Abstract
Vitamin D testing and treatment is a subject of controversial scientific discussions, and it is challenging to navigate through the expanding vitamin D literature with heterogeneous and partially opposed opinions and recommendations. In this narrative review, we aim to provide an update on vitamin D guidelines and the current evidence on the role of vitamin D for human health with its subsequent implications for patient care and public health issues. Vitamin D is critical for bone and mineral metabolism, and it is established that vitamin D deficiency can cause rickets and osteomalacia. While many guidelines recommend target serum 25-hydroxyvitamin D (25[OH]D) concentrations of ≥50 nmol/L (20 ng/mL), the minimum consensus in the scientific community is that serum 25(OH)D concentrations below 25–30 nmol/L (10–12 ng/mL) must be prevented and treated. Using this latter threshold of serum 25(OH)D concentrations, it has been documented that there is a high worldwide prevalence of vitamin D deficiency that may require public health actions such as vitamin D food fortification. On the other hand, there is also reason for concern that an exploding rate of vitamin D testing and supplementation increases costs and might potentially be harmful. In the scientific debate on vitamin D, we should consider that nutrient trials differ from drug trials and that apart from the opposed positions regarding indications for vitamin D treatment we still have to better characterize the precise role of vitamin D for human health.
Keywords: guideline, vitamin D, evidence-based medicine, recommendation
Introduction
Vitamin D is critical for bone and mineral metabolism and is effective in the prevention and treatment of rickets and osteomalacia (1, 2, 3, 4, 5). Given that vitamin D receptors (VDRs) are expressed in almost every tissue and cell, there have been numerous investigations on potential extra-skeletal effects of vitamin D (6, 7, 8, 9, 10, 11, 12, 13, 14). Epidemiological studies showed that low 25-hydroxyvitamin D (25[OH]D) concentrations are associated with various acute and chronic diseases, thus raising a high interest in vitamin D (15, 16). Randomized controlled trials (RCTs) have, however, largely failed to show significant effects of vitamin D supplementation on various health outcomes (17, 18, 19, 20). As a consequence, there are nowadays controversial scientific discussions and heterogeneous approaches in clinical routine and in public health actions regarding vitamin D testing and treatment (17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28).
In this brief narrative review, we give an overview on clinical and nutritional vitamin D guidelines and summarize the current evidence on the role of vitamin D for human health with its subsequent implications for patient care and public health issues. We start with a brief introduction on vitamin D physiology and its clinical effects and summarize nutritional clinical vitamin D guidelines. Then, we provide some insights and guidance regarding vitamin D testing and supplementation, followed by a critical appraisal of vitamin D research. Finally, we present our conclusions with an outlook on future directions in the field of vitamin D.
Vitamin D physiology
Vitamin D was initially described as a substance that was able to cure rickets and was termed ‘D’ as it was the fourth in the sequence of vitamins discovered (29). The main two isoforms are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) that share a similar metabolism so that we will not differentiate between these isoforms unless otherwise stated. It has been roughly estimated that ultraviolet-B (UV-B)-induced production of vitamin D in the skin accounts for about 80% of vitamin D supply, whereas dietary intake (e.g. fish, eggs or vitamin D-fortified food) plays usually only a minor role (30). The vitamin D supply from different sources is of course subject to significant variation based on genetic, environmental and lifestyle factors (30, 31, 32, 33). Classification of vitamin D status is based on serum 25(OH)D that is mainly derived from hydroxylation of vitamin D in the liver. Compared to vitamin D, 25(OH)D has a much higher serum concentration and a longer half-life (about 3 weeks versus 1 day) and is therefore considered the best parameter to indicate vitamin D supply from all different sources. 1,25-dihydroxyvitamin D (1,25[OH]2D) is the so-called active vitamin D hormone or calcitriol that has the highest affinity to the almost ubiquitously expressed VDR. Serum concentrations of 1,25(OH)2D are mainly derived from renal hydroxylation of 25(OH)D and are rather dependent on regulators of mineral metabolism (e.g. parathyroid hormone (PTH), phosphate or fibroblast growth factor-23 (FGF-23)) or kidney function, than on substrate availability of 25(OH)D, so that they do not well reflect vitamin D supply. In the circulation, vitamin D metabolites are mainly bound to vitamin D-binding protein (DBP) and to a lesser extent to albumin and lipoproteins with only a small fraction (less than 1%) circulating in its unbound (free) form (34). Although some tissues can take up DBP-bound vitamin D metabolites by the megalin–cubilin system, most cells seem to be dependent on free vitamin D metabolites that diffuse through the cell membrane to get access to the intracellularly located VDR. Therefore, measurements of free 25(OH)D might be useful in special conditions with significantly altered DBP levels (e.g. pregnancy, liver cirrhosis or hormonal contraceptive intake), but more data are needed to clarify the clinical significance of free 25(OH)D (34, 35). Vitamin D catabolism is initiated by 24-hydroxylation of vitamin D metabolites that are finally excreted in the bile and urine. For a more detailed description of vitamin D metabolism, we refer the reader to other excellent reviews (1, 6, 14, 22) (Fig. 1).
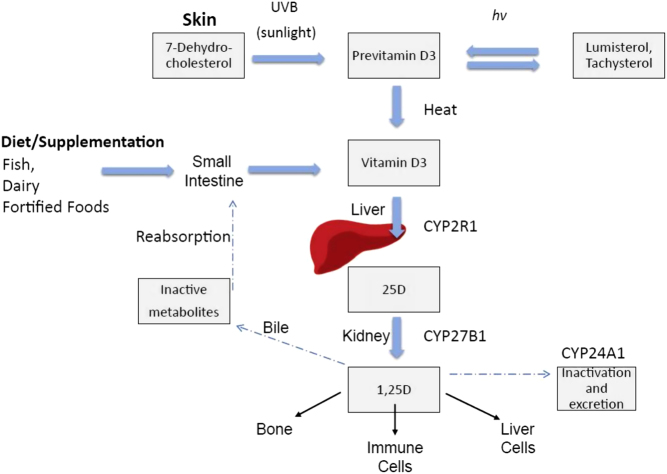
Figure 1.
Vitamin D endogenous synthesis and metabolism. Endogenous vitamin D synthesis occurs primarily through sunlight exposure which produces pre-vitamin D3. It is hydroxylated in the liver and then in the kidney, producing 1,25D (1,25 dihydroxyvitamin D), the physiologically active form of vitamin D which acts in target sites in bone and immune cells, as well as liver cells. Abbreviations: CYP (cytochrome P450), UV-B (ultraviolet-B), hν (denotes photochemical reaction). Reproduced from Keane et al. (14) under the terms of the CC Attribution 4.0 International (CC BY 4.0) licence.
Clinical effects of vitamin D
Physiologic effects of vitamin D and its metabolites are mainly exerted by binding to the VDR with subsequent downstream regulation of hundreds of genes, but there are also non-genomic rapid effects including a direct stabilizing effect on the endothelium (1, 36). Vitamin D has a critical role in the regulation of calcium and phosphate metabolism by effects on the intestine, bone and the kidneys. Breaking it down to a simple concept, an adequate vitamin D status is required to maintain normal calcium and phosphate levels and prevents secondary hyperparathyroidism. In this context, vitamin D is particularly important for optimal intestinal calcium absorption and exerts major effects on bone by maintaining mineral homeostasis but also by direct pleiotropic effects on bone cells (37, 38). Historically, the discovery of vitamin D was essential for the successful prevention and treatment of epidemic rickets in the early 20th century (39). This was achieved by increasing the vitamin D supply to the general population by public health actions such as intake of cod liver oil, UV radiation, vitamin D food fortification and, finally, also vitamin D supplementation (39). Nutritional rickets is characterized by bone deformities (Fig. 2) as a result of reduced apoptosis of hypertrophic chondrocytes in the growth plate and reduced mineralization (2, 3, 4, 5, 39). Additional symptoms are muscle weakness and developmental delay, and in severe cases, rickets may be fatal due to life-threatening heart failure and cardiac arrest (2, 3, 4, 5, 39). While vitamin D deficiency can cause rickets in bones with open growth plates, osteomalacia constitutes defective mineralization of existing bone leading to reduced bone stiffness and is frequently associated with bone pain and muscle weakness (4, 40). Treatment of nutritional rickets and osteomalacia with vitamin D plus calcium is associated with great improvements of bone mineral density (BMD), but data from RCTs and meta-analyses on vitamin D supplementation in unselected populations show either no or only slight increases in BMD (41, 42, 43, 44, 45). In subgroup analyses of RCTs, it has been documented that moderate improvements of BMD by vitamin D supplementation may be restricted to individuals with 25(OH)D serum concentrations ≤30 nmol/L (multiply by 2.496 to convert ng/mL to nmol/L) with no significant effect at higher 25(OH)D levels (43, 44). On the other hand, vitamin D deficiency is not necessarily associated with rickets or osteomalacia, suggesting that other factors such as those related to phosphate and calcium homeostasis play a role and apparently determine the individual sensitivity to detrimental effects of vitamin D deficiency. Regarding the effects of vitamin D supplementation on falls and fractures, the current meta-analyses of RCTs draw inconsistent conclusions with either a neutral or a small beneficial effect (46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57). Beyond methodological issues of meta-analyses, these inconsistent results may be attributed to the fact that only sensitive persons may significantly benefit, for example, those with low 25(OH)D receiving an adequate dose of vitamin D and those at high fracture/fall risk such as institutionalized individuals (46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57).
Figure 2.
Three children with rickets (reproduced, with permission, from Wellcome Library, London. Wellcome Images images@wellcome.ac.uk http://wellcomeimages.org; Three children with rickets; anon., Friends’ Relief Mission, Vienna XII, n.d.; Photograph circa 1920–1930; reproduced under the terms of the CC Attribution 4.0 International (CC BY 4.0) licence).
Beyond musculoskeletal effects, several studies investigated the potential extra-skeletal actions of vitamin D. Cell culture and animal studies as well as observational data support the hypothesis that vitamin D is critical for a variety of common diseases including for example, cardiovascular, autoimmune, and neurological diseases, infections, pregnancy complications and cancer (1, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20). By contrast, RCTs have largely shown no effect of vitamin D supplementation on nonskeletal health outcomes (7, 17, 18, 19, 20, 58, 59, 60, 61, 62, 63, 64, 65, 66). Nevertheless, some meta-analyses of RCTs documented beneficial vitamin D effects on certain health outcomes such as respiratory tract infections, asthma exacerbations, some pregnancy outcomes and mortality (67, 68, 69, 70, 71, 72). These data should, however, be interpreted with caution due to some limitations such as heterogeneity, different sources of potential bias, data quality of original trials and partially small effect sizes.
Of particular interest is the association between vitamin D status and cancer, with several observational studies showing an inverse association between serum 25(OH)D concentrations and cancer incidence as well as mortality (73, 74, 75, 76, 77). Meta-analyses of RCTs largely report a moderate, yet significant reduction in cancer mortality by vitamin D supplementation (19, 20, 65, 72). Vitamin D effects on cancer were also evaluated in the VITamin D and OmegA-3 TriaL (VITAL), a RCT in 25,871 older participants in the United States who were randomized to 50 µg (1 µg equals 40 international units (IU)) of vitamin D daily or placebo (78). After a median follow-up time of 5.3 years, the hazard ratios (with 95% confidence intervals (95% CI)) were 0.83 (0.67–1.02) for death from cancer, 1.02 (0.79–1.31) for breast cancer, 0.88 (0.72–1.07) for prostate cancer, and 1.09 (0.73–1.62) for colorectal cancer. In analyses excluding 1 year and 2 years of follow-up, neither of which was pre-specified, the hazard ratios (95% CI) for death from cancer were 0.79 (0.63–0.99) and 0.75 (0.59–0.96), respectively. Furthermore, in a subgroup analysis of study participants with a BMI below 25 kg/m2, cancer mortality was significantly reduced by vitamin D supplementation with a hazard ratio (95% CI) of 0.76 (0.63–0.90). In the entire study cohort, the mean ± standard deviation serum 25(OH)D concentration at baseline was 77 ± 25 nmol/L, and follow-up measurements in a subgroup of participants after 1 year indicated an increase in serum 25(OH)D concentrations in the treatment group of 30 nmol/L. The findings from the VITAL trial support a potential beneficial effect of vitamin D supplementation on cancer mortality, but they do not confirm the reductions in cancer incidence such as those for breast cancer that would have been expected from previous observational studies (73, 74, 75, 76, 77, 78).
Vitamin D guidelines
Several vitamin D guidelines and guidance papers have been published with heterogeneous and partially opposed opinions and recommendations regarding vitamin D requirements (79, 80, 81, 82, 83, 84). To avoid confusion and misinterpretations, it is essential to differentiate nutritional vitamin D guidelines targeted for the general population from clinical vitamin D guidelines intended for patients care.
Nutritional vitamin D guidelines use the terms dietary reference intakes (DRIs) or dietary reference values (DRVs) to describe the distribution of dietary vitamin D requirements in the population (84). Understanding of DRV/DRI in terms of their definition (Table 1), the process of their development, as well as their intended implications is essential for their use as public health policy instruments (84, 85). For deeper insights into these issues we refer the reader to other excellent publications, but we wish to briefly describe some of the key aspects of DRV/DRI (84, 85). A critical point regarding vitamin D requirements is that they are currently mainly based on musculoskeletal outcomes for which serum 25(OH)D concentrations have been used to characterize the dose–response relationship. As part of this process, the Institute of Medicine (IOM) in North America has defined target serum 25(OH)D concentrations at the estimated average requirement (EAR) and at the recommended dietary allowance (RDA) that should meet the vitamin D requirements in 50 and 97.5% of the population, respectively (86). The EAR and the RDA for vitamin D, that is, the dietary intakes of vitamin D to achieve the ‘EAR-like’ and ‘RDA-like’ serum 25(OH)D concentrations, were then calculated according to meta-regression analyses of ‘winter’ vitamin D RCTs. Winter RCTs were chosen because DRV/DRI apply to conditions with minimal or no sunlight exposure with consequently hardly any UV-B-induced endogenous vitamin D synthesis in the skin. Major health agencies have used similar approaches, and the resulting DRV/DRI for vitamin D are listed in Tables 2 and 3 (86, 87, 88, 89, 90, 91). While the RDA is traditionally adopted for planning intakes of individuals, as it meets the vitamin D requirements of 97.5% of individuals within a population, it must be differentiated between the individual and the population perspective. When taking care of an individual, the RDA is the intake target for this individual, but in terms of public health actions, the goal is not, and should not be, to assure that 97.5% of the population exceeds the RDA equivalent serum concentration, that is, 50 nmol/L when using the IOM RDA. Shifting the population vitamin D intake distribution to the point at which 97.5% of the population exceed the RDA like serum 25(OH)D concentration would consequently shift the higher end of the intake distribution toward potentially harmful levels (84, 92). In this context, it should also be noted that the RDA was calculated based on meta-regression analyses indicating that the lower end of the 95% CI for the median intake is ≥50 nmol/L, that is, we can be sure that at least 50% of the individuals will achieve ≥50 nmol/L at an RDA vitamin D intake. Such conventional meta-regression analyses using aggregate (group) data are suitable for establishing EARs as they well indicate mean responses and CI around these mean responses. They are not ideal for calculating RDAs, because they do not adequately capture between-individual variability as it can be done using regression analyses based on individual participant data (IPD) (84, 85). Using the same dataset for different statistical approaches, it has been documented in IPD analyses that a vitamin D intake of about 30 µg (1200 IU) per day is required to achieve a serum 25(OH)D concentration of ≥50 nmol/L in 97.5% of the population, whereas 12.7 µg (508 IU) per day were calculated according to the use of the lower end of the 95% CI of the mean response using aggregate data (84, 85). Such statistical considerations are crucial for the understanding and dealing with DRV/DRI. A simplified summary of nutritional guidelines is that target serum 25(OH)D concentrations range from ≥25 to ≥50 nmol/L corresponding to a daily vitamin D intake of 10–20 µg (400–800 IU). General populations around the world generally fail to meet these vitamin D intakes and target serum 25(OH)D concentrations pointing to the need for public health actions such as systematic vitamin D food fortification (93, 94, 95, 96, 97, 98). In Europe, for example, serum 25(OH)D concentrations <30 nmol/L and <50 nmol/L are reported in 13.0 and 40.4% of the general population, respectively (93). Therefore, some countries have already introduced systematic vitamin D food fortification to improve vitamin D intakes in the general population (99, 100, 101, 102, 103). While systematic vitamin D food fortification in countries such as the United States or Canada has improved vitamin D status in the general population, further actions have to be taken to optimize their food fortification approaches (80). In Finland, however, systematic vitamin D food fortification was highly effective by reducing the prevalence of individuals with serum 25(OH)D concentrations <30 nmol/L below 1% (99).
Table 1.
Definitions for the constituent dietary reference intakes and dietary reference values (reproduced from Cashman (85) under the terms of the CC Attribution 4.0 International (CC BY 4.0) licence).
| Institute of Medicine’s Dietary Reference Intakes | European Food Safety Authority’s Dietary Reference Values |
|---|---|
| Estimated average requirement (EAR): The average daily nutrient intake level that is estimated to meet the requirements of half of the healthy individuals in a particular life stage and gender group | Average requirement (AR): The level of (nutrient) intake estimated to satisfy the physiological requirement or metabolic demand, as defined by the specified criterion for adequacy for that nutrient, in half of the people in a population group, given a normal distribution of requirement |
| Recommended dietary allowance (RDA): The average daily dietary intake level that is sufficient to meet the nutrient requirements of nearly all (97.5 percent) healthy individuals in a particular life stage and gender group | Population reference intake (PRI): The level of (nutrient) intake that is adequate for virtually all people in a population group. On the assumption that the individual requirements for a nutrient are normally distributed within a population and the inter-individual variation is known, the PRI is calculated on the basis of the AR plus twice its standard deviation (s.d.). This will meet the requirements of 97.5% of the individuals in the population |
| Adequate intake (AI): The recommended average daily intake level of a nutrient based on observed or experimentally determined approximations or estimates of intakes that are assumed to be adequate for a group (or groups) of apparently healthy people; used when the RDA cannot be determined | Adequate intake (AI): The value estimated when a PRI cannot be established because an AR cannot be determined. An AI is the average observed or experimentally determined approximations or estimates of nutrient intake by a population group (or groups) of apparently healthy people that is assumed to be adequate |
| Tolerable upper intake level (UL): The highest average daily nutrient intake level that is likely to pose no risk of adverse health effects to almost all individuals in the general population. As intake increases above the UL, the potential risk of adverse effects may increase | Tolerable upper intake level (UL): The maximum level of total chronic daily intake of a nutrient (from all sources) judged to be unlikely to pose a risk of adverse health effects to humans |
Table 2.
Dietary reference values (DRV)/dietary reference intakes (DRI) for vitamin D (reproduced from Pilz et al. (81) under the terms of the CC Attribution 4.0 International (CC BY 4.0) licence).
| Country (health authority) | United States and Canada (IOM) | Europe (EFSA) | Germany, Austria and Switzerland (DACH) | UK (SACN) | Nordic European countries (NORDEN) | |
|---|---|---|---|---|---|---|
| DRV/DRI | EAR | RDA | AI | AI | RNI | RI |
| Target 25(OH)D in nmol/L | 40 | 50 | 50 | 50 | 25 | 50 |
| Age group | Vitamin D intakes in µg (international units, IU) per day (1 µg = 40 IU) | |||||
| 0–6 months | 10 (400) | 10 (400) | 8.5–10 (300–400) | |||
| 7–12 months | 10 (400) | 10 (400) | 10 (400) | 8.5–10 (300–400) | 10 (400) | |
| 1–3 years | 10 (400) | 15 (600) | 15 (600) | 20 (800) | 10 (400) | 10 (400) |
| 4–6 years | 10 (400) | 15 (600) | 15 (600) | 20 (800) | 10 (400) | 10 (400) |
| 7–8 years | 10 (400) | 15 (600) | 15 (600) | 20 (800) | 10 (400) | 10 (400) |
| 9–10 years | 10 (400) | 15 (600) | 15 (600) | 20 (800) | 10 (400) | 10 (400) |
| 11–14 years | 10 (400) | 15 (600) | 15 (600) | 20 (800) | 10 (400) | 10 (400) |
| 15–17 years | 10 (400) | 15 (600) | 15 (600) | 20 (800) | 10 (400) | 10 (400) |
| 18–69 years | 10 (400) | 15 (600) | 15 (600) | 20 (800) | 10 (400) | 10 (400) |
| 70–74 years | 10 (400) | 20 (600) | 15 (600) | 20 (800) | 10 (400) | 10 (400) |
| 75 years and older | 10 (400) | 20 (600) | 15 (600) | 20 (800) | 10 (400) | 20 (800) |
| Pregnancy | 10 (400) | 15 (600) | 15 (600) | 20 (800) | 10 (400) | 10 (400) |
| Lactation | 10 (400) | 15 (600) | 15 (600) | 20 (800) | 10 (400) | 10 (400) |
IOM, Institute of Medicine; EFSA, European Food Safety Authority; DACH, Germany, Austria and Switzerland; SACN, Scientific Advisory Committee on Nutrition; EAR, Estimated Average Requirement; RDA, Recommended Dietary Allowance; AI, Adequate Intake; RNI, Reference; Nutrient Intake; RI, Recommended Intake; 25(OH)D, 25-hydroxyvitamin D.
Table 3.
Tolerable upper intake levels for vitamin D (adapted from Pilz et al. (80) under the terms of the CC Attribution 4.0 International (CC BY 4.0) licence).
| Country (health authority) | United States and Canada (IOM) | Europe (EFSA) |
|---|---|---|
| Age group | Vitamin D in µg (international units, IU) per day (1 µg = 40 IU) | |
| 0–6 months | 25 (1000) | 25 (1000) |
| 6–12 months | 37.5 (1500) | 35 (1400)* |
| 1–3 years | 62.5 (2500) | 50 (2000) |
| 4–8 years | 75 (3000) | 50 (2000) |
| 9–10 years | 100 (4000) | 50 (2000) |
| 11–17 years | 100 (4000) | 100 (4000) |
| 18 years and older | 100 (4000) | 100 (4000) |
| Pregnancy | 100 (4000) | 100 (4000) |
| Lactation | 100 (4000) | 100 (4000) |
EFSA, European Food Safety Authority; IOM, Institute of Medicine. *recently updated (180)
Apart from nutritional guidelines on vitamin D requirements in the general population, there are also clinical vitamin D guidelines that aim to guide clinicians, when taking care of specific patient populations or individuals. A selection of these guidelines is presented in this paragraph. The ‘Global Consensus Recommendation on Prevention and Management of Nutritional Rickets’ recommends for the prevention of rickets the supplementation of 10 µg (400 IU) of vitamin D daily from birth to 12 months, and thereafter, vitamin D intakes through diet and supplements to meet the nutritional requirement according to the IOM report (i.e. 15–20 µg (600–800 IU) per day) (5). For treatment of nutritional rickets, the minimum recommended dose is 50 µg (2000 IU) of vitamin D per day for a minimum of 3 months plus oral calcium intake of 500 mg per day (5). Regarding vitamin D supplementation of osteoporosis patients, the recommendations are not fully consistent but 20 µg (800 IU) of vitamin D per day can be recommended in the general management of osteoporosis patients (104, 105, 106). Higher vitamin D intakes up to 50 µg (2000 IU) of vitamin D per day may also be used in specific patients but do not represent the common consensus of major osteoporosis guidelines (104, 105, 106). Some experts argue that in older individuals (aged ≥65 years), a general intake of a daily vitamin D supplement with 20 µg (800 IU) is a reasonable approach to ensure a sufficient vitamin D status (82). In patients with chronic kidney disease (CKD), it is suggested by the ‘Kidney Disease: Improving Global Outcomes (KDIGO) 2017 Clinical Practice Guideline’ that vitamin D deficiency and insufficiency be corrected by vitamin D supplementation using treatment strategies recommended for the general population (107). Parathyroid diseases also require particular attention regarding vitamin D status and represent an indication for measurement of serum 25(OH)D concentrations (108, 109, 110). Patients with primary hyperparathyroidism and 25(OH)D concentrations <50 nmol/L should be repleted with vitamin D doses (e.g. 15–25 µg (600–1000 IU) daily) aiming to bring 25(OH)D ≥50 nmol/L at a minimum, but a goal of 75 nmol/L also is reasonable (108). In primary hypoparathyroidism, it is also recommended to ensure a serum 25(OH)D concentration >50 nmol/L with a suggested supplemental vitamin D dose of 10–20 µg (400–800 IU) per day (109, 110). One major vitamin D guideline for patient care is the ‘Endocrine Society Clinical Practice Guideline’ for evaluation, treatment and prevention of vitamin D deficiency that supports the IOM recommendations for vitamin D intake to maximize bone health and muscle function in the general population (24). However, the ‘Endocrine Society Clinical Practice Guideline’ significantly differs from the IOM report as it is suggested to measure serum 25(OH)D concentrations in individuals at risk of vitamin D deficiency (Table 4). If vitamin D deficiency, classified as serum 25(OH)D <50 nmol/L, is detected in such individuals, it is recommended to supplement vitamin D to achieve serum 25(OH)D concentrations of at least 75 nmol/L. In detail, vitamin D-deficient adults should be treated with 1250 µg (50,000 IU) vitamin D once a week for 8 weeks or its equivalent of 150 µg (6000 IU) daily, followed by a maintenance dose of 37.5–50 µg (1500–2000 IU) daily. In obese patients, patients with malabsorption syndromes (in particular patients after bariatric surgery), and patients on medications affecting vitamin D metabolism, a higher dose (e.g. two to three times higher) is suggested to treat vitamin D deficiency. There has been an intensive scientific debate on the differences in the recommendations regarding vitamin D requirements from the IOM report and the Endocrine Society Clinical Practice guideline that is beyond the scope of this review (23, 24, 25, 26). In simple terms, the IOM report does not conclude that there is additional benefit of achieving serum 25(OH)D concentrations of 75 nmol/L when compared to 50 nmol/L. Furthermore, whether or which differences exist regarding vitamin D requirements in general populations and in certain patient populations or at-risk individuals is an unresolved issue (23, 24, 25, 26).
Table 4.
Indications for 25-hydroxyvitamin D measurements (candidates for screening) (reproduced, with permission, from Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Hassan Murad M & Weaver CM; Evaluation, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline; Journal of Clinical Endocrinology & Metabolism; 2011; 96(7) 1911–1930; by permission of Oxford University Press (24)).
| Rickets |
| Osteomalacia |
| Osteoporosis |
| Chronic kidney disease |
| Hepatic failure |
| Malabsorption syndromes |
| Cystic fibrosis |
| Inflammatory bowel disease |
| Crohn’s disease |
| Bariatric surgery |
| Radiation enteritis |
| Hyperparathyroidism |
| Medications |
| Antiseizure medications |
| Glucocorticoids |
| AIDS medications |
| Antifungals, e.g. ketoconazole |
| Cholestyramine |
| African–American and Hispanic children and adults |
| Pregnant and lactating women |
| Older adults with history of falls |
| Older adults with history of nontraumatic fractures |
| Obese children and adults (BMI 30 kg/m2) |
| Granuloma-forming disorders |
| Sarcoidosis |
| Tuberculosis |
| Histoplasmosis |
| Coccidiomycosis |
| Berylliosis |
| Some lymphomas |
Practical vitamin D testing and supplementation
There is a consensus that population-wide screening for vitamin D deficiency by measuring serum 25(OH)D concentrations in asymptomatic low-risk patients should not be done (111, 112, 113, 114). There is, however, no consensus on indications for 25(OH)D testing in patients at risk of vitamin D deficiency with suggested indications ranging from almost no testing to relatively wide testing according to the Endocrine Society Clinical Practice Guideline (Table 4). Making the long story short, no study has shown the effectiveness of 25(OH)D screening in certain groups so that any recommendations regarding 25(OH)D testing have a relatively low evidence base and are mostly derived from expert opinions. A high suspicion or diagnosis of rickets or osteomalacia does definitely justify the measurement of serum 25(OH)D concentrations. As mentioned earlier, several guidelines and experts argue that serum 25(OH)D concentrations should be measured in patients with hyper- and hypoparathyroidism as well as in CKD patients (107, 108, 109, 110). Although serum 25(OH)D concentrations are widely measured in patients with osteoporosis, there is some controversy on whether such a testing should be done in all patients, just selected high-risk patients or not at all. While there is definitely uncertainty regarding precise indications for vitamin D testing in at-risk individuals, there is evidence available that an uncritical high use of serum 25(OH)D measurements is performed in clinical routine that significantly increases healthcare costs (112, 113, 114, 115). Clinicians should be aware that laboratory measurements of serum 25(OH)D have shown significant inter-assay and inter-laboratory differences leading to efforts for standardization and a pressure toward well-validated gold standard measurements by mass spectrometry (115). There is, of course, a seasonal variation in serum 25(OH)D concentrations with the highest levels at the end of summer and the lowest levels at the end of winter, but the tracking of serum 25(OH)D concentrations over time reveals that a single measurement of serum 25(OH)D at a given time point provides an estimate of future 25(OH)D levels (even if years apart) which is similar to the tracking of blood pressure or blood lipids (116).
Apart from testing issues and the uncertainty regarding target concentrations, it is crucial to be aware on the dose–response relationship of vitamin D intakes and serum 25(OH)D concentrations. It should be considered that the average nutritional vitamin D intake in the general population is typically below 5 µg (200 IU) per day (80, 98). Using data from vitamin D RCTs in winter, Cashman et al. have calculated in an IPD regression analysis that with an overall (diet plus supplements) vitamin D intake of 10 µg (400 IU) per day, the percentages of individuals with serum 25(OH)D concentrations ≥25, ≥30 and ≥50 nmol/L, would be 97.5, 95 and about 50%, respectively (84, 85). To ensure that 97.5% of the individuals would achieve serum 25(OH)D concentrations ≥50 nmol/L would require an overall vitamin D intake of approximately 30 µg (1200 IU) per day (84, 85). These estimates are, for example, supported by studies on food fortification in Finland as well as by vitamin D RCTs showing that a vitamin D supplement with 20 µg (800 IU) per day is sufficient to achieve serum 25(OH)D concentrations ≥50 nmol/L in almost all participants (117, 118, 119). In pregnant women, it was calculated that a daily overall vitamin D intake of about 30 µg (1200 IU) ensured that almost all women had serum 25(OH)D concentrations ≥50 nmol/L and that cord 25(OH)D concentrations were >25 nmol/L in 99% and ≥30 nmol/L in 95% of the newborns (120).
Regarding the precise vitamin D intake serum 25(OH)D dose–response curve, there are slightly inconsistent results in the literature (88, 91). As a frequently quoted rough summary, it can be estimated that per intake of about 2.5 µg (100 IU) of vitamin D per day, the serum 25(OH)D concentrations may increase by about 2.5–5 nmol/L but with quite significant variability of such estimates in the literature (88, 91). Although not clearly established, there are data indicating that the dose–response curve is not linear and flattens at higher intakes (88, 91). Furthermore, several studies suggest that achieved increases in serum 25(OH)D are significantly higher in individuals with lower compared to higher baseline levels and are lower in persons with a higher BMI (88, 91). Although there is no clear recommendation to perform follow-up measurements of serum 25(OH)D after starting with a daily vitamin D supplement, it bears mentioning that re-measurements of serum 25(OH)D should not be done earlier than after 8 weeks on treatment because this is approximately the time required to reach a steady state (88, 91). Of note, some studies indicate that it may take even 12 weeks or longer to reach a steady state in serum 25(OH)D (121).
It should be noted that daily, weekly or monthly vitamin D dosing regimens can be used because they result in the same serum 25(OH)D concentrations (122, 123). Nevertheless, some experts recommend to prefer daily doses as vitamin D itself may be biologically relevant, but has only a half-life of about a day and because some RCTs on intermittent high-dose vitamin D supplementation have reported adverse effects such as increased falls and fractures (124, 125, 126, 127). In detail, an annual dose of 12,500 µg (500,000 IU) of vitamin D for 3–5 years in 2256 community-dwelling women aged 70 years or older resulted in an increased risk of fractures and falls with incident rate ratios (with 95% CI) compared to placebo of 1.15 (1.02–1.30; P = 0.03) and 1.26 (1.00–1.59; P = 0.47), respectively (124). Interestingly, post hoc analyses showed that increased risk of falls was exacerbated in the 3-month period following the annual vitamin D dose, with a similar trend for fractures (124). This also means that risk was particularly increased during the period with the highest serum 25(OH)D concentrations during the year in the intervention group with a median concentration, 1 month after the annual dose, that was slightly higher than 120 nmol/L including 24% of the participants with levels ≥150 nmol/L (124). Importantly, another RCT over 1 year in 200 community-dwelling men and women aged 70 years and older with a prior fall reported that risk of falls was significantly increased in participants allocated to monthly doses of 1500 µg (60,000 IU) of vitamin D compared to monthly doses of 600 µg (24,000 IU) of vitamin D (mean number of falls per participant: 1.47 vs 0.94; P = 0.02) (125). By contrast, other RCTs on intermittent high-dose vitamin D supplementation such as the Vitamin D Assessment (ViDA) Study in 5108 older individuals randomized to 2500 µg (100,000 IU) of vitamin D per month or placebo did not report on increased risk of fractures or falls (128, 129). In line with this, a recent meta-analysis on vitamin D supplementation and musculoskeletal health outcomes did not find differences for daily versus intermittent vitamin D doses (57). Interestingly, there are also data suggesting that there may be a U-shaped association of serum 25(OH)D and risk of falls (130). Anyway, we believe that some caution is warranted with intermittent high-dose vitamin D supplementation and with potential adverse effects of very high serum 25(OH)D concentrations.
From a clinical perspective, vitamin D intoxication is characterized by hypercalcemia, which is preceded by hypercalciuria (80, 127). Hypercalcemia induced by vitamin D intoxication does, however, usually only occur at serum 25(OH)D concentrations above 375 nmol/L and is very rare (80, 127, 131). Nevertheless, in view of limited data on high serum 25(OH)D concentrations and some observational studies reporting U- or J-shaped curves on the association between serum 25(OH)D and outcomes such as mortality, the IOM report classified serum 25(OH)D concentrations greater than 125 nmol/L, if sustained, as potentially harmful (25). It has been argued that the increased risk at high serum 25(OH)D concentrations might have been partially attributed to patients with previous vitamin D deficiency who therefore received vitamin D supplements, but whenever discussing associations between serum 25(OH)D and outcome, it must be stressed that such data should be based on surveys with standardization of 25(OH)D measurements. Current meta-analyses of observational studies do not report on significantly elevated risk of adverse events at serum 25(OH)D concentrations higher than 125 nmol/L, so that it is still unclear which serum 25(OH)D concentrations should be used as a threshold level for vitamin D toxicity (132, 133). To get some deeper insights into the association of serum 25(OH)D concentrations and clinical outcomes such as mortality, we show the results of an IPD meta-analysis on standardized serum 25(OH)D concentrations in Fig. 3 (16). Importantly, in the VITAL trial, there were no safety concerns with regard to hypercalcemia, kidney stones or kidney failure with a daily supplementation of 50 µg (2000 IU) vitamin D (78).
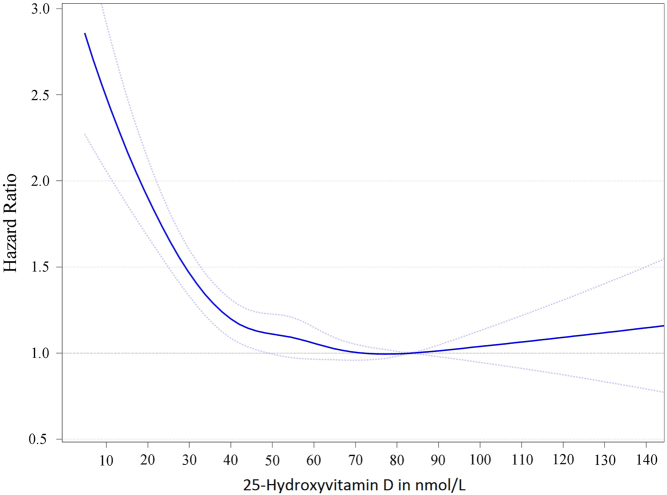
Figure 3.
Dose–response trend of hazard ratios of death from all causes by standardized 25-hydroxyvitamin D. Dose–response trend of hazard ratios of all-cause mortality by standardized 25-hydroxyvitamin D were adjusted for age, sex, BMI and season of blood drawing concentrations. Hazard ratios (blue line with 95% confidence interval as the dotted blue lines) are referring to the 25-hydroxyvitamin D concentration of 83.4 nmol/L (i.e. the median 25-hydroxyvitamin D concentration for the group with 25-hydroxyvitamin D concentrations from 75 to 99.99 nmol/L). Reproduced from Gaksch et al. (16) under the terms of the CC0 1.0 Universal (CC0 1.0) Public Domain Dedication.
Apart from oral intake, vitamin D can also be administered intramuscularly with a similar, yet delayed, increase compared to oral intakes (134, 135, 136). Transdermal applications of vitamin D do also raise serum 25(OH)D, but more data on this topic are needed (137, 138). Apart from vitamin D, there are also 25(OH)D preparations available for treatment that are about 3.2–5-fold as effective as vitamin D in raising serum 25(OH)D concentrations (139, 140). Regarding vitamin D3 and D2, most experts argue to rather prefer vitamin D3, as it is the endogenous form that may be more potent in increasing serum 25(OH)D concentrations compared to vitamin D2 (141). The lower affinity to DBP of vitamin D2 metabolites compared to vitamin D3 metabolites may contribute to a more rapid clearance of vitamin D2 (142, 143, 144). Reviewing the current literature on this topic, Bouillon et al. concluded that vitamin D2 can be considered as a good analog of vitamin D3 rather than as being truly bioequivalent (144). As part of the discussion on optimal strategies for vitamin D supplementation, it should also be emphasized that a healthy lifestyle with moderate sunlight exposure, a healthy diet (including fish) and avoiding or treating obesity can also effectively increase serum 25(OH)D concentrations (145, 146, 147, 148, 149).
Critical appraisal of vitamin D research
Several vitamin D RCTs have been published and are currently ongoing that have or will substantially increase our knowledge on vitamin D. Robert Heaney and other scientists pointed out that the evidence-based medicine (EBM) guidelines, developed specifically for drugs, have been applied to nutrients and their trials without considering major differences between nutrients and drugs (150, 151, 152, 153, 154, 155, 156, 157). One key point is that the dose–response curve of nutrient intake and outcomes is generally not linear, and it requires an accurate interpretation and study design of nutrient trials considering this dose–response curve. Assessment of nutrient status at baseline and study end and aiming for a change in nutrient intake that is associated with a significant change in outcomes on the dose–response curve is important. RCTs including participants regardless of their prevailing vitamin D status or with high serum 25(OH)D concentrations may miss to report significant vitamin D effects in ‘sensitive’ populations such as vitamin D-deficient individuals (158, 159). It should appear logical that when even established treatments such as aspirin are not effective in terms of improved clinical outcomes when given to everyone in the population, vitamin D will also fail and is not a ‘wonder drug’ (160). Unfortunately, many vitamin D RCTs have a similar study design as previous disappointing nutrient trials with no selection of sensitive (e.g. vitamin D deficient) individuals, and may, therefore likewise show no effect or might even be harmful (158, 159, 161). Subgroup analyses of vitamin D-deficient individuals, even if showing beneficial effects, will likewise not be widely accepted and will definitely not be able to compete with drug trials results that are not derived from unselected participants but rather from very large cohorts of carefully selected and ‘sensitive’ populations (158). Assessment of calcium intake is also crucial in vitamin D RCTs because it seems that individuals with a poor calcium intake may be more sensitive to adverse effects of vitamin D deficiency and vice versa.
It is important to point out that EBM is not exclusively based on RCTs but also on other study designs including, apart from classic observational studies, also Mendelian Randomization (MR) studies (157). These MR studies evaluate whether genetically determined serum 25(OH)D levels are associated with outcome and have the advantage over RCTs that they assess lifelong exposure (162, 163). While observational studies on vitamin D have been very useful to generate hypotheses that have to be further tested in RCTs or MR studies, they are definitely prone to bias or reverse causation as for example, DBP decreases due to critical illness thus consequently decreasing total serum 25(OH)D (164). It is also important to note that serum 25(OH)D concentrations in observational studies are mainly derived from sunlight-induced vitamin D synthesis in the skin, while interventional studies rather supplement vitamin D than increasing UV-induced vitamin D synthesis that may also exert vitamin D-independent effects on human health. Moreover, there are still several knowledge gaps regarding the role and regulation of DBP or regarding data on potential vitamin D toxicity at high serum 25(OH)D concentrations (164, 165, 166, 167). As an important task for future vitamin D research, Sempos et al. have proposed to develop an international ‘Rickets Registry’ based on standardized serum 25(OH)D and a standardized case definition of rickets (166).
Better education for professionals and the lay public is also required to reduce the overuse of high-dose vitamin D supplements (in particular doses that exceed the tolerable upper intake levels according to Table 3) in those who do not need it and to improve the underuse of vitamin D supplements in those individuals in whom it is indicated (168, 169, 170, 171, 172, 173). Infrequent use of vitamin D supplements in individuals with a low socioeconomic status has to be considered (170, 171, 172, 173). Importantly, rickets is still a worldwide public health problem causing morbidity and mortality and is even increasing in Europe with immigrants from Middle East, Africa and Asia being at particularly high risk (174). Prevalence and incidence of vitamin D deficiency-associated nutritional rickets is difficult to assess due to incomplete reporting and inconsistent case definition, but even if conservative estimates of only a few single-digit cases per 100,000 is true, vitamin D-deficient rickets and the therewith associated infant deaths are preventable and require adequate public health actions (174, 175, 176, 177).
Conclusions
It is established that vitamin D deficiency can cause rickets and osteomalacia with a significant risk increase at serum 25(OH)D concentrations <25–30 nmol/L, pointing to the need for prevention and treatment of such low serum 25(OH)D concentrations on an individual and population level. Adequate vitamin D supplementation may also have moderate beneficial effects on BMD, fractures and falls. These effects seem to be only evident in vitamin D-sensitive populations, that is, in particular in those with serum 25(OH)D concentrations <30 nmol/L and older or at-risk individuals. Importantly, high calcium intake may partially compensate for reduced serum 25(OH)D concentrations. Meta-analyses of RCTs indicated that vitamin D supplementation may also reduce extra-skeletal outcomes such as infections, asthma exacerbations, pregnancy outcomes and mortality, but more data are required to clearly establish causality. Anyway, effect sizes on any of these outcomes, if truly present, are only small but may eventually be significant on a population level. Clinicians are confronted with an overwhelming testing and self-supplementation of vitamin D in the general population. It is therefore recommended that vitamin D testing should not be misused as a universal population-wide screening tool, but rather be applied only in selected individuals at high risk of vitamin D deficiency. Being aware of inconsistent guidelines and recommendations, we suggest that a reasonable approach for clinicians is to supplement vitamin D in those individuals with measured serum 25(OH)D <50 nmol/L and osteoporosis patients. A supplemental dose of 20 µg (800 IU) of vitamin D per day should be sufficient for almost all individuals to safely achieve serum 25(OH)D concentrations ≥50 nmol/L and to exert beneficial clinical effects according to some dose–response analyses of RCTs (24, 49, 56). Even if following more conservative approaches, there is an imperative requirement for vitamin D treatment in individuals with serum 25(OH)D concentrations <25–30 nmol/L, a level that can be prevented by a supplemental vitamin D dose of 10 µg (400 IU) per day. We believe that upcoming large vitamin D RCTs will likewise not show significant beneficial effects as they did not target vitamin D-deficient or sensitive high-risk individuals, but will provide important safety data for relatively high doses of vitamin D supplementation in the general older population (158, 161, 178, 179).
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author contribution statement
All authors contributed to the drafting and critical review of this manuscript.
References
- 1.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiological Reviews 2016. 96 365–408. ( 10.1152/physrev.00014.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carpenter TO, Shaw NJ, Portale AA, Ward LM, Abrams SA, Pettifor JM. Rickets. Nature Reviews Disease Primers 2017. 3 17101 ( 10.1038/nrdp.2017.101) [DOI] [PubMed] [Google Scholar]
- 3.Uday S, Fratzl-Zelman N, Roschger P, Klaushofer K, Chikermane A, Saraff V, Tulchinsky T, Thacher TD, Marton T, Högler W. Cardiac, bone and growth plate manifestations in hypocalcemic infants: revealing the hidden body of the vitamin D deficiency iceberg. BMC Pediatrics 2018. 18 183 ( 10.1186/s12887-018-1159-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uday S, Högler W. Nutritional rickets and osteomalacia in the twenty-first century: revised concepts, public health, and prevention strategies. Current Osteoporosis Reports 2017. 15 293–302. ( 10.1007/s11914-017-0383-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, Michigami T, Tiosano D, Mughal MZ, Mäkitie O, et al Global consensus recommendations on prevention and management of nutritional rickets. Journal of Clinical Endocrinology and Metabolism 2016. 101 394–415. ( 10.1210/jc.2015-2175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocrine Reviews 2008. 29 726–776. ( 10.1210/er.2008-0004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, Murad MH, Kovacs CS. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocrine Reviews 2012. 33 456–492. ( 10.1210/er.2012-1000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilz S, Verheyen N, Grübler MR, Tomaschitz A, März W. Vitamin D and cardiovascular disease prevention. Nature Reviews Cardiology 2016. 13 404–417. ( 10.1038/nrcardio.2016.73) [DOI] [PubMed] [Google Scholar]
- 9.Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Reviews in Endocrine and Metabolic Disorders 2017. 18 153–165. ( 10.1007/s11154-017-9424-1) [DOI] [PubMed] [Google Scholar]
- 10.Trummer C, Pilz S, Schwetz V, Obermayer-Pietsch B, Lerchbaum E. Vitamin D, PCOS and androgens in men: a systematic review. Endocrine Connections 2018. 7 R95–R113. ( 10.1530/EC-18-0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muscogiuri G, Altieri B, Annweiler C, Balercia G, Pal HB, Boucher BJ, Cannell JJ, Foresta C, Grübler MR, Kotsa K, et al Vitamin D and chronic diseases: the current state of the art. Archives of Toxicology 2017. 91 97–107. ( 10.1007/s00204-016-1804-x) [DOI] [PubMed] [Google Scholar]
- 12.Zittermann A, Pilz S, Hoffmann H, März W. Vitamin D and airway infections: a European perspective. European Journal of Medical Research 2016. 21 14 ( 10.1186/s40001-016-0208-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner CL, Hollis BW. The implications of vitamin D status During pregnancy on mother and her developing child. Frontiers in Endocrinology 2018. 9 500 ( 10.3389/fendo.2018.00500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keane JT, Elangovan H, Stokes RA, Gunton JE. Vitamin D and the liver-correlation or cause? Nutrients 2018. 10 496 ( 10.3390/nu10040496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, Khan H, Baena CP, Prabhakaran D, Hoshen MB, et al Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 2014. 348 g1903 ( 10.1136/bmj.g1903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaksch M, Jorde R, Grimnes G, Joakimsen R, Schirmer H, Wilsgaard T, Mathiesen EB, Njølstad I, Løchen ML, März W, et al Vitamin D and mortality: individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS ONE 2017. 12 e0170791 ( 10.1371/journal.pone.0170791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes and Endocrinology 2014. 2 76–89. ( 10.1016/S2213-8587(13)70165-7) [DOI] [PubMed] [Google Scholar]
- 18.Swart KM, Lips P, Brouwer IA, Jorde R, Heymans MW, Grimnes G, Grübler MR, Gaksch M, Tomaschitz A, Pilz S, et al Effects of vitamin D supplementation on markers for cardiovascular disease and type 2 diabetes: an individual participant data meta-analysis of randomized controlled trials. American Journal of Clinical Nutrition 2018. 107 1043–1053. ( 10.1093/ajcn/nqy078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Autier P, Mullie P, Macacu A, Dragomir M, Boniol M, Coppens K, Pizot C, Boniol M. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes and Endocrinology 2017. 5 986–1004. ( 10.1016/S2213-8587(17)30357-1) [DOI] [PubMed] [Google Scholar]
- 20.Rejnmark L, Bislev LS, Cashman KD, Eiríksdottir G, Gaksch M, Grübler M, Grimnes G, Gudnason V, Lips P, Pilz S, et al Non-skeletal health effects of vitamin D supplementation: a systematic review on findings from meta-analyses summarizing trial data. PLoS ONE 2017. 12 e0180512 ( 10.1371/journal.pone.0180512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolland MJ, Avenell A, Grey A. Should adults take vitamin D supplements to prevent disease? BMJ 2016. 355 i6201 ( 10.1136/bmj.i6201) [DOI] [PubMed] [Google Scholar]
- 22.Ebeling P, Adler R, Jones G, Liberman UA, Mazziotti G, Minisola S, Munns C, Napoli N, Pittas A, Giustina A, et al MANAGEMENT of ENDOCRINE DISEASE: Therapeutics of vitamin D. European Journal of Endocrinology 2018. 179 R239–R259. ( 10.1530/EJE-18-0151) [DOI] [PubMed] [Google Scholar]
- 23.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. Journal of Clinical Endocrinology and Metabolism 2012. 97 1153–1158. ( 10.1210/jc.2011-2601) [DOI] [PubMed] [Google Scholar]
- 24.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. & Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2011. 96 1911–1930. ( 10.1210/jc.2011-0385) [DOI] [PubMed] [Google Scholar]
- 25.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, et al The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. Journal of Clinical Endocrinology and Metabolism 2011. 96 53–58. ( 10.1210/jc.2010-2704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, et al IOM committee members respond to Endocrine Society vitamin D guideline. Journal of Clinical Endocrinology and Metabolism 2012. 97 1146–1152. ( 10.1210/jc.2011-2218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid IR. Calcium and vitamin D: to supplement or not? Cleveland Clinic Journal of Medicine 2018. 85 693–698. ( 10.3949/ccjm.85a.18026) [DOI] [PubMed] [Google Scholar]
- 28.Pludowski P, Holick MF, Grant WB, Konstantynowicz J, Mascarenhas MR, Haq A, Povoroznyuk V, Balatska N, Barbosa AP, Karonova T, et al Vitamin D supplementation guidelines. Journal of Steroid Biochemistry and Molecular Biology 2018. 175 125–135. ( 10.1016/j.jsbmb.2017.01.021) [DOI] [PubMed] [Google Scholar]
- 29.Deluca HF. History of the discovery of vitamin D and its active metabolites. BoneKEy Reports 2014. 3 479 ( 10.1038/bonekey.2013.213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macdonald HM, Mavroeidi A, Fraser WD, Darling AL, Black AJ, Aucott L, O'Neill F, Hart K, Berry JL, Lanham-New SA, et al Sunlight and dietary contributions to the seasonal vitamin D status of cohorts of healthy postmenopausal women living at northerly latitudes: a major cause for concern? Osteoporosis International 2011. 22 2461–2472. ( 10.1007/s00198-010-1467-z) [DOI] [PubMed] [Google Scholar]
- 31.Jiang X, O'Reilly PF, Aschard H, Hsu YH, Richards JB, Dupuis J, Ingelsson E, Karasik D, Pilz S, Berry D, et al Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nature Communications 2018. 9 260 ( 10.1038/s41467-017-02662-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouillon R. Genetic and racial differences in the vitamin D endocrine system. Endocrinology and Metabolism Clinics of North America 2017. 46 1119–1135. ( 10.1016/j.ecl.2017.07.014) [DOI] [PubMed] [Google Scholar]
- 33.Martinaityte I, Kamycheva E, Didriksen A, Jakobsen J, Jorde R. Vitamin D stored in fat tissue during a 5-year intervention affects serum 25-hydroxyvitamin D levels the following year. Journal of Clinical Endocrinology and Metabolism 2017. 102 3731–3738. ( 10.1210/jc.2017-01187) [DOI] [PubMed] [Google Scholar]
- 34.Bikle DD, Malmstroem S, Schwartz J. Current controversies: are free vitamin metabolite levels a more accurate assessment of vitamin D status than total levels? Endocrinology and Metabolism Clinics of North America 2017. 46 901–918. ( 10.1016/j.ecl.2017.07.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pilz S, Obeid R, Schwetz V, Trummer C, Pandis M, Lerchbaum E, Pieber TR, Obermayer-Pietsch B, Wilhelm M, Hahn A, et al Hormonal contraceptive use is associated With higher total but unaltered free 25-hydroxyvitamin D serum concentrations. Journal of Clinical Endocrinology and Metabolism 2018. 103 2385–2391. ( 10.1210/jc.2018-00336) [DOI] [PubMed] [Google Scholar]
- 36.Gibson CC, Davis CT, Zhu W, Bowman-Kirigin JA, Walker AE, Tai Z, Thomas KR, Donato AJ, Lesniewski LA, Li DY. Dietary vitamin D and its metabolites non-genomically stabilize the endothelium. PLoS ONE 2015. 10 e0140370 ( 10.1371/journal.pone.0140370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson PH. Vitamin D activity and metabolism in bone. Current Osteoporosis Reports 2017. 15 443–449. ( 10.1007/s11914-017-0394-8) [DOI] [PubMed] [Google Scholar]
- 38.van de Peppel J, van Leeuwen JP. Vitamin D and gene networks in human osteoblasts. Frontiers in Physiology 2014. 5 137 ( 10.3389/fphys.2014.00137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajakumar K, Greenspan SL, Thomas SB, Holick MF. Solar ultraviolet radiation and vitamin D: a historical perspective. American Journal of Public Health 2007. 97 1746–1754. ( 10.2105/AJPH.2006.091736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhan A, Rao AD, Rao DS. Osteomalacia as a result of vitamin D deficiency. Endocrinology and Metabolism Clinics of North America 2010. 39 321–331. ( 10.1016/j.ecl.2010.02.001) [DOI] [PubMed] [Google Scholar]
- 41.Reid IR. Vitamin D effect on bone mineral density and fractures. Endocrinology and Metabolism Clinics of North America 2017. 46 935–945. ( 10.1016/j.ecl.2017.07.005) [DOI] [PubMed] [Google Scholar]
- 42.Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet 2014. 383 146–155. ( 10.1016/S0140-6736(13)61647-5) [DOI] [PubMed] [Google Scholar]
- 43.Macdonald HM, Reid IR, Gamble GD, Fraser WD, Tang JC, Wood AD. 25-Hydroxyvitamin D threshold for the effects of vitamin D supplements on bone density: secondary analysis of a randomized controlled trial. Journal of Bone and Mineral Research 2018. 33 1464–1469. ( 10.1002/jbmr.3442) [DOI] [PubMed] [Google Scholar]
- 44.Reid IR, Horne AM, Mihov B, Gamble GD, Al-Abuwsi F, Singh M, Taylor L, Fenwick S, Camargo CA, Stewart AW, et al Effect of monthly high-dose vitamin D on bone density in community-dwelling older adults substudy of a randomized controlled trial. Journal of Internal Medicine 2017. 282 452–460. ( 10.1111/joim.12651) [DOI] [PubMed] [Google Scholar]
- 45.Al-Ali H, Fuleihan GE. Nutritional osteomalacia: substantial clinical improvement and gain in bone density posttherapy. Journal of Clinical Densitometry 2000. 3 97–101. ( 10.1385/JCD:3:1:097) [DOI] [PubMed] [Google Scholar]
- 46.Bolland MJ, Grey A, Gamble GD, Reid IR. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes and Endocrinology 2014. 2 573–580. ( 10.1016/S2213-8587(14)70068-3) [DOI] [PubMed] [Google Scholar]
- 47.Murad MH, Elamin KB, Abu Elnour NO, Elamin MB, Alkatib AA, Fatourechi MM, Almandoz JP, Mullan RJ, Lane MA, Liu H, et al Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis. Journal of Clinical Endocrinology and Metabolism 2011. 96 2997–3006. ( 10.1210/jc.2011-1193) [DOI] [PubMed] [Google Scholar]
- 48.Bolland MJ, Grey A, Reid IR. Differences in overlapping meta-analyses of vitamin D supplements and falls. Journal of Clinical Endocrinology and Metabolism 2014. 99 4265–4272. ( 10.1210/jc.2014-2562) [DOI] [PubMed] [Google Scholar]
- 49.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, Wong JB, Egli A, Kiel DP, Henschkowski J. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ 2009. 339 b3692 ( 10.1136/bmj.b3692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cameron ID, Gillespie LD, Robertson MC, Murray GR, Hill KD, Cumming RG, Kerse N. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database of Systematic Reviews 2012. 12 CD005465 ( 10.1002/14651858.CD005465.pub3) [DOI] [PubMed] [Google Scholar]
- 51.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC. Issues of trial selection and subgroup considerations in the recent meta-analysis of Zhao and colleagues on fracture reduction by calcium and vitamin D supplementation in community-dwelling older adults. Osteoporosis International 2018. 29 2151–2152. ( 10.1007/s00198-018-4587-5) [DOI] [PubMed] [Google Scholar]
- 52.Kahwati LC, Weber RP, Pan H, Gourlay M, LeBlanc E, Coker-Schwimmer M, Viswanathan M. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2018. 319 1600–1612. ( 10.1001/jama.2017.21640) [DOI] [PubMed] [Google Scholar]
- 53.Zhao JG, Zeng XT, Wang J, Liu L. Association Between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA 2017. 318 2466–2482. ( 10.1001/jama.2017.19344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weaver CM, Alexander DD, Boushey CJ, Dawson-Hughes B, Lappe JM, LeBoff MS, Liu S, Looker AC, Wallace TC, Wang DD. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporosis International 2016. 27 367–376. ( 10.1007/s00198-015-3386-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Avenell A, Mak JC, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database of Systematic Reviews 2014. 4 CD000227 ( 10.1002/14651858.CD000227.pub4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, Flicker L, Wark J, Jackson RD, Cauley JA, et al A pooled analysis of vitamin D dose requirements for fracture prevention. New England Journal of Medicine 2012. 367 40–49. ( 10.1056/NEJMoa1109617) [DOI] [PubMed] [Google Scholar]
- 57.Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes and Endocrinology 2018. 6 847–858. ( 10.1016/S2213-8587(18)30265-1) [DOI] [PubMed] [Google Scholar]
- 58.Scragg R, Stewart AW, Waayer D, Lawes CMM, Toop L, Sluyter J, Murphy J, Khaw KT, Camargo CA., Jr. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study: a randomized clinical trial. JAMA Cardiology 2017. 2 608–616. ( 10.1001/jamacardio.2017.0175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lappe J, Watson P, Travers-Gustafson D, Recker R, Garland C, Gorham E, Baggerly K, McDonnell SL. Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA 2017. 317 1234–1243. ( 10.1001/jama.2017.2115) [DOI] [PubMed] [Google Scholar]
- 60.Kubiak J, Thorsby PM, Kamycheva E, Jorde R. Vitamin D supplementation does not improve CVD risk factors in vitamin D-insufficient subjects. Endocrine Connections 2018. 7 840–849. ( 10.1530/EC-18-0144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roth DE, Morris SK, Zlotkin S, Gernand AD, Ahmed T, Shanta SS, Papp E, Korsiak J, Shi J, Islam MM, et al Vitamin D supplementation in pregnancy and lactation and infant growth. New England Journal of Medicine 2018. 379 535–546. ( 10.1056/NEJMoa1800927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sluyter JD, Camargo CA, Waayer D, Lawes CMM, Toop L, Khaw KT, Scragg R. Effect of monthly, high-dose, long-term vitamin D on lung function: a randomized controlled trial. Nutrients 2017. 9 1353 ( 10.3390/nu9121353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saleem J, Zakar R, Zakar MZ, Belay M, Rowe M, Timms PM, Scragg R, Martineau AR. High-dose vitamin D3 in the treatment of severe acute malnutrition: a multicenter double-blind randomized controlled trial. American Journal of Clinical Nutrition 2018. 107 725–733. ( 10.1093/ajcn/nqy027) [DOI] [PubMed] [Google Scholar]
- 64.Hibbs AM, Ross K, Kerns LA, Wagner C, Fuloria M, Groh-Wargo S, Zimmerman T, Minich N, Tatsuoka C. Effect of vitamin D supplementation on recurrent wheezing in black infants who were born preterm: the D-wheeze randomized clinical trial. JAMA 2018. 319 2086–2094. ( 10.1001/jama.2018.5729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goulão B, Stewart F, Ford JA, MacLennan G, Avenell A. Cancer and vitamin D supplementation: a systematic review and meta-analysis. American Journal of Clinical Nutrition 2018. 107 652–663. ( 10.1093/ajcn/nqx047) [DOI] [PubMed] [Google Scholar]
- 66.Beveridge LA, Khan F, Struthers AD, Armitage J, Barchetta I, Bressendorff I, Cavallo MG, Clarke R, Dalan R, Dreyer G, et al Effect of vitamin D supplementation on markers of vascular function: A systematic review and individual participant meta-analysis. Journal of the American Heart Association 2018. 7 e008273 ( 10.1161/JAHA.117.008273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, et al Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017. 356 i6583 ( 10.1136/bmj.i6583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jolliffe DA, Greenberg L, Hooper RL, Griffiths CJ, Camargo CA, Jr, Kerley CP, Jensen ME, Mauger D, Stelmach I, Urashima M, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respiratory Medicine 2017. 5 881–890. ( 10.1016/S2213-2600(17)30306-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bi WG, Nuyt AM, Weiler H, Leduc L, Santamaria C, Wei SQ. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatrics 2018. 172 635–645. ( 10.1001/jamapediatrics.2018.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roth DE, Leung M, Mesfin E, Qamar H, Watterworth J, Papp E. Vitamin D supplementation during pregnancy: state of the evidence from a systematic review of randomised trials. BMJ 2017. 359 j5237 ( 10.1136/bmj.j5237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Putzu A, Belletti A, Cassina T, Clivio S, Monti G, Zangrillo A, Landoni G. Vitamin D and outcomes in adult critically ill patients. A systematic review and meta-analysis of randomized trials. Journal of Critical Care 2017. 38 109–114. ( 10.1016/j.jcrc.2016.10.029) [DOI] [PubMed] [Google Scholar]
- 72.Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database of Systematic Reviews 2014. 1 CD007470 ( 10.1002/14651858.CD007470.pub3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McDonnell SL, Baggerly CA, French CB, Baggerly LL, Garland CF, Gorham ED, Hollis BW, Trump DL, Lappe JM. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations ≥60 vs <20 ng/mL (150 vs 50 nmol/L): pooled analysis of two randomized trials and a prospective cohort. PLoS ONE 2018. 13 e0199265 ( 10.1371/journal.pone.0199265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McDonnell SL, Baggerly C, French CB, Baggerly LL, Garland CF, Gorham ED, Lappe JM, Heaney RP. Serum 25-hydroxyvitamin D concentrations ≥40 ng/mL are associated with >65% lower cancer risk: pooled analysis of randomized trial and prospective cohort study. PLoS ONE 2016. 11 e0152441 ( 10.1371/journal.pone.0152441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grant WB, Boucher BJ. Randomized controlled trials of vitamin D and cancer incidence: a modeling study. PLoS ONE 2017. 12 e0176448 ( 10.1371/journal.pone.0176448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pilz S, Dobnig H, Winklhofer-Roob B, Riedmüller G, Fischer JE, Seelhorst U, Wellnitz B, Boehm BO, März W. Low serum levels of 25-hydroxyvitamin D predict fatal cancer in patients referred to coronary angiography. Cancer Epidemiology, Biomarkers and Prevention 2008. 17 1228–1233. ( 10.1158/1055-9965.EPI-08-0002) [DOI] [PubMed] [Google Scholar]
- 77.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. Journal of the National Cancer Institute 2006. 98 451–459. ( 10.1093/jnci/djj101) [DOI] [PubMed] [Google Scholar]
- 78.Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, D’Agostino D, et al Vitamin D supplements and prevention of cancer and cardiovascular disease. New England Journal of Medicine 2019. 380 33–44. doi:10.1056/NEJMoa1809944.30415629 [Google Scholar]
- 79.Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nature Reviews Endocrinology 2017. 13 466–479. ( 10.1038/nrendo.2017.31) [DOI] [PubMed] [Google Scholar]
- 80.Pilz S, März W, Cashman KD, Kiely ME, Whiting SJ, Holick MF, Grant WB, Pludowski P, Hiligsmann M, Trummer C, et al Rationale and plan for vitamin D food fortification: a review and guidance. Frontiers in Endocrinology 2018. 9 373 ( 10.3389/fendo.2018.00373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.US Preventive Services Task Force, Grossman DC, Curry SJ, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW, Jr, Kemper AR, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US Preventive Services Task Force Recommendation Statement. JAMA 2018. 319 1592–1599. ( 10.1001/jama.2018.3185) [DOI] [PubMed] [Google Scholar]
- 82.Brouwer-Brolsma EM, Bischoff-Ferrari HA, Bouillon R, Feskens EJ, Gallagher CJ, Hyppönen E, Llewellyn DJ, Stoecklin E, Dierkes J, Kies AK, et al Vitamin D: do we get enough? A discussion between vitamin D experts in order to make a step towards the harmonisation of dietary reference intakes for vitamin D across Europe. Osteoporosis International 2013. 24 1567–1577. ( 10.1007/s00198-012-2231-3) [DOI] [PubMed] [Google Scholar]
- 83.Pilz S, Trummer C, Pandis M, Schwetz V, Aberer F, Grübler M, Verheyen N, Tomaschitz A, März W. Vitamin D: current guidelines and future outlook. Anticancer Research 2018. 38 1145–1151. ( 10.21873/anticanres.12333) [DOI] [PubMed] [Google Scholar]
- 84.Cashman KD. Vitamin D requirements for the future-lessons learned and charting a path forward. Nutrients 2018. 10 533 ( 10.3390/nu10050533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cashman KD, Ritz C, Kiely M. & Odin Collaborators. Improved dietary guidelines for vitamin D: application of individual participant data (IPD)-level meta-regression analyses. Nutrients 2017. 9 469 ( 10.3390/nu9050469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, et al The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. Journal of Clinical Endocrinology and Metabolism 2011. 96 53–58. ( 10.1210/jc.2010-2704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Institute of Medicine (US). Committee to review dietary reference intakes for vitamin D and calcium. In Dietary Reference Intakes for Calcium and Vitamin D. Eds AC Ross, Taylor CL, Yaktine AL. & Del Valle HB. Washington DC, USA: National Academies Press, 2011. [PubMed] [Google Scholar]
- 88.EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific opinion on dietary reference values for vitamin D. EFSA Journal 2016. 14 4547. [Google Scholar]
- 89.Nordic Council of Ministers. Nordic Nutrition Recommendation 2012. Integrating Nutrition and Physical Activity. Copenhagen, Denmark: Nordic Council of Ministers, 2014. [Google Scholar]
- 90.German Nutrition Society. New reference values for vitamin D. Annals of Nutrition and Metabolism 2012. 60 241–246. ( 10.1159/000337547) [DOI] [PubMed] [Google Scholar]
- 91.Scientific Advisory Committee on Nutrition. Report on vitamin D and health. London, UK: SACN & Public Health England, 2016. (available at: https://www.gov.uk/government/groups/scientific-advisory-committee-on-nutrition) [Google Scholar]
- 92.Allen L, de Benoist B, Dary O. & Hurrell R. Guidelines on Food Fortification with Micronutrients. Geneva, Switzerland: WHO/Food and Agriculture Organization of the United Nations, 2006. (available at: http://www.who.int/iris/handle/10665/43412) [Google Scholar]
- 93.Cashman KD, Dowling KG, Škrabáková Z, Gonzalez-Gross M, Valtueña J, De Henauw S, Moreno L, Damsgaard CT, Michaelsen KF, Mølgaard C, et al Vitamin D deficiency in Europe: pandemic? American Journal of Clinical Nutrition 2016. 103 1033–1044. ( 10.3945/ajcn.115.120873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Schoor N, Lips P. Global overview of vitamin D status. Endocrinology and Metabolism Clinics of North America 2017. 46 845–870. ( 10.1016/j.ecl.2017.07.002) [DOI] [PubMed] [Google Scholar]
- 95.Schleicher RL, Sternberg MR, Lacher DA, Sempos CT, Looker AC, Durazo-Arvizu RA, Yetley EA, Chaudhary-Webb M, Maw KL, Pfeiffer CM, et al The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. American Journal of Clinical Nutrition 2016. 104 454–461. ( 10.3945/ajcn.115.127985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schleicher RL, Sternberg MR, Looker AC, Yetley EA, Lacher DA, Sempos CT, Taylor CL, Durazo-Arvizu RA, Maw KL, Chaudhary-Webb M, et al National estimates of serum total 25-hydroxyvitamin D and metabolite concentrations measured by liquid chromatography-tandem mass spectrometry in the US population during 2007–2010. Journal of Nutrition 2016. 146 1051–1061. ( 10.3945/jn.115.227728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manios Y, Moschonis G, Lambrinou CP, Tsoutsoulopoulou K, Binou P, Karachaliou A, Breidenassel C, Gonzalez-Gross M, Kiely M, Cashman KD. A systematic review of vitamin D status in southern European countries. European Journal of Nutrition 2018. 57 2001–2036. ( 10.1007/s00394-017-1564-2) [DOI] [PubMed] [Google Scholar]
- 98.Spiro A, Buttriss JL. Vitamin D: an overview of vitamin D status and intake in Europe. Nutrition Bulletin 2014. 39 322–350. ( 10.1111/nbu.12108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jääskeläinen T, Itkonen ST, Lundqvist A, Erkkola M, Koskela T, Lakkala K, Dowling KG, Hull GL, Kröger H, Karppinen J, et al The positive impact of general vitamin D food fortification policy on vitamin D status in a representative adult Finnish population: evidence from an 11-y follow-up based on standardized 25-hydroxyvitamin D data. American Journal of Clinical Nutrition 2017. 105 1512–1520. ( 10.3945/ajcn.116.151415) [DOI] [PubMed] [Google Scholar]
- 100.Calvo MS, Whiting SJ. Survey of current vitamin D food fortification practices in the United States and Canada. Journal of Steroid Biochemistry and Molecular Biology 2013. 136 211–213. ( 10.1016/j.jsbmb.2012.09.034) [DOI] [PubMed] [Google Scholar]
- 101.Wilson LR, Tripkovic L, Hart KH, Lanham-New SA. Vitamin D deficiency as a public health issue: using vitamin D2 or vitamin D3 in future fortification strategies. Proceedings of the Nutrition Society 2017. 76 392–399. ( 10.1017/S0029665117000349) [DOI] [PubMed] [Google Scholar]
- 102.Moulas AN, Vaiou M. Vitamin D fortification of foods and prospective health outcomes. Journal of Biotechnology 2018. 285 91–101. ( 10.1016/j.jbiotec.2018.08.010) [DOI] [PubMed] [Google Scholar]
- 103.Itkonen ST, Erkkola M, Lamberg-Allardt CJE. Vitamin D fortification of fluid milk products and their contribution to vitamin D intake and vitamin D status in observational studies – a review. Nutrients 2018. 10 E1054 ( 10.3390/nu10081054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, Hope S, Kanis JA, McCloskey EV, Poole KES, et al UK clinical guideline for the prevention and treatment of osteoporosis. Archives of Osteoporosis 2017. 12 43 ( 10.1007/s11657-017-0324-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY, on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) & The Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporosis International 2013. 24 23–57. ( 10.1007/s00198-012-2074-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dawson-Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GE, Josse RG, Lips P, Morales-Torres J, Yoshimura N. IOF position statement: vitamin D recommendations for older adults. Osteoporosis International 2010. 21 1151–1154. ( 10.1007/s00198-010-1285-3) [DOI] [PubMed] [Google Scholar]
- 107.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney International Supplements 2017. 7 1–59. ( 10.1016/j.kisu.2017.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bilezikian JP, Brandi ML, Eastell R, Silverberg SJ, Udelsman R, Marcocci C, Potts JT., Jr. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the fourth International Workshop. Journal of Clinical Endocrinology and Metabolism 2014. 99 3561–3569. ( 10.1210/jc.2014-1413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bollerslev J, Rejnmark L, Marcocci C, Shoback DM, Sitges-Serra A, van Biesen W, Dekkers OM. & European Society of Endocrinology. European Society of Endocrinology Clinical Guideline: treatment of chronic hypoparathyroidism in adults. European Journal of Endocrinology 2015. 173 G1–G20. ( 10.1530/EJE-15-0628) [DOI] [PubMed] [Google Scholar]
- 110.Brandi ML, Bilezikian JP, Shoback D, Bouillon R, Clarke BL, Thakker RV, Khan AA, Potts JT., Jr. Management of hypoparathyroidism: summary statement and guidelines. Journal of Clinical Endocrinology and Metabolism 2016. 101 2273–2283. ( 10.1210/jc.2015-3907) [DOI] [PubMed] [Google Scholar]
- 111.Avenell A, Bolland MJ, Grey A. 25-Hydroxyvitamin D – should labs be measuring it? Annals of Clinical Biochemistry 2018. [epub]. ( 10.1177/0004563218796858) [DOI] [PubMed] [Google Scholar]
- 112.Rockwell M, Kraak V, Hulver M, Epling J. Clinical management of low vitamin D: a scoping review of physicians’ practices. Nutrients 2018. 10 493 ( 10.3390/nu10040493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Basatemur E, Hunter R, Horsfall L, Sutcliffe A, Rait G. Costs of vitamin D testing and prescribing among children in primary care. European Journal of Pediatrics 2017. 176 1405–1409. ( 10.1007/s00431-017-2986-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Woodford HJ, Barrett S, Pattman S. Vitamin D: too much testing and treating? Clinical Medicine 2018. 18 196–200. ( 10.7861/clinmedicine.18-3-196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Binkley N, Dawson-Hughes B, Durazo-Arvizu R, Thamm M, Tian L, Merkel JM, Jones JC, Carter GD, Sempos CT. Vitamin D measurement standardization: the way out of the chaos. Journal of Steroid Biochemistry and Molecular Biology 2017. 173 117–121. ( 10.1016/j.jsbmb.2016.12.002) [DOI] [PubMed] [Google Scholar]
- 116.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. American Journal of Epidemiology 2010. 171 903–908. ( 10.1093/aje/kwq005) [DOI] [PubMed] [Google Scholar]
- 117.Pilz S, Hahn A, Schön C, Wilhelm M, Obeid R. Effect of two different multimicronutrient supplements on vitamin D status in women of childbearing age: a randomized trial. Nutrients 2017. 9 30 ( 10.3390/nu9010030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lehmann U, Riedel A, Hirche F, Brandsch C, Girndt M, Ulrich C, Seibert E, Henning C, Glomb MA, Dierkes J, et al Vitamin D3 supplementation: response and predictors of vitamin D3 metabolites – a randomized controlled trial. Clinical Nutrition 2016. 35 351–358. ( 10.1016/j.clnu.2015.04.021) [DOI] [PubMed] [Google Scholar]
- 119.Gallagher JC, Sai A, Templin T, 2nd, Smith L. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Annals of Internal Medicine 2012. 156 425–437. ( 10.7326/0003-4819-156-6-201203200-00005) [DOI] [PubMed] [Google Scholar]
- 120.O'Callaghan KM, Hennessy Á, Hull GLJ, Healy K, Ritz C, Kenny LC, Cashman KD, Kiely ME. Estimation of the maternal vitamin D intake that maintains circulating 25-hydroxyvitamin D in late gestation at a concentration sufficient to keep umbilical cord sera ≥25–30 nmol/L: a dose-response, double-blind, randomized placebo-controlled trial in pregnant women at northern latitude. American Journal of Clinical Nutrition 2018. 108 77–91. ( 10.1093/ajcn/nqy064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bacon CJ, Gamble GD, Horne AM, Scott MA, Reid IR. High-dose oral vitamin D3 supplementation in the elderly. Osteoporosis International 2009. 20 1407–1415. ( 10.1007/s00198-008-0814-9) [DOI] [PubMed] [Google Scholar]
- 122.Jorde R, Grimnes G. Serum cholecalciferol may be a better marker of vitamin D status than 25-hydroxyvitamin D. Medical Hypotheses 2018. 111 61–65. ( 10.1016/j.mehy.2017.12.017) [DOI] [PubMed] [Google Scholar]
- 123.Ish-Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. Journal of Clinical Endocrinology and Metabolism 2008. 93 3430–3435. ( 10.1210/jc.2008-0241) [DOI] [PubMed] [Google Scholar]
- 124.Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 2010. 303 1815–1822. ( 10.1001/jama.2010.594) [DOI] [PubMed] [Google Scholar]
- 125.Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R, Dick W, Willett WC, Egli A. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Internal Medicine 2016. 176 175–183. ( 10.1001/jamainternmed.2015.7148) [DOI] [PubMed] [Google Scholar]
- 126.Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women – a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology 2007. 46 1852–1857. ( 10.1093/rheumatology/kem240) [DOI] [PubMed] [Google Scholar]
- 127.European Food Safety Authority. Scientific opinion on the tolerable upper intake level of vitamin D. EFSA Journal 2012. 10 2813. [Google Scholar]
- 128.Khaw KT, Stewart AW, Waayer D, Lawes CMM, Toop L, Camargo CA, Jr, Scragg R. Effect of monthly high-dose vitamin D supplementation on falls and non-vertebral fractures: secondary and post-hoc outcomes from the randomised, double-blind, placebo-controlled ViDA trial. Lancet Diabetes and Endocrinology 2017. 5 438–447. ( 10.1016/S2213-8587(17)30103-1) [DOI] [PubMed] [Google Scholar]
- 129.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 2003. 326 469 ( 10.1136/bmj.326.7387.469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Smith LM, Gallagher JC, Suiter C. Medium doses of daily vitamin D decrease falls and higher doses of daily vitamin D3 increase falls: a randomized clinical trial. Journal of Steroid Biochemistry and Molecular Biology 2017. 173 317–322. ( 10.1016/j.jsbmb.2017.03.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marcinowska-Suchowierska E, Kupisz-Urbańska M, Łukaszkiewicz J, Płudowski P, Jones G. Vitamin D toxicity – a clinical perspective. Frontiers in Endocrinology 2018. 9 550 ( 10.3389/fendo.2018.00550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schöttker B, Jorde R, Peasey A, Thorand B, Jansen EH, Groot Ld, Streppel M, Gardiner J, Ordóñez-Mena JM, Perna L, et al Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ 2014. 348 g3656 ( 10.1136/bmj.g3656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Garland CF, Kim JJ, Mohr SB, Gorham ED, Grant WB, Giovannucci EL, Baggerly L, Hofflich H, Ramsdell JW, Zeng K, et al Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. American Journal of Public Health 2014. 104 e43–e50. ( 10.2105/AJPH.2014.302034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Whyte MP, Haddad JG, Jr, Walters DD, Stamp TC. Vitamin D bioavailability: serum 25-hydroxyvitamin D levels in man after oral, subcutaneous, intramuscular, and intravenous vitamin D administration. Journal of Clinical Endocrinology and Metabolism 1979. 48 906–911. ( 10.1210/jcem-48-6-906) [DOI] [PubMed] [Google Scholar]
- 135.Wylon K, Drozdenko G, Krannich A, Heine G, Dölle S, Worm M. Pharmacokinetic evaluation of a single intramuscular high dose versus an oral long-term supplementation of cholecalciferol. PLoS ONE 2017. 12 e0169620 ( 10.1371/journal.pone.0169620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gupta N, Farooqui KJ, Batra CM, Marwaha RK, Mithal A. Effect of oral versus intramuscular vitamin D replacement in apparently healthy adults with vitamin D deficiency. Indian Journal of Endocrinology and Metabolism 2017. 21 131–136. ( 10.4103/2230-8210.196007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bubshait DA, Al-Dakheel DA, Alanii FM. Topical vitamin D3: a randomized controlled trial (RCT). Clinical Nutrition ESPEN 2018. 27 16–19. ( 10.1016/j.clnesp.2018.05.009) [DOI] [PubMed] [Google Scholar]
- 138.Sadat-Ali M, Bubshait DA, Al-Turki HA, Al-Dakheel DA, Al-Olayani WS. Topical delivery of vitamin d3: a randomized controlled pilot study. International Journal of Biological and Medical Science 2014. 10 21–24. [PMC free article] [PubMed] [Google Scholar]
- 139.Quesada-Gomez JM, Bouillon R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporosis International 2018. 29 1697–1711. ( 10.1007/s00198-018-4520-y) [DOI] [PubMed] [Google Scholar]
- 140.Hayes A, Cashman KD. Food-based solutions for vitamin D deficiency: putting policy into practice and the key role for research. Proceedings of the Nutrition Society 2017. 76 54–63. ( 10.1017/S0029665116000756) [DOI] [PubMed] [Google Scholar]
- 141.Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, Chope G, Hyppönen E, Berry J, Vieth R, et al Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. American Journal of Clinical Nutrition 2012. 95 1357–1364. ( 10.3945/ajcn.111.031070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chun RF, Hernandez I, Pereira R, Swinkles L, Huijs T, Zhou R, Liu NQ, Shieh A, Guemes M, Mallya SM, et al Differential responses to vitamin D2 and vitamin D3 are associated With variations in free 25-hydroxyvitamin D. Endocrinology 2016. 157 3420–3430. ( 10.1210/en.2016-1139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shieh A, Chun RF, Ma C, Witzel S, Meyer B, Rafison B, Swinkels L, Huijs T, Pepkowitz S, Holmquist B, et al Effects of high-dose vitamin D2 Versus D3 on total and free 25-hydroxyvitamin D and markers of calcium balance. Journal of Clinical Endocrinology and Metabolism 2016. 101 3070–3078. ( 10.1210/jc.2016-1871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bouillon R, Verlinden L, Verstuyf A. Is vitamin D2 really bioequivalent to vitamin D3? Endocrinology 2016. 157 3384–3387. ( 10.1210/en.2016-1528) [DOI] [PubMed] [Google Scholar]
- 145.Jager N, Schöpe J, Wagenpfeil S, Bocionek P, Saternus R, Vogt T, Reichrath J. The impact of UV-dose, body surface area exposed and other factors on cutaneous vitamin D synthesis measured as serum 25(OH)D concentration: systematic review and meta-analysis. Anticancer Research 2018. 38 1165–1171. ( 10.21873/anticanres.12336) [DOI] [PubMed] [Google Scholar]
- 146.Seckmeyer G, Schrempf M, Wieczorek A, Riechelmann S, Graw K, Seckmeyer S, Zankl M. A novel method to calculate solar UV exposure relevant to vitamin D production in humans. Photochemistry and Photobiology 2013. 89 974–983. ( 10.1111/php.12074) [DOI] [PubMed] [Google Scholar]
- 147.Lehmann U, Gjessing HR, Hirche F, Mueller-Belecke A, Gudbrandsen OA, Ueland PM, Mellgren G, Lauritzen L, Lindqvist H, Hansen AL, et al Efficacy of fish intake on vitamin D status: a meta-analysis of randomized controlled trials. American Journal of Clinical Nutrition 2015. 102 837–847. ( 10.3945/ajcn.114.105395) [DOI] [PubMed] [Google Scholar]
- 148.Himbert C, Ose J, Delphan M, Ulrich CM. A systematic review of the interrelation between diet- and surgery-induced weight loss and vitamin D status. Nutrition Research 2017. 38 13–26. ( 10.1016/j.nutres.2016.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, Cooper JD, Dastani Z, Li R, Houston DK, et al Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Medicine 2013. 10 e1001383 ( 10.1371/journal.pmed.1001383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lappe JM, Heaney RP. Why randomized controlled trials of calcium and vitamin D sometimes fail. Dermato-Endocrinology 2012. 4 95–100. ( 10.4161/derm.19833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Heaney RP. Vitamin D – baseline status and effective dose. New England Journal of Medicine 2012. 367 77–78. ( 10.1056/NEJMe1206858) [DOI] [PubMed] [Google Scholar]
- 152.Heaney RP. Nutrients, endpoints, and the problem of proof. Journal of Nutrition 2008. 138 1591–1595. ( 10.1093/jn/138.9.1591) [DOI] [PubMed] [Google Scholar]
- 153.Heaney RP. The nutrient problem. Nutrition Reviews 2012. 70 165–169. ( 10.1111/j.1753-4887.2011.00469.x) [DOI] [PubMed] [Google Scholar]
- 154.Heaney RP. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutrition Reviews 2014. 72 48–54. ( 10.1111/nure.12090) [DOI] [PubMed] [Google Scholar]
- 155.Hébert JR, Frongillo EA, Adams SA, Turner-McGrievy GM, Hurley TG, Miller DR, Ockene IS. Perspective: randomized controlled trials are not a panacea for diet-related research. Advances in Nutrition 2016. 7 423–432. ( 10.3945/an.115.011023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ioannidis JPA. The challenge of reforming nutritional epidemiologic research. JAMA 2018. 320 969–970. ( 10.1001/jama.2018.11025) [DOI] [PubMed] [Google Scholar]
- 157.Scragg R. Limitations of vitamin D supplementation trials: why observational studies will continue to help determine the role of vitamin D in health. Journal of Steroid Biochemistry and Molecular Biology 2018. 177 6–9. ( 10.1016/j.jsbmb.2017.06.006) [DOI] [PubMed] [Google Scholar]
- 158.Pilz S, Rutters F, Dekker JM. Disease prevention: vitamin D trials. Science 2012. 338 883 ( 10.1126/science.338.6109.883-c) [DOI] [PubMed] [Google Scholar]
- 159.Scragg R. Emerging evidence of thresholds for beneficial effects from vitamin D supplementation. Nutrients 2018. 10 561 ( 10.3390/nu10050561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, Kirpach B, Shah RC, Ives DG, Storey E, et al Effect of aspirin on all-cause mortality in the healthy elderly. New England Journal of Medicine 2018. 379 1519–1528. doi:10.1056/NEJMoa1803955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kupferschmidt K. Uncertain verdict as vitamin D goes on trial. Science 2012. 337 1476–1478. ( 10.1126/science.337.6101.1476) [DOI] [PubMed] [Google Scholar]
- 162.Trajanoska K, Morris JA, Oei L, Zheng HF, Evans DM, Kiel DP, Ohlsson C, Richards JB, Rivadeneira F. GEFOS/GENOMOS consortium and the 23andMe research team. Assessment of the genetic and clinical determinants of fracture risk: genome wide association and Mendelian randomisation study. BMJ 2018. 362 k3225 ( 10.1136/bmj.k3225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Afzal S, Brøndum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: mendelian randomisation analysis in three large cohorts. BMJ 2014. 349 g6330 ( 10.1136/bmj.g6330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jassil NK, Sharma A, Bikle D, Wang X. Vitamin D binding protein and 25-hydroxyvitamin D levels: emerging clinical applications. Endocrine Practice 2017. 23 605–613. ( 10.4158/EP161604.RA) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kroll MH, Bi C, Garber CC, Kaufman HW, Liu D, Caston-Balderrama A, Zhang K, Clarke N, Xie M, Reitz RE, et al Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS ONE 2015. 10 e0118108 ( 10.1371/journal.pone.0118108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sempos CT, Heijboer AC, Bikle DD, Bollerslev J, Bouillon R, Brannon PM, DeLuca HF, Jones G, Munns CF, Bilezikian JP, et al Vitamin D assays and the definition of hypovitaminosis D: results from the first International Conference on Controversies in Vitamin D. British Journal of Clinical Pharmacology 2018. 84 2194–2207. ( 10.1111/bcp.13652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zittermann A, Prokop S, Gummert JF, Börgermann J. Safety issues of vitamin D supplementation. Anti-Cancer Agents in Medicinal Chemistry 2013. 13 4–10. ( 10.2174/187152013804487290) [DOI] [PubMed] [Google Scholar]
- 168.Rooney MR, Harnack L, Michos ED, Ogilvie RP, Sempos CT, Lutsey PL. Trends in use of high-dose vitamin D supplements exceeding 1000 or 4000 international units daily, 1999–2014. JAMA 2017. 317 2448–2450. ( 10.1001/jama.2017.4392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dwyer JT, Coates PM, Smith MJ. Dietary supplements: regulatory challenges and research resources. Nutrients 2018. 10 41 ( 10.3390/nu10010041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Darling AL, Blackbourn DJ, Ahmadi KR, Lanham-New SA. Vitamin D supplement use and associated demographic, dietary and lifestyle factors in 8024 South Asians aged 40–69 years: analysis of the UK biobank cohort. Public Health Nutrition 2018. 21 2678–2688. ( 10.1017/S1368980018001404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Weernink MG, van Wijk RM, Groothuis-Oudshoorn CG, Lanting CI, Grant CC, van Vlimmeren LA, Boere-Boonekamp MM. Insufficient vitamin D supplement use during pregnancy and early childhood: a risk factor for positional skull deformation. Maternal and Child Nutrition 2016. 12 177–188. ( 10.1111/mcn.12153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Looman M, van den Berg C, Geelen A, Samlal RAK, Heijligenberg R, Klein Gunnewiek JMT, Balvers MGJ, Leendertz-Eggen CL, Wijnberger LDE, Feskens EJM, et al Supplement use and dietary sources of folate, vitamin D, and n-3 fatty acids during preconception: the GLIMP2 study. Nutrients 2018. 10 962 ( 10.3390/nu10080962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jun S, Cowan AE, Tooze JA, Gahche JJ, Dwyer JT, Eicher-Miller HA, Bhadra A, Guenther PM, Potischman N, Dodd KW, et al Dietary supplement use among U.S. Children by family income, food security level, and nutrition assistance program participation status in 2011–2014. Nutrients 2018. 10 1212 ( 10.3390/nu10091212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Creo AL, Thacher TD, Pettifor JM, Strand MA, Fischer PR. Nutritional rickets around the world: an update. Paediatrics and International Child Health 2017. 37 84–98. ( 10.1080/20469047.2016.1248170) [DOI] [PubMed] [Google Scholar]
- 175.Creo AL, Tebben PJ, Fischer PR, Thacher TD, Pittock ST. Cardiac arrest in a vitamin D-deficient infant. Global Pediatric Health 2018. 5 2333794X18765064 ( 10.1177/2333794X18765064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chehade H, Girardin E, Rosato L, Cachat F, Cotting J, Perez MH. Acute life-threatening presentation of vitamin D deficiency rickets. Journal of Clinical Endocrinology and Metabolism 2011. 96 2681–2683. ( 10.1210/jc.2011-1112) [DOI] [PubMed] [Google Scholar]
- 177.Uday S, Högler W. Prevention of rickets and osteomalacia in the UK: political action overdue. Archives of Disease in Childhood 2018. 103 901–906. ( 10.1136/archdischild-2018-314826) [DOI] [PubMed] [Google Scholar]
- 178.Zittermann A, Ernst JB, Prokop S, Fuchs U, Dreier J, Kuhn J, Knabbe C, Birschmann I, Schulz U, Berthold HK, et al Effect of vitamin D on all-cause mortality in heart failure (EVITA): a 3-year randomized clinical trial with 4000 IU vitamin D daily. European Heart Journal 2017. 38 2279–2286. ( 10.1093/eurheartj/ehx235) [DOI] [PubMed] [Google Scholar]
- 179.Bolland MJ, Grey A, Avenell A. Assessment of research waste part 2: wrong study populations – an exemplar of baseline vitamin D status of participants in trials of vitamin D supplementation. BMC Medical Research Methodology 2018. 18 101 ( 10.1186/s12874-018-0555-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.EFSA NDA Panel. Turck D, Bresson J-L, Burlingame B, Dean T, Fairweather‐Tait S, Heinonen M, Hirsch-Ernst KI, Mangelsdorf I, McArdle HJ.et al Update of the tolerable upper intake level for vitamin D for infants. EFSA Journal 2018. 16 e05365 ( 10.2903/j.efsa.2018.5365) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a