Abstract
Background
Peanut allergy is an increasing problem in Singapore and strict avoidance is difficult as peanut is ubiquitous in Asian cuisine.
Objective
We aimed to assess the efficacy and safety of peanut oral immunotherapy (OIT) in children with obvious peanut allergy in Singapore.
Methods
This was an open-label study of peanut OIT in children living in Singapore, with 2 weekly dose escalation until final maintenance dose of 3,000 mg of peanut protein and a maintenance phase of 12 months. An oral food challenge was performed at 6 months to assess for desensitisation and at 4 weeks after discontinuation of OIT having completed 12 months of maintenance therapy to assess for possible sustained unresponsiveness. The adverse events were monitored using the symptom diaries.
Results
Nine subjects were started on OIT, with 7 managing to complete maintenance phase of therapy. Of these 7, all were able to tolerate at least 3,000 mg of peanut protein by 6 months of maintenance therapy, showing that the OIT was effective. Of these 7, 3 patients complied with the 4-week abstinence period after completion of OIT before another peanut challenge; 2 of the 3 subjects showed a significant decrease from the initial ability to tolerate 3,000 mg of peanut protein. Side effects were mainly gastrointestinal in nature and were more common during the updosing phase than the maintenance phase. No episodes of anaphylaxis were observed in this study.
Conclusion
Peanut OIT seemed to be effective and safe in our cohort of Singaporean children.
Keywords: Peanut, Allergy, Oral immunotherapy, Trial, Probiotics
INTRODUCTION
Peanut allergy is often severe and children seldom outgrow it. Its prevalence in Singapore among school-going children is 0.5%–0.6% [1], it accounts for 42% of the auto-injector prescriptions and it increasingly represents the top cause of anaphylaxis presenting to Paediatric Emergency Departments [2]. Strict avoidance is ideal, but challenging as peanut is ubiquitous in Asian cuisine. Half of our children with peanut allergy report accidental ingestion after their diagnosis, with a short median time to first accidental ingestion of 4 months and half of those accidental ingestions leading to reactions more severe than the first time [3].
The only study done in an Asian context is the pilot study done in Hong Kong which included omalizumab [4]. Omalizumab, due to the subcutaneous route of administration, is less desirable for use in young children. Hence, more evidence is needed on the efficacy and safety of peanut oral immunotherapy (OIT) in the Asian setting before it can be considered for clinical use.
We sought to assess the efficacy and safety of peanut OIT in children in Singapore.
MATERIALS AND METHODS
We did a pilot open-label study in Singaporean children, with 2 weekly dose escalation and maintenance phase lasting 12 months. Primary outcome was efficacy, assessed by oral food challenge (OFC) at 6 months into the maintenance phase and at 4 weeks after discontinuation of peanut OIT after having completed 12 months of maintenance therapy. Safety was assessed by symptom diaries.
Study population
Children aged 4 to 18 years, with peanut allergy (as defined below), were recruited from the Allergy Outpatient Clinic, Department of Paediatrics, National University Hospital, Singapore.
(1) Positive food challenge to peanut in the last 1 year OR
(2) Convincing reaction to peanut, AND one of the following: (a) a positive skin prick test (SPT) > 3 mm, (b) specific IgE to peanuts > 15 kU/L, or (c) specific IgE > 7 kU/L with a history of a recent reaction to peanut within the past 6 months
They were excluded if they had history of anaphylaxis, presence of severe or uncontrolled asthma (defined as previous intensive care unit admissions/intubations, or increasing doses of inhaled corticosteroids needed to control asthma, or peak expiratory flow/spirometry values less than 70% of normal), comorbidities which could preclude them from undergoing an OFC, current use of medications which may interfere with treatment of a severe reaction, or inability to comply with the desensitisation protocol.
Schedule
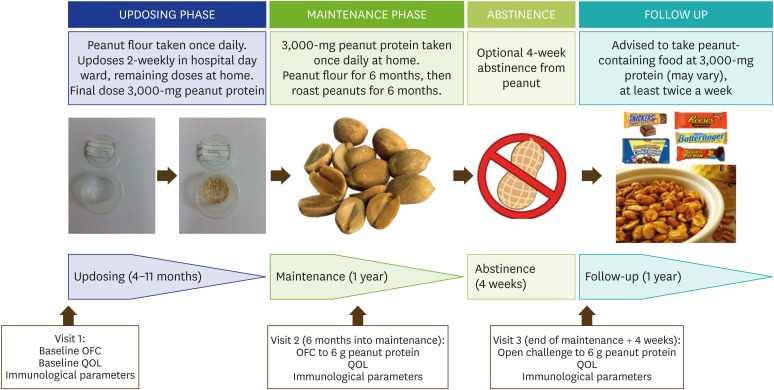
At visit 1, all subjects underwent a baseline OFC to peanut protein in the form of partially defatted peanut flour (12% fat light roast; Golden Peanut Co., Alpharetta, GA, USA; 2-g flour = 1-g peanut protein). The dose steps (in peanut protein dose) were: 0.5, 1, 2, 5, 12, 25, 50, 100, 200, 400, 800, 1,600, 3,000. The interval between doses was 2 weeks, and the doses were increased until the maintenance dose of 3,000-mg peanut protein was reached. The first OFC was at 6 months into the maintenance phase (visit 2). After completion of treatment, subjects reverted to strict peanut avoidance for 4 weeks and then attended visit 3 to undergo second OFC (Fig. 1).
Fig. 1. Schedule of oral immunotherapy. OFC, oral food challenge; QOL, quality of life.
All subjects also took daily intake of 2 × 1010 Lactobacillus rhamnosus GG (ATCC 53103) as an adjuvant during the maintenance phase. This recommendation follows the findings of improved efficacy and possible sustained unresponsiveness in a peanut OIT schedule which included Lactobacillus rhamnosus GG as an adjuvant [5].
Outcome measures
The primary outcomes were efficacy and safety.
Efficacy
Efficacy was assessed by desensitisation, defined as percentage of subjects who passed the OFCs to 6,000-mg peanut protein at 6 months into maintenance therapy. Sustained unresponsiveness was defined as percentage of subjects who passed the second OFC to 6,000-mg peanut protein at visit 3.
Safety
Safety was assessed with symptom diaries reviewed at each 2 weekly visit to the hospital. All adverse events reported by subjects were categorized into 6 systems as; skin, oral, upper respiratory tract, lower respiratory tract, gastrointestinal and cardiovascular, and classified as mild, moderate or severe. Skin manifestations include rash and angioedema, upper respiratory tract symptoms include rhinitis and cough, lower respiratory tract symptom refers to wheeze, gastrointestinal symptoms include abdominal pain and vomiting, and cardiovascular symptoms include palpitations and dizziness. Participants were withdrawn in the case of anaphylaxis.
Secondary outcomes were immunological parameters and quality of life (QoL).
Immunological parameters
Major peanut allergen-specific IgE and IgG4 levels were measured in the subjects' plasma samples using ImmunoCAP 100 instrument (Phadia AB, Uppsala, Sweden) according to the manufacturer's instructions. These included peanut extract, Ara h 1, Ara h 2, Ara h 3, Ara h 8 and Ara h 9. Blood was taken for these at baseline before starting peanut OIT (visit 1), 2 weeks after tolerating maintenance therapy (visit 2) and 4 weeks after discontinuation of peanut OIT (visit 3). SPTs (Greer Lab, Allergy Management) (SPTs) to peanut were also performed at these 3 visits.
Quality of life assessments
At the same 3 visits, QoL was assessed using age-specific and validated QoL questionnaires: Food Allergy Quality of Life Questionnaire – Parent Form (FAQLQ-PF) for parents of children aged below 12 years of age, Food Allergy Quality of Life Questionnaire – Child Form (FAQLQ-CF) for children aged 8–12 years, and Food Allergy Quality of Life Questionnaire – Teenager Form (FAQLQ-TF) for adolescents aged 13–17 years [6,7,8]. These were scored out of a total of 6 for FAQLQ-PF and CF, and out of 7 for FAQLQ-TF.
Ethical considerations
Ethical approval was granted by the Domain Specific Review Board, National Healthcare Group number 2013/00672. Informed consent was sought from all parents, with assent from the children as appropriate.
RESULTS
Baseline characteristics
Sixty-eight patients were screened for recruitment, of which, 11 were eligible. Two declined to participate hence 9 patients were recruited between December 2013 and January 2015. The median age of the patients was 8 years (range, 8–14 years); 2 were female. There were 3 Chinese patients, 2 Eurasian, and 4 Caucasian. The median baseline SPT wheal size to peanut extract was 15 mm (range, 8–25 mm), and specific IgE to peanut was 29.35 kUA/L for 4 patients and above the laboratory upper limit of measurement (>100 kUA/L) for 5 patients. Five patients had concomitant food allergies or other atopic diseases. The other food allergies included egg, seafood and garlic. The median starting dose of peanut protein for OIT was 5 mg (range, 0.5–50 mg) (Table 1).
Table 1. Subject characteristics and challenge outcomes.
| Subject No. | Age at starting (yr) | Sex | Baseline skin prick test wheal (mm) to peanut | Latest peanut sIgE prior to enrolment (kUA/L) | Comorbidities | Starting dose of peanut protein (mg) | Peanut protein (mg) tolerated, first challenge | Peanut protein (mg) tolerated, abstention for 4 weeks prior to second challenge |
|---|---|---|---|---|---|---|---|---|
| 1 | 8 | M | 20 | 28.7 | Other food allergies, asthma | 0.5 | 6,000 | 6,000 |
| 2 | 8 | M | 20 | 72.6 | Other food allergies, eczema | 0.5 | 6,000 | - |
| 3 | 14 | F | 9 | 26.3 | Asthma, allergic rhinitis | 12 | 6,000 | 3,690 |
| 4 | 7 | M | 10 | 30 | Other food allergies, asthma | 0.5 | 3,000* | - |
| 5 | 8 | M | 25 | >100 | Nil | 5 | Withdrawn | Withdrawn |
| 6 | 6 | M | 15 | >100 | Nil | 1 | 6,000 | - |
| 7 | 7 | F | 8 | >100 | Other food allergy, asthma | 5 | Withdrawn | Withdrawn |
| 8 | 10 | M | 15 | >100 | Asthma | 5 | 6,000 | - |
| 9 | 10 | M | 11 | >100 | Nil | 50 | 6,000 | 267 |
| Median | - | - | 15 | 29.35 | - | 5 | - | - |
*Subject was afraid of ingesting more than 3,000 mg of protein.
Primary outcomes: efficacy
Seven of the 9 patients completed the OIT protocol. One subject withdrew almost immediately after enrolment due to noncompliance, and the other subject withdrew soon after the start of the maintenance phase due to the amount of psychological burden the child suffered from ingesting peanuts. The proportion of missed doses was 0.26%.
Of the 7 who completed OIT, 6 tolerated 6,000 mg of peanut protein at the first OFC at 6 months of maintenance phase; the last patient was afraid of consuming more than 3,000 mg of peanut protein but passed the challenge with 3,000 mg. After 12 months of maintenance therapy, only 3 of the 7 subjects consented to 4 weeks of abstinence. Of these, only 1 passed the challenge with 6,000 mg of peanut protein. The threshold of peanut protein tolerated decreased from 6,000 mg at first OFC to 3,690 mg for subject 3, and from 6,000 mg to 257 mg for subject 9 (Table 1).
Primary outcomes: safety
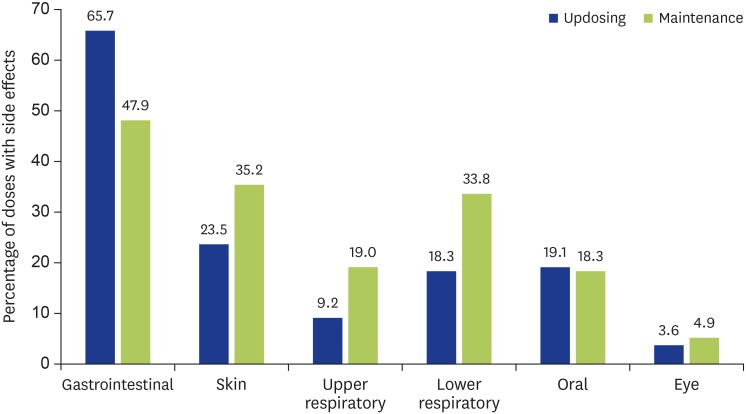
The median frequency of side effects was 12% (range, 2.9%–28.5%) out of all doses taken during the updosing phase which decreased to 1.65% (range, 0%–18.8%) during the maintenance phase. The most frequent side effects involve the gastrointestinal system (65.7% of all side effects during updosing phase and 47.9% during maintenance phase), especially transient abdominal cramps, followed by skin in the form of urticarial or itch (Fig. 2). Less frequent were upper respiratory, lower respiratory, oral and eye-related. Skin manifestations include rash and angioedema, upper respiratory tract symptoms include rhinitis and cough, lower respiratory tract symptom refers to wheeze, gastrointestinal symptoms include abdominal pain and vomiting, and cardiovascular symptoms include palpitations and dizziness. All side effects were mild, with no incident of anaphylaxis attributable to peanut OIT during treatment.
Fig. 2. Frequency of attributable side effects classified by system affected.
Side effects occurred more frequently during the first week after an updosing compared to the second week (58% reduction, p < 0.05). They were also most commonly associated with intercurrent illness. Other contributory factors included lack of sleep, missing the previous daily dose, taking a hot bath just before ingestion of the daily dose and in 1 instance, just after an episode of anaphylaxis due to accidental ingestion of another food that the subject was known to be allergic to.
Secondary outcomes: immunological parameters
There was a significant change in the median SPT wheal size to peanut extract, from 13 mm at baseline to 4 mm at visit 2 (p < 0.05), and 5 mm at visit 3 (p < 0.05).
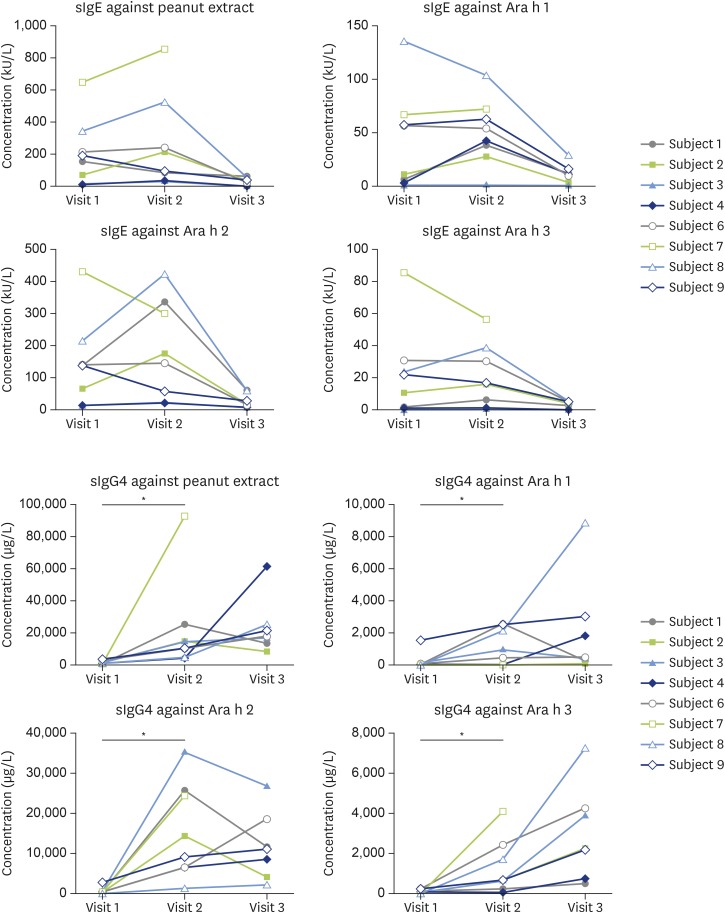
There was a trend towards lower specific IgE to peanut extract, Ara h 1, Ara h 2, and Ara h 3 between visits 1 and 3 (Fig. 3). There were no differences in specific IgE to Ara h 8 and Ara h 9 (data not shown).
Fig. 3. Specific IgE and specific IgG4 levels at visits 1, 2, and 3. Visit 1, baseline before starting peanut oral immunotherapy (OIT); visit 2, 6 weeks into maintenance therapy; visit 3, 4 weeks after discontinuation of peanut OIT.
There was an increase of peanut extract, Ara h 1, Ara h 2, and Ara h 3-specific IgG4 in the subjects' plasma from visit 1 to visit 2 (p < 0.05), but not between visits 2 and 3. There were no differences in Ara h 8 and Ara h 9-specific IgG4 between all visits (data not shown).
Secondary outcomes: QoL
Seven parents of children aged below 12 years completed the FAQLQ-PF at baseline and visit 2, and 5 at visit 3 (one withdrew and one was lost to follow up). Five children between 8 to 12 years of age completed the FAQLQ-CF and one 14-year-old patient completed the FAQLQ-TF at all 3 visits. The median baseline scores were 3.8, 4.1, and 7 out of a maximal possible 7 for PF, CF, and TF respectively.
At the end of OIT at visit 3, there was a trend towards improving QoL scores, with FAQLQ-PF scores decreasing by 0.5 (n = 5), FAQLQ-CF scores increasing by 0.1 (n = 5) and FAQLQ-TF score decreasing by 0.6 (n = 1).
Follow-up
The patients were followed up for 2–3 years after the end of their peanut OIT. Of the 5 patients who were still in contact with the study team at the time of manuscript preparation, 4 were still consuming peanuts regularly at a dose that was acceptable to the child.
DISCUSSION
This pilot study done in small cohort of children with peanut allergy, OIT was found to be efficacious and safe. The main side effects, gastrointestinal, followed by skin- and respiratory tract-related, were similar to previous studies [4, 5, 9].
Sustained unresponsiveness of 82%, as defined by discontinuation of therapy for a median of 2–3 weeks after completion of OIT, has been reported [5]. Of our 3 patients who consented to abstinence from peanut for 4 weeks after completion of OIT, only one child could tolerate 6 g of peanut protein upon rechallenge. When that child attempted to continue taking peanut at a frequency of once a month, mild symptoms reappeared by the next intake after the rechallenge, and persisted until the child increased the frequency of peanut intake to at least once a week. This supports the concept that tolerance does wear off over time even if there initially appears to be sustained unresponsiveness [10].
The withdrawal rate (2 out of 9 patients) in our cohort was similar to other studies, but instead of being directly related to the number of side effects these patients experienced, they were due to noncompliance and psychological burden. This highlights the importance of patient selection in the decision to embark on OIT for any food allergy.
Our baseline FAQLQ scores were higher than reported in other papers, reflective of greater impairment of QoL associated with peanut allergy [11]; this could be associated with the prevalence of peanut as an ingredient in Asian cooking, as well as lack of comprehensive food labelling. There was a general trend towards an improvement in QoL at the end of peanut OIT, but this was not statistically significant, likely due to the small sample size. In addition, a longer follow-up time would have been ideal, since it is reported that the QoL continues to improve 3 and 12 months after peanut OIT [11].
The small sample size limited our power to perform statistical analyses. However, the longitudinal nature of the study is its main strength, despite being pilot study with small sample size.
In conclusion, OIT for peanut allergy appears to be safe and effective in children in Singapore. Children who have completed peanut OIT should diligently continue to consume peanut as sustained unresponsiveness was not typical.
ACKNOWLEDGEMENTS
We would like to acknowledge the clinicians who helped look after these children with peanut allergy; Dr Lydia Wong, Dr Mohana D/O Rajakulendran, Dr Alison Joanne Lee and Dr Elizabeth Tham. We would also like to acknowledge Joanna Ling, biostatistician from the Singapore Clinical Research Institute for her invaluable advice and Dr Dimple Rajgor for her assistance in editing, formatting, reviewing, and in submitting the manuscript for publication. The study received funding from KTP-NUCMI Annual Grant call.
Footnotes
Author Contributions: Conceptualization: Jian Yi Soh. Data curation: Michelle Meiling Tan, Jian-Ming Chew Lamoney, Jian Yi Soh. Formal analysis: Youjia Zhong, Jian-Ming Chew Lamoney. Funding acquisition: Jian Yi Soh. Investigation: Youjia Zhong, Jian-Ming Chew Lamoney. Project administration: Michelle Meiling Tan. Resources: Michelle Meiling Tan. Supervision: Jian Yi Soh. Validation: Youjia Zhong, Jian Yi Soh. Writing - original draft: Youjia Zhong, Jian-Ming Chew Lamoney, Jian Yi Soh. Writing - review & editing: Youjia Zhong, Jian Yi Soh.
References
- 1.Shek LP, Cabrera-Morales EA, Soh SE, Gerez I, Ng PZ, Yi FC, Ma S, Lee BW. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol. 2010;126:324–331. 331.e1–331.e7. doi: 10.1016/j.jaci.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Tham EH, Tay SY, Lim DL, Shek LP, Goh AE, Giam YC, Chng HH, Lee BW. Epinephrine auto-injector prescriptions as a reflection of the pattern of anaphylaxis in an Asian population. Allergy Asthma Proc. 2008;29:211–215. doi: 10.2500/aap.2008.29.3102. [DOI] [PubMed] [Google Scholar]
- 3.Chiang WC, Pons L, Kidon MI, Liew WK, Goh A, Wesley Burks A. Serological and clinical characteristics of children with peanut sensitization in an Asian community. Pediatr Allergy Immunol. 2010;21(2 Pt 2):e429–e438. doi: 10.1111/j.1399-3038.2009.00930.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee TH, Chan J, Lau VW, Lee WL, Lau PC, Lo MH. Immunotherapy for peanut allergy. Hong Kong Med J. 2014;20:325–330. doi: 10.12809/hkmj144243. [DOI] [PubMed] [Google Scholar]
- 5.Tang ML, Ponsonby AL, Orsini F, Tey D, Robinson M, Su EL, Licciardi P, Burks W, Donath S. Administration of a probiotic with peanut oral immunotherapy: A randomized trial. J Allergy Clin Immunol. 2015;135:737–744.e8. doi: 10.1016/j.jaci.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 6.DunnGalvin A, de BlokFlokstra BM, Burks AW, Dubois AE, Hourihane JO. Food allergy QoL questionnaire for children aged 0-12 years: content, construct, and cross-cultural validity. Clin Exp Allergy. 2008;38:977–986. doi: 10.1111/j.1365-2222.2008.02978.x. [DOI] [PubMed] [Google Scholar]
- 7.Flokstra-de Blok BM, DunnGalvin A, Vlieg-Boerstra BJ, Oude Elberink JN, Duiverman EJ, Hourihane JO, Dubois AE. Development and validation of the self-administered Food Allergy Quality of Life Questionnaire for adolescents. J Allergy Clin Immunol. 2008;122:139–144. 144.e1–144.e2. doi: 10.1016/j.jaci.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Flokstra-de Blok BM, DunnGalvin A, Vlieg-Boerstra BJ, Oude Elberink JN, Duiverman EJ, Hourihane JO, Dubois AE. Development and validation of a self-administered Food Allergy Quality of Life Questionnaire for children. Clin Exp Allergy. 2009;39:127–137. doi: 10.1111/j.1365-2222.2008.03120.x. [DOI] [PubMed] [Google Scholar]
- 9.Pajno GB, Fernandez-Rivas M, Arasi S, Roberts G, Akdis CA, Alvaro-Lozano M, Beyer K, Bindslev-Jensen C, Burks W, Ebisawa M, Eigenmann P, Knol E, Nadeau KC, Poulsen LK, van Ree R, Santos AF, du Toit G, Dhami S, Nurmatov U, Boloh Y, Makela M, O'Mahony L, Papadopoulos N, Sackesen C, Agache I, Angier E, Halken S, Jutel M, Lau S, Pfaar O, Ryan D, Sturm G, Varga EM, van Wijk RG, Sheikh A, Muraro A EAACI Allergen Immunotherapy Guidelines Group. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy. 2018;73:799–815. doi: 10.1111/all.13319. [DOI] [PubMed] [Google Scholar]
- 10.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, Berglund JP, Tsai M, Maecker H, O'Riordan G, Galli SJ, Nadeau KC. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) J Allergy Clin Immunol. 2014;133:500–510. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn Galvin A, McMahon S, Ponsonby AL, Hsiao KC, Tang MLK, PPOIT study team The longitudinal impact of probiotic and peanut oral immunotherapy on health-related quality of life. Allergy. 2018;73:560–568. doi: 10.1111/all.13330. [DOI] [PubMed] [Google Scholar]