Abstract
Background/Aim: Periodontitis is a chronic inflammatory disease linked to various systemic age-related conditions. It is known that α,β-unsaturated carbonyl compounds such as dietary cinnamates (β-phenyl acrylates) and related (meth)acrylates can have various positive and negative health effects, including cytotoxicity, allergic activity, pro-and anti-inflammatory activity, and anticancer activity. To clarify the anti-inflammatory properties of α,β-unsaturated carbonyl compounds without a phenolic group in the context of periodontal tissue inflammation and alveolar bone loss, we investigated the cytotoxicity and up-regulatory/down-regulatory effect of three trans-cinnamates (trans-cinnamic acid, methyl cinnamate, trans-cinnamaldehyde), two acrylates (ethyl acrylate, 2-hydroxyethyl acrylate), and three methacrylates (methyl methacrylate, 2-hydroxyethyl methacrylate, and triethyleneglycol dimethacrylate) using RAW264.7 cells. Materials and Methods: Cytotoxicity was determined using a cell counting kit (CCK-8) and mRNA expression was determined using real-time reverse transcriptase-polymerase chain reaction (RT-PCR). Pro-inflammatory and anti-inflammatory properties were assessed in terms of expression of mRNAs for cyclo-oxygenase-2 (Cox2), nitric oxide synthase 2 (Nos2), tumor necrosis factor-alpha (Tnfa) and heme oxygenase 1 (Ho1). Results: The most cytotoxic compound was 2-hydroxyethyl acrylate, followed by ethyl acrylate and cinnamaldehyde (50% lethal cytotoxic concentration, LC50=0.2-0.5 mM). Cox2 mRNA expression was up-regulated by cinnamaldehyde and 2-hydroxyethyl acrylate, particularly by the former. In contrast, the up-regulatory effect on Nos2 mRNA expression was in the order: cinnamaldehyde >> ethyl acrylate ≈ triethyleneglycol dimethacrylate >> methyl methacrylate ≈ methyl cinnamate. On the other hand, cinnamic acid and 2-hydroxyethyl methacrylate had no effect on gene expression. The two acrylates, but not cinnamates and methacrylates, up-regulated the expression of Ho1 mRNA at a non-cytotoxic concentration of 0.1 mM. Expression of Cox2, Nos2 and Tnfa mRNAs induced by Porphyromonas gingivalis lipopolysaccharide was greatly suppressed by cinnamaldehyde, methyl cinnamate and the two acrylates at 0.1 mM (p<0.05), and slightly, but significantly suppressed by cinnamic acid and methacrylates at 0.1-1 mM (p<0.05). Conclusion: Cinnamaldehyde and acrylates exhibited both anti-inflammatory and pro-inflammatory properties, possibly due to their marked ability to act as Michael reaction acceptors, as estimated from the beta-carbon 13C-nuclear magnetic resonance spectra. Methyl cinnamate exhibited potent anti-inflammatory activity with less cytotoxicity and pro-inflammatory activity, suggesting that this compound may be useful for treatment of periodontal disease and related systemic diseases
Keywords: Cinnamates, (meth)acrylates, cytotoxicity, pro-/antiinflammatory properties, RAW2647 cells, Cox2, Nos2 and Ho1 mRNA, 13C-NMR spectra, β-carbon
Cinnamates, acrylates and methacrylates, and α,β-unsaturated carbonyl compounds act as Michael reaction acceptors (Figure 1). Dietary cinnamates with a phenylpropanoid structure (C6-C3) such as cinnamaldehyde, methyl cinnamate and cinnamic acid, are β-phenyl acrylates. These compounds are known to have interesting multifunctional properties including cytotoxicity, anti-ultraviolet (UV) activity, antioxidant activity, pro-and anti-inflammatory activity, and anti-ulcerogenic, antipyretic, antimicrobial, antidiabetic and antitumor activity (1-7). Acrylates and methacrylates are also widely used clinically as polymer materials in the medical and dental fields. Especially in the context of dentistry, these compounds can produce low amounts of monomer residues when used in dentures, restorative resins and adhesives, thereby possibly having adverse effects such as cytotoxicity, skin sensitization, mutagenicity, carcinogenicity, respiratory allergy, and organ toxicity (8-11). The adverse effects of cinnamates and (meth)acrylates may be due to their ability to induce oxidative stress and their covalent interactions with cellular nucleophiles such as proteins, histidine, lysine, glutathione (GSH) and DNA bases (7,8,11,12). The Michael addition of electrophilic cinnamates and (meth)acrylates to endogenous cysteine thiol plays a role in pathologies associated with oxidative stress (13), in the anti-inflammatory activity of cyclopentane prostaglandins (14), and in the induction of enzymes that protect against carcinogenesis (15-17).
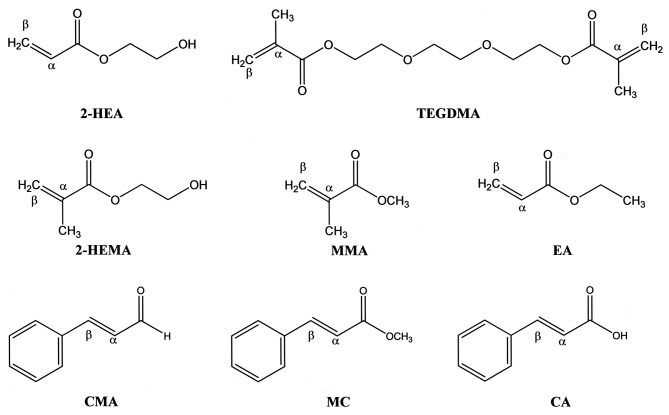
Figure 1. The chemical structures of 2-hydroxyethyl acrylate (2-HEA), triethyleneglycol dimethacrylate (TEGDMA), 2-hydroxyethyl methacrylate(2-HEMA), methyl methacrylate (MMA), ethyl acrylate (EA), trans-cinnamaldehyde (CMA), methyl cinnamate (MC) and trans-cinnamic acid (CA).α, Alpha carbon; β, beta carbon.
Cinnamates, acrylates and methacrylates, which are thiol-reactive electrophiles, induce enzymes that are involved in their metabolism, particularly phase II detoxication enzymes such as glutathione-S-transferase, uridine diphosphate glucuronosyl transferase and nicotinamide adenine dinucleotide phosphate [NAD(P)H:quinone oxide reductase (NQOR1)] (15,18-21). The NQOR1-inductive effects of α,β-unsaturated carbonyl compounds may be related to their anti-inflammatory activity in most cells and tissues.
In general, inflammatory activity is accompanied by overexpression of nitric oxide synthase 2 (NOS2), leading to production of nitric oxide, which enhances the catalytic activity of cyclooxygenase-2 (COX2) via formation of the peroxinitrite anion (22). COX2 is a downstream target of NOS2. In addition, heme oxygenase-1 (HO1), the inducible isoform of HO, catalyzes the degradation of heme into biliverdin, iron, and carbon monoxide, and inhibits immune responses and inflammation in vivo. Biliverdin and bilirubin are potent antioxidants that attenuate oxidative stress (23), and HO1 has anti-inflammatory, antioxidant, and antiproliferative effects (22,24,25).
Chronic periodontal inflammation is a risk factor for systemic problems such as cardiovascular disease, diabetes mellitus and osteoporosis because of the transport of contributory factors via the blood circulation (26). The rod-shaped, gram-negative anaerobic bacterium Porphyromonas gingivalis is considered to be the major causative agent of periodontitis. P. gingivalis lipopolysaccharide (LPS), a component of the cell wall, acts as a powerful activator of macrophages through the production of pro-inflammatory cytokines (27). Therefore, inhibition of COX2, NOS2 and tumor necrosis factor-alpha (TNFa) protein or gene expression in P. gingivalis LPS-stimulated gingival fibroblasts and RAW264.7 cells may be one way to screen anti-inflammatory antioxidant agents for their effects on age-related chronic diseases such as periodontitis with systemic problems. In this context, HO1 expression may be useful as a target for anti-inflammatory antioxidant drugs and preventive agents against such age-related chronic diseases such as periodontitis (28-31). We previously reported that the inhibitory effects of phenylpropanoids, including eugenol, bis-eugenol (the ortho-dimer of eugenol), magnolol, honokiol and curcumin on Escherichia coli LPS-induced RAW264.7 cells in terms of the release of pro-inflammatory mediators and cytokines, suggested that these compounds had potent anti-inflammatory activities (30,31).
In the present study, firstly we investigated the cytotoxicity of α,β-unsaturated carbonyl compounds, namely three cinnamates (cinnamaldehyde, methyl cinnamate, and cinnamic acid), two acrylates (2-hydroxyethyl acrylate, ethyl acrylate) and three methacrylates (methyl methacrylate, 2-hydroxyethyl methacrylate, triethyleneglycol dimethacrylate) towards RAW264.7 cells using a Cell Counting Kit-8 (CCK-8). RAW 264.7 cells were used to elucidate implications of these compounds in periodontal tissue inflammation and alveolar bone loss. Secondly, we then investigated the stimulatory effects of these compounds on expression of Nos2, Cox2 and Ho1 mRNA expression in this cell line. Subsequently, we investigated whether they these compounds inhibited the expression of mRNAs for Nos2, Cox2 and Tnfa in P. gingivalis LPS-stimulated RAW264.7 cells. On the basis of our results, we considered whether the cytotoxicity of cinnamates and (meth)acrylates is dependent on the pi-electron density of the α,β-carbon in these compounds. The higher the pi-electron density of the β-carbon, the higher the magnetic field at which the nuclear magnetic resonance (NMR) peak is observed, leading to a reduction of the NMR chemical shift. On this premise, we examined the relationship between the cytotoxicity, pro-inflammatory properties or anti-inflammatory properties and the NMR chemical shift of the β-carbons, and the site of electrophilic activity of cinnamates and (meth)acrylates.
Materials and Methods
Materials. Acrylates (ethyl acrylate, 2-hydroxyethyl acrylate), methacrylates [methyl methacrylate, 2-hydroxyethyl methacrylate, triethyleneglycol demethacrylate (TEGDMA)] and N-acetyl-L-cysteine (NAC) were purchased from Tokyo Kasei Co. (Tokyo, Japan). Cinnamates (trans-cinnamic acid, methyl cinnamate, trans-cinnamaldehyde) were also purchased from Tokyo Kasei Co. The chemical structures of these compounds are shown in Figure 1. Solutions of these compounds were prepared by dissolving each of them in dimethyl sulfoxide, followed by dilution to the required concentrations using serum-free RPMI-1640 (Invitrogen Co., Carlsbad, CA, USA) as test samples. Fetal bovine serum (FBS) was obtained from HyClone (Logan, UT, USA). P. gingivalis ATCC33277 LPS was obtained from Wako Pure Chemical Industries (Osaka, Japan).
Cell culture. The murine macrophage-like cell line RAW264.7, obtained from Dainippon Sumitomo Pharma Biomedical Co. Ltd. (Osaka, Japan), was used. The cells were cultured to a subconfluent state in RPMI-1640 medium supplemented with 10% FBS at 37˚C and 5% CO2 in air, washed, and then incubated overnight in serum-free RPMI-1640. They were then washed again and treated with the test samples for cytotoxicity and real-time polymerase chain reaction.
Cytotoxicity. In brief, RAW264.7 cells (3×104 per well) were cultured in NUNC 96-well plates (flat-well-type microculture plates) (100 μl) or 48 h, after which the cells were incubated with acrylates, methacrylates or cinnamates at a concentration of 0.001-100 mM for 24 h. The relative number of viable cells was then determined using a Cell Counting Kit-8 (CCK-8) (Dojindo Co., Kumamoto, Japan) (32). Ten microliters of CCK-8 solution was added to each well of the plate, which was then was incubated for1 hour and then the absorbance was measured at 450 nm with a microplate reader (Biochromatic, Helsinki, Finland). LC50 values were determined from the dose–response curves. Data are expressed as means of three independent experiments. Statistical analyses were performed using Student’s t-test and one-way ANOVA.
Effects of antioxidant NAC on ethyl acrylate or cinnamaldehyde effects. The cells were cultured for 48 hours, and then incubated with 1 mM ethyl acrylate or cinnamaldehyde, with or without NAC at 1:1 molar ratio for 24 h. CCK-8 solution was added to each well and then the absorbance was measured at 450 nm with a microplate reader in a similar manner as described above.
Preparation of total RNA and real-time polymerase chain reaction (PCR). The preparation of total RNA and the procedure for real-time PCR have been described previously (33). In brief, RAW264.7 cells in NUNC 96-flat-well-type microculture plates (105 cells per well) were pretreated for 30 min with or without acrylates, methacrylates and cinnamates at a concentration of 10-10,000 μM, and then incubated for 3.5 h with or without P. gingivalis LPS at 100 ng/ml. Total RNA was then isolated using an RNeasy Plus Micro Kit (Qiagen Japan Co. Ltd., Tokyo, Japan) in accordance with the instruction manual. cDNA was synthesized from 2 μg total RNA of each sample by random priming using a High Capacity RNA-to-cDNA Kit (Life Technologies Japan, Tokyo, Japan). Reaction mixtures without the reverse transcriptase were used as a negative control. An aliquot of each cDNA synthesis reaction mixture was diluted and used for real-time PCR quantification. An equal-volume aliquot of each cDNA was mixed, serially diluted, and used as a standard. TaqMan probes/primers for Cox2, Nos2, Ho1 and 18s rRNA and the PCR enzyme mix for real-time PCR were purchased from Life Technologies Japan. Real-time PCR quantification was performed in triplicate using the GeneAmp Sequence Detection System 5700 software (Life Technologies Japan) in accordance with the instruction manual. The relative amount of target was calculated from standard curves generated in each PCR, and quantitative data with a coefficient of variance of less than 10% were used for further analyses. Each calculated amount of mRNA was standardized by reference to that for 18s rRNA. Data are expressed as means of three independent experiments. Statistical analyses were performed using Student’s t-test and one-way ANOVA.
Results and Discussion
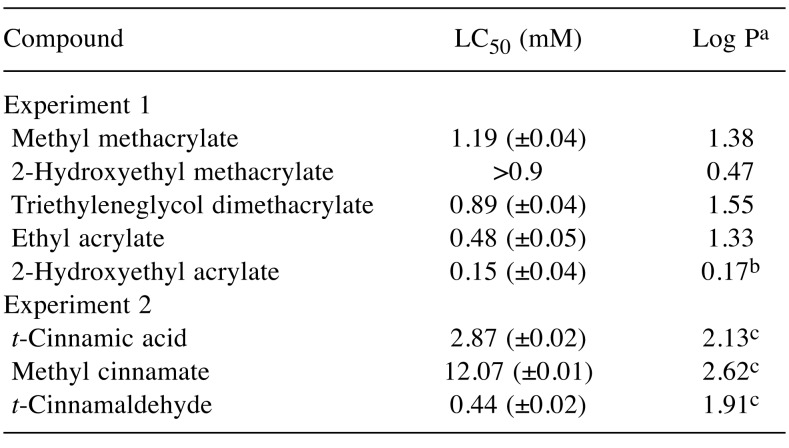
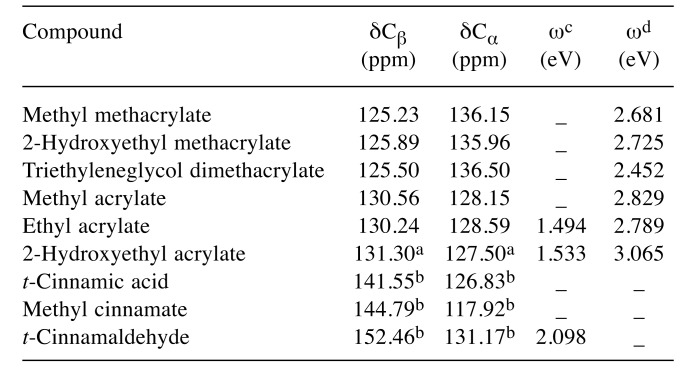
Cytotoxicity and 13C-NMR chemical shifts. LC50 values and 13C-NMR chemical shifts of the α,β-carbon (δCα, δCβ) for (meth)acrylates and cinnamates are shown in Table I and Table II, respectively. The rank order of cytotoxicity potency for (meth)acrylates was 2-hydroxyethyl acrylate > ethyl acrylate > TEGDMA > 2-hydroxyethyl methacrylates > methyl methacrylate; that for cinnamates was cinnamaldehyde> cinnamic acid > methyl cinnamate.
Table I. 50% Lethal cytotoxic concentration (LC50) and hydrophobicity(log P) for acrylates, methacrylates and cinnamates used in this study.
aTaken from Fujisawa and Kadoma (38); bTaken from Chan andO’Brien (10); chhtps://pubchem.ncbi.nim.nih.gov/t-cinnamaldehyde,methyl cinnamate, t-cinnamic acid.
Table II. 13C-Nuclear magnetic spectroscopy (NMR) chemical shifts (δ)of β-carbon (Cβ), α-carbon (Cα) and electrophilicity (ω) for acrylates,methacrylates and cinnamates used in this study.
13C-NMR chemical shifts of alpha and beta carbons for (meth)acrylateswere taken from Ishihara and Fujisawa (50). The chemical shift wasdetermined in CDCl3 and was converted to the tetramethylsilane scale.aAcrylic monomers, The Dow Chemical Company msds search dowcom/Published literature DOW/COM/. bhttps://www.chemicalbook.com/SpectrumEN_140-10-3, 103-26-4 and 14371-10-9_13C-NMR.htm.cTaken from Enoch et al. (53). dTaken from Ishihara and Fujisawa (52).
The cytotoxicity of 2-hydroxyethyl acrylate and ethyl acrylate was markedly greater than that of methacrylates. The LC50 value for 2-hydroxyethyl acrylate against RAW264.7 cells was similar to that against hepatocytes (10). Ethyl acrylate and methyl methacrylate had similar hydrophobicity (log P) but the cytotoxicity of the former was greater than that of the latter. As shown in Table II, the δCβ value for ethyl acrylate was greater than that for methyl methacrylate, indicating that the electrophilicity of the former is greater than that of the latter. 2-Hydroxyethyl methacrylate was found to be far less cytotoxic than 2-hydroxyethyl acrylate, possibly due to the high electrophilic reactivity of the latter, resulting from the great difference of the δCβ value between these compounds. Next, we investigated the relationship between the LC50 and the δCβ value for (meth)acrylates. A good linear relationship between the two parameters was observed, as shown below:
LC50=18.43 (±0.16) − 0.14 (±0.03) δCβ
(n=4, r2=0.92, p<0.05) (Eq. 1)
2-Hydroxyethyl methacrylate was omitted from Eq. 1 because its cytotoxicity altered the reaction time.
The cytotoxicity was closely positively correlated with the relative pi-electron density (δCβ) of the beta-carbon, i.e. the site of electrophilic activity. In addition, a linear relationship was observed between the LC50 and δCα values for these compounds (r2=0.87) (Table II), as δCα decreased, the cytotoxicity increased.
In a similar context, it was shown previously that when acrylates and methacrylates are separated, the LC50 of an acrylate series against hepatocytes was linearly correlated with the partial charges of the carbon atoms that make up the α,β-carbonyl structure; the partial charge of the carbon atoms was determined using semiempirical calculations. The hepatotoxicity of five acrylates in that study was linearly correlated with the increasing partial charge at the β-carbon (p<0.05) and more significantly with the negative partial charge on the α-carbon (p<0.01) (10). However, the hepatotoxicity of acrylates was shown not to correlate with their lipophilicity (log P) (10). Organic chemistry has shown that nucleophilic addition to the electrophilic (electron-deficient) double bond generates a significant negative charge on the α-carbon which is delocalized into the adjacent carbonyl moiety by pi-resonance (34). This suggests that the biological activity of acrylates without substituents at the α-carbon may be more influenced by the α-carbon than that by a β-carbon. Next, we investigated the relationship between the LC50 and δCβ or δCα values for cinnamates, and a good positive linear relationship was demonstrated between the LC50 and δCα (r2=0.91). The cytotoxicity of cinnamates may be attributed mainly to the pi-electron density at the alpha carbon. This was thought to be reasonable in view of the similar molecular structures of acrylates and cinnamates (β-phenyl acrylates) but the LC50 for acrylates was found to be negatively correlated with their δCα value (10). The cytotoxicity of cinnamates and (meth)acrylates against RAW264.7 cells was also not affected by their hydrophobicity (log P).
In contrast, the cytotoxicity of methacrylates can be affected by a combination of electronic and steric factors introduced by methyl substitution on α-carbons. We previously investigated the possible link between this cytotoxicity and Ca2+ mobilization in a human salivary gland carcinoma cell line and human gingival fibroblasts by (meth)acrylates. This revealed that hydrophilic 2-hydroxyethyl methacrylate, as well as acrylic acid and methacrylic acid, have low cytotoxicity and elicit only a small elevation of intracellular calcium concentration [Ca2+]i, whereas hydrophobic (meth)acrylates are cytotoxic and elicit a large [Ca2+]i elevation (35). It is well established that variations in cytosolic calcium concentration, [Ca2+]c, trigger key cellular functions, and that cellular Ca2+ overload is highly toxic, being related to the induction of apoptosis (36). The cytotoxicity of (meth)acrylates against human salivary gland carcinoma cell line and human gingival fibroblasts is related to their log P (35), the cytotoxic mechanisms being dependent on the cell species and inducers involved. We also previously investigated the relationship between the in vivo toxicity of (meth)acrylates in mice (oral and intraperitoneal 50% lethal dose) and GSH reactivities predicted by their δCβ values, demonstrating a good relationship between these parameters in series of both acrylates and methacrylates (37,38). Despite a possible discrepancy between the in vivo and in vitro toxicities, the cytotoxicity of (meth)acrylates in RAW264.7 cells is affected by the electrophilicity of the monomers.
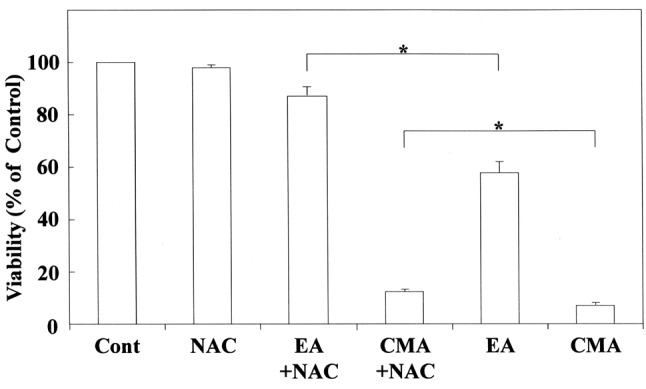
Next, to clarify the cytotoxic effects of GSH reactivity, the cytotoxicity of ethyl acrylate and cinnamaldehyde with or without the antioxidant NAC was investigated and the effect of NAC was evaluated. The results are shown in Figure 2. The cytotoxicity of both compounds was greatly reduced by addition of NAC. Cinnamaldehyde and ethyl acrylate probably interact spontaneously and more rapidly in the presence of NAC. This interaction may involve Michael-type addition between the nucleophilic NAC and electrophilic cinnamaldehyde or ethyl acrylate, and NAC may block the induction of monomer-mediated DNA and apoptosis. The mechanism of cinnamaldehyde cytotoxicity was investigated previously in isolated F344 rat hepatocytes, and the results suggested that cinnamaldehyde, but not cinnamic acid, reacted spontaneously with reduced GSH in vitro (39). Taken together with our results, the evidence suggests that the toxicity of cinnamaldehyde and ethyl acrylate is attenuated through interaction between the β-carbons of these compounds and NAC in vitro. However, at the cellular level, cinnamaldehyde and ethyl acrylate may induce a variety of stress responses in RAW264.7 cells, including GSH depression, oxidative stress, and mitochondrial dysfunction, as well as release of inflammatory mediators and pro-inflammatory cytokines, resulting in cell death.
Figure 2. Inhibition of ethyl acrylate (EA)-and trans-cinnamaldehyde(CMA)-induced cytotoxicity by N-acetyl-L-cysteine (NAC) in RAW264.7cells. The experimental procedure is described in the Materials andMethods. The results are presented as the means±standard error (SE)of three independent experiments, SE<15%. *Significantly different atp<0.01. Cont: Control without any additive.
Cox2 mRNA expression in RAW264.7 cells stimulated with cinnamates and (meth)acrylates. It has been well documented that the inflammatory response to E. coli and P. gingivalis LPS or P. gingivalis fimbriae involves the production of COX2, NOS2 and proinflammatory cytokines such as TNFα, interleukin (IL)-6, and IL1β in RAW264.7 cells (28,30,40). Contrary to the pro-inflammatory effects of LPS, the inflammatory mediators COX2 and NOS2 and cytokines such as TNFα at both the protein and gene levels may also be expressed in macrophages stimulated by α,β-unsaturated carbonyl compounds.
Therefore, we investigated the expression of Cox2 mRNA in RAW264.7 cells stimulated by cinnamates, acrylates and methacrylates. The results are shown in Figure 3 and Figure 4, respectively. Cinnamaldehyde stimulated Cox2 mRNA expression at 0.1 mM, whereas 2-hydroxyethyl acrylate stimulated its gene expression at 1 mM. Other compounds such as methyl cinnamates, ethyl acrylate and methacrylates had no effect on Cox2 expression. The concentration of cinnamaldehyde required for induction of Cox2 mRNA expression was approximately 10-fold higher than that of 2-hydroxyacrylate. In this study, despite the relatively high δCβ value for cinnamates, cinnamaldehyde was the most cytotoxic compound and potently elicited Cox2 gene expression, possibly reflecting the fact that aldehydes tend to have higher inductive potency. Among (meth)acrylates, 2-hydroxyethyl acrylate was the most cytotoxic and had the highest δCβ value, possibly due to the induction of Cox2 gene expression. Macrophages and other activated inflammatory cells secrete high amounts of prostaglandin E2 (PGE2), nitric oxide (NO), and cytokines such as IL6, TNFα, and IL1β. COX2 is a key mediator of the inflammatory process, being responsible for production of PGE2 from arachidonic acid. Down-regulation of Cox2 is a condition for inhibition of PGE2, which is expressed in all processes that lead to major features of inflammation, i.e. swelling, redness, and pain (41,42). On the other hand, it is suggested that up-regulation of Cox2 might lead to an increase of pro-inflammatory activity. It was shown that low concentrations (up to 1 μg/ml) of cinnamaldehyde induce a slight increase in nuclear factor-ĸB (NF-ĸB) activation (4), suggesting a degree of intrinsic pro-inflammatory activity. It has also occasionally been reported that cinnamaldehyde can induce intraoral allergic contact dermatitis; the most commonly implicated allergens are metals that are incorporated into dental appliances, but cinnamaldehyde is widely used as a flavoring agent in foods and dentifrices (43). Our results suggest that cinnamaldehyde (β-phenyl acrolein) and 2-hydroxyethyl acrylate may exert cytotoxic/genotoxic cell damage in mammalian cells due to their high reactivity with cellular nucleophiles (e.g. Michael adduct formation with DNA bases and with GSH) (44-46).
Figure 3. Stimulating effect of 2-hydroxyethyl methacrylate (2-HEMA),2-hydroxyethyl acrylate (2-HEA), ethyl acrylate (EA), triethylenglycoldimethacrylate (TEGDMA) and methyl methacrylate (MMA) on cyclooxygenase-2 (Cox2) mRNA expression in RAW264.7 cells. The cellswere incubated for 3.5 h with each compound at a concentration of10-1,000 μM, and then their total RNAs were prepared. Each cDNA wassynthesized, and the expression level of Cox2 mRNA was determined byreal-time polymerase chain reaction and standardized against theexpression of 18s rRNA. The results are presented as means±standarderror (SE) of three independent experiments, SE<15%. *Significantlydifferent at p<0.01 vs. control group.
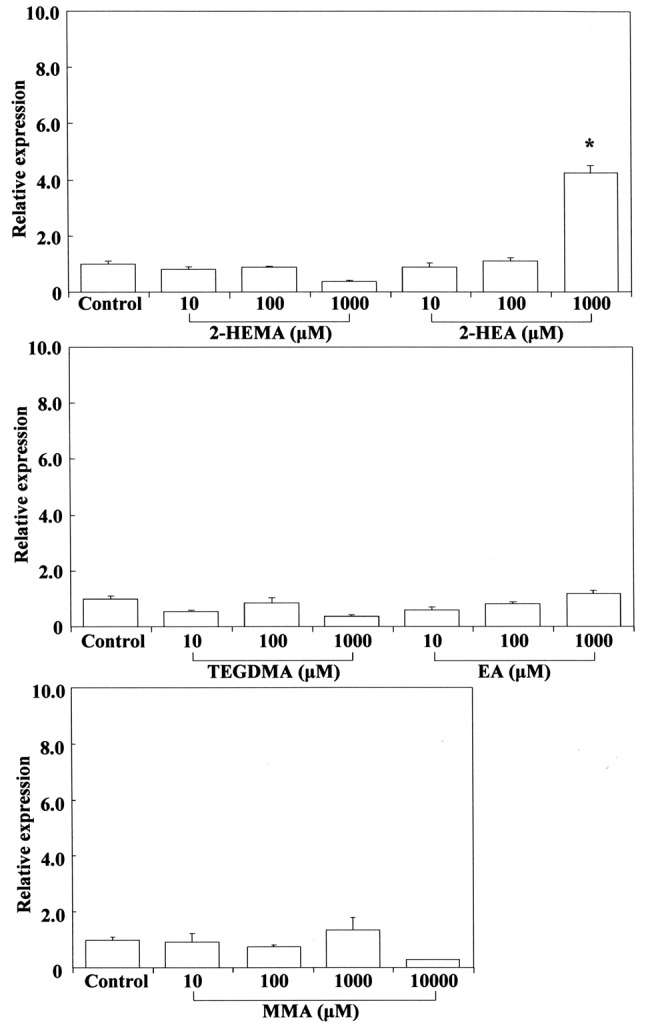
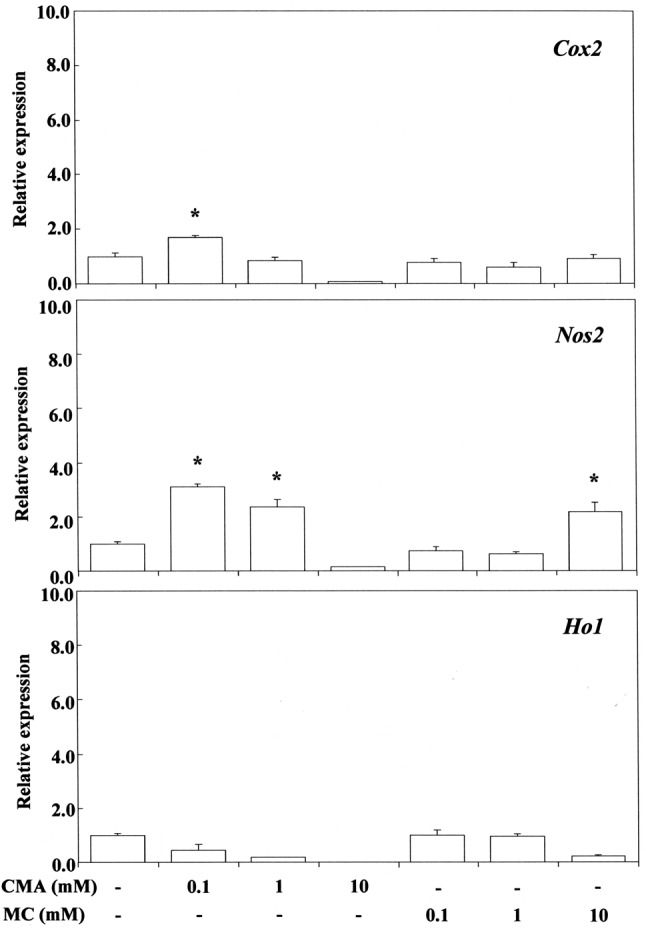
Figure 4. Stimulating effect of trans-cinnamaldehyde (CMA) and methylcinnamate (MC) on cyclo-oxygenase-2 (Cox2), nitric oxide synthase 2(Nos2) and heme oxygenase 1 (Ho1) gene expression in RAW264.7 cells.The cells were incubated for 3.5 h with each compound at aconcentration of 0.1-10 mM, and then their total RNAs were prepared.Each cDNA was synthesized, and the expression level of each mRNA wasdetermined by real-time polymerase chain reaction and standardizedagainst the expression of 18s rRNA. The results are presented asmeans±standard error (SE) of three independent experiments, SE<15%.*Significantly different at p<0.01 vs. control group .
In other contexts, cinnamaldehyde has been reported to exert marked antimutagenic effects against mutations induced by UV-mimic mutagens, but not those induced by N-methyl-N’-nitro-N-nitrosoguanidine or ethyl methanesulfonate, suggesting that cinnamaldehyde may interfere with the inducible error-prone DNA-repair pathway (47). In contrast, 2-hydroxyethyl acrylate, ethyl acrylate and TEGDMA have been listed as mutagenic (45).
Stimulation of Nos2 mRNA expression by cinnamates and (meth)acrylates. COX2 expression is selectively induced by pro-inflammatory cytokines at sites of inflammation, and NOS2 is also involved in the inflammatory process (48). NOS2 may be expressed in macrophages after induction of oxidative stress due to cinnamates and (meth)acrylates. Cinnamaldehyde elicited Nos2 mRNA expression at 0.1 mM, whereas methyl cinnamate did so at 10 mM, indicating that the inductive ability of the former is approximately two orders of magnitude greater than that of the latter. This may be due to the large electrophilicity of cinnamaldehyde, which has a reactive aldehyde moiety. 2-Hydroxyethyl acrylate, ethyl acrylate and TEGDMA elicited Nos2 mRNA expression at the cytotoxic concentration of 1 mM (Figure 5). Methyl methacrylate elicited Nos2 mRNA expression at a high cytotoxic concentration of 10 mM, whereas 2-hydroxyethyl methacrylate and cinnamic acid were ineffective over a wide concentration range of 1-10 mM. These findings suggest that the up-regulatory effect of monomers on Nos2 gene expression may not necessarily be controlled by their ω potency alone, and that the relative hydrophobic/hydrophilic balance of monomers is also important. Although methyl cinnamate and cinnamic acid have higher δCβ values than 2-hydroxyethyl or ethyl acrylate, they elicited weak Nos2 gene expression and were not effective. Up-regulation of Nos2 expression by TEGDMA, a dimethacrylate, may be attributable to the double Michael reaction acceptor. Production of PGE2 stimulated by TEGDMA, but not by 2-hydroxyethyl methacrylate, has been reported previously in RAW264.7 cells (49). TEGDMA and 2-hydroxyethyl methacrylate do not affect the expression of inducible nitric oxide synthase (Inos) mRNA (49).
Figure 5. Stimulating effects of 2-hydroxyethyl methacrylate (2-HEMA),2-hydroxyethyl acrylate (2-HEA), ethyl acrylate (EA), triethylenglycoldimethacrylate (TEGDMA) and methyl methacrylate (MMA) on nitricoxide synthase 2 (Nos2) mRNA expression in RAW264.7 cells. The cellswere incubated for 3.5 h with each acrylate-related compound at aconcentration of 10-1,000 μM then their total RNAs were prepared. EachcDNA was synthesized, and the expression level of each mRNA wasdetermined by real-time polymerase chain reaction and standardizedagainst the expression of 18s rRNA. The results are presented asmeans±standard error (SE) of three independent experiments, SE<15%.*Significantly different at p<0.01 vs. control group.
We previously investigated the cytotoxicity mechanism of (meth)acrylates (50), and eugenol-related compounds, using computational methods (30,51). The energy values of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energy are defined as ELUMO and EHOMO, respectively. Chemical hardness (η), electronegativity (χ), electrophilicity (ω) and softness (σ) are calculated using equations 2-5, respectively:
η=(ELUMO – EHOMO)/2 (Eq. 2)
χ =–(ELUMO + EHOMO)/2 (Eq. 3)
ω=χ2/2η (Eq. 4)
σ=1/η (Eq. 5)
The log LC50 for phenyl propanoids, which are eugenol-related compounds, was reported to be linearly and positively related to their ω value calculated using the B3LYP/6-31G* level (p<0.001) (30). In the present study, we also investigated the relationship between the ω and δCβ values for cinnamaldehyde, 2-hydroxy acrylates and ethyl acrylate using cited data (Table II), and found that the ω value of these compounds was markedly linearly negatively correlated with their δCβ value (n=3, r2=0.999, p<0.01). We also calculated the ω value of (meth)acrylates using Eq.4 based on our previous data (52) (Table II), and examination of the relationship between the ω and δCβ values for these compounds revealed a moderate positive linear correlation (n=6, r2=0.662, p<0.05). These findings suggested that there is a linear relationship between the ω and δCβ value for cinnamaldehyde and (meth)acrylates. It may therefore be possible to estimate the electrophilicity (ω) of α,β-unsaturated carbonyl compounds from their δCβ values. The ω value is a higher-order parameter that combines softness with electronegativity (χ) and represents a sensitive measure of electrophilic reactivity. The ω value has been used as a parameter of allergic dermatitis and allergic sensitization associated with α, β-unsaturated carbonyl compounds (53), allergic potential increasing with the ω value. Induction of inflammatory mediators and cytokines by α, β-unsaturated carbonyl compounds may be related to other parameters in addition to the ω value. The η value (HOMO-LUMO energy gap) for (meth)acrylates was also calculated from our previous data using Eq. 2 (38,50); the rank order magnitude of η (eV) is ethyl acrylate (5.495) > methyl acrylate (5.492) > methyl methacrylate (5.245)> 2-hydroxyethyl methacrylate (5.233) > 2-hydroxyethyl acrylate (5.174) > TEGDMA (5.092). A lower η value for bioactive compounds indicates that the molecules are more easily excitable. Therefore, their σ values (softness) were calculated using Eq. 5.
The σ value (eV) for methyl methacrylates, 2-hydroxyethyl methacrylate, 2-hydroxyethyl acrylate and TEGDMA was 0.1907, 0.1911, 0.1932 and 0.1936, respectively, being grouped as having the most softness among the (meth)acrylates we tested. The σ value indicates the ease with which electron redistribution takes place during covalent bonding, i.e. donation of electrons by nucleophiles and acceptance by electrophiles. Therefore, with respect to electrophilic species, it is often the case that softness (i.e. a higher σ value) is correlated with the ease of adduct formation (54), that is, the σ energy serves as a measure of molecular excitability. Up-regulation of Nos2 mRNA expression by TEGDMA and 2-hydroxyethyl acrylate may be related to their high softness (55). Although no stimulation of Nos2 mRNA expression by 2-hydroxyethyl methacrylate was observed in the present study, it may be induced under certain conditions in view of its relatively high softness.
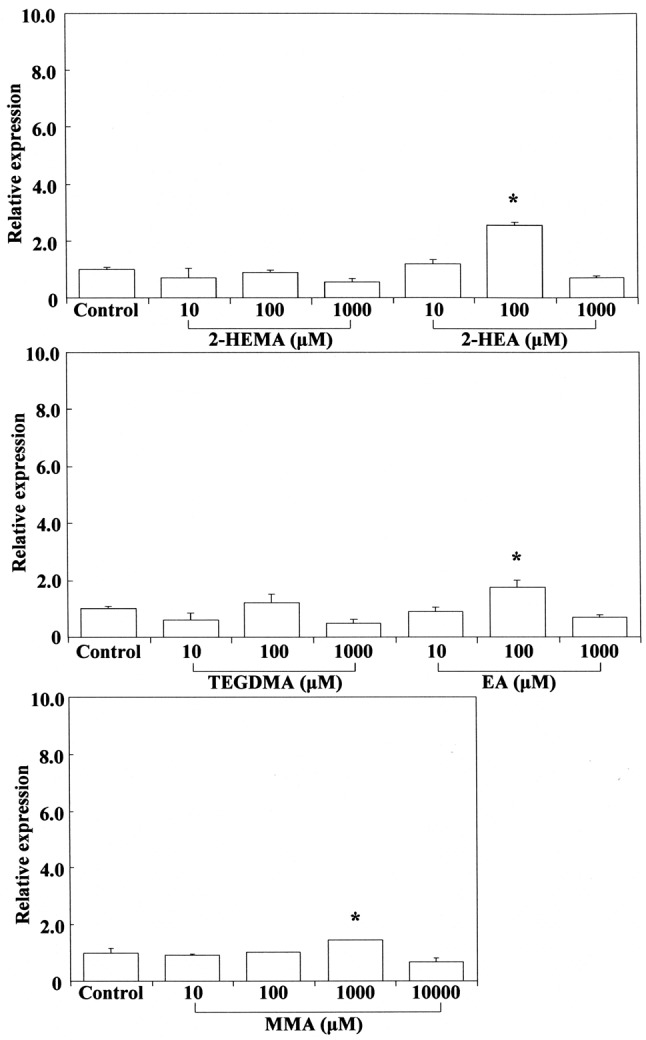
Stimulation of Ho1 mRNA expression by cinnamates and (meth)acrylates. The results are shown in Figure 4 and Figure 6. HO1 expression is enhanced not only by free heme, but also by various pro-inflammatory agents such as NO, LPS, cytokines, heavy metals, and other oxidants (56-59). Cinnamaldehyde and methyl cinnamate did not elicit any Ho1 mRNA expression over a wide concentration range of 0.1-10 mM. In contrast, 2-hydroxyethyl acrylate and ethyl acrylate at 0.1 mM potently elicited expression of Ho1 mRNA whereas methyl methacrylate did so poorly at the high concentration of 1 mM. Cinnamates with phenyl substituents at the β-carbons (β-phenyl acrylates) and methacrylates with methyl substituents at the α-carbons did not elicit Ho1 gene expression at non-cytotoxic concentrations. In contrast, acrylates without any substituents at both the α-and β-carbons elicited potent Ho1 gene expression. Michael addition of methane thiol (CH3SH), a model nucleophile of GSH, was performed previously at the B3LYP/6-31G* level for 47 Michael reaction acceptors, including α,β-unsaturated aldehydes, ketones and esters, focusing on the 1,2-olefin addition pathway without and with initial protonation. Michael reaction acceptors such as acrylates may be formed preferentially by direct 1,2 addition across the electron-poor double bond Cβ=Cα of Michael reaction acceptors (60). Induction of Ho1 gene expression by acrylates may be attributable to the high electrophilicity of their Cβ=Cα bond, which lacks substituents at the α-and β-carbons. These findings suggest that induction of Ho1 gene expression may be attributable to respective differences in the electrophilicity of β-arbon (δCβ) and steric interactions, as cinnamates with β-phenyl substituents and methacrylates with α-methyl substituents at the Cβ=Cα bonds did not elicit Ho1 mRNA expression in the present study. Interestingly, methyl methacrylate weakly elicited Ho1 mRNA expression. Therefore, we also calculated the σ values for some (meth)acrylates using the B3LYP/6-31G* level (30), which yielded a σ value (eV) rank order of methyl methacrylates (0.329) > TEGDMA (0.327) > ethyl acrylate (0.323) > 2-hydroxyethyl methacrylates (0.322). Methyl methacrylate had a relatively high σ value. This compound may be an excitable molecule, although its ω value is relatively small (Table II).
Figure 6. Stimulating effects of 2-hydroxyethyl methacrylate (2-HEMA),2-hydroxyethyl acrylate (2-HEA), ethyl acrylate (EA), triethylenglycoldimethacrylate (TEGDMA) and methyl methacrylate (MMA) on hemeoxygenase 1 (Ho1) mRNA expression in RAW264.7 cells. The cells wereincubated for 3.5 h with each acrylate-related compound at aconcentration of 10-1,000 μM, and then their total RNAs were prepared.Each cDNA was synthesized, and the expression level of Ho1 mRNA wasdetermined by real-time polymerase chain reaction and standardizedagainst the expression of 18s rRNA. The results are presented asmeans±standard error (SE) of three independent experiments, SE<15%.*Significantly different at p<0.01 vs. control group.
Talalay et al. (15) reported that methyl acrylate is a potent inducer of NQOR1 in Hepa 1c1c7 cells, whereas cinnamaldehyde and methyl cinnamates are weakly effective and methyl methacrylate and cinnamic acid are inactive. Induction of NQOR1 expression in the human hepatoma cell line HepG2 was measured at both enzyme activity and RNA level after exposure to methyl acrylates and ethyl acrylate (61), the results suggesting that both compounds are potent inducers of HepG2 gene expression. The inductive potency is reliable, and may be related to anti-inflammatory activity via induction of HO1 expression. Expression of the phase II detoxication gene NQOR1 is related to expression the HO1 gene and protein.
A PubMed search of recent articles on nuclear factor-erythroid related factor-2 (NRF2), HO and Ketch-like ECH-associated protein (KEAP1), an oxidative stress sensor, suggested that under normal physiological conditions, NRF2 in the cytoplasm is bound to its repressor, KEAP1. Upon activation, NRF2 is translocated to the nucleus and binds to the antioxidant response element located in the promoter region of some anti-oxidant genes, including that for the cytoprotective protein HO. Since the HO1 gene harbors a binding site for NRF2, mutual stimulatory and regulatory interactions between NRF2 and HO1 have been reported, and in fact the interaction between NRF2 and HO1 has been implicated in the regulation of many physiological antioxidants, including superoxide dismutase, catalase, glutathione S-transferase, peroxidase, NQOR1, and thioredoxin (62,63).
In other contexts, research on HO1 induction by cinnamaldehyde has revealed that the latter up-regulated the cellular protein level of HO1 and promoted translocation of NRF2 to the nucleus in human dental pulp cells. Cinnamaldehyde-mediated NRF2/HO1 activation reduced the level of reactive oxygen species (ROS) and protected human dental pulp cells from H2O2-induced oxidative stress, which induced apoptosis (64). In contrast, it has been reported that overexpression of NFκB p65 mRNA induced by high levels of glucose was markedly and dose-dependently attenuated by cinnamaldehyde in an in vitro dorsal root ganglion neuron model of diabetic neuropathy, whereas the expression of NRF2 and HO1 was not up-regulated (65). NRF2/HO-1 activation and the signaling mechanisms involved may be dependent on the cell species, inducers employed and reaction time.
In the present study, 2-hydroxyethyl acrylate and ethyl acrylate potently elicited Ho1 mRNA expression at low non-cytotoxic concentrations, suggesting that these electrophilic compounds may be protective against inflammation, oxidative damage, and cell death. Carbon monoxide, another byproduct of heme degradation by HO, inhibits NO secretion and reduces inflammation. Up-regulation of Ho1 mRNA expression for α,β-unsaturated carbonyl compounds may be associated with induction of NQOR1 (15,16). HO1 exerts a strong antioxidant and antiapoptotic effect favoring cancer cell growth (58). However, these active acrylates show marked cytotoxicity and pro-inflammatory activity and are well known to be toxic, allergic and mutagenic. Further studies of these compounds will be necessary to clarify the effects of HO1 expression at the gene and protein level.
Inhibitory effects on P. gingivalis LPS-stimulated expression of Cox2, Nos2 and Tnfa mRNA. LPS is known to induce high levels of ROS, thus promoting cytotoxicity, apoptosis, and pro-inflammatory activity. A high level of ROS modulates a number of cell signaling pathways and regulates the expression of multiple genes such as those for COX2 and NOS2 in vitro and in vivo (66). In general, inflammatory activity is accompanied by overexpression of inducible nitric oxide synthase, INOS, leading to production of nitric oxide, which enhances the catalytic activity of COX2 via formation of the peroxiynitrite anion (22). COX2 is a downstream target of NOS2.
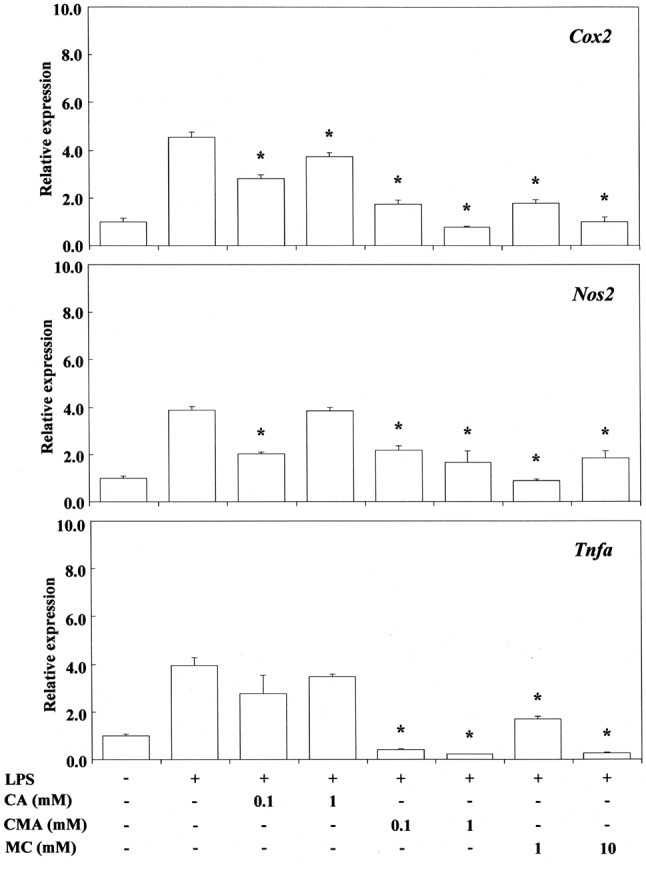
The inhibitory activity of cinnamates against the expression of P. gingivalis LPS-stimulated Cox2, Nos2 or Tnfa mRNA is shown in Figure 7. Cinnamaldehyde suppressed Cox2, Nos2 or Tnfa mRNA expression at a concentration of 0.1-1 mM, and methyl cinnamate did so at concentrations of 1-10 mM. In contrast, cinnamic acid had no effect. This may be due to the fact that free carboxyl groups weaken the efficiency of Michael reaction acceptors. Cinnamaldehyde, but not cinnamic acid, cinnamic alcohol and coumarin, inhibits the production of NO, TNFα and PGE2 by LPS-stimulated RAW264.7 cells (1). Cinnamaldehyde was also reported to inhibit the inflammatory activity of LPS-stimulated macrophages via suppression of mitogen-activated protein kinase (MAPK) phosphorylation and pro-inflammatory gene expression (67). Furthermore, it has been reported that when RAW264.7 macrophages were treated with cinnamaldehyde together with E. coli LPS, significant concentration-dependent inhibition of NO, TNFα, and PGE2 production was detected (1). It was also reported that cinnamaldehyde exerts a suppressive effect on toll-like-receptor-4 (TLR4)-mediated signaling and that this effect occurs through inhibition of receptor oligomerization (68). In contrast, cinnamic acid showed only low anti-inflammatory activity in a LPS-inducible inflammatory model in vitro (1), similarly to the findings of the present study.
Figure 7. Effects of trans-cinnamic acid (CA), trans-cinnamaldehyde(CMA) and methyl cinnamate (MC) on Porphyromonas gingivalislipopolysaccharide (LPS)-stimulated cyclo-oxygenase-2 (Cox2), nitricoxide synthase 2 (Nos2) and tumor necrosis factor-alpha (Tnfa) mRNAexpression in RAW264.7 cells. The cells were pretreated for 30 min withthe indicated doses of compounds, then they were incubated for 3 h withor without P. gingivalis LPS at 100 ng/ml, and their total RNAs wereprepared. Each cDNA was synthesized, and the expression level of eachmRNA was determined by real-time polymerase chain reaction andstandardized against the expression of 18s rRNA. The results are presentedas means±standard error (SE) of three independent experiments, SE<15%.*Significantly different at p<0.01 vs. LPS-treated group.
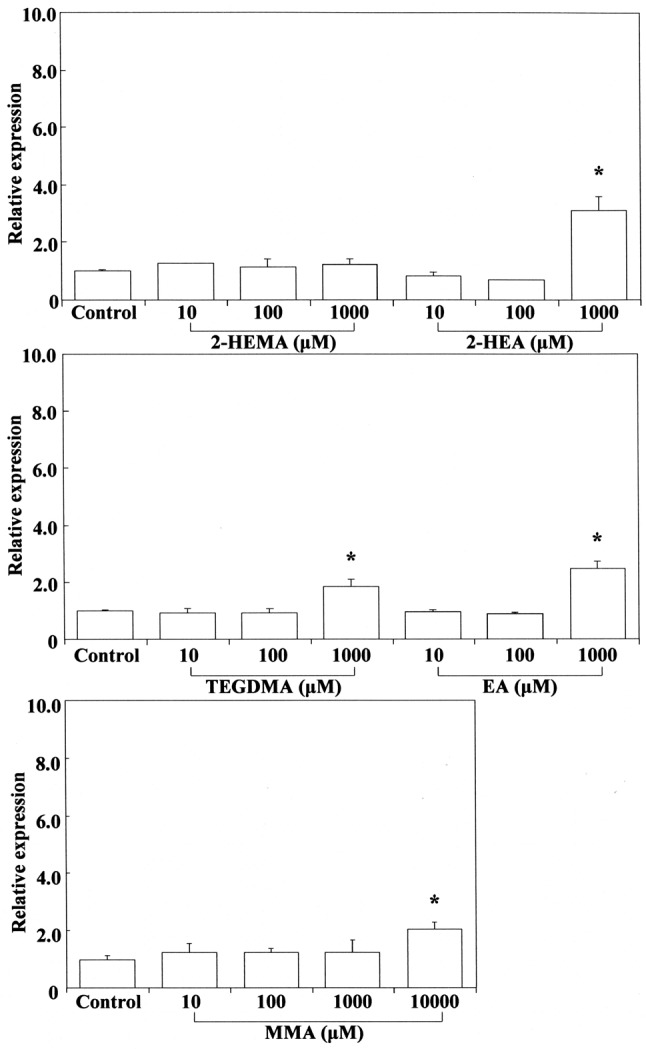
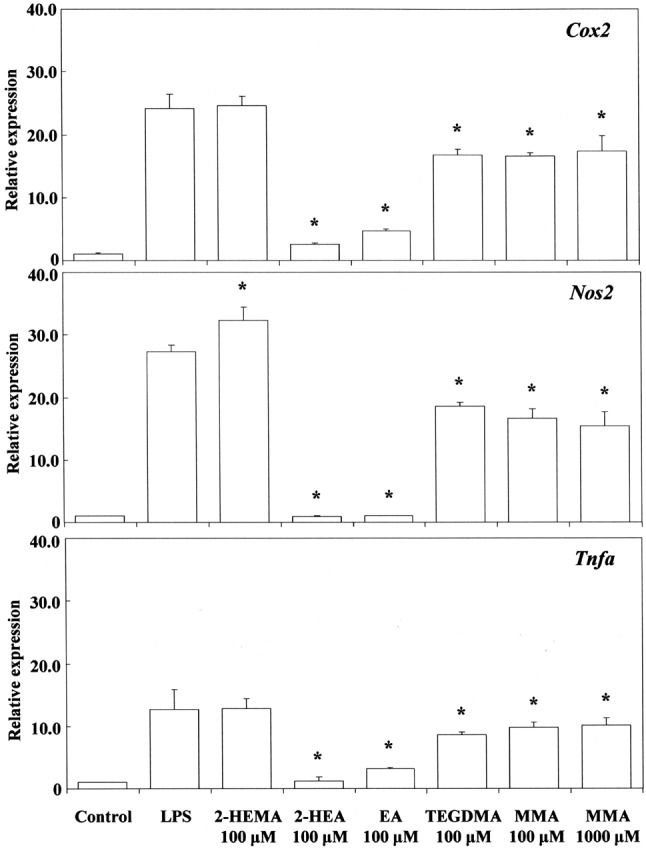
Next, we investigated the effects of acrylates and methacrylates on P. gingivalis LPS-stimulated expression of Cox2, Nos2 and Tnfa mRNA. The results are shown in Figure 8. Expression of Cox2, Nos2 and Tnfa mRNA was markedly and significantly suppressed by 0.1 mM 2-hydroxyethyl acrylates and ethyl acrylate, particularly the former, but only weakly by TEGDMA and methyl methacrylate, even at a highly cytotoxic concentration of 1 mM, the inhibitory effect being less than 50%. Cell-surface antigens and cytokines in macrophages have been reported to be up-regulated after exposure to LPS, whereas TEGDMA causes significant down-regulation dependent on exposure time. LPS and TEGDMA act differently on MAPK (69). In the present study, 2-hydroxyethyl methacrylate did not suppress the expression of Cox2 and Tnfa mRNA, but elicited overexpression of Nos2 mRNA. The reason is not known. Comparison of the anti-inflammatory activity of cinnamates with that of (meth)acrylates in terms of suppression of Cox2, Nos2 and Tnfa mRNA expression suggested that cinnamaldehyde and methyl cinnamate preferentially suppressed the expression of Tnfa in comparison with Nos2 or Cox2, whereas 2-hydroxyethy acrylate and ethyl acrylate appeared to preferentially suppress Nos2 gene expression in comparison with Cox2 or Tnfa (Figures 7 and 8). It is well known that LPS can directly activate macrophages, which trigger the production of inflammation mediators such as NO and TNFα at the protein level. Cinnamaldehyde suppresses LPS-induced production of NO and the expression of inflammatory protein products such as INOS, COX2 and TNFα. Pro-inflammatory cytokines such as TNFα are small secreted proteins which mediate and regulate immunity and inflammation. Production of TNFα is crucial for the synergistic induction of NO synthesis in LPS-stimulated macrophages in the presence of synapic acid (70). Cinnamates preferentially inhibit TNFα with LPS (71). When RAW264.7 cells are treated with cinnamaldehyde together with LPS for 24 h, the IC50 value required for inhibition of PGE2 production was about 37.7 μM, whereas that for TNFα production was about 30 μM (1). Cinnamaldehyde inhibited production of TNFα more strongly than that of PGE2 at the protein level. This marked ability of cinnamaldehyde to inhibit the production of TNFα at the protein level was similar to that of cinnamaldehyde at the gene level in the present study. Although cinnamaldehyde, 2-hydroxyethyl acrylate and ethyl acrylate had excellent anti-inflammatory properties, they also had potent pro-inflammatory properties. In contrast, methyl cinnamate had excellent anti-inflammatory properties together with slight cytotoxic and pro-inflammatory properties. Therefore, methyl cinnamate may have potential anti-inflammatory applications against periodontal disease and related systemic conditions.
Figure 8. Effects of 2-hydroxyethyl methacrylate (2-HEMA), 2-hydroxyethylacrylate (2-HEA), ethyl acrylate (EA), triethylenglycol dimethacrylate(TEGDMA) and methyl methacrylate (MMA) on Porphyromonas gingivalislipopolysaccharide (LPS)-stimulated cyclo-oxygenase-2 (Cox2), nitric oxidesynthase 2 (Nos2) and tumor necrosis factor-alpha (Tnfa) mRNA expressionin RAW264.7 cells. The cells were pretreated for 30 min with the indicateddoses of compounds, they were then incubated for 3 h with or without P.gingivalis LPS at 100 ng/ml, and their total RNAs were prepared. EachcDNA was synthesized, and the expression level of each mRNA wasdetermined by real-time polymerase chain reaction and standardizedagainst the expression of 18s rRNA. The results are presented asmeans±standard error (SE) of three independent experiments, SE<15%.*Significantly different at p<0.01 vs. LPS-treated group.
α,β-Unsaturated carbonyl compounds may exert dual pro-and anti-inflammatory properties. These compounds may not be ideal for drug design because of their tendency to undergo the Michael addition reaction leading to undesirable side-effects such as cytotoxicity, skin allergy, mutagenicity and carcinogenicity. Despite such side-effects, these agents may exert a range of beneficial effects similar to those of non-steroidal anti-inflammatory drugs, including anti-inflammatory and anticancer activity (72).
Cinnamaldehyde, a major chemical component of the cinnamon tree, has been shown to induce cellular ROS generation, leading to expression of the COX2 and NOS2 genes, and possibly apoptotic cell death. Cinnamaldehyde has an active Michael acceptor pharmacophore and is generally considered safe, with approval for use in the United States (73). It is noteworthy that some nucleophiles such as water, hydroxy anion (OH−), O2•− radical, peroxy radical (ROO•), nitric oxide (NO•) and GSH may be able to interact with the electrophilic β-carbon of cinnamates. LPS-treated cells generate a large amount of ROS/reactive nitrogen species (RNS) due to oxidative stress. It has been reported that cinnamaldehyde attenuates the release of ROS release from LPS-stimulated J774A.1 macrophages (71). Cinnamates without a phenolic O-H group may also be capable of scavenging more cellular ROS/RNS. Cinnamaldehyde scavenged 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals, NO and superoxide radicals (74). Cinnamic acid also scavenged DPPH radicals (data not shown). Cinnamaldehyde and cinnamic acid possess anti-oxidative and anti-peroxidative properties. However, induction of Ho1 gene expression by these electrophilic antioxidative compounds was not observed in the present study using RAW264.7 cells. Cinnamaldehyde also was a prooxidative compound because of its up-regulation of Cox2 and Nos2. This may be attributed to a great prooxidative activity due to the highly electrophilic aldehyde moiety present in this compound. Cinnamaldehyde might suppress LPS-stimulated Cox2, Nos2 and Tnfa gene expression through inactivation of the NF-ĸB pathway, but not through activation of NF2/HO1 in RAW264.7 cells. Further studies will be needed to clarify the signaling mechanisms involved in the anti-inflammatory and pro-inflammatory activities of α,β-unsaturated carbonyl compounds.
In conclusion, α,β-unsaturated carbonyl compounds such as cinnamates (CMA, MC) and acrylates (2-HEA, EA) but not methacrylates (2-HEMA, MMA, TEGDMA) potently suppressed P. gingivalis LPS-stimulated Cox2, Nos2 and Tnfa mRNA expression. Treatment with two acrylates up-regulated Ho1 mRNA expression. The Michael addition in biological systems is a likely molecular mechanism for the toxicity and pro-/anti-inflammatory property of such compounds. MC had little cytotoxicity and anti-inflammatory activity.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP15K11266 from the Ministry of Education, Science, and Culture of Japan.
References
- 1.Liao JC, Deng JS, Chiu CS, Hou WC, Huang SS, Shie PH, Huang GJ. Anti-inflammatory activities of Cinnamomum cassia constituents in vitro and in vivo. Evid Based Complement Alternat Med. 2012;2012:429320. doi: 10.1155/2012/429320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee HS, Kim BS, Kim MK. Suppression effect of Cinnamomum cassia bark-derived component on nitric oxide synthase. J Agric Food Chem. 2002;50:7700–7703. doi: 10.1021/jf020751f. [DOI] [PubMed] [Google Scholar]
- 3.Hancı D, Altun H, Çetinkaya EA, Muluk NB, Cengiz BP, Cingi C. Cinnamaldehyde is an effective anti-inflammatory agent for treatment of allergic rhinitis in a rat model. Int J Pediatr OtorhinolaryngoI. 2016;84:81–87. doi: 10.1016/j.ijporl.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Roth-Walter F, Moskovskich A, Gomez-Casado C, Diaz-Perales A, Oida K, Singer J, Kinaciyan T, Fuchs HC, Jensen-Jarolim E. Immune-suppressive effect of cinnamaldehyde due to inhibition of proliferation and induction of apoptosis in immune cells: implications in cancer. PLoS One. 2014;9:e108402. doi: 10.1371/journal.pone.0108402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Cássia da Silveira E Sá R, Andrade LN, Dos Reis Barreto de Oliveira R, de Sousa DP. A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules. 2014;19:1459–1480. doi: 10.3390/molecules19021459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunawardena D, Karunaweera N, Lee S, van Der Kooy F, Harman DG, Raju R, Bennett L, Gyengesi E, Sucher NJ, Münch G. Anti-inflammatory activity of cinnamon (C. zeylanicum and C. cassia) extracts – identification of E-cinnamaldehyde and o-methoxy cinnamaldehyde as the most potent bioactive compounds. Food Funct. 2015;6:910–919. doi: 10.1039/c4fo00680a. [DOI] [PubMed] [Google Scholar]
- 7.Niero EL, Machado-Santelli GM. Cinnamic acid induces apoptotic cell death and cytoskeleton disruption in human melanoma cells. J Exp Clin Cancer Res. 2013;32:31. doi: 10.1186/1756-9966-32-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheinman PL. Allergic contact dermatitis to fragrance: A review. Am J Contact Dermat. 1996;7:65–76. [PubMed] [Google Scholar]
- 9.Aptula AO, Roberts DW. Mechanistic applicability domains for nonanimal-based prediction of toxicological end points: General principles and application to reactive toxicity. Chem Res Toxicol. 2006;19:1097–1105. doi: 10.1021/tx0601004. [DOI] [PubMed] [Google Scholar]
- 10.Chan K, O’Brien PJ. Structure–activity relationships for hepatocyte toxicity and electrophilic reactivity of alpha,beta-unsaturated esters, acrylates and methacrylates. J Appl Toxicol. 2008;28:1004–1015. doi: 10.1002/jat.1366. [DOI] [PubMed] [Google Scholar]
- 11.Gosavi SS, Gosavi SY, Alla RK. Local and systemic effects of unpolymerised monomers. Dent Res J. 2010;7:82–87. [PMC free article] [PubMed] [Google Scholar]
- 12.LoPachin RM, Gavin T. Molecular mechanisms of aldehyde toxicity: a chemical perspective. Chem Res Toxicol. 2014;27:1081–1091. doi: 10.1021/tx5001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson PA, Widen JC, Harki DA, Brummond KM. Covalent Modifiers: A chemical perspective on the reactivity of α,β-unsaturated carbonyls with thiols via hetero-Michael addition reactions. J Med Chem. 2017;60:839–885. doi: 10.1021/acs.jmedchem.6b00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 15.Talalay P, De Long MJ, Prochaska HJ. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc Natl Acad Sci USA. 1988;85:8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci USA. 2001;98:3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, Williams C, Risingsong R, Honda T, Gribble GW, Sporn MB, Talalay P. Extremely potent triterpenoid inducers of the phase 2 response: Correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci USA. 2005;102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Dinkova-Kostova AT, Talalay P. Coordinate regulation of enzyme markers for inflammation and for protection against oxidants and electrophiles. Proc Natl Acad Sci USA. 2008;105:15926–15931. doi: 10.1073/pnas.0808346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kensler TW. Chemoprevention by inducers of carcinogen detoxication enzymes. Environ Health Perspect. 1997;105:965–970. doi: 10.1289/ehp.97105s4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Long MJ, Prochaska HJ, Talalay P. Induction of NAD(P)H:quinone reductase in murine hepatoma cells by phenolic antioxidants, azo dyes, and other chemoprotectors: A model system for the study of anticarcinogens. Proc Natl Acad Sci USA. 1986;83:787–791. doi: 10.1073/pnas.83.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez JL, Jayaprakasha GK, Cadena A, Martinez E, Ahmad H, Patil BS. In vivo induction of phase II detoxifying enzymes, glutathione transferase and quinone reductase by citrus triterpenoids. BMC Complement Altern Med. 2010;10:51. doi: 10.1186/1472-6882-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salvemini D, Kim SF, Mollace V. Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: relevance and clinical implications. Am J Physiol Regul Integr Comp Physiol. 2013;304:R473–487. doi: 10.1152/ajpregu.00355.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal A, Bolisetty S. Adaptive responses to tissue injury: Role of heme oxygenase-1. Trans Am Clin Climatol Assoc. 2013;124:111–122. [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet. 2000;66:187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabunia K, Ellison SP, Singh H, Datta P, Kelemen SE, Rizzo V, Autieri MV. Interleukin-19 (IL-19) induces heme oxygenase-1 (HO1) expression and decreases reactive oxygen species in human vascular smooth muscle cells. J Biol Chem. 2012;287:2477–2484. doi: 10.1074/jbc.M111.312470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13:3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 27.Nicholas C, Batra S, Vargo MA, Voss OH, Gavrilin MA, Wewers MD, Guttridge DC, Grotewold E, Doseff AI. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kappaB through the suppression of p65 phosphorylation. J Immunol. 2007;179:7121–7127. doi: 10.4049/jimmunol.179.10.7121. [DOI] [PubMed] [Google Scholar]
- 28.Ryu EY, Park SY, Kim SG, Park DJ, Kang JS, Kim YH, Seetharaman R, Choi YW, Lee SJ. Anti-inflammatory effect of heme oxygenase-1 toward Porphyromonas gingivalis lipopolysaccharide in macrophages exposed to gomisins A, G, and J. J Med Food. 2011;14:1519–1526. doi: 10.1089/jmf.2011.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motterlini R, Foresti R. Heme oxygenase-1 as a target for drug discovery. Antioxid Redox Signal. 2014;20:1810–1826. doi: 10.1089/ars.2013.5658. [DOI] [PubMed] [Google Scholar]
- 30.Murakami Y, Kawata A, Fujisawa S. Expression of cyclooxygenase-2, nitric oxide synthase 2 and heme oxygenase-1 mRNA induced by bis-eugenol in RAW264.7 cells and their antioxidant activity determined using the induction period method. In Vivo. 2017;31:819–831. doi: 10.21873/invivo.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujisawa S, Murakami Y. Eugenol and its role in chronic diseases. Adv Exp Med Biol. 2016;929:45–66. doi: 10.1007/978-3-319-41342-6_3. [DOI] [PubMed] [Google Scholar]
- 32.Ishiyama M, Miyazono Y, Sasamoto K, Ohkura Y, Ueno K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta. 1997;44:1299–1305. doi: 10.1016/s0039-9140(97)00017-9. [DOI] [PubMed] [Google Scholar]
- 33.Murakami Y, Kawata A, Seki Y, Koh T, Yuhara K, Maruyama T, Machino M, Ito S, Kadoma Y, Fujisawa S. Comparative inhibitory effects of magnolol, honokiol, eugenol and bis-eugenol on cyclooxygenase-2 expression and nuclear factor-kappa B activation in RAW264.7 macrophage-like cells stimulated with fimbriae of Porphyromonas gingivalis. In Vivo. 2012;26:941–950. [PubMed] [Google Scholar]
- 34.Frederick CB, Reynolds CH. Modeling the reactivity of acrylic acid and acrylate anion with biological nucleophiles. Toxicol Lett. 1989;47:241–247. doi: 10.1016/0378-4274(89)90142-2. [DOI] [PubMed] [Google Scholar]
- 35.Atsumi T, Fujisawa S, Tonosaki K. (Meth)acrylate monomer-induced cytotoxicity and intracellular Ca(2+) mobilization in human salivary gland carcinoma cells and human gingival fibroblast cells related to monomer hydrophobicity. Biomaterials. 2006;27:5794–5800. doi: 10.1016/j.biomaterials.2006.07.041. [DOI] [PubMed] [Google Scholar]
- 36.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujisawa S, Kadoma Y. Prediction of the reduced glutathione (GSH) reactivity of dental methacrylate monomers using NMR spectra – Relationship between toxicity and GSH reactivity. Dent Mater J. 2009;28:722–729. doi: 10.4012/dmj.28.722. [DOI] [PubMed] [Google Scholar]
- 38.Fujisawa S, Kadoma Y. Mechanisms of action of (meth)acrylates in hemolytic activity, in vivo toxicity and dipalmitoylphosphatidylcholine (DPPC) liposomes determined using NMR spectroscopy. Int J Mol Sci. 2012;13:758–773. doi: 10.3390/ijms13010758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swales NJ, Caldwell J. Studies on trans-cinnamaldehyde II: Mechanisms of cytotoxicity in rat isolated hepatocytes. Toxicol In Vitro. 1996;10:37–42. doi: 10.1016/0887-2333(95)00105-0. [DOI] [PubMed] [Google Scholar]
- 40.Guimarães MR, de Aquino SG, Coimbra LS, Spolidorio LC, Kirkwood KL, Rossa C Jr. Curcumin modulates the immune response associated with LPS-induced periodontal disease in rats. Innate Immun. 2012;18:155–163. doi: 10.1177/1753425910392935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 42.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Isaac-Renton M, Li MK, Parsons LM. Cinnamon spice and everything not nice: many features of intraoral allergy to cinnamic aldehyde. Dermatitis. 2015;26:116–121. doi: 10.1097/DER.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 44.Janzowski C, Glaab V, Mueller C, Straesser U, Kamp HG, Eisenbrand G. Alpha, beta-unsaturated carbonyl compounds: induction of oxidative DNA damage in mammalian cells. Mutagenesis. 2003;18:465–470. doi: 10.1093/mutage/geg018. [DOI] [PubMed] [Google Scholar]
- 45.Pérez-Garrido A, Helguera AM, Rodríguez FG, Cordeiro MN. QSAR models to predict mutagenicity of acrylates, methacrylates and alpha,beta-unsaturated carbonyl compounds. Dent Mater. 2010;26:397–415. doi: 10.1016/j.dental.2009.11.158. [DOI] [PubMed] [Google Scholar]
- 46.Mohammad MK, Avila D, Zhang J, Barve S, Arteel G, McClain C, Joshi-Barve S. Acrolein cytotoxicity in hepatocytes involves endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress. Toxicol Appl Pharmacol. 2012;265:73–82. doi: 10.1016/j.taap.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohta T, Watanabe K, Moriya M, Shirasu Y, Kada T. Antimutagenic effects of cinnamaldehyde on chemical mutagenesis in Escherichia coli. Mutat Res. 1983;107:219–227. doi: 10.1016/0027-5107(83)90164-1. [DOI] [PubMed] [Google Scholar]
- 48.Tracey KJ, Cerami A. Tumor necrosis factor: A pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 49.Lee DH, Kim NR, Lim BS, Lee YK, Yang HC. Effects of TEGDMA and HEMA on the expression of COX2 and iNOS in cultured murine macrophage cells. Dent Mater. 2009;25:240–246. doi: 10.1016/j.dental.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 50.Ishihara M, Fujisawa S. A structure–activity relationship study on the mechanisms of methacrylate-induced toxicity using NMR chemical shift of beta-carbon, RP-HPLC log P and semiempirical molecular descriptor. Dent Mater J. 2009;28:113–120. doi: 10.4012/dmj.28.113. [DOI] [PubMed] [Google Scholar]
- 51.Fujisawa S, Kadoma Y. Anti-and pro-oxidant effects of oxidized quercetin, curcumin or curcumin-related compounds with thiols or ascorbate as measured by the induction period method. In Vivo. 2006;20:39–44. [PubMed] [Google Scholar]
- 52.Ishihara M, Fujisawa S. Quantum-chemical descriptors for estimating hemolytic activity of aliphatic and aromatic methacrylates. Chemosphere. 2008;70:1898–1902. doi: 10.1016/j.chemosphere.2007.07.070. [DOI] [PubMed] [Google Scholar]
- 53.Enoch SJ, Madden JC, Cronin MT. Identification of mechanisms of toxic action for skin sensitisation using a SMARTS pattern based approach. SAR QSAR Environ Res. 2008;19:555–578. doi: 10.1080/10629360802348985. [DOI] [PubMed] [Google Scholar]
- 54.LoPachin RM, Gavin T, Geohagen BC, Zhang L, Casper D, Lekhraj R, Barber DS. β-Dicarbonyl enolates: A new class of neuroprotectants. J Neurochem. 2011;116:132–143. doi: 10.1111/j.1471-4159.2010.07091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LoPachin RM, Barber DS, Gavin T. Molecular mechanisms of the conjugated alpha,beta-unsaturated carbonyl derivatives: relevance to neurotoxicity and neurodegenerative diseases. Toxicol Sci. 2008;104:235–249. doi: 10.1093/toxsci/kfm301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Terry CM, Clikeman JA, Hoidal JR, Callahan KS. Effect of tumor necrosis factor-alpha and interleukin-1 alpha on heme oxygenase-1 expression in human endothelial cells. Am J Physiol. 1998;274:H883–891. doi: 10.1152/ajpheart.1998.274.3.H883. [DOI] [PubMed] [Google Scholar]
- 57.Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest. 2008;118:239–247. doi: 10.1172/JCI32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 59.Srisook K, Cha YN. Super-induction of HO1 in macrophages stimulated with lipopolysaccharide by prior depletion of glutathione decreases iNOS expression and NO production. Nitric Oxide. 2005;12:70–79. doi: 10.1016/j.niox.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Mulliner D, Wondrousch D, Schüürmann G. Predicting Michael-acceptor reactivity and toxicity through quantum chemical transition-state calculations. Org Biomol Chem. 2011;9:8400–8012. doi: 10.1039/c1ob06065a. [DOI] [PubMed] [Google Scholar]
- 61.Winner EJ, Prough RA, Brennan MD. Human NAD(P)H:quinone oxidoreductase induction in human hepatoma cells after exposure to industrial acrylates, phenolics, and metals. Drug Metab Dispos. 1997;25:175–181. [PubMed] [Google Scholar]
- 62.Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 63.Ndisang JF. Synergistic interaction between heme oxygenase (HO) and nuclear-factor E2-related factor-2 (NRF2) against oxidative stress in cardiovascular related diseases. Curr Pharm Des. 2017;23:1465–1470. doi: 10.2174/1381612823666170113153818. [DOI] [PubMed] [Google Scholar]
- 64.Kim NY, Ahn SG, Kim SA. Cinnamaldehyde protects human dental pulp cells against oxidative stress through the NRF2/HO1-dependent antioxidant response. Eur J Pharmacol. 2017;815:73–79. doi: 10.1016/j.ejphar.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 65.Yang D, Liang XC, Shi Y, Sun Q, Liu D, Liu W, Zhang H. Anti-oxidative and anti-inflammatory effects of cinnamaldehyde on protecting high glucose-induced damage in cultured dorsal root ganglion neurons of rats. Chin J Integr Med. 2016;22:19–27. doi: 10.1007/s11655-015-2103-8. [DOI] [PubMed] [Google Scholar]
- 66.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked. Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim ME, Na JY, Lee JS. Anti-inflammatory effects of trans-cinnamaldehyde on lipopolysaccharide-stimulated macrophage activation via MAPKs pathway regulation. Immunopharmacol Immunotoxicol. 2018;22:1–6. doi: 10.1080/08923973.2018.1424902. [DOI] [PubMed] [Google Scholar]
- 68.Youn HS, Lee JK, Choi YJ, Saitoh SI, Miyake K, Hwang DH, Lee JY. Cinnamaldehyde suppresses toll-like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochem Pharmacol. 2008;75:494–502. doi: 10.1016/j.bcp.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 69.Schmalz G, Krifka S, Schweikl H. Toll-like receptors, LPS, and dental monomers. Adv Dent Res. 2011;23:302–306. doi: 10.1177/0022034511405391. [DOI] [PubMed] [Google Scholar]
- 70.Yun KJ, Koh DJ, Kim SH, Park SJ, Ryu JH, Kim DG, Lee JY, Lee KT. Anti-inflammatory effects of sinapic acid through the suppression of inducible nitric oxide synthase, cyclooxygase-2, and proinflammatory cytokines expressions via nuclear factor-kappaB inactivation. J Agric Food Chem. 2008;56:10265–10272. doi: 10.1021/jf802095g. [DOI] [PubMed] [Google Scholar]
- 71.Chao LK, Hua KF, Hsu HY, Cheng SS, Lin IF, Chen CJ, Chen ST, Chang ST. Cinnamaldehyde inhibits pro-inflammatory cytokines secretion from monocytes/macrophages through suppression of intracellular signaling. Food Chem Toxicol. 2008;46:220–231. doi: 10.1016/j.fct.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 72.Arshad L, Jantan I, Bukhari SN, Haque MA. Immunosuppressive Effects of Natural α,β-unsaturated carbonyl-based compounds, and their analogs and derivatives, on immune cells: A review. Front Pharmacol. 2017;8:22. doi: 10.3389/fphar.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cabello CM, Bair WB 3rd, Lamore SD, Ley S, Bause AS, Azimian S, Wondrak GT. The cinnamon-derived Michael acceptor cinnamic aldehyde impairs melanoma cell proliferation, invasiveness, and tumor growth. Free Radic Biol Med. 2009;46:220–231. doi: 10.1016/j.freeradbiomed.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Subash-Babu P, Alshatwi AA, Ignacimuthu S. Beneficial antioxidative and antiperoxidative effect of cinnamaldehyde protect streptozotocin-induced pancreatic β-cell damage in Wistar rats. Biomol Ther. 2014;22:47–54. doi: 10.4062/biomolther.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]