, respectively. Figure 1 shows the changes in daily MTs before stimulation. The MTs of both outer canthus (a) and inner canthus (b) in the ES group decreased as the days of stimulation increased (P < 0.05), and there were significant differences between the ES group and the Sham group (a, **P < 0.01; b, ***P < 0.001). The most prominent time-dependent decrease was seen in vibrissal pad (c, P < 0.001). MTs of vibrissal pad in the ES group were significantly lower than those of the Sham group (***P < 0.001), beginning from Day 3 (##P < 0.01) and further lowering during Days 5 to 7 (###P < 0.001). (d) Collectively, there were significant differences in MTs among outer canthus, inner canthus, and vibrissal pad in the ES group (***P < 0.001), with MTs of vibrissal pad apparently lower than that of outer canthus beginning from Day 3 (Day 3: ^^P < 0.01; Day 4: ^P < 0.05; Days 5 to 7: ^^^P < 0.001).
Figure 1.
Daily MTs before stimulation decreased during the seven-day experiments. Data are represented by mean ± standard deviation, n = 6 for each group. (a) Outer canthus, (b) inner canthus, (c) vibrissal pad, and (d) comparing MTs of three facial areas: vibrissal pad showed fastest, and the most pronounced decrease during the seven-day experiment. Repeated measures analysis of variance test comparing between (among) groups: **P < 0.01, ***P < 0.001. Compared with the Sham group: #P <0.05; ##P<0.01; ###P < 0.001. Compared with outer canthus: ^P < 0.05; ^^P < 0.01; ^^^P<0.001.
ES: electrical stimulation.
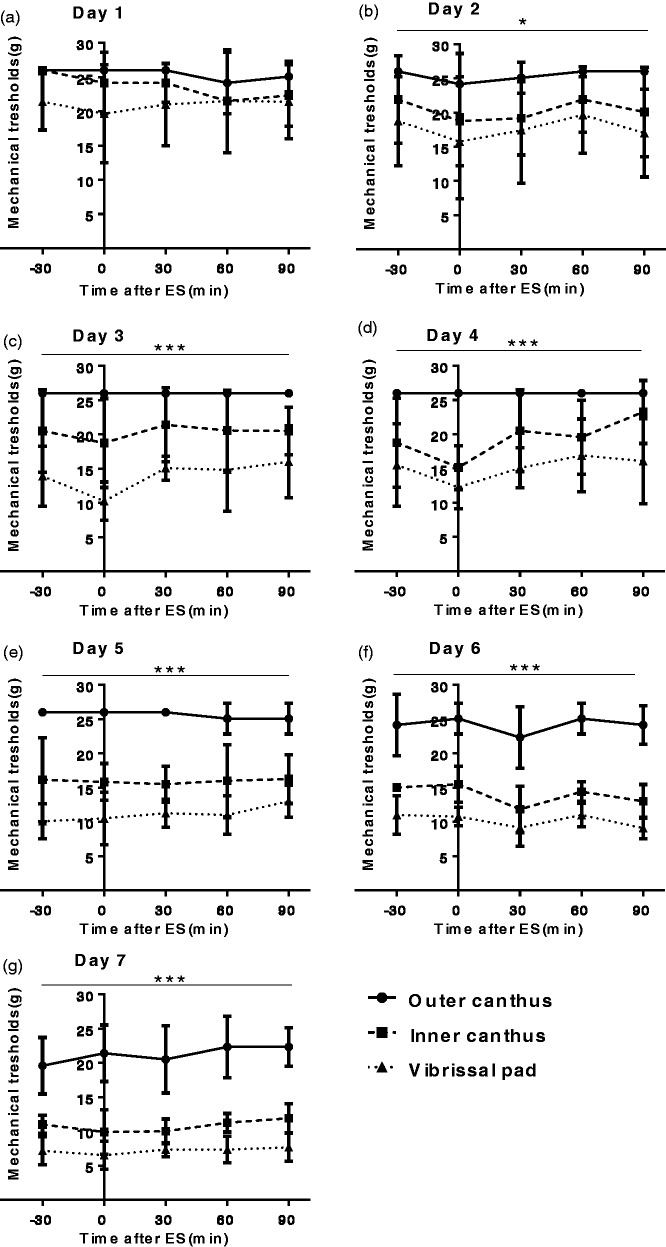
Figure 2.
Daily stimulation did not significantly alter facial MTs within 90 min. Data are represented by mean ± standard deviation, n = 6 for each group. (a) to (g) MTs of outer canthus, inner canthus, and vibrissal pad during 90 min after stimulation from Day 1 to Day 7. No significant relationships between time and MTs were found during the experimental period except on Day 4 (P < 0.01) and Day 7 (P < 0.05). There were significant differences among three areas since Day 2: *P < 0.05; ***P < 0.001.
ES: electrical stimulation.
To investigate whether acute effects of stimulation contributed to the gradual decrease in MTs in the ES group, we measured MTs of outer canthus, inner canthus, and vibrissal pad within 90 min after daily stimulation (Figure 2). Similarly, there were significant differences among three areas in the ES group starting from Day 2 (*P < 0.05) to Day 7 (Days 3 to 7: ***P < 0.001). However, no significant relationships between MTs and poststimulus time were found during the experimental period, except on Day 4 (P < 0.01) and Day 7 (P < 0.05). In other words, ES was not able to effectively lower MTs within 90 min after stimulation. Collectively, these results suggest that the repetitive ES can decrease the facial MTs potentially via chronic effects.
Nociceptive behaviors were induced in the repetitive ES model
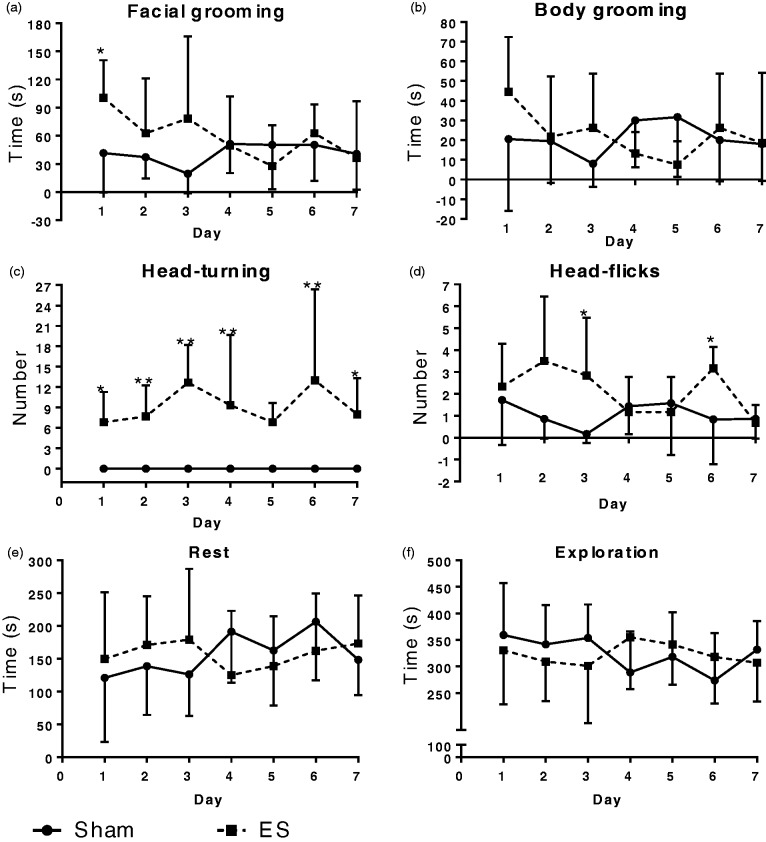
To evaluate whether the repetitive ES model can simulate the frequent onset of acute migraine, we investigated four kinds of nociceptive behaviors in conscious rats as described in our previous works33,43: facial grooming, body grooming, head-flicks, and head-turning. As shown in Figure 3(a), facial grooming time of the ES group was significantly higher than that of the Sham group on Day 1 (*P < 0.05), while no apparent differences were observed thereafter. In preexperiments, we noticed that body grooming was always observed alternatively with facial grooming. Given that facial grooming has been accepted as a kind of nociceptive behavior in the ES rat model of migraine,33 we investigated whether body grooming behavior could also be affected in the repetitive ES model. As expected, the trends of body grooming time were very similar to those of facial grooming time. However, no significant differences between groups were found during the experimental period (Figure 3(b)). Head-turning, characterized by turning head to one side of the body and keeping this position for 1 to 3 s, was only observed in the ES group (*P < 0.05, Figure 3(c)). Head-flicks is characterized by nonrhythmic quick shaking of head, and it often occurs before or after facial and body grooming. The overall numbers of the head-flicks in the ES group were not less than those of the Sham group, with significantly greater numbers on Days 3 and 6 (*P < 0.05, Figure 3(d)). We also analyzed exploration time during stimulation but did not find apparent differences between groups in rest and exploration behaviors (Figure 3(e) and (f)).
Figure 3.
Nociceptive behaviors were induced in the repetitive ES model. Data are represented by mean ± standard deviation, n = 6 for each group. (a) The ES group spent significant more time on facial grooming on Day 1. (b) No apparent differences in the body grooming time between groups were found. (c) The ES group exhibited head-turning behaviors throughout the experimental period. (d) The ES group had more head-flicks behavior than the Sham group. (e) and (f) No differences between groups were found in rest and exploration behaviors. Compared with Sham group: *P < 0.05; **P < 0.01.
ES: electrical stimulation.
Dynamic expressions of CGRP, PACAP, PAC1, VPAC1, and VPAC2 receptors in the TG and the TNC during repetitive ES
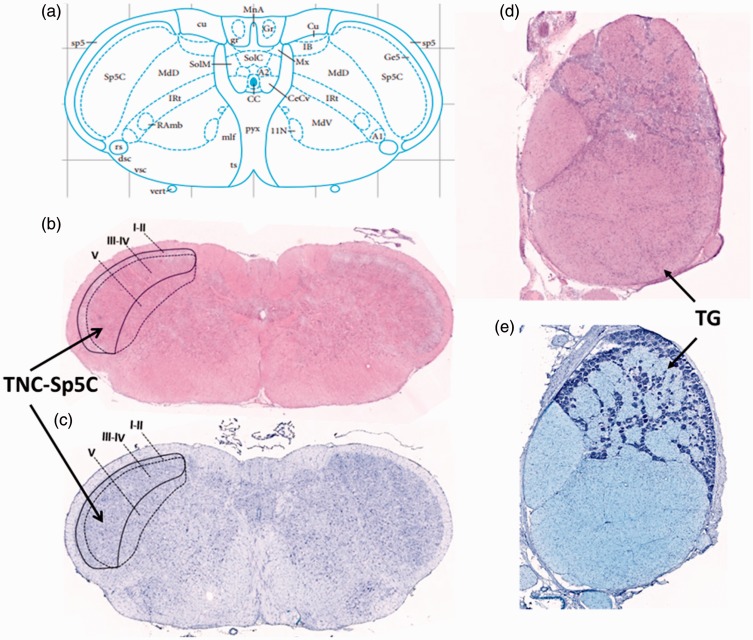
Based on the repetitive ES model, we investigated the number of cells that express CGRP, PACAP and its three receptors, namely, PAC1, VPAC1, VPAC2, in the TG and the TNC via IHC. We proposed to investigate the role of PACAP in migraine from two perspectives: (1) compare the expression patterns of CGRP and PACAP and further evaluate the model by dynamic expressions of CGRP and (2) investigate the expression patterns of PACAP receptors. Central afferent projections from the TG terminate in the spinal trigeminal nucleus caudalis (Sp5C) of the TNC.4 Recent study in rats also showed that descending cortical projections innervated by the ophthalmic (V1) branch of the trigeminal nerve, originating from contralateral insular and primary somatosensory (S1) cortices, terminate, respectively, in laminae I to II and III to V of the Sp5C.44 Therefore, the present study analyzed expression patterns of CGRP, PACAP, and its receptors in lamina III to V of the Sp5C (Figures 445and 5).
Figure 4.
Coordinates and structures of the TG and the TNC. (a) The stereotaxic coordinates of Sp5C in rat brain (adapted from Paxinos and Watson’s work). The H&E (b) and Nissl (c) staining of the TNC-Sp5C. The present study analyzed expression patterns of CGRP, PACAP, and its receptors in lamina III to V of the Sp5C. The H&E (d) and Nissl (e) staining of the TG.
TG: trigeminal ganglion; TNC: trigeminal nucleus caudalis; H&E: Hematoxylin and eosin; Sp5C :spinal trigeminal nucleus caudalis.
Figure 5.
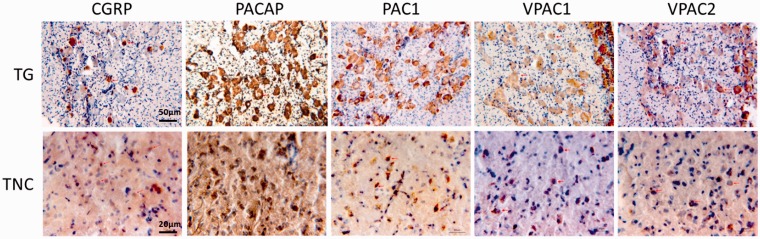
IHC staining of CGRP, PACAP, PAC1, VPAC1, and VPAC2 in the TG and the TNC. Images shown are randomly selected from the TG at 20× magnification and from the TNC at 50× magnification. PACAP-expressing fibers were found at lamina III to V of the Sp5C. CGRP-expressing fibers were mostly located at lamina I to II of the Sp5C (data not shown).
TG: trigeminal ganglion; TNC: trigeminal nucleus caudalis; CGRP: calcitonin gene-related peptide; PACAP: pituitary adenylate cyclase-activating peptide; PAC1: ■; VPAC1: ■; VPAC2: ■.
The number of cells expressing CGRP in both TG and TNC increased steadily as the stimuli repeated (Figure 6(a) and (b)): the number of positive cells of the ES group on Day 3 (**P < 0.01) and Day 7 (TG: ***P < 0.001; TNC: **P < 0.01) was significantly higher than that of the Sham group. Similar trends were seen in the expression patterns of PACAP (Figure 6(c) and (d)). PACAP+ cells in the TG built up as times of stimuli increased, with ES on Day 1, Day 3, and Day 7 significantly higher than the Sham group (***P < 0.001). Similarly, PACAP+ cells in the TNC were also elevated by the increasing stimuli: Day 3 (*P < 0.05) and Day 7 (**P < 0.01) were significantly higher than the Sham group.
Figure 6.
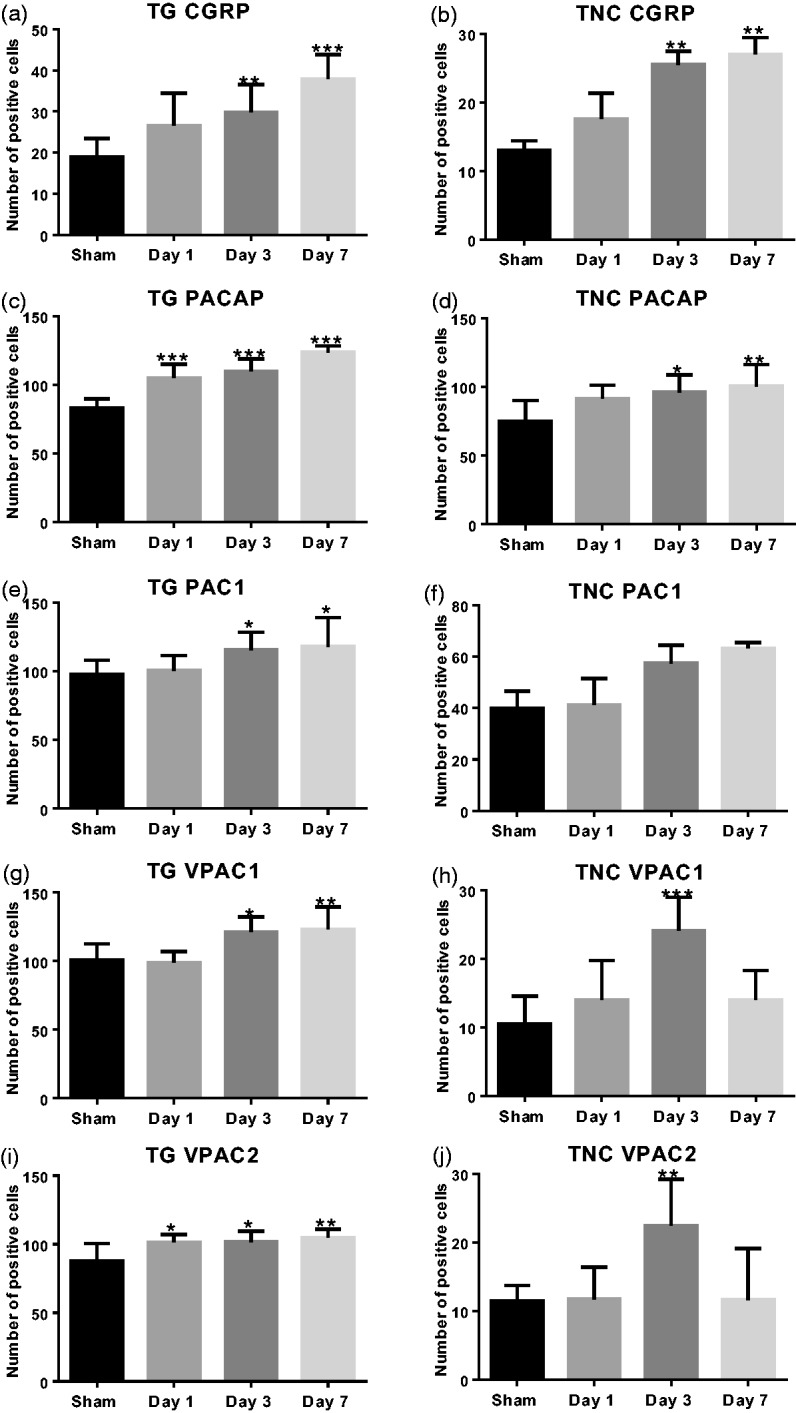
Dynamic expressions of CGRP, PACAP, PAC1, VPAC1, and VPAC2 receptors in the TG and the TNC during repetitive ES. Y-axis shows the average number of positive cells (six images per animal, six animals per group) at 20× magnification of the TG and at 50× magnification of the TNC as specified in “Materials and Methods” section. Data are represented by mean ± standard deviation. (a and b) The numbers of CGRP+ cells were increased in both TG and TNC during repetitive ES. (c and d) PACAP showed a similar pattern as CGRP: increased in both TG and TNC. (e and f) PAC1 receptor was increased from Day 3 to Day 7 in both TG and TNC. (g and h) VPAC1 receptor was increased from Day 3 to Day 7 in the TG, while in the TNC, it also peaked on Day 3 and then decreased by Day 7. (i and j) VPAC2 receptor reached a steady level from Day 1 to Day 7 in the TG, while in the TNC, it also peaked on Day 3 and then decreased by Day 7. Compared with the Sham group: *P < 0.05; **P < 0.01; ***P < 0.001.
TG: trigeminal ganglion; TNC: trigeminal nucleus caudalis; CGRP: calcitonin gene-related peptide; PACAP: pituitary adenylate cyclase-activating peptide; PAC1: ■; VPAC1: ■; VPAC2: ■.
Correspondingly, PAC1 receptor was also increased during the experimental period in both TG and TNC (Figure 6(e) and (f)). PAC1 was increased slowly, with levels on Day 1 being similar to the Sham group in both TG and TNC. Nevertheless, numbers of PAC1+ cells in both areas were higher on Day 3 (TG: *P < 0.05) and Day 7 (TG: *P < 0.05) compared with the Sham group. Interestingly, VPAC1 receptor and VPAC2 receptor exhibited more complex expression patterns, especially in the TNC. There were overt increases in VPAC1 expressing cells on Day 3 (*P < 0.05) and Day 7 (**P < 0.01) compared with the Sham group in the TG (Figure 6(g)). In the TNC, however, it went up during the first three days, reaching top on Day 3 (***P < 0.001), then went down on Day 7 (Figure 6(f)). In the TG, VPAC2 on Day 1 was significantly higher than the Sham group and remained at this steady level till Day 7 (Figure 6(i): *P < 0.05, **P < 0.01). The expression pattern of VPAC2 in the TNC was surprisingly similar with that of VPAC1: reached top on Day 3 (Figure 6(j): **P < 0.01) and went down on Day 7.
PACAP was colocalized with NeuN, PAC1 receptor, and CGRP in both TG and TNC
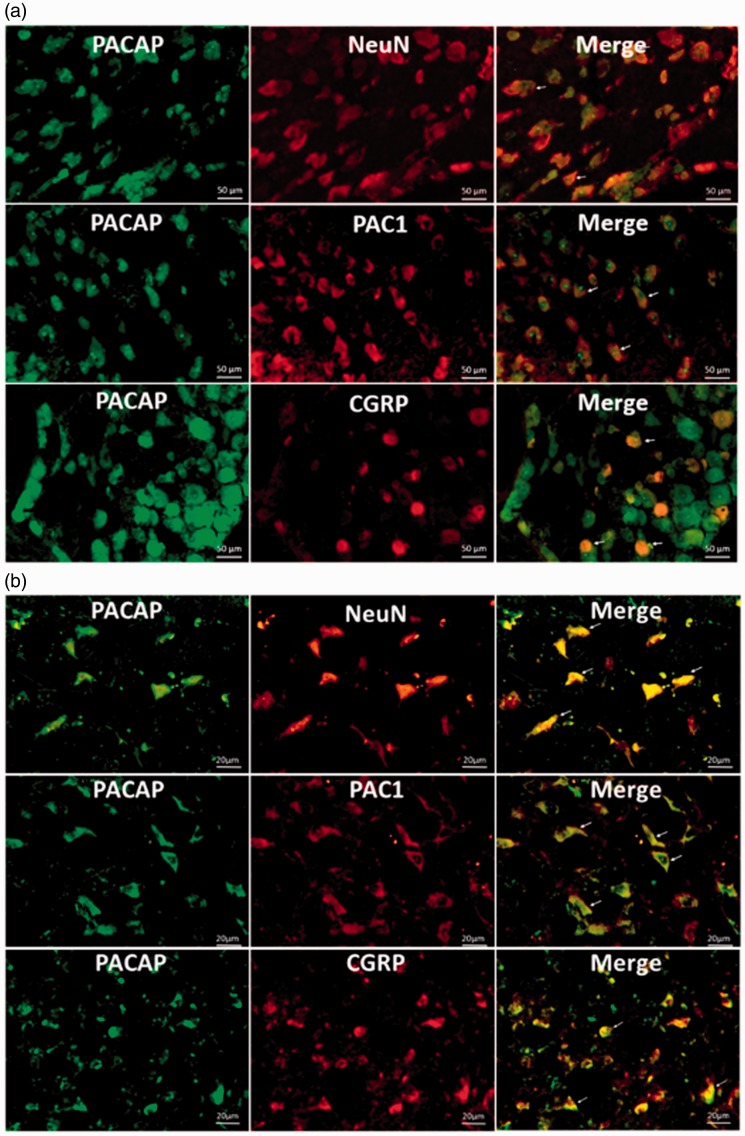
Based on the similar expression patterns of PACAP, CGRP, and PAC1 in both TG and TNC, we further asked: (1) whether they are colocalized with each other? (2) Is PACAP mainly expressed in neurons? Hence, we conducted IF to study the colocalizing relationships in the TG and the TNC. NeuN is widely used as the marker of mature neurons throughout the central nervous system. As shown in Figures 7(a) and 8(a), PACAP and NeuN were colocalized in the TG and the coexpression cells increased steadily with the repetitive stimuli: numbers on Days 3 and 7 were significantly higher than that of the Sham group (***P < 0.001). PACAP was also colocalized with PAC1 receptor in the TG (Figures 7(a) and 8(b)), and the number of positive cells on Days 3 and 7 was significantly higher than that of the Sham group (**P < 0.01). The colocalization of PACAP and CGRP was even more interesting (Figures 7(a) and 8(c)). There were much more PACAP positive cells than CGRP positive cells in the TG; and intriguingly, the majority of CGRP positive cells also expressed PACAP. This pattern was consistent with Edvinsson group’s findings in rat TG,38 and we also observed CGRP positive fibers lacking PACAP. The number of coexpressing cells rose steadily as the stimuli increased, with numbers on Day 3 and Day 7 significantly higher than the Sham group (**P < 0.01). Colocalization study in the TNC also showed that most cells that expressed NeuN, PAC1, or CGRP highly expressed PACAP (Figure 7(b)).
Figure 7.
PACAP was colocalized with NeuN, PAC1 receptor, and CGRP in both TG (a) and TNC (b). Images shown are randomly selected from the TG at 20× magnification and from the TNC at 50× magnification.
TG: trigeminal ganglion; TNC: trigeminal nucleus caudalis; CGRP: calcitonin gene-related peptide; PACAP: pituitary adenylate cyclase-activating peptide; PAC1: ■; NeuN: ■.
Figure 8.
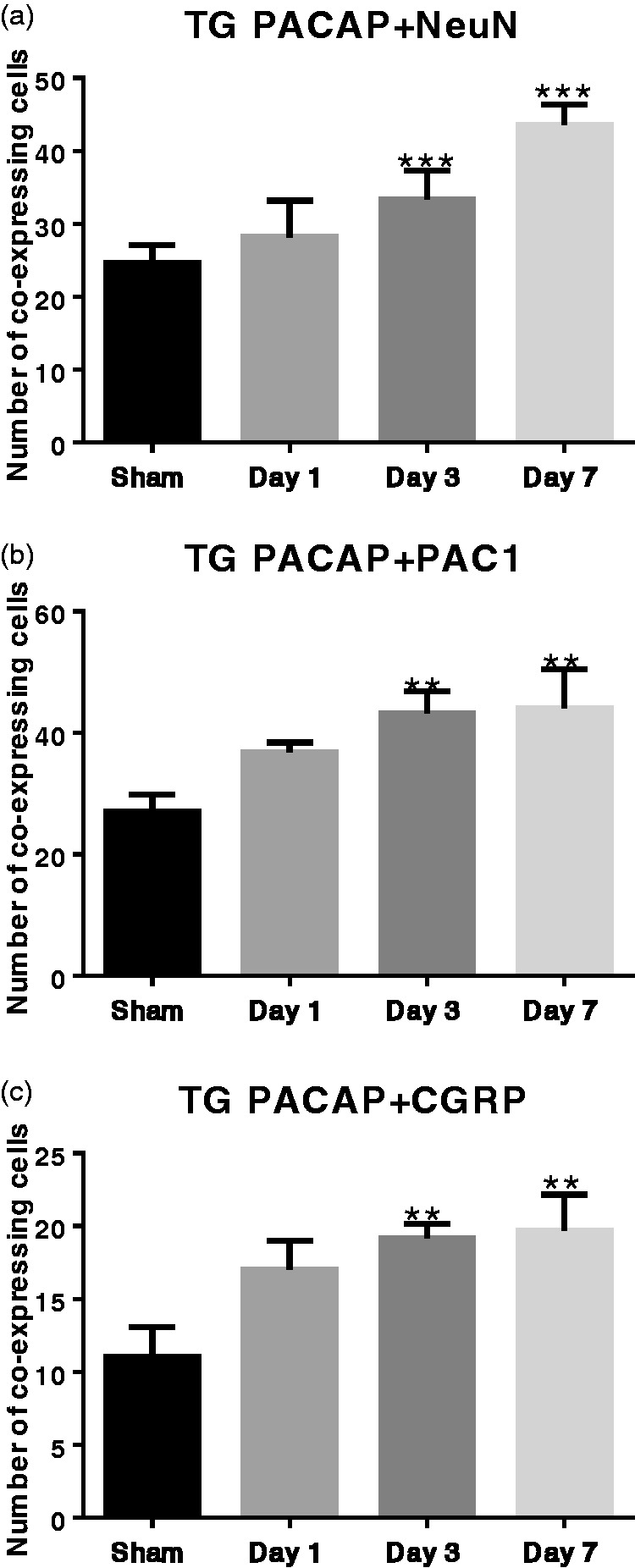
Coexpression levels of PACAP + NeuN, PACAP + PAC1, and PACAP + CGRP in the TG increased during repetitive ES. Data are represented by mean ±standard deviation, n = 6 for each group. Compared with the Sham group: **P<0.01; ***P<0.001.
TG: trigeminal ganglion; TNC: trigeminal nucleus caudalis; CGRP: calcitonin gene-related peptide; PACAP: pituitary adenylate cyclase-activating peptide; PAC1: ■; NeuN: ■.
Discussion
The novel repetitive ES model exhibits characteristics of the chronification of acute migraine
To investigate the relation between CGRP and PACAP as well as the role of PACAP in migraine, we first established a novel repetitive ES model by stimulating the dura mater in conscious rats and evaluated this model from perspectives of cutaneous allodynia and nociceptive behaviors.
Cutaneous allodynia (CA), characterized by pain provoked by nonnoxious stimuli of the normal skin, is commonly found in migraine patients.46,47 CA is also known as a hallmark of central sensitization and an independent predictor of migraine chronification in migraine patients.48 Chemical simulation or ES of the dura mater are the two major methods for intracranial stimulation models in studying migraine.49 Several studies, including ours, have demonstrated that repetitive chemical stimulations caused a gradual worsening and spreading of CA.22,50,51 Although ES is not particular translational to clinical migraine, it does directly activate the trigeminovascular system, which is involved in the pathogenesis of migraine. We propose that repetitive ES of the dura mater will facilitate migraine chronification in a time-dependent manner, which can be indicated by the development of CA. The chronic observation showed that repetitive ES successfully elicited facial CA, with the order of sensitivity being vibrissal pad >inner canthus >outer canthus (***P < 0.001). This temporal pattern may be resulted from different sensitivities of the tested regions, as the vibrissal pad has dense mechanoreceptors that transduce deformations and convey the input to primary sensory neurons in the TG.52 Although the stimulated dura mater was innervated by the ophthalmic (V1) branch of the trigeminal nerve, the range of CA at least had spread to the maxillary (V2) branch. The acute observation, however, did not yield apparent influences, indicating that the development of CA is not likely a transient process. There indeed were reductions in MTs upon ES from Day 1 to Day 4, whereas MTs became close to the baseline after 90 min. The variations in the time course of developing stable sensitization within groups may render the reductions inconsistent between 90 min and 24 h, while more apparent after 24 h. Collectively, our results propose that repetitive ES can elicit the CA through chronic effects.
Based on preliminary observations and previous studies,33,43 we analyzed four putative nociceptive behaviors: facial grooming, body grooming, head-flicks, and head-turning. Consistent with previous findings in our single stimulation study,33 facial grooming and head-flicks were more apparent in the ES group. Importantly, head-turning was only observed in the ES group. Therefore, it is reasonable to assume that head-flicks is a less serious pain-related behavior, whereas head-turning is a more severe and persistent pain-related behavior in the repetitive ES model. Taken together, our results suggest that this novel repetitive ES model exhibits some characteristics of the chronification of acute migraine.
CGRP and PACAP are increased during repetitive ES
CGRP has been widely acknowledged as an important neuropeptide in the pathophysiology of migraine.25 In this study, we found that CGRP was significantly increased in both TG and TNC correlatively with the repeats of stimuli, which provides a reasonable cause for the elevated CGRP in the saliva and circulation of migraine patients.23,53–55 On one hand, over release of CGRP from terminals may trigger the over expression of CGRP in the TG and the TNC to maintain the reserve pool. On the other hand, we found that CGRP was gradually elevated as stimuli increased, which is consistent with previous findings that peripheral CGRP levels were increased during intervals of migraine attacks in chronic migraine.56 Therefore, this novel repetitive ES model exhibits characteristics of the frequent onset of migraine from the perspective of CGRP involvement.
PACAP showed very similar patterns with CGRP in both TG and TNC; and surprisingly, it began to increase on Day 1, suggesting that the expression of PACAP started to increase at the beginning of migraine. Trigeminal nerves innervating the dura mater release CGRP and PACAP upon activation.5,6 Our results suggest that the over release of PACAP from the TG could trigger the over expression of PACAP, which provides a reasonable explanation for the further elevation of PACAP in the plasma during migraine attacks.13
PACAP might be a novel therapeutic target for migraine: Insights from the relationship with CGRP
Based on the IHC findings, we conducted IF staining. First, we found that PACAP was mainly expressed in neurons of both TG and TNC. Results in the TG suggested that increasing numbers of neurons were involved in migraine-like headache in conscious rats by producing PACAP.
As the highlight of this study, we demonstrated that CGRP and PACAP were colocalized in both TG and TNC, which is consistent with previous findings in the TG,38 and that the coexpression level in the TG increased as stimuli repeated. Interestingly, most CGRP was colocalized with PACAP, while only a part of PACAP was colocalized with CGRP. We assume that both types of PACAP+ cells may have important roles: (1) the PACAP+/CGRP- cells might be the majority that participate in migraine. They enhance the expression of PACAP upon stimulation and then increase synthesis as the stimuli repeat. (2) In addition to the first role, PACAP in the PACAP+/CGRP+ cells, the minority, may also facilitate the release of CGRP upon migraine attacks. Jansen-Olesen et al. found that exogenous administration of PACAP could induce the release of CGRP from isolated TG and TNC in a dose-dependent manner.17 Hence, it is reasonable to assume that the coexpressed PACAP may promote the release of CGRP from the same cell in a dose-dependent manner during repetitive stimuli.
Collectively, we propose that PACAP may be an important neuropeptide, like CGRP, in the pathophysiology of migraine. Three facts support this assumption: (1) PACAP has a similar expression pattern and a higher expression level compared with CGRP, (2) PACAP shows a more obvious increasing trend during repetitive stimuli, and (3) CGRP is mostly expressed in PACAP+ cells, which only account for a small part of all PACAP+ cells. In the light of recent findings26–28 that antibodies of CGRP and CGRP antagonists are effective in migraine prevention and acute treatment, our results highlight PACAP as a potential therapeutic target of migraine. As female sex hormones have been shown to regulate various mechanisms in migraine including CGRP and 5-hydroxytryptamine,57 it would be interesting to investigate whether PACAP is also influenced by sex hormones in further studies.
PACAP is involved in migraine potentially through PAC1 receptor
We propose that PACAP is involved in migraine potentially through PAC1 receptor. From the perspective of expression pattern, PAC1 receptor was more parallel to PACAP, whereas VPAC1 and VPAC2 were similar with each other. From the perspective of positive-cell numbers, PAC1 was much higher than VPAC1/2 in the TNC, showing a closer level to that of PACAP. Chaudhary and Baumann previously reported a very low mRNA level of VPAC1 in the TG compared with VPAC2 and PAC1.58 Indeed, mRNA levels and protein levels are not always consistent due to various transcriptional and translational regulations. Our recent study also found a lower mRNA level of VPAC1 despite comparable protein levels of three receptors in the TG.22 Hence, PAC1 might be the specific receptor of PACAP in migraine pathophysiology. At the beginning of the stimulation, all three receptors are increased due to the structural similarity. However, as the stimuli repeat, only the specific receptor PAC1 continues building up to catch up with the increasing PACAP. Furthermore, the TNC might be one of the specific areas in the trigminovascular system where PACAP participates in migraine through PAC1 receptor.
Based on the IHC findings, we selectively conducted IF staining. We found that most PAC1 receptors were colocalized with PACAP in the TG and the TNC. Consistent with the IHC findings, the coexpression cells increased as the stimuli repeated. We propose that upon stimulation, neurons begin to synthesize PACAP and PAC1 receptors at the same time, and then PACAP binds to PAC1 receptor to induce downstream effects. PAC1 can act as an autoreceptor or a heteroreceptor, regulating the presynaptic release of PACAP as well as modulating the postsynaptic events through GPCR-related downstream effects. Our previous study revealed a decrease in PACAP level and a selective increase in PAC1 level in a chronic migraine model (21 days),22 which is supportive to this “autoreceptor hypothesis” that the further release of PACAP is regulated by a negative feedback. However, colocalization does not necessarily represent direct binding; therefore, further studies are warranted to find direct evidence elucidating the relationship between PACAP and PAC1 receptor involved in migraine.
Here, we propose a dynamic model that upon stimuli of the dura mater, the TG begins to increase the synthesis of PACAP. Some PACAP is released from periphery terminals of the TG to innervating areas such as the dura mater, resulting in vasodilation. Other PACAP is transported through central terminals to the TNC, where PACAP binds to PAC1 receptor and triggers the excitation of nociceptive neurons as well as the further increase in PACAP.
Conclusion
The novel repetitive ES model established by stimulating the dura mater in conscious rats can simulate the chronification of frequent onset of acute migraine, from the perspectives of cutaneous allodynia and nociceptive behaviors. PACAP plays a role in the pathogenesis of migraine potentially via PAC1 receptor. PACAP is coexpressed with CGRP and has the potential to be a novel therapeutic target for migraine.
Acknowledgments
The authors are grateful to professor Ruisheng Li from the 302 Hospital of the PLA General Hospital and professors Yongming Yao, Qinghong Zhang, and Jiwei Hao from the 304 Hospital of the PLA General Hospital to support our work.
Author Contributions
The final manuscript was read and approved by all authors. Qing Zhang performed the experiments, analyzed the data, generated the figures, and drafted the manuscript. Xun Han performed the preexperiments, analyzed the data, generated the figures, and drafted the manuscript. Hangfei Wu performed the preexperiments, analyzed the data, and generated the figures. Mingjie Zhang and Guanqun Hu analyzed the data and generated the figures. Shengyuan Yu and Zhao Dong designed and monitored this study and edited the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grants 81471147, 81671077, 81471146, 81500966, 81500943, and 81600952), Beijing Science and Technology Project (grant Z161100002616013), the Capital Development Scientific Research (grant 2014–4-5013), and Beijing Natural Science Foundation (grant 7162178).
References
- 1.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1545–1602. [DOI] [PMC free article] [PubMed]
- 2.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007; 68: 343–349. [DOI] [PubMed] [Google Scholar]
- 3.Yu S, Liu R, Zhao G, Yang X, Qiao X, Feng J, Fang Y, Cao X, He M, Steiner T. The prevalence and burden of primary headaches in China: a population-based door-to-door survey. Headache 2012; 52: 582–591. [DOI] [PubMed] [Google Scholar]
- 4.Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev 2017; 97: 553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uddman R, Goadsby PJ, Jansen I, Edvinsson L. PACAP, a VIP-like peptide: immunohistochemical localization and effect upon cat pial arteries and cerebral blood flow. J Cereb Blood Flow Metab 1993; 13: 291–297. [DOI] [PubMed] [Google Scholar]
- 6.Petersen KA, Birk S, Doods H, Edvinsson L, Olesen J. Inhibitory effect of BIBN4096BS on cephalic vasodilatation induced by CGRP or transcranial electrical stimulation in the rat. Br J Pharmacol 2004; 143: 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vollesen ALH, Amin FM, Ashina M. Targeted pituitary adenylate cyclase-activating peptide therapies for migraine. Neurotherapeutics 2018; 15: 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen-Olesen I, Hougaard Pedersen S. PACAP and its receptors in cranial arteries and mast cells. J Headache Pain 2018; 19: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strother LC, Srikiatkhachorn A, Supronsinchai W. Targeted orexin and hypothalamic neuropeptides for migraine. Neurotherapeutics 2018; 15: 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zagami AS, Edvinsson L, Goadsby PJ. Pituitary adenylate cyclase activating polypeptide and migraine. Ann Clin Transl Neurol 2014; 1: 1036–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schytz HW, Birk S, Wienecke T, Kruuse C, Olesen J, Ashina M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain 2009; 132: 16–25. [DOI] [PubMed] [Google Scholar]
- 12.Vollesen AL, Guo S, Ashina M. PACAP38 dose-response pilot study in migraine patients. Cephalalgia 2017; 37: 391–395. [DOI] [PubMed] [Google Scholar]
- 13.Tuka B, Helyes Z, Markovics A, Bagoly T, Szolcsanyi J, Szabo N, Toth E, Kincses ZT, Vecsei L, Tajti J. Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia 2013; 33: 1085–1095. [DOI] [PubMed] [Google Scholar]
- 14.Han X, Dong Z, Hou L, Wan D, Chen M, Tang W, Yu S. Interictal plasma pituitary adenylate cyclase-activating polypeptide levels are decreased in migraineurs but remain unchanged in patients with tension-type headache. Clin Chim Acta 2015; 450: 151–154. [DOI] [PubMed] [Google Scholar]
- 15.Amin FM, Asghar MS, Guo S, Hougaard A, Hansen AE, Schytz HW, van der Geest RJ, de Koning PJ, Larsson HB, Olesen J, Ashina M. Headache and prolonged dilatation of the middle meningeal artery by PACAP38 in healthy volunteers. Cephalalgia 2012; 32: 140–149. [DOI] [PubMed] [Google Scholar]
- 16.Markovics A, Kormos V, Gaszner B, Lashgarara A, Szoke E, Sandor K, Szabadfi K, Tuka B, Tajti J, Szolcsanyi J, Pinter E, Hashimoto H, Kun J, Reglodi D, Helyes Z. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol Dis 2012; 45: 633–644. [DOI] [PubMed] [Google Scholar]
- 17.Jansen-Olesen I, Baun M, Amrutkar DV, Ramachandran R, Christophersen DV, Olesen J. PACAP-38 but not VIP induces release of CGRP from trigeminal nucleus caudalis via a receptor distinct from the PAC1 receptor. Neuropeptides 2014; 48: 53–64. [DOI] [PubMed] [Google Scholar]
- 18.Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, Vaudry D, Vaudry H, Waschek JA, Said SI. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol 2012; 166: 4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amin FM, Hougaard A, Schytz HW, Asghar MS, Lundholm E, Parvaiz AI, de Koning PJ, Andersen MR, Larsson HB, Fahrenkrug J, Olesen J, Ashina M. Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain 2014; 137: 779–794. [DOI] [PubMed] [Google Scholar]
- 20.Akerman S, Goadsby PJ. Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: relevance to migraine. Sci Transl Med 2015; 7: 308ra157. [DOI] [PubMed] [Google Scholar]
- 21.Chan KY, Baun M, de Vries R, van den Bogaerdt AJ, Dirven CMF, Danser AHJ, Jansen-Olesen I, Olesen J, Villalón CM, MaassenVanDenBrink A, Gupta S. Pharmacological characterization of VIP and PACAP receptors in the human meningeal and coronary artery. Cephalalgia 2011; 31: 181–189. [DOI] [PubMed] [Google Scholar]
- 22.Han X, Ran Y, Su M, Liu Y, Tang W, Dong Z, Yu S. Chronic changes in pituitary adenylate cyclase-activating polypeptide and related receptors in response to repeated chemical dural stimulation in rats. Mol Pain 2017; 13: 1744806917720361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 1990; 28: 183–187. [DOI] [PubMed] [Google Scholar]
- 24.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 1993; 33: 48–56. [DOI] [PubMed] [Google Scholar]
- 25.Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol 2010; 6: 573–582. [DOI] [PubMed] [Google Scholar]
- 26.Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, Leibensperger H, Froman S, Assaid C, Lines C, Koppen H, Winner PK. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet 2008; 372: 2115–2123. [DOI] [PubMed] [Google Scholar]
- 27.Sun H, Dodick DW, Silberstein S, Goadsby PJ, Reuter U, Ashina M, Saper J, Cady R, Chon Y, Dietrich J, Lenz R. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2016; 15: 382–390. [DOI] [PubMed] [Google Scholar]
- 28.Deen M, Correnti E, Kamm K, Kelderman T, Papetti L, Rubio-Beltran E, Vigneri S, Edvinsson L, Maassen Van Den Brink A. Blocking CGRP in migraine patients – a review of pros and cons. J Headache Pain 2017; 18: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaiser EA, Russo AF. CGRP and migraine: could PACAP play a role too? Neuropeptides 2013; 47: 451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bigal ME, Lipton RB. The epidemiology, burden, and comorbidities of migraine. Neurol Clin 2009; 27: 321–334. [DOI] [PubMed] [Google Scholar]
- 31.Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- 32.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 33.Dong Z, Jiang L, Wang X, Wang X, Yu S. Nociceptive behaviors were induced by electrical stimulation of the dura mater surrounding the superior sagittal sinus in conscious adult rats and reduced by morphine and rizatriptan benzoate. Brain Res 2011; 1368: 151–158. [DOI] [PubMed] [Google Scholar]
- 34.Jiang L, Dong Z, Li F, Liu R, Qiu E, Wang X, Yu S. Microarray analysis of gene expression after electrical stimulation of the dura mater surrounding the superior sagittal sinus in conscious adult rats. Chin Med J 2014; 127: 734–741. [PubMed] [Google Scholar]
- 35.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 36.Malatesta M. Histological and histochemical methods – theory and practice. Eur J Histochem 2016; 60: 2639. [Google Scholar]
- 37.Castorina A, Scuderi S, D’Amico AG, Drago F, D’Agata V. PACAP and VIP increase the expression of myelin-related proteins in rat schwannoma cells: involvement of PAC1/VPAC2 receptor-mediated activation of PI3K/Akt signaling pathways. Exp Cell Res 2014; 322: 108–121. [DOI] [PubMed] [Google Scholar]
- 38.Eftekhari S, Salvatore CA, Johansson S, Chen TB, Zeng Z, Edvinsson L. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res 2015; 1600: 93–109. [DOI] [PubMed] [Google Scholar]
- 39.Kanasaki H, Mijiddorj T, Sukhbaatar U, Oride A, Miyazaki K. Pituitary adenylate cyclase-activating polypeptide (PACAP) increases expression of the gonadotropin-releasing hormone (GnRH) receptor in GnRH-producing GT1-7 cells overexpressing PACAP type I receptor. Gen Comp Endocrinol 2013; 193: 95–102. [DOI] [PubMed] [Google Scholar]
- 40.Csati A, Tajti J, Kuris A, Tuka B, Edvinsson L, Warfvinge K. Distribution of vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, nitric oxide synthase, and their receptors in human and rat sphenopalatine ganglion. Neuroscience 2012; 202: 158–168. [DOI] [PubMed] [Google Scholar]
- 41.Kortesi T, Tuka B, Tajti J, Bagoly T, Fulop F, Helyes Z, Vecsei L. Kynurenic acid inhibits the electrical stimulation induced elevated pituitary adenylate cyclase-activating polypeptide expression in the TNC. Front Neurol 2017; 8: 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borsani E, Albertini R, Labanca M, Lonati C, Rezzani R, Rodella LF. Peripheral purinergic receptor modulation influences the trigeminal ganglia nitroxidergic system in an experimental murine model of inflammatory orofacial pain. J Neurosci Res 2010; 88: 2715–2726. [DOI] [PubMed] [Google Scholar]
- 43.Zhang M, Dai W, Liang J, Chen X, Hu Y, Chu B, Pan M, Dong Z, Yu S. Effects of UCMS-induced depression on nociceptive behaviors induced by electrical stimulation of the dura mater. Neurosci Lett 2013; 551: 1–6. [DOI] [PubMed] [Google Scholar]
- 44.Noseda R, Constandil L, Bourgeais L, Chalus M, Villanueva L. Changes of meningeal excitability mediated by corticotrigeminal networks: a link for the endogenous modulation of migraine pain. J Neurosci 2010; 30: 14420–14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6 ed. UK: Elsevier Inc., 2007, p.348
- 46.Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol 2000; 47: 614–624. [PubMed] [Google Scholar]
- 47.Lipton RB, Bigal ME, Ashina S, Burstein R, Silberstein S, Reed ML, Serrano D, Stewart WF. Cutaneous allodynia in the migraine population. Ann Neurol 2008; 63: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Louter MA, Bosker JE, van Oosterhout WP, van Zwet EW, Zitman FG, Ferrari MD, Terwindt GM. Cutaneous allodynia as a predictor of migraine chronification. Brain 2013; 136: 3489–3496. [DOI] [PubMed] [Google Scholar]
- 49.Storer RJ, Supronsinchai W, Srikiatkhachorn A. Animal models of chronic migraine. Curr Pain Headache Rep 2015; 19: 467. [DOI] [PubMed] [Google Scholar]
- 50.Boyer N, Dallel R, Artola A, Monconduit L. General trigeminospinal central sensitization and impaired descending pain inhibitory controls contribute to migraine progression. Pain 2014; 155: 1196–1205. [DOI] [PubMed] [Google Scholar]
- 51.Hu G, Zhang M, Su M, Zhang Q, Wu H, Wang X, Dong Z, Yu S. Wider range of allodynia in a rat model of repeated dural nociception compared with infraorbital nerve chronic constriction injury. Neurosci Lett 2018; 666: 120–126. [DOI] [PubMed] [Google Scholar]
- 52.Ebara S, Kumamoto K, Matsuura T, Mazurkiewicz JE, Rice FL. Similarities and differences in the innervation of mystacial vibrissal follicle-sinus complexes in the rat and cat: a confocal microscopic study. J Comp Neurol 2002; 449: 103–119. [DOI] [PubMed] [Google Scholar]
- 53.Bellamy JL, Cady RK, Durham PL. Salivary levels of CGRP and VIP in rhinosinusitis and migraine patients. Headache 2006; 46: 24–33. [DOI] [PubMed] [Google Scholar]
- 54.Cady RK, Vause CV, Ho TW, Bigal ME, Durham PL. Elevated saliva calcitonin gene-related peptide levels during acute migraine predict therapeutic response to rizatriptan. Headache 2009; 49: 1258–1266. [DOI] [PubMed] [Google Scholar]
- 55.Juhasz G, Zsombok T, Modos EA, Olajos S, Jakab B, Nemeth J, Szolcsanyi J, Vitrai J, Bagdy G. NO-induced migraine attack: strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain 2003; 106: 461–470. [DOI] [PubMed] [Google Scholar]
- 56.Cernuda-Morollon E, Larrosa D, Ramon C, Vega J, Martinez-Camblor P, Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology 2013; 81: 1191–1196. [DOI] [PubMed] [Google Scholar]
- 57.Gupta S, Mehrotra S, Villalon CM, Perusquia M, Saxena PR, MaassenVanDenBrink A. Potential role of female sex hormones in the pathophysiology of migraine. Pharmacol Ther 2007; 113: 321–340. [DOI] [PubMed] [Google Scholar]
- 58.Chaudhary P, Baumann TK. Expression of VPAC2 receptor and PAC1 receptor splice variants in the trigeminal ganglion of the adult rat. Brain Res Mol Brain Res 2002; 104: 137–142. [DOI] [PubMed] [Google Scholar]