Abstract
Background
Hematuria can be either grossly visible (macrohematuria) or only detectable under a microscope (microhematuria). Microhematuria is often asymptomatic and has a prevalence of 4–5% in routine clinical practice. It may be due to an underlying disease of the kidneys or the urogenital tract. In this article, we provide an overview of the causes of hematuria and of the recommendations of current guidelines for its diagnostic evaluation. A risk-adapted diagnostic strategy for the evaluation of asymptomatic microhematuria (aMH) is presented.
Methods
This review is based on pertinent publications retrieved by a selective search in PubMed, as well as on guidelines from Germany and abroad.
Results
Hematuria has many causes, and a broad urological and nephrological differential diagnosis must be considered. In the absence of high-quality scientific evidence, the recommendations of current guidelines for the diagnostic evaluation of hematuria are not uniform; this is particularly so for aMH. Microhematuria is said to be present when urine microscopy reveals three or more erythrocytes per high-power field. The basic diagnostic evaluation consists of a thorough history and physical examination, measurement of inflammatory parameters and renal function tests, and ultrasonography of the kidneys and bladder. Patients with non-glomerular aMH who have risk factors such as smoking, advanced age, and male sex are more likely to have relevant underlying conditions and should therefore undergo augmented, risk-adapted diagnostic evaluation with urethrocystoscopy, urine cytology, and, when indicated, CT urography. Patients with isolated glomerular hematuria are at elevated risk for renal disease and should undergo follow-up checks at six-month intervals.
Conclusion
Although hematuria is common, there is no uniform, internationally accepted, evidence-based algorithm for its diagnostic evaluation. All potential causes of hematuria must be considered, and all individual risk factors taken into account, so that an underlying disease requiring treatment can be identified or ruled out.
Hematuria is a term put together from the Greek words haima (blood) and ouron (urine) to refer to the presence of blood in the urine. The blood may be visible to the naked eye (macrohematuria, gross or frank hematuria) or only under the microscope (microhematuria). In some patient groups it is a frequent finding. The reported prevalence of asymptomatic microhematuria (aMH) ranges between 1.7% and 31.1%; in routine clinical practice, a prevalence of 4% to 5% appears realistic (1– 4). It is dependent on various factors in the population studied, such as the definition threshold, frequency of testing, age, and sex, as well as risk factors.
Macrohematuria
Macrohematuria always requires investigation. The hematuria may be visible from a concentration as low as 1 mL blood per liter of urine. The color and the intensity of the color correlate with the amount of blood content: fresh arterial blood (bright red, ranging from pink to ketchup-colored) can be distinguished from venous blood (dark red, Bordeaux red) and from old blood (dark brown or black). Rarely, urine may be colored red or dark owing to myoglobinuria (due to rhabdomyolysis) or hemoglobinuria (due to hemolysis). A diagnosis of hematuria is confirmed by demonstration of red blood cells in the urinary sediment as shown by qualitative and quantitative microscopy (5).
Microhematuria
In microhematuria, there is a microscopic increase in red blood cell content above the physiological threshold. The threshold is given as either ≥ or >3 red blood cells per high-power field in microscopic assessment of the urinary sediment in two out of three correctly collected urine samples. Some medical specialty societies regard evidence from one dipstick as adequate, while others require more than just one (e.g., positive results on two out of three dipsticks) for a diagnosis of “significant” microhematuria (eTable).
eTable. Comparison of important guidelines and further recommendations on the investigation of asymptomatic microhematuria (adapted from [2]).
| Parameter |
Canada Wollin et al. 2009 (20) |
Japan Horie et al. 2014 (16) |
Scotland SIGN 2008 (14) |
AUA Davis et al. 2012 (1) |
Loo et al. 2009 (21) |
BAUS Anderson et al. 2008 (15) |
Netherlands Hovius 2010 (17) |
Sweden Malmström 2003 (19) |
Health Technol Assess Rodgers et al. 2006 (8) |
| Definition of microhematuria |
>2 RBC/HPF in two microscopic urinalyses |
5 RBC/HPF or 20 RBC/µL = +1 dipstick test |
A single positive dipstick test is insufficient to show the presence of pathology. | >3 RBC/HPF | >3 RBC/HPF in two of three properly collected urine samples | 2 out of 3 dipsticks (significant hematuria +1) | >3 RBC/HPF | Not specified | ≥ 3 RBC/HPF |
| Exclusion criteria | Recent sports activity, menstruation, sexual activity, or instrumentation | Vigorous sports activity | UTI | UTI, menstruation, recent urological treatment, very intensive sports activity, trauma, renal disease, viral disease | Urine culture (UTI), creatinine, flank pain | UTI, sport, menstruation | UTI, menstruation | Not specified | UTI, very intensive sport, trauma, menstruation |
| Nephrology referral | Proteinuria, red cell casts, RBC under microscopy, and/or raised creatinine | All patients with proteinuria and microhematuria, uncertain distinction between glomerular and nonglomerular hematuria | Proteinuria together with abnormal creatinine | eGFR, creatinine, urea, dysmorphic RBC, proteinuria, red cell casts, and/or renal failure | Creatinine | Elevated BP, creatinine, eGFR, PCR, ACR | Elevated BP, MDRD-GFR, proteinuria, albuminuria, dysmorphic RBC | Not specified | Proteinuria, red cell casts, creatinine, elevated BP |
| Age threshold (years) |
>40 | >40 | >50 | >35 | Not specified | >40 | 50 | Not specified | 40 |
| Risk stratification | History of smoking, occupational exposure to chemicals or dyes [benzenes, aromatic amines), irritative voiding, painkiller abuse (phenacetin)], pelvis irradiation, or history of cyclophosphamide treatment | Male, >40 years, smoker, exposure to chemicals, history of urological disease, emergency patients, history of UTI, frequent use of NSAIDs, pelvic irradiation, cyclophosphamide treatment | Urgent assessment >50 | >35 years, (ex-) smoker, history of chemotherapy with alkylating agents such as cyclophosphamides, exposure to chemicals or dyes (benzenes, aromatic amines), pelvic irradiation, macrohematuria, urological disorder or disease, chronic UTI | Not specified | Age >40 | Male, (ex-)smoker, exposure to benzenes or aromatic amines, macrohematuria, irritative voiding, history of pelvic irradiation, urothelial carcinoma, recurrent UTIs, analgesic abuse (phenacetin) | Not specified | (Ex-)smoker, occupational exposure to chemicals (benzenes, aromatic amines), age >40, history of urological disease, UTI, macrohematuria, analgesic abuse, pelvic irradiation |
| Cytology | All patients referred for lower urinary tract investigation | Recommended for patients without risk factors | Not specified | Not recommended | No consensus on cytology | Not specified | For all patients >50 with negative US and cystoscopy: consider cytology and CTU | Not specified | For all high-risk patients, optional for low-risk patients (prioritize tumor markers) |
| Imaging | US for all patients (suboptimal: CTU, IVP) | US for patients without risk factors | US | CTU for all patients (suboptimal: MRI, US) | For all patients: modified CTU (or IVP with US), US for patients allergic to contrast media | For all patients >40 without underlying nephrological disease | All patients undergo US and cystoscopy, those >50 with positive US and/or cystoscopy findings and/or risk factors undergo CTU | Not specified | All patients undergo US; high-risk patients: CT scan if initial investigation negative; low-risk patients: consider CT if initial investigation positive |
| Cystoscopy | >40 years or at-risk patients, patients with atypical or positive cytology | All patients with risk factors | All patients >50 years, to rule out tumors of the urogenital tract | All patients >35; <35 at physician’s discretion; all patients with risk factors | Urology referral | For all patients >40 without underlying nephrological disease | All patients >50; all patients <50 at physician’s discretion, all patients with risk factors | Not specified | All high-risk patients; low-risk patients: if cytology positive or tumor markers found |
| Follow-up | After negative assessment: urinalysis, cytology, blood pressure, investigations after 6, 12, 24, and 36 months; repeat urological investigation if: macrohematuria, positive or atypical cytology, irritative voiding | Patients in whom a urinary tract tumor is not detected do not need monitoring unless they have macrohematuria or irritative voiding; annual checkups are recommended in cases of persistent hematuria. | Not specified | After negative urological workup, annual urinalysis for at least 2 years | Clinicians may consider whether to reassess every patient with persistent microhematuria and a history of smoking after 2 to 5 years. | Long-term monitoring for patients who do not fulfill the criteria for urology or nephrology re‧ferral or those in whom the investigation showed no abnormal findings | Patients >40, (ex-)smoker, history of chemical exposure, urinalysis, cytology, and BP check at 6, 12, 24, and 36 months after a negative initial investigation | Not specified | High-risk patients: ‧repeat renal investigations after initial negative investigation; if screening is negative, further screening (urinalysis, BP check); low-risk patients: urinalysis at 3 and 6 months after a negative initial investigation |
| Guideline aimed at: | Not specified | Residents, attending physicians | All health professionals in primary and secondary care within the National Health Service who are involved in the diagnosis and treatment of patients with chronic kidney disease | Hospitals | Primary care physicians | Not specified | Healthcare service personnel | Not specified | Those who use, manage and provide care in the National Health Service |
| General screening for aMH | Not specified | Urinalysis is included in the annual medical checkup | Not specified | Not specified | Routine screening not recommended for aMH | Not recommended | Not recommended | No recommended assessment of aMH | Not specified |
ACR, albumin–creatinine ratio; aMH, asymptomatic microhematuria; AUA, American Urological Association; BAUS, British Association of Urological Surgeons; BP, blood pressure; CTU, CT urography; eGFR, estimated glomerular filtration rate; HPF, high-power field (area visible under the microscope at 400× magnification); IVP, intravenous pyelogram; MDRD-GFR, Modification of Diet in Renal Disease GFR (formula for calculating renal function); MRI, magnetic resonance imaging; NSAIDs, nonsteroidal antiinflammatory drugs; PCR, protein–creatinine ratio; RBC, red blood cells; US, ultrasonography; UTI, urinary tract infection
The test strips or dipsticks used to demonstrate hematuria are very sensitive and can show positive even at physiological levels of red blood cells in the urine, so after a weak positive result a sediment test should always be carried out before embarking on any further diagnostic investigations. A false negative dipstick result can be caused by ingestion of high doses of vitamin C. Molecular urine marker tests are not recommended during the initial assessment of aMH (6). A study of red blood cell morphology can be helpful in identifying the origin of the hematuria (glomerular versus nonglomerular). Depending on the study or diagnostic procedure (light microscopy, phase contrast microscopy, or automated cell count), the cutoffs for the percentage of dysmorphic cells required to demonstrate a glomerular origin can vary considerably (5, 7).
The aim of this review article is to present urological and nephrological causes of hematuria together with recommendations from the different guidelines of various medical associations. Based on an assessment of costs and benefits, we propose a basic diagnostic process, further diagnostic investigations to be undertaken on a risk-adapted basis, and follow-up monitoring of hematuria or asymptomatic microhematuria (aMH).
Causes and differential diagnosis
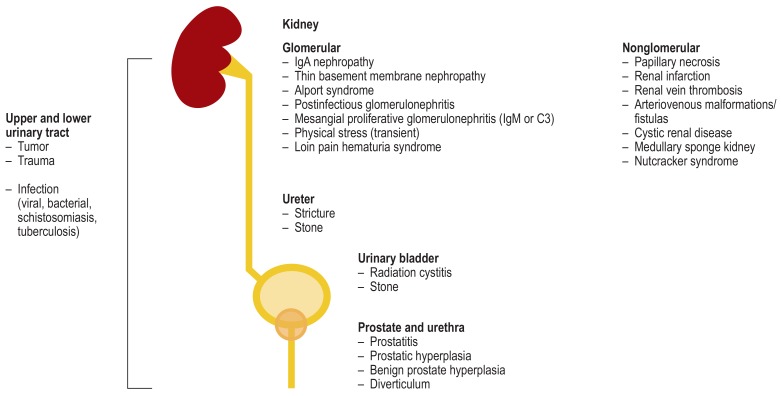
Among the most common causes of hematuria are infections of the lower urinary tract, especially the bladder. Other causes to consider are stones (urolithiasis) and, especially in older patients, tumors or benign prostatic hyperplasia (Figure 1). Among patients with asymptomatic nonglomerular microhematuria, 80% have so-called “idiopathic constitutional microhematuria” of no clinical significance (1, 2, 8); the remainder show findings requiring treatment. Because of its high prevalence and because of the differential diagnoses, aMH presents the greatest challenge clinically. In younger patients persistent microhematuria is associated with an increased risk of terminal kidney failure; the increase in risk is believed to be due to primary glomerular disease (9).
Figure 1.
Causes of hematuria in the region of the kidneys and urogenital organs
In addition to a detailed history and physical examination, it is important to distinguish between glomerular and nonglomerular hematuria. Hematuria that causes no symptoms and is clearly of glomerular origin makes serious urological disease unlikely and generally requires no further urological diagnostic investigation. Marked albuminuria (>500 mg/24 h) tends to indicate a glomerular cause of the blood in the urine. However, in a patient with known proteinuria and no previous recorded history of hematuria, it is important to exclude a urological cause (7).
Red blood cell morphology
Glomerular hematuria can result from immune-mediated damage to glomerular capillaries (e.g., IgA nephropathy) or from noninflammatory defects of the glomerular capillary wall (e.g., thin basement membrane nephropathy). In urinary sediment, glomerular hematuria is shown by the presence of red blood cell casts or, more often, dysmorphic red cells (Figure 2a). It is the passage through the glomerular basal membrane (mechanical damage) and then through the nephron (osmotic damage) that brings about these changes in red blood cell morphology. The presence of a sizable proportion of dysmorphic red cells tends to indicate glomerular hematuria. Acanthocytes (also known as G1 cells) show vesicle-like protrusions, and their assessment is generally more reproducible than a count of all dysmorphic cells (Figure 2b). If the percentage of acanthocytes is greater than 5%, this is a highly specific (98%) indicator of glomerular disease and requires further nephrological investigation (10). Automatic cell counters determine the mean corpuscular volume (MCV) of red blood cells; an MCV <74 femtoliters (fL) tends to indicate the presence of glomerular hematuria (sensitivity 76%, specificity 74%) (11).
Figure 2.
Red blood cell morphology in patients with glomerular hematuria
a) Urinary sediment showing a red blood cell cast and a few red blood cells. Red cell casts are diagnostic of glomerulonephritis or vasculitis.
b) Ring-shaped acanthocytes (G1 cells) with bleb-like protrusions. Acanthocytes are typical of glomerular damage in glomerulonephritis or vasculitis. Phase contrast microscopy, magnification 640×
The decision as to whether a renal biopsy is indicated in a patient with glomerular hematuria is affected by the individual risk–benefit calculation. If any additional risk factors are present (e.g., proteinuria, kidney failure, and/or systemic autoimmune disease), it should be carried out (12). Several long-term studies have shown that in patients with isolated glomerular hematuria, which does not require renal biopsy, regular monitoring will allow progression of the renal disease to be detected. Among asymptomatic adults identified in mass screening programs as having isolated hematuria without kidney failure, one in ten developed proteinuria after 5.8±4.4 years (13). In a retrospective cohort study of more than 1 million young adults, 0.3% of those included had persistent asymptomatic isolated microhematuria that in the long term was associated with an increased risk of terminal kidney failure compared with the control group (incidence rates: 19.6 versus 0.55 per 100 000 person-years; hazard ratio 32.4; 95% confidence interval: [18.9; 55.7]) (9). This underscores the importance of regular monitoring.
Guideline recommendations for the investigation of hematuria
The clinical importance of investigating hematuria is clear from the detail with which it is treated in clinical guidelines. A systematic review identified a total of nine relevant recommendations (1, 8, 14– 20): seven guidelines issued by medical specialist societies, one recommendation from a cost carrier (21), and one “Health Technology Assessment” (8). Five recommendations contain general advice for patients with hematuria (8, 15, 19, 21, 22), while the other four are concerned exclusively with asymptomatic microhematuria. The recommendations are summarized in the eTable.
In assessing the scientific basis of the recommendations, it must be borne in mind that they all are based on expert opinion, and so far none of the guidelines has been validated. Most of the recommendations follow much the same pathway. As a first step, a history is taken to rule out causes that do not require treatment, such as (previous) urinary tract infections, menstruation, strenuous sport activity, or medical interventions in the urinary tract: Patients with a history of any of these are excluded from further investigation. If the history is negative, indicators of a nephrological cause are sought, usually by testing for albuminuria, sediment testing to assess red blood cell morphology, and measuring blood pressure and renal function. If no sign of glomerular kidney disease is found, further urological investigation with imaging of the upper urinary tract and cystoscopy is recommended on the basis of a further risk calculation.
The risk calculation is done individually in each case. Because of the lack of scientific evidence regarding the investigation of postrenal hematuria, definitions, exclusion criteria, and recommendations vary considerably: In some recommendations, the risk calculation is based exclusively on patient age, whereas others take several risk factors into account. The suggested age threshold for an investigation ranges between >35 to >50 years. As regards the next steps, most of the guidelines are similar. In patients at increased risk of urothelial carcinoma (risk factors include higher age, male sex, macrohematuria, exposure to toxins) (Figure 3), the upper urinary tract is investigated using imaging techniques. Because of their sensitivity, the preferred modalities are CT urography (CTU) or, alternatively (e.g., where CTU is contraindicated), magnetic resonance urography (MRU). Some guidelines suggest ultrasonography as the primary imaging technique for the urinary tract. If no abnormalities are found in the upper urinary tract, the diagnostic investigations may, depending on the individual risk profile, be completed by urethrocystoscopy. A recent analysis of the cost-effectiveness of CT, urethrocystoscopy, and renal ultrasonography concluded that ultrasound and cystoscopy together form the most cost-effective way of investigating asymptomatic microhematuria (23). Attitudes toward urine cytology vary: some recommend its risk-adapted use while others reject it entirely.
Figure 3.
Algorithm for risk-adapted investigation of asymptomatic microhematuria depending on the presence of risk factors for relevant diseases including cancers of the urinary system
* Macrohematuria, by contrast, always requires both basic and further investigation.
The first S3 guideline for urinary bladder cancer, published in 2016 under the auspices of the German Urology Society (Deutsche Gesellschaft für Urologie), is short on detail regarding the further investigation of aMH (24). It recommends that “repeatedly confirmed asymptomatic microhematuria should prompt urological evaluation.” After the first evidence has been obtained, the aMH should (it says) be confirmed by a second test—but the extent of the diagnostic investigation is not defined, being left to the discretion of the treating physician. The Swedish guidelines are the only ones that do not recommend routine investigation of patients with aMH (19, 22), justifying this with the low predictive value of a positive dipstick test for the presence of urothelial carcinoma.
Drawing on the guideline recommendations described above, we have summarized suggested definitions and recommended actions for the investigation of asymptomatic microhematuria (aMH) in the Table.
Table. Definitions and recommended actions to investigate asymptomatic microhematuria (aMH), based on international guideline recommendations (1, 8, 14 – 20).
| Parameter | Definition/recommended actions |
| Microhematuria | ≥ 3 red blood cells per high-power field* |
| Nephrology referral | If proteinuria, albuminuria, red blood cell casts, and/or dysmorphic red blood cells shown by microscopy and/or renal failure/raised creatinine present |
| Age threshold | Investigate in patients >40 years |
| Urine cytology | For all patients >50 years with negative ultrasound and cystoscopy findings |
| Urethrocystoscopy | Age >40 years or other risk factors (figure 3), patients with atypical or positive cytology |
| Imaging | According to guideline recommendation, CT urography if basic investigation or urethrocystoscopy fails to show a correlate (Figure 3), or in patients with positive ultrasound findings In the opinion of the present authors, CT urography is only justified in patients with multiple risk factors. |
| Monitoring – who? | Patients >40 years, (ex-)smokers, history of exposure to chemicals |
| Monitoring – what? | Urinanalysis, cytology, and blood pressure measurement at 6, 12, 24, and 36 months after a negative initial investigation |
* Area visible under the microscope at 400× magnification
Investigation of hematuria in the “real world”
What does the investigation of hematuria look like in the primary care setting? The guideline of the German College of General Practitioners and Family Physicians (Deutsche Gesellschaft für Allgemeinmedizin) recommends that hematuria should be investigated if certain risk factors are present, such as patient age over 40, a history of abdominal/pelvic irradiation, the presence of symptoms, or a history of cyclophosphamide treatment (25). A questionnaire-based study by a Canadian working group showed that 47% of general practitioners carry out annual hematuria screening for all patients with hematuria, whereas 26% do not, even in the at-risk patients (26). Another study showed that 42% of patients with hematuria in a high-risk group (age over 50, smoker, exposure to toxic agents) were not referred for appropriate investigation (27).
Between 5% and 10% of all patients referred to a urological department attend because of hematuria. One health services research study evaluated data relating to 1049 patients of a urology group practice in the Ruhr area of Germany who showed evidence of micro- or macrohematuria in the period 2011 to 2012 (28). The study included 570 women and 479 men (median age 58 years). Asymptomatic microhematuria was found in 689 of those referred. Cancer was diagnosed in 99 of the 1049 patients (9.4%) (renal cancer, 7 cases; cancer of the renal pelvis, 6; ureteral cancer, 4; bladder cancer, 65; prostate cancer, 17). Macrohematuria, male sex, and age over 60 years were independent risk factors for the presence of cancer. Also shown was that renal cancer, renal pelvis cancer, and ureteral cancer are not reliably identified by an excretory urogram. Overall, the reported tumor detection rate appears to justify a more extensive diagnostic workup in this population of patients with micro- or macrohematuria. This is confirmed by a recent prospective study of 3556 patients referred to a urologist for investigation of micro- or macrohematuria: The incidence of malignant tumors in this patient group was 10.0% (8.0% bladder cancer, 1.0% renal cell carcinoma, 0.7% urothelial carcinoma of the upper urinary tract, and 0.3% prostate cancer) (29).
Should the investigation of micro- or macrohematuria be repeated within a certain period? In several studies cancer of the bladder or upper urinary tract was found in fewer than 1% of patients, even over the long term (30– 32); only in one series of 687 aMH patients who were reassessed after 4 years was a slightly higher incidence shown (10 tumors, 1.5%) (33). The US and Canadian guidelines take a pragmatic approach here, recommending that patients with persistent aMH should be reassessed after 3 years by ultrasound of the urogenital tract and urethrocystoscopy (1, 2, 20).
Risk-adapted investigation
Because of the lack of evidence, there is also a lack of consensus at present about the extent of diagnostic investigation required for hematuria. Routine clinical practice often diverges from the guidelines that recommend, even for hematuria confirmed by microscopy, diagnostic investigation of renal function including red cell morphology, urethrocystoscopy, and CTU (1). Several studies have shown that a less comprehensive diagnostic workup suffices to adequately confirm or exclude significant diagnoses that need to be treated (18, 23, 34, 35).
What risk factors should prompt further investigation of hematuria? Cancer diagnoses in the urogenital tract show a clear association with age and are rare below the age of 40 (27, 29). Several studies have identified clinical parameters that can indicate the presence of malignant disease in a patient with hematuria (18, 29, 34). These include higher age, macrohematuria, male sex, exposure to tobacco smoke, and exposure to occupational toxins. The studies cited above tend to suggest that particular risk groups should be referred for rapid and comprehensive specialist diagnosis (28, 29). This applies particularly to smokers and ex-smokers, since smoking is recognized as one of the main risk factors for urothelial carcinoma (24). According to current guideline recommendations, patients on anticoagulants for other conditions should not be treated differently to noncoagulated patients in terms of diagnostic workup for hematuria (6). It is true that a higher prevalence of urogenital tumors (especially bladder cancer) was found in patients with hematuria who were taking anticoagulants for atrial fibrillation than in those not taking anticoagulants. No difference was found between vitamin K antagonists, aspirin, and clopidogrel (36).
The motto “Investigate everyone with hematuria, but don’t always investigate everything!” seems sensible in view of the frequent occurrence of idiopathic, i.e., isolated, nonglomerular hematuria and the large number of trivial causes that can often be identified simply from the history.
In view of the above-mentioned risk factors, patients with macrohematuria should always be investigated unless an obvious benign cause is present (e.g., hemorrhagic cystitis). It should be borne in mind that even in such situations the presence of malignant disease cannot be definitely ruled out, so further tests should be carried out if the hematuria persists (29). Several studies have shown the high diagnostic value of renal ultrasound combined with urethrocystoscopy. For this reason, given the exposure to radiation and contrast agents and the additional costs involved, the decision to refer for CTU should not be made lightly (23, 37). Nevertheless, CTU does offer the highest sensitivity for the detection of urothelial carcinoma, especially in the upper urinary tract (24, 35), and so when the relevant risk factors are present (e.g., in the case of smokers), or when the cause is not revealed by a basic investigation and there are continuing grounds for suspicion (e.g., persistent aMH), CTU should always be carried out.
In the presence of aMH, a “basic investigation” could be distinguished from “further investigation” for patients with risk factors, and could be carried out on an individual basis (Figure 3). The basic investigation is carried out for every patient with glomerular or nonglomerular hematuria. This includes a detailed history with physical examination and laboratory tests (inflammation parameters, renal retention values) together with renal and bladder ultrasonography. If risk factors or abnormal findings are shown in the basic workup, “further investigation” should follow, involving urethrocystoscopy, urine cytology (as indicated) and, if neither the basic investigation nor urethrocystoscopy has identified a correlate, further imaging of the kidneys and the upper urinary tract (CTU or alternative). According to current data, CTU is not required if ultrasound shows no abnormality in the kidneys and bladder (35).
Key messages.
Every case of hematuria requires investigation. Macrohematuria requires more extensive investigation.
Because of the lack of high-quality scientific evidence, there are no consistent guideline recommendations for the investigation of hematuria, especially asymptomatic microhematuria.
Findings of red cell casts, large numbers of dysmorphic red blood cells, or more than 5% acanthocytes indicate the presence of glomerular hematuria, which requires a nephrology referral.
For a basic investigation, for all patients a history should be taken and clinical and laboratory tests carried out, and possibly also red cell morphology studies and renal and bladder ultrasonography.
Patients who have been exposed to exogenous toxins (including tobacco smoke), are older, are of male sex, or have macrohematuria, should be further investigated by urethrocystoscopy and perhaps CT urography.
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Davis R, Jones JS, Barocas DA, et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol. 2012;188:2473–2481. doi: 10.1016/j.juro.2012.09.078. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz-Drager BJ, Kuckuck EC, Zuiverloon TC, et al. Microhematuria assessment an IBCN consensus-based upon a critical review of current guidelines. Urol Oncol. 2016;34:437–451. doi: 10.1016/j.urolonc.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 3.Hiatt RA, Ordonez JD. Dipstick urinalysis screening, asymptomatic microhematuria, and subsequent urological cancers in a population-based sample. Cancer Epidemiol Biomarkers Prev. 1994;3:439–443. [PubMed] [Google Scholar]
- 4.Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy—part I: definition, detection, prevalence, and etiology. Urology. 2001;57:599–603. doi: 10.1016/s0090-4295(01)00919-0. [DOI] [PubMed] [Google Scholar]
- 5.Horstmann M, Franiel T, Grimm MO. [Differential diagnosis of hematuria] Urologe A. 2014;53:1215–1226. doi: 10.1007/s00120-014-3506-4. [DOI] [PubMed] [Google Scholar]
- 6.Linder BJ, Bass EJ, Mostafid H, Boorjian SA. Guideline of guidelines: asymptomatic microscopic haematuria. BJU Int. 2018;121:176–183. doi: 10.1111/bju.14016. [DOI] [PubMed] [Google Scholar]
- 7.Feldman A, Hsu C, Kurtz M, Cho K. Etiology and evaluation of hematuria in adults Wolters Kluwer Health, www.uptodate.com/contents/etiology-and-evaluation-of-hematuria-in-adults (last accessed on 15 April 2018) 2013 [Google Scholar]
- 8.Rodgers M, Nixon J, Hempel S, et al. Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation. Health Technol Assess. 2006;10 doi: 10.3310/hta10180. [DOI] [PubMed] [Google Scholar]
- 9.Vivante A, Calderon-Margalit R, Skorecki K. Hematuria and risk for end-stage kidney disease. Curr Opin Nephrol Hypertens. 2013;22:325–330. doi: 10.1097/MNH.0b013e32835f7241. [DOI] [PubMed] [Google Scholar]
- 10.Kohler H, Wandel E, Brunck B. Acanthocyturia—a characteristic marker for glomerular bleeding. Kidney Int. 1991;40:115–120. doi: 10.1038/ki.1991.188. [DOI] [PubMed] [Google Scholar]
- 11.Angulo JC, Lopez-Rubio M, Guil M, Herrero B, Burgaleta C, Sanchez-Chapado M. The value of comparative volumetric analysis of urinary and blood erythrocytes to localize the source of hematuria. J Urol. 1999;162:119–126. doi: 10.1097/00005392-199907000-00028. [DOI] [PubMed] [Google Scholar]
- 12.McGregor DO, Lynn KL, Bailey RR, Robson RA, Gardner J. Clinical audit of the use of renal biopsy in the management of isolated microscopic hematuria. Clin Nephrol. 1998;49:345–348. [PubMed] [Google Scholar]
- 13.Yamagata K, Yamagata Y, Kobayashi M, Koyama A. A long-term follow-up study of asymptomatic hematuria and/or proteinuria in adults. Clin Nephrol. 1996;45:281–288. [PubMed] [Google Scholar]
- 14.Scottish Intercollegiate Guidelines Network (SIGN) Diagnosis and management of chronic kidney disease, www.seqc.es/download/gpc/62/3729/1043258133/1073968/cms/sign-diagnosis-and-management-of-chronic-kidney-disease_guideline.pdf/ (last accessed on 15 April 2018) 2008 [Google Scholar]
- 15.Anderson J, Fawcett D, Feehally J, Goldberg L, Kelly J, MacTier R. Joint consensus statement on the initial assessment of haematuria Prepared on behalf of the renal association and British Association of Urological Surgeons (BAUS), www.baus.org.uk/_userfiles/pages/files/News/haematuria_consensus_guidelines_July_2008.pdf (last accessed on 15 April 2018) [Google Scholar]
- 16.Horie S, Ito S, Okada H, et al. Japanese guidelines of the management of hematuria 2013. Clin Exp Nephrol. 2013;18:679–689. doi: 10.1007/s10157-014-1001-2. [DOI] [PubMed] [Google Scholar]
- 17.Hovius MC (Vorsitz) Richtlijn Hematurie; Nederlandse Vereniging voor Urologie. https://www.nvu.nl/en-us/kwaliteit/richtlijnen/actuelerichtlijnen.aspx [Google Scholar]
- 18.Loo RK, Lieberman SF, Slezak JM, et al. Stratifying risk of urinary tract malignant tumors in patients with asymptomatic microscopic hematuria. Mayo Clin Proc. 2013;88:129–138. doi: 10.1016/j.mayocp.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Malmström PU. Time to abandon testing for microscopic haematuria in adults? BMJ. 2003;326:813–815. doi: 10.1136/bmj.326.7393.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wollin T, Laroche B, Psooy K. Canadian guidelines for the management of asymptomatic microscopic hematuria in adults. Can Urol Assoc J. 2009;3:77–80. doi: 10.5489/cuaj.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loo R, Whittaker J, Rabrenivich V. National practice recommendations for hematuria: how to evaluate in the absence of strong evidence? Perm J. 2009;13:37–46. doi: 10.7812/tpp/08-083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varenhorst E, Norlén BJ, Malmström PU, et al. Mikroskpisk hematuri hos vuxna; Svensk urologisk förening (SUF) http://urologi.org/wp-content/uploads/2016/12/SOTA-mikrohematuri.pdf (last accessed on 15 April 2018) [Google Scholar]
- 23.Halpern JA, Chughtai B, Ghomrawi H. Cost-effectiveness of common diagnostic approaches for evaluation of asymptomatic microscopic hematuria. JAMA Intern Med. 2017;177:800–807. doi: 10.1001/jamainternmed.2017.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF) S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Harnblasenkarzinoms; Langversion 1.1, 2016. www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Blasenkarzinom/LL_Harnblasenkarzinom_Langversion_1.1.pdf (last accessed on 15 April 2018) [Google Scholar]
- 25.Mainz A. Nicht-sichtbare Hämaturie - weniger ist mehr! Neue S1-Handlungsempfehlungen der Deutschen Gesellschaft für Allgemeinmedizin und Familienmedizin (DEGAM) Z Allg Med. 2014;90 [Google Scholar]
- 26.Yafi FA, Aprikian AG, Tanguay S, Kassouf W. Patients with microscopic and gross hematuria: practice and referral patterns among primary care physicians in a universal health care system. Can Urol Assoc J. 2011;5:97–101. doi: 10.5489/cuaj.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khadra MH, Pickard RS, Charlton M, Powell PH, Neal DE. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol. 2000;163:524–527. [PubMed] [Google Scholar]
- 28.Eisenhardt A, Heinemann D, Rubben H, Hess J. Haematuria work-up in general care—a German observational study. Int J Clin Pract. 2017;71 doi: 10.1111/ijcp.12982. e12982. [DOI] [PubMed] [Google Scholar]
- 29.Tan WS, Feber A, Sarpong R, et al. Who should be investigated for haematuria? Results of a contemporary prospective observational study of 3556 patients. Eur Urol. 2018;74:10–14. doi: 10.1016/j.eururo.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Mishriki SF, Nabi G, Cohen NP. Diagnosis of urologic malignancies in patients with asymptomatic dipstick hematuria: prospective study with 13 years‘ follow-up. Urology. 2008;71:13–16. doi: 10.1016/j.urology.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 31.Pichler R, Heidegger I, Leonhartsberger N, et al. The need for repeated urological evaluation in low-risk patients with microscopic hematuria after negative diagnostic work-up. Anticancer Res. 2013;33:5525–5530. [PubMed] [Google Scholar]
- 32.Madeb R, Golijanin D, Knopf J, et al. Long-term outcome of patients with a negative work-up for asymptomatic microhematuria. Urology. 2010,;75:20–25. doi: 10.1016/j.urology.2009.06.107. [DOI] [PubMed] [Google Scholar]
- 33.Edwards TJ, Dickinson AJ, Gosling J, McInerney PD, Natale S, McGrath JS. Patient-specific risk of undetected malignant disease after investigation for haematuria, based on a 4-year follow-up. BJU Int. 2010;107:247–252. doi: 10.1111/j.1464-410X.2010.09521.x. [DOI] [PubMed] [Google Scholar]
- 34.Ark JT, Alvarez JR, Koyama T, et al. Variation in the diagnostic evaluation among persons with hematuria: influence of gender, race and risk factors for bladder cancer. J Urol. 2017;198:1033–1038. doi: 10.1016/j.juro.2017.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan WS, Sarpong R, Khetrapal P, et al. Can renal and bladder ultrasound replace CT urogram in patients investigated for microscopic hematuria? J Urol. 2018;200:973–980. doi: 10.1016/j.juro.2018.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu HT, Kim TH, Uhm JS, et al. Clinical significance of hematuria in atrial fibrillation with oral anticoagulation therapy. Circ J. 2017;81:158–164. doi: 10.1253/circj.CJ-16-0917. [DOI] [PubMed] [Google Scholar]
- 37.Yecies T, Bandari J, Fam M, Macleod L, Jacobs B, Davies B. Risk of radiation from computerized tomography urography in the evaluation of asymptomatic microscopic hematuria. J Urol. 2018;200:967–972. doi: 10.1016/j.juro.2018.05.118. [DOI] [PubMed] [Google Scholar]